At the beginning of the last century, George Whipple reported a case of disease in a medical missionary who died after suffering from chronic arthralgias, diarrhea, weight loss, abdominal discomfort, cough, fever, hypotension, increased skin pigmentation, and severe anemia 62. The pathologic findings showed fat deposition in intestinal and mesenteric lymph nodes 62. Nowadays, Whipple's disease is being recognized with increasing frequency as the result of greater awareness of the entity, the improvement of diagnostic tools, and a possible true increase in incidence. Patients suffering from the disease often present with malabsorption and other gastrointestinal symptoms. However, articular, cardiac, and central nervous system involvement is not uncommon and may be more prominent clinically. These various manifestations reflect the systemic nature of a chronic infection associated with rod-shaped organisms. The traditional laboratory diagnosis is based on light microscopy, which shows diastase-resistant, periodic acid-Schiff (PAS)-positive, non-acid-fast granules in macrophages of intestinal biopsy specimens. The greatest concentration of these typical foamy macrophages, considered the hallmark of the disease, is in the mucosa of the small intestine and regional intestinal lymph nodes, but they have been found in a wide distribution of systemic sites, the most common being neurologic, pulmonary, or cardiovascular. In 1991, a portion of the 16S rRNA gene of the bacterium was sequenced by Wilson et al. 63, allowing the classification of the Whipple's disease bacterium within the Actinomycetes clade. One year later, these findings were confirmed and extended by Relman et al. 50. Since then, PCR has become a useful tool for the diagnosis of Whipple's disease 47. Culture of the bacterium has been an elusive goal for many generations of microbiologists 27, 55. In 2000, we reported the successful isolation and establishment of a strain of Whipple's disease bacterium obtained from the mitral valve of a patient with blood culture-negative endocarditis, the generation of antibodies against the bacterium in mice, the detection of the bacterium in the patient's mitral valve by immunochemistry with these antibodies, and the detection of specific antibodies against the bacterium in the patient's serum 48. At the beginning of this century, with the possible culture of the Whipple's disease bacterium and the new tools such as PCR, we believe that a new area has begun for the epidemiology and the diagnosis of Whipple's disease, accompanied by a more complete understanding of the infection, improved therapy, and better clinical outcomes. The past, the present, and the future of Whipple's disease are reviewed in this article.
HISTORICAL BACKGROUND
On May 9, 1907, George Hoyt Whipple, then an instructor in pathology at Johns Hopkins University, performed an autopsy on a 36-year-old physician who had been domiciled at Constantinople (Istanbul), Turkey 62. He presented with gradual loss of weight and strength, stools consisting chiefly of neutral fat and fatty acids, undefined abdominal signs, and arthritis in multiple joints 62. The findings at autopsy consisted of polyserositis, aortic valve lesions, and prominent deposition of fat within intestinal mucosa and mesenteric lymph nodes, with marked infiltration by foamy macrophages, as well as the presence of rod-like bacilli, approximately 2 μm long, in the lamina propria of the intestine, but Whipple did not consider the rod-like bacilli to be the etiology of the disease. With special stains, he noted the presence of fatty acids, but not neutral fat, and thus incorrectly concluded that the condition arose from an abnormality of fat metabolism; hence, he coined the term “intestinal lipodystrophy” 62. Similar pathological findings were reported in the English literature by Allchin and Hebb, 18 years before Whipple's description, and under the name “lymphangiectasis intestini.” The similarity between these two reports went unnoticed until 1961, when Morgan 41 reviewed the original tissue blocks, restained the sections, and demonstrated PAS-positive macrophages. The first demonstration that the foamy macrophages were diastase resistant and PAS positive was by Hendrix et al. in 1950 25. This finding of PAS-positive macrophages filling the lamina propria of the small intestine was considered then pathognomonic of Whipple's disease. In 1947, the first report of Whipple's disease prior to death was reported 43. In 1952, Pauley 44 was the first to successfully use systemic antibiotics in the treatment of Whipple's disease. In 1958, the use of a peroral small-bowel biopsy specimen to perform the diagnosis of the disease was reported 5.
In 1961, the rod-shaped structures in the intestinal mucosa and within intestinal macrophages were demonstrated by electron microscopy to have the structural characteristics of bacteria 56. Numerous attempts have been made to identify the causative organism for Whipple's disease. Many investigators have isolated a variety of species in pure culture from intestinal biopsy specimens including Haemophilus 60, Corynebacterium 7, and Streptococcus 10. These conflicting results were probably due to the constant bacterial colonization of the intestine. In 1991, Wilson et al. 63 reported on the 16S RNA gene of a novel bacterium. Indeed, the partial sequence of this novel bacterium was identified in bacterial DNA extracted from duodenal and lymph nodes biopsy specimens from patients in whom Whipple's disease had been diagnosed according to histopathological criteria. In 1992, these data were confirmed and the causative bacterium constituted the novel, not-yet-validated taxon “Tropheryma whippelii” to acknowledge Whipple's contribution and to allude to the malabsorption caused by the disease 50. This result of PCR and sequence analysis has classified the bacterium as belonging to the gram-positive Actinomycetes clade. A successful attempt to culture the causative organism was first reported in 1997 55, when human macrophages inactivated by interleukin-4 were used to grow the bacterium from two patients with culture-negative endocarditis, but unfortunately, the work could not be pursued or reproduced 27. In 2000, we reported on the successful isolation and establishment of a strain of Whipple's disease bacterium obtained from the valve of a patient with blood culture-negative endocarditis 48.
THE DISEASE
Whipple's disease is considered a rare pathology, with less than 1,000 cases having been reported to date. In postmortem studies, the frequency of the disease is quoted as being less than 0.1% 17. Males are more frequently affected than females 60. Indeed, 80% of the cases occur in males. All age groups can contract the disease, although the 40- to 50-year-old age group predominates and children are only extremely rarely affected 14, 17. The disease occurs worldwide, but most of the patients are Caucasian 14. It has been speculated that some kind of immune defect may predispose individuals to the development of the disease 54. It appears also that a certain genetic predisposition could be involved. Indeed, HLA-B-27 is detectable in 28 to 44% of those suffering from the disease, while it is found in only approximately 8% of the healthy population 18. Furthermore, even if Whipple's disease is not familial, two set of brothers, a brother-sister pair, and a father-daughter pair with the disorder have been reported 13, 16, 22, 46. This again suggests that Whipple's disease might be associated with immunogenetic factors. In addition, most patients have immune defects characterized by deficiency in the production of interleukin-12 by monocytes macrophages associated with a reduced capability to produce gamma interferon by T cells and subsequently to a decreased activation and function of macrophages 39.
