Abstract
There is a consensus that epigenetic alterations play a key role in cancer initiation and its biology. Studies evaluating the modification in the DNA methylation and chromatin remodeling patterns, as well as gene regulation profile by non-coding RNAs (ncRNAs) have led to the development of novel therapeutic approaches to treat several tumor types. Indeed, despite clinical and translational challenges, combinatorial therapies employing agents targeting epigenetic modifications with conventional approaches have shown encouraging results. However, for rare neoplasia such as uterine leiomyosarcomas (LMS) and endometrial stromal sarcomas (ESS), treatment options are still limited. LMS has high chromosomal instability and molecular derangements, while ESS can present a specific gene fusion signature. Although they are the most frequent types of “pure” uterine sarcomas, these tumors are difficult to diagnose, have high rates of recurrence, and frequently develop resistance to current treatment options. The challenges involving the management of these tumors arise from the fact that the molecular mechanisms governing their progression have not been entirely elucidated. Hence, to fill this gap and highlight the importance of ongoing and future studies, we have cross-referenced the literature on uterine LMS and ESS and compiled the most relevant epigenetic studies, published between 2009 and 2022.
Keywords: uterine leiomyosarcoma, endometrial stromal sarcoma, epigenetics mechanisms, ncRNA, DNA methylation, histones modifications
1. Introduction
The body of the uterus is composed of a mucosa muscular interface derived from the Müllerian embryonic ducts and constituted of internal endometrium and external myometrium (MM) tissue layers [1,2,3,4]. The internal endometrium is composed of luminal epithelium, glandular epithelium, and endometrial stroma whereas the MM consists mainly of smooth muscle cells [2,3,4]. Cellular and molecular alterations in the endometrial stroma and smooth muscle cell layers can lead to uterine sarcoma (US) development [4,5,6].
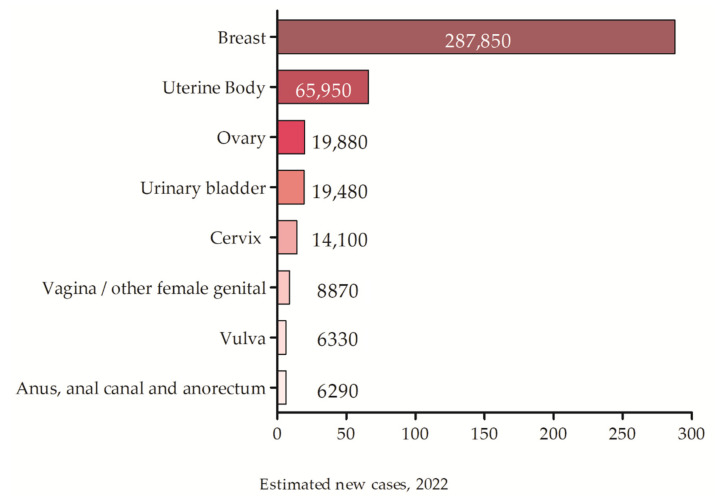
US accounts for 3–9% of all uterine malignancies and shows high rates of recurrence and metastasis [7,8], occupying the second place among all gynecological tumors [7,9]. The American Cancer Society (ACS) registered a total of 66,570 new cases of uterine tumors with about 12,940 related deaths in 2021 [10] and estimates 65,950 new cases for 2022 (Figure 1) [11].
Figure 1.
The estimated incidence of gynecological tumors for 2022 according to the ACS.
“Pure” sarcomas are composed exclusively of mesenchymal cells and include the leiomyosarcomas (LMS) and endometrial stromal sarcomas (ESS), which are morphologically classified mainly based on the tumor cells phenotype [12]. LMS arises from the smooth muscle compartment, while ESS arises from the stroma supporting the endometrial glands [8]. LMS and ESS are the most frequent uterine mesenchymal tumors in adult age [13].
For LMS and ESS, the disease stage is the single most important prognostic factor [14]. The International Federation of Gynecology and Obstetrics (FIGO) classification and staging system has been specifically designed for these tumors [15]. In 2018, the ACS published the latest revision of the definitions and clinical staging of LMS and ESS (Table 1), based on the FIGO system and the American Joint Committee on Cancer (AJCC) TNM staging system [14,16,17,18].
Table 1.
Staging of LMS and ESS (FIGO 1 and AJCC 2).
| Stage | Features | Description |
|---|---|---|
| I | T1 | Tumor limited to the uterus (T1). |
| N0 | ||
| M0 | ||
| IA | T1a | Tumor restricted to the uterus (less than 5 cm) (T1a). |
| N0 | ||
| M0 | ||
| IB | T1b | Tumor restricted to the uterus (more than 5 cm) (T1b). |
| N0 | ||
| M0 | ||
| II | T2 | Tumor growing outside the uterus but is restricted to the pelvis (T2). |
| N0 | ||
| M0 | ||
| IIIA | T3a | Tumor growing in a single tissue located in the abdomen (T3a). |
| N0 | ||
| M0 | ||
| IIIB | T3b | Involvement of other extrauterine pelvic tissues, 2 or more sites (T3b). |
| N0 | ||
| M0 | ||
| IIIC | T1–T3 | Tumor invades abdominal tissues (does not protrude from the abdomen) but does not grow into the bladder or rectum (T1 to T3). The cancer has spread to nearby lymph nodes (N1). |
| N1 | ||
| M0 | ||
| IVA | T4 | Tumor spread to the rectum or urinary bladder (T4). It might or might not have spread to nearby lymph nodes (Any N). |
| Any N | ||
| M0 | ||
| IVB | Any T | Tumor spread to distant sites (lungs, bones, or liver) (M1). It may or may not have grown into tissues in the pelvis and/or abdomen (any T) and it might or might not have spread to lymph nodes (Any N). |
| Any N | ||
| M1 |
1 FIGO: International Federation of Gynecology and Obstetrics classification (2009). 2 AJCC: American Joint Committee on Cancer TNM staging system (2018).
It is well known that several molecular events may lead to tumor development. Among these, epigenetic mechanisms such as DNA methylation, post-translational modifications (PTMs), and non-coding RNA (ncRNA) regulation (e.g., microRNAs) can significantly affect the expression of relevant genes, leading to dramatic cell changes [19,20,21]. Epigenetic alterations are characterized by reversibility and susceptibility to external factors and are the main regulatory events governing the development and progression of uterine sarcomas [19,20,22]. Here, we reviewed and summarized the scientific and clinical reports from the past twelve years regarding epigenetic events and their role in the pathophysiology of the ESS and LMS. The most relevant articles written in English were meticulously reviewed and included in this review, and no restrictions for geographic location were applied. Articles without tumor type description or any identification as “uterine” were excluded.
1.1. LMS Etiology, Prognosis, and Treatment
LMS arises from the myometrium (MM) and often does not reach the endometrial cavity surface [9,23]. Its incidence is 0.36 per 100,000 women a year, affecting mainly women of ≥40 years of age, and representing approximately 70% of all US [24,25,26,27,28,29]. LMS is a very heterogeneous tumor and represents the most common sarcoma of the uterine body [14,17,25,26,30,31,32]. Its pathogenesis is poorly understood, but several studies focusing on tumor clonality indicate that many of these tumors are de novo entities [33,34,35,36,37,38]. Even though it is an extremely rare event [37], some authors defend the hypothesis that LMSs could arise from the malignant transformation of a pre-existing leiomyoma (LM) [15,30,35,39,40]. However, most of the patients do not exhibit predisposing factors such as prior radiation therapy to the pelvis (10–25%), tamoxifen use (1–2%), genetic syndromes (e.g., retinoblastoma and Li–Fraumeni syndrome), postmenopausal status, and ethnicity (African American) [41].
Clinically, LMS is associated with a poor prognosis even when diagnosed in the early stages, consequently leading to a significant increase in uterine cancer-associated deaths [13,17,26,28,29]. The recurrence rate of LMS reaches 53–75%, even at the initial stages of the disease, with locoregional or distant recurrence in the first two years after diagnosis [7,14,26,28,42,43,44,45,46]. The overall survival expectancy of LMS is 2.6 years, and the survival at 2, 5, and 10 years are approximately 57%, 24%, and 12%, respectively [26,28,42,44]. The survival rates for patients with LMS decrease as the disease progresses; thus, for localized disease (i.e., restricted to the uterus) the estimated survival rates are 64%, for regional disease (afflicting nearby and adjacent tissues, i.e., lymph nodes) the survival rates are 36%, and for disseminated disease [44,47,48,49,50] or metastatic disease (i.e., lungs and liver) the survival is 14% [51].
LMS-related symptoms are associated with vaginal bleeding in 56% of the cases, increased pelvic mass in 54% of the cases, and/or pelvic pain in 22% of the cases [14,15,17,37,39]. Typically, 75% of the patients present a large tumor mass with an average diameter of 10 cm at the time of diagnosis [14,17]. Although LMSs occur primarily in postmenopausal women [52], both progesterone receptor (PR) and estrogen receptor (ER) are found to be expressed in 40% and 70% of the cases, respectively [43,52,53,54,55,56]. A recent review has suggested that hormonal therapy applied to LMS expressing ER/PR is effective and presents favorable tolerance and reliability [57].
There are preoperative methods that allow the differential diagnosis of benign and malignant uterine disease. Magnetic resonance imaging (MRI) remains the optimal imaging modality to characterize pelvic masses originating from the uterus, but distinguishing LMS from LM remains a challenge [17].
LMS histopathological analysis is characterized by the presence of spindle cells, with ruptured nuclei, perinuclear vacuolization, and eosinophilic cytoplasm arranged in intersecting fascicles within the analyzed sample. Meeting the Stanford criteria, LMS should be deemed intrinsically as a high-grade tumor [58]. Cell atypia can vary from moderate to severe, while nuclear atypia is always severe, large areas of tumor cell necrosis with variable mitotic index and atypical mitosis are often observed [15,24,37,59,60]. There are two uncommon subtypes of uterine LMS: myxoid and epithelioid LMS. These present mild or focal nuclear pleomorphism and lower mitotic degree, compared to typical LMS. Diagnostic mistakes between these types of LMS and other smooth muscle tumors are often common [61,62].
Immunohistochemical co-expression of Desmin, h-Caldesmon, smooth muscle actin (SMA), and HDAC8 can assist with LMS diagnosis [39,40,56,63,64,65]. Several other potential biomarkers such as PDGFRA, WT1, GNRHR, BCL2, ESR, PGR, and LAMP2 have also been evaluated to distinguish LMS from other tumors, mainly from LM [63]. The cell proliferative index (determined by Ki-67 protein expression), the protein expression levels of the tumor suppressors p16 and p53, and the expression of several isoforms of the CD44 (hyaluronan receptor) are, however, routinely used [8,65]. Additionally, some patients show high amounts of lactate dehydrogenase (LDH) [12] and/or CA125 levels [13], but these markers are quite unspecific [17].
Most recently, gene expression profile analysis has enabled the classification of LMS into two subtypes according to their molecular signature. The subtype I recapitulated the low-grade LMS and was enriched for LMOD1, SLMAP, MYLK, and MYH11, all of them smooth muscle-specific markers. Subtype II of LMS included tumors with worse prognosis and expressed genes associated with cell cycle, proliferation, and tumorigenesis (CDK6, BMP1, MAPK13, PDGFRL, and HOXA1) [66,67].
The gold standard treatment for LMS is still the tumor surgical excision. Total hysterectomy and bilateral salpingo-oophorectomy are recommended for early-stage tumors [17,32,46,47,48,56,58,68]. Adjuvant chemo and radiotherapies are indicated to avoid recurrences, or for early-stage disease, but their effectiveness is still unclear [17,44,49,69] and they do not offer a significant advantage to improve overall survival [22,43,49,55,70,71]. Recently, new chemotherapies, targeted therapies (pazopanib), and immunotherapies (nivolumab or pembrolizumab) seem to be promising new approaches to treat drug-resistant LMS [41].
1.2. ESS Etiology, Prognosis, and Treatment
ESS is the second most common type of US [72] and arises from the uterine stroma. It is composed of endometrial stromal cells reminiscent of proliferative phase endometrium [7,13,15,73]. It is predominantly intramural, showing both a myometrial invasion and myometrial lymphovascular space permeation [7]. ESS pathogenesis is unknown, but tamoxifen exposure and some medical conditions (e.g., polycystic ovary syndrome) may contribute to its development [73,74]. Although a rare tumor, representing less than 1% of all uterine tumors, ESS accounts for up to 25% of all uterine sarcomas [58]. Symptoms related to ESS development and progression include abnormal uterine bleeding (about 90% of patients), uterine enlargement (70% of cases), pelvic pain, and dysmenorrhea. In 25% of the cases, however, the patients can be asymptomatic [73,75].
The most recent World Health Organization (WHO) classification (2020) for ESS is based on both cytogenetic and molecular analyses (i.e., gene fusion or alterations [76]) where the tumors are divided into benign endometrial stromal nodules (ESN), low-grade endometrial stromal sarcoma (LG-ESS), high-grade endometrial stromal sarcoma (HG-ESS), and undifferentiated uterine sarcomas (UUS) (Table 2) [76,77,78]. Morphologically, ESN is differentiable from LG-ESS only for the absence of lymphovascular invasion and myometrial infiltration. LG-ESS is usually positive for CD10, ER, and PR and can express actin, keratins, and calretinin [79], which differ from the HG–ESS tumors carrying the YWAE-NUTM2 fusion that do not express these markers. HG-ESS shows high expression of Cyclin-D1, c-KIT, and BCOR, and when ZC3H7B-BCOR fusion is present, CD10 and variable staining for ER and PR are also observed. Finally, UUS exhibits myometrial invasion, severe nuclear pleomorphism, high mitotic activity and/or necrosis, and loss of differentiation. This tumor, however, does not show a specific immunohistochemical profile, instead showing a diffused and atypical staining for CD10 as well as heterogeneous patterns of ER and PR staining [14,80].
Table 2.
