ABSTRACT
Cyclin-dependent kinases (CDKs) and cyclins are critical cell cycle regulators in eukaryotes. In this study, we functionally characterized a CDK-related kinase (CRK5) of the human malaria parasite Plasmodium falciparum. P. falciparum CRK5 (PfCRK5) was expressed in asexual blood stages and sexual gametocyte stages, but showed male gametocyte- specific expression. In contrast to previous findings, we showed that gene deletion Pfcrk5− parasites grew normally as asexual stages and underwent normal gametocytogenesis to stage V gametocytes. However, Pfcrk5− parasites showed a severe defect in male gametogenesis, which was evident by a significant reduction in the emergence of male gametes (exflagellation). This defect caused a severe reduction of parasite transmission to the mosquito. Genetic crosses performed using sex-specific sterile transgenic parasites revealed that Pfcrk5− parasites suffered a defect in male fertility but female gametes were fertile. Taken together, these results demonstrate that PfCRK5 is a critical sexual stage kinase which regulates male gametogenesis and transmission to the mosquito.
KEYWORDS: CRK5, gametocyte, exflagellation, mosquito, transmission
INTRODUCTION
Plasmodium falciparum remains the main causative agent of malaria, a disease with significant mortality and morbidity in developing countries across the world. P. falciparum is an obligate intracellular parasite with its life cycle alternating between a human host and Anopheline mosquitoes. Inside the red blood cells of a human host, the parasite cyclically replicates asexually over ~48 h periods, undergoing development as rings, trophozoites, and schizonts, ultimately forming new infectious merozoites. Some of the asexually replicating parasites commit and differentiate into sexual stages called gametocytes and develop through a number of morphologically distinct stages (stage I-V) over a 2-week period. Stage V gametocytes are taken up by the mosquito in blood meal where they rapidly get activated to form gametes (female; macrogametes and male; microgametes). A male gametocyte forms 8 flagellar microgametes, while a female gametocyte forms a single macrogamete (1, 2). These gametes fuse to form a short-lived zygote, which transforms into a motile ookinete. These stages penetrate the mosquito midgut epithelium and further develop as oocysts and eventually produce transmissible sporozoites over a 2-week period.
Factors controlling gametogenesis include increase in pH (1), a drop in temperature (1, 2), and/or exposure to xanthurenic acid (XA), a metabolite of tryptophan (3, 4). Gametogenesis is further linked to mobilization of intracellular Calcium (Ca2+) via protein kinase G (PKG) (5), and Calcium-dependent protein kinases (CDPKs), CDPK1 (6), CDPK2 (7), and CDPK4 (8). Several other proteins implicated in this process include a mitogen-activated protein kinase, MAP2 (9), and an ARID-domain containing protein, PfARID (10).
In most eukaryotes, typical cell cycle stages include cell growth (interphase), replication of its chromosomes (S phase) and cell divisions (M phase), and 2 gap phases called G1 and G2 flanking S phase. Cell cycle progression relies upon post-translational mechanisms including cell cycle kinases and phosphatases. The cyclin-dependent kinases (CDKs) are important signaling proteins regulating the cell cycle in various organisms (11, 12). CDK kinase activity is regulated by their interactions with cyclins and CDK inhibitors (CKIs) (12). Mammalian CDK1-4 and CDK6 regulate cell cycle progression, while CDK5 is involved in neuronal/synaptic functions, circadian clocks, DNA damage, cell cycle reentry, and mitochondrial dysfunction (13). Other CDKs such as CDK8-11 regulate gene expression and the cell cycle (14–16). The Cyclin proteins were initially discovered and named because their expression levels markedly fluctuate during the cell cycle. Cyclins control the kinase activity and substrate specificity of CDKs (16). The cell cycle in Plasmodium spp., deviates significantly from the typical eukaryotic cell cycle and produces hundreds of new daughter cells during a single replicative cycle. Plasmodium genomes encode 8 members of the CDK protein kinase family namely, CRK1 (PF3D7_0417800), CRK2/Protein kinase 5 (PF3D7_1356900), CRK3 (PF3D7_0415300), CRK4 (PF3D7_0317200), CRK5 (PF3D7_0615500), Protein kinase 6 (PF3D7_1337100), MO15-related protein kinase, MRK (PF3D7_1014400), an unannotated CDK kinase PF3D7_1338900 (17), and 3 cyclins Cyc1 (PF3D7_1463700), Cyc3 (PF3D7_0518400) and Cyc4 (PF3D7_1304700). The cyclin-dependence for in vitro kinase activity has been demonstrated for 2 of these, PfPK5 (18) and PfMRK (19), but their functional dependence in the parasite is not known.
During erythrocytic schizogony, nuclear division are asynchronous and independent often leading to formation of odd number of nuclei per schizont (20). PfCRK4 is known to be an essential S phase regulatory factor required for initial and subsequent rounds of DNA replication (21). PfCRK5 was reported to be important for the asexual proliferation and nuclear divisions of parasite (22). We have revisited the role of CRK5 in P. falciparum sexual stage development. We created Pfcrk5− parasites and in contrast to previous findings (22), Pfcrk5− parasites do not show a growth defect in asexual stages. We show that PfCRK5 has a function in male gametogenesis and thus parasite transmission to the mosquito vector.
RESULTS
PfCRK5 is expressed in the asexual and sexual stages of the parasite.