For a long time, Whipple's disease has been considered a gastrointestinal disease. In reality, the clinical manifestations of Whipple's disease are myriad and nonspecific. The various manifestations of Whipple's disease are summarized in Table 1. Typical Whipple's disease is characterized by a prodromal period of migratory polyarthritis, fatigue, weight loss, and anemia, followed by a progressive syndrome of abdominal pain, distention, steatorrhea, and severe cachexia 9, 35). On physical examination, lymphadenopathy and hyperpigmentation are frequent findings 19, 60. The spontaneous evolution of this disease is frequently long. During many years, progress is marked by repeated remissions and relapses, with gradual worsening and eventual death 29.
TABLE 1.
Various manifestations of Whipple's disease
| Manifestation | |
|---|---|
| Classical manifestations | |
| Polyarthritis | |
| Weight loss | |
| Abdominal pain | |
| Steatorrhea | |
| Cachexia | |
| Lymphadenopathy | |
| Hyperpigmentation | |
| Fever of unknown origin | |
| Sarcoidosis-like syndrome | |
| Central nervous system manifestations | |
| Mental change, dementia | |
| Eye movement disorder | |
| Myoclonus | |
| Hypothalamic damage | |
| Epilepsy | |
| Focal cerebral and cerebellar syndromes | |
| Ophthalmologic manifestations | |
| Uveitis | |
| Retinitis | |
| Keratitis | |
| Optic neuritis | |
| Papilloedema | |
| Cardiovascular manifestations | |
| Endocarditis | |
| Pericarditis | |
| Myocarditis | |
| Coronary arteritis | |
| Congestive heart failure | |
| Sudden death | |
| Lung | |
| Cough | |
| Pleural effusion |
The central nervous system, lungs, heart, eyes, and skin may be involved, and the disease may first manifest in these organs. Some patients could present with fever of unknown origin, lymphadenopathy, and a sarcoidosis-like syndrome (W. O. Dobbins, Editorial, N. Engl. J. Med. 332:390–392, 1995). A recent study has shown that 15% of patients do not have gastrointestinal symptoms throughout their illness 60, and jejunal biopsy specimens may present as normal on histopathological examination 37. Central nervous system symptoms are frequent and varied. Perhaps in as many as 5% of all cases of Whipple's disease, the presentation is neurological and the disease remains confined to the nervous system for the most part of the evolution of the disease 2. Irrespective of disease elsewhere in the body, the neurological manifestations of Whipple's disease are known and sufficiently well described to enable the recognition of some patterns which point to the diagnosis. Dementia, disturbances of ocular movements, abnormal involuntary movements, particularly myoclonus, and deranged function of the hypothalamus are most often found. Epilepsy, focal cerebral signs, ataxia, and parkinsonian and meningitic features may also be present 2, 4. Headaches are a very common symptom. It is not usual to get involvement of the spinal cord or of muscle or peripheral nerve, although myelopathy has been described 24. It should be noted that the proportion of patients with asymptomatic central nervous system infection is probably quite high, as indicated by the report of von Herbay et al. 61 showing that 7 of 10 patients without neurological symptoms nevertheless had cerebrospinal fluid findings consistent with Whipple's disease. The ophthalmologic manifestations are usually associated with central nervous system manifestations, but are sometimes isolated 2. Uveitis, retinitis, keratitis, optic neuritis, and papilloedema may be found 2. Cardiovascular manifestations are also frequent in patient's with Whipple's disease. Since the first report in 1952, cardiac involvement has been reported in 20 to 55% of patients 58–60, whereas certain autopsy studies have shown an almost constant involvement of at least one of the three cardiac layers 52. Constrictive pericarditis, endocarditis, myocarditis, coronary arteritis, congestive heart failure, and sudden death have been documented 29–31. Furthermore, Whipple's disease endocarditis without gastrointestinal symptoms has been more and more frequently described, and duodenal biopsy specimens of some of these patients were negative by histopathology and PCR 8, 23. Another characteristic finding in Whipple's disease is chronic cough, which is probably a symptom of pleural involvement 17. New manifestations of Whipple's disease, such as spondylodiscitis 1, prosthetic joint infection 20, intractable immune thrombocytopenic purpura 40, granulomatous nephritis (I. Marie, F. Lecomte, and H. Levesque, Letter, Ann. Intern. Med. 132:94–95, 2000), hypertrophic osteoarthropathy 38, and hypopituitarism 6, continue to be reported in the literature.
THE AGENT
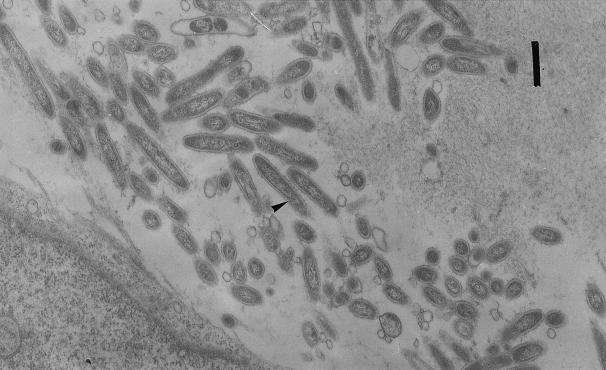
The etiology and pathogenesis of Whipple's disease have remained elusive for many years, even if a bacterial cause could have been suggested since the original description, as Whipple stated: “studied with 1/12 objective these sections show numbers of a rod-shaped organism (?)” 62. Since 1961, electron microscopy studies had documented the presence of a bacterium in involved tissues 56. The plasma membrane was surrounded by a thin homogeneous wall, itself surrounded by a plasma membrane-like structure, giving a trilamellar appearance (Fig. 1). The latter feature is more characteristic of gram-negative bacteria. Many laboratories have shown a bacterium of 0.25 by 1 to 2 μm in infected tissues, both intracellularly and extracellularly. The Whipple's disease bacterium is present within a variety of cells, including macrophages, intestinal epithelial cells, lymphatic and capillary endothelial cells, smooth-muscle cells, polymorphonuclear leukocytes, plasma cells, mast cells, and even intraepithelial lymphocytes 3. The intracellular bacteria are often intact in structure, which suggests that these organisms may be intracellular pathogens, but it has also been recognized as degraded to various degrees within macrophages 15. The difficulties experienced in isolating the microorganism stimulated the application of molecular techniques to the search and led to the identification of a single 16S RNA gene sequence from small-bowel biopsy specimens of several patients with Whipple's disease. Positive results have been obtained from various tissues including the heart, vitreous fluid, peripheral blood cells, pleural effusion cells, and cerebrospinal fluid by PCR gene amplification. By 16S RNA gene amplification, a study has suggested that the bacterium is an environmental agent present especially in water, and it seems to be a rather common environmental agent in certain geographic areas 34. This is in accordance with the phylogenetic relationship with the Actinomycetes clade of this bacterium. That could also explain the high proportion of farmers among patients (14; R. M. J. Donaldson, Editorial, N. Engl. J. Med. 327:346–348, 1992). An oral infectious route of the bacillus is then suspected. A possible carriage of the Whipple's disease bacterium in the gastrointestinal tract has been suggested by the fact that in a prospective blinded study by PCR of gastrointestinal biopsy specimens of 105 patients without any sign of Whipple's disease, the authors found positive results for 11.4% of the gastric fluid specimens and 4.8% of the duodenal biopsy specimens (H. U. Ehrbar, P. Bauerfeind, F. Dutly, H. R. Koelz, and M. Altwegg, Letter, Lancet, 353:2214, 1999). Furthermore, another recent PCR study has shown positive rates of 35% for saliva from a random sample of 40 healthy people (S. Street, H. D. Donoghue, and G. H. Neild, Letter, Lancet 354:1178–1179, 1999). We could then suspect that the Whipple's disease bacterium or closely related bacteria are present in a substantial fraction of the population in the absence of Whipple's disease, and it could be speculated that it is an oral commensal organism or that it is associated with currently unknown clinical manifestations. However, the data from these two studies should be confirmed, as they are PCR based and the PCR was possibly contaminated. Nevertheless, when we tested sera from blood donor controls with no identified Whipple's disease, the majority (29 of 40) exhibited antibodies of the immunoglobulin G(IgG) type to the Whipple's disease bacterium 48. This could be related to unspecific cross-reacting antibodies or to a previous contact with the bacterium.
