Abstract
Simple Summary
Though gastrointestinal stromal tumor is the most common mesenchymal neoplasm of the gastrointestinal tract, it is a rare entity among pediatric patients. It is usually characterized by a different molecular biology, histology and clinical course. Therefore, different handling of pediatric GIST is needed. Herein, we review the latest updates to the management of pediatric gastrointestinal tumors with a particular focus on the advances in molecular biology of the disease and emerging treatment with kinase inhibitors that could serve as targeted therapy.
Abstract
Gastrointestinal stromal tumor is the most common mesenchymal neoplasm of the gastrointestinal tract, usually found in elderly adults. It is infrequent among pediatric patients and usually differs biologically from adult-type diseases presenting mutations of KIT and PDGFR genes. In this population, more frequent is the wild-type GIST possessing SDH, TRK, RAS, NF1 mutations, among others. Both tumor types require individualized treatment with kinase inhibitors that are still being tested in the pediatric population due to the different neoplasm biology. We review the latest updates to the management of pediatric gastrointestinal tumors with a particular focus on the advances in molecular biology of the disease that enables the definition of possible resistance. Emerging treatment with kinase inhibitors that could serve as targeted therapy is discussed, especially with multikinase inhibitors of higher generation, the effectiveness of which has already been confirmed in the adult population.
Keywords: gastrointestinal stromal tumor, pediatric GIST, kinase inhibitors, molecular diagnostics
1. Introduction
Gastrointestinal stromal tumor (GIST) arises from Cajal cells present in the gastrointestinal tract. It is the most common mesenchymal tumor arising in this localization; however, it is an extremely rare entity in the pediatric population. Only up to 2% of GISTs are diagnosed in children and adolescents [1]. Usually, pediatric GIST are present as sporadic tumors and do not harbor c-KIT or PDGFRA (platelet-derived growth factor receptor α) mutations [2]. They may be associated with abnormalities of the SDH gene encoding gene (including SDHB) or the IGF-1R gene encoding insulin-like growth factor 1 receptor [3]. Histopathologically, the tumor tends to have epithelioid or mixed histology, which is different from classical spindle cell adult histology [3,4]. The tumor is often localized in the stomach, yet it may be present in any gastrointestinal localization or outside it: cases of the peritoneal, abdominal wall or pulmonary GISTs are reported in the literature, among others. Clinically, it usually manifests with anemia, bleedings or occult stool blood and its course may be indolent, yet it also metastasizes more often than in adults [3]. However, the rate has not been compared objectively and adequately, and this remark is based on case series with a long follow-up of the patients.
The basic management of pediatric GIST involves a slightly modified imaging diagnostic approach as the use of computed tomography is discouraged, favoring the use of MRI, USG and PET, therefore lowering the child’s exposure to ionizing radiation, as well as ensuring adequate diagnostics [5,6]. An incisional biopsy should be undertaken to validate the primary diagnosis. Then, R0 surgical procedure without lymph node removal is performed [7] with curate intention. It is vital to perform genetic testing on the specimen to get to know its biology and possible mutations to personalize further treatment if needed. It also allows for the classification of GIST: some children have an adult-type tumor that possesses KIT or PDGFRA mutations, which may allow for the use of imatinib (especially exon 9 or 11 KIT mutations that are sensitive to imatinib treatment) [8]. Pediatric-type GIST may be treated with different agents compatible with the exact genetic alterations [9,10].
2. Molecular Diagnostics of Pediatric GIST and Its Selected Consequences
There is a great need to state the molecular type of pediatric GIST, detecting genetic abnormalities, which is possible with DNA and RNA sequencing and immunohistochemistry methods [11,12,13,14]. As the single-molecule molecular inversion probe (smMIP) technology allows easy adaptation of panels, detection of the clue mutations in GIST can proceed with smMIP-based next-generation sequencing (NGS) panels [15]. However, whole exome sequencing (WES) and transcriptome analysis on formalin-fixed paraffin-embedded tissue allows the more complex analysis of the biology of GIST [16] and to apply targeted therapy when it comes both to the substance and its dose. The whole laboratory process should be strongly combined with the appropriate genetic counseling of patients and their families before embarking on a surveillance program.
2.1. NTRK Genetics in Wild-Type Pediatric GIST
Around 85% of pediatric GISTs are wild-type (WT-GIST), which makes up for 10% of all diagnosed tumors of this type. They are often characterized by mutations or silencing of four genes encoding the subunits of the SDH enzyme complex [7]. Moreover, the analyses show the frequent presence of NTRK/TRK (neurotrophic tyrosine kinase receptor) mutations (NRTK1, NTRK2, NTRK3), including fusions (e.g., ETV6::NTRK3, TPM3::NTRK1, TPR::NTRK1, LMNA::NTRK1, SPECC1L::NTRK3), in cases of WT-GISTs (5–25%) [17,18]. They can lead to different manifestations but also determine a specific treatment method. In parallel, they are characterized by the lack of canonical KIT, PDGFRA, SDHx or RAS pathway components (KRAS, BRAF, NF1) alterations, which eliminates the possibility of the use of a range of multikinase inhibitors [13]. Therefore, it is necessary to individualize treatment and apply other types of kinase inhibitors.
Thanks to the good antitumor efficacy of NTRK inhibitors, both in adults and children, there is a great need for individualized prognosis of pediatric GIST with genetic studies, including NTRK genes fusion status study, e.g., using hybrid capture DNA-based targeted panels (UCSF500 Cancer Gene Panel and OncoPanel) that include probes for exons and select introns of NTRK1, NTRK2, and NTRK3, as well as ETV6 exonic and intronic probe [17,19,20]. The World Sarcoma Network recommends NRTK testing in locally advanced and unresectable or metastatic sarcoma, with very high priority, classifying WT-GIST as sarcoma with low frequency of NTRK fusions. In the case of positive Pan-TRK immunochemistry, massive parallel sequencing is recommended to consider TRK inhibitors. Otherwise, additional NTRK testing is unnecessary. However, immunohistochemistry is not advised when myogenic and neural differentiation is present in histological diagnosis due to the high rate of false positivity [21].
2.2. FGFR Genetics in Wild-Type Pediatric GISTs
Some GISTs are connected with FGFR (fibroblast growth factor receptor) genetic abnormalities, including oncogenic fusion FGFR1::HOOK3, FGFR1::TACC1, activating FGFR1 missense mutations p.K656E and p.N546K, FGF4 overexpression; FGF2 overexpression or gain in FGFR2 in imatinib-resistant GIST cells [13,22,23,24,25]. Therefore, overcoming imatinib resistance with FGF pathways inhibitors, e.g., via the molecular mechanism of sensitization to DNA damaging agents, for example, DNA-topoisomerase II inhibitors can be a novel pathway in studies. It is possible thanks to the attenuation of DNA double-strand break repair after the application of a selective FGFR inhibitor. Therefore, doxorubicin treatment with the standard chemotherapeutic agents (i.e., doxorubicin) increases apoptosis [26,27].
2.3. RAS/NF1-Related Genetics in Wild-Type Pediatric GISTs
Searching for mutations in GIST remains necessary not only to predict a tumor’s malignancy but also to find molecular treatment targets, e.g., BRAF mutations to use BRAF inhibitors (e.g., dabrafenib) or NF1 mutations to use MEK inhibitors.
It is worth remembering that due to the NF1 (neurofibromin 1) participation in RAS-mediated pathways and possible RAS-related mutations in GISTs, NF1-mutant GIST SDHB IHC retained (+) is one of the WT-GIST. It is mainly located in a small intestine, however small, and multicentric, with low mitotic activity and a good prognosis. Therefore, it is worth monitoring children with RASopathies to detect the possible occurrence of GIST soonest because of the poor prognosis of metastatic disease and to treat them more efficiently with RAS-related inhibitors due to the potential resistance to imatinib and sunitinib. Likewise, BRAF-mutant and RAS-mutant SDBH IHC retained (+) GIST can also be detected independently of age (like NF1-mutants) but characterized more often with spindle cell morphology. The clinical course of this type of WT-GIST varies among the patients [28]. BRAF mutations in GISTs don’t present prognostic value themselves but remain a possible target for therapeutic options to control disease, especially in the case of an advanced stage [29].
