Abstract
Simple Summary
The immunopeptidome of cancer cells is a treasure trove of neoantigens bound to MHC molecules, thus a great source for mining immunopeptides for immunotherapy applications, including cancer vaccines. Immunopeptides may encompass post-translational modifications that are overlooked by genomic and transcriptomic tools. We review post-translational modifications that have been uncovered, and how this information could be harnessed for cancer vaccines.
Abstract
Although harnessing the immune system for cancer therapy has shown success, response to immunotherapy has been limited. The immunopeptidome of cancer cells presents an opportunity to discover novel antigens for immunotherapy applications. These neoantigens bind to MHC class I and class II molecules. Remarkably, the immunopeptidome encompasses protein post-translation modifications (PTMs) that may not be evident from genome or transcriptome profiling. A case in point is citrullination, which has been demonstrated to induce a strong immune response. In this review, we cover how the immunopeptidome, with a special focus on PTMs, can be utilized to identify cancer-specific antigens for immunotherapeutic applications.
Keywords: immunopeptidome, PTM, immunotherapy, cancer vaccine
1. Introduction
Identifying novel antigens in cancer is highly relevant for immunotherapeutic applications including chimeric antigen receptor (CAR)-T and NK cell, pulsed-dendritic cell therapy, and therapeutic and preventative cancer vaccines [1]. Mass spectrometry provides an important means for deciphering the immunopeptidome repertoire of tumor cells [2]. Whereas much emphasis has been placed on mutations as a source of neoantigens, the occurrence of specific mutations in peptides bound to the major histocompatibility complex (MHC) is quite variable from patient to patient [3,4,5]. Thus, there is a need to identify antigenic peptides that are commonly expressed in a cancer type that are presented through MHC class I for activation of cytotoxic CD8+ T cells [6,7]. Moreover, there is also an emerging interest in immune peptides bound to MHC class II that induce a B cell response [8,9,10].
Since the early days of profiling the immunopeptidome using mass spectrometry (MS) some three decades ago [11], there has been substantial improvement in the overall approach, including the application of machine learning [12,13,14]. The detection and prediction of immunogenic peptides through genomic and transcriptomic data is challenging and overlooks protein aberrations that occur after transcription. These include translational errors, post-translational modifications (PTMs) and peptide splicing that can be uncovered through analysis of the immunopeptidome [15,16,17]. Remarkably, PTMs have been discovered to induce immunogenicity more than their unmodified counterparts. Prior studies by our group have identified citrullination as a source of immunogenicity in cancer [18]. Other notable PTMs include phosphorylation, acetylation, deamination, and glycosylation [19,20,21,22]. However, not all PTMs are stable and presented by MHC, given their enzymatic reversibility as in the case of acetylation [23]. This review covers how interrogating the immunopeptidome can yield novel cancer-specific antigens, with an emphasis on PTMs and their applications.
2. The Immunopeptidome as a Source of Different Types of Neoantigens
The immunoediting concept has been critical for our understanding of the mechanisms through which the immune system responds to cancer and how tumor cells can evade the immune response [24]. A key factor in the immune response is the recognition of tumor antigens. T cells, through their TCRs, can interact with the myriad of peptides bound to MHC, sorting out self from non-self. Non-canonical tumor antigens, derived from sequences outside of exons or by alternate protein-processing mechanisms, are of increasing interest for immunotherapy [25]. PTMs are mediated by multiple enzymes, some of which may be dysregulated in tumor cells, rendering them potentially tumor specific. Post-translationally modified proteins undergo processing through the proteasome, resulting in peptides that bind to MHC-I for endogenous proteins or MHC-II for exogenous proteins [26]. Dendritic cells (DCs) are antigen-presenting cells (APCs) in cancer that are essential for T and B cell responses via immunopeptides and native protein presentation, respectively [27,28]. PTMs that are restricted to tumor cells have potential as a source of immunopeptides for immunotherapy.
3. Post-Translational Modifications as a Source of Tumor Antigens
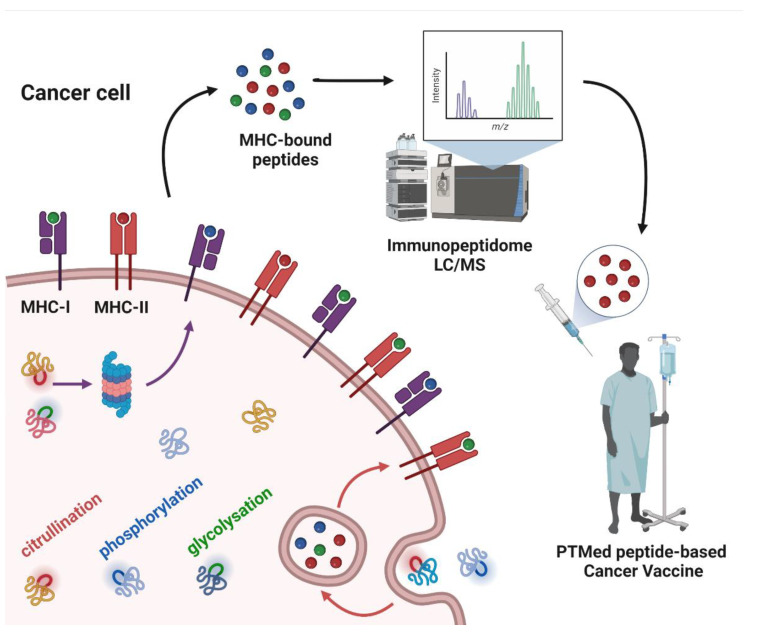
Whereas a multitude of PTMs are known to occur, most have not been previously investigated in cancer. Nanoscale liquid chromatography coupled mass spectrometry (nanoLC-MS) has contributed significantly to the identification of PTMs in the immunopeptidome through matching the peptide parent mass (MS1) and the fragment mass (MS2) to sequences in the human genome database, allowing for mass shift due to modified amino acids (e.g., +0.984 Da on Arg) for citrullination; (+97.976 Da on Ser, Thr, and Tyr) for phosphorylation; and (+203.079 Da on Ser and Thr) for O-GlcNAc. In this review, we cover PTMs that have been identified in the immunopeptidome with demonstrated immunogenicity in cancer (Figure 1).
Figure 1.
Post-translationally modified peptide-based cancer vaccine workflow. The figure depicts cancer cell antigen processing of intracellular and extracellular proteins, subsequently as peptides bound to MHC-I or MHC-II. Some of the proteins have PTMs in their structure, which are sketched in colors (citrullination: red; phosphorylation: blue; glycosylation: green) as well as in MHC-bound peptides. The MHC-bound peptides are identified by means of liquid chromatography-mass spectrometry (LC/MS), to derive the cancer cell immunopeptidome. From the immunopeptidome data, peptides with PTMs can be selected as antigens for cancer vaccines.
3.1. Citrullination
Several studies have explored citrullination as a source of antigenicity in cancer [29,30,31]. Citrullination occurs on arginine residues and is enzymatically driven by peptidyl arginine deiminases (PADI), which are dysregulated in multiple cancer types [18]. The dysregulated citrullination pathway was initially linked to autoimmune diseases, mainly rheumatoid arthritis. More recently, its role in cancer has attracted interest [31,32]. Citrullinated peptides are principally presented by MHC-II, eliciting a CD4+ T cell and B cell response [10,33]. Citrullination in cancer cells occurs as a result of cellular stress, exemplified by autophagy, nutrient starvation, and hypoxia [31,34,35,36], which induces PADI expression. The PADI family consists of five members, with PADI2 and PADI4 being predominantly expressed in cancer [37].
