Abstract
The polymorphonuclear neutrophils (PMNs) of patients infected with human immunodeficiency virus type 1 (HIV-1) show impaired microbicidal responses. The present study assessed the functional integrity of PMN degranulation responses and the expression of specific receptors that mediate these responses in a group of children vertically infected with HIV-1. PMN degranulation in response to interleukin-8 (IL-8) and complement 5a (C5a) was measured in a group of HIV-1-infected children with mild and severe clinical disease and in an uninfected control group. In addition, the expression of CXCR1, CXCR2, and CD88 on whole-blood PMNs was quantified by flow cytometry. Although CXCR1 expression was found to be largely unaltered in the HIV-1-infected children relative to that in the control children, the intensity of CXCR2 expression was significantly reduced in those with severe disease. Furthermore, there was a significant reduction in the percentage of cells expressing CD88 and in the intensity of CD88 fluorescence in the HIV-1-infected children compared to that in control children, with CD88 fluorescence intensity more significantly reduced in the presence of severe disease. PMNs from a large proportion of the HIV-1-infected children either showed reciprocal degranulation responses or were unresponsive to IL-8 and C5a, whereas the PMNs from the uninfected children showed positive responses. Inefficient agonist-induced degranulation may contribute to the increased susceptibility of HIV-1-infected children to secondary microbial infections. Furthermore, reduced expression of CXCR2 and CD88 may be suggestive of defects in other functions of PMNs from HIV-1-infected children.
Polymorphonuclear neutrophils (PMNs) are key effector cells in the nonspecific host defense 33 and kill phagocytosed organisms by oxygen-dependent and oxygen-independent mechanisms. When neutrophils undergo respiratory burst a series of toxic oxygen intermediates are produced, while nonoxidative mechanisms rely on the actions of potent antimicrobial polypeptides contained within cytoplasmic granules 17. A number of these responses are mediated by a variety of molecules, the most important being interleukin-8 (IL-8), leukotriene B4, anaphylatoxin complement 5a (C5a), N-formylmethionyl-leucyl-phenylalanine, and platelet-activating factor. IL-8, a member of the C-X-C chemokine subfamily, has a number of important biologic actions on neutrophils, including the induction of chemotaxis 19, induction of respiratory burst 41, and promotion of the release of lysosomal enzymes. C5a, a potent mediator of neutrophil, basophil, and T-lymphocyte chemotaxis 7, elicits responses in neutrophils similar to those elicited by IL-8 including the promotion of cellular aggregation 5 and the release of secretory constituents such as reactive oxygen intermediates 20 and lysosomal enzymes 2, 8. IL-8 mediates its effects on neutrophils via CXCR1 and CXCR2, which share 77% amino acid identity with the C5a receptor, CD88 13. CXCR1 and CXCR2 have been found to be functionally different, with changes in cytosolic calcium and granule enzyme release being mediated by both receptors, while the activation of phospholipase D and respiratory burst are triggered exclusively via CXCR1 15.
Patients infected with human immunodeficiency virus (HIV) type 1 (HIV-1) display a variety of immune abnormalities, including various defects in the microbicidal responses of phagocytic cells, which could contribute to the impaired host defense against various opportunistic pathogens that characterize AIDS. Functional defects in neutrophils from HIV-1-infected adults have also been observed in a number of studies, and these include defects in phagocytosis 16, 35, chemotaxis 6, 24, 40, oxidative burst 4, 29, 35, bacterial killing 6, 26, and degranulation 23. Furthermore, dysregulation of IL-8 production 18, 22, 38 and the altered expression of both IL-8 receptors 24 have been reported in HIV-1-infected patients.
A number of studies have also been conducted to look at the functional capacities of neutrophils in HIV-1-infected children. Both neutrophil antifungal 31 and bactericidal 32 activities have been reported to be impaired in children infected with HIV-1. Roilides et al. 31 observed reduced neutrophil activity against the hyphae of Aspergillus fumigatus in HIV-1-infected children of various ages. They also found that serum from these children suppressed the antifungal action of neutrophils from uninfected individuals, although incubation with recombinant HIV proteins (gp120, gp41, and p24) did not reduce neutrophil activity. Defective bactericidal activity against Staphylococcus aureus has also been reported 32, although in vitro, this bactericidal defect could be partially reversed by granulocyte-macrophage colony-stimulating factor. In addition, phagocytic cells from HIV-1-infected children have been found to have impaired oxidative burst capacity 4, 10. Neutrophils from HIV-1-infected children incubated with hyperimmune HIV immune globulin have also been shown to have significantly lower antibody-dependent cytotoxicities than neutrophils from healthy children 37.
We have previously shown an impaired IL-8-induced degranulation of PMNs from HIV-1-infected adults, but this was only in part associated with the reduced levels of expression of CXCR1 and CXCR2 23. In addition, results from a study by Wenisch et al. 42 suggested that the inability of neutrophils from HIV-1-infected individuals to kill Candida spp. was likely to be due to an ineffective nonoxidative defense armature. In neonates both PMN production and function are immature. Various studies have demonstrated defective adhesion and chemotaxis 1 of neonatal PMNs, although phagocytosis 36 and oxidative burst 27, 36 were found to be comparable to those found for adult PMNs. To our knowledge, little is known about the integrity of degranulation responses in both HIV-1-infected and HIV-1-uninfected children. The study described here was undertaken to determine the effect of HIV-1 infection on the expression of various receptors which mediate PMN function and to monitor specific receptor-dependent cellular responses. Degranulation of PMNs from a group of HIV-1 infected children and a group of HIV-1-uninfected children in response to two important in vivo agonists, IL-8 and C5a, was therefore measured. In addition, the expression of both IL-8 receptors, CXCR1 and CXCR2, and the receptor for C5a, CD88, on whole blood PMNs was quantified by flow cytometry.
MATERIALS AND METHODS
Patient samples.