2.4. SDH Genetics in Wild-Type Pediatric GISTs
Considering the familial GIST type (hereditary GIST) associated with SDH mutations, the interview and active screening remain beneficial to be able to suspect Carney–Stratakis syndrome (CSS). Mutations in the mitochondrial tumor suppressor gene pathway involve the succinate dehydrogenase subunits SDHA (most frequently), SDHD, SDHC (c.24delC in the exon 1 of SDHC and resulting in the frameshift variant p.His8GlnfsTer39, with a mutant allele burden >90%) and SDHB (c.287-1G > C in intron 3 with a mutant allele burden >95%) [30,31].
However, more characteristic of pediatric GISTs is the type with SDHB IHC loss, usually located in the stomach. Their traits are multinodular/plexiform growth, joint lymphatic invasion and lymph node metastases, and unpredictable behavior, including indolent clinical course (also in a metastatic state) [30,31,32].
SDH-deficient GISTs can be associated with Carney’s triad syndrome, a non-heritable syndrome related to GIST, pulmonary chondroma, and paraganglioma, mainly seen in girls and young women. The potential time from GIST diagnosis to the appearance of other components visible on CT may be more than 8 years, but possibly even 3 decades. Therefore, pediatric patients should receive regular clinical examinations to detect possible extra-adrenal paraganglioma and pulmonary chondroma rapidly. During the molecular diagnostic process of pediatric GIST, associations with hereditary paraganglioma-pheochromocytoma syndromes are worth searching [33,34].
It has been shown in some tumors that SDH complex is inactivated by hypoxia-inducible factor (HIF), which is understandable given its role in aerobic respiration. HIF is not degraded and is stabilized due to earlier succinate accumulation. Inactivated SDH thus promotes increased angiogenesis via the pseudo-hypoxic patchway. VEGF (vascular endothelial growth factor), GLUT1 (glucose transporter 1) and M-CSF (macrophage colony-stimulating factor) are also described to be upregulated. These all features serve as potential targets for the subsequent targeted therapy [35,36].
2.5. New Biomarkers of High-Risk GIST
HAND1 presents higher expression in metastatic or high-risk KIT-mutant GIST and supports KIT expression. Moreover, HAND1(+) tumor cells express proliferative markers. HAND1, expressed solely in small intestine interstitial cells of Cajal (ICCs), is a marker of aggressive behavior of GIST in the gastrointestinal tract out of the small intestine in contrast to S100A associated strictly with small intestine origin. BARX1 expression is restricted to gastric ICCs and is positive in the PDGFRA-mutated and WT-GIST groups, which also show more indolent clinical presentation and, usually, no recurrence. Thus, HAND1 and BARX1 expression in tumor cells might determine prognosis and the need for adjuvant imatinib therapy because of shorter progression-free survival in such GIST [37].
3. Perspectives in Pharmacological Management of Pediatric GIST with Use of Multikinase Inhibitors
3.1. Imatinib
Imatinib is still the most widely used agent in first-line therapy for either KIT/PDGFR or wild-type pediatric GIST. Imatinib is often administered after R1 surgery as adjuvant treatment (in the high-risk group) and before surgical treatment as neoadjuvant treatment with an additional adjuvant dose in the high-risk group. It is also useful in the management and prolonging the overall survival of patients with metastatic disease. Despite all that, researchers seek other favorable options for pediatric patients. The rarity of this type of tumor makes it difficult to construct a proper clinical trial, which is additionally ethically concerning in pediatric patients [2,38,39,40,41]. However, one of the NCT01738139 trial’s goals is to check the best dose of ipilimumab [42,43,44] and imatinib mesylate in treating patients with GIST over 15 years old.
3.2. Sunitinib
In the case of imatinib-resistant tumors, the second choice is usually sunitinib, a multikinase inhibitor of higher potency, especially in KIT(wt) GIST. The starting and maximum tolerated dose in children is 15 mg/m2 (study ADVL0612; NCT00387920; A6181196; NCT01396148), yet pharmacodynamic simulations show 25 mg/m2 dose as more efficient, providing the drug’s plasma concentrations similar to those observed in the adult population with 50 mg/m2 dose. The ongoing trial in phase I/II (NCT01396148) shows the tolerable dose of sunitinib in first-line therapy to be a minimum of 20 mg/m2 every 4 weeks with a rest period of 2 weeks. This provides an acceptable safety profile as observed in adults on 50 mg/m2 dose and the same schedule, and 5.8 months median PFS (progression-free survival). According to European and US records, only 9 children were treated with sunitinib until 2017 [1]. They were administered sunitinib 50 mg/day on a 4/2 schedule. An alternative was a continuous treatment with 37.5 mg/day. In the NCT01396148 study, stabilization of the disease was observed in three patients (50%) by day 15, cycle 1, with no additional accumulation across cycles [45,46,47,48,49,50]. Moreover, Forsythe et al. postulated MCV elevations during treatment with sunitinib may be an apparent drug-related epiphenomenon and possible valuable marker for therapeutic drug monitoring and treatment adherence indicator for pediatric and adult patients with no adverse events, but they considered it as an indicator of therapeutic toxicity [51].
3.3. Regorafenib
Another accessible alternative for imatinib-resistant tumors is the administration of regorafenib, a multikinase inhibitor targeting VEGFR (vascular endothelial growth factor), TIE-2 (angiopoietin-1 receptor), FGFR, PDGFR, KIT, RET oncogene and RAF kinases among others. While it is well-known in adults, it was only administered in a limited number of pediatric GIST cases. Its use with a dose of 160 mg/day 3 weeks in a month is beneficial. Moreover, regorafenib has been observed to possess radio- and chemosensitizing outcomes, especially in PDGFR-mutated GIST. It can also be beneficial in KIT-mutated tumors. In preclinical GIST models carrying a mutated KIT oncogene, partial regressions were observed after regorafenib application. Moreover, in vivo regorafenib exhibits significant antitumor activity in various pediatric malignancies, independent of histological tumor type, through inhibition of angiogenesis [52,53,54,55,56,57].
3.4. Avapritinib
An emerging drug dedicated to patients with a mutation in exon 18 of the PDGFRA gene (such as D842V) is avapritinib, applied in the NCT03862885 trial in patients with locally advanced unresectable or metastatic GIST over 16 years old. One of the other main inclusion criteria is the foregoing reception of 3 or more TKI therapies, including imatinib. Some higher-generation tyrosine kinase inhibitors are less effective in adult GISTs, e.g., nilotinib [58], though it hasn’t been proven in pediatric GISTs.
3.5. Vandetanib and Guadecitabine
Vandetanib, a small molecule inhibitor of VEGFR2, EGFR (epidermal growth factor), and RET is one of the new potential drugs in children and adults with succinate dehydrogenase deficient (dSDH) GIST. However, neither partial nor complete responses nor meaningful changes in the tumor growth or density rate were observed (median number of cycles 4, range 2–18, median PFS of participants-5,1 months). Regardless, improved molecular characterization of dSDH GISTs (including mutations, promoter methylation status or global DNA methylation status (e.g., hypermethylation), will be important in designing future therapeutic trials to apply such type of inhibitor [59]. In this type of pediatric GISTs, loss of activity of SDH in the Krebs cycle and, therefore, accumulation of succinate inhibiting α-ketoglutarate-dependent dioxygenases leads to DNA hypermethylation. Therefore, one of the new potential chemotherapeutics, guadecitabine, which is a small molecule DNA methyltransferase inhibitor, can reverse DNA hypermethylation in tumors with Krebs cycle abnormalities. Phase II study (NCT03165721) showed good tolerance by the majority of patients with SDH-deficient GIST, however, no complete or partial responses were observed [32].
