Abstract
Lactobacilli play an important role in maintaining vaginal health. However, during bacterial vaginosis lactobacilli decrease for unknown reasons. Our preliminary study showed that phages could infect vaginal lactobacilli. Therefore, the aim of this study was to analyze the distribution, virulence, and types of vaginal Lactobacillus phages isolated from women of two countries: the United States and Turkey. A total of 209 vaginal lactobacilli were isolated from reproductive-aged women in the United States (n = 107) and Turkey (n = 102). By analysis of 16S rRNA gene sequence and by comparison of protein profiles, most lactobacilli were identified as L. crispatus, L. gasseri, and L. jensenii. After mitomycin C induction, 28% of American lactobacilli and 36% of Turkish lactobacilli released phages. A total of 67 phages were isolated and further characterized by their host range, electron microscopy, and DNA homology. All 67 phages were infective against lactobacilli from both collections. The host ranges of most phages were broad, including multiple Lactobacillus species. Even though the phages were all temperate, they were able to cause lytic infection in various strains. The electron micrographs of these phages showed a hexagon-shaped head and a long tail with or without a contractile tail sheath. Based on their morphology, these phages belonged to Bradley's phage groups A and B, and could be further classified into four morphotypes. All four types were found among American phages, but only three were found among Turkish isolates. DNA hybridization with labeled probes of the four types of phages revealed that additional genetic types existed within each morphotype among these phages. The phage genomic sizes ranged between 34 and 55 kb. Many of the lysogenic Lactobacillus strains released phages spontaneously at a high frequency of 10−3 to 10−4 PFU/cell. In conclusion, lysogeny in vaginal lactobacilli is widely spread. Some lysogenic lactobacilli spontaneously release phages with a broad host range, which can be lytic against other vaginal lactobacilli regardless of their geographic origin.
Lactobacilli indigenous to the human vagina are beneficial to women's health 35. These bacteria can inhibit other potentially harmful microorganisms by producing lactic acid, hydrogen peroxide (H2O2), and antimicrobial substances 12, 23, 43. In most healthy women, lactobacilli are the dominant species in the vagina. Theoretically, the anaerobic bacteria are suppressed by lactobacilli 12, 23 and cannot replace lactobacilli unless the latter is first diminished. However, the group of anaerobic bacteria commonly outnumber lactobacilli, causing a microbial imbalance called bacterial vaginosis (BV) 3, 9, 10, 15, 38, 40.
BV is a clinical condition that is characterized by decreased lactobacilli and an increased number of anaerobic gram-negative rods, Gardnerella species, and genital mycoplasmas 10, 38, 40. Women who suffer from BV may have an increased discharge that often has an unpleasant fishy odor. BV has been associated with many health risks, including preterm birth of low-birth-weight infants, midtrimester pregnancy loss, amniotic fluid infection, postpartum endometritis, pelvic inflammatory disease, and gynecologic postoperative infections 14, 16, 17, 28, 29. Recently, a lack of vaginal lactobacilli or the presence of BV was found to promote human immunodeficiency virus transmission 8, 27, 37.
The cause of BV is currently unknown, and it is unclear what causes the decrease of vaginal lactobacilli. Several possible mechanisms by which vaginal lactobacilli decrease have been proposed. These include douching 13; the use of spermicide, such as nonoxynol-9 18; and treatment with antibiotics for other infections. It is important to examine the possibility that vaginal lactobacilli may decrease due to natural causes, such as phages or viruses.
Lactobacillus phages have been isolated from various sources, including dairy products 22, sausage 30, human intestines 34, and sewage 24. Recently, we reported the isolation of phages from human vaginal lactobacilli and documented their infectivity in vitro against lactobacilli isolated from the same and/or different women 32, 41. This suggested that reduction of vaginal lactobacilli may be caused by phages. It is important to further study and characterize these phages. In this study, we analyzed 67 vaginal Lactobacillus phages isolated from women in the United States and in Turkey based on their morphology, host range, spontaneous induction rate, DNA homology, and prevalence.
MATERIALS AND METHODS
Bacterial strains and growth media.
Vaginal samples were obtained from reproductive-aged women visiting obstetrics and gynecology clinics at the Truman Medical Center in Kansas City, Mo., and at the medical schools of Karadeniz Technical University, Trabzon, Turkey, and Firat University, Elazig, Turkey. These included healthy women and women with vaginal infections, such as BV and candidiasis. Both the Amsel criteria 3 and Nugent scoring system 31 were used for diagnosis of vaginosis. Vaginal pH was measured with pH paper (Fisher Scientific). Microscopic examination of the Gram-stained vaginal sample slides was used to confirm the initial clinical diagnosis. During sampling, two sterile cotton swabs were inserted into the vagina, rotated a few turns along the vaginal sidewall, and allowed to absorb for a few seconds before being withdrawn. One swab was used for Gram staining. The other swab was placed into a test tube containing the RTF-glycerol transport buffer and sent to the laboratory for analysis. The transport buffer included (wt/vol) 0.045% K2HPO4, 0.045% KH2PO4, 0.09% NaCl, 0.09% (NH4)SO4, 0.018% MgSO4(or MgCl2), 0.038% EDTA, 0.04%Na2CO3, 0.02% dithiothreitol, and 10% glycerol. Samples were either analyzed immediately or kept at −20°C for several weeks before processing. To isolate lactobacilli, the samples were streaked onto Lactobacillus Rogosa (Difco, Detroit, Mich.) agar plates (pH 5.2) and incubated at 37°C for 48 h under anaerobic conditions. The MRS medium (Difco) was subsequently used to grow lactobacilli. Lactobacilli were initially identified by their ability to grow on the selective Rogosa agar, gram-positive staining, rod shape, and catalase-negative phenotype. Purified cultures were maintained at −80°C in MRS broth with 10% glycerol. Biochemical analyses, including sugar fermentation profile and gas production in MRS broth, were conducted as described in Bergey's Manual of Systematic Bacteriology 21. Lactobacillus type strains used in the study included Lactobacillus acidophilus ATCC 4356 and 4357, Lactobacillus brevis ATCC 14869, Lactobacillus buchneri ATCC 4005, Lactobacillus casei subsp. casei ATCC 393 and 27139, Lactobacillus crispatus ATCC 33197 and 33820, Lactobacillus fermentum ATCC 14931 and 23271, Lactobacillus gasseri ATCC 9857, Lactobacillus jensenii ATCC 25258, Lactobacillus johnsonii ATCC 33220, Lactobacillus plantarum ATCC 8014 and 14917, Lactobacillus reuteri ATCC 23272, Lactobacillus rhamnosus ATCC 7489, Lactobacillus ruminis ATCC 25644, Lactobacillus salivarius subsp. salivarius ATCC 11741, and Lactobacillus vaginalis ATCC 49540.
