Abstract
Simple Summary
While the controlling nutritional status (CONUT) score and sarcopenia are both objective indices of different aspects of a patient’s general condition, few studies have comprehensively examined their mutual relationship as prognostic factors. In the present, retrospective study, we examined this question using a cohort of 200 patients with advanced urothelial carcinoma (aUC). No significant association was found between the CONUT score and sarcopenia, and most patients with sarcopenic aUC had normal or only slightly impaired nutritional status. The CONUT score and sarcopenia were significant, mutually independent, prognostic biomarkers and they outperformed performance the status as a prognostic factor in our cohort. Incorporating the CONUT score, sarcopenia or both into current established prognostic models increased their predictive accuracy. Our study corroborated the prognostic relevance of the CONUT score and sarcopenia and suggested the importance of separately evaluating these prognostic biomarkers in patients with aUC.
Abstract
Background: While the controlling nutritional status (CONUT) score and sarcopenia are objective indices of different aspects of a patient’s general condition, few studies have comprehensively examined their mutual relationship in patients with advanced cancer. Methods: This retrospective study included 200 Japanese patients with advanced urothelial carcinoma (aUC). Sarcopenia was diagnosed using Prado’s definition. The CONUT score and sarcopenia were examined for their possible association, and their prognostic value was analyzed. Results: The CONUT score and sarcopenia were not significantly associated. While sarcopenia occurred in 168 patients (84%), more than half of them had normal or only slightly impaired nutritional status, as indicated by a CONUT score of 0–2. During follow-up (median: 13.3 months), 149 patients died. The CONUT score and sarcopenia were independent prognostic factors (hazard ratio 1.22 and 2.23, respectively; both p < 0.001), whereas performance status was not. Incorporating the CONUT score, sarcopenia, and both into Bajorin’s and Apolo’s prognostic models increased their concordance index as follows: 0.612 for Bajorin’s original model to 0.653 (+the CONUT score), 0.631 (+sarcopenia), and 0.665 (+both), and 0.634 for Apolo’s original model to 0.655 (+the CONUT score), 0.653 (+ sarcopenia), and 0.668 (+both). Conclusion: The CONUT score and sarcopenia were mutually independent in terms of their prognostic value in patients with aUC. These objective indices of a patient’s general condition may help in decision-making when considering treatment for patients with aUC.
Keywords: advanced urothelial carcinoma, controlling nutritional status score, sarcopenia, performance status, prognosis
1. Introduction
Urothelial carcinoma (UC) is mainly comprised of bladder UC and upper tract (UT) UC (UTUC), and account for 90–95% and 5–10% of all UC cases, respectively [1]. Despite the recent advent of novel treatments, such as immune checkpoint inhibitors (ICIs) and enfortumab-vedotin, the prognosis of patients with advanced urothelial carcinoma (aUC) remains poor. Platinum-based regimens have been the standard of care for aUC since the late 1980s [2]. The median overall survival (OS) remained around 15 months, despite the efficacy of platinum-based chemotherapy [3,4,5,6]. While pembrolizumab and enfortumab-vedotin significantly prolonged the median OS, by approximately three months, as compared to second- and third-line chemotherapy after failure of first-line chemotherapy and second-line ICIs [7,8], respectively, only a small proportion of patients were able to extend their survival significantly. While several prognostic models of aUC have been proposed and externally validated [9,10], novel prognostic factors capable of better predicting the prognosis of aUC would help patients and their physicians to choose the optimal therapy.
Assessment of the general condition of a patient with advanced cancer is also crucial for decision-making and prognostication. Performance status (PS) is commonly used for these purposes. Despite its simplicity, the PS is a subjective assessment and thus includes possible problems with objectivity, accuracy, and interobserver variation [11]. Therefore, more objective and reliable tools for assessing the general condition of patients with advanced cancer are needed. The controlling nutritional status (CONUT) score [12] and sarcopenia may serve as such assessment tools from the perspective of nutritional status and body composition, respectively.
The CONUT score, based on the serum albumin level, lymphocyte count, and total cholesterol level (Table 1), is a simple, validated, objective tool for assessing nutritional status [12,13]. The CONUT score has been used as a prognostic factor in patients with various malignancies [14,15,16,17], including aUC [18,19].
Table 1.
Scoring and interpretation of the controlling nutritional status (CONUT) score.
| Parameter | Range of Values and Scores Per Parameter | |||
|---|---|---|---|---|
| Albumin (g/dL) | ≥3.50 | 3.00–3.49 | 2.50–2.99 | <2.50 |
| Score | 0 | 2 | 4 | 6 |
| Lymphocyte count (/μL) | ≥1600 | 1200–1599 | 800–1199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) * | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| Interpretation | ||||
| CONUT score (sum of above scores) | 0–1 | 2–4 | 5–8 | 9–12 |
| Degree of malnutrition | None | Light | Moderate | Severe |
* Ranges of total cholesterol (mmol/L) are ≥4.65, 3.62–4.64, 2.58–3.61, and <2.58, respectively.
Sarcopenia is defined as a decrease in skeletal muscle mass and function [20]. Most of the numerous studies examining sarcopenia [21,22,23,24,25] used the skeletal muscle index (SMI), based on computed tomography (CT) images. As with the CONUT score, sarcopenia has been used as a prognostic factor in a variety of cancers, including hepatocellular carcinoma, lung cancer, breast cancer, and aUC [26,27,28,29]. Moreover, sarcopenia is associated with an increased risk of adverse events related to chemotherapy and major surgery [30,31,32,33].
While both the CONUT score and sarcopenia reflect the deterioration in patients’ general condition associated with tumor-induced, chronic inflammation and cachexia and can provide prognostic information independently of canonical, prognostic factors in patients with various malignancies, no study has comprehensively investigated whether the CONUT score and sarcopenia are associated or are mutually independent prognostic factors. In the present study, we assessed the prognostic value of the CONUT score and sarcopenia in 200 patients with aUC, most of whom had received platinum-based systemic chemotherapy.