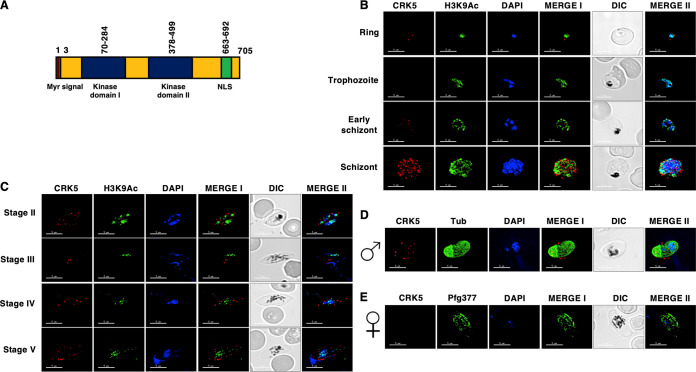
The domain architecture of PfCRK5 shows that it has N-terminal myristoylation signal, a single kinase domain with a spacer dividing it into 2 kinase subdomains, and a c-terminal nuclear localization signal (NLS) (Fig. 1A). To analyze the expression of PfCRK5, a previously generated peptide antisera was used (22). Western blotting performed on WT and Pfcrk− parasites using anti-PfCRK5 showed no PfCRK5 signal in Pfcrk5− parasites, confirming the absence of PfCRK5 protein and also the specificity of the antisera (Fig. S1A). Indirect immunofluorescence assays (IFAs) performed on thin blood smears of in vitro cultured PfNF54 revealed that PfCRK5 is expressed in the ring and schizont stages with a peri-nuclear localization (Fig. 1B), which is consistent with previous studies (22). PfCRK5 expression was also detected in gametocytes from stage II through stage V in the cytoplasm, near-nucleus, and membrane (Fig. 1C). Dual fluorescence IFAs with male (anti-tubulin) or female (anti-Pfg377) gametocyte specific antibodies revealed that PfCRK5 is expressed in male gametocytes (Fig. 1D and E), suggesting a male-specific function.
FIG 1.
Expression and localization of PfCRK5 in asexual and sexual stages. (A) Schematic for various motifs and domains of PfCRK5 showing an N-terminal myristoylation signal (red) followed by 2 bipartite domains (blue) and nuclear localization signal (NLS) (in green). (B) Immunofluorescence assays were performed on WT NF54 asexual blood stages (ring, trophozoite and schizont) to colocalize PfCRK5 (red) in combination with Histone marker H3K9Ac (green). The parasite nucleus was localized with 4′,6-diamidino-2-phenylindole (DAPI) (in blue). Scale bar = 5 μm. (C) Immunofluorescence assays were performed on WT NF54 sexual (stage II-V gametocytes) using thin culture smears and anti-PfCRK5 antisera (in red) in combination with H3K9Ac (green). (D) and (E) Immunofluorescence assays were performed on stage V gametocytes using thin smears and anti-PfCRK5 antisera (in red) either in combination with α-Tubulin (marker for male gametocytes, in green) or anti- Pfg377 (marker for female gametocytes, in green). Parasite nucleus was visualized with DAPI (blue). Scale bar = 5 μm.
(A) Western blot analysis of PfCRK5 in WT PfNF54 and Pfcrk5− gametocytes, showing absence of PfCRK5 in Pfcrk5− parasites. Actin abundance is shown as the loading control. (B) The Giemsa-stained thin culture smears were prepared for observing mature schizont stages for WT PfNF54 and Pfcrk5− parasites at 1,000×magnifcation. Representative Giemsa-stained images of WT PfNF54 and Pfcrk5− schizonts are shown. (C) The Giemsa-stained thin culture smears were used for quantitative assessment of number of daughter merozoites per schizont for WT PfNF54 and Pfcrk5− parasites. (Duplicate experiments; n = 50 cells per experiment; bars are SD). NS, Not significant. Download FIG S1, TIF file, 0.9 MB (971.5KB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PfCRK5 is not required for intra-erythrocytic parasite development.
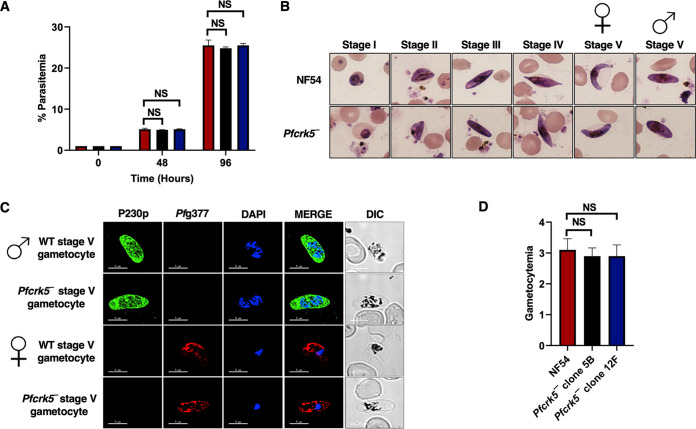
For functional analysis, the endogenous PfCRK5 gene was deleted using CRISPR/Cas9 (Fig. 2A). Gene deletion parasites (Pfcrk5−) were confirmed by a set of diagnostic PCRs with oligonucleotides specific for the PfCRK5 locus and its upstream (5′) and downstream (3′) regions (Fig. 2A to C). Two individual clones for Pfcrk5− parasites (clone 5B and 12F) were used for phenotypic characterization. To analyze the role of PfCRK5 in asexual parasite stages, a comparative growth assay was set up using Pfcrk5− parasites (clone 5B and 12F) along with wildtype (WT) NF54 parasites. Parasite growth was monitored over 2 asexual replication cycles. Giemsa-stained thin smears prepared every 48 h from the in vitro culture indicated that the growth rate of Pfcrk5− parasites was similar to WT NF54 parasites (Fig. 3A). We quantified the number of daughter merozoites per schizont for Pfcrk5− in comparison to WT NF54 parasites. This revealed that the average number of merozoites per schizont in Pfcrk5− was similar to WT PfNF54 parasites (Fig. S1B and C).
FIG 2.