Whole-cell protein analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of whole-cell proteins of lactobacilli was performed to help identify bacterial species. Approximately 50 mg of cells (wet weight)/ml was lysed by boiling in SDS sample buffer 25 for 10 min and then centrifuged at 10,000 × g for 15 min to remove any precipitates. The gel system of Laemmli 25 was used. Proteins were visualized by staining with Coomassie blue. Marker proteins were obtained from Sigma (St. Louis, Mo.).
16S rRNA gene sequence analysis.
The extraction of the genomic DNA of lactobacilli was performed as described by Chassy et al. 6. The amplification of the 16S ribosomal DNA (rDNA) by PCR and the determination of the sequences were described previously (Pavlova et al., Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. C-94, 2000). Analysis of genes encoding 16S rRNA of vaginal lactobacilli from women in different countries reveals multiple novel species (unpublished data). The sequences were used for comparison with data from GenBank.
Phage induction.
Mitomycin C (Sigma) was used to induce phages from vaginal lactobacilli as previously described 22, 32. The induction of Lactobacillus prophages was indicated by the lysis of a Lactobacillus culture 4 to 7 h after the addition of mitomycin C. These lysates were then centrifuged, filtered through a 0.45-μm-pore-size filter, and maintained at 4°C with a drop of chloroform.
Spontaneous phage induction.
Each lysogenic vaginal Lactobacillus strain was grown in 2 ml of MRS broth to mid-exponential phase without mitomycin C treatment. One milliliter of the culture was diluted and plated on MRS agar plates for cell count. Another 1 ml was centrifuged to harvest the supernatant, which was filtered through a sterile 0.45-μm-pore-size filter. The supernatant was diluted and used to infect its indicator strain by the soft-agar overlay method as described before 32. Plaques were enumerated after 24 h of incubation at 37°C. The frequency of spontaneous phage induction was calculated as the total number of phage plaques per milliliter of culture divided by the number of CFU and the burst size of the phage, which was calculated by one-step growth curves as described before 22, 32.
Phage infectivity assay.
Phage infectivity was determined by the agar spot method as previously described 32. All of the 67 phages were used to infect the two collections of vaginal Lactobacillus strains of a total of 209 isolates. The positive results were verified by single plaque formation.
Electron microscopy.
One drop of the purified phage in 0.1 M ammonium acetate (pH 7.0) was spotted on grids with a carbon-coated Formvar film (Ladd Research Industry, Burlington, Vt.). After drying for 30 s, the sample was negatively stained with 2% uranyl acetate (pH 4.2). Electron microscopy was performed with the CM12 transmission electron microscope (Philips Electronic Instruments, Inc., Mahwah, N.J.) at 80 kV.
Phage DNA isolation and restriction analysis.
The Lactobacillus phages were purified from 1 liter of mitomycin-induced lysate by a procedure described by Maniatis et al. 26. The phage DNA was extracted with the QIAGEN (Chatsworth, Calif.) lambda phage DNA isolation kit. Restriction enzyme (EcoRI) digests of the phage DNA were subjected to gel electrophoresis on a 0.8% agarose gel at 40 V for 3 h. The gel was stained with ethidium bromide and photographed under a UV light.
Phage genomic DNA hybridization.
The genomic DNA from representative phages was isolated and labeled with the nonradioactive biotinylated labeling kit from GIBCO-BRL as probes (Life Technologies, Inc., Rockville, Md.). The DNA from target phages was processed by two methods. The first method was to digest the DNA with restriction enzymes. The digested DNA was then subjected to agarose gel electrophoresis and Southern hybridization with the labeled probes. The second method was to perform a simple dot hybridization with undigested DNA.
Phage classification by PCR.
To obtain sequence data for the PCR analysis, the genomic DNA of four phages representing each morphotype was digested with Sau3A1. The digested DNA fragments were cloned into the pUC18 plasmid. A pUC18 plasmid that carries a random insert of about 1 to 2 kb was selected for each phage. The sequence of the cloned DNA was determined by the automated sequencing facility at the University of Missouri—Kansas City. The sequence data were analyzed by the BLAST program and used to design PCR primers. The primers used are listed Table 1. The DNA of target phages was isolated and used as template DNA. PCR was performed by using a thermal cycler (Techne, Princeton, N.J.). The reaction mixture (final volume of 50 μl) contained 100 ng of template DNA; 1 U of Taq DNA polymerase (Biolase; Bioline, Reno, Nev.); 1× reaction buffer (buffer J; pH 9.5, from the Invitrogen PCR optimizer kit; Invitrogen, Carlsbad, Calif.); 2 mM MgCl2; deoxynucleoside triphosphates, 0.1 mM each; primers, 50 pmol each; and bovine serum albumin, 2 μg. The thermal cycling program used was as follows: initial denaturation at 94°C for 2 min and 35 cycles of 94°C for 1 min, 50°C for 2 min, and 72°C for 3 min. Finally, there was an extension step at 72°C for 7 min. The PCR DNA products were analyzed for correct sizes and for purity by agarose gel electrophoresis.