2. Materials and Methods
2.1. Study Design, Data Collection, and CONUT Score Calculation
Our institutional ethics committee approved the present retrospective study (approval number: 2894). In total, 247 consecutive Japanese patients with inoperable (cT4 or lymph node metastasis) and/or metastatic UC of the bladder or UT were treated at a single, designated cancer center between December 2002 and December 2021. Of the 247 patients, 47 were excluded owing to missing data, which was required to calculate the CONUT score (n = 30) or missing CT imaging studies (n = 17). Finally, 200 patients were enrolled. Data at the diagnosis of aUC on age, sex, Eastern Cooperative Oncology Group (ECOG) PS, body mass index (BMI), the primary tumor site (bladder or UT), hydronephrosis, lymph node or visceral metastasis, curative surgery before and after the aUC diagnosis, first line therapy for aUC, hemoglobin, neutrophil and lymphocyte counts, creatinine, albumin, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), corrected calcium, C-reactive protein (CRP), total cholesterol, SMI, the CONUT score, and sarcopenia were collected from the medical records. The CONUT score was calculated using values for serum albumin, total lymphocyte count, and total cholesterol concentration (Table 1) [12]. BMI was calculated using the formula: BMI (kg/m2) = ((weight)/(height)2). The first line treatment of aUC was classified into: (1) Platinum-based chemotherapy; (2) chemoradiotherapy with a curative intent; and (3) best supportive care (BSC). Chemoradiotherapy was mainly offered to patients with locally advanced disease who were unfit for systemic chemotherapy. Low-dose cisplatin or fluorouracil was given concurrently with chemoradiotherapy as a radiosensitizer.
The primary aim of this study was to assess whether the CONUT score and sarcopenia are mutually independent, prognostic biomarkers in aUC.
2.2. Image Analysis Evaluating Sarcopenia
CT was performed for diagnosis or follow-up. Axial CT images taken within 30 days of diagnosis of aUC were used. The third lumbar vertebra (L3) was chosen as a landmark, and one slice was selected to measure the cross-sectional area of skeletal muscle using Hounsfield unit thresholds of −29 to +150 [21,34]. Skeletal muscle at the L3 level included the psoas, paraspinal muscles (erector spinae and quadratus lumborum), and abdominal wall muscles (transversus abdominus, external and internal obliques, and rectus abdominus). The total, cross-sectional area of lumbar skeletal muscle was linearly related to the cross-sectional area of the whole-body muscle [35]. To assess for sarcopenia, the total muscle area was normalized for stature, as is usually done for BMI and body composition assessments using the formula: Skeletal muscle index (SMI) (cm2/m2) = ((skeletal muscle cross-sectional area at L3)/(height)2) [21,34]. Images were analyzed using OsiriX Lite (Pixmeo, Geneva, Switzerland; https://www.osirix-viewer.com/ (accessed on 1 July 2022.)). Image analysis was performed by three investigators (M.U., H.S. and H.F.), who were blinded to other variables and patient outcomes. The representative definitions used to diagnose sarcopenia based on SMI included Prado’s definition (SMI < 52.4 cm2/m2 for male patients and <38.5 cm2/m2 for female patients) Martin’s definition (SMI < 43 cm2/m2 for male patients with a BMI < 25; SMI < 53 cm2/m2 for male patients with BMI ≥ 25; and SMI < 41 cm2/m2 for female patients), and the international definition (SMI < 55 cm2 /m2 for male patients and <39 cm2/m2 for female patients) (14–16). The prognostic value of sarcopenia was assessed according to each definition, and the definition in which sarcopenia was found to have the greatest prognostic value (the lowest p-value and highest Harrell’s concordance index [c-index]) was adopted.
2.3. Statistical Analysis
Differences in the distribution of the variables between the groups were evaluated using Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. OS was defined as the time from aUC diagnosis to either death or the last follow-up (data cutoff date: 31 December 2021). Martingale residuals were plotted for the CONUT score to judge the goodness of linear fit of the prognostic effects [29]. Patients were divided into three groups using two CONUT score cut-off values showing the greatest between-group differences in the OS curves. The survival curves were estimated using the Kaplan-Meier method, and differences between the groups were evaluated using the log-rank test. Univariate and multivariate Cox proportional hazards were used to test for any association between the variables and OS. The c-index was used to estimate the predictive accuracy of the prognostic models. To validate Apolo’s and Bajorin’s models, both of which include Karnofsky PS (K-PS) < 80% as one of the variables [9], K-PS < 80% was substituted with ECOG PS < 2 [36]. All statistical analyses were conducted using JMP 14.0.0. (SAS Institute Inc., Cary, NC, USA) and R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Patient Characteristics
Table 2 shows the demographic data of all 200 patients with aUC. The median (range) age at diagnosis was 71 (38–94) years, and the primary tumor site was the bladder and UT in 109 (55%) and 91 patients (45%), respectively. The ECOG PS was 0, 1, ≥2 in 136 (68%), 42 (21%), and 22 patients (11%), respectively. Forty-seven patients (23%) had previously undergone curative surgery. Metastases to lymph nodes and visceral organs and unresectable T4 disease were observed in 82 (41%), 78 (39%), and 44 patients (22%), respectively. In total, 163 (82%) patients received first-line therapy, such as platinum-based systemic chemotherapy (n = 148, 74%; n = 117, 59% for cisplatin and n = 31, 16% for carboplatin) or chemoradiation (n = 15, 8%). Meanwhile, 37 (19%) patients were placed on BSC. Pembrolizumab was given as a second or later line systemic therapy in 32 patients (16%), and 34 patients (17%) underwent curative surgery after achieving an objective response to systemic chemotherapy (n = 31, 16%) or chemoradiation (n = 3, 2%).