In a recent study, we found PADI2 to be highly expressed in several cancer types, notably in triple-negative breast cancer (TNBC) [18]. PADI2 expression was correlated with accumulation of citrullinated proteins and, with MHC-II-bound citrullinated peptides, and with a B cell immune response. PADI2 is also overexpressed in HER2+ breast cancer, hepatocellular carcinoma, esophageal cancer, gastric adenocarcinoma, and castration-resistant prostate cancer [38,39,40]. PADI2 is variably expressed in colorectal cancer (CRC) [41,42]. It is intriguing that low PADI2 expression seems to correlate with poor prognosis, possibly due to a lack of immunogenic citrullinated peptides [41,43]. Overexpression of PADI2 in skin tumors is associated with elevated inflammatory cell infiltration [31,44,45]. CRC with high PADI2 and PADI4 expression is associated with increased overall survival [46]. PADI4 expression in benign tumors and non-tumor inflamed tissues was found to be restricted to malignant tumors in gastric, liver, and ovarian cancers [47,48,49,50,51].
Citrullination has been investigated in relation to tumor biology and metastasis and as a source of cancer biomarkers [31]. For applications to immunotherapy usage, some citrullinated proteins have been tested as antigen candidates for therapeutic cancer vaccines. Among several hundred citrullinated proteins [52], α-enolase (ENO1), vimentin (VIM), nucleophosmin (NPM1), matrix metalloproteinase-21 (MMP21), cytochrome p450 (Cp450), and glutamate receptor ionotropic (GRI) citrullinated peptides have been selected for immunization for melanoma, lung, pancreas, and ovarian cancers [29,30,33,53,54,55].
3.2. Phosphorylation
The majority of phosphorylation research in cancer is focused on signaling pathways [56]. However, there is interest in phosphorylation as a neoantigen target. The most abundant of the enzymatically modified cancer proteins are represented by phosphoproteins, resulting from dysregulation of kinase-mediated signaling pathways triggering the synthesis of cancer-associated phosphopeptides [57]. Importantly, MHC-I and MHC-II present these phosphorylated peptides in cancer cells [58,59,60]. Moreover, there are reports of tumor-specific phosphorylated peptides stimulating CD8 and CD4 T cells and even phosphopeptide-specific T cells in cancer [61,62,63]. There is also evidence of a B cell response against phosphorylated proteins [64]. These findings elicit interest in phosphopeptides as a source of cancer antigens [65,66].
Two phosphopeptides are currently being tested in melanoma patients, one derived from the insulin receptor substrate 2 (IRS2) and the other from breast cancer antiestrogen resistance 3 (BCAR3) (NCT01846143). In CRC, 120 phosphopeptides were identified, some of which were tumor restricted. TILs from CRC patients recognized three of the identified phosphopeptides from IRS2, tensin 3 (TNS3), and selenoprotein H (SELH) [67]. Other phosphopeptides with potential utility as immunotherapeutic targets are derived from beta-catenin and CDC25b [58,62]. Approaches to enrich for phosphopeptides in the immunopeptidome include the use of immobilized metal affinity chromatography and titanium dioxide nanoparticles [68,69,70]. In development, is a predictor of interactions between MHC-I and phosphopeptides. [71].
3.3. Glycosylation
Protein glycosylation occurs in the endoplasmatic reticulum and Golgi apparatus and is commonly associated with secreted and extracellular membrane proteins. Considering that there are several types of glycans that could be covalently conjugated to proteins, there are a myriad of possible combinations. O-linked-N-Acetylglucosamine (O-GlcNAc) plays an important role in signal transduction, transcription, cell division, metabolism, and the cytoskeleton in cancer cells [72]. Given that glycosylation creates novel epitopes, its occurrence in peptides presented by MHC has been of interest as a source of targets for immunotherapy [73].
An important source of glycopeptides is represented by mucins (MUC), which are highly glycosylated in cancer. They have been utilized as a study model for carbohydrates’ immunogenicity [74,75]. Tumor cells overexpress MUC1 and MUC4 on the surface, both of which exhibit altered glycosylation, potentially representing tumor-specific targets. Common antigens expressed in cancer mucins are the O-glycans Tn and T antigens, which have been utilized as vaccines. Several vaccine strategies using glycopeptides from mucins in combination with adjuvant have been utilized to induce an anti-tumoral response in vitro and in vivo. These strategies are being put to the test for different cancer types, including breast, prostate, lung, pancreatic, and renal cancer, as well as melanoma and lymphoma [76]. The glycosylation pattern of MUC proteins has proven to be a useful target for single-domain antibodies overcoming tumor growth, invasion, and metastasis in a mouse model [77].
Other glycosylated immunopeptides have also been investigated as vaccines, tumor-selective antibodies, CAR T cells, nanoparticles, and DC therapy [78,79,80,81,82,83,84,85,86,87,88]. Critical to this effect is knowledge of the structure of glycoproteins for their synthesis and antibody binding properties and their surface localization and occurrence in the immunopeptidome [89,90,91,92,93,94,95,96,97,98,99,100]. Interestingly, MHC-I also undergoes glycosylation, which must be taken into account for antigen presentation [101]. Additionally, of interest is the finding that deaminated MHC-I-bound peptides are derived from glycopeptides, which has relevance to antigen presentation to T cells [97]. At present, there is a surge of interest in glycopeptides presented by MHC-II on DCs, resulting from a deeper understanding of how glycosylated proteins are presented [102]. These advances and diversified strategies around the glycoproteogenome and its immunopeptidome are promising, not only for glycopeptides but also for various peptide PTMs in cancer that could be utilized as tumor-specific targets.
4. Peptide PTMs as a Source of Cancer Vaccines
Taking into account that the immunopeptidome represents the whole spectrum of peptides presented in a cell, there is a need to identify the most promising cancer targets. Thus, there is a need to determine the structure of an MHC-bound peptide and its level of expression for vaccine development. There are numerous ongoing clinical trials utilizing different antigens and adjuvants as therapeutic cancer vaccines. Focusing on peptides with PTMs as vaccines, promising findings have resulted from the use of citrullinated peptides (Table 1). A citrullinated VIM peptide has been utilized as an antigen in combination with an adjuvant induced IFN-γ and granzyme B-secreting CD4+ T cells. Citrullinated VIM-specific Th1 cells induced by the vaccine had a potent antitumor response against established skin and lung tumors, as well as a long-term memory response [30]. Similarly, a citrullinated ENO1 peptide-based vaccine elicited a potent citrulline-specific Th1 cell response in pancreatic, skin, and lung cancers [29]. Additionally, ENO1 is commonly overexpressed in different tumor types, including melanoma, pancreatic, breast, and lung cancer, thus citrullinated ENO1 peptides are plausible antigens for a wide cancer spectrum [18,29,55]. Furthermore, the combination of citrullinated VIM and ENO1 peptides in a vaccine, designated Modi-1, induced a significant antitumoral response in a mouse model of ovarian cancer. Importantly, a substantial citrulline-specific T cell response was observed in more than half of ovarian cancer patients [103]. Moreover, analysis of the melanoma immunopeptidome led to the identification of MHC-II-bound citrullinated peptides are derived from MMP21, Cp450, and GRI proteins [33]. A combination of these citrullinated peptides did not induce a greater antitumoral response than citrullinated MMP21 and GRI peptides individually, pointing to the potential of a reduced response with multiple peptides with different MHC-II binding specificities [33]. Another source of citrullinated peptides is the NPM protein. Vaccination with a PADI2-mediated-citrullinated NPM peptide induced an antitumoral response which was therapeutic, increasing survival and resulting in protection against a second tumor challenge in melanoma and lung cancer mouse models. Interestingly, PADI4-mediated-citrullination of NPM peptide did not elicit a citrulline-specific Th1 response, in contrast to PADI2-mediated-citrullination [53]. The CD4 responses observed may result from binding of citrullinated peptides primarily by MHC-II [18] in HLA-DP4 and HLA-DR4 transgenic mice. Nevertheless, the vaccine-induced CD4 response was sufficient to inhibit tumor progression, indicating the effectiveness of responses that do not involve CD8+ T cells [10].
