Abstract
Simple Summary
The incidence of liver cancer is increasing worldwide. When detected early, the most common form of primary liver cancer (hepatocellular carcinoma, or HCC) can be treated with surgery and organ transplantation (when feasible). However, in most cases, HCC is detected at advanced stages, and the survival benefit of current treatments (e.g., systemic therapy with kinase inhibitors) is very limited. The advent of immune checkpoint inhibitors (ICIs) has changed the treatment paradigm for multiple types of cancer, including HCC. The success of ICIs, especially in combination with anti-angiogenic drugs, has extended survival times for a subset of patients with HCC and has stimulated further preclinical and clinical development of immunotherapies, not just ICIs, but also T cell therapy and oncolytic immunotherapy. Because the immunosuppressive tumor microenvironment in HCC often allows cancer cells to escape destruction by the immune system and develop resistance to immunotherapy, combinations with other agents that could sensitize HCC to immunotherapy are actively pursued.
Abstract
Liver cancer is a life-threatening disease, and its incidence is increasing globally. The most common form of liver cancer is hepatocellular carcinoma (HCC). Approximately half of patients with HCC, especially those at advanced disease stages, receive systemic therapies, including the tyrosine kinase inhibitors sorafenib and lenvatinib. Over the past few years, immune checkpoint inhibitors (ICIs) have changed the landscape of HCC treatment. In particular, the combination therapy with atezolizumab (an anti-PD-L1 antibody) and bevacizumab (an anti-VEGF antibody) significantly improved survival benefits compared with sorafenib as a single agent, a finding that has stimulated further preclinical and clinical development of immunotherapeutic approaches for treating HCC. In addition to ICIs, oncolytic immunotherapy and adoptive T cell therapy have also emerged as immunotherapeutic strategies. A major challenge is that the tumor microenvironment of HCC is usually immunosuppressive, leading to immune escape and immunotherapy resistance. Hence, combination therapies that could sensitize HCC to immunotherapy have become a growing area of investigation. In this review, we summarize recent advances in HCC immuno-oncology and review immunotherapeutic strategies that are under development for treating HCC.
Keywords: hepatocellular carcinoma, immunotherapy, immune checkpoint inhibitors, CAR T cells, resistance, combination immunotherapy
1. Introduction
The liver, which is the largest internal organ in humans, performs many crucial functions. Liver cancer, estimated to reach more than one million cases per year by 2025, is one of the most common cancers worldwide and is among the fastest-growing cancer types in Western countries [1]. According to the American Cancer Society (www.cancer.org; accessed in July 2022), the 5-year survival rate for patients with liver cancer in the United States is approximately 20%. When the disease is diagnosed at an early stage, the 5-year survival rate is higher (~35%); for patients with metastatic liver cancer, the 5-year survival rate is less than 5%. The subtypes of primary liver cancer include hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (iCCA), fibrolamellar carcinoma, and hepatoblastoma [2]. HCC accounts for ~90% of all liver cancer cases [1]. Chronic viral hepatitis (types B and C), alcohol consumption, and aflatoxin exposure are the main risk factors for liver cancer, whereas low-dose aspirin usage is associated with decreased risk of HCC [3,4].
Hepatic resection and liver transplantation are potentially curative for early-stage HCC [3]. Unfortunately, many patients with liver cancer are not eligible for hepatic resection, and the number of liver donors is quite small. For intermediate-stage HCC, transarterial chemoembolization (TACE) is a standard of care [1]. For advanced-stage HCC, the US Food and Drug Administration (FDA) approved the use of the tyrosine kinase inhibitor (TKI) sorafenib in 2007, and since then, systemic therapies have become available [5]. Over the past 5 years, three additional TKIs—lenvatinib, regorafenib, and cabozantinib—have received worldwide approval [6]. As first-line systemic treatment options for unresectable HCC, lenvatinib and sorafenib showed comparable survival benefits [7]. When compared with placebo, regorafenib and cabozantinib could extend overall survival as second-line treatment for HCC [8,9]. Over the past decade, the advent of immunotherapy has changed the way that cancers are treated. For patients with advanced-stage HCC, treatment with the immune checkpoint inhibitor atezolizumab (an anti-PD-L1 antibody) plus the angiogenesis inhibitor bevacizumab (an anti-VEGFA antibody) yielded better overall and progression-free survival outcomes than sorafenib as a single agent [10,11], representing a breakthrough in the management of HCC. In this review, we summarize the molecular mechanisms of HCC immune responses and discuss recent developments in HCC immunotherapy.