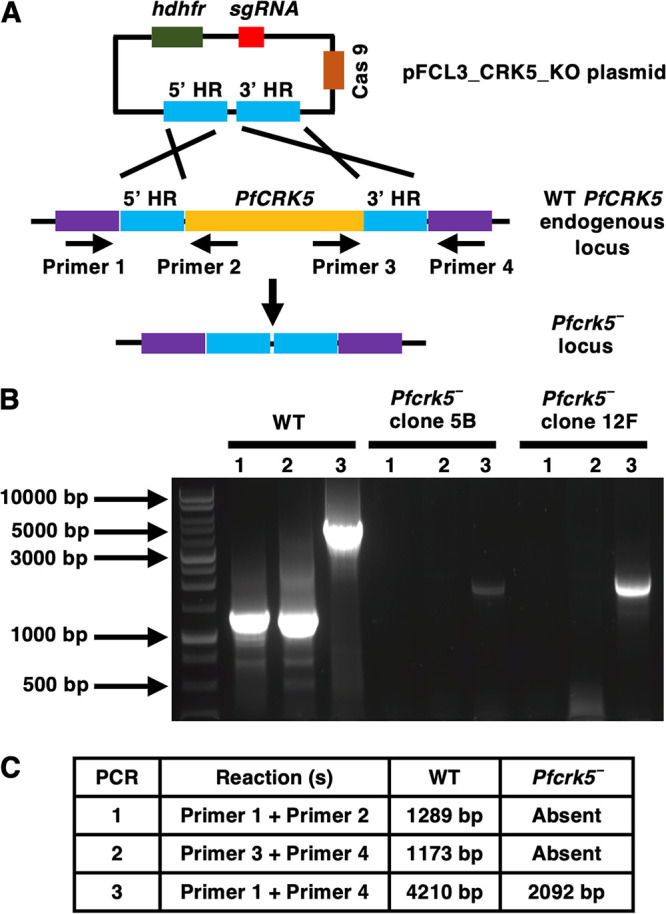
Generation of Pfcrk5− parasites. The schematic shows the strategy for deleting PfCRK5. The pFCL3_CRK5_KO plasmid has homology regions 5′ (5’HR) and 3′ (3’HR) not of the PfCRK5 locus, a guide RNA sequence (sgRNA) and human dihydrofolate reductase (hDHFR) locus and Cas 9 cloned. The oligonucleotides were designed from outside 5’HR and 3’HR and PfCRK5 locus and positions are indicated by arrows in (A). (B) Confirmation of PfCRK5 deletion by diagnostic PCR. The expected sizes for different set of PCRs are indicated in (C).
FIG 3.
Pfcrk5− asexual stages grow normally and undergo gametocytogenesis. (A) Ring stage synchronous cultures for WT and two clones of Pfcrk5− (clone 5B and 12F) were plated to measure parasite growth over the course of 2 erythrocytic cycles. Total parasitemia was determined by counting the parasites from Giemsa-stained thin blood smears. Data were averaged from three biological replicates and presented as the mean ± standard deviation (SD). ns, not significant unpaired two-tailed Student's t test. (B) Ring stage synchronous cultures for WT and 2 different clones of Pfcrk5− (clone 5B and 12F) were tested for their potential to form gametocytes. Light microscopy of Giemsa-stained smears showing development of WT PfNF54 and Pfcrk5− gametocytes and the 5 (I-V) distinct morphological stages. 1,000×magnifcation. Symbols for female and male gametocytes are shown on top of stage V gametocytes. (C) IFAs were performed on WT PfNF54 and Pfcrk5− mature stage V gametocytes thin culture smears using anti-PfP230p antisera, a marker for stage V male gametocytes (in green), in combination with anti-Pfg377 antisera, a marker for female gametocytes (in red). Representative images are shown. The parasite DNA was visualized with DAPI (blue). Scale bar = 5 μm. Merge I- merged image for red and green panels. Merge II- merged image for red, green, and DAPI (blue) channel. DIC, differential interference contrast. DAPI, 4′,6-diamidino-2-phenylindole. Symbols for male and female gametocytes are shown on left side of the image panels. (D) Gametocytemia was measured on day 15 using thin Giemsa-stained smears. Data were averaged from 3 biological replicates and presented as the mean ± standard deviation (SD). NS, Not significant.
Pfcrk5− parasites undergo gametocytogenesis but fail to form microgametes.
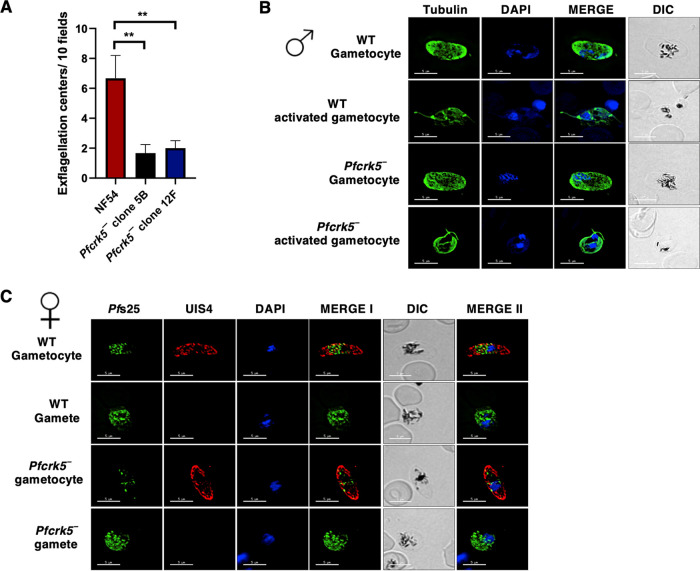
We next analyzed the ability of Pfcrk5− parasites to generate gametocytes. For this, Pfcrk5− parasites (clone 5B and 12F) along with WT NF54 parasites were used, and gametocytemia was scored for all cultures on day 15 of in vitro culture using Giemsa-stained culture smears and microscopic inspection. Pfcrk5− parasites were able to undergo gametocytogenesis, developing through stage I-V gametocytes, and could develop into mature stage V male and female gametocytes (Fig. 3B and C) with the gametocytemia being similar to WT NF54 (Fig. 3D). We next analyzed whether Pfcrk5− gametocytes undergo gametogenesis. Day 15 gametocyte cultures for WT NF54 and Pfcrk5− were activated by addition of O+ human serum and dropping the temperature from 37°C to room temperature (RT). Activated gametocytes were used to prepare a temporary live, wet mount of cultures and exflagellation centers were measured in 15 random fields of microscopic view at ×40 magnification. Strikingly, the number of exflagellation centers for Pfcrk5− (Fig. 4A) were significantly reduced, indicating an exflagellation defect. To confirm this defect, IFAs were performed with thin culture smears for WT NF54 and Pfcrk5− activated gametocytes 15 min post activation, and parasites were stained with an anti-tubulin antibody. The lack of release of observable male gamete exflagella from the gametocyte body confirmed an exflagellation defect in Pfcrk5− (Fig. 4B). Female Pfcrk5− gametes were stained with Pfs25 antibody and UIS4 antibody, which marks parasitophorous vacuole membranes. No observable defect was seen in Pfcrk5− (Fig. 4C). These results indicate PfCRK5 is critical for male gametogenesis.