Table 1.
Summary of the post-translational modified peptides used in immunotherapy.
| Post-Translational Modification |
Protein | Cancer Type | Immunotherapy | MHC Class | Reference |
|---|---|---|---|---|---|
| Citrullination | ENO1 | SKCM, PAAD, LUAD, OV | Vaccine | II | [18,29,55,103] |
| VIM | SKCM, LUAD, PAAD, OV | Vaccine | II | [30,103] | |
| MMP21 | SKCM | Vaccine | II | [33] | |
| GRI | SKCM | Vaccine | II | [33] | |
| Cp450 | SKCM | Vaccine | II | [33] | |
| NPM | SKCM, LUAD | Vaccine | II | [53] | |
| Phosphorylation | ISR2 | SKCM | Vaccine, ACT | I | [62,104] |
| BCAR | SKCM | Vaccine | I | [104] | |
| CDC25b | SKCM | ACT | I | [62] | |
| Glycosylation | MUC1 | BRCA, PRAD | Vaccine, DCTher | I, II | [77,80,81,82] |
| MUC4 | NA | Vaccine | II | [85] | |
| PHOX2B | Neuroblastoma | CAR T cell | I | [105] |
ACT: Adoptive Cell Therapy; DCTher: Dendritic Cell Therapy; ENO1: α-enolase 1; VIM: vimentin; MMP21: Matrix Metalloproteinase-21; GRI: Glutamate Receptor Ionotropic; NPM: Nucleophosmin; ISR2: Insulin Receptor Substrate 2; BCAR: Breast Cancer Antiestrogen Resistance 3; SKCM: Skin Cutaneous Melanoma; PAAD: Pancreatic Adenocarcinoma; PRAD: Prostate Adenocarcinoma; BRCA: Breast Invasive Carcinoma; LUAD: Lung Adenocarcinoma; OV: Ovarian Serous Cystadenocarcinoma; NA; Not Applicable.
There is a more limited number of studies utilizing phosphorylation as a PTM for peptide vaccines, although immunopeptidome analysis has pointed to a substantial number of phosphorylated peptides. Immunopeptidome analysis of melanoma, ovarian carcinoma, B lymphoblastoid, and leukemia resulted in the identification of a large number of phosphopeptides that were cancer specific with CD8 T cell antigen specificity in patients [58,63]. Some of the identified phosphopeptides were derived from ISR2, BCAR, TNS2, SELH, CDC25b, and beta-catenin [58,62,63], concordant with the identification of phosphorylated ISR2, TNS2, and SELH peptides in the colorectal cancer immunopeptidome [67]. At present, phosphopeptides from ISR2 and BCAR are being explored as a cancer vaccine for melanoma patients [104]. The phase I trial confirmed that the vaccines using these peptides are safe and capable of inducing an immune response, justifying future studies for their use as vaccines (NCT01846143).
As for glycopeptide-based cancer vaccines, an initial source was the glycosylated MUC protein displaying the Tn antigen. Immunization of mice with a desialylated ovine MUC with substantial representation of the Tn antigen elicited primarily a CD4+ T cell response specific to the Tn antigen. Conversely, immunization with a deglycosylated MUC did not induce an immune response [73]. The induction of an immune response specifically against the PTM protein suggests that glycosylation may be a useful source of cancer-specific antigens given the findings of aberrant glycosylation in many cancers, notably breast cancer [78,106]. A case in point is a fully synthetic cancer vaccine, a dendrimeric multiple antigenic glycopeptide displaying a trimer of Tn antigens (MAG-Tn3) associated with a promiscuous CD4 epitope, the tetanus toxoid-derived P2 peptide, that has been shown to induce an antitumoral Tn-specific T cell response in monkeys [78]. This MAG-Tn3 vaccine has been used in a phase I clinical trial for high-risk relapsed breast cancer patients (NCT02364492). Another cancer vaccine in clinical trial is based on MUC1 bearing Tn antigens (Tn-MUC1) pulsed with autologous DCs [81]. This phase I/II clinical trial follows the same strategy used in rhesus macaques, which resulted in five out of seven castrate-resistant prostate cancer patients having a CD4 and/or CD8 response (NCT00852007).
Other studies involving MUC1 glycopeptide vaccines induced a monoclonal IgG specific to mammary tumors [80]. Likewise, a synthetic MUC1 glycopeptide linked to a B-cell and a T-cell epitope together with poly I:C as an adjuvant elicited significant IgG titers against tumor-associated MUC1 expressed in breast cancer cells [82]. Recently, a novel approach was developed using a MUC1 glycopeptide that consists of a fluorinated nanoliposomal vaccine that is self-adjuvanated. This novel vaccine induced a high level of antigen-specific IgG in mice [107]. These glycopeptide-based vaccines were designed to induce primarily a humoral response. However, there are cancer vaccines using MUC1 glycopeptides that bind to MHC-I, inducing a cytotoxic CD8 response observed in healthy donors as well as in breast cancer patients [108]. There are also some promising glycopeptide candidates identified from leukemia MHC-I immunopeptidomes, five of which have been associated with a memory T cell response in healthy subjects [96]. Likewise, MUC4 glycopeptide candidates have been identified for pancreatic cancer immunotherapy [85,109]. Given the complexity of glycan modifications, there has been a surge of various approaches to identify and develop glycopeptides as vaccines, as reviewed above, including the use of glyco-antigen microarrays to investigate immune responses to cancer vaccines [79,91]. Another development is the use of an antigen delivery system based on gold nanoparticles with Dectin-1 to target DC, conjugated with MHC-II glycopeptides. This gold nanoparticle glycopeptide vaccine elicited a strong humoral and cellular immune response in mice [84]. In all, much progress has been made in the identification of glycopeptides and their structural and other properties to enhance their effectiveness as cancer vaccines [92,93,95,98].
5. Conclusions
It is evident that the potential of harnessing the immunopeptidome with its PTM peptides for cancer therapy and vaccines is quite substantial. Reliance on PTM modifications in tumor antigens further enhances the specificity of the epitopes and their restricted expression to cancer. Such PTMs do not occur in the thymus, resulting in a lack of negative selection for corresponding T cells. Although this review covered citrullination, phosphorylation, and glycosylation PTMs, there are numerous other modifications, some consisting of fusion peptides resulting from aberrant proteasomal function, known as proteasomal splicing. These spliced peptides result from the fusion of two unrelated fragments presented by MHC in cancer cells but may not be cancer specific [15,110]. An interesting development is harnessing the immunopeptidome to generate personalized oncolytic cancer vaccines, as demonstrated using a murine colon cancer model. This impressive immunopeptidomic-based pipeline harnesses the entire MHC-bound peptidome to develop an oncolytic cancer vaccine coated with tumor antigen peptides as a tool for immunotherapy [111]. The immunopeptidome also provides a basis for CAR T cell therapy, [83,112,113,114], targeting glycopeptides with promising results [86,115,116]. An example is the development of a peptide-centric CAR T cell using the immunopeptidome of neuroblastoma. Remarkably, computational modeling predicted that this peptide-centric CAR T cell was capable of recognizing peptides with different MHC-I polymorphisms, resulting in a strong and specific killing of neuroplastoma cells and complete tumor regression in mice [105,117].