Molecular features of endometrial stromal tumors (ESTs).
| Category EST | Fusion/Gene Alteration [72,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] |
|---|---|
| Endometrial Stromal Nodule (ESN) |
JAZF1-SUZ121 [86,87] MEAF6-PHF1 [86,87] |
| Low-Grade Endometrial Stromal Sarcoma (LG-ESS) |
JAZF1-SUZ121 [88] JAZF1-PHF1 [88] MEAF6-PHF1 [88] EPC1-PHF1 [89] MBTD1-EZHIP [89] JAZF1-BCORL1 [89] MAGED2-PLAG1 [90] MEAF6-SUZ12 [91] EPC2-PHF1 [92] BRD8-PHF1 [72] EPC1-BCOR [72] EPC1-SUZ12 [72] |
| High-Grade Endometrial Stromal Sarcoma (HG-ESS) |
YWHAE-NUTM2A/B1 [93] BCOR-rearrangement [94] ZC3H7B-BCOR [72,95] EPC1-BCOR [96] EPC1-SUZ12 [96] BCOR-ITD [72] LPP-BCOR [72] BRD8-PHF1 [97] |
| Undifferentiated Uterine Sarcoma (UUS) |
JAZF1-SUZ12 [97] YWHAE-NUTM2 [97] ZC3H7B-BCOR [97] YWHAE-rearrangement [97] HMGA-RAD51B [98] SMARCA4-deficient [99] |
| NTRK-Rearranged Uterine Sarcomas (HG-ESS) |
RBPMS-NTRK3 [100,101] TPR-NTRK1 [100,101] LMNA-NTRK1 [100,101] TPM3-NTRK1 [100,101] EML4-NTRK3 [100,101] STRN-NTRK3 [100,101] |
1 Most common alterations.
HG-ESS and UUS can be difficult to diagnostically differentiate from LMS since the latter can mimic both ESS and UUS. In this case, the immunohistochemical markers and morphological features can be useful, but not accurate. Tumor location can also provide important information because LMS is exclusively related to MM, while UUS may also involve the endometrium. Furthermore, ESS is also diagnosed only post-surgery, but unlike LMS, they present an indolent course with relapses occurring up to 20 years after diagnosis [81].
Overall, patients with ESS have a better life expectancy than other sarcomas. Their five-year survival rates are higher than 80%. For disease stages I and II the five-year survival is approximately 90% whereas for stages III and IV (i.e., advanced disease) the survival rate for the same interval of time is significantly reduced, according to the FIGO stage system [75,82].
Treatment protocols are defined based on the grade and stage of the tumor at the time of diagnosis. Total hysterectomy with bilateral salpingo-oophorectomy remains the standard treatment for ESS, and lymphadenectomy does not appear to improve overall survival rates [83]. Adjuvant radiotherapy and hormone therapy are not well-established therapeutic options yet, even though some studies have shown that hormonal agents can be an alternative to the management of LG-ESS [82,84]. In contrast, HG-ESS is generally detected in advanced stages with no effective adjuvant therapy available. In this case, immunotherapy with adoptive T cells transfer, targeting tumor fusion proteins, can be useful. Such an approach has been proved to be efficient in inhibiting tumor recurrences in other cancer types, thus inducing long-term memory cells and the persistent presence of these cells in the patient’s blood [85].
2. Genetics and Epigenetics Mechanisms in LMS and ESS
Genetic changes are related to alterations in the DNA sequences, whereas epigenetic modifications involve specific regulatory events apart from DNA codification [102,103,104,105,106,107,108]. Epigenetic events play an important role in several normal cellular processes, including embryonic development, genetic imprinting, and X-chromosome inactivation. When altered, epigenetic mechanisms may lead to several diseases, including cancer initiation and progression [106]. Epigenetic dysregulation affects gene functions by altering the gene expression mainly by (1) DNA methylation, (2) PTMs, and (3) RNA-mediated gene silencing by ncRNA (e.g., microRNA) (Figure 2) [102,109]. The main clinical and scientific interest in epigenetic events resides in the fact that they are reversible mechanisms [110,111].
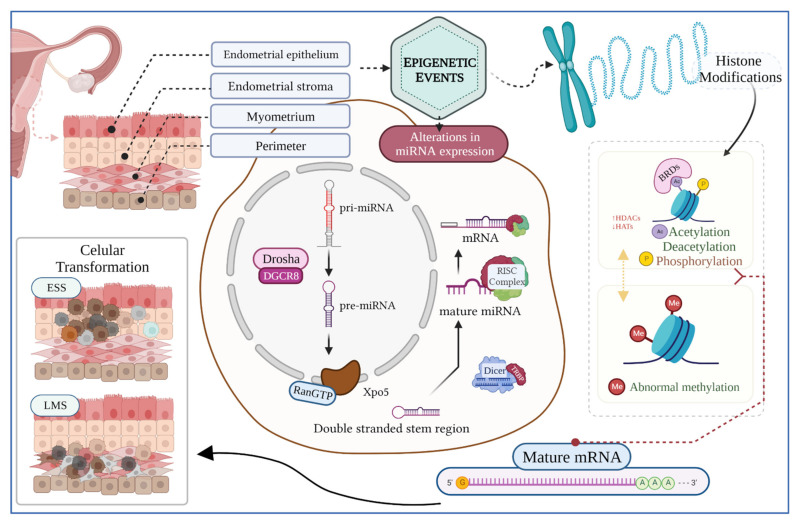
Figure 2.
Graphical representation of epigenetic events potentially involved in the initiation and development of the tumors, including uterine LMS and ESS. The biogenesis of miRNAs starts in the nucleus and ends in the cytoplasm. This process includes the participation of several enzymes and protein complexes that regulate the production of mature molecules capable of regulating gene expression, both through induction of mRNA degradation and translational repression. Likewise, dynamic alterations of histone modifications, including acetylation, methylation, and phosphorylation, modify gene expression, thus affecting DNA replication and repair, chromatin compaction, and cell cycle control. In addition, histone modification readers such as BRDs can recognize modified histones, therefore altering gene expression and responding to different signals. Dysregulation in the epigenetic machinery leads to malignant transformation of cells culminating in the development of cancer. Created with BioRender.com.
2.1. DNA Methylation
DNA methylation is the most studied and understood epigenetic event described to date. Found in more than 70% of the human genome, DNA methylation is crucial for cellular differentiation and normal development [112]. It consists of the addition of a methyl radical (CH3) to the 5-carbon on cytosine residues (5mC) in CpG dinucleotides [103,104,108,109,110,111,112,113,114]. DNA methyltransferases (DNMTs)—enzymes responsible for DNA methylation—are known to act in cancer cells by either hypomethylation or hypermethylation of specific CpG regions in the DNA [114].
Global DNA hypomethylation or loss of methylation has been associated with genomic instability as well as aneuploidy, loss of imprinting, reactivation of transposable elements, and endogenous retrovirus (ERVs) [108,113,115]. In cancer, hypomethylation is commonly followed by hypermethylation of localized CpG islands at the promoter and regulatory regions of target genes, which remain unmethylated in normal cells [109,115,116]. Hypermethylation of regulatory regions leads to transcriptional silencing by directly blocking the transcription factors binding to the promoter region, or by the binding of proteins with a high affinity for methylated DNA that compete with the transcription factors binding sites (Figure 3) [108,117].
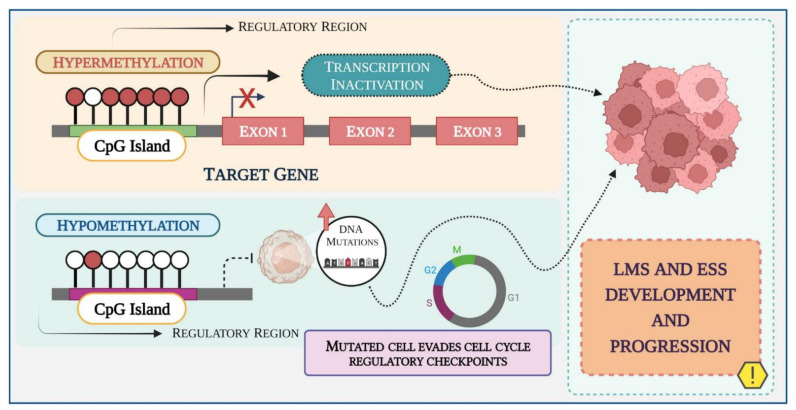
Figure 3.
Schematic representation of the DNA methylation process in LMS and ESS during cancer development and progression. Methyl groups are added to the DNA molecule and change its activity. Promoter hypermethylation has been shown to silence tumor suppressor genes in cancer cells, leading to either dysregulation of cell growth or inducing resistance to cancer therapies. Hypomethylation promotes genomic instability causing missegregation of chromosomes during cell division. Created with BioRender.com.
DNMTs are commonly found overexpressed in tumors, constituting an attractive target for specific therapy. The FDA has approved “epidrugs” such as 5-azacitidine (5-Aza), 5-aza-2-deoxycytidine (DAC), and the second-generation of the demethylation agent guadecitabine [116,118]. In LMS, Fischer et al. (2018) assessed the therapeutic potential of nucleoside analogs 5-Aza, DAC, and guadecitabine, using both in vitro and in vivo experiments. Their results show guadecitabine as a more effective inhibitor of both cell survival and colony formation in vitro. Additionally, animals who received this treatment showed a decrease in the tumor burden and increased survival [116].
Our group recently assessed the impact of DNMT inhibition on the Hedgehog (HH) signaling pathway with 5′-Aza-dc, in uterine LMS cells. We observed a reduction in the GLI1 mRNA, and SMO and GLI1 protein in response to the treatment. Moreover, GLI1 and GLI2 nuclear translocation were also decreased while nuclear translocation of GLI3 was increased. Our data showed that DNMT inhibitor, alone or in combination with pharmacological treatment, was able to block the HH pathway and showed a high inhibitory effect on the LMS malignant cells phenotype [119].
MGMT silencing due to its promoter hypermethylation has been commonly observed in several malignancies, including uterine sarcomas [120,121]. The methylation of the MGMT promoter region, which contributes to genome instability and sensitizes the cells to alkylating agents (such as Temozolomide (TMZ)), has been correlated with improved prognosis and as a potential factor of response to TMZ-based therapy prediction [121].
Global DNA methylation studies have also found methylation patterns or signatures that have been associated with different cancer hallmarks [122]. Braný et al. in 2019 observed differences in the methylation levels between MM and LM samples in the KLF4 and DLEC1 genes. Higher levels of methylation were found in LM compared to LMS cases, suggesting that methylation of KLF4 and DLEC1 are potential biomarkers to distinguish LM from LMS [123].
Hasan et al. (2021) identified differentially methylated and differentially expressed genes associated with LMS. Among the 77 hypermethylated genes, chromatin-modifying enzymes, including KAT6A, KMT2A and EZH2, and chromatin/DNA binding proteins such as CTNNB1, PBX3, SATB1, MEIS and COMMD1-BMI1 were observed. The findings indicate the possible involvement of chromatin modulation in regulating the DNA methylation of these genes [124].
A higher DNA damage response and hypomethylation of estrogen receptor 1 (ESR1) target genes were both observed when comparing uterine to extra uterine LMS [13].
Gene silencing through methylation can occur as frequently as mutations or deletions, leading to aberrant silencing of tumor suppressor genes [125]. In an LMS experimental model, the lack of BRCA1 function was associated with tumor initiation and development. This protein expression was next investigated in human samples, and a loss of 29% was associated with promoter methylation [126]. Methylation in the CDKN2A gene, using uterine LMS samples with a rhabdomyosarcomatous component, has also been described [127]. The authors identified both methylated and unmethylated alleles, originating mainly from the smooth muscle component. Moreover, the loss of heterozygosity in the rhabdomyosarcomatous component has been described exclusively in the cells expressing p16 and p14 [127].
Hierarchical clustering based on the hypermethylation of ALX1, CBLN1, CORIN, DUSP6, FOXP1, GATA2, IGLON5, NPTX2, NTRK2, STEAP4, PART1, and PRL allowed differentiation among several uterine tumors with up to 70% accuracy [128,129]. Clusters of distinct DNA methylation patterns have also been useful in distinguishing tumor types regardless of the number of CpG sites. Thus, LM and LMS were separated into two different clusters each, while ESS samples (LG- and HG-ESS) were grouped into two subtypes with specific profiles. The HG-ESS cluster included YWHAE and BCOR-rearranged tumors, distinct from LG-ESS and LMS [128,129]. Although LMS and HG-ESS are morphologically similar, the results show that the DNA methylation profile may be useful to discriminate against these closely related tumors [128].
DNA methylation plays a key role in gene expression regulation, inducing functional changes in key genes that regulate endometrial homeostasis [113]. Li et al. (2017) showed that the KLF4 promoter was hypermethylated in the ESS. They also found PCDHGC5 was highly methylated in the ESS samples when compared to endometrioid and endometrial serous carcinoma. sFRPs 1-5 are tumor suppressors that downregulate Wnt/β-catenin signaling [130,131,132,133]. Their consistent promoter hypermethylation and subsequent gene expression suppression were described in ESN, LG-ESS, and UUS.
Although the true role of DNA methylation patterns in the LMS and ESS initiation and progression is not completely understood, DNA methylation in normal endometrial stromal cells has been useful to identify signatures that indicate changes during decidualization or cell transformations [134,135,136,137]. Further studies to determine the precise methylation profile in pure mesenchymal tumors are certainly necessary to enable the characterization and differentiation of these tumors as well as to establish new therapeutic options.
2.2. Chromatin Remodeling
The chromatin is composed of DNA molecules tightly coiled around proteins called histones. This structure condensation degree is directly associated with greater or lesser RNA synthesis, with greater condensation (higher chromatin closing) being the state of more transcriptional repression [138]. The basic unit of chromatin, called nucleosome, is constituted by two copies of each core histone (H2A, H2B, H3, and H4) enveloped by DNA molecules [139]. Chromatin regulation occurs through PTMs of core histones and can involve phosphorylation, acetylation, methylation, ubiquitination, SUMOylation, and GlcNAcylation [139].
Currently, the two most studied and best understood mechanisms of chromatin regulation are histones acetylation and methylation. Such events are regulated by very specialized proteins called writers, erasers, and readers—which respectively add, remove, or recognize these PTMs [110,111,112,140,141,142]. Included in the writers’ group are histone acetyltransferases (HATs), DNA methyltransferases (DNMTs), histone lysine methyltransferases (HKMTs), and histone methyltransferases (HMTs). The erasers’ group includes histone deacetylase (HDACs) and histone demethylases (HDMs), ten eleven translocations (TETs), and histone lysine demethylases (HKDMs). In the reader’s group are methyl-CpG-binding domains (MBDs) and bromodomains [143].