FIG 4.
The Pfcrk5− parasites do not undergo male gametogenesis. (A) Number of exflagellation centers per field at 15 min post activation were enumerated. Data were averaged from 3 biological replicates and presented as the mean ± standard deviation (SD). (B) and (C) IFAs performed on thin blood smears of mature stage V gametocytes activated for 20 min in vitro for WT or Pfcrk5− (clone 12F) and were stained for α-tubulin (green), a male-specific marker, and Pfs25 (green), a marker for female gametes in an IFA. Anti-PfUIS4 was used to stain parasitophorous vacuolar membrane. α-Tubulin staining showed male gametes emerging from an exflagellating male gametocyte in the WT parasite. The Pfcrk5− gametocytes were defective for male gametocyte exflagellation. Female gametes did not show any defect in egress from gametocyte body.
The Pfcrk5− male defect causes a severe reduction in transmission to the mosquito vector.
We next examined the transmissibility of Pfcrk5− gametocytes to female Anopheles stephensi mosquitoes. Infectious blood meals of WT and Pfcrk5− stage V gametocytes were prepared using standard methods and fed to mosquitoes via membrane feeders. Mosquito midguts were dissected on Day 7 post feed, which revealed that Pfcrk5− parasites displayed a severe reduction in number of oocysts in comparison to well-infected WT controls (Fig. 5A). These results revealed that PfCRK5 is important for transmission to the mosquito vector via a crucial function in male gametogenesis. After determining the role of PfCRK5 in male gametocyte exflagellation, we sought to determine the fertility of individual sex. Since it is not possible to separate male and female gametocytes in in vitro culture, we further analyzed the fertility of male and female Pfcrk5−gametes utilizing genetic crosses as described previously (10). For this, we used Pfcrk5− parasites and transgenic parasite lines, which are sex-sterile for one sex forming either fertile female gametes only (Pfcdpk4−) (8) or fertile male gametes (Pfmacfet−) only (23), as described previously (10). WT NF54, Pfcrk5−, Pfcdpk4−, and Pfmacfet− gametocytes were generated in vitro in culture for 15 days, and cultures were first fed individually to mosquitoes. For crosses, the gametocytes from these parasites were mixed in equal ratio as follows: Pfcrk5− × Pfcdpk4−, Pfcrk5− × Pfmacfet−, Pfcdpk4− × Pfmacfet−. Mosquitoes were dissected on day 7 post feeding to enumerate midgut oocysts for all the feeds. While WT PfNF54 gametocytes infected mosquito midguts robustly, Pfcrk5− gametocytes showed a strong reduction in oocyst numbers (Fig. 5B), and the Pfcdpk4− and Pfmacfet− did not show any infection as expected. The Pfcrk5− × Pfcdpk4− cross showed highly reduced number of oocysts. However, in the Pfcrk5− × Pfmacfet− cross, oocysts were observed. This indicated productive fertilization of Pfcrk5− female gametes by Pfmacfet− male gametes (Fig. 5B). Oocyst development was also observed in Pfcdpk4− × Pfmacfet− (positive control) cross. These experiments demonstrate that PfCRK5 is important for male gametogenesis.
FIG 5.
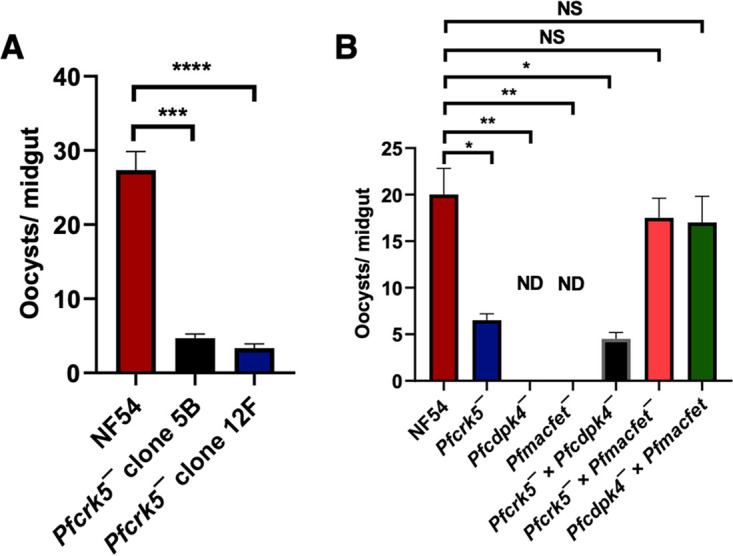
The Pfcrk5− parasites show robust reduction in infection in mosquitoes. (A) A. stephensi mosquitoes were dissected on day 7 post feed and number of oocysts were enumerated per midgut. Data were averaged from 3 biological replicates with a minimum of 50 mosquito guts and presented as the mean ± standard deviation (SD). (B) A. stephensi mosquitoes were dissected on day 7 post feed and number of oocysts were enumerated per midgut for WT PfNF54, Pfcrk5−, Pfcdpk4−, Pfmacfet−, Pfcrk5− × Pfcdpk4−, Pfcrk5− × Pfmacfet−, Pfcdpk4− × Pfmacfet−. In vitro genetic crosses revealed that the Pfcrk5− showed productive cross-fertilization with the female sterile Pfmacfet− parasites, while it was strongly reduced with the male sterile Pfcdpk4− parasites (error bar indicates mean ± SD; n = 2). ND, Not detected. NS, Not significant.