2. The Immune Microenvironment of HCC
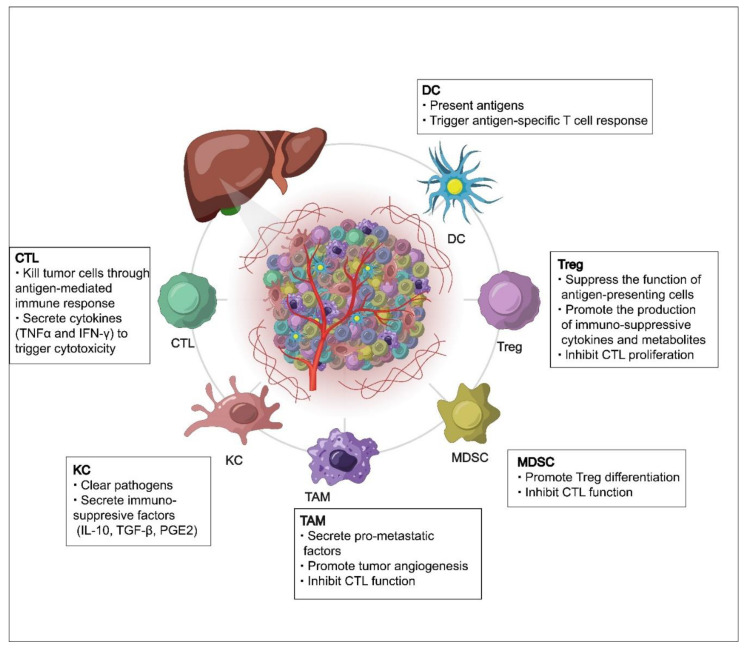
The tumor immune microenvironment plays pivotal roles in regulating tumor progression and therapy resistance [12]. In HCC, the immune microenvironment is dominated by immunosuppressive cells and signals that promote immune evasion and metastasis [13,14]. The main immune-suppressive cells in HCC are Kupffer cells, M2-type tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) [13,14] (Figure 1). Kupffer cells are liver-resident macrophages that are responsible for the phagocytic clearance of pathogens [15,16]; on the other hand, Kupffer cells also induce T cell tolerance by secreting immunosuppressive factors such as interleukin (IL)-10, transforming growth factor (TGF)-β, and prostaglandin E2 [17,18]. During the progression of HCC, hepatic macrophages convert from an M1 phenotype to an M2 phenotype characteristic of cancer-promoting TAMs, which function as immune suppressor cells to support tumor growth, metastasis, and resistance to targeted therapy and immunotherapy [19,20]. Mechanistically, Kupffer cells and M2-polarized TAMs induce immune escape in HCC through the expression of PD-L1, the downregulation of MHC-II, the secretion of immunosuppressive cytokines, and the recruitment of Tregs and CD4+ cells [13,14,21]. Tregs, which are characterized by the expression of the transcription factor forkhead box p3 (Foxp3), promote immune tolerance in HCC by targeting effector T cells or by modulating antigen-presenting cells (APCs). It has been shown that Tregs can target APCs through the expression of negative regulatory cell surface receptors, such as cytotoxic T lymphocyte antigen 4 (CTLA-4), CD39, and CD73, or directly kill APCs by producing perforin and granzyme B [22]. In addition, Tregs can alter the immune microenvironment and suppress the immune response through the secretion of anti-inflammatory factors and the inhibition of IL-2 production by effector T cells [13,14,23]. MDSCs are usually present in cancer or other pathological conditions, but not in healthy individuals, although they are morphologically and phenotypically similar to neutrophils and monocytes. MDSCs can be classified into two groups: monocytic and polymorphonuclear. The monocytic MDSCs inhibit T cell responses through the production of anti-inflammatory cytokines and nitric oxide, whereas the polymorphonuclear MDSCs suppress T cells by generating peroxynitrite, which nitrates T cell receptors to reduce their responsiveness to antigen-MHC complexes [24,25,26].
Figure 1.
A schematic overview of the tumor immune microenvironment of hepatocellular carcinoma (HCC). Liver cancer cells and immune cells interact dynamically through cell–cell contact and the secretion and recognition of soluble factors, such as cytokines. Among various immune cells in the tumor microenvironment of HCC, regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), M2-type tumor-associated macrophages (TAMs), and Kupffer cells (KCs) suppress anti-tumor immunity, whereas cytotoxic T lymphocytes (CTLs) and dendritic cells (DCs) promote immune tumor rejection.
In HCC, CD8+ cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, and dendritic cells are the major cell types for immunosurveillance [27] (Figure 1). CTLs, also known as effector T (Teff) cells, kill cancer cells through granule exocytosis and Fas ligand-mediated apoptosis. CTLs also secrete interferon-γ (IFN-γ) and tumor necrosis factor α (TNFα) to trigger cytotoxicity in cancer cells. The activated T cells then start to express immune co-inhibitory receptors, such as programmed death-1 receptor (PD-1), which dampens the effector function of CTLs [28]. Similarly, NK cells exert their anti-cancer effects by secreting perforin and granulein to induce tumor cell apoptosis or by releasing pro-inflammatory cytokines and chemokines [29,30,31]. Dendritic cells are antigen-presenting cells that activate CTLs by presenting antigens to T cells and secreting immune co-stimulatory factors. Hence, dendritic cell vaccination against HCC may represent a promising immunotherapeutic strategy [27,32,33,34]. In addition, it has been suggested that M1-polarized macrophages may play a role in cancer immunosurveillance [35], but evidence supporting this role in HCC is still lacking.
3. HCC Immunotherapy
3.1. Immune Checkpoint Inhibitors
The immune checkpoint is an immune regulation mechanism by which immune co-inhibitory receptor signaling prevents strong immune responses from destroying healthy cells. Cancer cells exploit immune checkpoints to escape immunosurveillance. Several inhibitory immunoreceptors, including but not limited to PD-1, CTLA-4, T cell immunoglobulin, and mucin domain containing-3 (TIM3), lymphocyte-activation gene 3 (LAG3), and T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT), have been identified and characterized in cancer [36,37,38]. Immune checkpoint inhibitors (ICIs) target inhibitory immunoreceptor signaling to reprogram the tumor immune microenvironment from pro-cancer to anti-cancer, and many ICIs have been translated into therapies for cancer patients [36,37,38].