Histone modifications affect the chromatin structure providing binding sites for several transcriptional factors. Its modification has a direct influence on gene expression, DNA replication and repair, chromatin compaction, and cell cycle control. Thus, loss of regulation of the histone modifications can lead to cancer pathogenesis and several developmental defects (Figure 4) [143].
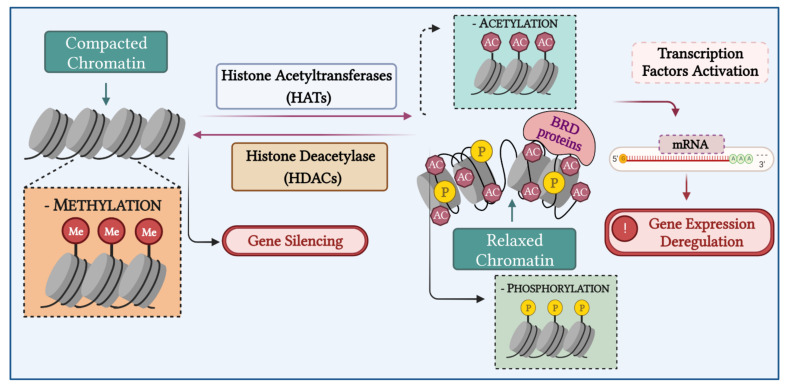
Figure 4.
Chromatin remodeling process in LMS and ESS. Chromatin remodeling is an important mechanism of gene expression regulation. In the histone acetylation induced by HATs, the condensed chromatin is transformed into a more relaxed structure (euchromatin) that is associated with greater levels of gene transcription, while histone hypoacetylation induced by HDAC activity is associated with more condensed chromatin (heterochromatin), inducing gene silencing. Altered expression and mutations of genes that encode HDACs have been linked to tumor development. Created with BioRender.com.
The lysine residues of histones H3 and H4 are targeted for methylation by site-specific enzymes, culminating in activation or repression of the gene expression. [144]. This molecular mechanism is uniquely able to originate three methylation levels: me1 (mono), me2 (di), and me3 (trimethylation). Lysine methylations may lead to both transcriptional activation and repression, depending on the lysine residue’s location [143].
In endometrial stromal cells, the transition from a proliferative to a decidual phenotype occurs due to the loss of the EZH2-dependent methyltransferase activity, which is part of the chromatin remodeling process [145]. The decidualization process in those cells down-regulates EZH2, resulting in lower levels of H3K27me3 at the promoter region of PRL and IGFBP1 (two decidual marker genes). The H3K27me3 loss, associated with acetylation enrichment in the same lysine residue, indicates the transition from a transcriptionally repressive chromatin form to a permissive one [145].
Little is known about the specific underlying mechanism of histone acetylation or methylation associated with the “pure” sarcoma pathobiology. A unique study available in the literature describes the fatty acid synthase (FASN)-enhanced expression inducing cell proliferation, migration, and invasion, in transfected cells of uterine LMS. It has been observed that FASN promotes H3K9me3 and H3K27ac by alteration in the HDAC, HDM, HMT, and HAT trimethylation activity. Thus, in the uterine LMS cells, the epigenome reprogramming by chromatin remodeling seems to induce a higher malignant phenotype [146].
The polycomb group (PcG) proteins are well-characterized transcriptional repressors that are essential for the regulation of physiological processes in several organisms. PcG proteins are known to form two distinct complexes with defined enzymatic activities: polycomb repressive complex 1 (PRC1), a histone ubiquitin ligase related to chromatin compaction; and PRC2, an HMT that mediates both H3K27me3 and target genes repression [147,148,149,150]. In several cancer types, the expression and function of PcG proteins are often found dysregulated [148,151,152,153], and their targeted deletions generally induce lethal phenotypes [153].
PRC1 catalytic core has two related E3 ubiquitin ligases, the RING1 (RING1A) or RNF2 (RING1B) that catalyze ubiquitination and BMI1 (polycomb ring finger oncogene), and one of six PCGF orthologues. The latter constitutes a PRC1 variant containing BCOR and KDM2B [154,155,156,157]. Translocations or chromosomal rearrangements, involving fusion proteins have also been implicated in PRCs mechanisms. Fusions such as KDM2B-CREBBP [158], ZC3H7-BCOR [79,155,156,159,160], JAZF1-BCORL1 [79,91,161], EPC1-BCOR [72,95], LPP-BCOR [72] and BCOR internal tandem duplications (BCOR-ITD) are frequently found in ESS, and have recently been also found in LMS samples [80,160,162,163]. Additionally, gene fusion such as MBTD1-CXorf67 [79,91,151,164], MBTD1-EZHIP [90] and MBTD1-PHF1 in ESS are found as products of PRC1-associated protein [152].
PRC2 has in its core subunits the EZH2 (or its homolog EZH1), EED, SUZ12, and RbAp46 (or 48). EZH1 and EZH2 are responsible for the H3K27me3 generation. EED binds to H3K27me3, enhancing the EZH2 catalytic activity, while SUZ12 is essential for the PRC2 activity, and bAp46/48 acts as a chaperone. H3K27 trimethylation leads to the suppression of several relevant genes, including tumor suppressors [165].
There are two well-characterized PcG proteins, the BMI1 and EZH2, which are required for the regulation of the PRC activity but are also known to display oncogenic functions in several cancers. BMI1 was the first identified PcG protein that was described as a proto-oncogene, and although there are no specific studies regarding the BMI1 role in uterine LMS [157,166]. Gao et al. (2021) have described one CD133 cell subpopulation that was derived from SK-UT-1 (uterine LMS cells) with enhanced levels of this protein. The authors found BMI1, among other CSCs-related (cancers stem cell) markers, up regulated in the CD133+ cells when compared to the negative cell population [167].
Zhang et al. (2018) investigated both gene and protein expressions of the four PRC2 subunits (EZH2, SUZ12, EED, and RbAp46) in extra-uterine and uterine LMS samples. The authors observed 91% of sensitivity and 100% of specificity for EZH2 positive staining in well-differentiated LMS, suggesting this expression is a specific marker for this tumor. Furthermore, the increased expression of EZH2 was inversely correlated with SUZ12 and EED expressions, leading to PRC2 suppression and H3K27me3 decrease [168].
Chromosomal rearrangements in genes belonging to the PRC2 complex, or in proteins that interact with them, have previously been described in the ESS [131]. JAZF1-SUZ12 (previously named JAZF1-JJAZ1) has been frequently reported as the most common feature of ESS [131,164,169,170,171,172]. Additionally, several other modifications such as EPC1-PHF1 [158,172,173], MEAF6-PHF1 [87,161,162,170], EPC1-ZUZ12 [72,95], MEAF6-SUZ12 [91,174], BRD8-PHF1 [169], JAZF1-PHF1 [88,158] and YWHAE-NUTM2A/B (previously known as FAM22A/B) [79,132,175] have also been reported. Panagopoulos et al. in 2012 observed that the rearrangement of genes involved in acetylation and methylation can be associated with ESS pathogenesis. LG-ESS harboring the EPC1-PHF1 fusion gene has decreased levels of H3K27me3 and a concomitant increase of H3K36me3 [176]. PHF1 acts in cell proliferation through the modulation of histone H3 methylation [171].
EZH2 can interact with HDAC1 and HDAC2, through the EED protein, suggesting that the transcriptional repression by the PRC2 complex may be mediated by HDACs [177]. These enzymes act in the acetylation control of transcription factors [178], and their classification (Class I, IIa, IIb, III, and IV) is based on their activity, structural similarity, subcellular localization, and expression patterns [179]. In LMS patients, strong expression of HDACs 1, 2, 3, 4, 6, and 8 were associated with unfavorable prognosis, while HDACs 5, 7, or 9 weak expressions, together with p53 expression, were associated with favorable disease-free survival (DFS). HDACs 5, 7, and 9 were associated with better survival outcomes, whereas HDAC5 expression was an independent predictor for DFS in epithelioid subtype tumors [180]. In vitro analysis using SK-UT-1, SK-LMS-1, MES-SA, and DMR cell lines demonstrated that HDAC9 (Class IIa) transcription is under MEF2D direct control, and this axis sustains cell proliferation and survival through FAS repression [177].
In ESS, it has been observed that high expression of HDACs 1, 4, 6, 7, and 8 is associated with lower DFS [181] whereas, in UUS, distant tumor recurrence was associated with a strong expression of HDAC6 [140,182].
The increased HDAC activity often observed in cancers justifies the number of current studies investigating HDAC inhibitors as novel therapeutic agents [183,184]. These studies have shown promising results for metastatic LMS [140,180,181,185]. In this context, mocetinostat acts by turning on tumor suppressor genes, restoring their normal function, and reducing tumor growth [140,180]. Its use as mono- or combinatorial therapy has been evaluated in metastatic extra-uterine and uterine LMS with resistance to gemcitabine and found to induce regression of tumors with acquired chemoresistance. Romidepsin, LBH589, belinostat, SAHA, and valproate (other HDAC inhibitors) have shown good results alone or in combination with decitabine [180,186].
Combinatorial therapy using SAHA, LY294002 (PI3K inhibitor), and rapamycin (mTOR inhibitor) were tested in ESS cell culture [186]. The results show that SAHA combined with either LY294002 or rapamycin, or both, reduce specifically phospho-p70S6K and 4E-BP1 levels, inhibiting the tumor cell proliferation [186,187]. A strong reduction of mTOR and phospho-mTOR levels has been reported after treatment with either SAHA or rapamycin, by targeting phospho-S6rp, in ESS cells [188]. Fröhlich et al. (2014) showed the benefit of SAHA treatment associated with TRAIL/Apo-2L in two US cell lines [189].
Another study assessed the effects of combined therapy with valproic acid (VPA, a weak histone deacetylase inhibitor), bevacizumab (mAb against VEGF), gemcitabine, and docetaxel, for extra- and intrauterine unresectable or metastatic soft tissue sarcomas [184]. This study found partial response in one case of carcinosarcoma, two extrauterine LMSs, two undifferentiated pleomorphic sarcomas, and one uterine LMS patient. This pharmacological combination was well tolerated and overall safe, showing that the combination of traditional medication and “epidrugs” may truly represent a new treatment strategy for sarcoma [184].
New therapeutic strategies to specifically treat US, such as regional hyperthermia, combined with chemotherapy, radiotherapy, and/or immunotherapy have emerged in the last few years. Pazopanib (a multitargeted tyrosine kinase inhibitor with antiangiogenic effects) combined with hyperthermia has demonstrated synergistic effects mainly for LMS growth inhibition, in vitro and in vivo [185]. This approach induces HAT1 downregulation by suppressing Clock which, in turn, is responsible for H3 and H4 acetylation [190].
Histone phosphorylation, which takes place predominantly but not exclusively on serine, threonine, and tyrosine residues at the histone tails [142], has gained considerable attention, especially regarding the histone H3, due to its close association with mitotic chromosome condensation in mammalian cells [191]. A preliminary study evaluating the mitotic index, based on H3 phosphorylation in LMS, found Phospho Histone H3 (PHH3) positive staining to be a promising mitosis-specific marker for this tumor [192].
Bromodomain-containing proteins (BRDs), as the “readers” of lysine acetylation are responsible for transducing regulatory signals carried by acetylated lysine residues into various biological phenotypes [193]. BRDs can exert a wide variety of functions via multiple gene regulatory mechanisms [194] and the deregulation of BRDs is involved in many diseases, including cancer [195,196,197]. BRD9 is a newly identified subunit of the noncanonical barrier-to-autointegration factor (ncBAF) complex and a member of the bromodomain family IV [198]. Studies have demonstrated that BRD9 plays an oncogenic role in multiple cancer types, by regulating tumor cell growth. Furthermore, the connection of BRD9 with the PI3K pathway [199], microRNAs [200], and STAT5 [201] is implicated in cancer progression. It has been shown that BRD9 is aberrantly overexpressed in uterine LMS tissues, compared to adjacent myometrium. [202]. In addition, BRD9 expression was upregulated in uterine LMS cell lines compared to benign LM and myometrium cell lines. Notably, targeting the BRD9 with a specific chemical inhibitor (TP-472) can suppress the LMS cell growth, concomitantly sculpting the transcriptome of uterine LMS cells, altering the important pathways, reprogramming the oncogenic epigenome, and inducing the miRNA-mediated gene regulation. These studies reveal that BRD9 constitutes a specific vulnerability in malignant LMS and that targeting non-bromodomain and extra-terminal BRDs in uterine LMS may provide a promising and novel strategy for treating patients with this aggressive uterine cancer [202].
In summary, histone modifications are frequently found in US, thus representing potential targets for new therapeutic strategies development. Several studies have demonstrated that HDAC inhibitors could modulate several signaling pathways, activating, or inhibiting numerous cascades that lead to an antitumor response. Moreover, combination with chemo- or targeted- therapies is likely to strengthen the activity of HDAC inhibitors. However, it is still necessary to further elucidate how the histone modifications are regulated, as well as to understand the mechanism of action of their available inhibitors in LMS and ESS. This information will enable more efficient clinical trials, which could lead to an improvement in patients’ response to treatment and overall survival [140].
2.3. Non-Coding RNA (ncRNAs)
ncRNA is known to regulate gene expression both at transcriptional and post-transcriptional levels. ncRNAs play an important role in epigenetic processes, including modulation of heterochromatin, histone modification, DNA methylation, and gene silencing (Figure 5) [108]. These molecules can be divided into housekeeping and regulatory ncRNAs [110,203]. Regulatory ncRNAs are classified according to their size in small non-coding RNAs (sncRNAs), with approximately 19-200 nucleotides (nt), and long non-coding RNAs (lncRNAs), with more than 200 nt [105,108,113,204,205]. The sncRNAs have a wide range of structural and functional roles in gene expression regulation, RNA splicing, and chromatin structure [206,207]. sncRNAs includes four different categories: (1) small interfering RNA (siRNA), (2) microRNA (miRNA), (3) PIWI-interfering RNA (piRNA, with approximately 19-31 nt), and (4) small nucleolar RNA (snoRNA, with 60-300 nt) [111,203]. miRNA and piRNA are probably the most studied sncRNAs categories to date, and their functions are well established in the literature [206]. Due to miRNAs’ broad roles, mainly at the post-transcriptional level, dysfunctions in their regulation have been associated with the development of several diseases, including cancer [205,206,208].