A group of children vertically infected with HIV-1 attending Chris Hani Baragwanath Hospital, Johannesburg, South Africa, were enrolled for this study. The children ranged in age from 3 months to 11 years. Two age-matched groups were selected to represent individuals with “severe” and “mild” clinical presentations of HIV-1 disease. Infants grouped in the mild category were well nourished, had no more than two prior hospital admissions for a non-life-threatening event, and had no previous prolonged oxygen requirements (i.e., <48 h). Infants were grouped in the severe category if they had previously had at least one life-threatening HIV-1-related illness (e.g., meningitis or proven sepsis) and/or more than two hospital admissions with HIV-associated conditions (e.g., pneumonia or gastroenteritis), showed a severe failure to thrive, and/or had a previous prolonged oxygen requirement (>48 h). Division of the HIV-1-infected children into the mild disease and the severe disease groups correlated well to standard immunological categories based on CD4 counts. Blood samples were also collected from a control group of 15 age-matched, uninfected children. The immunological characteristics and age distribution of the children are shown in Table 1. Blood was collected into EDTA-containing Vacutainer tubes (Becton Dickinson) and were processed within 6 h of collection. This study was approved by the University of the Witwatersrand Committee for Research on Human Subjects, and informed consent was obtained from the parents or legal guardians of all children enrolled in the study.
TABLE 1.
Immunological characteristics and age distribution of the study groupsa
| Characteristic | Control | Mild | Severe |
|---|---|---|---|
| No. of subjects | 15 | 27 | 25 |
| White cell count (no. of cells [103]/μl) | 9.0 ± 0.7 | 9.6 ± 0.9 | 7.9 ± 0.5 |
| PMNs | |||
| Count (no. of PMNs [103]/μl) | 2.5 ± 0.2 | 3.7 ± 0.5 | 2.8 ± 0.4 |
| Percentage | 28.6 ± 2.8 | 37.8 ± 2.3b | 34.9 ± 2.7 |
| CD4 cells | |||
| Count (no. of cells/μl) by age | 2,180 ± 320 | 1,143 ± 198b | 528 ± 105b |
| 0–24 mo | 2,819 ± 562 (n = 7) | 2,909 ± 304 (n = 6) | 903 ± 214 (n = 8)b |
| 24–60 mo | 1,847 ± 335 (n = 5) | 986 ± 185 (n = 9)b | 448 ± 161 (n = 10)b |
| 60+ mo | 1,244 ± 51 (n = 3) | 436 ± 65 (n = 12)b | 239 ± 55 (n = 7)b |
| Percentage by age | 38.2 ± 1.9 | 22.2 ± 1.8b | 12.6 ± 2.4b |
| 0–24 mo | 40.2 ± 3.2 (n = 7) | 33.9 ± 1.7 (n = 6) | 18.5 ± 5.2 (n = 8)b |
| 24–60 mo | 34.8 ± 0.8 (n = 5) | 25.3 ± 1.4 (n = 9)b | 8.3 ± 2.6 (n = 10)b |
| 60+ mo | 39.4 ± 6.2 (n = 3) | 14.0 ± 2.3 (n = 12)b | 8.9 ± 1.7 (n = 7)b |
| CD8 cells | |||
| Count (no. of cells/μl) | 1,468 ± 213 | 2,058 ± 240 | 2,322 ± 264b |
| Percentage | 26.6 ± 1.7 | 46.2 ± 2.5b | 58.0 ± 2.9b |
| CD4:CD8 ratio | 1.55 ± 0.15 | 0.60 ± 0.09b | 0.26 ± 0.06b |
| HIV viral load (log no. of RNA copies/ml) | 4.57 ± 0.15 | 5.33 ± 0.14 |
Results are expressed as the mean ± standard error of the mean.
Significant differences (P < 0.05) when results for the mild and severe disease groups are compared to those for the control group.
Reagents.
Recombinant human C5a, cytochalasin B, and p-nitrophenyl-β-d-glucuronide were obtained from Sigma Chemical Co. (St. Louis, Mo.). Recombinant human IL-8 was from Boehringer Mannheim (Mannheim, Germany). Fluorescein isothiocyanate (FITC)-conjugated CD88 was obtained from Serotec (Oxford, England). Mouse monoclonal antibodies to CXCR1 (9H1) and CXCR2 (10H2) were supplied by Genentech Inc. (San Francisco, Calif.). Mouse immunoglobulin G1 (IgG1) and IgG2a isotype antibodies from Serotec were used as controls for CXCR1 and CXCR2, respectively. Secondary antibody was FITC-conjugated goat anti-mouse (GAM-FITC) antibody obtained from Dako (Glostrup, Denmark). Fluorescence-activated cell sorter (FACS) lysing solution (10× concentrate) was from Becton Dickinson (San Jose, Calif.).
HIV-1 quantitation.
HIV-1 levels were quantitated in appropriately diluted patient plasma with the Quantiplex HIV RNA branched DNA system (Chiron Diagnostics, East Walpole, Mass.) according to the manufacturer's instructions.
Fluorescent labeling of PMNs in whole blood.
Labeling of cells for CD88 expression was performed by adding 5 μl of FITC-conjugated CD88 antibody to 50 μl of whole blood. As a control, 5 μl of IgG1-FITC was added to 50 μl of whole blood. Samples were incubated with the antibodies for 20 min at room temperature, and the red blood cells were lysed with 2 ml of 1× FACS lysing solution. The cells were then washed and resuspended in 200 μl of fixative, which was 1.5% (vol/vol) formaldehyde containing 2% (wt/vol) bovine serum albumin. Samples were stored at 4°C until analysis.
Labeling of cells with CXCR1 and CXCR2 antibodies was done by an indirect staining method as described previously 24. Briefly, 5 μl of CXCR1 or CXCR2 antibody was added to 50 μl of whole blood final concentration, 2.5 μg/ml. Control antibodies for CXCR1 and CXCR2 staining were 5 μl of mouse IgG1 or IgG2a, respectively, added to 50 μl of whole blood. The samples were then incubated with the antibodies for 20 min at room temperature and washed twice with 3 ml of wash solution. After the samples were washed, 5 μl of the secondary antibody, GAM-FITC, was then added to each of the samples, which were again incubated for 20 min at room temperature. The samples were then washed, the residual erythrocytes were lysed with 2 ml of 1× FACS lysing solution and washed again, and the cells were resuspended in 200 μl of fixative.
Flow cytometry.
All stained samples were acquired (10,000 events each) and analyzed on a Becton Dickinson FACSort flow cytometer with a 488-nm argon laser. Granulocyte populations were gated by using forward light scatter and side light scatter characteristics. Data were analyzed with Cellquest, version 3.1, software (Becton Dickinson) and were expressed as the percentage of cells expressing CXCR1, CXCR2, or CD88 and their respective fluorescence intensities.
Separation of neutrophils.