FIG. 1.
Transmission electron micrograph showing the bacterium (arrowhead). Bar, 500 nm.
Whether the same bacterium causes all forms of Whipple's disease and its multisystem manifestations remains to be determined. However, sequencing of the nested PCR products obtained with primers derived from the 16S-23S rRNA gene and domain III of the 23S rRNA gene has revealed four different genotypes 26, 27. In the absence of DNA-DNA hybridization data, it is uncertain whether the types found represent subtypes of a single species or different but closely related species. Now, with the possibility of cultivation of the Whipple's disease bacterium, new isolates can be obtained, allowing a better understanding of the physiopathology of the disease.
DIAGNOSIS
Nonspecific diagnosis.
Nonspecific biological findings often include signs of chronic inflammation with an elevated erythrocyte sedimentation rate and elevated C-reactive protein levels 60. Anemia is also frequently observed 60. Leukocytosis, leukopenia, and eosinophilia can be noted in the sample used for determination the white blood cell count, and signs of malabsorption may occur 9, 19, 35, 60.
Specific diagnosis.
The specific tools for the diagnosis of Whipple's disease are summarized in Table 2.
TABLE 2.
Diagnostic tools for diagnosis of Whipple's disease
| Time frame and technique | Sample |
|---|---|
| Present | |
| PAS staining | Biopsy, aspirate fluida |
| Electron microscopy | Biopsy, aspirate fluid |
| Genomic detection | Biopsy, aspirate fluid, blood |
| Future | |
| Culture | Biopsy, aspirate fluid |
| Serology | Serum |
| Immunodetection with polyclonal or monoclonal antibodies | Biopsy |
Aspirate fluid consists of gastric fluid, cerebrospinal fluid, pleural effusion, synovial fluid, bone marrow, or vitreous fluid.
(i) Pathological examination.
If the disease is suspected on the basis of the clinical profile, then duodenal biopsy specimens provide the answer in the majority of cases. The confirmatory finding is the presence of characteristic histological features on microscopic examination. In typical Whipple's disease, the most severe changes are seen in the small intestine and mesenteric lymph nodes, in which biopsy often reveals large, foamy macrophages. These macrophages were considered the hallmark of Whipple's disease and contain intracytoplasmic granules that are positive on PAS staining; they were then filled with either mucopolysaccharide or glycoprotein 25. These PAS-positive cells could also be detected in practically all organs (colon, stomach, esophagus, gall bladder, liver, pancreas, spleen, heart, lungs, kidneys, suprarenal glands, central nervous system, serous membranes, blood vessels, and joints). However, it is noteworthy that PAS-positive cells, which in the past were considered pathognomonic of Whipple's disease, were seen not only in healthy persons (in whom cells were few and their staining was faint) but also in patients with infection due to Mycobacterium avium-M. intracellulare 36, 53; Dobbins, Letter). The distinction could be made by acid-fast staining, which is positive for patients infected with M. avium and negative for those with Whipple's disease. In one case, a pulmonary infiltrate in a patient with AIDS also contained macrophages with PAS-positive granules which correspond in reality to a gram-positive coccobacillus (Rhodococcus equi) (H. H. Wang, D. Tollerud, D. Danar, P. Hanff, K. Gottesdiener, and S. Rosen, Letter, N. Engl. J. Med. 314:1577–1578, 1986).
With the successful isolation and cultivation of the Whipple's disease agent, generation of polyclonal antibodies to the bacterium in the mouse and the rabbit could be realized. By using these antibodies with immunohistology, we could detect the bacterium in tissues, as in a patient with Whipple's disease endocarditis 48 and a biopsy specimen of the small intestine, but the specificities of these antibodies remain to be shown with a larger series of patients (unpublished data). We have now designed monoclonal antibodies which are species specific and which could probably be used in the near future (unpublished data).
(ii) Culture.