Table 2.
Baseline characteristics of 200 patients with advanced urothelial carcinoma.
| Variable | Total, N (%) | CONUT Score, N (%) | p | Sarcopenia, N (%) | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–3 | ≥4 | Yes | No | |||||
| Total | 200 (100) | 103 (52) | 72 (36) | 25 (12) | 168 (84) | 32 (16) | |||
| Age (years) * | 71 (38–94) | 70 (38–86) | 72 (46–90) | 72 (57–94) | 0.334 | 71 (38–91) | 69 (47–94) | 0.188 | |
| Sex | 0.426 | 0.012 | |||||||
| Male | 138 (69) | 70 (68) | 53 (74) | 15 (60) | 122 (73) | 16 (50) | |||
| Female | 62 (31) | 33 (32) | 19 (26) | 10 (40) | 46 (27) | 16 (50) | |||
| ECOG PS | <0.001 | 0.141 | |||||||
| 0 | 136 (68) | 73 (71) | 54 (75) | 9 (36) | 115 (68) | 21 (66) | |||
| 1 | 42 (21) | 24 (23) | 12 (17) | 6 (24) | 32 (19) | 10 (31) | |||
| ≥2 | 22 (11) | 6 (6) | 6 (8) | 10 (40) | 21 (13) | 1 (3) | |||
| BMI (kg/m2) * | 22 (15–36) | 23 (15–36) | 22 (15–32) | 21 (17–27) | <0.001 | 22 (15–34) | 24 (18–36) | <0.001 | |
| Primary tumor site | 0.599 | 0.019 | |||||||
| Bladder | 109 (55) | 58 (56) | 36 (50) | 15 (60) | 98 (58) | 11 (34) | |||
| UT | 91 (45) | 45 (44) | 36 (50) | 10 (40) | 70 (42) | 21 (66) | |||
| Hydronephrosis | <0.001 | 0.127 | |||||||
| No | 106 (53) | 56 (54) | 44 (61) | 6 (24) | 85 (51) | 21 (66) | |||
| Yes | 94 (47) | 47 (46) | 28 (39) | 19 (76) | 83 (49) | 11 (34) | |||
| Lymph node metastasis | 0.716 | 0.245 | |||||||
| No | 118 (59) | 62 (60) | 40 (56) | 16 (64) | 96 (57) | 22 (69) | |||
| Yes | 82 (41) | 41 (40) | 32 (44) | 9 (36) | 72 (43) | 10 (31) | |||
| Visceral metastasis | 0.072 | 0.845 | |||||||
| No | 122 (61) | 67 (65) | 45 (63) | 10 (40) | 103 (61) | 19 (59) | |||
| Yes | 78 (39) | 36 (35) | 27 (38) | 15 (60) | 65 (39) | 13 (41) | |||
| Prior curative surgery | 0.819 | 0.066 | |||||||
| No | 153 (77) | 80 (78) | 55 (76) | 18 (72) | 133 (79) | 20 (63) | |||
| Yes | 47 (23) | 23 (22) | 17 (24) | 7 (28) | 35 (21) | 12 (38) | |||
| 1st line therapy for aUC | 0.044 | 0.546 | |||||||
| Platinum-based chemotherapy | cisplatin | 117 (59) | 67 (34) | 40 (20) | 10 (5) | 0.782 | 93(47) | 24(12) | 0.009 |
| carboplatin | 31 (16) | 17 (9) | 10 (5) | 4 (2) | 30(15) | 1(1) | |||
| Chemoradiation | 15 (7.5) | 5 (33) | 8 (54) | 2 (13) | 14 (93) | 1 (7) | |||
| BSC | 37 (19) | 14 (38) | 14 (38) | 9 (24) | 30 (81) | 7 (19) | |||
| Curative surgery after diagnosis of aUC | 0.117 | 0.774 | |||||||
| No | 166 (83) | 80 (48) | 64 (39) | 22 (13) | 140 (84) | 26 (16) | |||
| Yes | 34 (17) | 23 (68) | 8 (24) | 3 (8) | 28 (82) | 6 (18) | |||
| Administration of pembrolizumab for 2nd or later line therapy | 32 (16) | 19 (59) | 12 (38) | 1 (3) | 0.206 | 31 (18) | 1 (3) | 0.030 | |
| Hemoglobin (g/dL) * | 12 (3.1–18) | 13 (7.7–18) | 11 (6.2–16) | 11 (3.1–13) | <0.001 | 12 (3.1–18) | 12 (6.2–15) | 0.109 | |
| Neutrophil (×103/μL) * | 4.9 (1.1–56) | 5.2 (1.1–31) | 4.3 (1.8–12) | 6.7 (1.7–56) | 0.011 | 6.9 (1.0–59) | 8.0 (4.2–18) | 0.060 | |
| Lymphocyte (×103/µL) * | 1.5 (0.17–4.3) | 1.8 (1.2–4.3) | 1.1 (0.64–2.7) | 1.1 (0.17–2.3) | <0.001 | 1.4 (0.17–4.0) | 1.8 (0.52–4.3) | 0.030 | |
| Creatinine (mg/dL) * | 1.0 (0.49–16) | 1.1 (0.49–4.3) | 1.0 (0.50–3.1) | 1.1 (0.50–16) | 0.298 | 1.0 (0.50–16) | 1.0 (0.50–3.1) | 0.450 | |
| Albumin (g/dL) * | 4.0 (2.7–5.0) | 4.1 (3.6–5.0) | 4.0 (3.2–4.9) | 3.2 (2.7–4.2) | <0.001 | 4 (2.7–5.0) | 4 (2.8–4.7) | 0.979 | |
| ALP (U/L) * | 261 (92–3351) | 253 (92–1539) | 258 (103–3351) | 307 (212–682) | 0.006 | 266 (92–3351) | 237 (147–586) | 0.142 | |
| LDH (U/L) * | 191 (118–2970) | 191 (119–2482) | 191 (118–2970) | 212 (129–386) | 0.382 | 192 (129–2970) | 187 (118–880) | 0.288 | |