Anticoagulated whole blood was diluted 1:1 with phosphate-buffered saline (PBS), and the mixture was centrifuged for 30 min at room temperature on a Hypaque-Ficoll gradient. Following removal of the mononuclear cell layer, the remaining PMN-erythrocyte layer was overlaid onto a secondary Ficoll gradient and centrifuged as described above. The remaining steps were all carried out at 4°C. The PMN layer was removed from the second gradient, the residual erythrocytes were lysed with a solution of 0.15 M NH4Cl, 10 mM KHCO3, and 1 mM sodium EDTA (pH 7.2), and the PMNs were washed twice with PBS. The viabilities of the purified PMN suspensions were >98% as determined by trypan blue exclusion, and the cells were immediately used for assay of function.
β-Glucuronidase bioassay.
PMN degranulation in response to C5a and IL-8 was measured by determination of the amount of β-glucuronidase released, as described by Schröder et al. 34. Briefly, the concentration of PMNs was adjusted to 107/ml, and cytochalasin B was added to a final concentration of 5 μg/ml. Aliquots of 100 μl of the cell suspension were placed in a 96-well round-bottom plate, and the plate was incubated for 15 min at 37°C. Human C5a test samples with low (15.63 ng/ml) and high (1,000 ng/ml) input concentrations and human IL-8 test samples with low (15.63 ng/ml) and high (500 ng/ml) input concentrations, each in a total volume of 100 μl, were added to separate wells, and the plate was incubated for a further 30 min at 37°C. The cells were then pelleted at 200 × g for 10 min at 4°C, and 100 μl of the supernatant was transferred to the wells of a 96-well flat-bottom plate containing 100 μl of 0.01 M p-nitrophenyl-β-d-glucuronide in 0.1 M sodium acetate (pH 4.0). The plate was incubated overnight at 20°C, the reaction was stopped with 100 μl of 0.4 M glycine buffer (pH 10), and the absorbance was read at 405 nm. For the determination of the total β-glucuronidase content in PMNs, 5 × 105 cells were lysed in 100 μl of 1% (vol/vol) Triton X-100–PBS. The release of β-glucuronidase at different C5a and IL-8 concentrations was calculated as the optical density at 405 nm obtained at a particular C5a or IL-8 concentration divided by the total optical density at 405 nm of the PMN lysate, expressed as a percentage. Degranulation responses were defined as either positive, negative (reciprocal), or unresponsive, according to the criteria described in Table 2.
TABLE 2.
Frequencies of IL-8- and C5a-induced degranulation responses
| Group and response inducer | No. (%) of subjects
|
||
|---|---|---|---|
| Positive responsesa | Reciprocal responsesb | Unresponsivec | |
| Control (n = 15) | |||
| IL-8 | 15 (100) | 0 (0) | 0 (0) |
| C5a | 14 (93.3) | 0 (0) | 1 (6.6) |
| Mild disease (n = 25) | |||
| IL-8 | 10 (40.0) | 12 (48.0) | 3 (12.0) |
| C5a | 10 (40.0) | 10 (40.0) | 5 (20.0) |
| Severe disease (n = 23) | |||
| IL-8 | 8 (34.8) | 12 (52.2) | 3 (13.0) |
| C5a | 10 (43.5) | 12 (52.2) | 1 (4.3) |
Greater release of β-glucuronidase at the higher input concentration of agonist than at the lower: a normal response.
Greater release of β-glucuronidase at the lower input concentration of agonist than at the higher: a reciprocal response.
No difference in release of β-glucuronidase between the two concentrations of agonist used.
Statistical analysis.
All statistical analyses were performed with SPSS, version 8.0, software (SPSS Inc. Chicago, Ill.). Comparisons of various parameters between the different groups were done by the Mann-Whitney U test. Relationships between data within the control group and the mild and severe HIV-1 disease groups were determined by Spearman's rank correlations. Comparisons of frequencies of the various types of degranulation responses were done by the Kruskal-Wallis H test.
RESULTS
Immunological characteristics of study groups.
HIV-1-infected children with severe disease had significantly reduced CD4 counts, CD4 percentages, and CD4:CD8 ratios and significantly increased CD8 percentages (P < 0.01) and HIV-1 RNA copy numbers than HIV-1-infected children with a milder clinical course (Table 1). The percentage of PMNs, although significantly higher among HIV-1-infected children than among uninfected children, did not differ significantly between those with mild and severe disease (Table 1).
CXCR1, CXCR2, and CD88 expression on whole-blood PMNs.
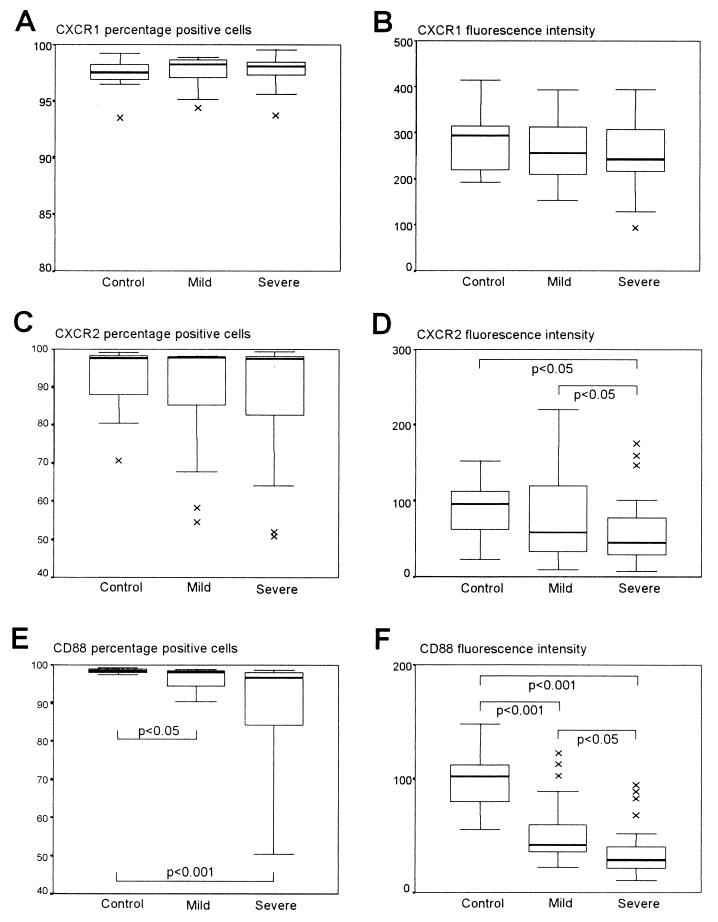
The expression of both IL-8 receptors (CXCR1 and CXCR2) and the C5a receptor (CD88) was determined on whole-blood PMNs by flow cytometry for 15 HIV-1-uninfected control children and 48 HIV-1-infected children, 24 in the mild disease group and 24 in the severe disease group. Figure 1A shows the proportions of PMNs expressing CXCR1, which did not differ between the three groups. The same was true in a comparison of the relative fluorescence intensities of CXCR1 on PMNs (Fig. 1B), although there was a trend toward reduced CXCR1 fluorescence intensity from normal levels in both the mild disease and the severe disease groups. Similar proportions of PMNs in all the groups expressed CXCR2 (Fig. 1C), but the intensity of CXCR2 fluorescence was reduced in the HIV-1-infected children relative to that in the controls and was lower among HIV-1-infected children with severe disease than in HIV-1-infected children with mild disease (Fig. 1D).