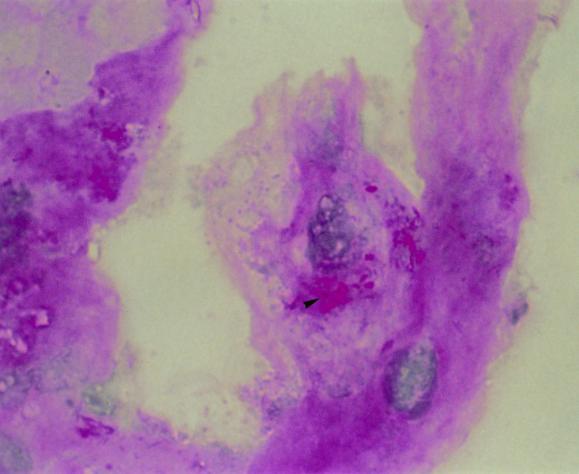
Attempts to cultivate the organism over the years have been considered unsuccessful, even if a culture of the causative organism was reported, because, unfortunately, the work could not be pursued, reproduced, or confirmed and no strain is available 27, 55. Our work has shown that a culture can be performed by using the human fibroblast cells lines HEL and MRC5, which are grown in minimal essential medium with 10% fetal calf serum and 2mM l-glutamine without antibiotics, and a control strain is available for the scientific community 48. By observing the flask monolayer with an inverted microscope, a cytopathic effect could be observed after several weeks; small coarse dark inclusions and large coarse round structures were detected within cells. Within this system, the generation time had been evaluated to be about 18 days. Cells appeared to be filled with coarse PAS-positive conglomerates and short slender PAS-positive rods (Fig. 2). Culture may then be performed with a biopsy sample or a fluid aspirate by the centrifugation shell vial technique with human fibroblast cell line HEL 48. It took 6 weeks before a cytopathic effect was observed by this technique 48. However, immunodetection or PCR could be performed, and the growth of the Whipple's disease bacterium in the cells could be detected earlier. All attempts of subculture on axenic medium with chocolate and Columbia sheep blood agars incubated at 32 and 37°C under 5% CO2 in a microaerophilic and anaerobic atmosphere and with cell culture medium and cell culture medium with lysates of HEL cells incubated at 32 and 37°C under 5% CO2 were unsuccessful 48. The limits of culture are the generation time of the bacteria, the necessity to have qualified personnel, and laboratory technical capacities. If the 18-day minimal doubling time is confirmed with other cell lines, it will explain why the bacterium could not be propagated either in human macrophages, which have a short life time in vitro, or in multiplying cells, which may dilute the bacterium.
FIG. 2.
PAS staining showing the bacterium in infected HEL cells (arrowhead). Magnification, ×1000.
(iii) PCR gene amplification.
On the basis of sequence analysis of the 16S rRNA gene, several diagnostic PCR assays targeting various parts of this gene were established 26. PCR has become an important diagnostic tool for establishment of the diagnosis of Whipple's disease, especially in patients with unusual presentations and if the diagnosis cannot be confirmed histologically (Dobbins, Letter). DNA amplification methods are considerably more sensitive, thus facilitating the laboratory diagnosis and monitoring of both typical and atypical cases of Whipple's disease. For all these situations, research on the Whipple's disease bacterium can now be performed on the basis of PCR with biopsy specimens tissue (i.e., duodenum, ileum, jejunum, lymph nodes, cardiac valve, cardiac muscle, or synovium) or fluid aspirate (i.e., gastric fluid, cerebrospinal fluid, pleural effusion, synovial fluid, bone marrow, or vitreous fluid) or by analysis of peripheral blood 32, 45, 47; Dobbins, Letter; S. A. Misbah, D. Stirzaker, B. Ozols, A. Franks, and N. Mapstone, Letter, Q. J. Med. 92:61, 1999; C. Muller, C. Stain, and O. Burghuber, Letter, Lancet 341:701, 1993). The sequences of the PCR primers available for the diagnosis of Whipple's disease are summarized in Table 3 12, 21, 26, 27, 28, 42, 47, 50, 51, 61. One of the important limits of PCR is its specificity, due to several problems. First, positive PCR results have been found in duodenal biopsy specimens, saliva, and gastric juice in people without clinical signs of Whipple's disease (Muller, C. Stain, and O. Burghuber, Letter, Lancet 341:701, 1993). Second, if the Whipple's disease bacterium is an environmental agent commonly found in water, PCR contamination may occur easily. Third, amplified bands of the presumably appropriate fragment length may be nonspecific. Therefore, a second step, direct sequencing or a hybridization step, is necessary after PCR to confirm the identities of the amplified products. Thus, interpretation of PCR results without histological confirmation should be regarded with prudence and in light of the clinical features (Muller et al., Letter). Although hybridization and sequencing of PCR products may provide further evidence for the presence of the DNA of the Whipple's disease bacterium, these techniques are rather tedious and time-consuming and do not reliably exclude the possibility of amplicon carryover contamination. However, an additional species-specific PCR with an independent target of the Whipple's disease bacterium might provide the necessary confirmation within a reasonable time frame for specimens with inconclusive histopathological findings. Hinrikson et al. 26 have recently proposed performance of such a study by nested PCR, targeting a part of 23S rRNA gene domain III of the Whipple's disease bacterium. This technique seems to be sensitive and specific. Furthermore, sequence data for 23S rRNA gene domain III amplicons were included in a proposed classification system for molecular variants 26. Very recently, a new PCR system targeting a heat shock protein (hsp65) has been described 42. In our laboratory, we have begun to sequence new genes such as rpoB (RNA polymerase beta subunit-encoding gene) which could be a useful tool for identification (unpublished data).
TABLE 3.
Sequences of PCR primers available for diagnosis of Whipple's disease
| Primer(s) | Sequence | Reference |
|---|---|---|
| W3FE | 5′-GGA ATT CCA GAG ATA CGC CCC CCG CAA-3′ | 50 |
| W2RB | 5′-CGG GAT CCC ATT CGC TCC ACC TTG CGA-3′ | 50 |
| W4RB | 5′-CGG GAT CCT GTG AGT CCC CGC CAT TAC GC-3′ | 51 |
| TW-1 and whip1 | 5′-AGA GAT ACG CCC CCC GCA A-3′ | 21, 61 |
| TW-3 | 5′-TCC TGT GAG TCC CCG CCA TTA GGC-3′ | 21 |
| W3AF | 5′-TAC CGG AAA GGC GTA GAG ATA CGC C-3′ | 47 |
| W4AR | 5′-CAG TCT CCT GTG AGT CCC CGC CAT T-3′ | 47 |
| Whip2 | 5′-ATT CGC TCC ACC TTG CGA-3′ | 61 |
| W185 | 5′-CGA CCC ATG AGG GCA TCC TC-3′ | 12 |
| RW830 | 5′-GCG GTG GAA CCA CCC CCA CG-3′ | 12 |
| tw1662f | 5′-ACT ATT GGG TTT TGA GAG GC-3′ | 28 |
| tw1662r | 5′-GCC TCT CAA AAC CCA ATA GT-3′ | 28 |
| tw1857r1 | 5′-TCC CGA GCC TTA TCC GAG A-3′ | 28 |
| tw1857r2 | 5′-TCC CGA GGC TTA TCG CAC A-3′ | 28 |