Several clinical trials have been carried out to test ICIs as single agents to treat HCC (selected mono-immunotherapy trials are listed in Table 1), some of which led to FDA approvals. Nivolumab and pembrolizumab were approved in 2017 and 2018, respectively, to treat patients with HCC who had been previously treated with sorafenib (i.e., for second-line treatment). Nivolumab is a fully human immunoglobulin G4 monoclonal antibody targeting PD-1. In a phase I/II trial (CheckMate 040), the objective response rate to nivolumab was 20% in patients with HCC [39]. However, a randomized multi-center phase III trial (CheckMate 459) indicated that first-line treatment with nivolumab did not significantly improve the overall survival of patients with advanced HCC when compared with sorafenib [40]. Pembrolizumab is a humanized antibody targeting PD-1. A phase II clinical trial (KEYNOTE-224) testing pembrolizumab as a second-line therapy demonstrated an objective response in 18 of 104 (17%) HCC patients who had previously been treated with sorafenib [41]. Subsequently, a phase III trial (KEYNOTE-240) showed that pembrolizumab improved progression-free survival and overall survival of Asian patients with advanced HCC who had previously been treated with sorafenib [42], supporting further evaluation of pembrolizumab as a second-line agent for HCC treatment. Moreover, camrelizumab (SHR-1210), another humanized anti-PD-1 antibody, showed manageable toxicity and an objective response rate of 14.7% (32 of 217) as a second-line agent in treating Chinese patients with advanced HCC, according to a multi-center randomized phase II trial [43]. In addition, tremelimumab is a humanized monoclonal antibody targeting CTLA-4 and is one of the earliest ICIs tested in clinical trials for treating HCC patients. Although a phase II trial demonstrated the safety of tremelimumab in HCC patients with chronic hepatitis C virus (HCV) [44], phase III trial data on tremelimumab for treating HCC patients with HCV are not yet available.
Table 1.
Selected clinical trials of single-agent immunotherapy for HCC.
| Drug | Trial ID | Phase | N | Population | mOS | ORR | Reference | |
|---|---|---|---|---|---|---|---|---|
| ICI | Nivolumab | NCT01658878 | I/II | 214 | Advanced HCC | 13.8 | 20% | [39] |
| NCT02576509 | III | 371 | Advanced HCC | 16.4 | 15.4% | [40] | ||
| Pembrolizumab | NCT02702414 | II | 104 | Advanced HCC | 12.9 | 17.3% | [41] | |
| Camrelizumab | NCT02989922 | II | 217 | Advanced HCC | N/A | 14.7% | [43] | |
| Tremelimumab | NCT02519348 | I/II | 69 | Unresectable HCC | 15.1 | 7.2% | [45] | |
| Oncolytic virus | Pexa-Vec | NCT00554372 | II | 30 | Unresectable HCC | 6.7 (low dose), 14.1 (high dose), | 15% | [46] |
| Vaccine | Ilixadencel | NCT01974661 | I | 11 | Advanced HCC | 2.7 (first-line), 10.9 (second-line) | N/A | [47] |
6ORR, objective response rate; mOS, median overall survival (months); N/A, not available.
3.2. Chimeric Antigen Receptor (CAR) T Cells
CAR T-cell therapy involves engineering a cancer patient’s own T cells to recognize and eliminate tumor cells [48,49]. To date, the FDA has approved CAR T cells targeting CD19 or B-cell maturation antigen for treating blood cancers, including acute lymphoblastic leukemia, non-Hodgkin lymphoma, multiple myeloma, and relapsed or refractory large B cell lymphoma [50,51,52]. Although CAR T-cell therapy has not yet been approved by the FDA for the treatment of HCC and other solid cancers, CAR T cells targeting different tumor-associated antigens have been developed, and some have been tested in clinical trials. For example, glypican-3 (GPC3) is overexpressed in HCC, but shows little or no expression in normal tissues, making it an excellent HCC antigen for CAR T-cell therapy [53]. GPC3 CAR T cells were found to eliminate GPC3-positive HCC cells in mouse models [54], and a phase I clinical trial demonstrated its safety, as well as some early signs of anti-tumor activity of GPC3 CAR T cells in patients with advanced HCC [55]. CAR T cells have also been engineered to target other HCC-associated antigens, such as α-fetoprotein (AFP) [56], CD147 [57], CD133 [58], HBV surface protein [59], NKG2D [60], and c-MET [61]. These CAR T cells have yet to be tested in clinical studies. In addition, further identification of novel HCC-specific antigens may help to improve the safety and efficacy of CAR T-based immunotherapy.
3.3. Oncolytic Immunotherapies
Oncolytic immunotherapy involves the use of oncolytic viruses that are naturally occurring or genetically altered to kill tumor cells by selectively replicating in cancer cells, but not in normal cells [62]. Like CAR T-cell-based HCC therapy, oncolytic immunotherapy for treating HCC is still in the early development stages. In 2013, the Kim laboratory reported a phase I/II dose-finding clinical trial for oncolytic immunotherapy in liver cancer [46]. In this early trial, the vaccinia virus (Wyeth vaccine strain) known as JX-594, or Pexa-Vec, showed safety, oncolytic activity, and a dose-related survival benefit in advanced HCC. However, in a subsequent phase IIb trial, Pexa-Vec did not improve overall survival as a second-line therapy for patients with advanced HCC who had previously been treated with sorafenib [63].
3.4. HCC Vaccines
Therapeutic cancer vaccines are dendritic cell-based immunotherapies that achieve vaccination through the administration of tumor-specific antigens in combination with dendritic cell-activating adjuvants, or the administration of dendritic cells loaded with specific tumor antigens [64]. Hence, cancer vaccines activate the patient’s adaptive immune system to eliminate cancer cells. Among the various types of antigens, cancer researchers have turned their attention to tumor “neoantigens,” which arise from non-synonymous somatic mutations in the protein-coding regions, frameshift mutations, endogenous retroviruses, or tumor-specific post-translational modifications, such as phosphorylation and glycosylation [64]. Because of their tumor specificity and immunogenic characteristics, neoantigens have become attractive targets for developing cancer vaccines. Both neoantigen peptides and dendritic cells loaded with neoantigen have been developed to treat HCC. For instance, in a recent clinical study, treating HCC patients with personalized neoantigen peptides led to improved postoperative recurrence-free survival [65]. In a phase I trial that enrolled 17 HCC patients, intratumoral injections of the immune primer ilixadencel (pro-inflammatory allogeneic dendritic cells) were safe and elicited tumor-specific immunologic responses [47]. In a phase I/II trial that included 22 patients diagnosed with early- to immediate-stage HCC, intradermal administrations of HepaVac-101, a combination of peptide antigens and an RNA-based immunostimulator, showed safety and immunogenicity [66]. Further evaluations of clinical outcomes are warranted.