DISCUSSION
The uptake of gametocytes by Anopheline mosquitoes is critical for the completion of the sexual phase of the parasite life cycle. Upon encountering cellular triggers in the mosquito midgut, gametocytes rapidly form gametes that move through the blood meal, undergo fertilization to form zygotes, followed by differentiation into other mosquito stages. However, proteins that are critical for the formation of fertilization-competent gametes and sexual reproduction, particularly for human malaria parasites, are unknown. Our study demonstrates that PfCRK5 is important for male gametogenesis and transmission to the mosquito.
Signaling proteins such as kinases are key regulators across various life cycle stages of the malaria parasite (24–27). Protein kinases such as PfCDPK1 (6), PfCDPK2 (7), and PfCDPK4 (8) are involved in gametogenesis and are critical for establishing infection of the mosquito vector. Other kinases such as PfPKG (28), PfMAP2 (9), and PfSRPK1 (Kumar et al., 2022; DOI: 10.1128/spectrum.02141-22) play a role in gametogenesis, indicating the importance of phospho-signaling events in sexual development of the parasite. Other proteins regulating gametogenesis in Pf include Pfg377 (29), M-TRAP (merozoite-thrombospondin-related anonymous protein) (30) and perforin-like protein (PPLP2) (31).
In this study, we show that PfCRK5 is expressed throughout asexual blood stage development and gametocyte development. PfCRK5 displays a peri-nuclear localization in asexual stages, while in sexual stages it shows more cytoplasmic or membrane localization. We further demonstrate that PfCRK5 exhibits a male gametocyte specific expression which can be relevant to its cellular function. PfCRK5 possesses an N-terminal myristoylation signal and a c-terminal nuclear localization signal. The myristoylation signal for various kinases has been shown to regulate their membranous localization in plants (32) and Plasmodium spp. (33). Therefore, both myristoylation signal and nuclear localization signals may be regulating PfCRK5 cellular localization in various stages which may be relevant to its function.
A previous report with Pfcrk5− parasites has shown that Pfcrk5− parasites show a growth defect in asexual blood stages due to a defective number of daughter merozoites per schizont (22). However, in our experiments, we did not observe any growth defect in Pfcrk5− parasites. It is possible that the growth defect may arise due to long-term parasite culture and/or culture conditions. Another study on the rodent malaria parasite Plasmodium berghei PbCRK5 have also shown that the gene deletion parasites do not exhibit any growth defect (34). Our experiments show that even if PfCRK5 is expressed in the asexual blood stage and throughout gametocyte development, it is not required for asexual blood stage proliferation or gametocyte development. There is a possibility of the compensation of CRK5 kinase activity during asexual schizogony by other kinases such as CRK4, since it is also important for DNA replication during this developmental stage (21). As a result, deletion of PfCRK5 is not lethal for asexual development which involves DNA replication. This hypothesis is based upon a previous study that demonstrates compensation of PfCDPK1 kinase activity in asexual stages through the action of another protein kinase PfPKG (35).
We found that Pfcrk5− parasites develop into mature stage V male and female gametocytes. While female Pfcrk5− gametocytes undergo gametogenesis, male gametocytes undergo activation and form spheroid cells but exhibit a severe defect in male gametogenesis. Mosquito feeding experiments revealed that Pfcrk5− parasites show a robust defect in transmission which can be attributed to a defect in male gametogenesis. Further genetic crosses experiments with sex-sterile transgenic parasite lines revealed that Pfcrk5− female gametes are fertile but male gametes suffer severe defects in fertility.
In eukaryotic cells, cell cycle progression is regulated by interplay between cyclins and cyclin-dependent protein kinases (CDKs), along with additional protein complexes such as the anaphase promoting complex (APC), which regulates cyclin degradation (36, 37). While still inside the erythrocytes, male gametocytes (which are in the G1 phase of the cell cycle) undergo 3 rapid rounds of DNA replication and start assembling the flagellum (38). During male gametogenesis, there is no detectable karyokinesis and cytokinesis is uncoupled from DNA replication, indicating a lack of cell cycle checkpoints (38). In fact, Plasmodium cyclins do not show oscillating expression profiles, though stable complexes of cyclins and CRKs have been reported (39), which may represent a parasite specific cell cycle machinery. Two of the four Plasmodium cyclins (PfCyc1 and PfCyc4) are known to interact with PfCRK5 in vitro and regulate its activity (22), suggesting a cyclin-dependence for PfCRK5 although coimmunoprecipitation of PfCyc1 from parasite lysates identified PfMAT1 and PfMRK as specific interactors (39). This suggests that PfCRK5 may interact with PfCyc4 or some other cyclin like protein in the parasite in a stage specific manner. In the rodent malaria parasite P. berghei, PbCRK5 is known to interact with a predicted parasite cyclin SOC2, although there is no evidence of SOC2 cycling by transcription, translation, or degradation (34). PbCRK5 phosphorylates components of pre-replicative complexes, such as proteins important in DNA replication (i.e., a licensing factor like protein chromatin licensing and DNA replication factor 1 [CDT1]), a possible orthologue of the DNA replication factor CDC6, and 2 ORC components (ORC2 and ORC4) (34). Previous studies have also indicated the role of PfCDPK4 in male gametogenesis possibly by phosphorylation of PfCDPK1, PfSOC3, PfSOC7 (a ribonucleoside-diphosphate reductase), and ATP-dependent6-phosphofructokinase (PFK9), replication components, such as replication factor C subunit 1, replication factor C subunit 4, DNA replication licensing factor CDT1, MCM4, DNA polymerase alpha catalytic subunit A, SAS6, and microtubule proteins Kinesin 13 and Kinesin 8B (8). This suggests that PfCRK5 and PfCDPK4 may target similar parasite proteins during gametogenesis. Therefore, it is reasonable to propose here that there may be a possible cross talk between CDPK4 and CRK5 which may be regulating phosphorylation of these key parasite proteins. These mechanisms may be responsible for the role of PfCRK5 in regulating DNA replication and exflagella formation during male gametogenesis.