| tws1,f | 5′-ATC GCA AGG TGG AGC GAA TCT-3′ | 27, 28 |
| tws2,r | 5′-CGC ATT CTG GCG CCC CAC-3′ | 27, 28 |
| tws3,f | 5′-CCG GTG ACT TAA CCT TTT TGG AGA-3′ | 27, 28 |
| tws4,r | 5′-TCC CGA GGC TTA TCG CAG ATT G-3′ | 27, 28 |
| twsA1/f | 5′-AAG TGA TAC CGC CAT AGT GCA CTG T-3′ | 27 |
| twsA2/f | 5′-AAG TGA TAC CGC CAT AGT GCA CTG C-3′ | 27 |
| twsB1/r | 5′-CTC CCG TGA GCT TGT GCC CAA AAC-3′ | 27 |
| twsB2/r | 5′-CTC CCG TGA GCT TGT GCC CAA AC-3′ | 27 |
| twsC1/r | 5′-AAT AGT GCA CAC AAG TGC ATA AGC A-3′ | 27 |
| twC2/r | 5′-AAT AGT GCA CAC AAG CGC ATA AGC A-3′ | 27 |
| HGC-23InsF | 5′-CGT AGT CGA TGG ACA ACG-3′ | 26 |
| TW-23InsR1 | 5′-TAG AAC CTT GTG TCG ATG C-3′ | 26 |
| TW-23InsF | 5′-GGT TGA TAT TCC CGT ACC GGC AAA G-3′ | 26 |
| TW-23InsR2 | 5′-GCA TAG GAT CAC CAA TTT CGC GCC-3′ | 26 |
| whipp-f | 5′-GCC TGC GCC TCG ATC TCT GC-3′ | 42 |
| whipp-frw1 | 5′-TGA CGG GAC CAC AAC ATC TG-3′ | 42 |
| whipp-frw2 | 5′-CGC GAA AGA GGT TGA GAC TG-3′ | 42 |
| whipp-rev | 5′-ACA TCT TCA GCA ATG ATA AGA AGT T-3′ | 42 |
| 16Spro1 | 5′-TTG AGA ACT CAA BAG YGT G-3′ | 42 |
| tw318r | 5′-CGA AGT TAT CCC AAA GTT AG-3′ | 42 |
| tw1581f | 5′-GTG ACT TAA CCT TTT TGG AGA-3′ | 33 |
| tw2015r | 5′-GCA TCC ACC ATT TGC TCT TAA A-3′ | 33 |
| tw1974f | 5′-GTA TTT GTG ATT CAA GCT AC-3′ | 33 |
| ms37ar | 5′-CTG CTT CTA AGC CAA CAT CCT-3′ | 33 |
| tw3002f | 5′-TGC CGG TAA GTT AGA GCG CA-3′ | 33 |
| ms38a | 5′-GAC AAG GAA TTT CGC TAC CTT A-3′ | 33 |
| tw3887f | 5′-GCC TGA GGC GTG ACG AG-3′ | 33 |
| co5189r | 5′-GCT TCC GGG TTC GGA ATG-3′ | 33 |
| tw5104f | 5′-CTT GAT GTG CGG CCC TTT GC-3′ | 33 |
| tw5745r | 5′-AAG ATC CCA CTG CAC TGA CAT CG-3′ | 33 |
| tw5068f | 5′-ATT GCT TGA AAC ACA TTT TG-3′ | 33 |
| NheI-RSO | 5′-AAT ACG ACT CAC TAT AGC N10GC TAG C-3′ | 33 |
PCR may also be used for the monitoring of Whipple's disease. Indeed, a negative result by PCR may predict a low likelihood of clinical relapse. A result that remains positive despite therapy may be associated with a poor clinical outcome 47, 61.
THE FUTURE
The disease.
Whipple's disease is considered rare, and postmortem studies have estimated the disease rate to be less than 0.1% 17. Paradoxically, by PCR, first studies have shown that the frequency of the Whipple's disease bacterium is high in healthy people as well as in the environment. Several hypotheses could be suggested to explain this discrepancy. The disease is rare, occurring only in patients with particular risk factors or genetic susceptibility. Some specific pathogenic strains of the Whipple's disease bacterium may cause the disease and others may not. The frequency of the disease could have been widely underestimated. Indeed, with the development of diagnosis by PCR, new clinical manifestations due to the Whipple's disease bacterium have been described with an increased frequency, such as endocarditis and uveitis. In addition, the characterization of the Whipple's disease bacterium and the associated diseases will be improved, as for endocarditis, for which two entities seem to exist: one in which valve involvement is a part of the disease and another in which it is the unique symptom 23, 48, 49. Finally, Whipple's disease could be only one manifestation of a much more frequent disease currently unidentified. The increasing number of available tools may help to identify such new clinical entities.
Serology.
Serological tests could be highly useful since a single blood sample could be used to make the diagnosis and could be life saving by allowing the institution of appropriate therapy. Now, with the possible cultivation of the Whipple's disease bacterium, some antigens could be produced to develop a serological test that would allow easier diagnosis of this disease that is currently difficult to diagnose. By using a monolayer infected with the bacillus of Whipple's disease, an immunofluorescence serological test has been developed 48. The serum samples are diluted in phosphate-buffered saline containing 3% nonfat dry milk, and the IgG and IgM titers are determined. To remove IgG, rheumatoid factor adsorbant is added before the determination of the IgM titer. Using this technique, we examined sera from 9 patients with Whipple's disease and 40 control subjects 48. When a cutoff value of 1:100 was selected, IgG antibodies against the bacillus were detected in the serum samples of all nine patients with Whipple's disease, as well as almost 75% of the samples from the control subjects. The specificity of the presence of IgM antibodies was greater; using a cutoff value of 1:50, we found that the results were positive for 7 of 9 patients with Whipple's disease, whereas they were positive for 3 of 40 control subjects. Also, higher titers of IgM antibodies (≥1:400) were present in three of seven patients with classic Whipple's disease and in both patients with Whipple's disease endocarditis but in none of the control subjects 48. The high frequency of IgG antibodies against the Whipple's disease isolate suggest that this pathogen is ubiquitous, causing illness only occasionally, perhaps because of differences in virulence among the strains or in host factors or as a result of the patient's exposure to other immunologically cross-reacting microorganisms. Large-scale studies are necessary to confirm these results. Moreover, Western blotting, by which the discriminative potential of proteins may be determined by a scoring method, may contribute to a specific diagnosis.
Monoclonal antibodies.
The culture of the Whipple's disease bacterium could allow the production of monoclonal antibodies. Monoclonal antibodies may provide a specific, simple, rapid, and low-cost tool that could be applied to tissues for the identification of the Whipple's disease bacterium and infection due to the microorganism. They are being developed in our laboratory.
Sequencing.
The establishment of a strain of the Whipple's disease bacterium has allowed its purification. It will also make it possible to start genetic studies. Thus, new target sequences will be available to perform PCR assays and to study the presence of molecular variants of the Whipple's disease bacterium. Sequencing would help with further epidemiological and clinical studies with the Whipple's disease bacterium and associated diseases and also with characterization of the mechanism of pathogenicity.