TABLE 1.
Morphotype-specific primers for vaginal Lactobacillus phage classification
| Type | Strain | Primer | Sequence | Product size (bp) |
|---|---|---|---|---|
| A1 | φkc5a | Forward | 5′-ATGCTGACGGAAGGTGTGGTCAATGCT-3′ | 480 |
| Reverse | 5′-AGTGCTACAACAGCCCTTGCACCGT-3′ | |||
| A2 | φkc12a | Forward | 5′-GCGGTTTATCTGGAAGTATAGCCCT-3′ | 326 |
| Reverse | 5′-CTGATGCCAACCTTCACCATGAAGCCT-3′ | |||
| B1 | φkc39 | Forward | 5′-CGAACTGGCGAATTTGTACCATCT-3′ | 237 |
| Reverse | 5′-GTCGCCAGTTGTTGAAGCAGTGATGT-3′ | |||
| B2 | φTL76 | Forward | 5′-CACCTCCGAGTGACATGGGCACAGCT-3′ | 250 |
| Reverse | 5′-GCAATTGCAAATACTGCACCA-3′ |
RESULTS
Isolation and identification of vaginal lactobacilli.
About 200 vaginal samples were obtained from reproductive-aged women in Turkey and about 100 were obtained from the United States. While the Turkish women were all Caucasian, the American group included black (55%), white (35%), Asian (5%), Hispanic (3%), and Native American (2%) women. Some Turkish isolates did not survive the oversea shipping, so only 102 Lactobacillus strains were obtained. From American women, 107 strains were obtained. Among the Turkish women, 43 cases of BV were diagnosed, but only 22 had culturable lactobacilli. Among the American women, 14 cases of BV were diagnosed, but only 4 had culturable lactobacilli. Storage of samples in the RTF-glycerol buffer at −20 to −70°C did not result in loss of Lactobacillus viability. Each collection had 10 obligate anaerobic strains (about 10%). All of the remaining strains were facultative anaerobes (Table 2).
TABLE 2.
Lactobacillus lysogens among different species and anaerobic groups in vaginal isolates from women in the United States and Turkey
| Species or group | No. in U.S. collection
|
No. in Turkish collection
|
Total no.
|
% Lysogen | |||
|---|---|---|---|---|---|---|---|
| Strain | Lysogen | Strain | Lysogen | Strain | Lysogen | ||
| L. crispatus | 27 | 4 | 34 | 7 | 61 | 11 | 18 |
| L. gasseri | 30 | 11 | 33 | 14 | 63 | 25 | 40 |
| L. jensenii | 30 | 12 | 30 | 14 | 60 | 26 | 43 |
| L. fermentum | 10 | 2 | 0 | 0 | 10 | 2 | 20 |
| L. vaginalis | 1 | 1 | 2 | 2 | 3 | 3 | 100 |
| Other Lactobacillus spp. | 9 | 0 | 3 | 0 | 12 | 0 | 0 |
| Facultative anaerobes | 97 | 23 | 92 | 31 | 189 | 54 | 29 |
| Obligate anaerobesa | 10 | 7 | 10 | 6 | 20 | 13 | 65 |
| Total | 107 | 30 | 102 | 37 | 209 | 67 | 32 |
Among Turkish lactobacilli, the obligate anaerobes were L. jensenii and L. crispatus, but among U.S. lactobaclli, the obligate anaerobes were L. gasseri and L. jensenii.
Species identification of lactobacilli.
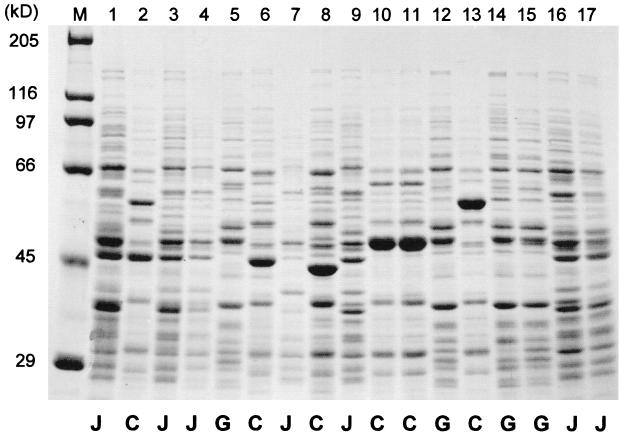
Since the traditional biochemical and physiological methods could not effectively classify these lactobacilli to the species level, we applied genetic and molecular methods. First, we grouped these strains based on their sugar fermentation pattern and whole-cell protein profiles. Then, we determined the sequence of the 16S rDNA of some representative strains from each group. Based on the sequence data, we identified their species. Finally, the whole cell protein profiles were analyzed among all of the remaining strains. Several representative strains from each group that shared the same cell morphology, sugar fermentation pattern and whole cell protein profile were selected to analyze their 16S rDNA sequences. The sequence data of 23 strains (9 from Turkey and 14 from the United States) have been deposited into GenBank with accession numbers from AF243150 to AF243166 and from AF243170 to AF143175. These data were compared to those for Lactobacillus type strains already in GenBank using the BLAST program 2. Once the species of the representative strains were identified, the identification for the remaining strains was achieved by comparison of their total protein profiles with those of the representative strains. The results of species designation of these strains are listed in Table 2. Figure 1 shows the result of one of the SDS-PAGE gels. Based on the 16S rDNA analysis and the protein profile comparison, most clinical vaginal strains belonged to three Lactobacillus species, L. crispatus, L. gasseri, and L. jensenii. The protein profiles of L. gasseri and L. jensenii were highly consistent among all isolates tested. Although a major band of L. crispatus was variable among different isolates (between 40 and 60 kDa on the SDS-PAGE gel), all of the other bands were consistent within the same species. Additional species included L. fermentum, L. vaginalis, and several unknown species. Interestingly, the fourth largest species among American isolates was L. fermentum (9%), while the Turkish isolates did not have any L. fermentum strains. As shown in Table 2, the majority of vaginal lactobacilli were facultative anaerobes.
