Abstract
Enteroviruses (EVs) are major pathogens in young infants. These viruses were traditionally classified into the following four subgenera: polio, coxsackie A and B, and echoviruses. Now that poliomyelitis seems to be controlled in most parts of the world, coxsackie and echoviruses are gaining more attention because (i) the structural and pathophysiological similarities and (ii) the consequent possibilities in translational medicine. Enteroviruses are transmitted mainly by oral and fecal–oral routes; the clinical manifestations include a viral prodrome including fever, feeding intolerance, and lethargy, which may be followed by exanthema; aseptic meningitis and encephalitis; pleurodynia; myopericarditis; and multi-system organ failure. Laboratory diagnosis is largely based on reverse transcriptase–polymerase chain reaction, cell culture, and serology. Prevention and treatment can be achieved using vaccination, and administration of immunoglobulins and antiviral drugs. In this article, we have reviewed the properties of these viruses, their clinical manifestations, and currently available methods of detection, treatment, and prognosis.
Keywords: Coxsackie virus, Enteroviruses, Neonate, Newborn
INTRODUCTION
Enteroviruses belong to the Picornaviridae family of viruses.1 In infants, these pathogens can cause varied clinical manifestations, including hand, foot, and mouth disease; respiratory illness; myocarditis; meningitis; and sepsis; and even lethal multi-system organ failure. These viruses are transmitted primarily from one person to another.2
Enteroviruses were identified as a distinct class of viruses in 1957.3 These pathogens were named based on their natural enteric habitat.3 Many serotypes were identified based upon neutralization with specific antisera and with polymerase chain reactions,4 and were initially classified into the following four subgenera:2 (a) Polioviruses (serotypes 1–3); (b) Coxsackieviruses (CVs) group A (CV-A; serotypes 1–22 and 24); (c) CV-B; serotypes 1–6); and (d) echoviruses (serotypes 1–9, 11–21, 24–27, and 29–33). Newer classifications divide EVs into four species, A-D, based on the regions of the viral RNA that encode for the VP1 capsid protein.5 Serotypes added after 1970 are simply named as EVs with a species designation (such as EV-D68) (Table 1).6 New serotypes are being continuously added and the number now exceeds more than 100.7,8 Now that poliomyelitis seems to be better controlled, the CVs and the echoviruses are receiving more attention.
Table 1:
Genomic classification of EVs
| Species designation | Types |
|---|---|
| Human enterovirus A (HEV-A) | CV A2–8, A10, A12, A14, and A16 EV A71, A76, A89, A90, A91, A114, and A119 |
| Human enterovirus B (HEV-B) | CV A9 CV B1–6 Echovirus 1–9, 11–21, 24–27, and 29–33 Enterovirus 69, B73–B75, B77–B78, B93, B97, B98, B100, B101, B106, and B107 |
| Human enterovirus C (HEV-C) | Poliovirus 1–3 CV A1, A11, A13, A17, A19–22, and A24 Enterovirus C95, C96, C99, C102, C104, C105, C109, C113, C116–118 |
| Human enterovirus D (HEV-D) | Enterovirus D68, D70, D94, and D111 |
Virology
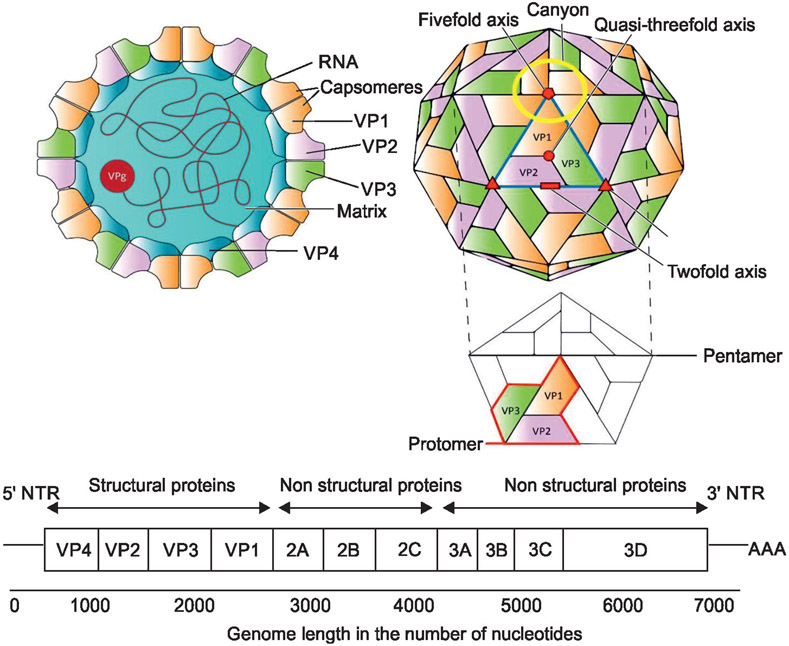
Enteroviruses are small (approximately 27 nm), non-enveloped virions with an icosahedral capsid with 60 subunits, each formed from four proteins (from VP1 to VP4).9 Each virion has a linear, single-stranded, positive-sense RNA genome of about 7.5 kB (Fig. 1).10 We have developed a standardized 16-component table to describe viral pathogens. Detailed information is available for some of these components in EVs (Table 2).