Antibiotic susceptibility testing.
Almost all antibiotics have been used for the treatment of Whipple's disease. However, the optimum treatment for Whipple's disease remains controversial with respect to both the choice of drug and the duration of treatment. Care must be taken to use those antibiotics that readily cross the blood-brain barrier, as organisms sequestered in the central nervous system can be a cause of disease recrudescence 57. The recommended treatment currently is daily parenteral administration of streptomycin (1 g) and benzylpenicillin (penicillin G; 1.2 × 10b units) over a period of 14 days, followed by oral co-trimoxazole (trimethoprim-sulfamethoxazole at 160/800 mg) twice daily for 1 year 57. However, it is documented that central nervous symptoms can also develop during its use 11. Dykman et al. 16 have suggested that this may be related to the fact that despite the high intracellular concentrations achieved with the use of this drug, it is only bacteriostatic. They have also added that the use of bactericidal drugs, such as ceftriaxone, initially followed by the use of an oral cephalosporin, such as cefixime, may be the most prudent strategy. With the recent possibility of cultivation of the Whipple's disease bacterium, tests for determination of the resistance of the bacterium to antibiotics could be developed and will allow a better definition of an antibiotic therapy strategy.
Pathophysiology.
With the culture of the Whipple's disease bacterium, pathophysiology studies can begin. An animal model which closely mimics pathological mechanisms of Whipple's disease should be developed. It is first necessary to determine the method of inoculation (intravenous, intraperitoneal, or another way) and the kind of animal to be used, mice (immunocompetent and/or immunosuppressed), guinea pigs, rabbits, rats, or some other animal. An experimental model with monocytes/macrophages could also be developed, leading to a better understanding of the phagocytosis and the survival of the Whipple's disease bacterium in these cells, as have already been described 55.
CONCLUSION
For numerous years, Whipple's disease was considered to be due to a bacterium which was responsible for a gastrointestinal disease. In reality, this bacterium seems to be more ubiquitous than once believed, and various sites of localization of Whipple's disease without gastrointestinal symptoms have recently been described. At present, with the development of new tools for performance of the diagnosis of Whipple's disease, such as PCR and culture, a new era is emerging. In the future, one can expect descriptions of a spectrum of new diseases due to the Whipple's disease bacterium. New tools such as monoclonal antibodies and serology could also be developed to improve the diagnosis. It is yet the case for Whipple's disease endocarditis. Furthermore, with the culture of the bacterium, pathophysiology studies could help us to better understand this complex disease.
ACKNOWLEDGMENT
We are indebted to Bernard La Scola for the picture demonstrating T. whippelii by electron microscopy and PAS staining.
REFERENCE
- 1.Altwegg M, Fleisch-Marx A, Goldenberg D, Hailemariam S, Schaffner A, Kissling R. Spondylodiscitis caused by Tropheryma whippelii. Schweiz Med Wochenschr. 1996;126:1495–1499. [PubMed] [Google Scholar]
- 2.Anderson M. Neurology of Whipple's disease. J Neurol Neurosurg Psychiatry. 2000;68:2–5. doi: 10.1136/jnnp.68.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin L L, Dobbins W O. Intraepithelial leukocytes of the intestinal mucosa in normal man and in Whipple's disease: a light- and electron-microscopic study. Dig Dis Sci. 1982;27:311–320. doi: 10.1007/BF01296750. [DOI] [PubMed] [Google Scholar]
- 4.Averbuch-Heller L, Paulson G W, Daroff R B, Leigh R J. Whipple's disease mimicking progressive supranuclear palsy: the diagnostic value of eye movement recording. J Neurol Neurosurg Psychiatry. 1999;66:532–535. doi: 10.1136/jnnp.66.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolt R J, Pollard H M, Tandaert L. Transoral small bowel biopsy as an aid in the diagnosis of malabsorption states. N Engl J Med. 1958;259:32–34. doi: 10.1056/NEJM195807032590107. [DOI] [PubMed] [Google Scholar]
- 6.Brandle M, Ammann P, Spinas G A, Dutly F, Galeazzi R L, Schmid C, Altwegg M. Relapsing Whipple's disease presenting with hypopituitarism. Clin Endocrinol (Oxford) 1999;50:399–403. [PubMed] [Google Scholar]
- 7.Caroli J, Julien C, Bonneville B. La maladie de Whipple. Revue générale et acquisitions récentes. Rev Franc Etudes Clin Biol. 1965;X:362–380. [PubMed] [Google Scholar]
- 8.Celard M, de Gevigney G, Mosnier S, Buttard P, Benito Y, Etienne J, Vandenesch F. Polymerase chain reaction analysis for diagnosis of Tropheryma whippelii infective endocarditis in two patients with no previous evidence of Whipple's disease. Clin Infect Dis. 1999;29:1348–1349. doi: 10.1086/313477. [DOI] [PubMed] [Google Scholar]
- 9.Chears W C, Hargrove M D, Verner J V, Smith A G, Ruffin J M. Whipple's disease. A review of twelve patients from one service. Am J Med. 1961;30:226–234. doi: 10.1016/0002-9343(61)90094-8. [DOI] [PubMed] [Google Scholar]
- 10.Clancy R L, Tomkins W A, Muckle T J, Richardson H, Rawls W E. Isolation and characterization of an aetiological agent in Whipple's disease. Br Med J. 1975;3:568–570. doi: 10.1136/bmj.3.5983.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper G S, Blades E W, Remler B F, Salata R A, Bennert K W, Jacobs G H. Central nervous system Whipple's disease: relapse during therapy with trimethoprim-sulfamethoxazole and remission with cefixime. Gastroenterology. 1994;106:782–786. doi: 10.1016/0016-5085(94)90716-1. [DOI] [PubMed] [Google Scholar]