Another promising avenue for immunopeptide-based immunotherapies is in combination with immune checkpoint inhibitors (ICI) with the potential for synergism as with anti-PD-1 immunotherapy [118]. Taking into account that ICI is mostly effective in the presence of tumor infiltrating lymphocytes, a prior immunization that can efficiently induce T cell tumor migration would enhance the efficiency of ICI therapy [119,120]. All things considered, the immunopeptidome field has crucial relevance for cancer interception.
Author Contributions
Conceptualization, R.A.L.-L.; writing—original draft preparation, R.A.L.-L. and H.K.; writing—review and editing, R.A.L.-L. and S.H.; supervision, S.H. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbott M., Ustoyev Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019;35:150923. doi: 10.1016/j.soncn.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Kote S., Pirog A., Bedran G., Alfaro J., Dapic I. Mass Spectrometry-Based Identification of MHC-Associated Peptides. Cancers. 2020;12:535. doi: 10.3390/cancers12030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada M., Shimizu K., Fujii S.I. Identification of Neoantigens in Cancer Cells as Targets for Immunotherapy. Int. J. Mol. Sci. 2022;23:2594. doi: 10.3390/ijms23052594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotsias F., Cebrian I., Alloatti A. Antigen processing and presentation. Int. Rev. Cell Mol. Biol. 2019;348:69–121. doi: 10.1016/bs.ircmb.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Freudenmann L.K., Marcu A., Stevanovic S. Mapping the tumour human leukocyte antigen (HLA) ligandome by mass spectrometry. Immunology. 2018;154:331–345. doi: 10.1111/imm.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters H.L., Tripathi S.C., Kerros C., Katayama H., Garber H.R., St John L.S., Federico L., Meraz I.M., Roth J.A., Sepesi B., et al. Serine Proteases Enhance Immunogenic Antigen Presentation on Lung Cancer Cells. Cancer Immunol. Res. 2017;5:319–329. doi: 10.1158/2326-6066.CIR-16-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Qi Y., Zhang Q., Liu W. Application of mass spectrometry-based MHC immunopeptidome profiling in neoantigen identification for tumor immunotherapy. Biomed. Pharm. 2019;120:109542. doi: 10.1016/j.biopha.2019.109542. [DOI] [PubMed] [Google Scholar]
- 8.Santambrogio L. Molecular Determinants Regulating the Plasticity of the MHC Class II Immunopeptidome. Front. Immunol. 2022;13:878271. doi: 10.3389/fimmu.2022.878271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson N., Jiang W., Adler L.N., Mellins E.D., Elias J.E. Tuning DO:DM Ratios Modulates MHC Class II Immunopeptidomes. Mol. Cell Proteom. 2022;21:100204. doi: 10.1016/j.mcpro.2022.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alspach E., Lussier D.M., Miceli A.P., Kizhvatov I., DuPage M., Luoma A.M., Meng W., Lichti C.F., Esaulova E., Vomund A.N., et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt D.F., Henderson R.A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A.L., Appella E., Engelhard V.H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 12.Yewdell J.W. MHC Class I Immunopeptidome: Past, Present & Future. Mol. Cell Proteom. 2022:100230. doi: 10.1016/j.mcpro.2022.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen M., Ternette N., Barra C. The interdependence of machine learning and LC-MS approaches for an unbiased understanding of the cellular immunopeptidome. Expert Rev. Proteom. 2022:1–12. doi: 10.1080/14789450.2022.2064278. [DOI] [PubMed] [Google Scholar]
- 14.Leko V., Rosenberg S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell. 2020;38:454–472. doi: 10.1016/j.ccell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liepe J., Sidney J., Lorenz F.K.M., Sette A., Mishto M. Mapping the MHC Class I-Spliced Immunopeptidome of Cancer Cells. Cancer Immunol. Res. 2019;7:62–76. doi: 10.1158/2326-6066.CIR-18-0424. [DOI] [PubMed] [Google Scholar]
- 16.Mishto M. Commentary: Are There Indeed Spliced Peptides in the Immunopeptidome? Mol. Cell Proteom. 2021;20:100158. doi: 10.1016/j.mcpro.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishto M. What We See, What We Do Not See, and What We Do Not Want to See in HLA Class I Immunopeptidomes. Proteomics. 2020;20:e2000112. doi: 10.1002/pmic.202000112. [DOI] [PubMed] [Google Scholar]
- 18.Katayama H., Kobayashi M., Irajizad E., Sevillarno A., Patel N., Mao X., Rusling L., Vykoukal J., Cai Y., Hsiao F., et al. Protein citrullination as a source of cancer neoantigens. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J., Zanker D., Di Carluccio A.R., Smelkinson M.G., Takeda K., Seedhom M.O., Dersh D., Gibbs J.S., Yang N., Jadhav A., et al. Varied Role of Ubiquitylation in Generating MHC Class I Peptide Ligands. J. Immunol. 2017;198:3835–3845. doi: 10.4049/jimmunol.1602122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beresford G.W., Boss J.M. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat. Immunol. 2001;2:652–657. doi: 10.1038/89810. [DOI] [PubMed] [Google Scholar]
- 21.McGinty J.W., Marre M.L., Bajzik V., Piganelli J.D., James E.A. T cell epitopes and post-translationally modified epitopes in type 1 diabetes. Curr. Diab. Rep. 2015;15:90. doi: 10.1007/s11892-015-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidney J., Vela J.L., Friedrich D., Kolla R., von Herrath M., Wesley J.D., Sette A. Low HLA binding of diabetes-associated CD8+ T-cell epitopes is increased by post translational modifications. BMC Immunol. 2018;19:12. doi: 10.1186/s12865-018-0250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drazic A., Myklebust L.M., Ree R., Arnesen T. The world of protein acetylation. Biochim. Biophys. Acta. 2016;1864:1372–1401. doi: 10.1016/j.bbapap.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Robert D., Schreiber L.J.O., Smyth M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 25.Chong C., Coukos G., Bassani-Sternberg M. Identification of tumor antigens with immunopeptidomics. Nat. Biotechnol. 2022;40:175–188. doi: 10.1038/s41587-021-01038-8. [DOI] [PubMed] [Google Scholar]