Fig. 1:
Schematic diagram showing the structure of EVs. Left-side of the schematic panel shows a cross-section with the location of the RNA, capsomeres, the matrix, and the viral proteins (VPs); Right-side of the schematic panel shows the surface with the location of structural and non-structural VPs on the surface of viral particles
Table 2:
List of major structural components of EVs have been listed (we have used a standardized table developed to describe viral pathogens)
| Structure | Available information |
|---|---|
| Lipid envelope | EVs are small, spherical viruses made up of an RNA genome surrounded by a protein shell.These viruses lack a “membrane.”87 |
| Glycoproteins | Proteins such as A9 and 3A are important; interact with host secretory carrier membrane protein 3 and participate in viral replication.87 |
| Receptor-binding motifs | The arginine–glycine–aspartic acid (RGD) motif found in the VP1 capsid protein of CV-A9 has a role in cell entry. This motif binds integrins to promote entry into the cells.88 |
| Envelope protein E | Either not expressed or relevance unclear in fetal/infantile disease. |
| Membrane protein | Either not expressed or relevance unclear in fetal/infantile disease. |
| MHC or HLA Proteins | Either not expressed or relevance unclear in fetal/infantile disease. |
| Spike protein | Either not expressed or relevance unclear in fetal/infantile disease. |
| Surface tubules | Lipid droplets (LDs) are transported to lysosomes by autophagy. Lipases are recruited to the LD surface for sequential hydrolysis of TGs stored within LDs. After enterovirus infection, TGs within LDs transformed into fatty acids.89 |
| Palisade layer | Either not expressed or relevance unclear in fetal/infantile disease. |
| Viral tegument | Either not expressed or relevance unclear in fetal/infantile disease. |
| Lateral bodies | Either not expressed or relevance unclear in fetal/infantile disease. |
| Capsid | EVs are small (approximately 27 nm), non-enveloped virions with an icosahedral capsid with 60 subunits, each formed from four proteins (VP1 to VP4).9 |
| Capsomeres | Viral polyprotein domains (from P1 to P3) are cleaved into 3–4 domains each; P1 is liberated from the polyprotein by 2A protein. Amino acids in the loops that extend from the β-barrel domain of VP1, VP2, and VP3 give the EVs their distinct antigenicity. |
| Core membrane | Either not expressed or relevance unclear in fetal/infantile disease.9 |
| Protein core | Details on genome-associated polyprotein are described below. |
| Core fibrils | Either not expressed or relevance unclear in fetal/infantile disease. |
| Matrix | Virions penetrating the cell surface get uncoated and the viral genome functions as mRNA for the viral polyprotein.90 |
| Enzymes | Details scant. Alter the expression of host enzymes.91 |
| RNA elements | Enteroviral 3’ non-translated regions (3’NTR) are comprised of two (X and Y) hairpin structures.92 |
| Nucleus | Either not expressed or relevance unclear in fetal/infantile disease. |
| Nucleosome | Either not expressed or relevance unclear in fetal/infantile disease. |
| DNA | No DNA genome exists |
| RNA | The enteroviral genome (7.5-8 kb) is flanked by a 5’-UTR that is composed of an RNA cloverleaf structure and an internal ribosomal entry site (IRES).5,9 |
| Genome-associated polyprotein | A single polyprotein is cleaved by the host and viral protease into 4 capsid VP proteins and 7 non-structural proteins. The capsid protein VP1 varies and confers antigenic properties.93 |
| RNA polymerase | The RNA-dependent RNA polymerase (RdRP), known as 3D protein, functions as a replica for viral RNA synthesis in infected cells.94 |
| Reverse transcriptase | Either not expressed or relevance unclear in fetal/infantile disease. |
| Head | Either not expressed or relevance unclear in fetal/infantile disease. |
| Base plate | Either not expressed or relevance unclear in fetal/infantile disease. |
| Integrase | Either not expressed or relevance unclear in fetal/infantile disease. |
| Tail | Either not expressed or relevance unclear in fetal/infantile disease. |
| Tail fiber | Either not expressed or relevance unclear in fetal/infantile disease. |
| Neck | Either not expressed or relevance unclear in fetal/infantile disease. |
HLA, human leukocyte antigens; MHC, major histocompatibility complex; TGs, triglycerides
Intracellular Replication
Enteroviruses replication is initiated by attachment to cell membrane receptors which determine host cell susceptibility.11 Penetration and uncoating of the virions lead to the release of RNA into the cell cytoplasm and synthesis of negative-strand RNA begins within 30 minutes.12 The newly formed positive-strand RNAs serve as a message for translation and are incorporated into newly formed virions. Complete virions can be seen by electron microscopy within hours.13
Coxsackieviruses and echoviruses are non-enveloped virions with an icosahedral capsid structure containing linear single-stranded RNA (Fig. 1).10 As described above, group A has 23 serotypes (1–22 and 24). Coxsackieviruses-B are usually classified into 6 serotypes (1–6), namely, the CV-B1, CV-B2, CV-B3, CV-B4, CV-B5, and CV-B6.14
Epidemiology
Seasonal and Demographic Distribution
Enteroviruses infections occur throughout the year in warmer regions. This differs from the temperate climates, where the incidence is higher in summer and fall.15 Infants are most susceptible, particularly males.16 The incubation periods for enterovirus infections vary with different clinical syndromes.17
Transmission
Transmission of EVs occurs mainly by oral and fecal–oral routes.13 It is enhanced by poor sanitary conditions, contaminated water, food, and fomites.18 Flies appear to be a significant vector in situations of poor sanitation and heavy human infection.19 Swimming pools are a major channel of spread during summer.20 Respiratory route is an important mode of transmission for some serotypes, including CV A21 and EV-D68.21 Moreover, EV-D70 is shed with tears and spreads via fingers and fomites.22 There is a high incidence of secondary infection in household contacts, especially in infants.23
Furthermore, CV-B affects infants of both genders with equal incidence. Compared to older children and adults, neonates and young infants may be affected more frequently and with higher severity of the disease.24 Also, CV-B4 has higher mortality than other serotypes. Moreover, CV-B virus is the major cause of viral myocarditis, especially in neonates and younger children.1,25 The prevalence of echovirus excretion in the community resembles the general population. Most transmission is vertical, from the mother to her fetus/newborn.26
Pathophysiology
These virions penetrate the cell surface, get uncoated, and the viral genome functions as mRNA for the viral polyprotein (Fig. 2). The polyprotein has three domains, from P1 to P3, which are cleaved into three to four proteins each. Domain P1 is liberated from the polyprotein by 2A protein and gets split into three proteins, VP0, VP1, and VP3, by 3C protease. Protein VP0 is processed further into smaller proteins, VP4 and VP2. They form eight-stranded antiparallel β-sheets. The amino acids in the loops that connect the β-strands and the N-terminal and C-terminal sequences that extend from the β-barrel domain of VP1, VP2, and VP3 give the EVs their distinct antigenicity.27
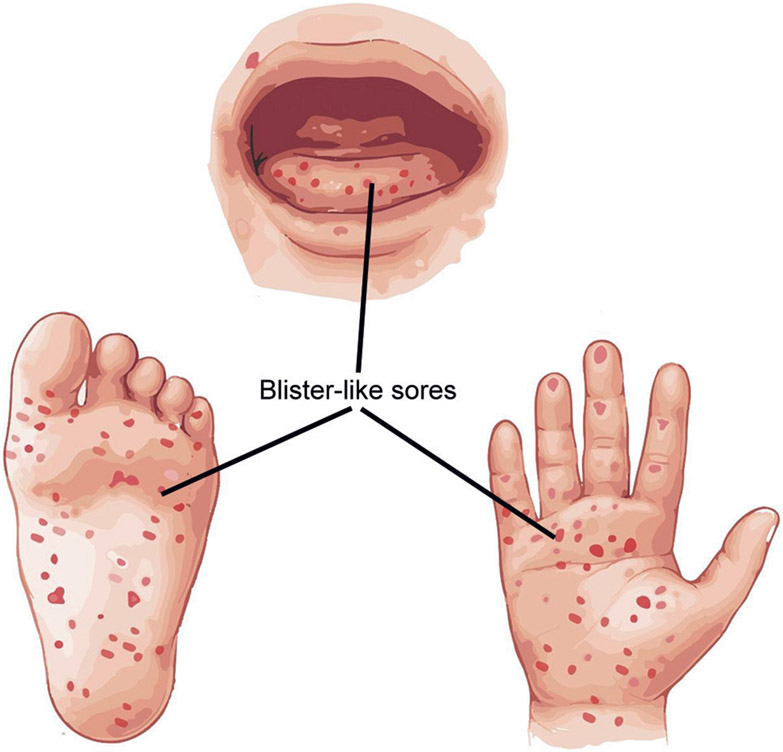
Fig. 2:
An artist’s recall of vesico–bullous (blister-like) sores in hand, foot, and mouth disease.