FIG. 1.
Protein profiles of some representative Lactobacillus strains on SDS-PAGE(10% polyacrylamide). Lane M contains protein molecular weight markers. Lanes 1 to 17 contain the indicated vaginal Lactobacillus strains: 1, KC23T; 2, TL152; 3, TL145a; 4, TL143b; 5, TL114; 6, TL127a; 7, TL109b; 8, TL60a; 9, TL27; 10, TL23b; 11, TL23a; 12, TL33a; 13, TL13; 14, TL102; 15, TL76; 16, TL74c; 17, TL34c. At the bottom of the gel, the species identification of each strain is indicated by a letter. C; L. crispatus; G; L. gasseri; J; L. jensenii.
Phage isolation.
Phage induction was performed by the mitomycin C method for 209 clinically isolated vaginal strains. The lysates were used to interact with these Lactobacillus strains to screen for phage-sensitive indicator strains. Sixty-seven lysates were confirmed to contain phages, because they formed single plaques on the agar plates of sensitive strains. Additionally, these phages were confirmed by DNA hybridization with labeled phage DNA probes and observation under an electron microscope to rule out possible bacterial inhibition effects due to bacteriocins, H2O2, and organic acids. Among the 67 phages, 30 were isolated from the American collection, while 37 were isolated from the Turkish collection.
Table 2 shows that the obligate anaerobes were more likely to carry a phage (65%) than the facultative anaerobic lactobacilli (29%). The difference was significant (P < 0.01). About 36% of vaginal lactobacilli from Turkish women released phages, while about 28% of lactobacilli from American women released phages. The difference was not statistically significant between the two groups (P > 0.05). Among six Lactobacillus strains isolated from the four American women with BV, four strains from two patients were lysogens. Among 22 Lactobacillus strains from Turkish women with BV, 11 were infected by phages (lysogens). Overall, about 50% of lysogenic lactobacilli were isolated from women with BV, but only about 30% of lysogens were isolated from women without BV. The difference was statistically significant (P < 0.05).
Spontaneous phage induction and burst size.
Phages can be spontaneously released without any inducing agent due to random errors during the host bacterial DNA replication 20. In this study, the lysogenic lactobacilli released infective phages at different rates, which were detected by observation of phage plaques on the indicator Lactobacillus plate cultures. Among American lactobacilli, 17% of lysogenic strains spontaneously released phages at a higher frequency of 10−3 to 10−4 PFU/cell, while 27% of lysogenic strains from the Turkish collection released phages at this level. About one-third of both collections released phages at an intermediate frequency (about 10−6 PFU/cell). Approximately one-half of the culture collections from both countries spontaneously released phages at a frequency of less than 10−8 PFU/cell. These data were repeated observations, and the frequency of phage release from each strain was highly stable. The burst sizes were between 60 and 300 phages per cell.
Phage host ranges and infection characteristics.
All 67 temperate phages isolated from vaginal lactobacilli infected vaginal lactobacilli in vitro by forming clear plaques on agar plates. As shown in Table 3, the 30 phages from the United States and 37 phages from Turkey infected most vaginal lactobacilli from both collections, including lysogenic strains. Overall, fewer lactobacilli isolated from Turkish women resisted phage infection than lactobacilli isolated from U.S. women. A group of vaginal lactobacilli sensitive to multiple phages was identified. They were used as indicator strains to display clear single plaques after the infection and used to screen for new phages. There were no apparent differences in phage sensitivity between lactobacilli isolated from healthy women and those from women with vaginal infections.
TABLE 3.
Infection of vaginal lactobacilli by 67 phages from the United States and Turkey
| Infection category | No. of vaginal lactobacillus strains froma:
|
Total | |
|---|---|---|---|
| U.S. women | Turkish women | ||
| Infected by both phage collections | 71 (20) | 86 (25) | 157 (45) |
| Infected only by American phages | 7 (5) | 2 (0) | 9 (5) |
| Infected only by Turkish phages | 3 (1) | 13 (11) | 16 (12) |
| Resisted all phages | 26 (4) | 1 (1) | 27 (5) |
| Total | 107 (30) | 102 (37) | 209 (67) |
The number in parentheses represents lysogenic strains in each group.
Many phages had a broad host range and infected vaginal Lactobacillus strains of multiple species, including L. crispatus, L. jensenii, L. gasseri, L. fermentum, and L. vaginalis. Among the obligate anaerobic lactobacilli, the American collection had mostly L. gasseri strains, while the Turkish collection had mostly L. jensenii strains. They were equally high in the rate of phage lysogeny. After infection of 100 million Lactobacillus cells by these phages (multiplicity of infection, 1:10), no survival colonies or lysogens could be observed, indicating lytic infection. Nearly all temperate phages in the two collections lytically infected other sensitive lactobacilli.
Phage morphology.