- 26.Sellars M.C., Wu C.J., Fritsch E.F. Cancer vaccines: Building a bridge over troubled waters. Cell. 2022;185:2770–2788. doi: 10.1016/j.cell.2022.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner A., Ruffell B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016;37:855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoitzner P., Romani N., Rademacher C., Probst H.C., Mahnke K. Antigen targeting to dendritic cells: Still a place in future immunotherapy? Eur. J. Immunol. 2022:1–16. doi: 10.1002/eji.202149515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook K., Daniels I., Symonds P., Pitt T., Gijon M., Xue W., Metheringham R., Durrant L., Brentville V. Citrullinated alpha-enolase is an effective target for anti-cancer immunity. Oncoimmunology. 2018;7:e1390642. doi: 10.1080/2162402X.2017.1390642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brentville V.A., Metheringham R.L., Gunn B., Symonds P., Daniels I., Gijon M., Cook K., Xue W., Durrant L.G. Citrullinated Vimentin Presented on MHC-II in Tumor Cells Is a Target for CD4+ T-Cell-Mediated Antitumor Immunity. Cancer Res. 2016;76:548–560. doi: 10.1158/0008-5472.CAN-15-1085. [DOI] [PubMed] [Google Scholar]
- 31.Yuzhalin A.E. Citrullination in Cancer. Cancer Res. 2019;79:1274–1284. doi: 10.1158/0008-5472.CAN-18-2797. [DOI] [PubMed] [Google Scholar]
- 32.Turunen S., Koivula M.K., Risteli L., Risteli J. Ureido group-specific antibodies are induced in rabbits immunized with citrulline or homocitrulline-containing antigens. Autoimmunity. 2016;49:459–465. doi: 10.3109/08916934.2016.1171853. [DOI] [PubMed] [Google Scholar]
- 33.Symonds P., Marcu A., Cook K.W., Metheringham R.L., Durrant L.G., Brentville V.A. Citrullinated Epitopes Identified on Tumour MHC Class II by Peptide Elution Stimulate Both Regulatory and Th1 Responses and Require Careful Selection for Optimal Anti-Tumour Responses. Front. Immunol. 2021;12:764462. doi: 10.3389/fimmu.2021.764462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ireland J.M., Unanue E.R. Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J. Exp. Med. 2011;208:2625–2632. doi: 10.1084/jem.20110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravanan P., Srikumar I.F., Talwar P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017;188:53–67. doi: 10.1016/j.lfs.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Sase T., Arito M., Onodera H., Omoteyama K., Kurokawa M.S., Kagami Y., Ishigami A., Tanaka Y., Kato T. Hypoxia-induced production of peptidylarginine deiminases and citrullinated proteins in malignant glioma cells. Biochem. Biophys. Res. Commun. 2017;482:50–56. doi: 10.1016/j.bbrc.2016.10.154. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Chen R., Gan Y., Ying S. The roles of PAD2- and PAD4-mediated protein citrullination catalysis in cancers. Int. J. Cancer. 2021;148:267–276. doi: 10.1002/ijc.33205. [DOI] [PubMed] [Google Scholar]
- 38.Guo W., Zheng Y., Xu B., Ma F., Li C., Zhang X., Wang Y., Chang X. Investigating the expression, effect and tumorigenic pathway of PADI2 in tumors. Onco. Targets. 2017;10:1475–1485. doi: 10.2147/OTT.S92389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L., Song G., Zhang X., Feng T., Pan J., Chen W., Yang M., Bai X., Pang Y., Yu J., et al. PADI2-Mediated Citrullination Promotes Prostate Cancer Progression. Cancer Res. 2017;77:5755–5768. doi: 10.1158/0008-5472.CAN-17-0150. [DOI] [PubMed] [Google Scholar]
- 40.Chandrashekar D.S., Bashel B., Balasubramanya S.A.H., Creighton C.J., Ponce-Rodriguez I., Chakravarthi B., Varambally S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cantarino N., Musulen E., Valero V., Peinado M.A., Perucho M., Moreno V., Forcales S.V., Douet J., Buschbeck M. Downregulation of the Deiminase PADI2 Is an Early Event in Colorectal Carcinogenesis and Indicates Poor Prognosis. Mol. Cancer Res. 2016;14:841–848. doi: 10.1158/1541-7786.MCR-16-0034. [DOI] [PubMed] [Google Scholar]
- 42.Funayama R., Taniguchi H., Mizuma M., Fujishima F., Kobayashi M., Ohnuma S., Unno M., Nakayama K. Protein-arginine deiminase 2 suppresses proliferation of colon cancer cells through protein citrullination. Cancer Sci. 2017;108:713–718. doi: 10.1111/cas.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guertin M.J., Zhang X., Anguish L., Kim S., Varticovski L., Lis J.T., Hager G.L., Coonrod S.A. Targeted H3R26 deimination specifically facilitates estrogen receptor binding by modifying nucleosome structure. PLoS Genet. 2014;10:e1004613. doi: 10.1371/journal.pgen.1004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohanan S., Horibata S., Anguish L.J., Mukai C., Sams K., McElwee J.L., McLean D., Yan A., Coonrod S.A. PAD2 overexpression in transgenic mice augments malignancy and tumor-associated inflammation in chemically initiated skin tumors. Cell Tissue Res. 2017;370:275–283. doi: 10.1007/s00441-017-2669-x. [DOI] [PubMed] [Google Scholar]
- 45.Yu H., Kortylewski M., Pardoll D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 46.Gijon M., Metheringham R.L., Toss M.S., Paston S.J., Durrant L.G. The Clinical and Prognostic Significance of Protein Arginine Deiminases 2 and 4 in Colorectal Cancer. Pathobiology. 2022;89:38–48. doi: 10.1159/000518414. [DOI] [PubMed] [Google Scholar]
- 47.Xin J., Song X. Role of peptidylarginine deiminase type 4 in gastric cancer. Exp. Med. 2016;12:3155–3160. doi: 10.3892/etm.2016.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang X., Han J., Pang L., Zhao Y., Yang Y., Shen Z. Increased PADI4 expression in blood and tissues of patients with malignant tumors. BMC Cancer. 2009;9:40. doi: 10.1186/1471-2407-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang X., Han J. Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol. Carcinog. 2006;45:183–196. doi: 10.1002/mc.20169. [DOI] [PubMed] [Google Scholar]
- 50.Fan T.T., Zhang C.S., Zong M., Zhao Q.D., Yang X., Hao C., Zhang H., Yu S.S., Guo J.H., Gong R.H., et al. Peptidylarginine deiminase IV promotes the development of chemoresistance through inducing autophagy in hepatocellular carcinoma. Cell Biosci. 2014;4:49. doi: 10.1186/2045-3701-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L., Chang X.T., Yuan G.Y., Zhao Y., Wang P.C. Expression of Peptidylarginine Deiminase Type 4 in Ovarian Tumors. Int. J. Biol. Sci. 2010;6:454–464. doi: 10.7150/ijbs.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee C.Y., Wang D., Wilhelm M., Zolg D.P., Schmidt T., Schnatbaum K., Reimer U., Ponten F., Uhlen M., Hahne H., et al. Mining the Human Tissue Proteome for Protein Citrullination. Mol. Cell Proteom. 2018;17:1378–1391. doi: 10.1074/mcp.RA118.000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choudhury R.H., Symonds P., Paston S.J., Daniels I., Cook K.W., Gijon M., Metheringham R.L., Brentville V.A., Durrant L.G. PAD-2-mediated citrullination of nucleophosmin provides an effective target for tumor immunotherapy. J. Immunother. Cancer. 2022;10:e003526. doi: 10.1136/jitc-2021-003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brentville V.A., Symonds P., Cook K.W., Daniels I., Pitt T., Gijon M., Vaghela P., Xue W., Shah S., Metheringham R.L., et al. T cell repertoire to citrullinated self-peptides in healthy humans is not confined to the HLA-DR SE alleles; Targeting of citrullinated self-peptides presented by HLA-DP4 for tumour therapy. Oncoimmunology. 2019;8:e1576490. doi: 10.1080/2162402X.2019.1576490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brentville V.A., Vankemmelbeke M., Metheringham R.L., Durrant L.G. Post-translational modifications such as citrullination are excellent targets for cancer therapy. Semin. Immunol. 2020;47:101393. doi: 10.1016/j.smim.2020.101393. [DOI] [PubMed] [Google Scholar]