FIG. 1.
CXCR1, CXCR2, and CD88 staining of PMNs from control children and HIV-1-infected children in the mild and severe disease groups. PMNs were gated, and the proportion of cells expressing CXCR1 (A), CXCR2 (C), and CD88 (E) were determined. The corresponding relative fluorescence intensities of CXCR1 (B), CXCR2 (D), and CD88 (F) are also shown. Data are presented as medians (horizontal bar), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars). Significant differences between groups are indicated. ×, outlier.
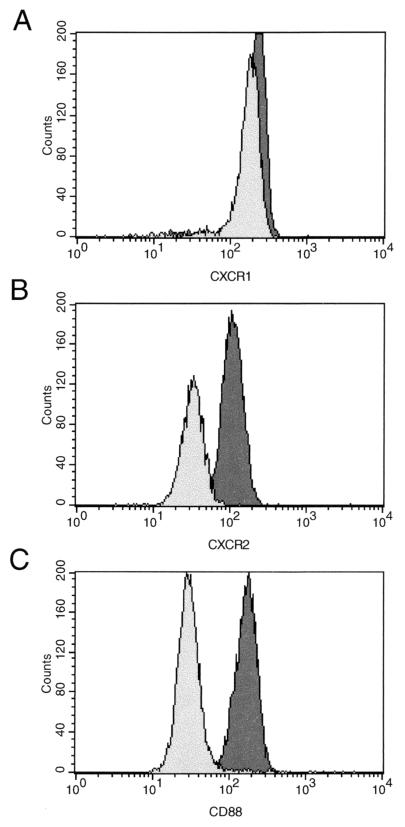
CD88 expression, on the other hand, measured as the proportion of PMNs expressing CD88, was significantly reduced in both the mild (P < 0.05) and the severe (P < 0.001) disease groups compared to that in the control group (Fig. 1E). Similarly, the fluorescence intensity of CD88 was significantly reduced in the mild disease group (P < 0.001) and to a greater extent in the severe disease group (P < 0.001) compared to that in the control group (Fig. 1F), although in this case the severe disease group showed a significantly lower CD88 fluorescence intensity than the mild disease group (P < 0.05). Representative histograms of the data obtained for control and HIV-1-infected children are shown in Fig. 2.
FIG. 2.
Histograms showing comparative fluorescent staining of CXCR1 (A), CXCR2 (B), and CD88 (C) on PMNs from representative control (dark shading) and HIV-1-infected (light shading) children.
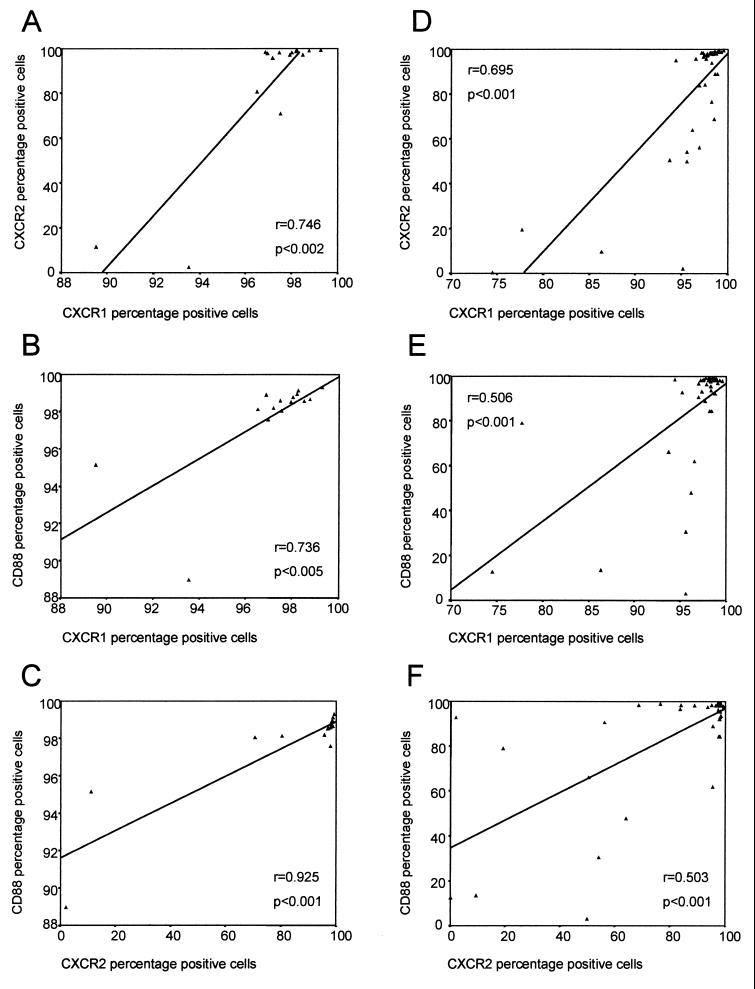
In addition to looking at differences in the levels of expression of these receptors between the study groups, we also compared the levels of expression of the different receptors relative to each other. Figure 3 shows the associations observed between the expression of these receptors on PMNs for the control group and the HIV-1-infected children (mild and severe disease groups combined). For the control uninfected children, there was a very strong association between the percentage of PMNs expressing CXCR1 and the proportion of PMNs expressing CXCR2 (r = 0.746; P < 0.002) (Fig. 3A), as might be expected. In addition, however, the proportion of PMNs expressing CD88 also strongly correlated to the proportion of PMNs expressing CXCR1 (r = 0.736; P < 0.005) (Fig. 3B) and especially CXCR2 (r = 0.925; P < 0.001) (Fig. 3C). These associations, although less strongly correlated, were also observed in the HIV-1-infected children (mild and severe disease groups combined), in whom the percentage of CXCR1-expressing PMNs correlated with the proportion of PMNs expressing CXCR2 (r = 0.695; P < 0.001) (Fig. 3D), while the proportion of PMNs expressing CD88 also correlated to the percentage of cells expressing both CXCR1 (r = 0.506; P < 0.001) (Fig. 3E) and CXCR2 (r = 0.503; P < 0.001) (Fig. 3F). Similar associations were found if the data for the HIV-1-infected children in the mild and severe disease groups were analyzed separately. CXCR1 fluorescence intensity also correlated with CXCR2 fluorescence intensity in the uninfected control group (r = 0.586; P < 0.05), the mild disease group (r = 0.636; P < 0.002), and the severe disease group (r = 0.502; P < 0.05). No significant correlations were found between the CD88 fluorescence intensity and the fluorescence intensity of either of the IL-8 receptors in any of the groups studied (P > 0.05).