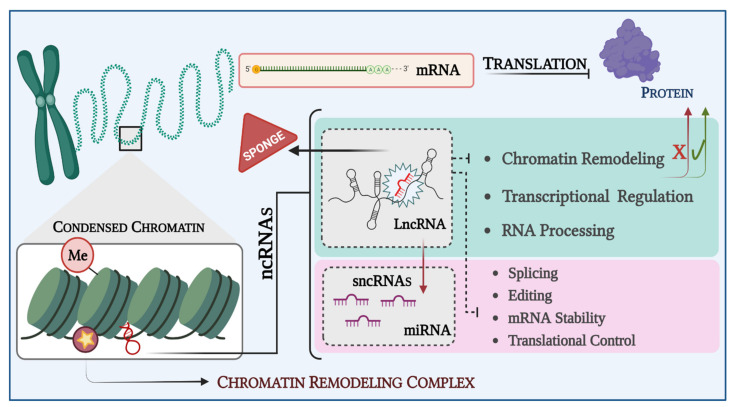
Figure 5.
Graphical representation of the ncRNAs dysregulation in LMS and ESS. ncRNAs have been identified as oncogenic drivers or tumor suppressors in cancer. LncRNAs often affect the expression of their target genes by interacting with miRNAs, which are the main post-transcriptional regulation factors. Some lncRNAs act like sponges, thereby preventing miRNAs from binding to their target mRNAs. As lncRNAs work as decoys for miRNAs, oncogene mRNA translation is allowed, starting the LMS and ESS carcinogenesis. Created with BioRender.com.
To understand the complex biology of sarcomas, numerous correlative and functional studies aiming to integrate gene expression patterns and miRNAs have been carried out [209]. As mentioned above, the differential diagnosis of LMS is still a challenge, and studies focusing on new biomarkers to help distinguish uterine LMS from LM are extremely important [210,211,212,213]. Yokoi et al. (2019) demonstrate the feasibility of circulating serum miRNAs detection as a preoperative clinical assay to detect US. They identified two miRNA signatures (miR-1246 and miR-191-5p) in uterine LMS (95% confidence interval of 0.91–1.00) [214]. Dvorská et al. (2019), and later, Wei et al. (2020) reviewed how liquid biopsies could increase the overall understanding of uterine LMS behavior and how its molecular profile could contribute to more accurate discrimination from LM [210,211].
Comparing LMS, LM, and MM, Anderson et al. (2014) found 37 miRNAs differentially expressed in uterine LMS. The lack of miR-10b in LMS samples was critical for tumor growth and metastasis. Indeed, rescuing miR-10b expression in the cell lines resulted in prominent inhibition of cell proliferation, migration, and invasion, and increased apoptosis. Similarly, stable miR-10b expression significantly reduced the number and size of tumor implants in vivo by reducing cell proliferation and increasing apoptosis [212].
Later, Schiavon et al. (2019) found that dysregulation of miR-148a-3p, 27b-3p, 124-3p, 183-5p, and 135b-5p expression was associated with tumor relapse, increased metastasis, and poor survival rates in uterine LMS patients [213]. De Almeida et al. (2017) evaluated the miRNAs expression profile in cell lines of MM, LM, and LMS. Thirteen molecules presented differential expression profiles in LM and LMS, compared to normal tissue (MM). Additionally, the authors observed that miR-1-3p, miR-130b-3p, miR-140-5p, miR-202, miR-205, and miR-7-5p presented similar expression patterns between the cell lines and 16 patients’ samples [215].
Zhang et al. (2014) demonstrated that miRNAs were significantly dysregulated among different types of uterine smooth muscle tumors (USMTs), including ordinary LM, mitotically active leiomyoma (MALM), cellular leiomyoma (CLM), atypical leiomyoma (ALM), uterine smooth muscle tumor of uncertain malignant potential (STUMP), and LMS samples. The miRNA expression profile showed that ALM and LMS shared similar signatures (including miR-34a-5p, miR-10b-5p, miR-21-5p, miR-490-3p, miR-26a-5p and miR-650). Unsupervised analysis divided the tumors into three clusters: LMS/ALM, LM/STUMP, and CLM/MM [216]. miR-200c was found to be significantly downregulated in LM, compared to MM [217], acting directly in ZEB1/2, VEGFA, FBLNS, and TIMP2 regulation. Next, the authors observed a significant reduction of miR-200c in the SK-LMS-1 cells, compared to isolated LM cells, indicating this miRNA is an important marker for LM progression and malignance risk [4,218].
To date, the differential expression (i.e., up- or down-regulation) of several miRNAs has been directly correlated with US patients’ prognosis. In 2018, Dos Anjos et al. analyzed the expression profile of 84 cancer-related miRNAs and associated their signatures with patients’ clinical and pathological data. In LMS, specifically, the authors found an association between miRNA dysregulation and lower cancer-specific survival (CSS) and aggressive tumor phenotype. In ESS samples, alterations in miRNA regulation were related to both lower CSS and metastasis [219].
Shi et al. (2009) found a significant inverse correlation between endogenous HMGA2 levels and let-7 expression in uterine LMS. Their study revealed that the ectopic expression of let-7a inhibits LMS proliferation by HMGA2 repression, suggesting that the let-7 loss of expression can represent a worse prognostic factor [220]. Zavadil et al. (2010) identified the way in which let-7s is responsible for the direct regulation of PPP1R12B, STARD13, TRIB1, BTG2, HMGA2, and ITGB3 genes (involved in the cell proliferation and extracellular matrix regulation) in LM samples. [221]. De Almeida et al. (2019), found that decreased expression of let-7 family members was directly correlated with worse prognosis, affecting both the overall survival (OS) and the DFS rates of the LMS patients [22].
Dysregulation of some miRNAs has also been correlated with acquired chemoresistance in uterine neoplasm. For instance, the loss of miR-34a expression and its release from LMS cells via exosomes contribute indirectly to the tumor doxorubicin chemoresistance. This mechanism seems to be mediated by MELK overexpression and the recruitment of M2 macrophages [216].
Although less studied than other ncRNAs, lncRNAs are known to interact with either DNA, RNA, or proteins, and play a significant regulatory function in several cellular processes [208]. lncRNAs are responsible for regulating transcription on three different levels: pre-transcriptional (chromatin remodeling), transcriptional, and post-transcriptional [203,222]. Some similarities can be found between lncRNAs and mRNAs, including size, transcription by RNA pol-II, 5′-capping, RNA splicing, and poly(A) tail (approximately 60% of all lncRNAs) [223]. LncRNAs can be stratified into five categories: (1) intergenic (present between two protein-coding genes), (2) intronic (between the introns of a protein-coding gene), (3) overlapping (a coding gene is located on the intron of a lncRNA), (4) antisense (the lncRNA is transcribed from the opposite strand of a protein-coding gene), and (5) processed lncRNAs (lacks an open reading frame ORF) [208,224]. lncRNA can be expressed in distinguished cell regions and their functions are directly related to their sub-cellular location. However, these epigenetic regulators may suffer molecular alterations that affect their expression and, consequently, their physiological function. Accumulated evidence shows that several differentially expressed lncRNAs are related to cancer development, progression, and metastasis [204,222].
Unfortunately, in uterine LMS and ESS, the molecular role of lncRNAs and their regulation remains unclear. Yet, Guo et al. (2014) performed a microarray-based genome-wide analysis of lncRNAs, including 35 LM and MM-matched samples. The authors showed, for the first time, the differential expression profile of the lncRNAs between these tissues. The expression pattern obtained was associated with the downregulation of the cytokine–cytokine receptor interaction pathway in large LM, and the upregulation of the fatty acid metabolism pathway in small LM. This study, although preliminary, sheds light on future studies that will attempt to elucidate the role of lncRNAs specifically in uterine mesenchymal tumors [225].
3. Conclusions
Uterine pure sarcomas constitute the most frequently diagnosed group of malignant neoplasms in the uterine body. LMS and ESS are distinct tumors with a variety of features similar to other uterine neoplasms. The high heterogeneity, morphological and molecular variations pose challenges to subtypes differentiation and diagnosis. The origin of these tumors remains unclear, as well as the molecular mechanisms that drive their clinical and biological behavior. However, genetic, and epigenetic mechanisms have been shown to directly and indirectly influence the USMT malignant transformation, but the high complexity of this group of tumors still represents a barrier to diagnosis and disease management. In this review, we provided insights into the most recent studies regarding epigenetic events in LMS and ESS, and their potential as novel biomarkers or for developing new therapeutic modalities to treat these tumors.
Acknowledgments
Not applicable.
Author Contributions
B.C.d.A. and L.G.d.A. literature review, data collection and manuscript preparation; A.S.D., E.C.B., Q.Y. and A.A.-H. manuscript review, intellectual and technical support; K.C.C. idea conception, data collection and manuscript preparation, and review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Fundação De Amparo à Pesquisa do Estado Do São Paulo (FAPESP 2019/01109-2) and Coordenação de Aperfeiçoamento de Pessoal De Nível Superior—Brasil (CAPES).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uduwela A.S., Perera M.A.K., Aiqing L., Fraser I.S. Endometrial-myometrial interface: Relationship to adenomyosis and changes in pregnancy. Obstet. Gynecol. Surv. 2000;55:390–400. doi: 10.1097/00006254-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Commandeur A.E., Styer A.K., Teixeira J.M. Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth. Hum. Reprod. Update. 2015;21:593–615. doi: 10.1093/humupd/dmv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nothnick W.B. Non-coding rnas in uterine development, function and disease. Adv. Exp. Med. Biol. 2016;886:171. doi: 10.1007/978-94-017-7417-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brany D., Dvorska D., Nachajova M., Slavik P., Burjanivova T. Malignant tumors of the uterine corpus: Molecular background of their origin. Tumor Biol. 2015;36:6615–6621. doi: 10.1007/s13277-015-3824-1. [DOI] [PubMed] [Google Scholar]
- 5.Kuperman T., Gavriel M., Gotlib R., Zhang Y., Jaffa A., Elad D., Grisaru D. Tissue-engineered multi-cellular models of the uterine wall. Biomech. Model. Mechanobiol. 2020;19:1629–1639. doi: 10.1007/s10237-020-01296-6. [DOI] [PubMed] [Google Scholar]
- 6.De Almeida T.G., da Cunha I.W., Maciel G.A.R., Baracat E.C., Carvalho K.C. Clinical and molecular features of uterine sarcomas. Med. Exp. 2014;1:291–297. doi: 10.5935/MedicalExpress.2014.06.02. [DOI] [Google Scholar]
- 7.Desar I.M.E., Ottevanger P.B., Benson C., van der Graaf W.T.A. Systemic treatment in adult uterine sarcomas. Crit. Rev. Oncol. Hematol. 2018;122:10–20. doi: 10.1016/j.critrevonc.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Koivisto-Korander R., Butzow R., Koivisto A.-M., Leminen A. Immunohistochemical studies on uterine carcinosarcoma, leiomyosarcoma, and endometrial stromal sarcoma: Expression and prognostic importance of ten different markers. Tumour Biol. 2011;32:451–459. doi: 10.1007/s13277-010-0138-1. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society What is Uterine Sarcoma? [(accessed on 21 April 2022)]. Available online: https://www.cancer.org/cancer/uterine-sarcoma/about/what-is-uterine-sarcoma.html.
- 10.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 11.Analysis Tool American Cancer Society—Cancer Facts & Statistics. [(accessed on 15 April 2022)]. Available online: https://cancerstatisticscenter.cancer.org/#!/data-analysis/module/BmVYeqHT?type=barGraph.
- 12.Parra-Herran C., Howitt B.E. Uterine mesenchymal tumors: Update on classification, staging, and molecular features. Surg. Pathol. Clin. 2019;12:363–396. doi: 10.1016/j.path.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Tsuyoshi H., Yoshida Y. Molecular biomarkers for uterine leiomyosarcoma and endometrial stromal sarcoma. Cancer Sci. 2018;109:1743–1752. doi: 10.1111/cas.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mbatani N., Olawaiye A.B., Prat J. Uterine sarcomas. Int. J. Gynaecol. Obstet. 2018;143:51–58. doi: 10.1002/ijgo.12613. [DOI] [PubMed] [Google Scholar]
- 15.D’Angelo E., Prat J. Uterine sarcomas: A review. Gynecol. Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society Uterine Sarcoma Stages. [(accessed on 21 April 2022)]. Available online: https://www.cancer.org/cancer/uterine-sarcoma/detection-diagnosis-staging/staging.html.