3.5. Immunotherapy Resistance in HCC
Despite promising clinical trials and recent FDA approvals of several immunotherapies, only a subset of HCC patients responded to this type of treatment [1,14]. Immunotherapy resistance in HCC can be attributed to the immune tolerance of the liver, as well as tumor-induced immune evasion [67]. As mentioned above, liver-resident macrophages, also known as Kupffer cells, are phagocytic cells that clear pathogens, and these cells also secrete immunosuppressive factors to induce tolerance to T cells [17,18]. In liver cancer, both tumor-intrinsic signaling, as well as crosstalk between cancer cells and the tumor microenvironment, contribute to immune suppression and immunotherapy resistance. For instance, HCC-intrinsic CDK20, also known as cell cycle-related kinase (CCRK), has been reported to induce immune escape through an NF-κB−EZH2−IL-6 axis [68]. HCC-secreted IL-6 recruits polymorphonuclear MDSCs, resulting in the suppression of T cell activity. In a preclinical model of liver cancer, inhibition of CCRK signaling led to increased sensitivity to anti-PD-L1 immunotherapy [68]. For HCC patients treated with ICIs, the activation of β-catenin signaling correlated with lower disease control rates, shorter progression-free survival, and shorter overall survival [69]. By using a transposon-mediated oncogene-induced HCC model, the Lujambio laboratory found that the activation of β-catenin signaling in HCC blocked CCL5 secretion, resulting in reduced dendritic cell recruitment, decreased T cell activity, and resistance to anti-PD-1 treatment [70]. In addition, the activation of PKCα−ZFP64−CSF1 signaling in HCC has been found to promote anti-PD-1 resistance via M2 macrophage polarization induced by CSF1 [71]. The rapid expansion of preclinical and clinical research on immune therapies for treating HCC is expected to reveal more insights into the mechanisms of immunotherapy resistance in HCC.
4. Combinatorial Therapeutic Strategies for HCC Treatment
As mentioned above, single-agent immunotherapies have shown promising results in treating HCC, but response rates remain low. To overcome HCC immunotherapy resistance, it is beneficial to identify targets that can transform the HCC tumor microenvironment from immunologically “cold” to “hot,” thereby enhancing responsiveness to immunotherapy. In fact, a growing body of evidence has demonstrated the benefits of combinatorial therapeutic strategies for treating HCC (Figure 2 and Table 2).
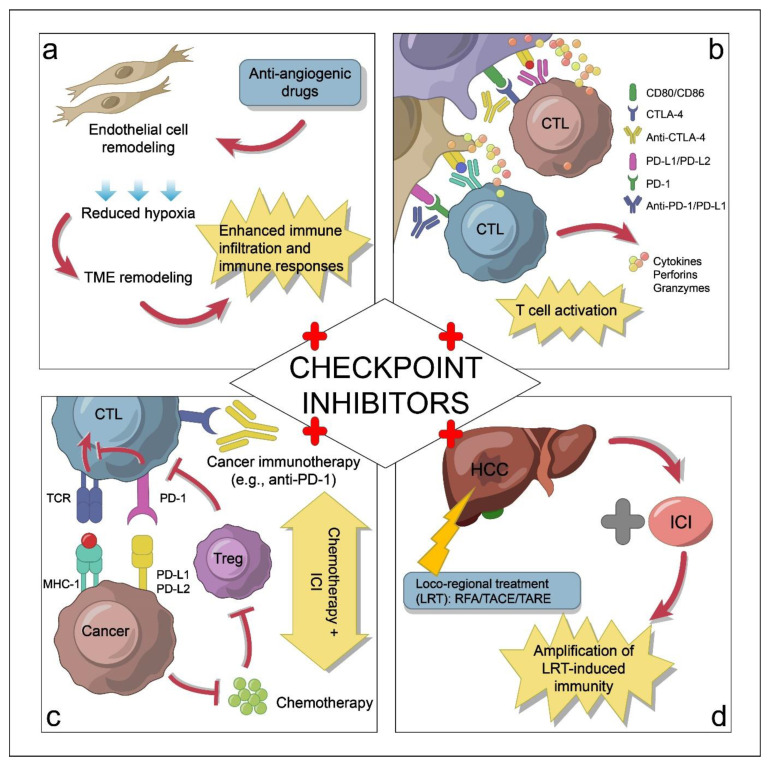
Figure 2.
Schematic illustrations of how immune checkpoint inhibitors (ICIs) interact with other forms of treatment as combinatorial therapeutic strategies for hepatocellular carcinoma (HCC) treatment. (a). Combining ICIs with anti-angiogenic drugs (e.g., anti-VEGF) blocks HCC angiogenesis and boosts anti-tumor immune responses. (b). Dual ICIs that target non-redundant pathways of T cell inactivation (e.g., PD-1 and CTLA-4 pathways) outperform single-agent ICI therapy. (c). Adding chemotherapeutic drugs to ICIs promotes immunogenic cell death by activating dendritic cells, increasing T cell cross-priming, and suppressing myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), which collectively boosts anti-tumor immunity. (d). Combining ICIs with neoadjuvant or adjuvant therapies can upregulate HCC antigens (e.g., glypican 3 and AFP), thereby enhancing immunotherapy efficacy. Abbreviations in this figure: CTL, cytotoxic T lymphocyte; MHC-1, major histocompatibility complex class I; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; TCR, T cell receptor; TME, tumor microenvironment.
Table 2.