FIG. 3.
Relationships between the proportions of PMNs expressing CXCR1, CXCR2, and CD88 for the uninfected controls (panels A, B, and C, respectively) and the HIV-1-infected children (panels D, E, and F, respectively).
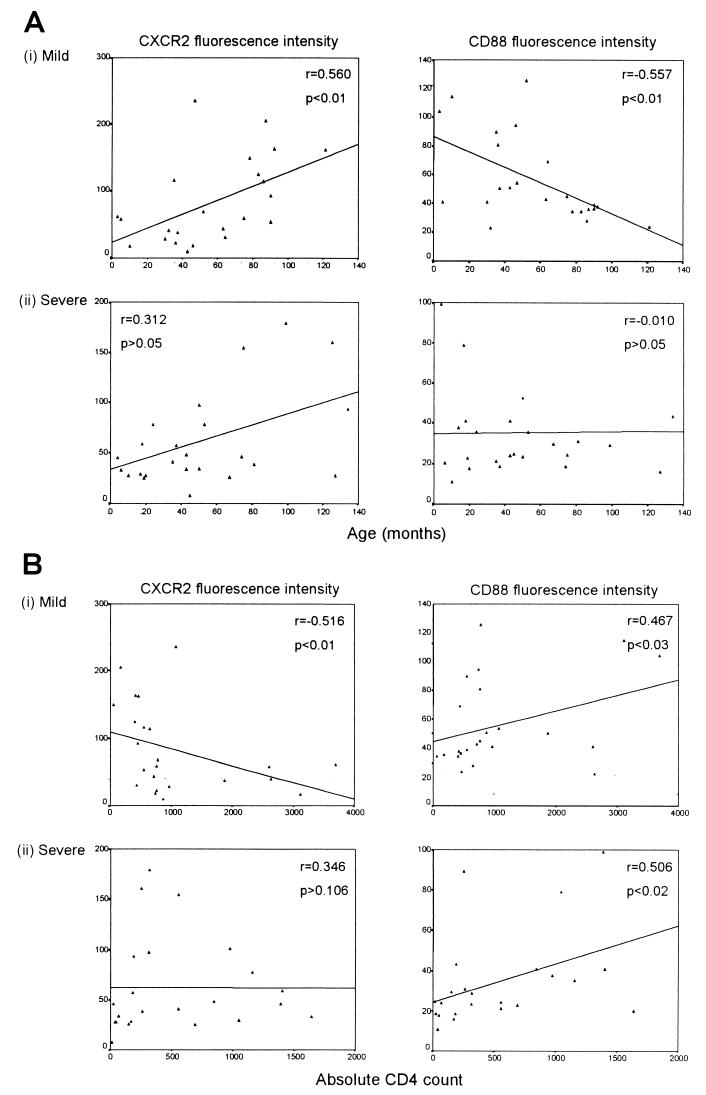
Relationship between receptor expression and age and CD4 count of the child.
The fluorescence intensities of CXCR1 and CXCR2 on PMNs increased with age and decreased with CD4 count among all three groups studied. CD88 fluorescence intensity, however, did not show these trends but, rather, tended to decrease with age and increase with CD4 counts. Representative data showing the associations between the fluorescence intensities of CXCR2 and CD88 and the age of the child (Fig. 4A) and CD4 count (Fig. 4B) for the mild and severe disease groups are shown. Data for the control uninfected children showed similar trends, but the associations found were not significant (data not shown).
FIG. 4.
Relationships between the fluorescence intensities of CXCR2 and CD88 and corresponding age (A) and CD4 counts (B) of the HIV-1-infected children in the mild and severe disease groups.
PMN degranulation in response to IL-8 and C5a.
PMN degranulation was evaluated in 15 HIV-1-uninfected children, 25 HIV-1-infected children with mild disease, and 23 HIV-1-infected children with severe disease. PMN degranulation in response to both IL-8 and C5a, each at a high and low concentration, was measured by quantitating the release of β-glucuronidase, an enzyme contained within PMN azurophilic granules. Table 2 shows the frequencies of IL-8- and C5a-induced degranulation responses for the different study groups. All the uninfected control children were found to have normal positive degranulation responses to IL-8, while 14 of 15 showed a positive response to C5a, with PMNs from 1 control child being unresponsive. In the mild disease group, however, the frequency of positive responses was substantially reduced, with more than half of the children in this group showing a reciprocal response or being unresponsive to both IL-8 and C5a. The frequency of positive degranulation responses in the mild disease group was found to be significantly less than that observed in the control group for both IL-8 (P < 0.001) and C5a (P < 0.005). Among the HIV-1-infected children with severe disease the proportion with impaired PMN degranulation responses was even greater, with the frequencies of positive responses being significantly lower than those for the control group for both IL-8 (P < 0.001) and C5a (P < 0.005). The number of positive responses to C5a was the same for the mild and severe disease groups, whereas the severe disease group had a lower proportion of positive responders to IL-8 than the mild disease group (8 versus 10 children, respectively).
We further measured the abilities of PMNs to degranulate spontaneously in the absence of any stimulus, and we found that there was a trend toward an increased spontaneous release of β-glucuronidase from the PMNs of children in the mild and severe disease groups compared to that for PMNs from children in the control group (P > 0.05) (data not shown).
Relationship between degranulation responses and receptor expression.