- 12.Dauga C, Miras I, Grimont P A. Strategy for detection and identification of bacteria based on 16S RNA genes in suspected cases of Whipple's disease. J Med Microbiol. 1997;46:340–347. doi: 10.1099/00222615-46-4-340. [DOI] [PubMed] [Google Scholar]
- 13.Dobbins W O. HLA antigens in Whipple's disease. Arthritis Rheum. 1987;30:102–105. doi: 10.1002/art.1780300115. [DOI] [PubMed] [Google Scholar]
- 14.Dobbins W O. Whipple's disease. Springfield, III: Charles C Thomas; 1987. [Google Scholar]
- 15.Dobbins W O, Kawanishi H. Bacillary characteristics in Whipple's disease: an electron microscopic study. Gastroenterology. 1981;80:1468–1475. [PubMed] [Google Scholar]
- 16.Dykman D, Cuccherini B A, Fuss I, Blum L, Wouters R S. Whipple's disease in a father-daughter. Dig Dis Sci. 2000;44:2542–2544. doi: 10.1023/a:1026607726745. [DOI] [PubMed] [Google Scholar]
- 17.Enzinger F M, Helwig E B. Whipple's disease: a review of the literature and report of fifteen patients. Virchows Arch Pathol Anat. 1963;336:238–269. [Google Scholar]
- 18.Feldman M. Whipple's disease. Am J Med Sci. 1986;291:56–67. doi: 10.1097/00000441-198601000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Fleming J L, Wiesner R H, Shorter R G. Whipple's disease: clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients. Mayo Clin Proc. 1988;63:539–551. doi: 10.1016/s0025-6196(12)64884-8. [DOI] [PubMed] [Google Scholar]
- 20.Fresard A, Guglielminotti C, Berthelot P, Ros A, Farizon F, Dauga C, Rousset H, Lucht F. Prosthetic joint infection caused by Tropheryma whippelii. Clin Infect Dis. 1996;22:575–576. doi: 10.1093/clinids/22.3.575. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberger D, Kunzli A, Vogt P, Zbinden R, Altwegg M. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol. 1997;35:2733–2739. doi: 10.1128/jcm.35.11.2733-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross J B, Wollaeger E E, Sauer W G, Huizenga K A, Dahlin D C, Power M H. Whipple's disease—report of four cases, including two in brothers, with observation on pathologic physiology, diagnosis, and treatment. Gastroenterology. 1959;36:65–93. [PubMed] [Google Scholar]
- 23.Gubler J G, Kuster M, Dutly F, Bannwart F, Krause M, Vogelin H P, Garzoli G, Altwegg M. Whipple endocarditis without overt gastrointestinal disease: report of four cases. Ann Intern Med. 1999;131:112–116. doi: 10.7326/0003-4819-131-2-199907200-00007. [DOI] [PubMed] [Google Scholar]
- 24.Helliwell T, Appleton R, Mapstone N, Davidson J, Walsh K. Dermatomyositis and Whipple's disease. Neuromusc Disord. 2000;10:46–51. doi: 10.1016/s0960-8966(99)00054-1. [DOI] [PubMed] [Google Scholar]
- 25.Hendrix J P, Black-Schaffer B, Withers R W, Handler P. Whipple's intestinal lipodystrophy. Report of four cases and discussion of possible pathogenic factors. Arch Intern Med. 1950;85:91–131. doi: 10.1001/archinte.1950.00230070113006. [DOI] [PubMed] [Google Scholar]
- 26.Hinrikson H P, Dutly F, Altwegg M. Evaluation of a specific nested PCR targeting domain III of the 23S rRNA gene of “Tropheryma whippelii” and proposal of a classification system for its molecular variants. J Clin Microbiol. 2000;38:595–599. doi: 10.1128/jcm.38.2.595-599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinrikson H P, Dutly F, Nair S, Altwegg M. Detection of three different types of ‘Tropheryma whippelii’ directly from clinical specimens by sequencing, single-strand conformation polymorphism (SSCP) analysis and type-specific PCR of their 16S–23S ribosomal intergenic spacer region. Int J Syst Bacteriol. 1999;49(Pt. 4):1701–1706. doi: 10.1099/00207713-49-4-1701. [DOI] [PubMed] [Google Scholar]
- 28.Hinrikson H P, Dutly F, Altwegg M. Homogeneity of 16S–23S ribosomal intergenic spacer regions of Tropheryma whippellii in Swiss patients with Whipple's disease. J Clin Microbiol. 1999;37:152–156. doi: 10.1128/jcm.37.1.152-156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James T N, Bulkley B H. Abnormalities of the coronary arteries in Whipple's disease. Am Heart J. 1983;105:481–491. doi: 10.1016/0002-8703(83)90367-8. [DOI] [PubMed] [Google Scholar]
- 30.Khairy P, Graham A F. Whipple's disease and the heart. Can Cardiol. 1996;12:831–834. [PubMed] [Google Scholar]
- 31.Kloos K. Über eine eigenartige Fettresorptionsstörung und ihre Beziehung zur Sprue. Arch Pathol Anat. 1939;304:625–658. [Google Scholar]
- 32.Lowsky R, Archer G L, Fyles G, Minden M, Curtis J, Messner H, Atkins H, Patterson B, Willey B M, McGeer A. Brief report: diagnosis of Whipple's disease by molecular analysis of peripheral blood. N Engl J Med. 1994;331:1343–1346. doi: 10.1056/NEJM199411173312004. [DOI] [PubMed] [Google Scholar]
- 33.Maiwald M, von Herbay A, Lepp P W, Relman D A. Organization, structure, and variability of the rRNA operon of the Whipple's disease bacterium (Tropheryma whippelii) J Bacteriol. 2000;182:3292–3297. doi: 10.1128/jb.182.11.3292-3297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maiwald M, Schuhmacher F, Ditton H J, von Herbay A. Environmental occurrence of the Whipple's disease bacterium (Tropheryma whippelii) Appl Environ Microbiol. 1998;64:760–762. doi: 10.1128/aem.64.2.760-762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maizel H, Ruffin J M, Dobbins W O. Whipple's disease: a review of 19 patients from one hospital and a review of the literature since 1950. Medicine (Baltimore) 1970;49:175–205. [PubMed] [Google Scholar]
- 36.Maliha G M, Hepps K S, Maia D M, Gentry K R, Fraire A E, Goodgame R W. Whipple's disease can mimic chronic AIDS enteropathy. Am J Gastroenterol. 1991;86:79–81. [PubMed] [Google Scholar]
- 37.Mansbach C M, Shelburne J D, Stevens R D, Dobbins W O. Lymph-node bacilliform bodies resembling those of Whipple's disease in a patient without intestinal involvement. Ann Intern Med. 1978;89:64–66. doi: 10.7326/0003-4819-89-1-64. [DOI] [PubMed] [Google Scholar]