Selected clinical trials of combinations of ICIs and other therapies for HCC.
| Drug | Trial ID | Phase | N | Population | mOS | ORR | Reference |
|---|---|---|---|---|---|---|---|
| Nivolumab + ipilimumab | NCT01658878 | I/II | 49 (arm A) | Advanced HCC | 22.8 (arm A) | 32% (arm A) | [72] |
| Atezolizumab + bevacizumab | NCT03434379 | III | 336 | Unresectable HCC | 19.2 | 30% | [10,11] |
| Camrelizumab + FOLFOX4 | NCT03605706 | III | 396 | Advanced HCC | N/A | N/A | N/A |
| Pembrolizumab + lenvatinib | NCT03006926 | Ib | 104 | Unresectable HCC | 22.0 | 36% | [75] |
| Pembrolizumab + lenvatinib | NCT03713593 | III | 794 | Advanced HCC | N/A | N/A | N/A |
| Nivolumab + TACE | NCT04268888 | II/III | Recruiting | Intermediate stage HCC | N/A | N/A | N/A |
| Durvalumab + bevacizumab + TACE | NCT03778957 | III | 724 | Locoregional HCC | N/A | N/A | N/A |
ORR, objective response rate; mOS, median overall survival (months); N/A, not available.
Liver tumors usually contain multiple immunosuppressive factors, and blocking one factor is unlikely to substantially improve HCC treatment. Therefore, the simultaneous blockade of non-redundant pathways of immune suppression is expected to have better efficacy than the blockading of a single immune checkpoint. Indeed, in the CheckMate 040 trial (NCT01658878), the concomitant inhibition of the PD-1 and CTLA-4 pathways by nivolumab plus ipilimumab (an anti-CTLA-4 antibody) showed manageable safety and an objective response rate of 32% in patients with advanced HCC previously treated with sorafenib [72]. This combination therapy was approved by the FDA in 2020 [73]. In another study of HCC patients with disease progression on prior single-agent ICI treatment, dual ICI therapy with ipilimumab plus either nivolumab or pembrolizumab was found to achieve durable anti-tumor response and encouraging survival outcomes [74].
Because HCC is a highly vascularized cancer, targeting angiogenesis has become a promising therapeutic strategy. Currently, many clinical trials are testing angiogenesis inhibitors for HCC treatment, and a few of them have been approved by the FDA [76]. Vascular endothelial growth factor (VEGF) is not only a pro-angiogenic protein, but also an immune-suppressive factor [77]. It has been shown that VEGF suppresses immune responses by modulating cytotoxic T cells, dendritic cells, Tregs, and MDSCs [78,79,80]. Thus, one plausible approach is to combine immunotherapy with anti-angiogenic drugs to block HCC angiogenesis and boost anti-tumor immune responses. In patients with unresectable HCC, atezolizumab (anti-PD-L1) plus bevacizumab (anti-VEGF) was the first systemic therapy demonstrating an overall survival benefit surpassing that of sorafenib, based on a global open-label phase III trial, IMbrave150 (NCT03434379) [10,11,81]. This combination therapy was approved by the FDA to treat unresectable or metastatic HCC in 2020 [82]. A subsequent study identified potential biomarkers for clinical responses to this combination therapy, including high CD274, low GPC3 and AFP, high intratumor CD8+ T cell density, and low Treg/Teff ratio [83].
Therapies that combine ICIs with chemotherapy, radiotherapy, targeted therapy, or TACE are also under active clinical investigation. For instance, camrelizumab (anti-PD-1) combined with a standard FOLFOX4 (infusional fluorouracil, leucovorin, and oxaliplatin) regimen was used as a first-line treatment for advanced HCC in a multi-center phase Ib/II clinical trial, and this combination showed promising anti-tumor responses with good safety and tolerability [84]. That study led to a phase III trial (NCT03605706), now underway, comparing camrelizumab plus the FOLFOX4 regimen with the placebo plus the FOLFOX4 regimen for treating advanced HCC. In a phase Ib study (NCT03006926) that enrolled patients with unresectable HCC, the combination of lenvatinib (a multi-kinase inhibitor) with pembrolizumab showed promising anti-tumor activity with a manageable safety profile [75], and a phase III trial is now underway to compare this combination therapy with lenvatinib alone as a first-line treatment for advanced HCC (NCT03713593). In addition, several clinical trials are now ongoing to test combinations of immunotherapy with TACE for HCC treatment (e.g., NCT03778957, NCT04268888, and NCT04246177).
5. Conclusions
Immunotherapy has opened a new era in HCC treatment, and many clinical trials are underway to test ICIs and combination therapies in liver cancer. Compared with other systemic therapies, such as chemotherapy and targeted therapy, immunotherapy has unique advantages in managing advanced HCC. In particular, immune therapy has the potential to achieve systemic and durable anti-cancer responses via immunological memory, and this is clinically beneficial against HCC, which often exhibits multicentric and metachronous occurrences. Currently, the best available first-line therapy for advanced-stage HCC is the combination of the PD-L1 blockade with atezolizumab and the VEGF blockade with bevacizumab.
A major challenge for HCC immunotherapy is the unique immunosuppressive microenvironment of HCC, in addition to the immune tolerance of the liver itself. Despite recent breakthroughs in clinical trials and FDA approvals, only a small subset of patients with HCC respond to immunotherapy. Therefore, it is important to better understand the mechanisms of immune regulation in HCC and to identify biomarkers that predict immunotherapy response and guide the clinical use of ICIs and their combinations for HCC treatment. Compared with ICIs, CAR T-cell-based immunotherapies, oncolytic immunotherapies, and HCC vaccines are still in the early development stages. Future research should not only focus on improving the efficacy and safety of these immunotherapies, but also continue to identify HCC-specific antigens. Moreover, efforts should be made to identify new immune checkpoints and to develop novel immunotherapeutic agents, such as bispecific antibodies, antibody-drug conjugates, and agonist antibodies targeting immune co-stimulatory receptors. Ultimately, therapies that combine immunotherapies with other treatments are anticipated to improve clinical outcomes in advanced HCC.