Since IL-8 mediates its activities via CXCR1 and CXCR2 and since C5a mediates its activities through CD88, we compared the levels of expression of these receptors to the response induced by a particular concentration of either agonist. For the control group, the amount of β-glucuronidase released at the low input concentration of IL-8 (15.63 ng/ml) correlated negatively with the percentage of PMNs expressing both CXCR1 (r = −0.529; P < 0.05) and CXCR2 (r = −0.646; P < 0.01). In addition, the release of β-glucuronidase induced by 1,000 ng of C5a per ml correlated with the CD88 fluorescence intensity (r = 0.524; P < 0.05). For the mild and severe disease groups, however, no significant associations were found between receptor expression and the magnitude of degranulation responses at either input concentration of agonist. In addition, when we looked at the types of responses in the HIV-1-infected children, we observed that PMNs from those children who had reciprocal or unresponsive IL-8- or C5a-induced degranulation responses expressed similar levels of the IL-8 receptors or CD88 compared to the levels of expression of IL-8 receptors and CD88 on PMNs from children with positive responses. For all three groups, IL-8-induced degranulation responses were found to be very strongly correlated to the responses induced by C5a for both magnitude and type of response (P < 0.001).
DISCUSSION
It is widely accepted that the defective functioning of phagocytic cells in HIV-1-infected individuals, particularly children, may contribute to the increased risk of serious bacterial and fungal infections. In this study we have shown that PMNs from children vertically infected with HIV-1 have a significantly altered expression of CXCR2 and CD88 but not of CXCR1 compared to the expression of CXCR1, CXCR2, and CD88 on PMNs from a group of HIV-1-uninfected children. Although there was a trend toward a reduced fluorescence intensity of CXCR1 in the mild and severe disease groups compared to that in the control group of children, this did not attain significance. CXCR2 expression on PMNs was, however, found to be significantly reduced in the HIV-1-infected children with a severe clinical course. We have previously shown a reduced expression of both CXCR1 and CXCR2 on PMNs from HIV-1-infected adults 24. We had observed that both the proportion of PMNs as well as the relative fluorescence intensities of CXCR1 and CXCR2 were significantly reduced in HIV-1-infected patients compared to the intensities for uninfected controls. These reductions were found to be present, irrespective of the patients' immunological status, as determined from their CD4 cell counts. Similarly, in our current study, the HIV-1-infected children, divided into those with mild disease and those with severe disease on the basis of clinical and immunological observations, showed no differences in CXCR1 expression when the levels of expression for the two groups were compared to each other. However, the fluorescence intensity of CXCR2 was found to be significantly reduced in children with severe HIV-1 disease than in those with a milder clinical course, indicating that expression of CXCR2 in children may be linked to disease progression. However, the observation that only CXCR2 fluorescence intensity and not proportions of cells is reduced in the HIV-1-infected children indicates that infection with HIV-1 may play a lesser role in the overall modulation of CXCR2, and especially that of CXCR1, in children than it does in adults.
The expression of the receptor for the classical chemoattractant C5a, CD88, was quantified in parallel to the expression of the IL-8 receptors on PMNs. Both the proportion of PMNs expressing CD88 and fluorescence intensities were found to be significantly reduced in the mild disease group and were found to be reduced even more in the severe disease group compared to those for the uninfected controls, thereby providing another possible indicator of disease severity. Monari et al. 25 have recently demonstrated a significant reduction of CD88 expression on PMNs from adults with late-stage HIV-1 infection (<200 CD4 cells/μl). Comparable levels of CD88 expression on PMNs were, however, observed in patients with early-stage HIV-1 disease (>400 CD4 cells/μl) and uninfected controls. They hypothesize that this reduced level of expression of CD88 would render PMNs unable to respond to C5a, resulting in the impairment of various PMN functions. Other studies have indicated the importance of CD88 for host defense in the lung, where CD88-expressing neutrophils mediate clearance of mucosal bacterial organisms. Höpken et al. 14 showed that mice deficient in the C5a receptor were unable to clear Pseudomonas aeruginosa given intratracheally and succumbed to pneumonia, whereas wild-type littermates rapidly cleared the bacterial burden.
Both the IL-8 receptors and CD88 belong to the family of seven transmembrane-spanning G-coupled protein receptors. Quantification of the expression of all these receptors on the same samples has allowed us to demonstrate a very strong association between the modulation of CD88 expression and that of CXCR1 and CXCR2 expression, which has not previously been described. We have observed that the proportion of PMNs expressing CD88 correlated with the percentage of cells expressing CXCR1 and CXCR2 in all three groups studied. Other studies have demonstrated interactions between these receptors on PMNs. A study that used scanning electron microscopy to detect immunogold-labeled CD88 and IL-8 receptors showed that these receptor populations are expressed in clusters on nonprojecting domains on the PMN membrane 9. A unidirectional heterologous receptor desensitization between CD88 and the IL-8 receptors has been reported by Blackwood et al. 3, in which prestimulation of PMNs with C5a resulted in a significant reduction in chemotaxis of these cells toward IL-8.
We further found that the expression of these receptors on PMNs is related to both the age and the CD4 percentage of the child. CXCR1 and CXCR2 were found to be modulated in the same way, with receptor density (fluorescence intensity) increasing with increasing age of the child. In contrast, however, the intensity of CD88 expression decreased with age. These results may indicate that in younger children CD88 may be a critical receptor in mediating PMN function, but over time, as the immune system matures and develops, other receptors such as the IL-8 receptors may become increasingly expressed and become more involved in PMN functioning and so compensate for the reduced level of CD88 expression.