In conclusion, our study shows that PfCRK5 plays a key role in male gametogenesis and transmission. Further studies are warranted to identify the molecular mechanisms via which it regulates male gametogenesis. Since PfCRK5 is significantly divergent from mammalian CDKs, it could be an attractive target for developing kinase inhibitors that block malaria transmission.
MATERIALS AND METHODS
Reagents and primary antibodies.
All molecular biology reagents were purchased from Millipore Sigma, unless otherwise stated. All oligonucleotides were purchased from IDT Inc. The following primary antibodies/antisera and dilutions were used: mouse anti-tubulin antibody (1:200, Millipore Sigma, cat# T5168); mouse anti-PfP230p (1:200, kindly gifted by Professor Kim C. Williamson, Uniformed Services University of the Health Sciences, USA) (40), mouse anti-Pfg377 (1:250, kindly gifted by Professor Pietro Alano at Istituto Superiore di Sanità, Italy), mouse anti-H3K9Ac (1:100, MABI0305, GeneTex), and PfCRK5 (1:50, kindly provided by Professor Christian Doerig, RMIT university, Australia) (22). All Alexa fluor conjugated secondary antibodies were purchased from ThermoFisher Scientific.
P. falciparum culture and transfection.
The P. falciparum NF54 and Pfcrk5− parasites, asexual and sexual cultures were maintained as described elsewhere (10). The oligonucleotides used for creation and genotyping analysis of Pfcrk5− parasites are detailed in Table S1. Deletion of PfCRK5 (PlasmoDB identifier Gene - PF3D7_0615500) was achieved as described elsewhere (10). Two individual clones for Pfcrk5− (clone 5B and 12F) were used for phenotypic analysis.
Oligonucleotides used in the study. Download Table S1, DOCX file, 0.02 MB (16KB, docx) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth assays and measurement of gametocyte development.
The comparative growth for asexual blood stage between the PfNF54 WT and Pfcrk5− parasites was assessed as described elsewhere (10). To compare gametocyte formation between WT PfNF54 and Pfcrk5−, gametocytes were cultured as described elsewhere (41), and gametocytemia was enumerated using Giemsa-stained thin culture smears on day 15 of in vitro culture.
Exflagellation, standard membrane feeding assay, and oocyst measurements.
The assessment of comparative exflagellation, standard membrane feeding assay (SMFA), and oocyst measurements were performed as described elsewhere (10).
Indirect immunofluorescence.
IFAs were performed on asexual and sexual blood stage parasites and exflagellating microgametocytes using thin smears prepared on Teflon coated slides as described elsewhere (42). Antigens were visualized using anti-species antibodies. Images were acquired using a 100 × 1.4 NA objective 90 (Olympus) on a Delta Vision Elite High-Resolution Microscope (GE Healthcare Life Sciences).
Statistical analysis.
All data related to phenotyping assays are expressed as mean ± SD. Statistical differences were deemed significant using P-values from an unpaired, two-tailed Student's t test. Values of P < 0.05 were considered statistically significant. Significances were calculated using GraphPad Prism and are represented in the Figures as follows: ns, not significant, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Data availability.
All other relevant data are available from the authors upon reasonable request.
ACKNOWLEDGMENTS
We acknowledge and thank William W. Betz, Kenza M.Z. Oualim, and Tess Seltzer for maintaining insectaries at the Center for Global Infectious Disease Research, Seattle Children’s Research Institute, and for timely providing uninfected mosquitoes for this research.
Seed funds from Seattle Children’s to SHIK.
Sudhir Kumar, Olivia R. Gargaro, and Stefan H.I. Kappe contributed this manuscript.
S.K. conceptualized this study and was in charge of the methodology, visualization, and writing the original draft of this study. S.K. and O.R.G. performed the investigation. S.H.I.K. took care of the resources and funding acquisition. Finally, S.K. and S.H.I.K. reviewed and edited the manuscript.
We declare no competing financial or non-financial interests.
Contributor Information
Sudhir Kumar, Email: sudhir.kumar@seattlechildrens.org.