- 56.Singh V., Ram M., Kumar R., Prasad R., Roy B.K., Singh K.K. Phosphorylation: Implications in Cancer. Protein J. 2017;36:1–6. doi: 10.1007/s10930-017-9696-z. [DOI] [PubMed] [Google Scholar]
- 57.Song G., Chen L., Zhang B., Song Q., Yu Y., Moore C., Wang T.L., Shih I.M., Zhang H., Chan D.W., et al. Proteome-wide Tyrosine Phosphorylation Analysis Reveals Dysregulated Signaling Pathways in Ovarian Tumors. Mol. Cell Proteom. 2019;18:448–460. doi: 10.1074/mcp.RA118.000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zarling A.L., Polefrone J.M., Evans A.M., Mikesh L.M., Shabanowitz J., Lewis S.T., Engelhardt V.H., Hunt D.F. Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. Proc. Natl. Acad. Sci. USA. 2006;103:14889–14894. doi: 10.1073/pnas.0604045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohammed F., Cobbold M., Zarling A.L., Salim M., Barrett-Wilt G.A., Shabanowitz J., Hunt D.F., Engelhard V.H., Willcox B.E. Phosphorylation-dependent interaction between antigenic peptides and MHC class I: A molecular basis for the presentation of transformed self. Nat. Immunol. 2008;9:1236–1243. doi: 10.1038/ni.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer V.S., Drews O., Gunder M., Hennenlotter J., Rammensee H.G., Stevanovic S. Identification of natural MHC class II presented phosphopeptides and tumor-derived MHC class I phospholigands. J. Proteome Res. 2009;8:3666–3674. doi: 10.1021/pr800937k. [DOI] [PubMed] [Google Scholar]
- 61.Depontieu F.R., Qian J., Zarling A.L., McMiller T.L., Salay T.M., Norris A., English A.M., Shabanowitz J., Engelhard V.H., Hunt D.F., et al. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc. Natl. Acad. Sci. USA. 2009;106:12073–12078. doi: 10.1073/pnas.0903852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarling A.L., Obeng R.C., Desch A.N., Pinczewski J., Cummings K.L., Deacon D.H., Conaway M., Slingluff C.L., Jr., Engelhard V.H. MHC-restricted phosphopeptides from insulin receptor substrate-2 and CDC25b offer broad-based immunotherapeutic agents for cancer. Cancer Res. 2014;74:6784–6795. doi: 10.1158/0008-5472.CAN-14-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cobbold M., De La Pena H., Norris A., Polefrone J.M., Qian J., English A.M., Cummings K.L., Penny S., Turner J.E., Cottine J., et al. MHC Class I-Associated Phosphopeptides Are the Targets of Memory-like Immunity in Leukemia. Sci. Transl. Med. 2013;5:203ra125. doi: 10.1126/scitranslmed.3006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomaino B., Cappello P., Capello M., Fredolini C., Sperduti I., Migliorini P., Salacone P., Novarino A., Giacobino A., Ciuffreda L., et al. Circulating Autoantibodies to Phosphorylated a-Enolase are a Hallmark of Pancreatic Cancer. J. Proteome Res. 2010;10:105–112. doi: 10.1021/pr100213b. [DOI] [PubMed] [Google Scholar]
- 65.Mohammed F., Stones D.H., Zarling A.L., Willcox C.R., Shabanowitz J., Cummings K.L., Hunt D.F., Cobbold M., Engelhard V.H., Willcox B.E. The antigenic identity of human class I MHC phosphopeptides is critically dependent upon phosphorylation status. Oncotarget. 2017;8:54160–54172. doi: 10.18632/oncotarget.16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alpizar A., Marino F., Ramos-Fernandez A., Lombardia M., Jeko A., Pazos F., Paradela A., Santiago C., Heck A.J., Marcilla M. A Molecular Basis for the Presentation of Phosphorylated Peptides by HLA-B Antigens. Mol. Cell Proteom. 2017;16:181–193. doi: 10.1074/mcp.M116.063800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Penny S.A., Abelin J.G., Malaker S.A., Myers P.T., Saeed A.Z., Steadman L.G., Bai D.L., Ward S.T., Shabanowitz J., Hunt D.F., et al. Tumor Infiltrating Lymphocytes Target HLA-I Phosphopeptides Derived From Cancer Signaling in Colorectal Cancer. Front. Immunol. 2021;12:723566. doi: 10.3389/fimmu.2021.723566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stopfer L.E., Conage-Pough J.E., White F.M. Quantitative Consequences of Protein Carriers in Immunopeptidomics and Tyrosine Phosphorylation MS(2) Analyses. Mol. Cell Proteom. 2021;20:100104. doi: 10.1016/j.mcpro.2021.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcilla M. Immunopeptidomic Analysis of the Phosphopeptidome Displayed by HLA Class I Molecules. Methods Mol. Biol. 2022;2420:149–158. doi: 10.1007/978-1-0716-1936-0_12. [DOI] [PubMed] [Google Scholar]
- 70.Chen R., Li J.J. Enrichment of Phosphorylated MHC Peptides with Immobilized Metal Affinity Chromatography and Titanium Dioxide Particles. Methods Mol. Biol. 2019;2024:259–268. doi: 10.1007/978-1-4939-9597-4_16. [DOI] [PubMed] [Google Scholar]
- 71.Solleder M., Guillaume P., Racle J., Michaux J., Pak H.S., Muller M., Coukos G., Bassani-Sternberg M., Gfeller D. Mass Spectrometry Based Immunopeptidomics Leads to Robust Predictions of Phosphorylated HLA Class I Ligands. Mol. Cell Proteom. 2020;19:390–404. doi: 10.1074/mcp.TIR119.001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ouyang M., Yu C., Deng X., Zhang Y., Zhang X., Duan F. O-GlcNAcylation and Its Role in Cancer-Associated Inflammation. Front. Immunol. 2022;13:861559. doi: 10.3389/fimmu.2022.861559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singhal A., Fohn M., Hakomori S. Induction of alpha-N-acetylgalactosamine-O-serine/threonine (Tn) antigen-mediated cellular immune response for active immunotherapy in mice. Cancer Res. 1991;51:1406–1411. [PubMed] [Google Scholar]
- 74.Mulder W.M.C., Stukart M.J., deWindt E., Wagstaff J., Scheper R.J., Bloemena E. Mucin-1-related T cell infiltration in colorectal carcinoma. Cancer Immunol. Immun. 1996;42:351–356. doi: 10.1007/s002620050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irazoqui F.J., Nores G.A. Thomsen-friedenreich disaccharide immunogenicity. Curr. Cancer Drug Targets. 2003;3:433–443. doi: 10.2174/1568009033481714. [DOI] [PubMed] [Google Scholar]
- 76.Brockhausen I., Melamed J. Mucins as anti-cancer targets: Perspectives of the glycobiologist. Glycoconj. J. 2021;38:459–474. doi: 10.1007/s10719-021-09986-8. [DOI] [PubMed] [Google Scholar]