3.6. NRTK Inhibitors
When it comes to TRK-fusion GISTs, first studies prove the good antitumor efficacy, a high and durable clinical response rate and good toleration of treatment with larotrectinib and entrectinib, NTRK inhibitors, both in adults and children, including recurrent or refractory neoplasms (e.g., NCT02122913, NCT02637687 and NCT02576431 involved 3 cases of GIST) [60,61].
3.7. Sorafenib and Other Multikinase Inhibitors
Several targeted therapies also play the role of multikinase inhibitors [37], such as sorafenib [40,62,63,64], avapritinib [65,66,67], ponatinib [68], whose effectiveness is confirmed in adult GIST treatment; however, there has been any cohort evidence in the literature of clinical effectiveness in the pediatric population. Sorafenib has been used with excellent efficacy in 2 patients with BRAF-mutated wildtype GIST resistant to imatinib, sunitinib and regorafenib and has proved ineffective in BRAF V600E mutated GIST [69]. Based on a case report published by Brinch et at., it may be effective in patients with D842 PDGFRA exon 18 mutations that are usually insensitive to imatinib [64]. Relying on the experience of its use in other pediatric tumors, it may be used in pediatric GIST.
3.8. Ripretinib
Finally, the effect of the recent development of KIT/PDGFRA inhibitors is ripretinib (formerly DCC-2618), whose effectiveness in inhibition of a wide range of KIT mutants in patients with drug-resistant GISTs has been confirmed in preclinical cancer models and preliminary clinical data [70]. PFS as a primary outcome measure is currently verified in a phase 3 study (NCT03353753 trial) in advanced adult GISTs treated with prior anticancer therapies. Moreover, multicenter, global, randomized, open-label Phase III INTRIGUE (NCT03673501 trial) compares the efficacy of ripretinib in comparison with sunitinib in advanced adult GISTs after treatment with imatinib.
3.9. Treatment of SDH-Mutated and Carney-Stratakis Syndrome Associated GIST
Succinate dehydrogenase (SDH) deficiency is one of the possible genetic alterations in wild-type pediatric GIST that is associated with this entity the most frequently. Such type of tumor should discard the use of imatinib. However, the patients could benefit from using the aforementioned kinase inhibitors with anti-angiogenic activity, i.e., sunitinib, vandetanib, sorafenib or regorafenib. The existing evidence is limited and based only on individual cases of patients with SDH-deficient tumors enrolled in more extensive trials. Unfortunately, they indicate limited efficacy of sunitinib, regorafenib, nilotinib and a lack of efficacy of vandetanib in this type of tumor [59,71,72,73,74]. Ganjoo et al. report a patient that took pazopanib showed long-lasting disease control [75]. Morever, such patients rarely profit from the neoadjuvant therapy [76]. There have been two clinical trials adjusted for SDH-deficient GIST among other neoplasms, NCT02071862 involving glutaminase inhibitor CB-839 and NCT03165721 with guadecitabine (SGI-110), though both were cessated and no results have been published.
Due to the rarity of this neoplasm in children, the scarcity of available data and the ethical considerations of clinical trials in the pediatric population, the aforementioned reviewed data relies on a tumor-agnostic approach. Unfortunately, there has not been a large multicenter clinical trial or head-to-head trial that could assess the actual effectiveness of these agents in children.
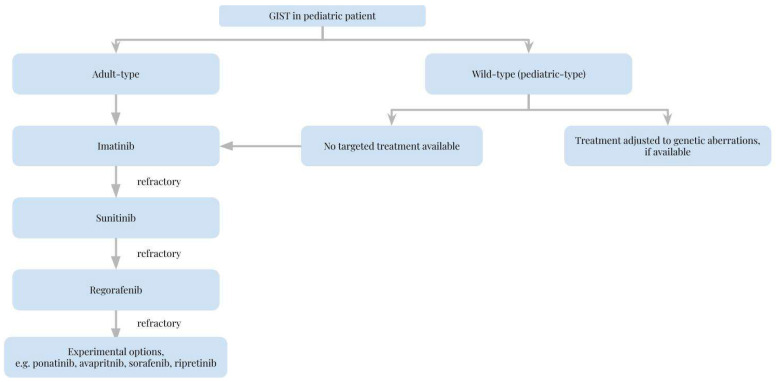
Table 1 lists the agents that are or might be used in pediatric GISTS and their molecular targets and provides short comments on their use. Figure 1 provides a suggested algorithm for the process of choosing a kinase inhibitor for the treatment of pediatric GIST.
Table 1.
Current application and perspectives in targeted therapies in pediatric GISTs treatment.
| Drug Substance | Molecular Target | Comments | Reference |
|---|---|---|---|
| Imatinib | PDGF, SCF, KIT, C-KIT, ABL (including BCR-ABL fusion) | First-line treatment after R1 surgery (including high-risk group); if needed- neoadjuvant treatment | [2,38,39,40,41] |
| Sunitinib | PDGFR-α, PDGFR-β, VEGFR1, VEGFR2, VEGFR3, KIT, FLT3, CSF-1R, RET | Usually the second-line treatment of imatinib-resistant GIST; leads to the tumor stabilization | [1,45,46,47,48,49,50] |
| Regorafenib | VEGF-1, VEGF-2, VEGF-3, VEGFR-1, VEGFR-2, VEGFR-3, TIE-2, KIT, RET, RAF-1, B-RAF, PDGFR(-β), FGFR-1, EGFR | Beneficial for KIT-mutated and PDGFR-mutated GIST, including radio- and chemosensitization in vitro; anti-angiogenic effect (limited data) | [52,53,54,55,56,57] |
| Nilotinib | BCR-ABL, KIT, LCK, EPHA3, EPHA8, DDR1, DDR2, PDGFRB, MAPK11, ZAK | Less effective in adult GIST than imatinib without evidence in the pediatric population | [58] |
| Vandetanib | VEGFR-2, VEGFR-3 (weak interaction), VEGF, EGFR(-3), EGF, RET | No meaningful changes observed after application so far | [59] |
| Larotrectinib | TRK A, B, C | High and durable clinical response rate, good toleration of treatment | [60,61] |
| Entrectinib | TRK A, B, C, ROS1, ALK | High and durable clinical response rate, well-tolerated | [60,61] |
| Sorafenib | RAF kinases (C-RAF > B-RAF), PDGFR kinases, PDGFRβ, VEGFR-1, VEGFR-2, VEGFR-3, FLT-3, C-KIT, RET, induction of autophagy | Effective in adult GIST treatment but ineffective in V600E BRAF mutation, no evidence of efficacy in pediatric GIST | [40,62,63,64,69] |
| Avapritinib | KIT, PDGFRA mutant (such as D816 V KIT and D842 V PDGFRA) | Effective in adult GIST treatment, no evidence of efficacy in pediatric GIST treatment, ongoing NCT03862885 trial with early access for avapritinib in patients >16 years old with locally advanced unresectable or metastatic GIST | [65,66,67] |
| Ponatinib | ABL (BCR-ABL mutants), VEGFR, PDGFR, EGFR, SRC kinase, KIT, RET, FLT3 | Effective in adult GIST treatment, no evidence of efficacy in pediatric GIST treatment yet | [68,77] |
| Ipilimumab | CTLA-4 | Good safety, limited data about the treatment efficacy in combination with other multikinase inhibitors, ongoing NCT01738139 trial in patients >15 years old | [42,43,44] |
| Ripretinib | KIT and PDGFRA kinases | Effective inhibition of a wide range of KIT mutants in patients with drug-resistant GISTs (preclinical models, phase III trial finished) | [70] |
Figure 1.
Choice of kinase inhibitors in the management of pediatric gastrointestinal stromal tumor.
4. Updates to Surgical Approach
4.1. Current Surgical Standards
Overall, the last years did not bring any major advances in the surgical approach to pediatric GISTs. Surgical resection remains the main treatment method that should be undertaken after the suspicion of GIST. However, it can be performed as wide local excision to total gastrectomy. Tumor size >5 cm, high mitotic index, and spindle morphology predict mortality. Pediatric GISTs have a more favorable prognosis in comparison to adult GISTs [4,78,79,80]. Frequent recurrence of GISTs is present despite complete resection and multiple surgeries.