| Corrected calcium (mg/dL) * | 8.8 (7.6–14) | 8.8 (7.6–14) | 8.7 (7.9–11) | 8.8 (7.6–11) | 0.829 | 8.8 (7.6–14) | 8.7 (7.9–9.3) | 0.168 | |
| CRP (mg/L) * | 6.0 (0.00–266) | 3.0 (0.40–115) | 5.7 (0.00–266) | 34 (0.60–139) | <0.001 | 6.4 (0.0–266) | 6.0 (0.60–65) | 0.632 | |
| Total cholesterol (mg/dL) * | 183 (98–275) | 195 (140–271) | 175 (98–275) | 167 (118–224) | <0.001 | 169 (126–240) | 187 (98–275) | 0.039 | |
| SMI (cm2/m2) * | 37 (16–64) | 38 (21–64) | 36 (16–62) | 33 (18–54) | 0.017 | 35 (16–51) | 53 (39–64) | <0.001 | |
| CONUT score * | 2 (0–8) | 1 (0–1) | 2 (2–3) | 5 (4–8) | - | 2 (0–8) | 1 (0–7) | 0.416 | |
| Sarcopenia | 0.219 | - | |||||||
| Yes | 168 (84) | 82 (80) | 64 (89) | 22 (88) | 168 (100) | 0 (0) | |||
| No | 32 (16) | 21 (20) | 8 (11) | 3 (12) | 0 (0) | 32 (100) | |||
CONUT, controlling nutritional status; ECOG PS, Eastern Cooperative Oncology Group performance status; BMI, body mass index; UT, upper tract; UC, urothelial carcinoma; BSC, best supportive care; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; CRP, C-reactive protein; SMI, skeletal mass index. * Median (range).
The CONUT score was 0, 1, 2, 3, and ≥4 in 37 (19%), 66 (33%), 43 (22%), 29 (14%), and 25 patients (12%), respectively, and was subgrouped into scores 0–1 (n = 103, 52%), 2–3 (n = 72, 36%), and ≥4 (n = 25, 12%) according to its association with OS. A higher CONUT score was significantly associated with a poorer ECOG PS (p < 0.001), lower BMI (p < 0.001), presence of hydronephrosis (p < 0.001), higher proportion of BSC (p = 0.044), lower hemoglobin (p < 0.001), higher neutrophil count (p = 0.011), higher ALP (p = 0.006), higher CRP (p < 0.001), lower SMI (p = 0.017), as well as lower albumin, lymphocyte count, and total cholesterol (all p < 0.001).
Table 3 shows that Prado’s definition had the highest prognostic value of the three definitions of sarcopenia. Based on Pardo’s definition, sarcopenia was observed in 168 patients (84%) and was more prevalent in male patients (p = 0.012) and patients with primary bladder UC (p = 0.019), in comparison to their counterparts, and was significantly associated with a lower BMI (p < 0.001), more frequent carboplatin use (p = 0.009), lower lymphocyte count (p = 0.030), and lower total cholesterol (p = 0.039, Table 2). No significant association was observed between sarcopenia and the CONUT score (p = 0.219); the median (range) CONUT score was 2 (0–8) and 1 (0–7) in patients with and without sarcopenia, respectively (p = 0.416).
Table 3.
Median overall survival and concordance index for each definition of Sarcopenia.
| Definition of Sarcopenia | Sarcopenia | Median OS (Range, Months) | p | C-Index |
|---|---|---|---|---|
| Prado’s definition | Absent | 20.5 (2–183) | 0.003 | 0.541 |
| Present | 12.5 (1–162) | |||
| Martin’s definition | Absent | 18.7 (1–183) | 0.214 | 0.516 |
| Present | 12.7 (1–162) | |||
| International definition | Absent | 20.9 (2–183) | 0.020 | 0.530 |
| Present | 12.7 (1–162) |
OS, overall survival; c-index, concordance-index.
3.2. Association of the CONUT Score and Sarcopenia with OS
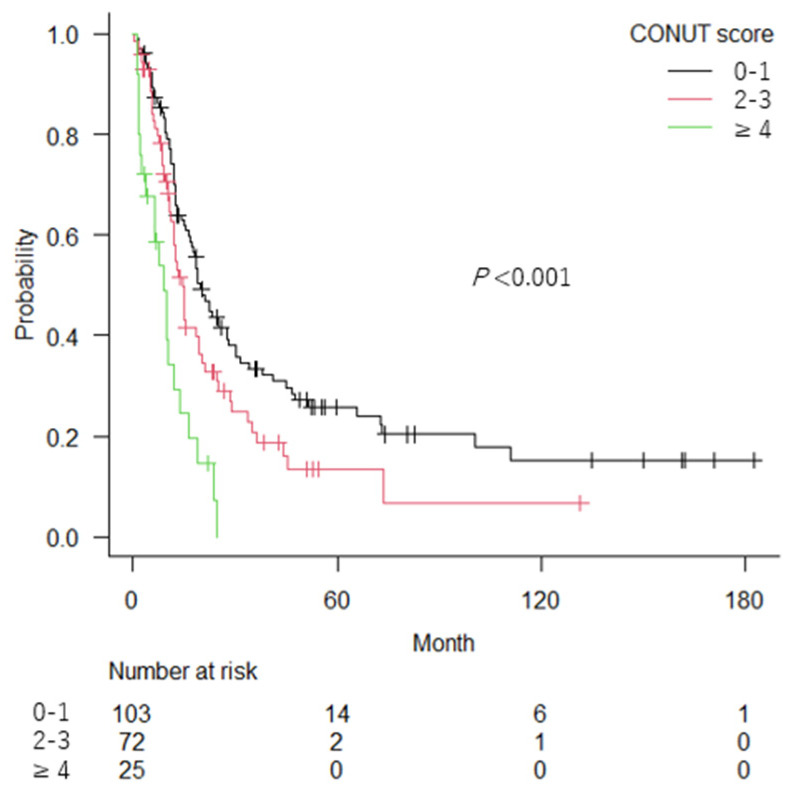
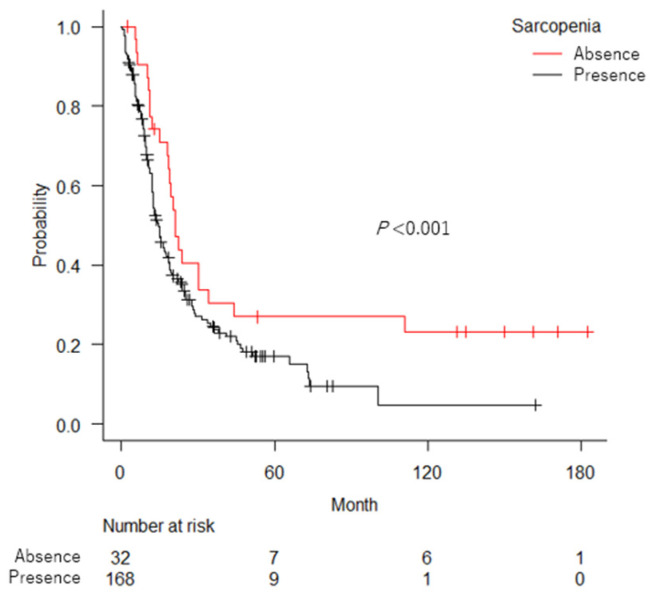
During follow-up (median: 13.3 months; range 1–183 months), 149 patients died. Figure 1 shows that the OS curves differed significantly among the CONUT scores 0–1, 2–3, and ≥4 with a median OS of 19.7 months, 14.5 months and 9.1 months, respectively (p < 0.001). Figure 2 shows that patients with sarcopenia had significantly shorter OS than those without sarcopenia (median OS: 14.5 vs. 20.9 months; p = 0.027).
Figure 1.
Kaplan–Meier curves for overall survival in patients with advanced urothelial carcinoma according to the controlling nutritional status (CONUT) score.