- 17.Roberts M.E., Aynardi J.T., Chu C.S. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol. Oncol. 2018;151:562–572. doi: 10.1016/j.ygyno.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Sociedade Brasileira de Patologia Útero Neoplasias Mesenquimais do Corpo Uterino. [(accessed on 20 January 2022)]. Available online: http://www.sbp.org.br/mdlhisto/utero-neoplasias-mesenquimais-corpo-uterino/
- 19.Laganà A.S., Vergara D., Favilli A., la Rosa V.L., Tinelli A., Gerli S., Noventa M., Vitagliano A., Triolo O., Rapisarda A.M.C., et al. Epigenetic and genetic landscape of uterine leiomyomas: A current view over a common gynecological disease. Arch. Gynecol. Obstet. 2017;296:855–867. doi: 10.1007/s00404-017-4515-5. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y., Chan Y.-T., Tan H.-Y., Li S., Wang N., Feng Y. Epigenetic regulation in human cancer: The potential role of epi-drug in cancer therapy. Mol. Cancer. 2020;19:79. doi: 10.1186/s12943-020-01197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro A., Yin P., Ono M., Monsivais D., Moravek M.B., Coon J.S.V., Dyson M.T., Wei J.-J., Bulun S.E. 5-Hydroxymethylcytosine promotes proliferation of human uterine leiomyoma: A biological link to a new epigenetic modification in benign tumors. J. Clin. Endocrinol. Metab. 2014;99:E2437. doi: 10.1210/jc.2014-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Almeida B.C., dos Anjos L.G., Uno M., Cunha I.W., Soares F.A., Baiocchi G., Baracat E.C., Carvalho K.C. Let-7 miRNA’s expression profile and its potential prognostic role in uterine leiomyosarcoma. Cells. 2019;8:1452. doi: 10.3390/cells8111452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tantitamit T., Huang K.-G., Manopunya M., Yen C.-F. Outcome and management of uterine leiomyosarcoma treated following surgery for presumed benign disease: Review of literature. Gynecol. Minim. Invasive Ther. 2018;7:47–55. doi: 10.4103/GMIT.GMIT_10_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benson C., Miah A.B. Uterine sarcoma—Current perspectives. Int. J. Womens Health. 2017;9:597–606. doi: 10.2147/IJWH.S117754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seagle B.L.L., Sobecki-Rausch J., Strohl A.E., Shilpi A., Grace A., Shahabi S. Prognosis and treatment of uterine leiomyosarcoma: A national cancer database study. Gynecol. Oncol. 2017;145:61–70. doi: 10.1016/j.ygyno.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Bogani G., Ditto A., Martineli F., Signorelli M., Chiappa V., Fonatella C., Sanfilippo R., Leone Roberti Maggiore U., Ferrero S., Lorusso D., et al. Role of bevacizumab in uterine leiomyosarcoma. Crit. Rev. Oncol. Hematol. 2018;126:45–51. doi: 10.1016/j.critrevonc.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Juhasz-Böss I., Gabriel L., Bohle R.M., Horn L.C., Solomayer E.F., Breitbach G.P. Uterine leiomyosarcoma. Oncol. Res. Treat. 2018;41:680–686. doi: 10.1159/000494299. [DOI] [PubMed] [Google Scholar]
- 28.Ray-Coquard I., Serre D., Reichardt P., Martín-Broto J., Bauer S. Options for treating different soft tissue sarcoma subtypes. Future Oncol. 2018;14:25–49. doi: 10.2217/fon-2018-0076. [DOI] [PubMed] [Google Scholar]
- 29.Meng Y., Yang Y., Zhang Y., Li X. Construction and validation of nomograms for predicting the prognosis of uterine leiomyosarcoma: A population-based study. Med. Sci. Monit. 2020;26:e922739-1. doi: 10.12659/MSM.922739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittal K.R., Chen F., Wei J.J., Rijhvani K., Kurvathi R., Streck D., Dermody J., Toruner G.A. Molecular and immunohistochemical evidence for the origin of uterine leiomyosarcomas from associated leiomyoma and symplastic leiomyoma-like areas. Mod. Pathol. 2009;22:1303–1311. doi: 10.1038/modpathol.2009.96. [DOI] [PubMed] [Google Scholar]
- 31.Yasutake N., Ohishi Y., Taguchi K., Hiraki Y., Oya M., Oshiro Y., Mine M., Iwasaki T., Yamamoto H., Kohashi K., et al. Insulin-like growth factor II messenger RNA-binding protein-3 is an independent prognostic factor in uterine leiomyosarcoma. Histopathology. 2017;12:3218–3221. doi: 10.1111/his.13422. [DOI] [PubMed] [Google Scholar]
- 32.Patel D., Handorf E., von Mehren M., Martin L., Movva S. Adjuvant chemotherapy in uterine leiomyosarcoma: Trends and factors impacting usage. Sarcoma. 2019;2019:3561501. doi: 10.1155/2019/3561501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittal K., Joutovsky A. Areas with benign morphologic and immunohistochemical features are associated with some uterine leiomyosarcomas. Gynecol. Oncol. 2007;104:362–365. doi: 10.1016/j.ygyno.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Banas T., Pitynski K., Okon K., Czerw A. DNA fragmentation factors 40 and 45 (DFF40/DFF45) and B-cell lymphoma 2 (Bcl-2) protein are underexpressed in uterine leiomyosarcomas and may predict survival. OncoTargets Ther. 2017;10:4579–4589. doi: 10.2147/OTT.S142979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams A.R.W. Uterine fibroids—What’s new? F1000Research. 2017;6:2109. doi: 10.12688/f1000research.12172.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang P., Zhang C., Hao J., Sung C.J., Quddus M.R., Steinhoff M.M., Lawrence W.D. Use of X-chromosome inactivation pattern to determine the clonal origins of uterine leiomyoma and leiomyosarcoma. Hum. Pathol. 2006;37:1350–1356. doi: 10.1016/j.humpath.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Oh J., Park S.B., Park H.J., Lee E.S. Ultrasound features of uterine sarcomas. Ultrasound Q. 2019;35:376–384. doi: 10.1097/RUQ.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 38.Chen L., Yang B. Immunohistochemical analysis of P16, P53, and Ki-67 expression in uterine smooth muscle tumors. Int. J. Gynecol. Pathol. 2008;27:326–332. doi: 10.1097/PGP.0b013e31815ea7f5. [DOI] [PubMed] [Google Scholar]
- 39.Loizzi V., Cormio G., Nestola D., Falagario M., Surgo A., Camporeale A., Putignano G., Selvaggi L. Prognostic factors and outcomes in 28 cases of uterine leiomyosarcoma. Oncology. 2011;81:91–97. doi: 10.1159/000331679. [DOI] [PubMed] [Google Scholar]
- 40.Devereaux K.A., Schoolmeester J.K. Smooth muscle tumors of the female genital tract. Surg. Pathol. Clin. 2019;12:397–455. doi: 10.1016/j.path.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Byar K.L., Fredericks T. Uterine leiomyosarcoma. J. Adv. Pract. Oncol. 2022;13:70–76. doi: 10.6004/jadpro.2022.13.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoang H.L.T., Ensor K., Rosen G., Leon Pachter H., Raccuia J.S. Prognostic factors and survival in patients treated surgically for recurrent metastatic uterine leiomyosarcoma. Int. J. Surg. Oncol. 2014;2014:919323. doi: 10.1155/2014/919323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman C.F., Hensley M.L. Options for adjuvant therapy for uterine leiomyosarcoma. Curr. Treat. Options Oncol. 2018;19:7. doi: 10.1007/s11864-018-0526-0. [DOI] [PubMed] [Google Scholar]
- 44.Stope M.B., Cernat V., Kaul A., Diesing K. Functionality of the tumor suppressor microrna-1 in malignant tissue and cell line cells of uterine leiomyosarcoma. Anticancer Res. 2018;1550:1547–1550. doi: 10.21873/anticanres.12383. [DOI] [PubMed] [Google Scholar]
- 45.Rizzo A., Pantaleo M.A., Saponara M., Nannini M. Current status of the adjuvant therapy in uterine sarcoma: A literature review. World J. Clin. Cases. 2019;7:1753. doi: 10.12998/wjcc.v7.i14.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y., Ren H., Wang J. Outcome of adjuvant radiotherapy after total hysterectomy in patients with uterine leiomyosarcoma or carcinosarcoma: A SEER-based study. BMC Cancer. 2019;19:697. doi: 10.1186/s12885-019-5879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amant F., Lorusso D., Mustea A., Duffaud F., Pautier P. Management strategies in advanced uterine leiomyosarcoma: Focus on trabectedin. Sarcoma. 2015;2015:704124. doi: 10.1155/2015/704124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barlin J.N., Zhou Q.C., Leitao M.M., Bisogna M., Olvera N., Shih K.K., Jacobsen A., Schultz N., Tap W.D., Hensley M.L., et al. Molecular subtypes of uterine leiomyosarcoma and correlation with clinical outcome. Neoplasia. 2015;17:183–189. doi: 10.1016/j.neo.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Ami E., Barysauskas C.M., Solomon S., Tahlil K., Malley R., Hohos M., Polson K., Loucks M., Severgnini M., Patel T., et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer. 2017;123:3285–3290. doi: 10.1002/cncr.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elvin J.A., Gay L.M., Ort R., Shuluk J., Long J., Shelley L., Lee R., Chalmers Z.R., Frampton G.M., Ali S.M., et al. Clinical benefit in response to palbociclib treatment in refractory uterine leiomyosarcomas with a common CDKN2A alteration. Oncologist. 2017;22:416–421. doi: 10.1634/theoncologist.2016-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Cancer Society Survival Rates for Uterine Sarcoma. [(accessed on 13 January 2022)]. Available online: https://www.cancer.org/cancer/uterine-sarcoma/detection-diagnosis-staging/survival-rates.html.
- 52.Kobayashi H., Uekuri C., Akasaka J., Ito F., Shigemitsu A., Koike N., Shigetomi H. The biology of uterine sarcomas: A review and update. Mol. Clin. Oncol. 2013;1:599–609. doi: 10.3892/mco.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baek M.-H., Park J.-Y., Park Y., Kim K.-R., Kim D.-Y., Suh D.-S., Kim J.-H., Kim Y.-M., Kim Y.-T., Nam J.-H. Androgen receptor as a prognostic biomarker and therapeutic target in uterine leiomyosarcoma. J. Gynecol. Oncol. 2018;29:e30. doi: 10.3802/jgo.2018.29.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slomovitz B.M., Taub M.C., Huang M., Levenback C., Coleman R.L. A randomized phase II study of letrozole vs. observation in patients with newly diagnosed uterine leiomyosarcoma (uLMS) Gynecol. Oncol. Rep. 2019;27:1. doi: 10.1016/j.gore.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiang S., Oliva E. Recent developments in uterine mesenchymal neoplasms. Histopathology. 2013;62:124–137. doi: 10.1111/his.12048. [DOI] [PubMed] [Google Scholar]
- 56.Ducie J.A., Leitao M.M., Jr. The role of adjuvant therapy in uterine leiomyosarcoma. Expert Rev. Anticancer Ther. 2016;16:45. doi: 10.1586/14737140.2016.1115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zang Y., Dong M., Zhang K., Gao C., Guo F., Wang Y., Xue F. Hormonal therapy in uterine sarcomas. Cancer Med. 2019;8:1339–1349. doi: 10.1002/cam4.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliva E., Zaloudek C.J., Soslow R.A. Blaustein’s Pathology of the Female Genital Tract. Springer International Publishing; Berlin/Heidelberg, Germany: 2019. Mesenchymal tumors of the uterus; pp. 535–647. [Google Scholar]
- 59.Gockley A.A., Rauh-Hain J.A., del Carmen M.G. Uterine leiomyosarcoma: A review article. Int. J. Gynecol. Cancer. 2014;24:1538–1542. doi: 10.1097/IGC.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 60.Arend R.C., Toboni M.D., Montgomery A.M., Burger R.A., Olawaiye A.B., Monk B.J., Herzog T.J. Systemic treatment of metastatic/recurrent uterine leiomyosarcoma: A changing paradigm. Oncologist. 2018;23:1–13. doi: 10.1634/theoncologist.2018-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parra-Herran C., Schoolmeester J.K., Yuan L., Dal Cin P., Fletcher C.D., Quade B.J., Nucci M.R. Myxoid leiomyosarcoma of the uterus: A clinicopathologic analysis of 30 cases and review of the literature with reappraisal of its distinction from other uterine myxoid mesenchymal neoplasms. Am. J. Surg. Pathol. 2016;40:285–301. doi: 10.1097/PAS.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 62.Prayson R.A., Goldblum J.R., Hart W.R. Epithelioid smooth-muscle tumors of the uterus: A clinicopathologic study of 18 patients. Am. J. Surg. Pathol. 1997;21:383–391. doi: 10.1097/00000478-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Guled M., Pazzaglia L., Borze I., Mosakhani N., Novello C., Benassi M.S., Knuutila S. Differentiating soft tissue leiomyosarcoma and undifferentiated pleomorphic sarcoma: A miRNA analysis. Genes Chromosomes Cancer. 2014;53:693–702. doi: 10.1002/gcc.22179. [DOI] [PubMed] [Google Scholar]
- 64.Ptáková N., Miesbauerová M., Kos J., Grossmann P., Henrieta Š. Immunohistochemical and selected genetic reflex testing of all uterine leiomyosarcomas and STUMPs for ALK gene rearrangement may provide an effective screening tool in identifying uterine ALK-rearranged mesenchymal tumors. Virchows Arch. 2018;473:583–590. doi: 10.1007/s00428-018-2428-8. [DOI] [PubMed] [Google Scholar]
- 65.DeLair D. Management of Gynecological Cancers in Older Women. Springer; London, UK: 2013. Pathology of gynecologic cancer; pp. 21–38. [Google Scholar]
- 66.Dos Anjos L.G., da Cunha I.W., Baracat E.C., Carvalho K.C. Genetic and epigenetic features in uterine smooth muscle tumors: An update. Clin. Oncol. 2019;4:1637. [Google Scholar]
- 67.An Y., Wang S., Li S., Zhang L., Wang D., Wang H., Zhu S., Zhu W., Li Y., Chen W., et al. Distinct molecular subtypes of uterine leiomyosarcoma respond differently to chemotherapy treatment. BMC Cancer. 2017;17:17639. doi: 10.1186/s12885-017-3568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui R., Wright J., Hou J. Uterine leiomyosarcoma: A review of recent advances in molecular biology, clinical management and outcome. BJOG Int. J. Obstet. Gynaecol. 2017;124:1028–1037. doi: 10.1111/1471-0528.14579. [DOI] [PubMed] [Google Scholar]