Approximately 30% of the pediatric GISTs develop regional lymph node metastases, which differs from GISTs in adults (≤2% of GIST with lymph node metastases). Therefore, some parts of pediatric GIST of high-risk lymph node metastases may require dissection of lymph nodes. A precise definition of this population should be discussed as this is not a common standard now [31,81,82]. Other gastrointestinal tumors can be mistaken in some cases as GIST [82] or found accidentally, e.g., in the material from an appendectomy [83].
No guidelines concerning the surgical approach to differently mutated GIST have been developed to date [74]. The recommended option of pediatric GISTs surgical treatment is a limited operation with wedge resection, as obtaining negative gross margins is indicated. Though, the laparoscopic surgery of gastric GIST and pediatric WT-GIST remains possible [5,84,85]. Simple enucleations generate a high risk of recurrence. Matsumoto et al. proposed a laparoscopic-endoscopic cooperative surgery-related procedure for tumor resection transorally using the non-exposed endoscopic wall-inversion surgery in the following steps: marking around the tumor on the mucosal and serosal surfaces, performing a submucosal injection of sodium hyaluronate with indigo carmine dye and afterward, circumferential seromuscular dissection with suture closure under the laparoscopic vision and finally a circumferential muco-submucosal incision under gastric endoscopic vision. Tumor resection can proceed from every position in the stomach. However, closure of the esophagogastric junction or the pyloric ring may be difficult [86]. The amount of tissue required to make the GIST diagnosis is 1 cm3 (about 5–10 core-needle biopsy specimens) [5]. EUS (endoscopic ultrasound)-guided fine-needle with 95% ethanol injection (FNI) therapy of GISTs in adults is an effective treatment method [87,88], however, its application in pediatric GISTs has not been set down yet.
4.2. Biopsy of GIST
One of the possible pediatric GIST symptoms, especially in the stomach, is bleeding from a tumor mass because of the central vessel bulging into the lumen. It also makes up the negative predictive factor for pediatric GIST treatment as so-called tumor rupture that can be defined by tumor fracture or spillage, blood-stained ascites, microscopic infiltration of an adjacent organ, intralesional dissection or piecemeal resection or incisional biopsy [89,90,91,92,93]. Jakob et al. performed a systematic review to determine the risk of needle tract seeding and abdominal recurrence of GIST after a pretreatment biopsy. Even though GIST is considered to be a fragile, easily-rupturing tumor, it has been found that EUS-FNA-guided biopsy does not augment the risk of the aforementioned events [94].
4.3. Benefits from Extensive GIST Resection
However, due to the indolence of pediatric WT-GISTs and their biology, there is no improvement in event-free survival, even after extensive or serial resections and in the presence of disease progression or recurrence. Therefore, resections of WT-GISTs should be limited to the initial procedure unless symptoms, like obstruction or bleeding, are present [84].
4.4. Hyperthermic Intraperitoneal Chemotherapy in Pediatric GIST
As peritoneal metastases may be present in pediatric GISTs, one of the methods used to remove the visible macroscopic disease is HIPEC (hyperthermic intraperitoneal chemotherapy) with complete cytoreduction (CRS) that can be proceeded even in infant treatment [95]. There is still little data concerning the use of HIPEC not only in pediatrics, but also adult patients, especially in respect to GIST, though its use in peritoneal sarcomatosis is gaining more attention. It was also used in palliative GIST-related ascites treatment. Importantly, HIPEC procedure in pediatric patients was linked with a smaller mortality and mortality as in adult population [96,97,98]. However, owing to the safety of the procedure patient selection and technique modification is necessary, and all important considerations were summarized by Garnier et al. CRS preceding HIPEC makes up the key factor in survival [95]. It is unknown, however, which cytostatic agent should be used in HIPEC treatment of GIST—there are reports on the use of mitomycin C.
4.5. Possible Future Amendments
Future development in the surgical management of GIST may be characterized by the search for the application of fluorescence markers to improve surgical procedures. To date, indocyanine green has proved to be inefficient in this matter [99]. Another promise is the future widespread use of 3D planning and augmented reality to better plan and execute the surgery, especially in tumors of difficult localization, including vessel- and neuroinvasive tumor. Until now, there has been no report of such practice in pediatric gastrointestinal stromal tumor, though this has already been used in different types of sarcomas [100,101,102].
5. Conclusions
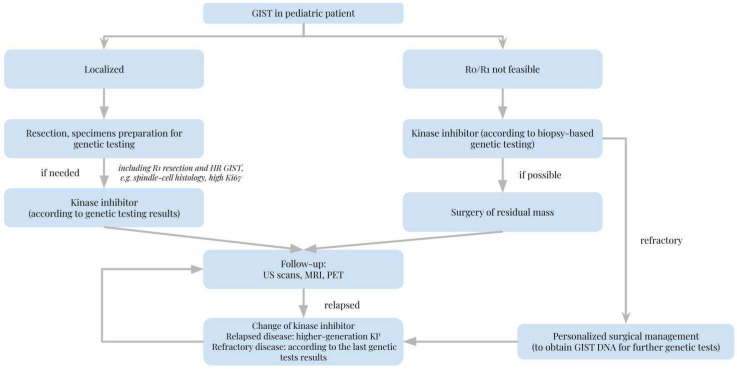
Pediatric GISTs are characterized by distinct biology that is still under numerous studies due to scarce study material. Due to little evidence from multicenter studies, we propose an oncoagnostic approach that could be of great importance. This entity requires complex genetic testing combined with histopathological diagnosis to apply the treatment properly. To the best of our knowledge, despite few studies, such a complex approach and tailoring the treatment to the molecular goal may lead to remission. We depict the importance of genetic studies that should contain not only KIT or PDGFR genes but also TRK, FGFR, RAS, SDH genes and potentially genes encoding for other multikinase inhibitors targets. The establishment of the NGS panel or even WES dedicated to pediatric GIST would enable the possibility of stating the prognosis and, if needed, administration of proper multikinase inhibitor (of proper generation). While imatinib remains the first-line agent for pediatric GIST, it should be recognized it is not suitable for every patient. Medications of higher generation (such as sunitinib, regorafenib) are important in overcoming imatinib resistance, though, in the future other targeted therapies (such as sorafenib, ponatinib, avapritinib) may play a significant role in the treatment of adult-type GISTs in children. As most pediatric GISTs are wild-type, non-standard kinase inhibitors, e.g., larotrectinib, entrectinib, could be applied in such cases. Nevertheless, there is still limited data regarding their administration, especially in pediatric patients. Finally, when it comes to surgical treatment in pediatric GISTs, we propose the application of laparoscopic surgery with lymph nodes excision as the method of choice with the MRI, USG and PET imaging control. Although pediatric GISTs belong to rare children’s neoplasms, unified diagnostics and treatment guidelines are necessary to systematize the approach in gastrointestinal stromal tumors in pediatric patients. We encourage a tumor agnostic approach, especially in wild-type/pediatric-type GIST, proposing a provisional management algorithm (Figure 2).
Figure 2.
Management algorithm when a gastrointestinal stromal tumor in a pediatric patient is diagnosed.