Figure 2.
Kaplan–Meier curves of overall survival in patients with advanced urothelial carcinoma according to the presence or absence of sarcopenia.
Table 4 shows the association of the variables with OS in univariable and multivariable analyses. In the univariable analysis, the CONUT score and sarcopenia were significantly associated with poor OS (hazard ratio [HR]: 1.18, p = 0.036; and HR: 2.28; p = 0.006, respectively). The following variables were also significantly associated with poor OS: age (HR: 1.03; p = 0.013), presence of hydronephrosis (HR: 1.50; p = 0.049), the UT primary tumor (HR: 1.90; p = 0.034), visceral metastasis (HR: 1.56; p = 0.034), previous curative surgery (HR: 1.11; p = 0.002), high neutrophil count (HR: 1.01; p = 0.013), high ALP (HR: 1.02; p < 0.001), and high LDH (HR: 1.01; p = 0.003). Multivariable analysis demonstrated that a high CONUT score (HR: 1.22; p < 0.001) and sarcopenia (HR: 2.23; p < 0.001), along with the following variables, were independently associated with poor OS: age (HR: 1.04; p = 0.002), presence of hydronephrosis (HR: 1.53; p = 0.016), UT primary tumor (HR: 1.69; p = 0.004), visceral metastasis (HR: 1.71; p = 0.004), high neutrophil count (HR: 1.01; p = 0.005), high ALP (HR: 1.02; p < 0.001), high LDH (HR: 1.01; p = 0.003), and high CRP (HR: 1.01; p = 0.033).
Table 4.
Univariable and multivariable analyses of overall survival in 200 patients with advanced urothelial carcinoma.
| Variables | Univariable | Multivariable (Final Model) | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p | HR | (95% CI) | p | |
| Age | 1.03 | (1.01–1.06) | 0.013 | 1.04 | (1.02–1.06) | 0.002 |
| Sex | ||||||
| Female (vs. male) | 1.38 | (0.89–2.13) | 0.134 | |||
| ECOG PS | ||||||
| 0 | ref | |||||
| 1 | 1.39 | (0.85–2.28) | 0.184 | |||
| ≥2 | 1.69 | (0.85–3.34) | 0.133 | |||
| BMI | 0.97 | (0.92–1.02) | 0.170 | |||
| Hydronephrosis | ||||||
| Yes (vs. no) | 1.50 | (1.00–2.26) | 0.049 | 1.53 | (1.08–2.17) | 0.016 |
| Primary site | ||||||
| UT (vs. bladder) | 1.90 | (1.26–2.85) | 0.034 | 1.69 | (1.18–2.41) | 0.004 |
| Lymph node metastasis | ||||||
| Yes (vs. no) | 0.94 | (0.64–1.37) | 0.758 | |||
| Visceral metastasis | ||||||
| Yes (vs. no) | 1.56 | (1.03–2.33) | 0.034 | 1.71 | (1.18–2.44) | 0.004 |
| Previous curative surgery | ||||||
| Yes (vs. no) | 1.11 | (1.26–2.85) | 0.002 | |||
| 1st line therapy for aUC | ||||||
| Chemotherapy | ref | |||||
| CRT | 1.32 | (0.61–2.67) | 0.452 | |||
| BSC | 1.05 | (0.59–1.82) | 0.862 | |||
| Curative surgery after diagnosis of aUC | ||||||
| Yes (vs. no) | 0.64 | (0.36–1.10) | 0.108 | |||
| Hemoglobin | 1.09 | (0.96–1.01) | 0.203 | |||
| Neutrophil † | 1.01 | (1.00–1.01) | 0.013 | 1.01 | (1.00–1.01) | 0.005 |
| Lymphocyte † | 1.00 | (0.96–1.03) | 0.900 | - | - | - |
| Creatinine | 1.13 | (0.97–1.29) | 0.103 | |||
| Albumin | 0.79 | (0.42–1.47) | 0.460 | - | - | - |
| ALP †† | 1.02 | (1.01–1.03) | <0.001 | 1.02 | (1.01–1.03) | <0.001 |
| LDH †† | 1.01 | (1.01–1.02) | 0.003 | 1.01 | (1.01–1.02) | 0.003 |
| Corrected calcium | 1.24 | (0.88–1.66) | 0.212 | |||
| CRP | 1.13 | (0.97–1.29) | 0.066 | 1.01 | (1.00–1.01) | 0.033 |
| Total cholesterol | 1.00 | (0.99–1.01) | 0.997 | - | - | - |
| Sarcopenia | ||||||
| Yes (vs. no) | 2.28 | (1.26–4.27) | 0.006 | 2.23 | (1.39–3.72) | <0.001 |
| CONUT score | 1.18 | (1.01–1.37) | 0.036 | 1.22 | (1.09–1.36) | <0.001 |
HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; BMI, body mass index; UT, upper tract; UC, urothelial carcinoma; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; CRP, C-reactive protein; CONUT, controlling nutritional status; NS, not significant. † every 100 units; †† every 10 units.