The coxsackie–adenovirus receptor (CAR) and the decay-accelerating factor (DAF) are receptors involved in the pathogenesis of coxsackie B virus infections.27 Interaction of CV-B with CAR and DAF leads to the pathogenesis of various clinical manifestations, especially acute and chronic myocarditis. The CAR is expressed in the intercalated discs in the heart. DAF is expressed mostly in epithelial and endothelial cells. Interaction of cardiotropic CV-B with DAF and CAR enhances viral entry into myocardial cells and is responsible for myocarditis.28 Pathogenesis of CNS infections may involve hematogenous spread or axonal transport. Also, CV-B is subdivided into the following two DAF-binding phenotypes: The CV-B that does not bind to DAF (CV-B2, 4, and 6) and the CV-B that binds to DAF and requires CAR for infection.
Echoviruses bind to integrin αvβ3 (vitronectin receptor; serotypes 1, 9),29 integrin α2β1 (serotypes 1 and 8), and the human neonatal Fc receptor (FcRn; binds serotypes 5–7, 9, 11, 13, and 30).30 Moreover, FcRn is a pan-echovirus receptor; it is expressed in the placenta, intestinal epithelium, hepatocytes, and cerebral endothelial cells. This pattern of expression is consistent with the organ sites targeted by echoviruses, as the primary entry site of infection is the intestinal, and secondary sites of infection include the liver and brain.31
Host Factors
Neonates are predisposed to infections with CV-B and certain serotypes of echovirus (such as 11). Vertical transmission is more common than postnatal transmission. Infection is more frequent in seasonal community outbreaks of CV-B disease. Neonates are predisposed to severe infections with EVs, but the involved mechanisms are still not known.32 The relative functional inability of neonatal macrophages and dysregulated cytokine/chemokine responses have been implicated.33
Passively acquired antibodies from mothers seem to be protective against serious disease and death.34 Infants with transplacental acquired antibodies have relatively asymptomatic infections. The timing of the mother’s infection determines the outcome of neonatal CV-B infection. Maternal infections beginning more than 5–7 days before delivery allow transplacental passage of specific immunoglobulin G (IgG) antibodies and prevents severe neonatal disease. Infants with maternal infections in the immediate peripartum period have a relatively poor prognosis. The clinical expression of neonatal CV-B disease depends on the timing of maternal infection, age, and passively acquired maternal antibodies.
Clinical Manifestations
These viruses can cause a range of clinical manifestations including fever, lethargy, myalgia, ileus, and diarrhea; exanthemata; aseptic meningitis and encephalitis; pleurodynia; and myopericarditis.
Hand, Foot, and Mouth Disease (HFMD)
The HFMD is a common illness in children characterized by fever, vesicles on the buccal mucosa and tongue, and tender cutaneous lesions on the hands, feet, buttocks, and genitalia (Flowchart 1).35 Moreover, EV-A71 is the most frequently seen causative organism and can be associated with encephalitis, pulmonary edema and heart failure.36,37 An atypical presentation of HFMD characterized by vesiculobullous lesions is caused by CV-A6.38,39 Echovirus serotypes 3 and 33 have also been isolated.40,41
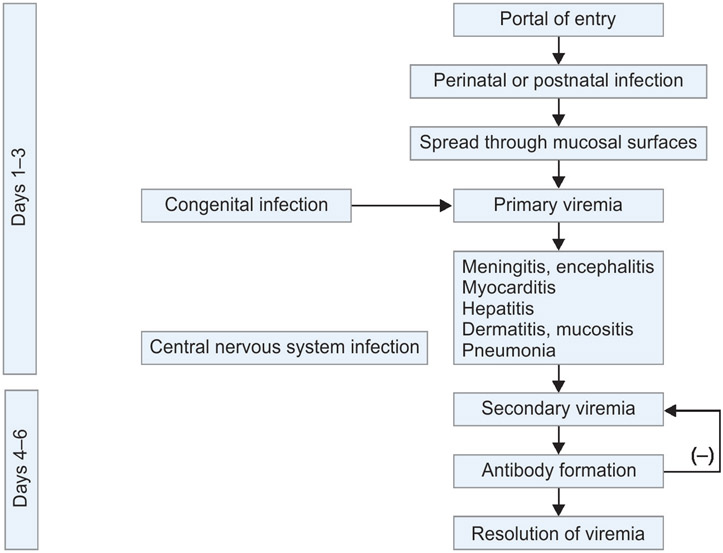
Flowchart 1:
Temporal evolution of enterovirus infections before and after birth
Herpangina
The CVs are a major cause of herpangina, a vesicular enanthem of the in the oral cavity.42 Echoviruses can also rarely be the causative agents. It affects infants only when they have reached the age of 3 years or more of age and is more common in summers.43 Sore throat, fever, and odynophagia are the predominant symptoms.