Author Contributions
Conceptualization—M.A., J.C. and K.D. Draft—M.A., J.C. and K.D. Revision—M.A., J.C. and K.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors received external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rutkowski P., Magnan H., Chou A.J., Benson C. Treatment of gastrointestinal stromal tumours in paediatric and young adult patients with sunitinib: A multicentre case series. BMC Cancer. 2017;17:1. doi: 10.1186/s12885-017-3727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida T., Blay J.Y., Hirota S., Kitagawa Y., Kang Y.K. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:1. doi: 10.1007/s10120-015-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rink L., Godwin A.K. Clinical and molecular characteristics of gastrointestinal stromal tumors in the pediatric and young adult population. Curr. Oncol. Rep. 2009;11:4. doi: 10.1007/s11912-009-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raitio A., Salim A., Mullassery D., Losty P.D. Current treatment and outcomes of pediatric gastrointestinal stromal tumors (GIST): A systematic review of published studies. Pediatr. Surg. Int. 2021;37:9. doi: 10.1007/s00383-021-04931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quiroz H.J., Willobee B.A., Sussman M.S., Fox B.R., Thorson C.M., Sola J.E., Perez E.A. Pediatric gastrointestinal stromal tumors-a review of diagnostic modalities. Transl. Gastroenterol. Hepatol. 2018;3:54. doi: 10.21037/tgh.2018.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzberg M., Beer M., Anupindi S., Vollert K., Kröncke T. Imaging pediatric gastrointestinal stromal tumor (GIST) J. Pediatr. Surg. 2018;53:9. doi: 10.1016/j.jpedsurg.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Casali P.G., Abecassis N., Aro H.T., Bauer S., Biagini R., Bielack S., Bonvalot S., Boukovinas I., Bovee J., Brodowicz T., et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:4. doi: 10.1093/annonc/mdy095. [DOI] [PubMed] [Google Scholar]
- 8.Tornillo L., Terracciano L.M. An update on molecular genetics of gastrointestinal stromal tumours. J. Clin. Pathol. 2006;59:6. doi: 10.1136/jcp.2005.031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappo A.S., Janeway K., Laquaglia M., Kim S.Y. Special considerations in pediatric gastrointestinal tumors. J. Surg. Oncol. 2011;104:8. doi: 10.1002/jso.21868. [DOI] [PubMed] [Google Scholar]
- 10.Willobee B.A., Quiroz H.J., Sussman M.S., Thorson C.M., Sola J.E., Perez E.A. Current treatment strategies in pediatric gastrointestinal stromal cell tumor. Transl. Gastroenterol. Hepatol. 2018;3:53. doi: 10.21037/tgh.2018.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naito Y., Mishima S., Akagi K., Igarashi A., Ikeda M., Okano S., Kato S., Takano T., Tsuchihara K., Terashima K., et al. Japan society of clinical oncology/Japanese society of medical oncology-led clinical recommendations on the diagnosis and use of tropomyosin receptor kinase inhibitors in adult and pediatric patients with neurotrophic receptor tyrosine kinase fusion-positive advanced solid tumors, cooperated by the Japanese society of pediatric hematology/oncology. Int. J. Clin. Oncol. 2020;25:3. doi: 10.1007/s10147-019-01610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocco E., Scaltriti M., Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018;15:12. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi E., Chmielecki J., Tang C.M., Wang K., Heinrich M.C., Kang G., Corless C.L., Hong D., Fero K.E., Murphy J.D., et al. FGFR1 and NTRK3 actionable alterations in ‘Wild-Type’ gastrointestinal stromal tumors. J. Transl. Med. 2016;14:1. doi: 10.1186/s12967-016-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss L.M., Funari V.A. NTRK fusions and Trk proteins: What are they and how to test for them. Hum. Pathol. 2021;112:59–69. doi: 10.1016/j.humpath.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Steeghs E., Kroeze L.I., Tops B., van Kempen L.C., Ter Elst A., Kastner-van Raaij A., Hendriks-Cornelissen S., Hermsen M., Jansen E., Nederlof P.M., et al. Comprehensive routine diagnostic screening to identify predictive mutations, gene amplifications, and microsatellite instability in FFPE tumor material. BMC Cancer. 2020;20:1. doi: 10.1186/s12885-020-06785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik F., Santiago T., Bahrami A., Davis E., McCarville B., Newman S., Azzato E.M., Davidoff A.M., Brennan R., Ellison D.W., et al. Dedifferentiation in SDH-Deficient Gastrointestinal Stromal Tumor: A Report With Histologic, Immunophenotypic, and Molecular Characterization. Pediatr. Dev. Pathol. 2019;22:5. doi: 10.1177/1093526619846222. [DOI] [PubMed] [Google Scholar]
- 17.Atiq M.A., Davis J.L., Hornick J.L., Dickson B.C., Fletcher C., Fletcher J.A., Folpe A.L., Mariño-Enríquez A. Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: A clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST) Mod. Pathol. 2021;34:1. doi: 10.1038/s41379-020-0623-z. [DOI] [PubMed] [Google Scholar]
- 18.Lee J.H., Shin S.-J., Choe E.-A., Kim J., Hyung W.J., Kim H.S., Jung M., Beom S.-H., Kim T.I., Ahn J.B., et al. Tropomyosin-Related Kinase Fusions in Gastrointestinal Stromal Tumors. Cancers. 2022;14:2659. doi: 10.3390/cancers14112659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia E.P., Minkovsky A., Jia Y., Ducar M.D., Shivdasani P., Gong X., Ligon A.H., Sholl L.M., Kuo F.C., MacConaill L.E., et al. Validation of OncoPanel: A Targeted Next-Generation Sequencing Assay for the Detection of Somatic Variants in Cancer. Arch. Pathol. Lab. Med. 2017;141:751–758. doi: 10.5858/arpa.2016-0527-OA. [DOI] [PubMed] [Google Scholar]
- 20.Garcia E.P., Minkovsky A., Jia Y., Ducar M.D., Shivdasani P., Gong X., Ligon A.H., Sholl L.M., Kuo F.C., MacConaill L.E., et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro. Oncol. 2017;19:5. doi: 10.1093/neuonc/now254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demetri G.D., Antonescu C.R., Bjerkehagen B., Bovée J., Boye K., Chacón M., Dei Tos A.P., Desai J., Fletcher J.A., Gelderblom H., et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: Expert recommendations from the World Sarcoma Network. Ann. Oncol. 2020;31:11. doi: 10.1016/j.annonc.2020.08.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napolitano A., Ostler A.E., Jones R.L., Huang P.H. Fibroblast Growth Factor Receptor (FGFR) Signaling in GIST and Soft Tissue Sarcomas. Cells. 2021;10:1533. doi: 10.3390/cells10061533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javidi-Sharifi N., Traer E., Martinez J., Gupta A., Taguchi T., Dunlap J., Heinrich M.C., Corless C.L., Rubin B.P., Druker B.J., et al. Crosstalk between KIT and FGFR3 Promotes Gastrointestinal Stromal Tumor Cell Growth and Drug Resistance. Cancer Res. 2015;75:5. doi: 10.1158/0008-5472.CAN-14-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boichuk S., Galembikova A., Dunaev P., Valeeva E., Shagimardanova E., Gusev O., Khaiboullina S. A Novel Receptor Tyrosine Kinase Switch Promotes Gastrointestinal Stromal Tumor Drug Resistance. Molecules. 2017;22:2152. doi: 10.3390/molecules22122152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbini M., Astolfi A., Indio V., Nannini M., Schipani A., Bacalini M.G., Angelini S., Ravegnini G., Calice G., Del Gaudio M., et al. Gene duplication, rather than epigenetic changes, drives FGF4 overexpression in KIT/PDGFRA/SDH/RAS-P WT GIST. Sci. Rep. 2020;10:1. doi: 10.1038/s41598-020-76519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boichuk S., Galembikova A., Mikheeva E., Bikinieva F., Aukhadieva A., Dunaev P., Khalikov D., Petrov S., Kurtasanov R., Valeeva E., et al. Inhibition of FGF2-Mediated Signaling in GIST-Promising Approach for Overcoming Resistance to Imatinib. Cancers. 