Louis M. Weiss, Albert Einstein College of Medicine
REFERENCES
- 1.Sinden RE. 1983. The cell biology of sexual development in plasmodium. Parasitology 86 (Pt 4):7–28. doi: 10.1017/s0031182000050824. [DOI] [PubMed] [Google Scholar]
- 2.Sinden RE, Croll NA. 1975. Cytology and kinetics of microgametogenesis and fertilization in Plasmodium yoelii nigeriensis. Parasitology 70:53–65. doi: 10.1017/s0031182000048861. [DOI] [PubMed] [Google Scholar]
- 3.Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. 1998. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 4.Garcia GE, Wirtz RA, Barr JR, Woolfitt A, Rosenberg R. 1998. Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. J Biol Chem 273:12003–12005. doi: 10.1074/jbc.273.20.12003. [DOI] [PubMed] [Google Scholar]
- 5.Balestra AC, Koussis K, Klages N, Howell SA, Flynn HR, Bantscheff M, Pasquarello C, Perrin AJ, Brusini L, Arboit P, Sanz O, Castaño LP, Withers-Martinez C, Hainard A, Ghidelli-Disse S, Snijders AP, Baker DA, Blackman MJ, Brochet M. 2021. Ca(2+) signals critical for egress and gametogenesis in malaria parasites depend on a multipass membrane protein that interacts with PKG. Sci Adv 7. doi: 10.1126/sciadv.abe5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal A, Molina-Cruz A, Brzostowski J, Liu P, Luo Y, Gunalan K, Li Y, Ribeiro JMC, Miller LH. 2018. PfCDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proc Natl Acad Sci USA 115:774–779. doi: 10.1073/pnas.1715443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal A, Molina-Cruz A, Brzostowski J, Mu J, Miller LH. 2017. Plasmodium falciparum calcium-dependent protein Kinase 2 is critical for male gametocyte exflagellation but not essential for asexual proliferation. mBio 8:e01656-17. doi: 10.1128/mBio.01656-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Haile MT, Hoopmann MR, Tran LT, Michaels SA, Morrone SR, Ojo KK, Reynolds LM, Kusebauch U, Vaughan AM, Moritz RL, Kappe SHI, Swearingen KE. 2021. Plasmodium falciparum calcium-dependent protein kinase 4 is critical for male gametogenesis and transmission to the mosquito vector. mBio 12:e0257521. doi: 10.1128/mBio.02575-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hitz E, Balestra AC, Brochet M, Voss TS. 2020. PfMAP-2 is essential for male gametogenesis in the malaria parasite Plasmodium falciparum. Sci Rep 10:11930. doi: 10.1038/s41598-020-68717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Baranwal VK, Haile MT, Oualim KMZ, Abatiyow BA, Kennedy SY, Vaughan AM, Kappe SHI. 2022. PfARID regulates P. falciparum malaria parasite male gametogenesis and female fertility and is critical for parasite transmission to the mosquito vector. mBio 13:e0057822. doi: 10.1128/mbio.00578-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satyanarayana A, Kaldis P. 2009. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28:2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 12.Lim S, Kaldis P. 2013. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 13.Pao PC, Tsai LH. 2021. Three decades of Cdk5. J Biomed Sci 28:79. doi: 10.1186/s12929-021-00774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garriga J, Graña X. 2004. Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene 337:15–23. doi: 10.1016/j.gene.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd RL, Urban V, Muñoz-Martínez F, Ayestaran I, Thomas JC, de Renty C, O'Connor MJ, Forment JV, Galanty Y, Jackson SP. 2021. Loss of cyclin C or CDK8 provides ATR inhibitor resistance by suppressing transcription-associated replication stress. Nucleic Acids Res 49:8665–8683. doi: 10.1093/nar/gkab628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loyer P, Trembley JH. 2020. Roles of CDK/Cyclin complexes in transcription and pre-mRNA splicing: cyclins L and CDK11 at the cross-roads of cell cycle and regulation of gene expression. Semin Cell Dev Biol 107:36–45. doi: 10.1016/j.semcdb.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Solyakov L, Halbert J, Alam MM, Semblat JP, Dorin-Semblat D, Reininger L, Bottrill AR, Mistry S, Abdi A, Fennell C, Holland Z, Demarta C, Bouza Y, Sicard A, Nivez MP, Eschenlauer S, Lama T, Thomas DC, Sharma P, Agarwal S, Kern S, Pradel G, Graciotti M, Tobin AB, Doerig C. 2011. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat Commun 2:565. doi: 10.1038/ncomms1558. [DOI] [PubMed] [Google Scholar]
- 18.Le Roch K, Sestier C, Dorin D, Waters N, Kappes B, Chakrabarti D, Meijer L, Doerig C. 2000. Activation of a Plasmodium falciparum cdc2-related kinase by heterologous p25 and cyclin H. Functional characterization of a P. falciparum cyclin homologue. J Biol Chem 275:8952–8958. doi: 10.1074/jbc.275.12.8952. [DOI] [PubMed] [Google Scholar]
- 19.Waters NC, Woodard CL, Prigge ST. 2000. Cyclin H activation and drug susceptibility of the Pfmrk cyclin dependent protein kinase from Plasmodium falciparum. Mol Biochem Parasitol 107:45–55. doi: 10.1016/s0166-6851(99)00229-7. [DOI] [PubMed] [Google Scholar]
- 20.Arnot DE, Ronander E, Bengtsson DC. 2011. The progression of the intra-erythrocytic cell cycle of Plasmodium falciparum and the role of the centriolar plaques in asynchronous mitotic division during schizogony. Int J Parasitol 41:71–80. doi: 10.1016/j.ijpara.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Ganter M, Goldberg JM, Dvorin JD, Paulo JA, King JG, Tripathi AK, Paul AS, Yang J, Coppens I, Jiang RH, Elsworth B, Baker DA, Dinglasan RR, Gygi SP, Duraisingh MT. 2017. Plasmodium falciparum CRK4 directs continuous rounds of DNA replication during schizogony. Nat Microbiol 2:17017. doi: 10.1038/nmicrobiol.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorin-Semblat D, Carvalho TG, Nivez M-P, Halbert J, Poullet P, Semblat J-P, Goldring D, Chakrabarti D, Mehra P, Dhar S, Paing MM, Goldberg DE, McMillan PJ, Tilley L, Doerig C. 2013. An atypical cyclin-dependent kinase controls Plasmodium falciparum proliferation rate. Kinome 1:4–16. doi: 10.2478/kinome-2013-0001. [DOI] [Google Scholar]