Maculopapular Eruptions
Generalized maculopapular eruptions are seen with EV infections.44,45 The “Boston exanthem” is a febrile 24–36-hour prodrome followed by the appearance of small, non-pruritic, pink maculopapular eruptions on the face and upper chest.46 Petechial and purpuric rashes have been associated with echoviruses 9 and 25 and CV-A9 infections.44,47,48
Urticaria-like Eruptions
Cutaneous manifestations of CV A9 and some echoviruses can range from urticarial, scarlatiniform, vesicular, pustular, and/or petechial lesions.49
Central Nervous System Infections
Acute CNS infection occurs at all ages. Aseptic meningitis is the most common CNS manifestation. Polioviruses, EV-D68, and EV-A71 target motor nuclei within the brainstem and spinal cord, causing acute paresis of cranial and spinal nerves. The CV-A2 and echovirus 9 have also been identified.50,51
Myocarditis
The CV-B types 2, 3, 4, and 5 are the most common causes of neonatal myocarditis.5,50,52,53 Onset of symptoms is generally before day 10 of life. It has a biphasic presentation. Non-specific signs and symptoms of lethargy, poor feeding, or mild respiratory distress precede the onset of myocarditis by 2–5 days.54 These infants continue to have respiratory distress, tachycardia, jaundice, and diarrhea. There may also be temperature instability, tachycardia, arrhythmias, hepatomegaly, and poor perfusion. The EKGs show low voltage and other electrophysiologic abnormalities. Echocardiographic studies indicate poor left ventricular or biventricular function.
Infants with CV-B myocarditis may have concomitant meningoencephalitis, pneumonia, hepatitis, pancreatitis, and adrenalitis. Mortality among infants with myocarditis alone is around 30–50% and is higher in cases with multisystem involvement. Meningoencephalitis may manifest with altered sensorium, seizures, flaccid paralysis, and coma.
Echovirus infections have been associated with neonatal hepatitis. Congenital infections with echovirus 11, 21, and 30 can cause fulminant neonatal hepatitis, which can be lethal.55,56 Echovirus 6 has been associated with fever, respiratory distress, sepsis-like syndrome, acute respiratory and renal failure, and disseminated intravascular coagulopathy. Autopsy studies have shown jaundice, anasarca, massive hepatic necrosis, adrenal hemorrhagic necrosis, renal medullary hemorrhage, hemorrhagic non-inflammatory pneumonia, and severe encephalomalacia.56
The histopathology of neonatal EV infections typically shows diffuse or scattered lesions with perivascular infiltration, consisting of mononuclear cells and polymorphonuclear leukocytes in the cerebrum, cerebellum, pons, medulla, and spinal cord.
Undifferentiated Fever and Aseptic Meningitis
Viral meningitis is most common in infants less than 1 year of age.15,16 More than 90% of viral meningitis in infants is due to species B EVs (group B CVs and most echoviruses).
Neonates infected with CV-B are at risk for a severe systemic illness especially meningitis or meningoencephalitis.53 Infection due to CV-B is a cause of 53–63% of the cases of fever without focus in infants less than 3 months of age.57,58 The CV-B serotypes 2, 4, and 5 are most commonly identified in these infants. Infants may present with irritability, lethargy, poor feeding, vomiting, diarrhea, exanthems,and respiratory distress.59 A sepsis screen can help rule out bacterial infection. The cerebrospinal fluid (CSF) study reveals aseptic meningitis in almost half of the infants with enterovirus infection.60
The CSF typically shows monocytic pleocytosis (100–1000 cells/mm3) with normal or decreased glucose and slightly increased protein levels. Most infants recover within 2–10 days without complications. Also, 10% of infants may progress to develop seizures, obtundation, or raised intracranial pressure. The short-term prognosis of enterovirus meningitis is good. It is not associated with long-term neurodevelopmental deficits in most patients.2
Overall, about 5% of all cases of acute encephalitis are caused by EVs.61 The CV serotypes A9, B2, and B5, and echovirus serotypes 6 and 9 are frequently associated with encephalitis.
Respiratory Tract Diseases
Many CV-B infections are accompanied by respiratory distress and non-specific radiological signs.62 Echovirus 6 and CV-A serotypes 4, 6, 9, and 10 are other causative agents.56
Ocular infections
Acute hemorrhagic conjunctivitis is a highly contagious but self-limited ocular infection. A CV-A24 variant is responsible for outbreaks.63 Transmission is by eye discharge, fingers,and fomites. Symptoms peak in 2–3 days and it resolves within 10 days without complication.
Hepatitis
Echoviruses, particularly serotypes 5–7, 9, 11, 14, 19–21, and 30 may cause severe hepatitis with foci of hepatic necrosis.64 Upper respiratory infection, general signs of sepsis-like illness, meningitis, gastroenteritis, aseptic meningitis, gastroenteritis, meningoencephalitis, and fatal interstitial pneumonia may be seen. No association was found between maternal echovirus serotype 9 infection and congenital malformations.65,66 Echoviral infections can cause considerable mortality in epidemics.
Cloud Baby
Echovirus 20 has been associated with Staphylococcal colonization and dissemination in nurseries. Eichenwald and associates identified this phenomenon and named these infants as “cloud babies.” Active staphylococcal dissemination occurred only during the time that echovirus 20 was recovered from the nasopharynx; hence, viral–bacterial synergism was postulated as a mechanism.
Diagnosis
Most EV cause self-limited illnesses and are diagnosed based on clinical manifestations. A laboratory diagnosis is required when the identification of the causative organism has management implications as in central nervous system infections, myopericarditis, and in neonates and immunocompromised patients. Laboratory diagnosis is also required in disease outbreaks.
Lab Diagnosis
Reverse Transcriptase–Polymerase Chain Reaction
Detection of virus in the blood, CSF, pericardial fluid, lacrimal fluid, urine, respiratory secretions, or tissue by reverse transcriptase polymerase chain reaction (RT-PCR) is diagnostic of infection.67-70 A positive RT-PCR test from stool may represent the carrier state. In CSF, RT-PCR is more rapid and sensitive than cell culture.
Viral Isolation (Cell Culture)
For serotype identification, specimens should be sent to a reference laboratory where an isolate can be amplified in cell culture and identified at the serotype level with special PCR primers or genomic sequencing.67-69
Cell culture is expensive and culture in multiple cell lines is required for optimal sensitivity. Recovery of an isolate in cell culture helps in its typing for clinical and epidemiologic purposes. The characteristic enterovirus cytopathic effect (CPE) requires 2–6 days to develop in primary cell culture.70,71 Indirect immunofluorescence may be used to confirm the virus causing the CPE.71
Serology
Serology is not generally used for the diagnosis of acute enteroviral illnesses except when infection with a specific serotype is suspected. The diagnosis of acute infection can be made retrospectively with a 4-fold or greater increase in antibody titers between acute and convalescent specimens separated by a minimum of 4 weeks. Serum IgM antibodies to the CV-B can often be detected early in the course of illness.72,73 Type-specific immunoassays that measure the antibody response against the more common enterovirus serotypes are of limited utility due to cross-reactivity and standardization issues.
Management
The management of CVB disease in the newborn is predominantly supportive care (Flowchart 2). The severity of disease and poor prognosis have generated interest in immunoglobulins (Ig) for the treatment of neonatal enterovirus infections.