2020;12:1674. doi: 10.3390/cancers12061674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sergei B., Pavel D., Aigul G., Firyuza B., Ilmira N., Ilshat M., Aida A., Refat K., Natalia A., Elena S., et al. Inhibition of FGFR2-Signaling Attenuates a Homology-Mediated DNA Repair in GIST and Sensitizes Them to DNA-Topoisomerase II Inhibitors. Int. J. Mol. Sci. 2020;21:352. doi: 10.3390/ijms21010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brčić I., Argyropoulos A., Liegl-Atzwanger B. Update on Molecular Genetics of Gastrointestinal Stromal Tumors. Diagnostics. 2021;11:194. doi: 10.3390/diagnostics11020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huss S., Pasternack H., Ihle M.A., Merkelbach-Bruse S., Heitkötter B., Hartmann W., Trautmann M., Gevensleben H., Büttner R., Schildhaus H.U., et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum. Pathol. 2017;62:206–214. doi: 10.1016/j.humpath.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Carney J.A. Carney triad: A syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. J. Clin. Endocrinol. Metab. 2009;94:10. doi: 10.1210/jc.2009-1156. [DOI] [PubMed] [Google Scholar]
- 31.Jing X., Meng X., Gao Y., Yu J., Liu B. A 4-month-old boy with gastrointestinal stromal tumor of mesocolon. Cancer Biol. Ther. 2019;20:1. doi: 10.1080/15384047.2018.1504719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Rivero J., Arnaldez F.I., Srinivasan R., Spencer M., Steinberg S.M., Pacak K., Killian K., Helman L.J., Meltzer P.S., Linehan W.M., et al. A phase II trial of the DNA methyl transferase inhibitor, SGI-110 (Guadecitabine), in children and adults with SDH-deficient GIST, pheochromocytoma, and paraganglioma, and HLRCC-associated kidney cancer. JCO. 2020;38:11540. doi: 10.1200/JCO.2020.38.15_suppl.11540. [DOI] [Google Scholar]
- 33.Belinsky M.G., Rink L., von Mehren M. Succinate dehydrogenase deficiency in pediatric and adult gastrointestinal stromal tumors. Front. Oncol. 2013;3:117. doi: 10.3389/fonc.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez J., Thomas J., Levy M., Patel N., Levy A., Smadi Y. Presyncope Leading to the Diagnosis of Gastrointestinal Stromal Tumor in a Pediatric Patient. J. Pediatr. Gastroenterol. Nutr. 2021;73:3. doi: 10.1097/MPG.0000000000003144. [DOI] [PubMed] [Google Scholar]
- 35.Roh T.H., Yim H., Roh J., Lee K.B., Park S.H., Jeong S.-Y., Kim S.-H., Kim J.-H. The Loss of Succinate Dehydrogenase B Expression Is Frequently Identified in Hemangioblastoma of the Central Nervous System. Sci. Rep. 2019;9:5873. doi: 10.1038/s41598-019-42338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nannini M., Rizzo A., Indio V., Schipani A., Astolfi A., Pantaleo M.A. Targeted therapy in SDH-deficient GIST. Ther. Adv. Med. Oncol. 2021;13:17588359211023278. doi: 10.1177/17588359211023278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemming M.L., Coy S., Lin J.R., Andersen J.L., Przybyl J., Mazzola E., Abdelhamid Ahmed A.H., van de Rijn M., Sorger P.K., Armstrong S.A., et al. HAND1 and BARX1 act as transcriptional and anatomic determinants of malignancy in gastrointestinal stromal tumor. Clin. Cancer Res. 2021;27:6. doi: 10.1158/1078-0432.CCR-20-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singeltary B., Ghose A., Sussman J., Choe K., Olowokure O. Durable response with a combination of imatinib and sorafenib in KIT exon 17 mutant gastrointestinal stromal tumor. J. Gastrointest. Oncol. 2014;5:1. doi: 10.3978/j.issn.2078-6891.2013.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer S., George S., von Mehren M., Heinrich M.C. Early and Next-Generation KIT/PDGFRA Kinase Inhibitors and the Future of Treatment for Advanced Gastrointestinal Stromal Tumor. Front. Oncol. 2021;11:672500. doi: 10.3389/fonc.2021.672500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brzozowska M., Wierzba W., Szafraniec-Buryło S., Czech M., Połowinczak-Przybyłek J., Potemski P., Śliwczyński A. Real-World Evidence of Patient Outcome Following Treatment of Advanced Gastrointestinal Stromal Tumor (GIST) with Imatinib, Sunitinib, and Sorafenib in Publicly Funded Health Care in Poland. Med. Sci. Monit. 2019;25:3846. doi: 10.12659/MSM.914517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benesch M., Wardelmann E., Ferrari A., Brennan B., Verschuur A. Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatr. Blood Cancer. 2009;53:7. doi: 10.1002/pbc.22123. [DOI] [PubMed] [Google Scholar]
- 42.Singh A.S., Hecht J.R., Rosen L., Wainberg Z.A., Wang X., Douek M., Hagopian A., Andes R., Sauer L., Brackert S.R., et al. A Randomized Phase II Study of Nivolumab Monotherapy or Nivolumab Combined with Ipilimumab in Patients with Advanced Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2022;28:1. doi: 10.1158/1078-0432.CCR-21-0878. [DOI] [PubMed] [Google Scholar]
- 43.Reilley M.J., Bailey A., Subbiah V., Janku F., Naing A., Falchook G., Karp D., Piha-Paul S., Tsimberidou A., Fu S., et al. Phase I clinical trial of combination imatinib and ipilimumab in patients with advanced malignancies. J. Immunother. Cancer. 2017;5:1–10. doi: 10.1186/s40425-017-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Angelo S.P., Shoushtari A.N., Keohan M.L., Dickson M.A., Gounder M.M., Chi P., Loo J.K., Gaffney L., Schneider L., Patel Z., et al. Combined KIT and CTLA-4 Blockade in Patients with Refractory GIST and Other Advanced Sarcomas: A Phase Ib Study of Dasatinib plus Ipilimumab. Clin. Cancer Res. 2017;23:12. doi: 10.1158/1078-0432.CCR-16-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agaram N.P., Laquaglia M.P., Ustun B., Guo T., Wong G.C., Socci N.D., Maki R.G., DeMatteo R.P., Besmer P., Antonescu C.R. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin. Cancer Res. 2008;14:10. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubois S.G., Shusterman S., Ingle A.M., Ahern C.H., Reid J.M., Wu B., Baruchel S., Glade-Bender J., Ivy P., Grier H.E., et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: A children’s oncology group study. Clin. Cancer Res. 2011;17:15. doi: 10.1158/1078-0432.CCR-11-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y., DuBois S.G., Wetmore C., Khosravan R. Physiologically Based Pharmacokinetic Modeling and Simulation of Sunitinib in Pediatrics. AAPS J. 2020;22:2. doi: 10.1208/s12248-020-0423-x. [DOI] [PubMed] [Google Scholar]
- 48.Wang E., DuBois S.G., Wetmore C., Verschuur A.C., Khosravan R. Population Pharmacokinetics of Sunitinib and its Active Metabolite SU012662 in Pediatric Patients with Gastrointestinal Stromal Tumors or Other Solid Tumors. Eur. J. Drug. Metab. Pharm. 2021;46:3. doi: 10.1007/s13318-021-00671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verschuur A.C., Bajčiová V., Mascarenhas L., Khosravan R., Lin X., Ingrosso A., Janeway K.A. Sunitinib in pediatric patients with advanced gastrointestinal stromal tumor: Results from a phase I/II trial. Cancer Chemother. Pharmacol. 2019;84:41–50. doi: 10.1007/s00280-019-03814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verschuur A.C., Bajčiová V., Mascarenhas L., Khosravan R., Lin X., Ingrosso A., Janeway K.A. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr. Blood Cancer. 2009;52:7. doi: 10.1002/pbc.21909. [DOI] [PubMed] [Google Scholar]