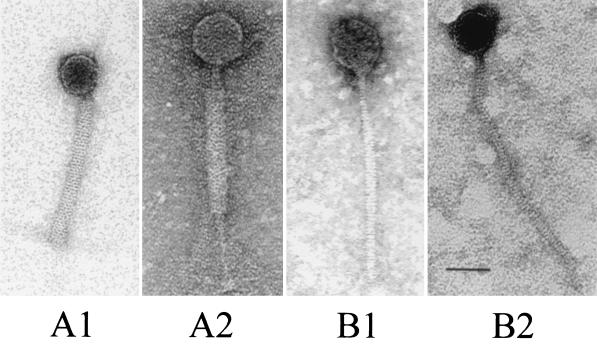
The electron micrograph (Fig. 2) showed two major morphotypes, Bradley 5 type A and B, among the 67 phages studied to date. Bradley type A is characterized by a hexagonal head and a tail with a contractile sheath. The first type, represented by φkc21T and φkc12a, belongs to Bradley phage type A 5, because both phages had a contractile tail sheath. However, there was a difference between the two phages in the head size and tail length. Additionally, φkc12a had a tail plate. Bradley type B is characterized by a hexagonal head and a tail without a contractile sheath. The second type, represented by φkc39 and φkc7a, belonged to Bradley phage type B, because both phages were lacking a contractile tail sheath, although they differed in head size and tail length. While all four types existed in the American phage collection, only three types (all but A2) were found among the phages in the Turkish collection. All four types had hexagonal heads but were of two sizes. The smaller one, type A1 and B2, was about 45 nm in diameter, and the larger one, type A2 and B1 was about 67 nm in diameter. The length and appearance of their tails were quite different. The type A1 phages had a shorter tail, about 160 nm long, which could be completely covered by a sheath with about 50 horizontal bands. The type A2 phages had a longer tail about 260 nm long and a tail plate. The sheath was about the same size as that in type A1 phages, but it had a dotted pattern instead of horizontal bands. The tail of the type B1 phages was about 250 nm in length with about 60 disks. The type B2 phages had the longest tails, about 300 nm long with about 80 disks, and also a tail fiber about 40 nm long.
FIG. 2.
Electron micrograph of vaginal Lactobacillus phages. A1, φkc21T; A2, φkc12a; B1, φkc39; B2, φkc7a. Bar = 50 nm.
Phage DNA restriction analysis.
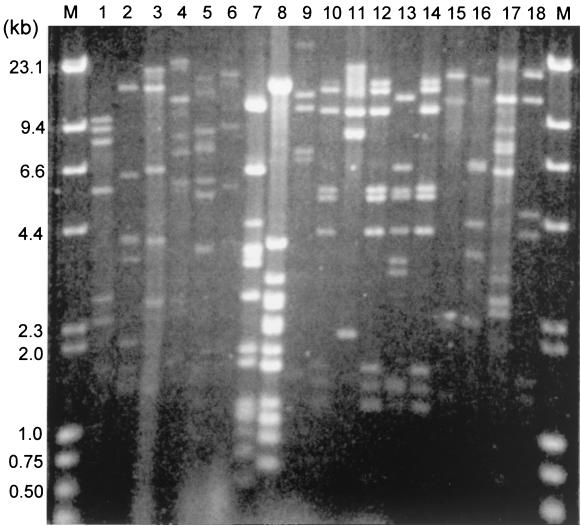
To further characterize these phages, DNA from phages representing different morphotypes were isolated, digested with EcoRI, and subjected to agarose gel electrophoresis (Fig. 3). The phage genomes ranged from 34 to 55 kb and were all double stranded and linear as determined by the DNA-heating agarose gel electrophoresis assay 22. The DNA fingerprints showed that most of the phages were genetically different, even among phages with the same morphotype. One identical pattern, however, was found among three phages isolated from different women. According to the protein profile analysis, the three lysogenic lactobacilli belonged to two different species. They were L. jensenii TL34 and TL74c and L. gasseri TL76.
FIG. 3.
DNA fingerprinting of vaginal Lactobacillus phages. The phage DNA were digested by EcoRI. Lanes: M, molecular weight DNA markers; 1, φkc5a; 2, φkc7a; 3, φkc12a; 4 φkc21T; 5, φkc23T; 6, φkc31; 7, φkc39; 8, φTL32b; 9, φTL33a; 10, φTL34; 11, φTL72a; 12, φTL74c; 13, φTL75a; 14, φTL76; 15, φTL122b; 16, φTL125, 17, φTL138; 18, φTL141. Note: lanes 10, 12, and 14 show identical DNA patterns.
Phage classification by DNA hybridization and PCR.
DNA probes were made of complete genomic DNA of four phages, each representing different morphotypes as shown in Fig. 2. The PCR primers were designed according to the sequence data from the shotgun-cloned phage DNA fragments representing four morphotypes. The BLAST analysis of these sequences did not yield any homology with existing data in GenBank. By Southern hybridization, we found that the genome of φkc5a was homologous to those of φkc21T, φTL32, and φTL138, representing phage type A1, and the genome of φTL76 was homologous to those of φTL34, φTL74c, and φTL75a, representing phage type B2. Several homology groups were identified by additional Southern hybridization and dot blot hybridization, as well as by PCR. The results are shown in Table 4. No correlations were found between these phage types and the vaginal health status of these women, because most women who suffered from BV had no detectable lactobacilli in their vaginal samples.
TABLE 4.