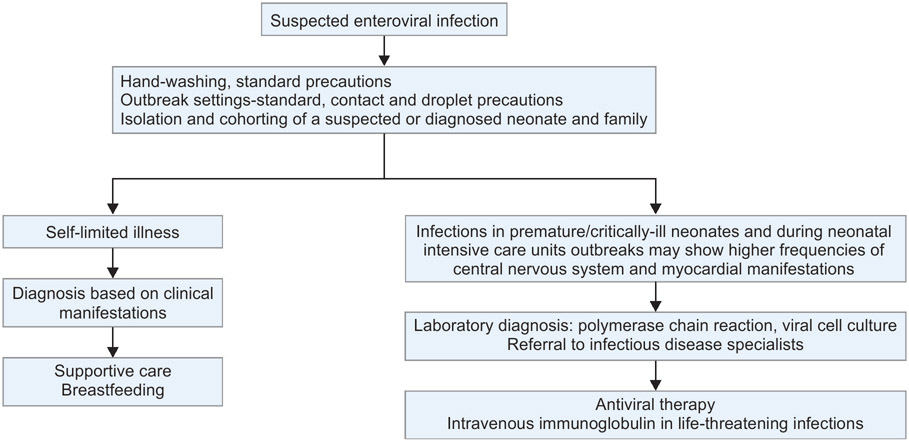
Flowchart 2:
Algorithm for management of a neonate with suspected enteroviral infection
Antiviral Therapy for Severe Cases
Most enteroviral infections are self-limited and do not require specific therapy. Exceptions are fulminant neonatal infection, severe myocarditis, chronic infection, and disseminated infections in B cell-immunodeficient patients and hematologic malignancies.
Antiviral Drugs
Antiviral drugs against EVs have limited availability. There are a few available options:
Capsid inhibitors are drugs that inhibit viral attachment and uncoating. They have been shown to have activity against EVs. Pocapavir is an orally administered drug under development to treat chronic enterovirus infections, although resistance was quick to develop.74 It is available only for poliovirus infections in B cell-deficient patients.
Pleconaril, an orally administered capsid inhibitor, has been tested clinically against enterovirus and rhinovirus infections but is not currently available for systemic administration.75
Intravenous (Ig)
There is no clear evidence of benefit. It may be used in life-threatening enteroviral infections but is not recommended for routine use.76 A retrospective study showed that intravenous immune globulin (IVIG) increases survival.76 However, a randomized controlled trial at a dose of 750 mg/kg found no clinical benefit.77 There is also no convincing evidence of benefit in acute myocarditis. There is anecdotal, not convincing, evidence for the use of IVIG and maternal plasma transfusions in echovirus infections.78,79
Prevention
General Measures
Simple hygienic measures, such as hand washing, are important to prevent the spread of infection.80 Alcohol-based hand sanitizers may not be optimally effective for EVs.81 In hospitalized patients, standard precautions are indicated to control outbreaks.82 In outbreak settings, standard contact and droplet precautions for suspect cases in healthcare settings have been recommended.
Vaccines
Three inactivated enterovirus A71 vaccines have been tested in China for use in pediatric patients.83-85 In a multi-center RCT in children aged from 2 to 71 months who received the B4 genotype-based enterovirus A71 vaccine, the vaccine efficacy was found to be 96.8%.86
Pregnant Women
Neonatal CV-B infections are acquired in the peripartum period either from the mother or nosocomial sources. The risk of maternal infection late in gestation can be avoided by hand washing, especially after changing diapers and after close contact with objects contaminated by feces, urine, or respiratory secretions. Strict enforcement of recommended infection control practices for health care workers is warranted to reduce transmission in newborn nurseries. If feasible, the delivery may be delayed till 5-10 days after symptoms onset to allow the transplacental transfer of maternal IgG antibodies to improve outcomes.
Future Directions
We need specific antivirals and vaccines targeting coxsackie virus infections in neonates. There is also a need for well-controlled trials to evaluate IVIG as a preventive measure against nosocomial transmission of EVs.
Source of support:
This project was supported in part by the NIH awards DK054016 and DK092441 (PKD).
Footnotes
Conflict of interest: None
References
- 1.Baggen J, Thibaut HJ, Strating J, et al. The life cycle of non-polio enteroviruses and how to target it. Nat Rev Microbiol 2018;16(6):368–381. DOI: 10.1038/s41579-018-0005-4. [DOI] [PubMed] [Google Scholar]
- 2.Hawkes MT, Vaudry W. Nonpolio enterovirus infection in the neonate and young infant. Paediatr Child Health 2005;10(7):383–388. [PMC free article] [PubMed] [Google Scholar]
- 3.Horstmann DM. Enterovirus infections: Etiologic, epidemiologic and clinical aspects. Calif Med 1965;103:1–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Oberste MS, Maher K, Williams AJ, et al. Species-specific RT-PCR amplification of human enteroviruses: A tool for rapid species identification of uncharacterized enteroviruses. J Gen Virol 2006;87(Pt. 1):119–128. DOI: 10.1099/vir.0.81179-0. [DOI] [PubMed] [Google Scholar]
- 5.Oberste MS, Maher K, Kilpatrick DR, et al. Molecular evolution of the human enteroviruses: Correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol 1999;73(3):1941–1948. DOI: 10.1128/JVI.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Collaborating Centre for Infectious Diseases C. EV-D68. University of Mannitoba. Available at: https://nccid.ca/debrief/ev-d68/ Accessed on: 23 August 2022. [Google Scholar]
- 7.Tapparel C, Siegrist F, Petty TJ, et al. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol 2013;14:282–293. DOI: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Pons–Salort M, Parker EP, Grassly NC. The epidemiology of non-polio enteroviruses: Recent advances and outstanding questions. Curr Opin Infect Dis 2015;28(5):479–487. DOI: 10.1097/QCO.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang P, Liu Y, Ma HC, et al. Picornavirus morphogenesis. Microbiol Mol Biol Rev 2014;78(3):418–437. DOI: 10.1128/MMBR.00012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne S. Introduction to RNA viruses. Viruses 2017:97–105. DOI: 10.1016/B978-0-12-803109-4.00010-6. [DOI] [Google Scholar]