Acknowledgments
We are grateful to Christine Wogan for critical reading and editing, and to members of the Yao Lab and the Ma Lab for discussions.
Author Contributions
W.S. and Y.C. wrote the draft and created the figures and tables; P.L., Y.S., F.Y. and L.M. provided intellectual input; M.S., Y.S., F.Y. and L.M. edited the manuscript. In response to reviewers’ comments and editors’ suggestions, L.M. revised the manuscript, with input from all other authors. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
F.Y. is supported by the National Natural Science Foundation of China (grant number: 31900935) and the Hubei Provincial Natural Science Foundation (grant number: 2022CFA108). L.M. is supported by a US National Institutes of Health (NIH) grant R01CA166051 and a Cancer Prevention and Research Institute of Texas (CPRIT) grant RP190029. Y.S. is supported by MD Anderson’s Cancer Center Support Grant P30CA016672 from the NIH.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Sia D., Villanueva A., Friedman S.L., Llovet J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Simon T.G., Duberg A.S., Aleman S., Chung R.T., Chan A.T., Ludvigsson J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020;382:1018–1028. doi: 10.1056/NEJMoa1912035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Sangro B., Sarobe P., Hervas-Stubbs S., Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., Cicin I., Merle P., Chen Y., Park J.W., et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 11.Cheng A.L., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Lim H.Y., Kudo M., Breder V., Merle P., et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Greten F.R., Grivennikov S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringelhan M., Pfister D., O’Connor T., Pikarsky E., Heikenwalder M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 14.Llovet J.M., Castet F., Heikenwalder M., Maini M.K., Mazzaferro V., Pinato D.J., Pikarsky E., Zhu A.X., Finn R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 15.Helmy K.Y., Katschke K.J., Jr., Gorgani N.N., Kljavin N.M., Elliott J.M., Diehl L., Scales S.J., Ghilardi N., van Lookeren Campagne M. CRIg: A macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Knolle P.A., Gerken G. Local control of the immune response in the liver. Immunol. Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 17.Krenkel O., Tacke F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 18.Breous E., Somanathan S., Vandenberghe L.H., Wilson J.M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z., Wu T., Zheng B., Chen L. Individualized precision treatment: Targeting TAM in HCC. Cancer Lett. 2019;458:86–91. doi: 10.1016/j.canlet.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Degroote H., van Dierendonck A., Geerts A., van Vlierberghe H., Devisscher L. Preclinical and Clinical Therapeutic Strategies Affecting Tumor-Associated Macrophages in Hepatocellular Carcinoma. J. Immunol. Res. 2018;2018:7819520. doi: 10.1155/2018/7819520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heymann F., Peusquens J., Ludwig-Portugall I., Kohlhepp M., Ergen C., Niemietz P., Martin C., van Rooijen N., Ochando J.C., Randolph G.J., et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. 2015;62:279–291. doi: 10.1002/hep.27793. [DOI] [PubMed] [Google Scholar]
- 22.Raffin C., Vo L.T., Bluestone J.A. Treg cell-based therapies: Challenges and perspectives. Nat. Rev. Immunol. 2020;20:158–172. doi: 10.1038/s41577-019-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T. Regulatory T cells: How do they suppress immune responses? Int. Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 24.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabrilovich D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Zhang T., Sun M., Ji X., Xie M., Huang W., Xia L. Therapeutic Values of Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma: Facts and Hopes. Cancers. 2021;13:5127. doi: 10.3390/cancers13205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao X., Sun G., Zhang Y., Kong X., Rong D., Song J., Tang W., Wang X. Targeting Immune Cells in the Tumor Microenvironment of HCC: New Opportunities and Challenges. Front. Cell Dev. Biol. 2021;9:775462. doi: 10.3389/fcell.2021.775462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farhood B., Najafi M., Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 29.Garnelo M., Tan A., Her Z., Yeong J., Lim C.J., Chen J., Lim K.H., Weber A., Chow P., Chung A., et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66:342–351. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillerey C., Huntington N.D., Smyth M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 31.Habif G., Crinier A., Andre P., Vivier E., Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol. Immunol. 2019;16:415–422. doi: 10.1038/s41423-019-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streba L.A., Streba C.T., Sandulescu L., Vere C.C., Mitrut P., Cotoi B.V., Popescu L.N., Ion D.A. Dendritic cells and hepatocellular carcinoma. Rom. J. Morphol. Embryol. 2014;55:1287–1293. [PubMed] [Google Scholar]
- 33.Teng C.F., Wang T., Wu T.H., Lin J.H., Shih F.Y., Shyu W.C., Jeng L.B. Combination therapy with dendritic cell vaccine and programmed death ligand 1 immune checkpoint inhibitor for hepatocellular carcinoma in an orthotopic mouse model. Ther. Adv. Med. Oncol. 2020;12:1758835920922034. doi: 10.1177/1758835920922034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cazzetta V., Franzese S., Carenza C., Della Bella S., Mikulak J., Mavilio D. Natural Killer-Dendritic Cell Interactions in Liver Cancer: Implications for Immunotherapy. Cancers. 2021;13:2184. doi: 10.3390/cancers13092184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prenen H., Mazzone M. Tumor-associated macrophages: A short compendium. Cell Mol. Life Sci. 2019;76:1447–1458. doi: 10.1007/s00018-018-2997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He X., Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma P., Siddiqui B.A., Anandhan S., Yadav S.S., Subudhi S.K., Gao J., Goswami S., Allison J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021;11:838–857. doi: 10.1158/2159-8290.CD-20-1680. [DOI] [PubMed] [Google Scholar]