- 38.Marie I, Levesque H, Levade M H, Cailleux N, Lecomte F, Francois A, Metayer J, Lerebours E, Courtois H. Hypertrophic osteoarthropathy can indicate recurrence of Whipple's disease. Arthritis Rheum. 1999;42:2002–2006. doi: 10.1002/1529-0131(199909)42:9<2002::AID-ANR29>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.Marth T, Neurath M, Cuccherini B A, Strober W. Defects of monocyte interleukin 12 production and humoral immunity in Whipple's disease. Gastroenterology. 1997;113:442–448. doi: 10.1053/gast.1997.v113.pm9247462. [DOI] [PubMed] [Google Scholar]
- 40.Misbah S A, Ozols B, Franks A, Mapstone N. Whipple's disease without malabsorption: new atypical features. O J Med. 1997;90:765–772. doi: 10.1093/qjmed/90.12.765. [DOI] [PubMed] [Google Scholar]
- 41.Morgan A D. The first recorded case of Whipple's disease. Gut. 1961;2:370–372. doi: 10.1136/gut.2.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgenegg S, Dutly F, Altwegg M. Cloning and sequencing of a part of the heat shock protein 65 (hsp65) gene of Tropheryma whippelii and its use for the detection of Tropheryma whippelii in clinical specimens by PCR. J Clin Microbiol. 2000;38:2248–2253. doi: 10.1128/jcm.38.6.2248-2253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliver-Pasqual E, Galan J, Oliver-Pasqual A. Un case de lipodistrofica intestinal con lesions gangliones mesentericas de granulomatosis lipofagica (Enfermed de Whipple) Rev Esp Enferm Apar Dig. 1947;6:213–226. [PubMed] [Google Scholar]
- 44.Paulley J W. A case of Whipple's disease (intestinal lipodystrophy) Gastroenterology. 1952;22:128–133. [PubMed] [Google Scholar]
- 45.Pron B, Poyart C, Abachin E, Fest T, Belanger C, Bonnet C, Capelle P, Bretagne J F, Fabianek A, Girard L, Hagege H, Berche P. Diagnosis and follow-up of Whipple's disease by amplification of the 16S rRNA gene of Tropheryma whippelii. Eur J Clin Microbiol Infect Dis. 1999;18:62–65. doi: 10.1007/s100960050228. [DOI] [PubMed] [Google Scholar]
- 46.Puite R, Teslik H. Whipple's disease. Am J Med. 1955;19:383–400. doi: 10.1016/0002-9343(55)90127-3. [DOI] [PubMed] [Google Scholar]
- 47.Ramzan N N, Loftus E J, Burgart L J, Rooney M, Batts K P, Wiesner R H, Fredricks D N, Relman D A, Persing D H. Diagnosis and monitoring of Whipple disease by polymerase chain reaction. Ann Intern Med. 1997;126:520–527. doi: 10.7326/0003-4819-126-7-199704010-00004. [DOI] [PubMed] [Google Scholar]
- 48.Raoult D, Birg M L, La Scola B, Fournier P E, Enea M, Lepidi H, Roux V, Piette J C, Vandenesch F, Vital-Durand D, Marrie T J. Cultivation of the bacillus of Whipple's disease. N Engl J Med. 2000;342:620–625. doi: 10.1056/NEJM200003023420903. [DOI] [PubMed] [Google Scholar]
- 49.Raoult D. Afebrile blood culture-negative endocarditis. Ann Intern Med. 1999;131:144–146. doi: 10.7326/0003-4819-131-2-199907200-00012. [DOI] [PubMed] [Google Scholar]
- 50.Relman D A, Schmidt T M, MacDermott R P, Falkow S. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med. 1992;327:293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 51.Rickman L S, Freeman W R, Green W R, Feldman S T, Sullivan J, Russack V, Relman D A. Brief report: uveitis caused by Tropheryma whippelii (Whipple's bacillus) N Engl J Med. 1995;332:363–366. doi: 10.1056/NEJM199502093320604. [DOI] [PubMed] [Google Scholar]
- 52.Rose A G. Mitral stenosis in Whipple's disease. Thorax. 1978;33:500–503. doi: 10.1136/thx.33.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roth R I, Owen R L, Keren D F, Volberding P A. Intestinal infection with Mycobacterium avium in acquired immune deficiency syndrome (AIDS). Histological and clinical comparison with Whipple's disease. Dig Dis Sci. 1985;30:497–504. doi: 10.1007/BF01318186. [DOI] [PubMed] [Google Scholar]
- 54.Schneider T, Stallmach A, von Herbay A, Marth T, Strober W, Zeltz M. Treatment of refractory Whipple's disease with interferon-γ. Ann Intern Med. 1998;129:875–877. doi: 10.7326/0003-4819-129-11_part_1-199812010-00006. [DOI] [PubMed] [Google Scholar]
- 55.Schoedon G, Goldenberger D, Forrer R, Gunz A, Dutly F, Hochli M, Altwegg M, Schaffner A. Deactivation of macrophages with interleukin-4 is the key to the isolation of Tropheryma whippelii. J Infect Dis. 1997;176:672–677. doi: 10.1086/514089. [DOI] [PubMed] [Google Scholar]
- 56.Silva M T, Macedo P M, Moura N J. Ultrastructure of bacilli and the bacillary origin of the macrophagic inclusions in Whipple's disease. J Gen Microbiol. 1985;131:1001–1013. doi: 10.1099/00221287-131-5-1001. [DOI] [PubMed] [Google Scholar]
- 57.Singer R. Diagnosis and treatment of Whipple's disease. Drugs. 1998;55:699–704. doi: 10.2165/00003495-199855050-00007. [DOI] [PubMed] [Google Scholar]
- 58.Tytgat G N, Hoogendijk J L, Agenant D, Schellekens P T. Etiopathogenetic studies in a patient with Whipple's disease. Digestion. 1977;15:309–321. doi: 10.1159/000198017. [DOI] [PubMed] [Google Scholar]
- 59.Upton A C. Histochemical investigation of the mesenchymal lesions in Whipple's disease. Am J Clin Pathol. 1952;22:755–764. doi: 10.1093/ajcp/22.8.755. [DOI] [PubMed] [Google Scholar]
- 60.Vital-Durand D, Lecomte C, Cathebras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Societe Nationale Francaise de Medecine Interne. Medicine (Baltimore) 1997;76:170–184. doi: 10.1097/00005792-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 61.von Herbay A, Ditton H J, Schuhmacher F, Maiwald M. Whipple's disease: staging and monitoring by cytology and polymerase chain reaction analysis of cerebrospinal fluid. Gastroenterology. 1997;113:434–441. doi: 10.1053/gast.1997.v113.pm9247461. [DOI] [PubMed] [Google Scholar]
- 62.Whipple G H. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull Johns Hopkins Hosp. 1907;198:383. [Google Scholar]
- 63.Wilson K H, Blitchington R, Frothingham R, Wilson J A. Phylogeny of the Whipple's-disease-associated bacterium. Lancet. 1991;338:474–475. doi: 10.1016/0140-6736(91)90545-z. [DOI] [PubMed] [Google Scholar]