To confirm the prognostic independence of the CONUT score and sarcopenia, a sensitivity analysis was conducted. For 168 patients with sarcopenia (Supplementary Materials, Figure S1A), the CONUT score was significantly associated with OS (univariable HR: 1.35; 95% CI: 1.20–1.51; p < 0.001). While the OS curves were similarly separated (Supplementary Materials, Figure S1B) in 32 patients without sarcopenia, statistical significance was not observed, probably due to the small sample size (univariable HR: 1.16; 95% CI: 0.92–1.46; p = 0.218). In 175 patients with the CONUT score 0–3, sarcopenia was significantly associated with shorter OS (p = 0.039, Supplementary Materials, Figure S2A). OS curves were similarly separated in 25 patients with the CONUT score ≥4, but no statistical difference was observed in this small subgroup (p = 0.394, Supplementary Materials, Figure S2B).
3.3. Role of the CONUT Score and Sarcopenia as Prognostic Factors
Next, we evaluated the role of the CONUT score and sarcopenia as prognostic factors in a subgroup of 148 patients who had received first-line platinum-based systemic chemotherapy using two established prognostic models for such patients. When adding the CONUT score instead of Alb, sarcopenia, and both of Apolo’s models, the c-index increased from 0.634 to 0.655, 0.653, and 0.668, respectively. Similarly, the c-index increased from 0.612 to 0.653, 0.631 and 0.665, respectively, with Bajorin’s model (Table 5).
Table 5.
C-index of the established prognostic models combined with the controlling nutritional status (CONUT) score or/and sarcopenia.
| Prognostic Model | C-Index | Prognostic Model | C-Index |
|---|---|---|---|
| Apolo’s model (KPS + visceral metastasis + Hb + Alb) | 0.634 | Bajorin’s model (KPS + visceral metastasis) | 0.612 |
| Apolo’s model + the CONUT score—Alb | 0.655 | Bajorin’s model + the CONUT score | 0.653 |
| Apolo’s model + sarcopenia | 0.653 | Bajorin’s model + sarcopenia | 0.631 |
| Apolo’s model + the CONUT score—Alb + sarcopenia | 0.668 | Bajorin’s model + the CONUT score + sarcopenia | 0.665 |
KPS, Karnofsky performance status; Hb, hemoglobin; Alb, albumin; the CONUT score, controlling nutritional status score.
4. Discussion
Both the CONUT score and sarcopenia are known to be objective indicators of a patient’s general condition and to provide prognostic information about patients with various malignancies. However, no study has examined whether there is any association between the two indicators or what their comprehensive prognostic role in patients with advanced cancer might be. To the best of our knowledge, the present study is the first to address these questions. First, our study found no significant association between the CONUT score and sarcopenia diagnosed using Prado’s definition in our cohort of 200 patients with aUC. Second, both the CONUT score and sarcopenia were independent prognostic factors of OS, and these indices were mutually independent in terms of their prognostic value. In fact, adding the CONUT score, sarcopenia, and both to Apolo’s and Bajorin’s prognostic models, improved the models’ predictive accuracy in 148 patients who had received first-line platinum-based chemotherapy. Our findings suggested that the CONUT score and sarcopenia, which are objective indices of a patient’s general condition in terms of nutritional status and body composition, respectively, are independently associated with the prognosis of patients with aUC. This study corroborated the prognostic value of a patient’s general condition and indicated the importance of separately evaluating the CONUT score and sarcopenia as prognostic factors in aUC.
PS is an established prognostic factor of aUC [9,10]. However, it demonstrated no prognostic value in the present study, possibly because the subjectivity of PS led to inaccuracies in its assessment by multiple physicians [37]. At any rate, the CONUT score and sarcopenia outperformed PS as prognostic factors in the present study.
According to the international consensus, cancer cachexia is defined as a multifactorial, complex, metabolic syndrome characterized by an ongoing loss of skeletal muscle mass that cannot be fully reversed by conventional nutritional support [22]. In patients with cancer cachexia, the metabolic balance shifts towards catabolism rather than anabolism, owing mainly to cancer-related systemic inflammation and anorexia, which lead to sarcopenia and malnutrition [38,39]. Thus, sarcopenia and the CONUT score can reflect the presence and severity of cancer cachexia, respectively. In the present study, the CONUT score was inversely associated with SMI, while sarcopenia was associated with lower lymphocyte counts and cholesterol levels. However, no significant association was observed between the CONUT score and sarcopenia. The lack of the association could be the consequence of the chosen cut-off of SMI according to the Prado’s definition. While sarcopenia was observed in 84% of aUC patients, more than half of the sarcopenic patients had normal or only slightly impaired nutritional status, as indicated by a CONUT score of 0–2, thus suggesting that malnutrition, particularly hypoalbuminemia, develops at a later phase of cachexia than sarcopenia in the majority of aUC patients. It is notable that more than 20% of patients with aUC without malnutrition (CONUT score 0–1) and those without sarcopenia survived five years or longer (Figure 1 and Figure 2). As less cachexic patients are also more tolerant of intensive therapy [40,41], they may be good candidates for intensive aUC treatment.