- 11.Laitinen OH, Svedin E, Kapell S, et al. Enteroviral proteases: Structure, host interactions and pathogenicity. Rev Med Virol Jul 2016;26(4):251–267. DOI: 10.1002/rmv.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groppelli E, Levy HC, Sun E, et al. Picornavirus RNA is protected from cleavage by ribonuclease during virion uncoating and transfer across cellular and model membranes. PLoS Pathog 2017;13(2):e1006197. DOI: 10.1371/journal.ppat.1006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells AI, Coyne CB. Enteroviruses: A gut–wrenching game of entry, detection, and evasion. Viruses 2019;11(5):460. DOI: 10.3390/v11050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone VM, Hankaniemi MM, Laitinen OH, et al. A hexavalent coxsackievirus B vaccine is highly immunogenic and has a strong protective capacity in mice and nonhuman primates. Sci Adv 2020;6(19):eaaz2433. DOI: 10.1126/sciadv.aaz2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugo D, Krogstad P. Enteroviruses in the early 21st century: New manifestations and challenges. Curr Opin Pediatr 2016;28(1):107–113. DOI: 10.1097/MOP.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olchawa–Czech A, Ptak K, Szymonska I, et al. Severe enterovirus infections in infants <3 months of age and the importance of medical history. J Mother Child 2021;24(3):37–44. DOI: 10.34763/jmotherandchild.20202403.2022.d-20-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bopegamage S. Enterovirus infections: Pivoting role of the adaptive immune response. Virulence 2016;7(5):495–497. DOI: 10.1080/21505594.2016.1175701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez–Lazaro D, Cook N, Ruggeri FM, et al. Virus hazards from food, water and other contaminated environments. FEMS Microbiol Rev 2012;36(4):786–814. DOI: 10.1111/j.1574-6976.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari S, Dhole TN. Assessment of enteroviruses from sewage water and clinical samples during eradication phase of polio in North India. Virol J 2018;15(1):157. DOI: 10.1186/s12985-018-1075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keswick BH, Gerba CP, Goyal SM. Occurrence of enteroviruses in community swimming pools. Am J Public Health. 1981;71(9):1026–1030. DOI: 10.2105/ajph.71.9.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imamura T, Oshitani H. Global reemergence of enterovirus D68 as an important pathogen for acute respiratory infections. Rev Med Virol 2015;25(2):102–114. DOI: 10.1002/rmv.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noor A, Krilov LR. Enterovirus Infections. Pediatr Rev 2016;37(12):505–515. DOI: 10.1542/pir.2016-0103. [DOI] [PubMed] [Google Scholar]
- 23.Chang LY, Tsao KC, Hsia SH, et al. Transmission and clinical features of enterovirus 71 infections in household contacts in Taiwan. JAMA 2004;291(2):222–227. DOI: 10.1001/jama.291.2.222. [DOI] [PubMed] [Google Scholar]
- 24.Yen M, Tsao H, Huang Y, et al. Viral load in blood is correlated with disease severity of neonatal coxsackievirus B3 infection: Early diagnosis and predicting isease severity is possible in severe neonatal enterovirus infection. Clin Infect Dis 2007;44(10):e78–e81. DOI: 10.1086/515399. [DOI] [PubMed] [Google Scholar]
- 25.Moore M, Kaplan MH, McPhee J, et al. Epidemiologic, clinical, and laboratory features of coxsackie B1–B5 infections in the United States, 1970–1979. Public Health Rep 1984;99(5):515–522. [PMC free article] [PubMed] [Google Scholar]
- 26.Modlin JF, Polk BF, Horton P, et al. Perinatal echovirus infection: Risk of transmission during a community outbreak. N Engl J Med 1981;305(7):368–371. DOI: 10.1056/NEJM198108133050703. [DOI] [PubMed] [Google Scholar]
- 27.Hendry E, Hatanaka H, Fry E, et al. The crystal structure of coxsackievirus A9: New insights into the uncoating mechanisms of enteroviruses. Structure 1999;7(12):1527–1538. DOI: 10.1016/S0969-2126(00)88343-4. [DOI] [PubMed] [Google Scholar]
- 28.Selinka HC, Wolde A, Sauter M, et al. Virus–receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol 2004;193(2–3):127–131. DOI: 10.1007/S00430-003-0193-y. [DOI] [PubMed] [Google Scholar]
- 29.Nelsen–Salz B, Eggers HJ, Zimmermann H. Integrin α(v)β3 (vitronectin receptor) is a candidate receptor for the virulent echovirus 9 strain Barty. J Gen Virol 1999;80(Pt. 9):2311–2313. DOI: 10.1099/0022-1317-80-9-2311. [DOI] [PubMed] [Google Scholar]
- 30.Jokinen J, White DJ, Salmela M, et al. Molecular mechanism of α2β1 integrin interaction with human echovirus 1. EMBO J 2010;29(1):196–208. DOI: 10.1038/emboj.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morosky S, Wells AI, Lemon K, et al. The neonatal Fc receptor is a pan-echovirus receptor. Proc Natl Acad Sci U S A 2019;116(9):3758–3763. DOI: 10.1073/pnas.1817341116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modlin JF. Treatment of neonatal enterovirus infections. J Pediatric Infect Dis Soc 2016;5(1):63–64. DOI: 10.1093/jpids/piv030. [DOI] [PubMed] [Google Scholar]
- 33.Gong X, Zhou J, Zhu W, et al. Excessive proinflammatory cytokine and chemokine responses of human monocyte-derived macrophages to enterovirus 71 infection. BMC Infect Dis 2012;12:224. DOI: 10.1186/1471-2334-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei X, Yang J, Gao L, et al. The transfer and decay of maternal antibodies against enterovirus A71, and dynamics of antibodies due to later natural infections in Chinese infants: A longitudinal, paired mother–neonate cohort study. Lancet Infect Dis S2021;21(3):418–426. DOI: 10.1016/S1473-3099(20)30480-1. [DOI] [PubMed] [Google Scholar]
- 35.Chen B, Yang Y, Xu X, et al. Epidemiological characteristics of hand, foot, and mouth disease in China: A meta-analysis. Medicine (Baltimore) 2021;100(20):e25930. DOI: 10.1097/MD.0000000000025930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander JP Jr, Baden L, Pallansch MA, et al. Enterovirus 71 infections and neurologic disease–United States, 1977–1991. J Infect Dis. 1994;169(4):905–908. DOI: 10.1093/infdis/169.4.905. [DOI] [PubMed] [Google Scholar]
- 37.Lum LC, Wong KT, Lam SK, et al. Fatal enterovirus 71 encephalomyelitis. J Pediatr 1998;133(6):795–798. DOI: 10.1016/s0022-3476(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 38.Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: A vesiculobullous eruption caused by coxsackie virus A6. Lancet Infect Dis 2014;14(1):83–86. DOI: 10.1016/S1473-3099(13)70264-0. [DOI] [PubMed] [Google Scholar]
- 39.Stewart CL, Chu EY, Introcaso CE, et al. Coxsackievirus A6-induced hand–foot–mouth disease. JAMA Dermatol 2013;149(12):1419–1421. DOI: 10.1001/jamadermatol.2013.6777. [DOI] [PubMed] [Google Scholar]