Our previous work has demonstrated that the altered expression of CXCR1 and CXCR2 can in part be associated with impaired IL-8-induced PMN functions, including chemotaxis, calcium mobilization 24, and degranulation 23. Therefore, in addition to quantifying the expression of CXCR1, CXCR2, and CD88 on PMNs we also assayed for IL-8- and C5a-induced degranulation in order to determine if functions dependent on sufficient receptor expression were also impaired in children. Adult HIV-1-infected patients had a reciprocal degranulation response to IL-8 compared to the response of uninfected persons, in that increasing IL-8 concentrations resulted in decreased levels of enzyme release. Similarly, in this study, a large proportion of the HIV-1-infected children had either reciprocal degranulation responses or were unresponsive to IL-8 and C5a. This was especially apparent in the severe disease group compared to the response of those in the mild disease group, suggesting an association with the severity of clinical disease. Studies carried out with two monoclonal antibodies raised against both IL-8 receptors of human PMNs have indicated that CXCR1 and CXCR2 are functionally different 15, although both can signal independently for enzyme release from the granule. The reciprocal degranulation responses observed in the HIV-1-infected children in this study could not, however, be associated with a reduced level of expression of either CXCR1 or CXCR2, indicating that a number of factors which result in impaired responses are probably involved 39. Although we observed that HIV-1-infected adults whose PMNs had the poorest ability to degranulate were also those who had the lowest density of CXCR1 on their PMNs 23, this could not explain the impaired IL-8-induced degranulation responses in the HIV-1-infected children in this study, since CXCR1 expression was largely unaltered in the groups with HIV-1 disease. Several lines of evidence suggest that PMNs from HIV-1-infected individuals are primed in vivo; that is, they have been stimulated by exposure to various proinflammatory cytokines, HIV-1 proteins, or circulating bacterial products. The altered expression of surface makers such as FcγRIII (CD16) 21 and CD11b 28, enhanced apoptosis of PMNs upon isolation 30, and enhanced phagocytosis of Escherichia coli 35 and Candida spp. 42 are all suggestive of a primed PMN phenotype in individuals infected with HIV-1. Further support is provided by an increased spontaneous exocytosis of PMN granules from HIV-1-infected individuals 23, which has been confirmed for the children in this study. In vitro, PMNs which have been desensitized by prestimulation have also been found to be unable to generate oxygen intermediates and to degranulate when induced 11, 12. Taken together, PMNs primed in vivo in HIV-1-infected individuals are likely to have reduced responsiveness when they are subsequently activated, which could provide an explanation for the results presented here.
In conclusion, this study has shown that PMN degranulation in HIV-1-infected children is impaired and that the expression of various receptors which mediate a number of essential PMN functions is altered. Since PMNs play such a vital role in the clearance of invasive organisms, these changes may be an important explanation for why HIV-1-infected children are more susceptible to secondary infections than their uninfected counterparts. As impairments in degranulation are often apparent early in HIV-1 infection, monitoring of this cell function as a measure of the recovery of important innate immune functions may be important for HIV-1-infected children treated with antiretroviral drugs.
ACKNOWLEDGMENTS
CXCR1- and CXCR2-specific monoclonal antibodies were kindly supplied by A. Chuntharapai and K. Jin Kim from the Department of Bioanalytical Technology, Genentech Inc. We thank Karin Simmank for help with the collection of blood specimens and clinical data.
REFERENCES
- 1.Anderson D C, Hughes B J, Smith C W. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Investig. 1981;68:863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker E L, Showell H J. The ability of chemotactic factors to induce lysosomal enzyme release. II. The mechanism of release. J Immunol. 1974;112:2055–2062. [PubMed] [Google Scholar]
- 3.Blackwood R A, Hartiala K T, Kwoh E E, Transue A T, Brower R C. Unidirectional heterologous receptor desensitization between both the fMLP and C5a receptor and the IL-8 receptor. J Leukoc Biol. 1996;60:88–93. doi: 10.1002/jlb.60.1.88. [DOI] [PubMed] [Google Scholar]
- 4.Chen T P, Roberts R L, Wu K G, Ank B J, Stiehm E R. Decreased superoxide anion and hydrogen peroxide production by neutrophils and monocytes in human immunodeficiency virus-infected children and adults. Pediatr Res. 1993;34:544–550. doi: 10.1203/00006450-199310000-00032. [DOI] [PubMed] [Google Scholar]
- 5.Damerau B, Grunefeld E, Vogt W. Aggregation of leukocytes induced by the complement-derived peptides C3a and C5a and by three synthetic formyl-methionyl peptides. Int Arch Allergy Appl Immunol. 1980;63:159–169. doi: 10.1159/000232622. [DOI] [PubMed] [Google Scholar]
- 6.Ellis M, Gupta S, Galant S, Hakim S, VandeVen C, Toy C, Cairo M S. Impaired neutrophil function in patients with AIDS or AIDS-related complex: a comprehensive evaluation. J Infect Dis. 1988;158:1268–1276. doi: 10.1093/infdis/158.6.1268. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez H N, Henson P M, Otani A, Hugli T E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978;120:109–115. [PubMed] [Google Scholar]
- 8.Goldstein I M, Weissmann G. Generation of C5-derived lysosomal enzyme-releasing activity (C5a) by lysates of leukocyte lysosomes. J Immunol. 1974;113:1583–1588. [PubMed] [Google Scholar]
- 9.Gray G D, Hasslen S R, Ember J A, Carney D F, Herron M J, Erlandsen S L, Nelson R D. Receptors for the chemoattractants C5a and IL-8 are clustered on the surface of human neutrophils. J Histochem Cytochem. 1997;45:1461–1467. doi: 10.1177/002215549704501103. [DOI] [PubMed] [Google Scholar]
- 10.Hayani K C, Verral S C, Pitrak D L. Impaired phagocyte oxidative capacity in human immunodeficiency virus-infected children. J Infect Dis. 1999;179:584–589. doi: 10.1086/314622. [DOI] [PubMed] [Google Scholar]
- 11.Henson P M, Schwartzman N A, Zanolari B. Intracellular control of human neutrophil secretion. II. Stimulus specificity of desensitization induced by six different soluble and particulate stimuli. J Immunol. 1981;127:754–759. [PubMed] [Google Scholar]
- 12.Henson P M, Zanolari B, Schwartzman N A, Hong S R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978;121:851–855. [PubMed] [Google Scholar]