Currently, the optimal definition of SMI-based sarcopenia has not been standardized. The present study compared the prognostic value of three major definitions of sarcopenia [21,22,23] and demonstrated that Prado’s definition most significantly stratified the prognosis (Table 3). The three definitions of sarcopenia originated from the same Canadian cohort. Given that the Japanese population generally have smaller body frame than their Canadian counterparts, Prado’s definition with its lower SMI thresholds may more accurately reflect sarcopenia in Japanese patients than the international definition. Indeed, a multiracial cohort study of healthy elderly people demonstrated that the prevalence of sarcopenia was higher in Asians than other racial groups including Whites, Blacks, and Hispanics when a common definition of sarcopenia was applied [42]. Some researchers have questioned the scientific basis of Martin’s definition, in which the SMI threshold for men depends on BMI and is therefore discontinuous [43]. In fact, a preliminary study of 64 Japanese patients with aUC demonstrated that, of the three major definitions, Prado’s definition was the best predictor of cancer-specific survival, while Martin’s definition failed to demonstrate a significant difference between patients with and without sarcopenia [44]. Given that body frame varies among racial groups, different definitions of SMI-based sarcopenia may be needed depending on racial groups.
The present study had several limitations. First, its retrospective, monocentric design may have introduced a bias. External validation using a large, multicentric cohort is needed to verify the generalizability of the mutual independence of the CONUT score and sarcopenia in aUC patients. Second, about 20% (47/250) of patients were excluded because some of the data pertaining to the CONUT sore (mainly total cholesterol) or sarcopenia in the initial cohort were missing. There may also be some bias associated with this exclusion. Third, use of statins, which influence the total cholesterol level, was not included as a variable because the relevant data were incomplete. Fourth, only 32 patients received pembrolizumab, the current, standard, second-line treatment for aUC and none received maintenance avelumab following platinum-based chemotherapy. As pembrolizumab and avelumab were approved in December 2017 and February 2021, respectively, in Japan, most of the patients were unable to receive ICIs. Different results might be obtainable in a new cohort receiving ICIs. Fifth, our study cohort consisted of only Japanese patients and thus further studies on other racial groups are needed to validate the generality of our findings.
5. Conclusions
The present study demonstrated the prognostic significance of the CONUT score and sarcopenia, which were found to be mutually independent in Japanese patients with aUC. These objective indices of a patient’s general condition in terms of nutritional status and body composition, respectively, may help in decision-making when choosing a treatment for aUC.
Acknowledgments
This study was supported by the Clinical Research Fund of the Tokyo Metropolitan Government (R04030318).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14205075/s1, Figure S1: Kaplan-Meier curves for overall survival in patients with (A) and without sarcopenia (B) according to the controlling nutritional status (CONUT) score. Figure S2: Kaplan–Meier curves of overall survival in patients with the CONUT score 0–3 (A) and ≥4 (B) according to the presence or absence of sarcopenia.
Author Contributions
Conceptualization, F.K.; methodology, M.U., M.I. and F.K.; software, M.U., H.S. and H.F.; validation, M.I., S.K. and M.T.; formal analysis, M.U. and M.I.; research, M.U. and M.I.; resources, F.K.; data curation, M.U. and M.I.; writing—original draft preparation, M.U. and M.I.; writing—review and editing, H.S., M.T., S.K., H.F. and F.K.; visualization, M.U.; supervision, F.K.; project administration, F.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital (protocol code 2894 and date of approval 10 May 2022).
Informed Consent Statement
Informed consent was waived because the study was retrospective and observational.