Phage classification by electron microscopic (EM) morphology, DNA hybridization, and PCR
| Phage | EM | DNA hybridizationa
|
PCRa
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| A1 φkc5a | A2 φkc12a | B1 φkc39 | B2 φkc7a | A1 φkc5a | A2 φkc12a | B1 φkc39 | B2 φTL76 | ||
| U.S. phages | |||||||||
| φkc5a | A1 | + | − | − | − | + | − | + | − |
| φkc6a, -bb | − | − | + | − | − | − | + | − | |
| φkc7a, -b, -cb | B2 | − | − | − | + | + | − | + | − |
| φkc12a | A2 | − | + | − | − | − | + | + | − |
| φkc13b | − | − | + | − | − | − | + | − | |
| φkc19b | − | + | − | − | − | + | − | − | |
| φkc31 | B1 | − | − | + | − | − | − | + | − |
| φkc39 | B1 | − | − | + | − | − | − | + | − |
| φkc58ab | + | − | − | + | − | − | − | − | |
| φkc59ab | + | − | − | + | + | − | − | − | |
| φkc60ab | + | − | − | + | + | − | − | − | |
| φkc102bb | − | − | + | − | − | − | + | − | |
| φkc109ab | − | − | − | + | − | − | − | − | |
| φkc148b | − | − | + | − | − | − | + | − | |
| φkc149b | − | − | + | − | − | − | + | − | |
| Turkish phages | |||||||||
| φkc21T | A1 | + | − | − | − | + | − | + | − |
| φkc23T | B1 | − | − | − | − | − | − | + | − |
| φkc26T | + | − | − | − | + | − | − | − | |
| φTL16 | − | − | + | − | − | − | − | − | |
| φTL32 | + | − | + | − | + | − | + | − | |
| φTL33a | A1 | + | − | − | − | − | − | − | − |
| φTL34 | B2 | − | − | − | + | − | − | + | + |
| φTL56b | A1 | + | − | − | − | + | − | − | − |
| φTL74c | B2 | − | − | − | + | − | − | + | − |
| φTL75a | − | − | − | + | − | − | − | + | |
| φTL76 | B2 | − | − | − | + | − | − | − | + |
| φTL87b | + | − | − | − | + | − | − | − | |
| φTL102b | A1 | + | − | − | + | + | − | − | − |
| φTL109a, -cb | − | − | − | + | − | − | − | − | |
| φTL110b | + | − | − | − | + | − | − | − | |
| φTL122b | B1 | − | − | + | − | − | − | + | − |
| φTL125 | B2 | − | − | − | + | − | − | − | − |
| φTL138 | + | − | − | − | + | − | − | − | |
| φTL139a, -cb | + | − | − | − | + | − | − | − | |
| φTL141 | B2 | − | − | − | + | + | − | − | − |
Phages that showed negative results included φkc36b, φkc38, φkc48, φkc55a, φkc58b, φkc72, φkc74, φTL25a, φTL35, φTL39b, φTL56c, φTL59c, φTL61a, φTL61b, φTL65, φTL72, φTL109c, φTL113, and φTL134.
Hybridization was performed with the lysogenic Lactobacillus chromosomal DNA.
DISCUSSION
BV is the most common vaginal disorder affecting women worldwide 38, 40. Since it can increase the risk of preterm delivery of low-birth-weight infants 14, 16, 17 and the risk of contracting human immunodeficiency virus in women 8, 27, 37, treatment and prevention of BV become an important issue 29. Unfortunately, the exact cause of BV is unknown. It has been well documented, however, that during BV, the normally predominant Lactobacillus vaginal flora is replaced with anaerobic bacteria 3, 9, 10 38, 40. Therefore, the question was raised of whether bacteriophages could inhibit lactobacilli in the vagina. We have previously reported the identification of phages in vaginal lactobacilli 32, 41. In this work, we report the study on the prevalence, genetic diversity, and infectivity of these phages from women in two geographically distant countries: the United States and Turkey.
To study whether the phage infection in vaginal lactobacilli was species specific, we first classified the species of these lactobacilli. By comparing the protein profiles of the strains of unknown species with those of known species and Lactobacillus type strains, most of the strains were characterized to the species level. The majority of strains from both countries belonged to three species, L. gasseri, L. jensenii, and L. crispatus, with almost equal proportions. These data largely agreed with previous studies performed by DNA-DNA hybridization 4, 11, 39. The protein patterns for L. gasseri and L. jensenii were mostly consistent and reliable. L. crispatus was distinguished from L. gasseri and L. jensenii by having a thick band, with sizes between 40 and 60 kDa among different isolates (Fig. 1). This thick band appeared to represent its S-layer protein 19. Not only could it serve as a potential marker to differentiate L. crispatus from L. gasseri and L. jensenii, but it may also be used to identify different strains within the species of L. crispatus due to its size variability. The overall correlation between 16S rDNA data and the protein profiles was strong. The combination of these two methods offered a reliable approach to identify species of a large number of lactobacilli.
By analyzing phage host ranges and Lactobacillus species data, we found that many phages infected multiple Lactobacillus species. However, some strains remained uninfected. This implied that the phage host range in vaginal lactobacilli might not be determined by species-specific markers. Instead, certain characteristic receptors on the cell surface might determine phage host ranges. Although we do not know what may be the phage receptor on these vaginal lactobacilli, our study (data not shown) revealed that it was not the rhamnose residue of the polysaccharide on the cell surface as in the case of L. casei 42. Further studies are needed to identify these phage receptor molecules. Normally, a lysogenic strain is immune from infection by the same phage or the same type of phages. This is called superinfection immunity 20. However, in this study, we found that many lysogenic strains were superinfected by different phages, and some were even infected by the same phage. This suggested that the superinfection immunity might not always function in the group of vaginal Lactobacillus lysogens.
Our phage classification studies included electron microscopy and DNA analysis. Based on current knowledge about phage taxonomy 1, phages with similar morphology may be genetically different, but phages with different morphology are usually different in their genomics. The differences in genomic sizes and restriction patterns among the four phages (Fig. 3: lane 3, φkc7a, 34.5 kb; lane 4, φkc12a, 47 kb; lane 5, φkc21T, 38 kb; and lane 8, φkc39, 41 kb) further indicate that these four phages may be genetically different species. Although only four phage morphotypes were noticed among the 67 phages studied, additional genetic types may exist within each morphotype, because many phages did not hybridize with the probes made of the genomic DNA of these four phages. Clearly, none of these phages displayed a prolate-shaped head like that of the dairy Lactobacillus phage φy8, which was released by a Lactobacillus starter strain in one of the name brand American yogurts 22. The most prevalent phage morphotype was type B. Three phages showed an identical DNA fingerprinting pattern (Fig. 3), suggesting that a prevalent phage might be transmitting among different women. Further studies will be needed to study phage transmissions.