- 77.Merikhian P., Darvishi B., Jalili N., Esmailinejad M.R., Khatibi A.S., Kalbolandi S.M., Salehi M., Mosayebzadeh M., Barough M.S., Majidzadeh A.K., et al. Recombinant nanobody against MUC1 tandem repeats inhibits growth, invasion, metastasis, and vascularization of spontaneous mouse mammary tumors. Mol. Oncol. 2022;16:485–507. doi: 10.1002/1878-0261.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laubreton D., Bay S., Sedlik C., Artaud C., Ganneau C., Deriaud E., Viel S., Puaux A.L., Amigorena S., Gerard C., et al. The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830-844 universal epitope provides anti-tumor immunity. Cancer Immunol. Immunother. 2016;65:315–325. doi: 10.1007/s00262-016-1802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Padler-Karavani V. Glycan Microarray Reveal the Sweet Side of Cancer Vaccines. Cell Chem. Biol. 2016;23:1446–1447. doi: 10.1016/j.chembiol.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palitzsch B., Gaidzik N., Stergiou N., Stahn S., Hartmann S., Gerlitzki B., Teusch N., Flemming P., Schmitt E., Kunz H. A Synthetic Glycopeptide Vaccine for the Induction of a Monoclonal Antibody that Differentiates between Normal and Tumor Mammary Cells and Enables the Diagnosis of Human Pancreatic Cancer. Angew. Chem. Int. Ed. Engl. 2016;55:2894–2898. doi: 10.1002/anie.201509935. [DOI] [PubMed] [Google Scholar]
- 81.Scheid E., Major P., Bergeron A., Finn O.J., Salter R.D., Eady R., Yassine-Diab B., Favre D., Peretz Y., Landry C., et al. Tn-MUC1 DC Vaccination of Rhesus Macaques and a Phase I/II Trial in Patients with Nonmetastatic Castrate-Resistant Prostate Cancer. Cancer Immunol. Res. 2016;4:881–892. doi: 10.1158/2326-6066.CIR-15-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glaffig M., Stergiou N., Schmitt E., Kunz H. Immunogenicity of a Fully Synthetic MUC1 Glycopeptide Antitumor Vaccine Enhanced by Poly(I:C) as a TLR3-Activating Adjuvant. ChemMedChem. 2017;12:722–727. doi: 10.1002/cmdc.201700254. [DOI] [PubMed] [Google Scholar]
- 83.He Y., Schreiber K., Wolf S.P., Wen F., Steentoft C., Zerweck J., Steiner M., Sharma P., Shepard H.M., Posey A., et al. Multiple cancer-specific antigens are targeted by a chimeric antigen receptor on a single cancer cell. JCI Insight. 2019;4 doi: 10.1172/jci.insight.135306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trabbic K.R., Kleski K.A., Barchi J.J., Jr. A Stable Gold Nanoparticle-Based Vaccine for the Targeted Delivery of Tumor-Associated Glycopeptide Antigens. ACS Biol. Med. Chem. Au. 2021;1:31–43. doi: 10.1021/acsbiomedchemau.1c00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trabbic K.R., Whalen K., Abarca-Heideman K., Xia L., Temme J.S., Edmondson E.F., Gildersleeve J.C., Barchi J.J., Jr. A Tumor-Selective Monoclonal Antibody from Immunization with a Tumor-Associated Mucin Glycopeptide. Sci. Rep. 2019;9:5662. doi: 10.1038/s41598-019-42076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou R., Yazdanifar M., Roy L.D., Whilding L.M., Gavrill A., Maher J., Mukherjee P. CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth. Front. Immunol. 2019;10:1149. doi: 10.3389/fimmu.2019.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu H., Wang K., Wang Z., Wang D., Yin X., Liu Y., Yu F., Zhao W. An efficient and safe MUC1-dendritic cell-derived exosome conjugate vaccine elicits potent cellular and humoral immunity and tumor inhibition in vivo. Acta Biomater. 2022;138:491–504. doi: 10.1016/j.actbio.2021.10.041. [DOI] [PubMed] [Google Scholar]
- 88.Del Bano J., Flores-Flores R., Josselin E., Goubard A., Ganier L., Castellano R., Chames P., Baty D., Kerfelec B. A Bispecific Antibody-Based Approach for Targeting Mesothelin in Triple Negative Breast Cancer. Front. Immunol. 2019;10:1593. doi: 10.3389/fimmu.2019.01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qu J., Yu H., Li F., Zhang C., Trad A., Brooks C., Zhang B., Gong T., Guo Z., Li Y., et al. Molecular basis of antibody binding to mucin glycopeptides in lung cancer. Int. J. Oncol. 2016;48:587–594. doi: 10.3892/ijo.2015.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Movahedin M., Brooks T.M., Supekar N.T., Gokanapudi N., Boons G.J., Brooks C.L. Glycosylation of MUC1 influences the binding of a therapeutic antibody by altering the conformational equilibrium of the antigen. Glycobiology. 2017;27:677–687. doi: 10.1093/glycob/cww131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marchiori M.F., Bortot L.O., Carvalho I., Campo V.L. Synthesis of MUC1-derived glycopeptide bearing a novel triazole STn analog. Carbohydr. Res. 2020;498:108155. doi: 10.1016/j.carres.2020.108155. [DOI] [PubMed] [Google Scholar]
- 92.Stergiou N., Urschbach M., Gabba A., Schmitt E., Kunz H., Besenius P. The Development of Vaccines from Synthetic Tumor-Associated Mucin Glycopeptides and their Glycosylation-Dependent Immune Response. Chem. Rec. 2021;21:3313–3331. doi: 10.1002/tcr.202100182. [DOI] [PubMed] [Google Scholar]
- 93.Asín A., García-Martín F., Busto J.H., Avenoza A., Peregrina J.M., Corzana F. Structure-based Design of Anti-cancer Vaccines: The Significance of Antigen Presentation to Boost the Immune Response. Curr. Med. Chem. 2022;29:1258–1270. doi: 10.2174/0929867328666210810152917. [DOI] [PubMed] [Google Scholar]
- 94.Doelman W., van Kasteren S.I. Synthesis of glycopeptides and glycopeptide conjugates. Org. Biomol. Chem. 2022 doi: 10.1039/D2OB00829G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toraskar S., Madhukar Chaudhary P., Kikkeri R. The Shape of Nanostructures Encodes Immunomodulation of Carbohydrate Antigen and Vaccine Development. ACS Chem. Biol. 2022;17:1122–1130. doi: 10.1021/acschembio.1c00998. [DOI] [PubMed] [Google Scholar]
- 96.Malaker S.A., Penny S.A., Steadman L.G., Myers P.T., Loke J.C., Raghavan M., Bai D.L., Shabanowitz J., Hunt D.F., Cobbold M. Identification of Glycopeptides as Posttranslationally Modified Neoantigens in Leukemia. Cancer Immunol. Res. 2017;5:376–384. doi: 10.1158/2326-6066.CIR-16-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mei S., Ayala R., Ramarathinam S.H., Illing P.T., Faridi P., Song J., Purcell A.W., Croft N.P. Immunopeptidomic Analysis Reveals That Deamidated HLA-bound Peptides Arise Predominantly from Deglycosylated Precursors. Mol. Cell Proteom. 2020;19:1236–1247. doi: 10.1074/mcp.RA119.001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferreira J.A., Relvas-Santos M., Peixoto A., Silva A.M.N., Lara Santos L. Glycoproteogenomics: Setting the Course for Next-generation Cancer Neoantigen Discovery for Cancer Vaccines. Genom. Proteom. Bioinform. 2021;19:25–43. doi: 10.1016/j.gpb.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukherjee S., Sanchez-Bernabeu A., Demmers L.C., Wu W., Heck A.J.R. The HLA Ligandome Comprises a Limited Repertoire of O-GlcNAcylated Antigens Preferentially Associated With HLA-B*07:02. Front. Immunol. 