- 51.Rihacek M., Selingerova I., Kocak I., Kocakova I., Rihackova E., Valik D., Sterba J. Sunitinib-Induced Elevation of Mean Corpuscular Volume (MCV)-Exploring Its Possible Clinical Relevance in Cancer Patients. Curr. Oncol. 2022;29:330. doi: 10.3390/curroncol29060330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mross K., Frost A., Steinbild S., Hedbom S., Büchert M., Fasol U., Unger C., Krätzschmar J., Heinig R., Boix O., et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin. Cancer Res. 2012;18:9. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 53.Demetri G.D., Reichardt P., Kang Y.K., Blay J.Y., Rutkowski P., Gelderblom H., Hohenberger P., Leahy M., von Mehren M., Joensuu H., et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demetri G.D., Reichardt P., Kang Y.K., Blay J.Y., Rutkowski P., Gelderblom H., Hohenberger P., Leahy M., von Mehren M., Joensuu, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: A multicenter phase II trial. J. Clin. Oncol. 2012;30:19. doi: 10.1200/jco.2012.30.18_suppl.lba10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilhelm S.M., Dumas J., Adnane L., Lynch M., Carter C.A., Schütz G., Thierauch K.H., Zopf D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer. 2011;129:1. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 56.Bruix J., Tak W.Y., Gasbarrini A., Santoro A., Colombo M., Lim H.Y., Mazzaferro V., Wiest R., Reig M., Wagner A., et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur. J. Cancer. 2013;49:16. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 57.Daudigeos-Dubus E., Le Dret L., Lanvers-Kaminsky C., Bawa O., Opolon P., Vievard A., Villa I., Pagès M., Bosq J., Vassal G., et al. Regorafenib: Antitumor Activity upon Mono and Combination Therapy in Preclinical Pediatric Malignancy Models. PLoS ONE. 2015;10:11. doi: 10.1371/journal.pone.0142612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blay J.Y., Shen L., Kang Y.K., Rutkowski P., Qin S., Nosov D., Wan D., Trent J., Srimuninnimit V., Pápai Z., et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): A randomised phase 3 trial. Lancet Oncol. 2015;16:5. doi: 10.1016/S1470-2045(15)70105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glod J., Arnaldez F.I., Wiener L., Spencer M., Killian J.K., Meltzer P., Dombi E., Derse-Anthony C., Derdak J., Srinivasan R., et al. A Phase II Trial of Vandetanib in Children and Adults with Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumor. Clin. Cancer Res. 2019;25:21. doi: 10.1158/1078-0432.CCR-19-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drilon A., Laetsch T.W., Kummar S., DuBois S.G., Lassen U.N., Demetri G.D., Nathenson M., Doebele R.C., Farago A.F., Pappo A.S., et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018;378:8. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong D.S., DuBois S.G., Kummar S., Farago A.F., Albert C.M., Rohrberg K.S., van Tilburg C.M., Nagasubramanian R., Berlin J.D., Federman N., et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:4. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heinrich M.C., Marino-Enriquez A., Presnell A., Donsky R.S., Griffith D.J., McKinley A., Patterson J., Taguchi T., Liang C.W., Fletcher J.A. Sorafenib Inhibits Many Kinase Mutations Associated with Drug-Resistant Gastrointestinal Stromal Tumors. Mol. Cancer Ther. 2012;11:1770–1780. doi: 10.1158/1535-7163.MCT-12-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rutkowski P., Jagielska B., Andrzejuk J., Bylina E., Lugowska I., Switaj T., Kosela-Paterczyk H., Kozak K., Falkowski S., Klimczak A. The analysis of the long-term outcomes of sorafenib therapy in routine practice in imatinib and sunitinib resistant gastrointestinal stromal tumors (GIST) Contemp. Oncol. 2017;21:4. doi: 10.5114/wo.2017.72393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brinch C., Dehnfeld M., Hogdall E., Poulsen T.S., Toxvaerd A., Al-Farra G., Bergenfeldt M., Krarup-Hansen A. Outstanding Response to Sorafenib in a Patient with Metastatic Gastrointestinal Stromal Tumour. Case Rep. Oncol. 2021;14:3. doi: 10.1159/000519747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henriques-Abreu M., Serrano C. Avapritinib in unresectable or metastatic gastrointestinal stromal tumor with PDGFRA exon 18 mutation: Safety and efficacy. Expert Rev. Anticancer. Ther. 2021;21:10. doi: 10.1080/14737140.2021.1963235. [DOI] [PubMed] [Google Scholar]
- 66.Jones R.L., Serrano C., von Mehren M., George S., Heinrich M.C., Kang Y.K., Schöffski P., Cassier P.A., Mir O., Chawla S.P., et al. Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumours: Long-term efficacy and safety data from the NAVIGATOR phase I trial. Eur. J. Cancer. 2021;145:132–142. doi: 10.1016/j.ejca.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.George S., Jones R.L., Bauer S., Kang Y.K., Schöffski P., Eskens F., Mir O., Cassier P.A., Serrano C., Tap W.D., et al. Avapritinib in Patients With Advanced Gastrointestinal Stromal Tumors Following at Least Three Prior Lines of Therapy. Oncologist. 2021;26:4. doi: 10.1002/onco.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George S., von Mehren M., Fletcher J.A., Sun J., Zhang S., Pritchard J.R., Hodgson J.G., Kerstein D., Rivera V.M., Haluska F.G., et al. Phase II Study of Ponatinib in Advanced Gastrointestinal Stromal Tumors: Efficacy, Safety, and Impact of Liquid Biopsy and Other Biomarkers. Clinical. Cancer Res. 2022;28:7. doi: 10.1158/1078-0432.CCR-21-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franck C., Rosania R., Franke S., Haybaeck J., Canbay A., Venerito M. The BRAF Status May Predict Response to Sorafenib in Gastrointestinal Stromal Tumors Resistant to Imatinib, Sunitinib, and Regorafenib: Case Series and Review of the Literature. Digestion. 2019;99:2. doi: 10.1159/000490886. [DOI] [PubMed] [Google Scholar]
- 70.Smith B.D., Kaufman M.D., Lu W.P., Gupta A., Leary C.B., Wise S.C., Rutkoski T.J., Ahn Y.M., Al-Ani G., Bulfer S.L., et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell. 2019;35:5. doi: 10.1016/j.ccell.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Boikos S.A., Pappo A.S., Killian J.K., LaQuaglia M.P., Weldon C.B., George S., Trent J.C., von Mehren M., Wright J.A., Schiffman J.D., et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016;2:7. doi: 10.1001/jamaoncol.2016.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ben-Ami E., Barysauskas C.M., von Mehren M., Heinrich M.C., Corless C.L., Butrynski J.E., Morgan J.A., Wagner A.J., Choy E., Yap J.T., et al. Long-Term Follow-up Results of the Multicenter Phase II Trial of Regorafenib in Patients with Metastatic and/or Unresectable GI Stromal Tumor after Failure of Standard Tyrosine Kinase Inhibitor Therapy. Ann. Oncol. 2016;27:1794–1799. doi: 10.1093/annonc/mdw228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pantaleo M.A., Nannini M., Saponara M., Gnocchi C., Di Scioscio V., Lolli C., Catena F., Astolfi A., Di Battista M., Biasco G., et al. Impressive long-term disease stabilization by nilotinib in two pretreated patients with KIT/PDGFRA wild-type metastatic gastrointestinal stromal tumours. Anticancer. Drugs. 2012;23:5. doi: 10.1097/CAD.0b013e328352cc50. [DOI] [PubMed] [Google Scholar]
- 74.Neppala P., Banerjee S., Fanta P.T., Yerba M., Porras K.A., Burgoyne A.M., Sicklick J.K. Current Management of Succinate Dehydrogenase–Deficient Gastrointestinal Stromal Tumors. Cancer Metastasis Rev. 2019;38:525–535. doi: 10.1007/s10555-019-09818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ganjoo K.N., Villalobos V.M., Kamaya A., Fisher G.A., Butrynski J.E., Morgan J.A., Wagner A.J., D’Adamo D., McMillan A., Demetri G.D., et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann. Oncol. 2014;25:1. doi: 10.1093/annonc/mdt484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gokozan H.N., Bomeisl P. Succinate dehydrogenase-deficient gastrointestinal stromal tumor of stomach diagnosed by endoscopic ultrasound-guided fine-needle biopsy: Report of a distinct subtype in cytology. Diagn. Cytopathol. 2020;48:12. doi: 10.1002/dc.24591. [DOI] [PubMed] [Google Scholar]