Normally, a bacteriophage may be spontaneously released at a frequency of 10−6 per cell 20. A high-frequency spontaneous phage release by many lysogenic vaginal lactobacilli (about 10−3 to 10−4 per cell) is of particular interest. It suggested that a large number of free phages can be spontaneously released from these strains and found present in the vaginal secretion. This characteristic may be clinically significant, because free phages can infect other lactobacilli in the same woman or be transmitted to different women to infect their lactobacilli. This matched the clinical observation that BV, or the lack of vaginal lactobacilli, is associated with sexual transmission 38, 40. Since many vaginal lactobacilli spontaneously released phages, it suggests that lysogenic Lactobacillus strains may be a source of potentially infectious phages.
Among lysogenic lactobacilli that had a low spontaneous induction frequency, phages were induced by mitomycin C. Some of these phages infected other Lactobacillus strains under in vitro conditions. These lysogenic strains might coexist with other phage-sensitive Lactobacillus strains in the same vaginal environment, because they rarely released phages. However, this condition could change when the vaginal environment encounters a phage-inducing agent. We have recently reported that trace amounts of cigarette smoke chemical benzo[a]pyrene diol epoxide promoted phage release from lysogenic vaginal lactobacilli 33. Among women who smoke, the cigarette-associated mutagenic chemicals could reach their vaginal secretions and cause phage induction in lysogenic lactobacilli.
All phages in the present study were temperate phages released from lysogenic strains. We have so far not been able to isolate lytic phages directly from women. Truly lytic or virulent phages are usually short lived. Once they appear, the virulent phages can rapidly eliminate their host bacteria; as a result, they lose their living shelter for self-reproduction. Therefore, phages that are temperate to some bacteria but lytic to others are of concern. It is well known that some temperate phages can become virulent due to genetic mutations 36, but it is unknown why so many temperate phages from vaginal lactobacilli can become lytic against other vaginal Lactobacillus strains. Probably, certain differences in the bacterial host background prohibit these phages from integrating their DNA into the chromosome of their new hosts to form lysogens 7.
In conclusion, we studied phages from vaginal lactobacilli of women in Turkey and the United States. We have determined that most of these Lactobacillus strains belonged to three species, L. crispatus, L. gasseri, and L. jensenii. Phages isolated from vaginal lactobacilli of some women lytically infected vaginal lactobacilli of other women regardless of their countries of origin. Four morphotypes were identified among these phages, and their host range was broad and beyond any particular Lactobacillus species. Most lysogenic lactobacilli spontaneously released phages into the environment at varied frequencies. This suggested that lysogenic lactobacilli could be a source of infective phages. Although the phage infection observed in vitro may not necessarily indicate that the same situation could happen in vivo, the results imply that vaginal lactobacilli may be eliminated or repressed by phages. This implication may be important for studying the etiology of BV due to its association with a decrease in vaginal lactobacilli. Apparently, further studies with an increased number of clinical samples will be needed to associate phage infections in vaginal lactobacilli with women's vaginal health.
ACKNOWLEDGMENTS
We are grateful to Susan Mou for her assistance in obtaining samples from American women. We also thank S. Robinson and D. Sackuvich for assisting with electron microscopy.
This work was supported in part by grant 02069-15-RG from the Concerned Parents for AIDS Research-AmFAR, grant K-3-40532 from the University of Missouri Research Board, Public Health Service grant R03 AI45127 from the National Institute of Allergy and Infectious Diseases, and grant 2-2-25521 from the Center for Research on Women and Gender, University of Illinois at Chicago.
REFERENCES
- 1.Ackermann H W, DuBow M S, Jarvis A W, Jones L A, Krylov V N, Maniloff J, Rocourt J, Safferman R S, Schneider J, Seldin L, Sozzi T, Stewart P R, Werquin M, Wunsche L. The species concept and its application to tailed phages. Arch Virol. 1992;124:69–82. doi: 10.1007/BF01314626. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Amsel R, Totten P A, Spiegel C A, Chen K S C, Eschenbach D A, Holmes K K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 4.Antonio M A, Hawes S E, Hillier S L. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 5.Bradley D E. Ultrastructure of bacteriophage and bacteriocin. Bacteriol Rev. 1967;31:230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassy B, Gibson M, Guifrida A. Evidence for extrachromosomal elements in Lactobacillus. J Bacteriol. 1976;127:1576–1578. doi: 10.1128/jb.127.3.1576-1578.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cluzel P J, Serio J, Accolas J P. Interaction of Lactobacillus bulgaricus temperate bacteriophage 0448 with host strains. Appl Environ Microbiol. 1987;53:1850–1854. doi: 10.1128/aem.53.8.1850-1854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen C R, Duerr A, Pruithithada N, Rugpao S, Hillier S L, Garcia P, Nelson K. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS. 1995;9:1093–1097. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Eschenbach D A, Davick P R, Williams B L, Klebanoff S J, Young-Smith K, Critchlow C M, Holmes K K. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eschenbach D A, Hillier S L, Critchlow C, Stevens C, DeRousen T, Holmes K K. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158:819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 11.Giorgi A, Torriani S, Dellaglio F, Bo G, Stola E, Bernuzzi L. Identification of vaginal lactobacilli from asymptomatic women. Microbiologica. 1987;10:377–384. [PubMed] [Google Scholar]