2021;12:796584. doi: 10.3389/fimmu.2021.796584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pont L., Kuzyk V., Benavente F., Sanz-Nebot V., Mayboroda O.A., Wuhrer M., Lageveen-Kammeijer G.S.M. Site-Specific N-Linked Glycosylation Analysis of Human Carcinoembryonic Antigen by Sheathless Capillary Electrophoresis-Tandem Mass Spectrometry. J. Proteome Res. 2021;20:1666–1675. doi: 10.1021/acs.jproteome.0c00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoek M., Demmers L.C., Wu W., Heck A.J.R. Allotype-Specific Glycosylation and Cellular Localization of Human Leukocyte Antigen Class I Proteins. J. Proteome Res. 2021;20:4518–4528. doi: 10.1021/acs.jproteome.1c00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parker R., Partridge T., Wormald C., Kawahara R., Stalls V., Aggelakopoulou M., Parker J., Powell Doherty R., Ariosa Morejon Y., Lee E., et al. Mapping the SARS-CoV-2 spike glycoprotein-derived peptidome presented by HLA class II on dendritic cells. Cell Rep. 2021;35:109179. doi: 10.1016/j.celrep.2021.109179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brentville V.A., Metheringham R.L., Daniels I., Atabani S., Symonds P., Cook K.W., Vankemmelbeke M., Choudhury R., Vaghela P., Gijon M., et al. Combination vaccine based on citrullinated vimentin and enolase peptides induces potent CD4-mediated anti-tumor responses. J. Immunother. Cancer. 2020;8:e000560. doi: 10.1136/jitc-2020-000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Engelhard V.H., Obeng R.C., Cummings K.L., Petroni G.R., Ambakhutwala A.L., Chianese-Bullock K.A., Smith K.T., Lulu A., Varhegyi N., Smolkin M.E., et al. MHC-restricted phosphopeptide antigens: Preclinical validation and first-in-humans clinical trial in participants with high-risk melanoma. J. Immunother. Cancer. 2020;8:e000262. doi: 10.1136/jitc-2019-000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yarmarkovich M., Marshall Q.F., Warrington J.M., Premaratne R., Farrel A., Groff D., Li W., di Marco M., Runbeck E., Truong H., et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature. 2021;599:477–484. doi: 10.1038/s41586-021-04061-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Vlad A.M., Finn O.J. Glycoprotein tumor antigens for immunotherapy of breast cancer. Breast. Dis. 2004;20:73–79. doi: 10.3233/BD-2004-20109. [DOI] [PubMed] [Google Scholar]
- 107.Dong P., Cheng S., Wang Y., Gao H., Zhang Y., Zhu T., Yu P., Meng X. A self-adjuvanting anti-tumor nanoliposomal vaccine based on fluorine-substituted MUC1 glycopeptide. Chem. Commun. 2022;58:8642–8645. doi: 10.1039/D2CC02143A. [DOI] [PubMed] [Google Scholar]
- 108.Pathangey L.B., Lakshminarayanan V., Suman V.J., Pockaj B.A., Mukherjee P., Gendler S.J. Aberrant Glycosylation of Anchor-Optimized MUC1 Peptides Can Enhance Antigen Binding Affinity and Reverse Tolerance to Cytotoxic T Lymphocytes. Biomolecules. 2016;6:31. doi: 10.3390/biom6030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gautam S.K., Kumar S., Dam V., Ghersi D., Jain M., Batra S.K. MUCIN-4 (MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy. Semin. Immunol. 2020;47:101391. doi: 10.1016/j.smim.2020.101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vigneron N., Ferrari V., Stroobant V., Abi Habib J., Van den Eynde B.J. Peptide splicing by the proteasome. J. Biol. Chem. 2017;292:21170–21179. doi: 10.1074/jbc.R117.807560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feola S., Chiaro J., Martins B., Russo S., Fusciello M., Ylosmaki E., Bonini C., Ruggiero E., Hamdan F., Feodoroff M., et al. A novel immunopeptidomic-based pipeline for the generation of personalized oncolytic cancer vaccines. Elife. 2022;11:e71156. doi: 10.7554/eLife.71156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ternette N., Olde Nordkamp M.J.M., Muller J., Anderson A.P., Nicastri A., Hill A.V.S., Kessler B.M., Li D. Immunopeptidomic Profiling of HLA-A2-Positive Triple Negative Breast Cancer Identifies Potential Immunotherapy Target Antigens. Proteomics. 2018;18:e1700465. doi: 10.1002/pmic.201700465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bilich T., Nelde A., Bauer J., Walz S., Roerden M., Salih H.R., Weisel K., Besemer B., Marcu A., Lubke M., et al. Mass spectrometry-based identification of a B-cell maturation antigen-derived T-cell epitope for antigen-specific immunotherapy of multiple myeloma. Blood Cancer J. 2020;10:24. doi: 10.1038/s41408-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marcu A., Bichmann L., Kuchenbecker L., Kowalewski D.J., Freudenmann L.K., Backert L., Muhlenbruch L., Szolek A., Lubke M., Wagner P., et al. HLA Ligand Atlas: A benign reference of HLA-presented peptides to improve T-cell-based cancer immunotherapy. J. Immunother. Cancer. 2021;9:e002071. doi: 10.1136/jitc-2020-002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heard A., Landmann J.H., Hansen A.R., Papadopolou A., Hsu Y.S., Selli M.E., Warrington J.M., Lattin J., Chang J., Ha H., et al. Antigen glycosylation regulates efficacy of CAR T cells targeting CD19. Nat. Commun. 2022;13:3367. doi: 10.1038/s41467-022-31035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raglow Z., McKenna M.K., Bonifant C.L., Wang W., Pasca di Magliano M., Stadlmann J., Penninger J.M., Cummings R.D., Brenner M.K., Markovitz D.M. Targeting Glycans for CAR Therapy: The Advent of Sweet CARs. Mol. Ther. 2022;30:2881–2890. doi: 10.1016/j.ymthe.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Irving M., Zoete V., Bassani-Sternberg M., Coukos G. A roadmap for driving CAR T cells toward the oncogenic immunopeptidome. Cancer Cell. 2022;40:20–22. doi: 10.1016/j.ccell.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 118.Leon-Letelier R.A., Bonifaz L.C., Fuentes-Panana E.M. OMIC signatures to understand cancer immunosurveillance and immunoediting: Melanoma and immune cells interplay in immunotherapy. J. Leukoc. Biol. 2019;105:915–933. doi: 10.1002/JLB.MR0618-241RR. [DOI] [PubMed] [Google Scholar]
- 119.Leon-Letelier R.A., Castro-Medina D.I., Badillo-Godinez O., Tepale-Segura A., Huanosta-Murillo E., Aguilar-Flores C., De Leon-Rodriguez S.G., Mantilla A., Fuentes-Panana E.M., Lopez-Macias C., et al. Induction of Progenitor Exhausted Tissue-Resident Memory CD8(+) T Cells Upon Salmonella Typhi Porins Adjuvant Immunization Correlates With Melanoma Control and Anti-PD-1 Immunotherapy Cooperation. Front. Immunol. 2020;11:583382. doi: 10.3389/fimmu.2020.583382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., Zhang W., Luoma A., Giobbie-Hurder A., Peter L., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]