- 39.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.Y., Choo S.P., Trojan J., Welling T.H.R., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yau T., Park J.W., Finn R.S., Cheng A.L., Mathurin P., Edeline J., Kudo M., Harding J.J., Merle P., Rosmorduc O., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhu A.X., Finn R.S., Edeline J., Cattan S., Ogasawara S., Palmer D., Verslype C., Zagonel V., Fartoux L., Vogel A., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 42.Kudo M., Lim H.Y., Cheng A.L., Chao Y., Yau T., Ogasawara S., Kurosaki M., Morimoto N., Ohkawa K., Yamashita T., et al. Pembrolizumab as Second-Line Therapy for Advanced Hepatocellular Carcinoma: A Subgroup Analysis of Asian Patients in the Phase 3 KEYNOTE-240 Trial. Liver Cancer. 2021;10:275–284. doi: 10.1159/000515553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin S., Ren Z., Meng Z., Chen Z., Chai X., Xiong J., Bai Y., Yang L., Zhu H., Fang W., et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 44.Sangro B., Gomez-Martin C., de la Mata M., Inarrairaegui M., Garralda E., Barrera P., Riezu-Boj J.I., Larrea E., Alfaro C., Sarobe P., et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Kelley R.K., Sangro B., Harris W., Ikeda M., Okusaka T., Kang Y.K., Qin S., Tai D.W., Lim H.Y., Yau T., et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heo J., Reid T., Ruo L., Breitbach C.J., Rose S., Bloomston M., Cho M., Lim H.Y., Chung H.C., Kim C.W., et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizell M., Sternby Eilard M., Andersson M., Andersson B., Karlsson-Parra A., Suenaert P. Phase 1 Trial With the Cell-Based Immune Primer Ilixadencel, Alone, and Combined With Sorafenib, in Advanced Hepatocellular Carcinoma. Front. Oncol. 2019;9:19. doi: 10.3389/fonc.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kingwell K. CAR T therapies drive into new terrain. Nat. Rev. Drug Discov. 2017;16:301–304. doi: 10.1038/nrd.2017.84. [DOI] [PubMed] [Google Scholar]
- 49.Wolf P., Alzubi J., Gratzke C., Cathomen T. The potential of CAR T cell therapy for prostate cancer. Nat. Rev. Urol. 2021;18:556–571. doi: 10.1038/s41585-021-00488-8. [DOI] [PubMed] [Google Scholar]
- 50.Mullard A. FDA approves first CAR T therapy. Nat. Rev. Drug Discov. 2017;16:669. doi: 10.1038/nrd.2017.196. [DOI] [PubMed] [Google Scholar]
- 51.Braendstrup P., Levine B.L., Ruella M. The long road to the first FDA-approved gene therapy: Chimeric antigen receptor T cells targeting CD19. Cytotherapy. 2020;22:57–69. doi: 10.1016/j.jcyt.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson L.D., Jr. Idecabtagene vicleucel (ide-cel) CAR T-cell therapy for relapsed and refractory multiple myeloma. Future Oncol. 2022;18:277–289. doi: 10.2217/fon-2021-1090. [DOI] [PubMed] [Google Scholar]
- 53.Zheng X., Liu X., Lei Y., Wang G., Liu M. Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma. Front. Oncol. 2022;12:824208. doi: 10.3389/fonc.2022.824208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao H., Li K., Tu H., Pan X., Jiang H., Shi B., Kong J., Wang H., Yang S., Gu J., et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin. Cancer Res. 2014;20:6418–6428. doi: 10.1158/1078-0432.CCR-14-1170. [DOI] [PubMed] [Google Scholar]
- 55.Shi D., Shi Y., Kaseb A.O., Qi X., Zhang Y., Chi J., Lu Q., Gao H., Jiang H., Wang H., et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin. Cancer Res. 2020;26:3979–3989. doi: 10.1158/1078-0432.CCR-19-3259. [DOI] [PubMed] [Google Scholar]
- 56.Liu H., Xu Y., Xiang J., Long L., Green S., Yang Z., Zimdahl B., Lu J., Cheng N., Horan L.H., et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin. Cancer Res. 2017;23:478–488. doi: 10.1158/1078-0432.CCR-16-1203. [DOI] [PubMed] [Google Scholar]
- 57.Tseng H.C., Xiong W., Badeti S., Yang Y., Ma M., Liu T., Ramos C.A., Dotti G., Fritzky L., Jiang J.G., et al. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat. Commun. 2020;11:4810. doi: 10.1038/s41467-020-18444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai H., Tong C., Shi D., Chen M., Guo Y., Chen D., Han X., Wang H., Wang Y., Shen P. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: A single-arm, open-label, phase II trial. Oncoimmunology. 2020;9:1846926. doi: 10.1080/2162402X.2020.1846926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou F., Tan J., Liu T., Liu B., Tang Y., Zhang H., Li J. The CD39(+) HBV surface protein-targeted CAR-T and personalized tumor-reactive CD8(+) T cells exhibit potent anti-HCC activity. Mol. Ther. 2021;29:1794–1807. doi: 10.1016/j.ymthe.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun B., Yang D., Dai H., Liu X., Jia R., Cui X., Li W., Cai C., Xu J., Zhao X. Eradication of Hepatocellular Carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol. Res. 2019;7:1813–1823. doi: 10.1158/2326-6066.CIR-19-0026. [DOI] [PubMed] [Google Scholar]