- 13.Holmes W E, Lee J, Kuang W J, Rice G C, Wood W I. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 14.Höpken U E, Lu B, Gerard N P, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 15.Jones S A, Wolf M, Qin S, Mackay C R, Baggiolini M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci USA. 1996;93:6682–6686. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazzarin A, Uberti Foppa C, Galli M, Mantovani A, Poli G, Franzetti F, Novati R. Impairment of polymorphonuclear leucocyte function in patients with acquired immunodeficiency syndrome and with lymphadenopathy syndrome. Clin Exp Immunol. 1986;65:105–111. [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrer R I, Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990;76:2169–2181. [PubMed] [Google Scholar]
- 18.Matsumoto T, Miike T, Nelson R P, Trudeau W L, Lockey R F, Yodoi J. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol. 1993;93:149–151. doi: 10.1111/j.1365-2249.1993.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushima K, Oppenheim J J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989;1:2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- 20.McPhail L C, Snyderman R. Activation of the respiratory burst enzyme in human polymorphonuclear leukocytes by chemoattractants and other soluble stimuli. Evidence that the same oxidase is activated by different transductional mechanisms. J Clin Investig. 1983;72:192–200. doi: 10.1172/JCI110957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meddows-Taylor S, Martin D J, Tiemessen C T. Altered expression of FcγRIII (CD16) on polymorphonuclear neutrophils from individuals with human immunodeficiency virus type 1 disease and pulmonary tuberculosis. Clin Diagn Lab Immunol. 1997;4:789–791. doi: 10.1128/cdli.4.6.789-791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meddows-Taylor S, Martin D J, Tiemessen C T. Dysregulated production of interleukin-8 in individuals infected with human immunodeficiency virus type 1 and Mycobacterium tuberculosis. Infect Immun. 1999;67:1251–1260. doi: 10.1128/iai.67.3.1251-1260.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meddows-Taylor S, Martin D J, Tiemessen C T. Impaired interleukin-8-induced degranulation of polymorphonuclear neutrophils from human immunodeficiency virus type 1-infected individuals. Clin Diagn Lab Immunol. 1999;6:345–351. doi: 10.1128/cdli.6.3.345-351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meddows-Taylor S, Martin D J, Tiemessen C T. Reduced expression of interleukin-8 receptors A and B on polymorphonuclear neutrophils from persons with human immunodeficiency virus type 1 disease and pulmonary tuberculosis. J Infect Dis. 1998;177:921–930. doi: 10.1086/515232. [DOI] [PubMed] [Google Scholar]
- 25.Monari C, Casadevall A, Pietrella D, Bistoni F, Vecchiarelli A. Neutrophils from patients with advanced human immunodeficiency virus infection have impaired complement receptor function and preserved Fcgamma receptor function. J Infect Dis. 1999;180:1542–1549. doi: 10.1086/315099. [DOI] [PubMed] [Google Scholar]
- 26.Murphy P M, Lane H C, Fauci A S, Gallin J I. Impairment of neutrophil bactericidal capacity in patients with AIDS. J Infect Dis. 1988;158:627–630. doi: 10.1093/infdis/158.3.627. [DOI] [PubMed] [Google Scholar]
- 27.Newburger P E. Superoxide generation by human fetal granulocytes. Pediatr Res. 1982;16:373–376. doi: 10.1203/00006450-198205000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Palmer S, Hamblin A S. Increased CD11/CD18 expression on the peripheral blood leucocytes of patients with HIV disease: relationship to disease severity. Clin Exp Immunol. 1993;93:344–349. doi: 10.1111/j.1365-2249.1993.tb08183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitrak D L, Bak P M, DeMarais P, Novak R M, Andersen B R. Depressed neutrophil superoxide production in human immunodeficiency virus infection. J Infect Dis. 1993;167:1406–1410. doi: 10.1093/infdis/167.6.1406. [DOI] [PubMed] [Google Scholar]
- 30.Pitrak D L, Tsai H C, Mullane K M, Sutton S H, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J Clin Investig. 1996;98:2714–2719. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roilides E, Holmes A, Blake C, Pizzo P A, Walsh T J. Impairment of neutrophil antifungal activity against hyphae of Aspergillus fumigatus in children infected with human immunodeficiency virus. J Infect Dis. 1993;167:905–911. doi: 10.1093/infdis/167.4.905. [DOI] [PubMed] [Google Scholar]
- 32.Roilides E, Mertins S, Eddy J, Walsh T J, Pizzo P A, Rubin M. Impairment of neutrophil chemotactic and bactericidal function in children infected with human immunodeficiency virus type 1 and partial reversal after in vitro exposure to granulocyte-macrophage colony-stimulating factor. J Pediatr. 1990;117:531–540. doi: 10.1016/s0022-3476(05)80684-5. [DOI] [PubMed] [Google Scholar]
- 33.Sandborg R R, Smolen J E. Early biochemical events in leukocyte activation. Lab Investig. 1988;59:300–320. [PubMed] [Google Scholar]
- 34.Schröder J M, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]
- 35.Shalekoff S, Tiemessen C T, Gray C M, Martin D J. Depressed phagocytosis and oxidative burst in polymorphonuclear leukocytes from individuals with pulmonary tuberculosis with or without human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1998;5:41–44. doi: 10.1128/cdli.5.1.41-44.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speer C, Johnston R B. Neutrophil function in newborn infants. In: Polin R A, Fox W W, editors. Fetal and neonatal physiology. 2nd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1997. pp. 1954–1960. [Google Scholar]
- 37.Szelc C M, Mitcheltree C, Roberts R L, Stiehm E R. Deficient polymorphonuclear cell and mononuclear cell antibody-dependent cellular cytotoxicity in pediatric and adult human immunodeficiency virus infection. J Infect Dis. 1992;166:486–493. doi: 10.1093/infdis/166.3.486. [DOI] [PubMed] [Google Scholar]
- 38.Thea D M, Porat R, Nagimbi K, Baangi M, St. Louis M E, Kaplan G, Dinarello C A, Keusch G T. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med. 1996;124:757–762. doi: 10.7326/0003-4819-124-8-199604150-00009. [DOI] [PubMed] [Google Scholar]
- 39.Tiemessen C T, Meddows-Taylor S, Shalekoff S, Martin D J. Impairment of neutrophil function contributes to increased morbidity and mortality in HIV-1 and Mycobacterium tuberculosis co-infection. S Afr J Sci. 2000;96:328–334. [Google Scholar]
- 40.Valone F H, Payan D G, Abrams D I, Goetzl E J. Defective polymorphonuclear leukocyte chemotaxis in homosexual men with persistent lymph node syndrome. J Infect Dis. 1984;150:267–271. doi: 10.1093/infdis/150.2.267. [DOI] [PubMed] [Google Scholar]
- 41.Walz A, Meloni F, Clark-Lewis I, von Tscharner V, Baggiolini M. [Ca2+] changes and respiratory burst in human neutrophils and monocytes induced by NAP-1/interleukin-8, NAP-2, and gro/MGSA. J Leukoc Biol. 1991;50:279–286. doi: 10.1002/jlb.50.3.279. [DOI] [PubMed] [Google Scholar]
- 42.Wenisch C, Parschalk B, Zedwitz-Liebenstein K, Graninger W, Rieger A. Dysregulation of the polymorphonuclear leukocyte—Candida spp. interaction in HIV-positive patients. AIDS. 1996;10:983–987. doi: 10.1097/00002030-199610090-00008. [DOI] [PubMed] [Google Scholar]