- 40.Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28(1):1–5. DOI: 10.1111/j.1525-1470.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Liu H, Zhao Y, et al. Identification of a new recombinant strain of echovirus 33 from children with hand, foot, and mouth disease complicated by meningitis in Yunnan, China. Virol J 2019;16(1):63. DOI: 10.1186/s12985-019-1164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bian L, Gao F, Mao Q, et al. Hand, foot, and mouth disease associated with coxsackievirus A10: more serious than it seems. Expert Rev Anti Infect Ther 2019;17(4):233–242. DOI: 10.1080/14787210.2019.1585242. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Gao HH, Zhang Q, et al. Large outbreak of herpangina in children caused by enterovirus in summer of 2015 in Hangzhou, China. Sci Rep 2016;6:35388. DOI: 10.1038/srep35388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabin AB, Krumbiegel ER, Wigand R. ECHO type 9 virus disease. AMA J Dis Child 1958;96(2):197–219. DOI: 10.1001/archpedi.1958.02060060199011. [DOI] [PubMed] [Google Scholar]
- 45.Bell EJ, Ross CA, Grist NR. ECHO 9 infection in pregnant women with suspected rubella. J Clin Pathol 1975;28(4):267–269. DOI: 10.1136/jcp.28.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neva FA. A second outbreak of Boston exanthem disease in Pittsburgh during 1954. N Engl J Med 1956;254(18):838–843. DOI: 10.1056/NEJM195605032541806. [DOI] [PubMed] [Google Scholar]
- 47.Frothingham TE. ECHO virus type 9 associated with three cases simulating meningococcemia. N Engl J Med 1958;259(10):484–485. DOI: 10.1056/NEJM195809042591007. [DOI] [PubMed] [Google Scholar]
- 48.Cherry JD, Jahn CL. Herpangina: The etiologic spectrum. Pediatrics 1965;36(4):632–634. [PubMed] [Google Scholar]
- 49.Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol 2013;6(3):34–39. [PMC free article] [PubMed] [Google Scholar]
- 50.Nagai T, Hanaoka N, Katano H, et al. A fatal case of acute encephalopathy in a child due to coxsackievirus A2 infection: A case report. BMC Infect Dis 2021;21(1):1167. DOI: 10.1186/s12879-021-06858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalwai A, Ahmad S, Pacsa A, et al. Echovirus type 9 is an important cause of viral encephalitis among infants and young children in Kuwait. J Clin Virol 2009;44(1):48–51. DOI: 10.1016/j.jcv.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Dancea AB. Myocarditis in infants and children: A review for the paediatrician. Paediatr Child Health 2001;6(8):543–545. DOI: 10.1093/pch/6.8.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaplan MH, Klein SW, McPhee J, et al. Group B coxsackievirus infections in infants younger than three months of age: A serious childhood illness. Rev Infect Dis 1983;5(6):1019–1032. DOI: 10.1093/clinids/5.6.1019. [DOI] [PubMed] [Google Scholar]
- 54.Eichenwald HF, Shinefield HR. Viral infections of the fetus and of the premature and newborn infant. Adv Pediatr 1962;12:249–305. [PubMed] [Google Scholar]
- 55.Pedrosa C, Lage MJ, Virella D. Congenital echovirus 21 infection causing fulminant hepatitis in a neonate. BMJ Case Rep 2013;2013 DOI: 10.1136/bcr-2012-008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ventura KC, Hawkins H, Smith MB, Walker DH. Fatal neonatal echovirus 6 infection: Autopsy case report and review of the literature. Mod Pathol 2001;14(2):85–90. DOI: 10.1038/modpathol.3880260. [DOI] [PubMed] [Google Scholar]
- 57.Leggiadro RJ, Darras BT. Viral and bacterial pathogens of suspected sepsis in young infants. Pediatr Infect Dis 1983;2(4):287–289. DOI: 10.1097/00006454-198307000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Krober MS, Bass JW, Powell JM, et al. Bacterial and viral pathogens cause fever in infants less than 3 months old. Am J Dis Child 1985;139(9):889–892. DOI: 10.1001/archpedi.1985.02140110043025. [DOI] [PubMed] [Google Scholar]
- 59.Rorabaugh ML, Berlin LE, Heldrich F, et al. Aseptic meningitis in infants younger than 2 years of age: Acute illness and neurologic complications. Pediatrics 1993;92(2):206–211. [PubMed] [Google Scholar]
- 60.Modlin JF, Rotbart HA. Group B coxsackie disease in children. Curr Top Microbiol Immunol 1997;223:53–80. DOI: 10.1007/978-3-642-60687-8_4. [DOI] [PubMed] [Google Scholar]
- 61.Fowlkes AL, Honarmand S, Glaser C, et al. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. J Infect Dis 2008;198(11):1685–1691. DOI: 10.1086/592988. [DOI] [PubMed] [Google Scholar]
- 62.Chonmaitree T, Menegus MA, Powell KR. The clinical relevance of ‘CSF viral culture’. A two-year experience with aseptic meningitis in Rochester, NY. JAMA 1982;247(13):1843–1847. [PubMed] [Google Scholar]
- 63.Zhang L, Zhao N, Huang X, et al. Molecular epidemiology of acute hemorrhagic conjunctivitis caused by coxsackie A type 24 variant in China, 2004–2014. Sci Rep 2017;7:45202. DOI: 10.1038/srep45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hughes JR, Wilfert CM, Moore M, et al. Echovirus 14 infection associated with fatal neonatal hepatic necrosis. Am J Dis Child 1972;123(1):61–67. DOI: 10.1001/archpedi.1972.02110070111017. [DOI] [PubMed] [Google Scholar]
- 65.Kleinman H, Prince JT, Mathey WE, et al. ECHO 9 virus infection and congenital abnormalities: A negative report. Pediatrics 1962;29:261–269. [PubMed] [Google Scholar]
- 66.Landsman JB, Grist NR, Ross CA. ECHO 9 virus infection and congenital malformations. Br J Prev Soc Med 1964;18:152–156. DOI: 10.1136/jech.18.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mirand A, Henquell C, Archimbaud C, et al. Prospective identification of enteroviruses involved in meningitis in 2006 through direct genotyping in cerebrospinal fluid. J Clin Microbiol 2008;46(1):87–96. DOI: 10.1128/JCM.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 2006;44(8):2698–2704. DOI : 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oberste MS, Maher K, Kilpatrick DR, et al. Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol 1999;37(5):1288–1293. DOI: 10.1128/JCM.37.5.1288-1293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dagan R, Menegus MA. A combination of four cell types for rapid detection of enteroviruses in clinical specimens. J Med Virol 1986;19(3):219–228. DOI: 10.1002/jmv.1890190304. [DOI] [PubMed] [Google Scholar]