- 69.Kim W.Y., Chang S.-J., Chang K.-H., Yoon J.-H., Kim J.H., Kim B.-G., Bae D.-S., Ryu H.-S. Uterine leiomyosarcoma: 14-year two-center experience of 31 cases. Cancer Res. Treat. 2009;41:24–28. doi: 10.4143/crt.2009.41.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cordoba A., Prades J., Basson L., Robin Y.M., Taïeb S., Narducci F., Hudry D., Bresson L., Chevalier A., le Tinier F., et al. Adjuvant management of operated uterine sarcomas: A single institution experience. Cancer Radiother. 2019;23:401–407. doi: 10.1016/j.canrad.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Ricci S., Giuntoli R.L., Eisenhauer E., Lopez M.A., Krill L., Tanner E.J., Gehrig P.A., Havrilesky L.J., Secord A.A., Levinson K., et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol. Oncol. 2013;131:629–633. doi: 10.1016/j.ygyno.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 72.Micci F., Heim S., Panagopoulos I. Molecular pathogenesis and prognostication of “low-grade’’ and ‘high-grade’ endometrial stromal sarcoma. Genes Chromosomes Cancer. 2021;60:160–167. doi: 10.1002/gcc.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puliyath G., Nair M.K. Endometrial stromal sarcoma: A review of the literature. Indian J. Med. Paediatr. Oncol. 2012;33:1–6. doi: 10.4103/0971-5851.96960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Angelos D., Anastasios L., Dimosthenis M., Roxani D., Alexis P., Konstantinos D. Endometrial stromal sarcoma presented as endometrial polyp: A rare case. Gynecol. Surg. 2020;17:1–3. doi: 10.1186/s10397-020-01073-4. [DOI] [Google Scholar]
- 75.Jabeen S., Anwar S., Fatima N. Endometrial stromal sarcoma: A rare entity. J. Coll. Physicians Surg. Pak. 2015;25:216–217. [PubMed] [Google Scholar]
- 76.WHO Classification of Tumours Editorial Board . Female Genital Tumours: WHO Classification of Tumours. 5th ed. IARC Publications; Lyon, France: 2020. [Google Scholar]
- 77.Zappacosta R., Fanfani F., Zappacosta B., Sablone F., Pansa L., Liberati M., Rosini S. Neoplasm. IntechOpen; London, UK: 2018. Uterine sarcomas: An updated overview part 2: Endometrial stromal tumor. [DOI] [Google Scholar]
- 78.Prat J., Mbatani N. Uterine sarcomas. Int. J. Gynaecol. Obstet. 2015;131:S105–S110. doi: 10.1016/j.ijgo.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 79.Hoang L., Chiang S., Lee C.H. Endometrial stromal sarcomas and related neoplasms: New developments and diagnostic considerations. Pathology. 2018;50:162–177. doi: 10.1016/j.pathol.2017.11.086. [DOI] [PubMed] [Google Scholar]
- 80.Subbaraya S., Murthy S.S., Devi G.S. Immunohistochemical and molecular characterization of endometrial stromal sarcomas. Clin. Pathol. 2020;13:2632010X20916736. doi: 10.1177/2632010X20916736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Conklin C.M.J., Longacre T.A. Endometrial stromal tumors: The new who classification. Adv. Anat. Pathol. 2014;21:383–393. doi: 10.1097/PAP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 82.Gangireddy M., Chan Gomez J., Kanderi T., Joseph M., Kundoor V. Recurrence of endometrial stromal sarcoma, two decades post-treatment. Cureus. 2020;12:e9249. doi: 10.7759/cureus.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DuPont N.C., Disaia P.J. Recurrent endometrial stromal sarcoma: Treatment with a progestin and gonadotropin releasing hormone agonist. Sarcoma. 2010;2010:353679. doi: 10.1155/2010/353679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serkies K., Pawłowska E., Jassem J. Systemic therapy for endometrial stromal sarcomas: Current treatment options. Ginekol. Pol. 2016;87:594–597. doi: 10.5603/GP.2016.0051. [DOI] [PubMed] [Google Scholar]
- 85.Tuyaerts S., Amant F. Endometrial stromal sarcomas: A Revision of their potential as targets for immunotherapy. Vaccines. 2018;6:56. doi: 10.3390/vaccines6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nomura Y., Tamura D. Detection of MEAF6-PHF1 translocation in an endometrial stromal nodule. Genes Chromosomes Cancer. 2020;59:702–708. doi: 10.1002/gcc.22892. [DOI] [PubMed] [Google Scholar]
- 87.Ali R.H., Rouzbahman M. Endometrial stromal tumours revisited: An update based on the 2014 WHO classification. J. Clin. Pathol. 2015;68:325–332. doi: 10.1136/jclinpath-2014-202829. [DOI] [PubMed] [Google Scholar]
- 88.Han L., Liu Y.J., Ricciotti R.W., Mantilla J.G. A novel MBTD1-PHF1 gene fusion in endometrial stromal sarcoma: A case report and literature review. Genes Chromosomes Cancer. 2020;59:428–432. doi: 10.1002/gcc.22845. [DOI] [PubMed] [Google Scholar]
- 89.Da Costa L.T., dos Anjos L.G., Kagohara L.T., Torrezan G.T., de Paula C.A.A., Baracat E.C., Carraro D.M., Carvalho K.C. The mutational repertoire of uterine sarcomas and carcinosarcomas in a Brazilian cohort: A preliminary study. Clinics. 2021;76:1–15. doi: 10.6061/clinics/2021/e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mansor S., Kuick C.H., Lim-Tan S.K., Leong M.Y., Lim T.Y.K., Chang K.T.E. 28. Novel fusion MAGED2-PLAG1 in endometrial stromal sarcoma. Pathology. 2020;52:S142–S143. doi: 10.1016/j.pathol.2020.01.029. [DOI] [Google Scholar]
- 91.Makise N., Sekimizu M., Kobayashi E., Yoshida H., Fukayama M., Kato T., Kawai A., Ichikawa H., Yoshida A. Low-grade endometrial stromal sarcoma with a novel MEAF6-SUZ12 fusion. Virchows Arch. 2019;475:527–531. doi: 10.1007/s00428-019-02588-8. [DOI] [PubMed] [Google Scholar]
- 92.Brunetti M., Gorunova L., Davidson B., Heim S., Panagopoulos I., Micci F. Identification of an EPC2-PHF1 fusion transcript in low-grade endometrial stromal sarcoma. Oncotarget. 2018;9:19203–19208. doi: 10.18632/oncotarget.24969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee C.H., Nucci M.R. Endometrial stromal sarcoma—The new genetic paradigm. Histopathology. 2015;67:1–19. doi: 10.1111/his.12594. [DOI] [PubMed] [Google Scholar]
- 94.Chiang S., Lee C.H., Stewart C.J.R., Oliva E., Hoang L.N., Ali R.H., Hensley M.L., Arias-Stella J.A., 3rd, Frosina D., Jungbluth A.A., et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod. Pathol. 2017;30:1251–1261. doi: 10.1038/modpathol.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akaev I., Yeoh C.C., Rahimi S. Update on endometrial stromal tumours of the uterus. Diagnostics. 2021;11:429. doi: 10.3390/diagnostics11030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dickson B.C., Lum A., Swanson D., Bernardini M.Q., Colgan T.J., Shaw P.A., Yip S., Lee C.H. Novel EPC1 gene fusions in endometrial stromal sarcoma. Genes Chromosomes Cancer. 2018;57:598–603. doi: 10.1002/gcc.22649. [DOI] [PubMed] [Google Scholar]
- 97.Cotzia P., Benayed R., Mullaney K., Oliva E., Felix A., Ferreira J., Soslow R.A., Antonescu C.R., Ladanyi M., Chiang S. Undifferentiated uterine sarcomas represent under-recognized high-grade endometrial stromal sarcomas. Am. J. Surg. Pathol. 2019;43:662–669. doi: 10.1097/PAS.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Momeni-Boroujeni A., Mohammad N., Wolber R., Yip S., Köbel M., Dickson B.C., Hensley M.L., Leitao M.M., Antonescu C.R., Benayed R., et al. Targeted RNA expression profiling identifies high-grade endometrial stromal sarcoma as a clinically relevant molecular subtype of uterine sarcoma. Mod. Pathol. 2021;34:1008–1016. doi: 10.1038/s41379-020-00705-6. [DOI] [PubMed] [Google Scholar]
- 99.Murakami I., Tanaka K., Shiraishi J., Yamashita H. SMARCA4-deficient undifferentiated uterine sarcoma: A case report. Gynecol. Obstet. Case Rep. 2021;7:128. [Google Scholar]
- 100.Boyle W., Williams A., Sundar S., Yap J., Taniere P., Rehal P., Ganesan R. TMP3-NTRK1 rearranged uterine sarcoma: A case report. Case Rep. Womens Health. 2020;28:e00246. doi: 10.1016/j.crwh.2020.e00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiang S., Cotzia P. NTRK fusions define a novel uterine sarcoma subtype with features of fibrosarcoma. Am. J. Surg. Pathol. 2018;42:791–798. doi: 10.1097/PAS.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nebbioso A., Tambaro F.P., Dell’Aversana C., Altucci L. Cancer epigenetics: Moving forward. PLoS Genet. 2018;14:e1007362. doi: 10.1371/journal.pgen.1007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jones P.A., Laird P.W. Cancer epigenetics comes of age. Nat. Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 104.Cavalli G., Heard E. Advances in epigenetics link genetics to the environment and disease. Nature. 2019;571:489–499. doi: 10.1038/s41586-019-1411-0. [DOI] [PubMed] [Google Scholar]
- 105.Verma M., Rogers S., Divi R.L., Schully S.D., Nelson S., Su L.J., Ross S.A., Pilch S., Winn D.M., Khoury M.J. Epigenetic research in cancer epidemiology: Trends, opportunities, and challenges. Cancer Epidemiol. Biomark. Prev. 2014;23:223–233. doi: 10.1158/1055-9965.EPI-13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herceg Z., Vaissière T. Epigenetic mechanisms and cancer an interface between the environment and the genome. Epigenetics. 2011;6:804–819. doi: 10.4161/epi.6.7.16262. [DOI] [PubMed] [Google Scholar]
- 107.Werner R.J., Kelly A.D., Issa J.P.J. Epigenetics and precision oncology. Cancer J. 2017;23:262–269. doi: 10.1097/PPO.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanwal R., Gupta K., Gupta S. Cancer epigenetics: An introduction. Methods Mol. Biol. 2015;1238:3–25. doi: 10.1007/978-1-4939-1804-1_1. [DOI] [PubMed] [Google Scholar]
- 109.Topper M.J., Vaz M., Marrone K.A., Brahmer J.R., Baylin S.B. The emerging role of epigenetic therapeutics in immuno-oncology. Nat. Rev. Clin. Oncol. 2020;17:75–90. doi: 10.1038/s41571-019-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei J.W., Huang K., Yang C., Kang C.S. Non-coding RNAs as regulators in epigenetics (Review) Oncol. Rep. 2017;37:3–9. doi: 10.3892/or.2016.5236. [DOI] [PubMed] [Google Scholar]
- 111.Hosseini A., Minucci S. Epigenetics in Human Disease. Elsevier; Amsterdam, The Netherlands: 2018. Alterations of histone modifications in cancer; pp. 141–217. [DOI] [Google Scholar]
- 112.Zhao Z., Shilatifard A. Epigenetic modifications of histones in cancer. Genome Biol. 2019;20:1–16. doi: 10.1186/s13059-019-1870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caplakova V., Babusikova E., Blahovcova E., Balharek T., Zelieskova M., Hatok J. DNA methylation machinery in the endometrium and endometrial cancer. Anticancer Res. 2016;36:4407–4420. doi: 10.21873/anticanres.10984. [DOI] [PubMed] [Google Scholar]
- 114.Kagohara L.T., Stein-O’Brien G.L., Kelley D., Flam E., Wick H.C., Danilova L.V., Easwaran H., Favorov A.V., Qian J., Gaykalova D.A., et al. Epigenetic regulation of gene expression in cancer: Techniques, resources and analysis. Brief Funct. Genom. 2018;17:49–63. doi: 10.1093/bfgp/elx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feinberg A.P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 116.Fischer C.D.C., Hu Y., Morreale M., Lin W.Y., Wali A., Thakar M., Karunasena E., Sen R., Cai Y., Murphy L., et al. Treatment with epigenetic agents profoundly inhibits tumor growth in leiomyosarcoma. Oncotarget. 2018;9:19379–19395. doi: 10.18632/oncotarget.25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bird A.P. DNA methylation–how important in gene control? Nature. 1984;307:503–504. doi: 10.1038/307503a0. [DOI] [PubMed] [Google Scholar]
- 118.Gnyszka A., Jastrzebski Z., Flis S. DNA methyltransferase inhibitors and their emerging role in epigenetic therapy of cancer. Anticancer Res. 2013;33:2989–2996. [PubMed] [Google Scholar]
- 119.Garcia N., Al-Hendy A., Baracat E.C., Carvalho K.C., Yang Q. Targeting hedgehog pathway and DNA methyltransferases in uterine leiomyosarcoma cells. Cells. 2020;10:53. doi: 10.3390/cells10010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferriss J.S., Atkins K.A., Lachance J.A., Modesitt S.C., Jazaeri A.A. Temozolomide in advanced and recurrent uterine leiomyosarcoma and correlation with O6-methylguanine DNA methyltransferase expression a case series. Int. J. Gynecol. Cancer. 2010;20:120–125. doi: 10.1111/IGC.0b013e3181c7fe53. [DOI] [PubMed] [Google Scholar]
- 121.Bujko M., Kowalewska M., Danska-Bidzinska A., Bakula-Zalewska E., Siedecki J.A., Bidzinski M. The promoter methylation and expression of the O6-methylguanine-DNA methyltransferase gene in uterine sarcoma and carcinosarcoma. Oncol. Lett. 2012;4:551–555. doi: 10.3892/ol.2012.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Romero-Garcia S., Prado-Garcia H., Carlos-Reyes A. Role of DNA methylation in the resistance to therapy in solid tumors. Front. Oncol. 2020;10:1152. doi: 10.3389/fonc.2020.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Braný D., Dvorská D., Grendár M., Ňachajová M., Szépe P., Lasabová Z., Žúbor P., Višňovský J., Halášová E. Different methylation levels in the KLF4, ATF3 and DLEC1 genes in the myometrium and in corpus uteri mesenchymal tumours as assessed by MS-HRM. Pathol. Res. Pract. 2019;215:152465. doi: 10.1016/j.prp.2019.152465. [DOI] [PubMed] [Google Scholar]
- 124.Hasan N.M., Sharma A., Ruzgar N.M., Deshpande H., Olino K., Khan S., Ahuja N. Epigenetic signatures differentiate uterine and soft tissue leiomyosarcoma. Oncotarget. 2021;12:1566–1579. doi: 10.18632/oncotarget.28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baylin S.B. DNA methylation and gene silencing in cancer. Nat. Clin. Pract. Oncol. 2005;2:S4–S11. doi: 10.1038/ncponc0354. [DOI] [PubMed] [Google Scholar]