- 77.Frankfurt O., Licht J.D. Ponatinib--a step forward in overcoming resistance in chronic myeloid leukemia. Clin. Cancer Res. 2013;19:21. doi: 10.1158/1078-0432.CCR-13-0258. [DOI] [PubMed] [Google Scholar]
- 78.Danti G., Addeo G., Cozzi D., Maggialetti N., Lanzetta M.M., Frezzetti G., Masserelli A., Pradella S., Giovagnoni A., Miele V. Relationship between diagnostic imaging features and prognostic outcomes in gastrointestinal stromal tumors (GIST) Acta Biomed. 2019;90:9. doi: 10.23750/abm.v90i5-s.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lino-Silva L.S., Segales-Rojas P., Aguilar-Cruz E., Salcedo-Hernández R.A., Zepeda-Najar C. Gastrointestinal Stromal Tumors Risk of Recurrence Stratification by Tumor Volume is a Best Predictor Compared with Risk Based on Mitosis and Tumor Size. J. Gastrointest. Cancer. 2019;50:3. doi: 10.1007/s12029-018-0115-2. [DOI] [PubMed] [Google Scholar]
- 80.Jumniensuk C., Charoenpitakchai M. Gastrointestinal stromal tumor: Clinicopathological characteristics and pathologic prognostic analysis. World J. Surg. Oncol. 2018;16:1. doi: 10.1186/s12957-018-1532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miettinen M., Lasota J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 2006;130:10. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 82.Sugai Y., Hirayama Y., Iinuma Y., Nakaya K., Aikou T., Taki S., Hashidate H., Kinoshita Y. A rare case of neonatal colonic obstruction caused by a solitary intestinal tumor. Surg. Case Rep. 2021;7:26. doi: 10.1186/s40792-021-01107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tran S., Dingeldein M., Mengshol S.C., Kay S., Chin A.C. Incidental GIST after appendectomy in a pediatric patient: A first instance and review of pediatric patients with CD117 confirmed GISTs. Pediatr. Surg. Int. 2014;30:4. doi: 10.1007/s00383-013-3432-3. [DOI] [PubMed] [Google Scholar]
- 84.Weldon C.B., Madenci A.L., Boikos S.A., Janeway K.A., George S., von Mehren M., Pappo A.S., Schiffman J.D., Wright J., Trent J.C., et al. Surgical Management of Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Pediatric and Wildtype GIST Clinic. J. Clin. Oncol. 2017;35:5. doi: 10.1200/JCO.2016.68.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi S.M., Kim M.C., Jung G.J., Kim H.H., Kwon H.C., Choi S.R., Jang J.S., Jeong J.S. Laparoscopic wedge resection for gastric GIST: Long-term follow-up results. Eur. J. Surg. Oncol. 2007;33:4. doi: 10.1016/j.ejso.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto S., Hosoya Y., Lefor A.K., Ino Y., Haruta H., Kurashina K., Saito S., Kitayama J., Sata N. Non-exposed endoscopic wall-inversion surgery for pediatric gastrointestinal stromal tumor: A case report. Asian J. Endosc. Surg. 2019;12:3. doi: 10.1111/ases.12641. [DOI] [PubMed] [Google Scholar]
- 87.Günter E., Lingenfelser T., Eitelbach F., Müller H., Ell C. EUS-guided ethanol injection for treatment of a GI stromal tumor. Gastrointest. Endosc. 2003;57:1. doi: 10.1067/mge.2003.39. [DOI] [PubMed] [Google Scholar]
- 88.Hernández-Ludeña L., Consiglieri C.F., Gornals J.B. EUS-guided ethanol ablation therapy for gastric stromal tumors. Rev. Esp. Enferm. Dig. 2018;110:1. doi: 10.17235/reed.2017.5361/2017. [DOI] [PubMed] [Google Scholar]
- 89.Valli P.V., Valli C., Pfammatter T., Bauerfeind P. Life-threatening bleeding of a duodenal gastrointestinal stromal tumor in a teenager: A rare case report. Endosc. Int. Open. 2016;4:12. doi: 10.1055/s-0042-115936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaemmer D.A., Otto J., Lassay L., Steinau G., Klink C., Junge K., Klinge U., Schumpelick V. The Gist of literature on pediatric GIST: Review of clinical presentation. J. Pediatr. Hematol. Oncol. 2009;31:2. doi: 10.1097/MPH.0b013e3181923cd8. [DOI] [PubMed] [Google Scholar]
- 91.Miranda M.E., Alberti L.R., Tatsuo E.S., Piçarro C., Rausch M. Gastrointestinal stromal tumor of the stomach in a child with a 3-year follow-up period-Case report. Int. J. Surg. Case Rep. 2011;2:6. doi: 10.1016/j.ijscr.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dave M., Jimenez A., Evans K., Leslie W. Treatment of recurrent pediatric gastrointestinal stromal tumors. Gastrointest. Cancer Res. 2012;5:4. [PMC free article] [PubMed] [Google Scholar]
- 93.Nishida T., Hølmebakk T., Raut C.P., Rutkowski P. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann. Surg. Oncol. 2019;26:6. doi: 10.1245/s10434-019-07297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jakob J., Salameh R., Wichmann D., Charalambous N., Zygmunt A.C., Kreisel I., Heinz J., Ghadimi M., Ronellenfitsch U. Needle tract seeding and abdominal recurrence following pre-treatment biopsy of gastrointestinal stromal tumors (GIST): Results of a systematic review. BMC Surg. 2022;22:1. doi: 10.1186/s12893-022-01648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garnier H., Murawski M., Jastrzebski T., Pawinska-Wasikowska K., Balwierz W., Sinacka K., Gorecki W., Izycka-Swieszewska E., Czauderna P. Case Report: Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Application in Intraperitoneally Disseminated Inflammatory Myofibroblastic Tumor and in the Youngest Patient in the World: New Indication and Modification of Technique. Front. Surg. 2021;8:746700. doi: 10.3389/fsurg.2021.746700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seitz G., Fuchs J., Königsrainer I., Warmann S., Königsrainer A., Beckert S. Zytoreduktive Chirurgie und HIPEC bei peritoneal-metastasierten Tumoren im Kindesalter. Zent. Chir. 2014;139:607–612. doi: 10.1055/s-0034-1383240. [DOI] [PubMed] [Google Scholar]
- 97.Bryan M.L., Fitzgerald N.C., Levine E.A., Shen P., Stewart J.H., Votanopoulos K.I. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in sarcomatosis from gastrointestinal stromal tumor. Am. Surg. 2014;80:9. doi: 10.1177/000313481408000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ong E., Diven C., Abrams A., Lee E., Mahadevan D. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for palliative treatment of malignant ascites from gastrointestinal stromal tumours. J. Palliat. Care. 2012;28:4. doi: 10.1177/082585971202800409. [DOI] [PubMed] [Google Scholar]
- 99.Kalisvaart G.M., Meijer R., Bijlstra O.D., Galema H.A., de Steur W.O., Hartgrink H.H., Verhoef C., de Geus-Oei L.F., Grünhagen D.J., Schrage Y.M., et al. Intraoperative Near-Infrared Fluorescence Imaging with Indocyanine Green for Identification of Gastrointestinal Stromal Tumors (GISTs), a Feasibility Study. Cancers. 2022;14:1572. doi: 10.3390/cancers14061572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Souzaki R., Ieiri S., Uemura M., Ohuchida K., Tomikawa M., Kinoshita Y., Koga Y., Suminoe A., Kohashi K., Oda Y., et al. An augmented reality navigation system for pediatric oncologic surgery based on preoperative CT and MRI images. J. Pediatr. Surg. 2013;48:12. doi: 10.1016/j.jpedsurg.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 101.Brookes M.J., Chan C.D., Baljer B., Wimalagunaratna S., Crowley T.P., Ragbir M., Irwin A., Gamie Z., Beckingsale T., Ghosh K., et al. Surgical Advances in Osteosarcoma. Cancers. 2021;13:388. doi: 10.3390/cancers13030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Privitera L., Paraboschi I., Cross K., Giuliani S. Above and Beyond Robotic Surgery and 3D Modelling in Paediatric Cancer Surgery. Front. Pediatr. 2021;9:777840. doi: 10.3389/fped.2021.777840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.