- 12.Hallen A, Jarstrand C, Påhlson C. Treatment of bacterial vaginosis with lactobacilli. Sex Transm Dis. 1992;19:146–148. doi: 10.1097/00007435-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Harwood B, Mittendorf R, Judge D, Dayal S, Walker C. Patterns of vaginal douching and their association with vaginal bacteriosis. Infect Dis Obstet Gynecol. 1996;4:51. [Google Scholar]
- 14.Hay P E, Lamont R F, Taylor-Robinson D, Morgan D J, Ison C A, Pearson J. Abnormal bacterial colonization of the genital tract and subsequent preterm delivery and late miscarriage. Br Med J. 1994;308:295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill G B. The microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:450–454. doi: 10.1016/0002-9378(93)90339-k. [DOI] [PubMed] [Google Scholar]
- 16.Hillier S L, Krohn M A, Cassen E, Easterling T R, Rabe L K, Eschenbach D A. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clin Infect Dis. 1995;20(Suppl. 2):S276–278. doi: 10.1093/clinids/20.supplement_2.s276. [DOI] [PubMed] [Google Scholar]
- 17.Hillier S L, Nugent R P, Eschenbach D A, Krohn M A, Gibbs R S, Martin D H, Cotch M F, Edelman R, Pastorek II J G, Rao A V, McNellis D, Regan J A, Carey J C, Klebanoff M A the Vaginal Infections and Prematurity Study Group. Association between bacterial vaginosis and preterm delviery of a low-birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 18.Hooton T M, Fennell C I, Clark A M, Stamm W E. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216–1219. doi: 10.1093/infdis/164.6.1216. [DOI] [PubMed] [Google Scholar]
- 19.Johnson M C, Ray B, Bhowmik T. Selection of Lactobacillus acidophilus strains for use in “acidophilus products.”. Antonie Leeuwenhoek. 1987;53:215–231. doi: 10.1007/BF00393929. [DOI] [PubMed] [Google Scholar]
- 20.Joklik W K, Willett H P, Amos D B. Zinsser microbiology. 17th ed. New York; N.Y: Appleton-Century-Crofts; 1980. p. 1178. [Google Scholar]
- 21.Kandler O, Weiss N. Genus Lactobacillus. In: Sneath P, editor. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore; md: Williams, & Wilkins; 1986. pp. 1209–1234. [Google Scholar]
- 22.Kilic A O, Pavlova S I, Ma W, Tao L. Analysis of Lactobacillus phages and bacteriocins in American dairy products and characterization of a phage isolated from yogurt. Appl Environ Microbiol. 1996;62:2111–2116. doi: 10.1128/aem.62.6.2111-2116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klebanoff S J, Hillier S L, Eschenbach D A, Waltersdorph A M. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis. 1991;164:94–100. doi: 10.1093/infdis/164.1.94. [DOI] [PubMed] [Google Scholar]
- 24.Kopeloff N. Dissociation and filtration of Lactobacillus acidophilus. J Infect Dis. 1934;55:368–372. [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:684–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 27.Martin H L, Richardson B A, Nyange P M, Lavreys L, Hillier S L, Chohan B, Mandaliya K, Ndinya-Achola J O, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 28.Martius J, Krohn M A, Hillier S L, Stamm W E, Holmes K K, Eschenbach D A. Relationship of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and vaginosis to preterm birth. Obstet Gynecol. 1988;71:89–95. [PubMed] [Google Scholar]
- 29.McGregor J A, French J I, Parker R, Draper D, Patterson E, Jones W, Thorsgard K, McFee J. Prevention of premature birth by screening and treatment for common genital tract infections. Results of a prospective controlled evaluation. Am J Obstet Gynecol. 1995;173:157–167. doi: 10.1016/0002-9378(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 30.Nes I F, Sorheim O. Effect of infection of a bacteriophage in a starter culture during the production of salami dry sausage: a model study. J Food Sci. 1984;49:337–340. [Google Scholar]
- 31.Nugent R P, Krohn M A, Hillier S L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlova S I, Kilic A O, Mou S M, Tao L. Phage infection in vaginal Lactobacillus: an in vitro study. Infect Dis Obstet Gynecol. 1997;5:36–44. doi: 10.1155/S1064744997000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlova S I, Tao L. Induction of vaginal Lactobacillus phages by the cigarette smoke chemical benzol[a]pyrene diol epoxide. Mutat Res. 2000;466:57–62. doi: 10.1016/s1383-5718(00)00003-6. [DOI] [PubMed] [Google Scholar]
- 34.Raya R R, Kleeman E G, Luchansky J B, Klaenhammer T R. Characterization of the temperate bacteriophage φadh and plasmid transduction in Lactobacillus acidophilus ADH. Appl Environ Microbiol. 1989;55:2206–2213. doi: 10.1128/aem.55.9.2206-2213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redondo-Lopez V, Cook R L, Sobel J D. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 36.Sechaud L, Cluzel P J, Pousseau M, Baumgartner A, Accolas J P. Bacteriophages of lactobacilli. Biochimie. 1988;70:401–410. doi: 10.1016/0300-9084(88)90214-3. [DOI] [PubMed] [Google Scholar]
- 37.Sewankambo N, Gray R H, Wawer M J, Paxton L, McNairn D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier S L, Rabe L, Gaydos C A, Quinn T C, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 38.Sobel J D. Vaginitis. N Engl J Med. 1997;337:1896–1903. doi: 10.1056/NEJM199712253372607. [DOI] [PubMed] [Google Scholar]
- 39.Song Y L, Kato N, Matsumiya Y, Liu C X, Kato H, Watanabe K. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J Clin Microbiol. 1999;37:3062–3064. doi: 10.1128/jcm.37.9.3062-3064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiegel C A. Bacterial vaginosis. Clin Microbiol Rev. 1991;4:485–502. doi: 10.1128/cmr.4.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao L, Pavlova S, Alpay S, Kilic A. Initial evidence for phage infection and transmission in vaginal lactobacilli. Int J Gynecol Obstet. 1999;67(Suppl):S49. [Google Scholar]
- 42.Watanabe K, Takesue S. Use of L-rhamnose to study irreversible adsorption of bacteriophage PL-1 to a strain of Lactobacillus casei. J Gen Virol. 1975;28:29–35. doi: 10.1099/0022-1317-28-1-29. [DOI] [PubMed] [Google Scholar]
- 43.Zheng H, Alcorn T M, Cohen M S. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J Infect Dis. 1994;170:1209–1215. doi: 10.1093/infdis/170.5.1209. [DOI] [PubMed] [Google Scholar]