- 126.Xing D., Scangas G., Nitta M., He L., Xu A., Ioffe Y.J.M., Aspuria P.J., Hedvat C.Y., Anderson M.L., Oliva E., et al. A role for BRCA1 in uterine leiomyosarcoma. Cancer Res. 2009;69:8231–8235. doi: 10.1158/0008-5472.CAN-09-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roncati L., Barbolini G., Sartori G., Siopis E., Pusiol T., Maiorana A. Loss of CDKN2A promoter methylation coincides with the epigenetic transdifferentiation of uterine myosarcomatous cells. Int. J. Gynecol. Pathol. 2016;35:309–315. doi: 10.1097/PGP.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 128.Kommoss F.K.F., Stichel D., Schrimpf D., Kriegsmann M., Tessier-Cloutier B., Talhouk A., McAlpine J.N., Chang K.T.E., Sturm D., Pfister S.M., et al. DNA methylation-based profiling of uterine neoplasms: A novel tool to improve gynecologic cancer diagnostics. J. Cancer Res. Clin. Oncol. 2020;146:97–104. doi: 10.1007/s00432-019-03093-w. [DOI] [PubMed] [Google Scholar]
- 129.Kommoss F.K., Chang K.T., Stichel D., Banito A., Jones D.T., Heilig C.E., Fröhling S., Sahm F., Stenzinger A., Hartmann W., et al. Endometrial stromal sarcomas with BCOR-rearrangement harbor MDM2 amplifications. J. Pathol. Clin. Res. 2020;6:178–184. doi: 10.1002/cjp2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li J., Xing X., Li D., Zhang B., Mutch D.G., Hagemann I.S., Wang T. Whole-Genome DNA methylation profiling identifies epigenetic signatures of uterine carcinosarcoma. Neoplasia. 2017;19:100–111. doi: 10.1016/j.neo.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chiang S., Oliva E. Cytogenetic and molecular aberrations in endometrial stromal tumors. Hum. Pathol. 2011;42:609–617. doi: 10.1016/j.humpath.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 132.Kurihara S., Oda Y., Ohishi Y., Kaneki E., Kobayashi H., Wake N., Tsuneyoshi M. Coincident expression of β-catenin and cyclin D1 in endometrial stromal tumors and related high-grade sarcomas. Mod. Pathol. 2010;23:225–234. doi: 10.1038/modpathol.2009.162. [DOI] [PubMed] [Google Scholar]
- 133.Hrzenjak A., Dieber-Rotheneder M., Moinfar F., Petru E., Zatloukal K. Molecular mechanisms of endometrial stromal sarcoma and undifferentiated endometrial sarcoma as premises for new therapeutic strategies. Cancer Lett. 2014;354:21–27. doi: 10.1016/j.canlet.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 134.Matsukura H., Aisaki K.I., Igarashi K., Matsushima Y., Kanno J., Muramatsu M., Sudo K., Sato N. Genistein promotes DNA demethylation of the steroidogenic factor 1 (SF-1) promoter in endometrial stromal cells. Biochem. Biophys. Res. Commun. 2011;412:366–372. doi: 10.1016/j.bbrc.2011.07.104. [DOI] [PubMed] [Google Scholar]
- 135.Maekawa R., Tamura I., Shinagawa M., Mihara Y., Sato S., Okada M., Taketani T., Tamura H., Sugino N. Genome-wide DNA methylation analysis revealed stable DNA methylation status during decidualization in human endometrial stromal cells. BMC Genom. 2019;20:324. doi: 10.1186/s12864-019-5695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tamura I., Maekawa R., Jozaki K., Ohkawa Y., Takagi H., Doi-Tanaka Y., Shirafuta Y., Mihara Y., Taketani T., Sato S., et al. Transcription factor C/EBPβ induces genome-wide H3K27ac and upregulates gene expression during decidualization of human endometrial stromal cells. Mol. Cell. Endocrinol. 2021;520:111085. doi: 10.1016/j.mce.2020.111085. [DOI] [PubMed] [Google Scholar]
- 137.Nie J., Liu X., Sun-Wei G. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod. Sci. 2010;17:995–1005. doi: 10.1177/1933719110377118. [DOI] [PubMed] [Google Scholar]
- 138.Gillette T.G., Hill J.A. Readers, writers, and erasers: Chromatin as the whiteboard of heart disease. Circ. Res. 2015;116:1245–1253. doi: 10.1161/CIRCRESAHA.116.303630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luger K., Hansen J.C. Nucleosome and chromatin fiber dynamics. Curr. Opin. Struct. Biol. 2005;5:188–196. doi: 10.1016/j.sbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 140.Mastoraki A., Schizas D., Vlachou P., Melissaridou N.M., Charalampakis N., Fioretzaki R., Kole C., Savvidou O., Vassiliu P., Pikoulis E. Assessment of synergistic contribution of histone deacetylases in prognosis and therapeutic management of sarcoma. Mol. Diagn. Ther. 2020;24:557–569. doi: 10.1007/s40291-020-00487-2. [DOI] [PubMed] [Google Scholar]
- 141.Gujral P., Mahajan V., Lissaman A.C., Ponnampalam A.P. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod. Biol. Endocrinol. 2020;18:1–11. doi: 10.1186/s12958-020-00637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Biswas S., Rao C.M. Epigenetic tools (the writers, the readers and the erasers) and their implications in cancer therapy. Eur. J. Pharmacol. 2018;837:8–24. doi: 10.1016/j.ejphar.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 144.Martin C., Zhang Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 145.Grimaldi G., Christian M., Steel J.H., Henriet P., Poutanen M., Brosens J.J. Down-Regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells. Mol. Endocrinol. 2011;25:1892. doi: 10.1210/me.2011-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guan M., Wu X., Chu P., Chow W.A. Fatty acid synthase reprograms the epigenome in uterine leiomyosarcomas. PLoS ONE. 2017;12:e0179692. doi: 10.1371/journal.pone.0179692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dunican D.S., Mjoseng H.K., Duthie L., Flyamer I.M., Bickmore W.A., Meehan R.R. Bivalent promoter hypermethylation in cancer is linked to the H327me3/H3K4me3 ratio in embryonic stem cells. BMC Biol. 2020;18:1–21. doi: 10.1186/s12915-020-0752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Piunti A., Shilatifard A. The roles of polycomb repressive complexes in mammalian development and cancer. Nat. Rev. Mol. Cell Biol. 2021;22:326–345. doi: 10.1038/s41580-021-00341-1. [DOI] [PubMed] [Google Scholar]
- 149.Kim H., Ekram M.B., Bakshi A., Kim J. AEBP2 as a transcriptional activator and its role in cell migration. Genomics. 2015;105:108–115. doi: 10.1016/j.ygeno.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tan J.Z., Yan Y., Wang X.X., Jiang Y., Xu H.E. EZH2: Biology, disease, and structure-based drug discovery. Acta Pharmacol. Sin. 2014;35:161–174. doi: 10.1038/aps.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Piunti A., Smith E.R., Morgan M.A.J., Ugarenko M., Khaltyan N., Helmin K.A., Ryan C.A., Murray D.C., Rickels R.A., Yilmaz B.D., et al. CATACOMB: An endogenous inducible gene that antagonizes H3K27 methylation activity of Polycomb repressive complex 2 via an H3K27M-like mechanism. Sci. Adv. 2019;5:2887. doi: 10.1126/sciadv.aax2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dewaele B., Przybyl J., Quattrone A., Ferreiro J.F., Vanspauwen V., Geerdens E., Gianfelici V., Kalender Z., Wozniak A., Moerman P., et al. Identification of a novel, recurrent MBTD1-CXorf67 fusion in low-grade endometrial stromal sarcoma. Int. J. Cancer. 2014;134:1112–1122. doi: 10.1002/ijc.28440. [DOI] [PubMed] [Google Scholar]
- 153.Levine S.S., Weiss A., Erdjument-Bromage H., Shao Z., Tempst P., Kingston R.E. The core of the polycomb repressive complex is compositionally and functionally conserved in flies and humans. Mol. Cell. Biol. 2002;22:6070. doi: 10.1128/MCB.22.17.6070-6078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oliviero G., Munawar N., Watson A., Streubel G., Manning G., Bardwell V., Bracken A.P., Cagney G. The variant polycomb repressor complex 1 component PCGF1 interacts with a pluripotency sub-network that includes DPPA4, a regulator of embryogenesis. Sci. Rep. 2015;5:1–11. doi: 10.1038/srep18388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Astolfi A., Fiore M., Melchionda F., Indio V., Bertuccio S.N., Pession A. BCOR involvement in cancer. Epigenomics. 2019;11:835. doi: 10.2217/epi-2018-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Panagopoulos I., Thorsen J., Gorunova L., Haugom L., Bjerkehagen B., Davidson B., Heim S., Micci F. Fusion of the ZC3H7B and BCOR genes in endometrial stromal sarcomas carrying an X;22-translocation. Genes Chromosomes Cancer. 2013;52:610–618. doi: 10.1002/gcc.22057. [DOI] [PubMed] [Google Scholar]
- 157.Chase A., Cross N.C. Aberrations of EZH2 in cancer. Clin. Cancer Res. 2011;17:2613–2618. doi: 10.1158/1078-0432.CCR-10-2156. [DOI] [PubMed] [Google Scholar]
- 158.Micci F., Gorunova L., Agostini A., Johannessen L.E., Brunetti M., Davidson B., Heim S., Panagopoulos I. Cytogenetic and molecular profile of endometrial stromal sarcoma. Genes Chromosomes Cancer. 2016;55:834–846. doi: 10.1002/gcc.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aldera A.P., Govender D. Gene of the month: BCOR. J. Clin. Pathol. 2020;73:314–317. doi: 10.1136/jclinpath-2020-206513. [DOI] [PubMed] [Google Scholar]
- 160.Juckett L.T., Lin D.I., Madison R., Ross J.S., Schrock A.B., Ali S. A pan-cancer landscape analysis reveals a subset of endometrial stromal and pediatric tumors defined by internal tandem duplications of BCOR. Oncology. 2019;96:101–109. doi: 10.1159/000493322. [DOI] [PubMed] [Google Scholar]
- 161.Allen A.J., Ali S.M., Gowen K., Elvin J.A., Pejovic T. A recurrent endometrial stromal sarcoma harbors the novel fusion JAZF1-BCORL1. Gynecol. Oncol. Rep. 2017;20:51–53. doi: 10.1016/j.gore.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Monaghan L., Massett M.E., Bunschoten R.P., Hoose A., Pirvan P.-A., Liskamp R.M.J., Jørgensen H.G., Huang X. The emerging role of H3K9me3 as a potential therapeutic target in acute myeloid leukemia. Front. Oncol. 2019;9:705. doi: 10.3389/fonc.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brahmi M., Franceschi T., Treilleux I., Pissaloux D., Ray-Coquard I., Dufresne A., Vanacker H., Carbonnaux M., Meeus P., Sunyach M.P., et al. Molecular classification of endometrial stromal sarcomas using RNA sequencing defines nosological and prognostic subgroups with different natural history. Cancers. 2020;12:2604. doi: 10.3390/cancers12092604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Przybyl J., Kidzinski L., Hastie T., Debiec-Rychter M., Nusse R., van de Rijn M. Gene expression profiling of low-grade endometrial stromal sarcoma indicates fusion protein-mediated activation of the Wnt signaling pathway. Gynecol. Oncol. 2018;149:388–393. doi: 10.1016/j.ygyno.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 165.Itoh Y., Takada Y., Yamashita Y., Suzuki T. Recent progress on small molecules targeting epigenetic complexes. Curr. Opin. Chem. Biol. 2022;67:102130. doi: 10.1016/j.cbpa.2022.102130. [DOI] [PubMed] [Google Scholar]
- 166.Piunti A., Pasini D. Epigenetic factors in cancer development: Polycomb group proteins. Future Oncol. 2011;7:57–75. doi: 10.2217/fon.10.157. [DOI] [PubMed] [Google Scholar]
- 167.Gao J., Yang T., Wang X., Zhang Y., Wang J., Zhang B., Tang D., Liu Y., Gao T., Lin Q., et al. Identification and characterization of a subpopulation of CD133+ cancer stem-like cells derived from SK-UT-1 cells. Cancer Cell Int. 2021;8:157. doi: 10.1186/s12935-021-01817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang N., Zeng Z., Li S., Wang F., Huang P. High expression of EZH2 as a marker for the differential diagnosis of malignant and benign myogenic tumors. Sci. Rep. 2018;8:12331. doi: 10.1038/s41598-018-30648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Micci F., Brunetti M., Cin P.D., Nucci M.R., Gorunova L., Heim S., Panagopoulos I. Fusion of the genes BRD8 and PHF1 in endometrial stromal sarcoma. Genes Chromosomes Cancer. 2017;56:841–845. doi: 10.1002/gcc.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hrzenjak A. JAZF1/SUZ12 gene fusion in endometrial stromal sarcomas. Orphanet J. Rare Dis. 2016;11:1–8. doi: 10.1186/s13023-016-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hodge J.C., Bedroske P.P., Pearce K.E., Sukov W.R. Molecular cytogenetic analysis of JAZF1, PHF1, and YWHAE in endometrial stromal tumors: Discovery of genetic complexity by fluorescence in situ Hybridization. J. Mol. Diagn. 2016;18:516–526. doi: 10.1016/j.jmoldx.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 172.Tavares M., Khandelwal G., Mutter J., Viiri K., Beltran M., Brosens J.J., Jenner R.G. JAZF1-SUZ12 dysregulates PRC2 function and gene expression during cell differentiation. bioRxiv. 2021;15:440052. doi: 10.1016/j.celrep.2022.110889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sudarshan D., Avvakumov N., Lalonde M.-E., Alerasool N., Jacquet K., Mameri A., Rousseau J., Lambert J.-P., Paquet E., Setty S.T., et al. Recurrent chromosomal translocations in sarcomas create a mega-complex that mislocalizes NuA4/TIP60 to polycomb target loci. bioRxiv. 2021;26:436670. doi: 10.1101/2021.03.26.436670. [DOI] [Google Scholar]
- 174.Davidson B., Matias-Guiu X., Lax S.F. The clinical, morphological, and genetic heterogeneity of endometrial stromal sarcoma. Virchows Arch. 2020;476:489–490. doi: 10.1007/s00428-020-02762-3. [DOI] [PubMed] [Google Scholar]