- 23.Kumar S, Abatiyow BA, Haile MT, Oualim KMZ, Leeb AS, Vaughan AM, Kappe SHI. 2022. A putative Plasmodium RNA-binding protein plays a critical role in female gamete fertility and parasite transmission to the mosquito vector. Front Cell Dev Biol 10:825247. doi: 10.3389/fcell.2022.825247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Kumar M, Ekka R, Dvorin JD, Paul AS, Madugundu AK, Gilberger T, Gowda H, Duraisingh MT, Keshava Prasad TS, Sharma P. 2017. PfCDPK1 mediated signaling in erythrocytic stages of Plasmodium falciparum. Nat Commun 8:63. doi: 10.1038/s41467-017-00053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, Bright AT, Westenberger S, Winzeler E, Blackman MJ, Baker DA, Wandless TJ, Duraisingh MT. 2010. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science 328:910–912. doi: 10.1126/science.1188191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam MM, Solyakov L, Bottrill AR, Flueck C, Siddiqui FA, Singh S, Mistry S, Viskaduraki M, Lee K, Hopp CS, Chitnis CE, Doerig C, Moon RW, Green JL, Holder AA, Baker DA, Tobin AB. 2015. Phosphoproteomics reveals malaria parasite protein kinase G as a signalling hub regulating egress and invasion. Nat Commun 6:7285. doi: 10.1038/ncomms8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar P, Tripathi A, Ranjan R, Halbert J, Gilberger T, Doerig C, Sharma P. 2014. Regulation of Plasmodium falciparum development by calcium-dependent protein kinase 7 (PfCDPK7). J Biol Chem 289:20386–20395. doi: 10.1074/jbc.M114.561670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA. 2008. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol 6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Koning-Ward TF, Olivieri A, Bertuccini L, Hood A, Silvestrini F, Charvalias K, Berzosa Díaz P, Camarda G, McElwain TF, Papenfuss T, Healer J, Baldassarri L, Crabb BS, Alano P, Ranford-Cartwright LC. 2008. The role of osmiophilic bodies and Pfg377 expression in female gametocyte emergence and mosquito infectivity in the human malaria parasite Plasmodium falciparum. Mol Microbiol 67:278–290. doi: 10.1111/j.1365-2958.2007.06039.x. [DOI] [PubMed] [Google Scholar]
- 30.Bargieri DY, Thiberge S, Tay CL, Carey AF, Rantz A, Hischen F, Lorthiois A, Straschil U, Singh P, Singh S, Triglia T, Tsuboi T, Cowman A, Chitnis C, Alano P, Baum J, Pradel G, Lavazec C, Ménard R. 2016. Plasmodium merozoite TRAP family protein is essential for vacuole membrane disruption and gamete egress from Erythrocytes. Cell Host Microbe 20:618–630. doi: 10.1016/j.chom.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirth CC, Glushakova S, Scheuermayer M, Repnik U, Garg S, Schaack D, Kachman MM, Weißbach T, Zimmerberg J, Dandekar T, Griffiths G, Chitnis CE, Singh S, Fischer R, Pradel G. 2014. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol 16:709–733. doi: 10.1111/cmi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martín ML, Busconi L. 2000. Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J 24:429–435. doi: 10.1046/j.1365-313x.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 33.Möskes C, Burghaus PA, Wernli B, Sauder U, Dürrenberger M, Kappes B. 2004. Export of Plasmodium falciparum calcium-dependent protein kinase 1 to the parasitophorous vacuole is dependent on three N-terminal membrane anchor motifs. Mol Microbiol 54:676–691. doi: 10.1111/j.1365-2958.2004.04313.x. [DOI] [PubMed] [Google Scholar]
- 34.Balestra AC, Zeeshan M, Rea E, Pasquarello C, Brusini L, Mourier T, Subudhi AK, Klages N, Arboit P, Pandey R, Brady D, Vaughan S, Holder AA, Pain A, Ferguson DJ, Hainard A, Tewari R, Brochet M. 2020. A divergent cyclin/cyclin-dependent kinase complex controls the atypical replication of a malaria parasite during gametogony and transmission. Elife 9:e56474. doi: 10.7554/eLife.56474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bansal A, Ojo KK, Mu J, Maly DJ, Van Voorhis WC, Miller LH. 2016. Reduced activity of mutant calcium-dependent protein kinase 1 is compensated in Plasmodium falciparum through the action of protein kinase G. mBio 7:e02011-16. doi: 10.1128/mBio.02011-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, Morgan DO, Tsai LH, Wolgemuth DJ. 2009. Cyclin-dependent kinases: a family portrait. Nat Cell Biol 11:1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modi PK, Komaravelli N, Singh N, Sharma P. 2012. Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Mol Biol Cell 23:3722–3730. doi: 10.1091/mbc.E12-02-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews H, McDonald J, Totanes FIG, Merrick CJ. 2021. Dynamics of DNA replication during male gametogenesis in the malaria parasite Plasmodium falciparum. bioRxiv. 10.1101/2021.12.18.473304. [DOI]
- 39.Robbins JA, Absalon S, Streva VA, Dvorin JD. 2017. The malaria parasite Cyclin H homolog PfCyc1 is required for efficient cytokinesis in blood-stage Plasmodium falciparum. mBio 8:e00605-17. doi: 10.1128/mBio.00605-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eksi S, Williamson KC. 2002. Male-specific expression of the paralog of malaria transmission-blocking target antigen Pfs230, PfB0400w. Mol Biochem Parasitol 122:127–130. doi: 10.1016/s0166-6851(02)00091-9. [DOI] [PubMed] [Google Scholar]
- 41.Tripathi AK, Mlambo G, Kanatani S, Sinnis P, Dimopoulos G. 2020. Plasmodium falciparum gametocyte culture and mosquito infection through artificial membrane feeding. J Vis Exp 3. doi: 10.3791/61426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar S, Leeb AS, Vaughan AM, Kappe SHI. 2022. Plasmodium falciparum cysteine rich secretory protein uniquely localizes to one end of male gametes. Mol Biochem Parasitol 248:111447. doi: 10.1016/j.molbiopara.2022.111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Western blot analysis of PfCRK5 in WT PfNF54 and Pfcrk5− gametocytes, showing absence of PfCRK5 in Pfcrk5− parasites. Actin abundance is shown as the loading control. (B) The Giemsa-stained thin culture smears were prepared for observing mature schizont stages for WT PfNF54 and Pfcrk5− parasites at 1,000×magnifcation. Representative Giemsa-stained images of WT PfNF54 and Pfcrk5− schizonts are shown. (C) The Giemsa-stained thin culture smears were used for quantitative assessment of number of daughter merozoites per schizont for WT PfNF54 and Pfcrk5− parasites. (Duplicate experiments; n = 50 cells per experiment; bars are SD). NS, Not significant. Download FIG S1, TIF file, 0.9 MB (971.5KB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotides used in the study. Download Table S1, DOCX file, 0.02 MB (16KB, docx) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
All other relevant data are available from the authors upon reasonable request.