- 71.Trabelsi A, Grattard F, Nejmeddine M, et al. Evaluation of an enterovirus group-specific anti-VP1 monoclonal antibody, 5-D8/1, in comparison with neutralization and PCR for rapid identification of enteroviruses in cell culture. J Clin Microbiol 1995;33(9):2454–2457. DOI: 10.1128/jcm.33.9.2454-2457.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bell EJ, McCartney RA, Basquill D, et al. Mu-antibody capture ELISA for the rapid diagnosis of enterovirus infections in patients with aseptic meningitis. J Med Virol 1986;19(3):213–217. DOI: 10.1002/jmv.1890190303. [DOI] [PubMed] [Google Scholar]
- 73.Pozzetto B, Gaudin OG, Aouni M, et al. Comparative evaluation of immunoglobulin M neutralizing antibody response in acute-phase sera and virus isolation for the routine diagnosis of enterovirus infection. J Clin Microbiol 1989;27(4):705–708. DOI: 10.1128/jcm.27.4.705-708.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torres–Torres S, Myers AL, Klatte JM, et al. First use of investigational antiviral drug pocapavir (v-073) for treating neonatal enteroviral sepsis. Pediatr Infect Dis J 2015;34(1):52–54. DOI: 10.1097/INF.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 75.Pevear DC, Tull TM, Seipel ME, et al. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother 1999;43(9):2109–2115. DOI: 10.1128/AAC.43.9.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yen MH, Huang YC, Chen MC, et al. Effect of intravenous immunoglobulin for neonates with severe enteroviral infections with emphasis on the timing of administration. J Clin Virol 2015;64:92–96. DOI: 10.1016/j.jcv.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 77.Abzug MJ, Keyserling HL, Lee ML, et al. Neonatal enterovirus infection: Virology, serology, and effects of intravenous immune globulin. Clin Infect Dis 1995;20(5):1201–1206. DOI: 10.1093/clinids/20.5.1201. [DOI] [PubMed] [Google Scholar]
- 78.Johnston JM, Overall JC Jr. Intravenous immunoglobulin in disseminated neonatal echovirus 11 infection. Pediatr Infect Dis J 1989;8(4):254–256. [PubMed] [Google Scholar]
- 79.Jantausch BA, Luban NL, Duffy L, et al. Maternal plasma transfusion in the treatment of disseminated neonatal echovirus 11 infection. Pediatr Infect Dis J 1995;14(2):154–155. [PubMed] [Google Scholar]
- 80.Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics 2011;127(4):e898–e904. DOI: 10.1542/peds.2010-1497. [DOI] [PubMed] [Google Scholar]
- 81.Chang SC, Li WC, Huang KY, et al. Efficacy of alcohols and alcohol-based hand disinfectants against human enterovirus 71. J Hosp Infect 2013;83(4):288–293. DOI: 10.1016/j.jhin.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Siegel JD, Rhinehart E, Jackson M, et al. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. Am J Infect Control 2007;35(10 Suppl. 2):S65–164. DOI: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Zhou Y, Cheng Y, et al. Effectiveness of EV-A71 vaccination in prevention of paediatric hand, foot, and mouth disease associated with EV-A71 virus infection requiring hospitalisation in Henan, China, 2017–18: A test-negative case–control study. Lancet Child Adolesc Health 2019;3(10):697–704. DOI: 10.1016/S2352-4642(19)30185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei M, Meng F, Wang S, et al. 2-Year efficacy, immunogenicity, and safety of Vigoo enterovirus 71 vaccine in healthy Chinese children: A randomized open-label study. J Infect Dis 2017;215(1):56–63. DOI: 10.1093/infdis/jiw502. [DOI] [PubMed] [Google Scholar]
- 85.Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014;370(9):829–837. DOI: 10.1056/NEJMoa1303224. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen TT, Chiu CH, Lin CY, et al. Efficacy, safety,and immunogenicity of an inactivated, adjuvanted enterovirus 71 vaccine in infants and children: a multiregion, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2022;399(10336):1708–1717. DOI: 10.1016/S0140-6736(22)00313-0. [DOI] [PubMed] [Google Scholar]
- 87.Lu JY, Brewer G, Li ML, et al. Secretory carrier membrane protein 3 interacts with 3A viral protein of enterovirus and participates in viral replication. Microbiol Spectr 2021;9(1):e0047521. DOI: 10.1128/Spectrum.00475-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shakeel S, Seitsonen JJ, Kajander T, et al. Structural and functional analysis of coxsackievirus A9 integrin alphavbeta6 binding and uncoating. J Virol 2013;87(7):3943–3951. DOI: 10.1128/JVI.02989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laufman O, Perrino J, Andino R. Viral generated inter-organelle contacts redirect lipid flux for genome replication. Cell 2019;178(2):275–289.e16. DOI: 10.1016/j.cell.2019.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Louten J. Virus replication. Essential Human Virol 2016:49–70. DOI: 10.1016/B978-0-12-800947-5.00004-1. [DOI] [Google Scholar]
- 91.Maciejewski S, Nguyen JH, Gomez–Herreros F, et al. Divergent requirement for a DNA repair enzyme during enterovirus infections. mBio 2015;7(1):e01931–15. DOI: 10.1128/mBio.01931-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Merkle I, van Ooij MJ, van Kuppeveld FJ, et al. Biological significance of a human enterovirus B-specific RNA element in the 3’ nontranslated region. J Virol 2002;76(19):9900–9909. DOI: 10.1128/jvi.76.19.9900-9909.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan J, Shen L, Wu J, et al. Enterovirus A71 proteins: Structure and function. Front Microbiol 2018;9:286. DOI: 10.3389/fmicb.2018.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee KM, Wu CC, Wu SE, et al. The RNA-dependent RNA polymerase of enterovirus A71 associates with ribosomal proteins and positively regulates protein translation. RNA Biol 2020;17(4):608–622. DOI: 10.1080/15476286.2020.1722448. [DOI] [PMC free article] [PubMed] [Google Scholar]