- 175.Lee C.H., Ou W.B., Mariño-Enriquez A., Zhu M., Mayeda M., Wang Y., Guo X., Brunner A.L., Amant F., French C.A., et al. 14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc. Natl. Acad. Sci. USA. 2012;109:929–934. doi: 10.1073/pnas.1115528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Panagopoulos I., Micci F., Thorsen J., Gorunova L., Eibak A.M., Bjerkehagen B., Davidson B., Heim S. Novel fusion of MYST/Esa1-associated factor 6 and PHF1 in endometrial stromal sarcoma. PLoS ONE. 2012;7:e39354. doi: 10.1371/journal.pone.0039354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fiskus W., Pranpat M., Balasis M., Herger B., Rao R., Chinnaiyan A., Atadja P., Bhalla K. Histone deacetylase inhibitors deplete enhancer of zeste 2 and associated polycomb repressive complex 2 proteins in human acute leukemia cells. Mol. Cancer Ther. 2006;5:3096–3104. doi: 10.1158/1535-7163.MCT-06-0418. [DOI] [PubMed] [Google Scholar]
- 178.Di Giorgio E., Dalla E., Franforte E., Paluvai H., Minisini M., Trevisanut M., Picco R., Brancolini C. Different class IIa HDACs repressive complexes regulate specific epigenetic responses related to cell survival in leiomyosarcoma cells. Nucleic Acids Res. 2020;48:646–664. doi: 10.1093/nar/gkz1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Park S.Y., Kim J.S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 2020;52:204–212. doi: 10.1038/s12276-020-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Choy E., Ballman K., Chen J., Dickson M.A., Chugh R., George S., Okuno S., Pollock R., Patel R.M., Hoering A., et al. SARC018-SPORE02: Phase II study of mocetinostat administered with gemcitabine for patients with metastatic leiomyosarcoma with progression or relapse following prior treatment with gemcitabine-containing therapy. Sarcoma. 2018;2018:2068517. doi: 10.1155/2018/2068517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miller H., Ike C., Parma J., Masand R.P., Mach C.M., Anderson M.L. Molecular targets and emerging therapeutic options for uterine leiomyosarcoma. Sarcoma. 2016;2016:7018106. doi: 10.1155/2016/7018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Baek M.-H., Park J.-Y., Rhim C.C., Kim J.-H., Park Y., Kim K.-R., Nam J.-H. Investigation of new therapeutic targets in undifferentiated endometrial sarcoma. Gynecol. Obstet. Investig. 2017;82:329–339. doi: 10.1159/000454769. [DOI] [PubMed] [Google Scholar]
- 183.Rafehi H., Karagiannis T.C., El-Osta A. Pharmacological histone deacetylation distinguishes transcriptional regulators. Curr. Top. Med. Chem. 2017;17:1611–1622. doi: 10.2174/1568026617666161104104341. [DOI] [PubMed] [Google Scholar]
- 184.Monga V., Swami U., Tanas M., Bossler A., Mott S.L., Smith B.J., Milhem M. A phase I/II study targeting angiogenesis using bevacizumab combined with chemotherapy and a histone deacetylase inhibitor (Valproic acid) in advanced sarcomas. Cancers. 2018;10:53. doi: 10.3390/cancers10020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yen M.S., Chen J.R., Wang P.H., Wen K.C., Chen Y.J., Ng H.T. Uterine sarcoma part III—Targeted therapy: The taiwan association of gynecology (TAG) systematic review. Taiwan. J. Obstet. Gynecol. 2016;55:625–634. doi: 10.1016/j.tjog.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 186.Quan P., Moinfar F., Kufferath I., Absenger M., Kueznik T., Denk H., Zatloukal K., Haybaeck J. Effects of targeting endometrial stromal sarcoma cells via histone deacetylase and PI3K/AKT/mTOR signaling. Anticancer Res. 2014;34:2883–2897. [PubMed] [Google Scholar]
- 187.Tang F., Choy E., Tu C., Hornicek F., Duan Z. Therapeutic applications of histone deacetylase inhibitors in sarcoma. Cancer Treat. Rev. 2017;59:33–45. doi: 10.1016/j.ctrv.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rikiishi H. Autophagic and apoptotic effects of HDAC inhibitors on cancer cells. J. Biomed. Biotechnol. 2011;2011:830260. doi: 10.1155/2011/830260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fröhlich L.F., Mrakovcic M., Smole C., Lahiri P., Zatloukal K. Epigenetic silencing of apoptosis-inducing gene expression can be efficiently overcome by combined saha and trail treatment in uterine sarcoma cells. PLoS ONE. 2014;9:e91558. doi: 10.1371/journal.pone.0091558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lin C.Y., Chao A., Wu R.C., Lee L.Y., Ueng S.H., Tsai C.L., Lee Y.S., Peng M.T., Yang L.Y., Huang H., et al. Synergistic effects of pazopanib and hyperthermia against uterine leiomyosarcoma growth mediated by downregulation of histone acetyltransferase 1. J. Mol. Med. 2020;98:1175–1188. doi: 10.1007/s00109-020-01888-w. [DOI] [PubMed] [Google Scholar]
- 191.Sawicka A., Seiser C. Histone H3 phosphorylation—A versatile chromatin modification for different occasions. Biochimie. 2012;94:2193. doi: 10.1016/j.biochi.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Veras E., Malpica A., Deavers M.T., Silva E.G. Mitosis-specific marker phospho-histone h3 in the assessment of mitotic index in uterine smooth muscle tumors: A pilot study. Int. J. Gynecol. Pathol. 2009;28:316–321. doi: 10.1097/PGP.0b013e318193df97. [DOI] [PubMed] [Google Scholar]
- 193.Jain A.K., Barton M.C. Bromodomain Histone Readers and Cancer. J. Mol. Biol. 2017;429:2003–2010. doi: 10.1016/j.jmb.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 194.Fujisawa T., Filippakopoulos P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat. Rev. Mol. Cell Biol. 2017;18:246–262. doi: 10.1038/nrm.2016.143. [DOI] [PubMed] [Google Scholar]
- 195.Sima X., He J., Peng J., Xu Y., Zhang F., Deng L. The genetic alteration spectrum of the SWI/SNF complex: The oncogenic roles of BRD9 and ACTL6A. PLoS ONE. 2019;14:e0222305. doi: 10.1371/journal.pone.0222305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Barma N., Stone T.C., Carmona Echeverria L.M., Heavey S. Exploring the Value of BRD9 as a Biomarker, Therapeutic Target and Co-Target in Prostate Cancer. Biomolecules. 2021;11:1794. doi: 10.3390/biom11121794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang X., Wang S., Troisi E.C., Howard T.P., Haswell J.R., Wolf B.K., Hawk W.H., Ramos P., Oberlick E.M., Tzvetkov E.P., et al. BRD9 defines a SWI/SNF sub-complex and constitutes a specific vulnerability in malignant rhabdoid tumors. Nat. Commun. 2019;10:1881. doi: 10.1038/s41467-019-09891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhu X., Liao Y., Tang L. Targeting BRD9 for Cancer Treatment: A New Strategy. OncoTargets Ther. 2020;13:13191–13200. doi: 10.2147/OTT.S286867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bell C.M., Raffeiner P., Hart J.R., Vogt P.K. PIK3CA Cooperates with KRAS to Promote MYC Activity and Tumorigenesis via the Bromodomain Protein BRD9. Cancers. 2019;11:1634. doi: 10.3390/cancers11111634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang H., Wang Y., Li Q., Fei X., Ma H., Hu R. miR-140-3p functions as a tumor suppressor in squamous cell lung cancer by regulating BRD9. Cancer Lett. 2019;446:81–89. doi: 10.1016/j.canlet.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 201.Del Gaudio N., Di Costanzo A., Liu N.Q., Conte L., Migliaccio A., Vermeulen M., Martens J.H.A., Stunnenberg H.G., Nebbioso A., Altucci L. BRD9 binds cell type-specific chromatin regions regulating leukemic cell survival via STAT5 inhibition. Cell Death Dis. 2019;18:338. doi: 10.1038/s41419-019-1570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang Q., Bariani M.V., Falahati A., Khosh A., Lastra R.R., Siblini H., Boyer T.G., Al-Hendy A. The Functional Role and Regulatory Mechanism of Bromodomain-Containing Protein 9 in Human Uterine Leiomyosarcoma. Cells. 2022;11:2160. doi: 10.3390/cells11142160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vafadar A., Shabaninejad Z., Movahedpour A., Mohammadi S., Fathullahzadeh S., Mirzaei H.R., Namdar A., Savardashtaki A., Mirzaei H. Long non-coding rnas as epigenetic regulators in cancer. Curr. Pharm. Des. 2019;25:3563–3577. doi: 10.2174/1381612825666190830161528. [DOI] [PubMed] [Google Scholar]
- 204.Hanly D.J., Esteller M., Berdasco M. Interplay between long non-coding RNAs and epigenetic machinery: Emerging targets in cancer? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373:20170074. doi: 10.1098/rstb.2017.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Romano G., Veneziano D., Acunzo M., Croce C.M. Small non-coding RNA and cancer. Carcinogenesis. 2017;38:485–491. doi: 10.1093/carcin/bgx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guisier F., Barros-Filho M.C., Rock L.D., Constantino F.B., Minatel B.C., Sage A.P., Marshall E.A., Martinez V.D., Lam W.L. Gene Expression Profiling in Cancer. IntechOpen; London, UK: 2019. Small noncoding rna expression in cancer. [Google Scholar]
- 207.Eddy S.R. Noncoding RNA genes. Curr. Opin. Genet. Dev. 1999;9:695–699. doi: 10.1016/S0959-437X(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 208.Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A., et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 209.Subramanian S., Kartha R.V. MicroRNA-mediated gene regulations in human sarcomas. Cell. Mol. Life Sci. 2012;69:3571–3585. doi: 10.1007/s00018-012-1127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wei J., Liu X., Li T., Xing P., Zhang C., Yang J. The new horizon of liquid biopsy in sarcoma: The potential utility of circulating tumor nucleic acids. J. Cancer. 2020;11:5293–5308. doi: 10.7150/jca.42816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dvorská D., Škovierová H., Braný D., Halašová E., Danková Z. Liquid biopsy as a tool for differentiation of leiomyomas and sarcomas of corpus uteri. Int. J. Mol. Sci. 2019;20:3825. doi: 10.3390/ijms20153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Anderson M.L., Kim G.E., Mach C.M., Creighton C.J., Lev D. Abstract 5242: miR-10b functions as a novel tumor suppressor in uterine leiomyosarcoma by promoting overexpression of SDC1. Cancer Res. 2014;74:5242. doi: 10.1158/1538-7445.AM2014-5242. [DOI] [Google Scholar]
- 213.Schiavon B.N., Carvalho K.C., Coutinho-Camillo C.M., Baiocchi G., Valieris R., Drummond R., da Silva I.T., De Brot L., Soares F.A., da Cunha I.W. MiRNAs 144-3p, 34a-5p, and 206 are a useful signature for distinguishing uterine leiomyosarcoma from other smooth muscle tumors. Surg. Exp. Pathol. 2019;2:1–8. doi: 10.1186/s42047-019-0032-0. [DOI] [Google Scholar]
- 214.Yokoi A., Matsuzaki J., Yamamoto Y., Tate K., Yoneoka Y., Shimizu H., Uehara T., Ishikawa M., Takizawa S., Aoki Y., et al. Serum microRNA profile enables preoperative diagnosis of uterine leiomyosarcoma. Cancer Sci. 2019;110:3718. doi: 10.1111/cas.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.De Almeida B., Garcia N., Maffazioli G., Gonzalez dos Anjos L., Chada Baracat E., Candido Carvalho K. Oncomirs expression profiling in uterine leiomyosarcoma cells. Int. J. Mol. Sci. 2017;19:52. doi: 10.3390/ijms19010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang Q., Ubago J., Li L., Guo H., Liu Y., Qiang W., Kim J.J., Kong B., Wei J.-J. Molecular analyses of 6 different types of uterine smooth muscle tumors: Emphasis in atypical leiomyoma. Cancer. 2014;120:3165–3177. doi: 10.1002/cncr.28900. [DOI] [PubMed] [Google Scholar]
- 217.Chuang T.-D., Panda H., Luo X., Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr. Relat. Cancer. 2012;19:541. doi: 10.1530/ERC-12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chuang T.-D., Ho M., Khorram O. The regulatory function of mir-200c on inflammatory and cell-cycle associated genes in SK-LMS-1, a leiomyosarcoma cell line. Reprod. Sci. 2015;22:563–571. doi: 10.1177/1933719114553450. [DOI] [PubMed] [Google Scholar]
- 219.Dos Anjos L.G., de Almeida B.C., de Almeida T.G., Rocha A.M.L., Maffazioli G.D.N., Soares F.A., da Cunha I.W., Baracat E.C., Carvalho K.C. Could miRNA signatures be useful for predicting uterine sarcoma and carcinosarcoma prognosis and treatment? Cancers. 2018;10:315. doi: 10.3390/cancers10090315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shi G., Perle M.A., Mittal K., Chen H., Zou X., Narita M., Hernando E., Lee P., Wei J.J. Let-7 repression leads to HMGA2 overexpression in uterine leiomyosarcoma. J. Cell Mol. Med. 2009;13:3898–3905. doi: 10.1111/j.1582-4934.2008.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zavadil J., Ye H., Liu Z., Wu J., Lee P., Hernando E., Soteropoulos P., Toruner G.A., Wei J.-J. Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PLoS ONE. 2010;5:e12362. doi: 10.1371/journal.pone.0012362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ayub A.L.P., D’Angelo Papaiz D., da Silva Soares R., Jasiulionis M.G. Non-Coding RNAs. IntechOpen; Rijeka, Croatia: 2020. The function of lncrnas as epigenetic regulators. [DOI] [Google Scholar]
- 223.Grillone K., Riillo C., Riillo C., Scionti F., Rocca R., Rocca R., Tradigo G., Guzzi P.H., Alcaro S., Alcaro S., et al. Non-coding RNAs in cancer: Platforms and strategies for investigating the genomic “dark matter”. J. Exp. Clin. Cancer Res. 2020;39:1–19. doi: 10.1186/s13046-020-01622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rasool M., Malik A., Zahid S., Basit Ashraf M.A., Qazi M.H., Asif M., Zaheer A., Arshad M., Raza A., Jamal M.S. Non-coding RNAs in cancer diagnosis and therapy. Non-Coding RNA Res. 2016;1:69–76. doi: 10.1016/j.ncrna.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Guo H., Zhang X., Dong R., Liu X., Li Y., Lu S., Xu L., Wang Y., Wang X., Hou D., et al. Integrated analysis of long noncoding RNAs and mRNAs reveals their potential roles in the pathogenesis of uterine leiomyomas. Oncotarget. 2014;5:8625. doi: 10.18632/oncotarget.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.