- 61.Huang X., Guo J., Li T., Jia L., Tang X., Zhu J., Tang Q., Feng Z. c-Met-targeted chimeric antigen receptor T cells inhibit hepatocellular carcinoma cells in vitro and in vivo. J. Biomed. Res. 2021;36:10–21. doi: 10.7555/JBR.35.20200207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kessler T., Wick W. Oncolytic virotherapy: Potentially a game-changing tumor treatment. Cancer Cell. 2021;39:753–755. doi: 10.1016/j.ccell.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 63.Moehler M., Heo J., Lee H.C., Tak W.Y., Chao Y., Paik S.W., Yim H.J., Byun K.S., Baron A., Ungerechts G., et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: A randomized multicenter Phase IIb trial (TRAVERSE) Oncoimmunology. 2019;8:1615817. doi: 10.1080/2162402X.2019.1615817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saxena M., van der Burg S.H., Melief C.J.M., Bhardwaj N. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 65.Cai Z., Su X., Qiu L., Li Z., Li X., Dong X., Wei F., Zhou Y., Luo L., Chen G., et al. Personalized neoantigen vaccine prevents postoperative recurrence in hepatocellular carcinoma patients with vascular invasion. Mol. Cancer. 2021;20:164. doi: 10.1186/s12943-021-01467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loffler M.W., Gori S., Izzo F., Mayer-Mokler A., Ascierto P.A., Konigsrainer A., Ma Y.T., Sangro B., Francque S., Vonghia L., et al. Phase I/II Multicenter Trial of a Novel Therapeutic Cancer Vaccine, HepaVac-101, for Hepatocellular Carcinoma. Clin. Cancer Res. 2022;28:2555–2566. doi: 10.1158/1078-0432.CCR-21-4424. [DOI] [PubMed] [Google Scholar]
- 67.Nakano S., Eso Y., Okada H., Takai A., Takahashi K., Seno H. Recent Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers. 2020;12:775. doi: 10.3390/cancers12040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou J., Liu M., Sun H., Feng Y., Xu L., Chan A.W.H., Tong J.H., Wong J., Chong C.C.N., Lai P.B.S., et al. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. 2018;67:931–944. doi: 10.1136/gutjnl-2017-314032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harding J.J., Nandakumar S., Armenia J., Khalil D.N., Albano M., Ly M., Shia J., Hechtman J.F., Kundra R., El Dika I., et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019;25:2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruiz de Galarreta M., Bresnahan E., Molina-Sanchez P., Lindblad K.E., Maier B., Sia D., Puigvehi M., Miguela V., Casanova-Acebes M., Dhainaut M., et al. beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei C.Y., Zhu M.X., Zhang P.F., Huang X.Y., Wan J.K., Yao X.Z., Hu Z.T., Chai X.Q., Peng R., Yang X., et al. PKCalpha/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J. Hepatol. 2022;77:163–176. doi: 10.1016/j.jhep.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Yau T., Kang Y.K., Kim T.Y., El-Khoueiry A.B., Santoro A., Sangro B., Melero I., Kudo M., Hou M.M., Matilla A., et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saung M.T., Pelosof L., Casak S., Donoghue M., Lemery S., Yuan M., Rodriguez L., Schotland P., Chuk M., Davis G., et al. FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncologist. 2021;26:797–806. doi: 10.1002/onco.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong J.S.L., Kwok G.G.W., Tang V., Li B.C.W., Leung R., Chiu J., Ma K.W., She W.H., Tsang J., Lo C.M., et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J. Immunother. Cancer. 2021;9:e001945. doi: 10.1136/jitc-2020-001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Finn R.S., Ikeda M., Zhu A.X., Sung M.W., Baron A.D., Kudo M., Okusaka T., Kobayashi M., Kumada H., Kaneko S., et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morse M.A., Sun W., Kim R., He A.R., Abada P.B., Mynderse M., Finn R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. 2019;25:912–920. doi: 10.1158/1078-0432.CCR-18-1254. [DOI] [PubMed] [Google Scholar]
- 77.Yang J., Yan J., Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voron T., Colussi O., Marcheteau E., Pernot S., Nizard M., Pointet A.L., Latreche S., Bergaya S., Benhamouda N., Tanchot C., et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alfaro C., Suarez N., Gonzalez A., Solano S., Erro L., Dubrot J., Palazon A., Hervas-Stubbs S., Gurpide A., Lopez-Picazo J.M., et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br. J. Cancer. 2009;100:1111–1119. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y., Huang H., Coleman M., Ziemys A., Gopal P., Kazmi S.M., Brekken R.A. VEGFR2 activity on myeloid cells mediates immune suppression in the tumor microenvironment. JCI Insight. 2021;6:e150735. doi: 10.1172/jci.insight.150735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salem R., Li D., Sommer N., Hernandez S., Verret W., Ding B., Lencioni R. Characterization of response to atezolizumab + bevacizumab versus sorafenib for hepatocellular carcinoma: Results from the IMbrave150 trial. Cancer Med. 2021;10:5437–5447. doi: 10.1002/cam4.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casak S.J., Donoghue M., Fashoyin-Aje L., Jiang X., Rodriguez L., Shen Y.L., Xu Y., Jiang X., Liu J., Zhao H., et al. FDA Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable or Metastatic Hepatocellular Carcinoma. Clin. Cancer Res. 2021;27:1836–1841. doi: 10.1158/1078-0432.CCR-20-3407. [DOI] [PubMed] [Google Scholar]
- 83.Zhu A.X., Abbas A.R., de Galarreta M.R., Guan Y., Lu S., Koeppen H., Zhang W., Hsu C.H., He A.R., Ryoo B.Y., et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022;28:1599–1611. doi: 10.1038/s41591-022-01868-2. [DOI] [PubMed] [Google Scholar]
- 84.Li H., Qin S., Liu Y., Chen Z., Ren Z., Xiong J., Meng Z., Zhang X., Wang L., Zhang X., et al. Camrelizumab Combined with FOLFOX4 Regimen as First-Line Therapy for Advanced Hepatocellular Carcinomas: A Sub-Cohort of a Multicenter Phase Ib/II Study. Drug Des. Devel. Ther. 2021;15:1873–1882. doi: 10.2147/DDDT.S304857. [DOI] [PMC free article] [PubMed] [Google Scholar]