Data Availability Statement
The data can be shared up on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding besides the Clinical Research Fund of the Tokyo Metropolitan Government (R04030318).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.De Santis M., Bellmunt J., Mead G., Kerst J.M., Leahy M., Maroto P., Gil T., Marreaud S., Daugaard G., Skoneczna I., et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012;30:191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von der Maase H., Sengelov L., Roberts J.T., Ricci S., Dogliotti L., Oliver T., Moore M.J., Zimmermann A., Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 4.Logothetis C.J., Dexeus F.H., Finn L., Sella A., Amato R.J., Ayala A.G., Kilbourn R.G. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J. Clin. Oncol. 1990;8:1050–1055. doi: 10.1200/JCO.1990.8.6.1050. [DOI] [PubMed] [Google Scholar]
- 5.Loehrer P.J., Einhorn L.H., Elson P.J., Crawford E.D., Kuebler P., Tannock I., Raghavan D., Stuart-Harris R., Sarosdy M.F., Lowe B.A. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J. Clin. Oncol. 1992;10:1066–1073. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J., von der Maase H., Mead G.M., Skoneczna I., De Santis M., Daugaard G., Boehle A., Chevreau C., Paz-Ares L., Laufman L.R., et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012;30:1107–1113. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellmunt J., de Wit R., Vaughn D.J., Fradet Y., Lee J.L., Fong L., Vogelzang N.J., Climent M.A., Petrylak D.P., Choueiri T.K., et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powles T., Rosenberg J.E., Sonpavde G.P., Loriot Y., Durán I., Lee J.L., Matsubara N., Vulsteke C., Castellano D., Wu C., et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021;384:1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajorin D.F., Dodd P.M., Mazumdar M., Fazzari M., McCaffrey J.A., Scher H.I., Herr H., Higgins G., Boyle M.G. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J. Clin. Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 10.Apolo A.B., Ostrovnaya I., Halabi S., Iasonos A., Philips G.K., Rosenberg J.E., Riches J., Small E.J., Milowsky M.I., Bajorin D.F. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J. Natl. Cancer Inst. 2013;105:499–503. doi: 10.1093/jnci/djt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takemura K., Yuasa T., Fujiwara R., Ito M., Suzuki H., Yonese J., Koga F. Prognostic Significance of the Controlling Nutritional Status (CONUT) Score in Patients with Advanced Renal Cell Carcinoma Treated with Nivolumab after Failure of Prior Tyrosine Kinase Inhibitors. J. Urol. 2020;204:1166–1172. doi: 10.1097/JU.0000000000001196. [DOI] [PubMed] [Google Scholar]
- 12.de Ulíbarri J.I., González-Madroño A., de Villar N.G., González P., González B., Mancha A., Rodríguez F., Fernández G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 13.González-Madroño A., Mancha A., Rodríguez F.J., Culebras J., de Ulibarri J.I. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: Comparison with two logistic regression models developed using SGA as the gold standard. Nutr. Hosp. 2012;27:564–571. doi: 10.1590/S0212-16112012000200033. [DOI] [PubMed] [Google Scholar]
- 14.Baysal M., Bas V., Demirci U., Gulsaran S.K., Umit E., Kirkizlar H.O., Demir A.M. The Utility of CONUT Score in Diffuse Large B Cell Lymphoma Patients. Niger J. Clin. Pract. 2021;24:1194–1199. doi: 10.4103/njcp.njcp_429_20. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda D., Sawayama H., Kurashige J., Iwatsuki M., Eto T., Tokunaga R., Kitano Y., Yamamura K., Ouchi M., Nakamura K., et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018;21:204–212. doi: 10.1007/s10120-017-0744-3. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto S., Ureshino H., Kidoguchi K., Kusaba K., Kizuka-Sano H., Sano H., Nishioka A., Yamaguchi K., Kamachi K., Itamura H., et al. Clinical impact of the CONUT score in patients with multiple myeloma. Ann. Hematol. 2020;99:113–119. doi: 10.1007/s00277-019-03844-2. [DOI] [PubMed] [Google Scholar]
- 17.Toyokawa T., Kubo N., Tamura T., Sakurai K., Amano R., Tanaka H., Muguruma K., Yashiro M., Hirakawa K., Ohira M. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC Cancer. 2016;16:722. doi: 10.1186/s12885-016-2696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki H., Ito M., Takemura K., Nakanishi Y., Kataoka M., Sakamoto K., Tobisu K.I., Koga F. Prognostic significance of the controlling nutritional status (CONUT) score in advanced urothelial carcinoma patients. Urol. Oncol. 2020;38:76.e11–76.e17. doi: 10.1016/j.urolonc.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H., Ito M., Takemura K., Kobayashi S., Kataoka M., Iida N., Sekiya K., Matsumoto T., Koga F. The Controlling Nutritional Status(CONUT) Score is a Prognostic Biomarkerin Advanced Urothelial Carcinoma PatientsTreated with First-Line Platinum-Based Chemotherapy. Bladder Cancer. 2021;7:13–21. doi: 10.3233/BLC-200354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim T.N., Choi K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013;20:1–10. doi: 10.11005/jbm.2013.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., Baracos V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 22.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., Jatoi A., Loprinzi C., MacDonald N., Mantovani G., et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 23.Martin L., Birdsell L., Macdonald N., Reiman T., Clandinin M.T., McCargar L.J., Murphy R., Ghosh S., Sawyer M.B., Baracos V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 24.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., van Kan G.A., Andrieu S., Bauer J., Breuille D., et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang H.Y., Choi G.H., Hwang S.H., Jang E.S., Kim J.W., Ahn J.M., Choi Y., Cho J.Y., Han H.S., Lee J., et al. Sarcopenia and visceral adiposity predict poor overall survival in hepatocellular carcinoma patients after curative hepatic resection. Transl. Cancer Res. 2021;10:854–866. doi: 10.21037/tcr-20-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pekařová A., Pekař M., Daniš D., Nováková Z. CT evaluated sarcopenia signals: Shorter survival for small cell lung cancer patients. Physiol. Res. 2021;70:S381–S386. doi: 10.33549/physiolres.934816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caan B.J., Cespedes Feliciano E.M., Prado C.M., Alexeeff S., Kroenke C.H., Bradshaw P., Quesenberry C.P., Weltzien E.K., Castillo A.L., Olobatuyi T.A., et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukushima H., Yokoyama M., Nakanishi Y., Tobisu K., Koga F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE. 2015;10:e0115895. doi: 10.1371/journal.pone.0115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayr R., Fritsche H.M., Zeman F., Reiffen M., Siebertz L., Niessen C., Pycha A., van Rhijn B.W.G., Burger M., Gierth M. Sarcopenia predicts 90-day mortality and postoperative complications after radical cystectomy for bladder cancer. World J. Urol. 2018;36:1201–1207. doi: 10.1007/s00345-018-2259-x. [DOI] [PubMed] [Google Scholar]
- 31.van der Kroft G., Olde Damink S.W.M., Neumann U.P., Lambertz A. Sarcopenia and Cachexia-associated Risk in Surgery. Zent. Chir. 2021;146:277–282. doi: 10.1055/a-1447-1259. [DOI] [PubMed] [Google Scholar]
- 32.Abe H., Takei K., Uematsu T., Tokura Y., Suzuki I., Sakamoto K., Nishihara D., Yamaguchi Y., Mizuno T., Nukui A., et al. Significance of sarcopenia as a prognostic factor for metastatic urothelial carcinoma patients treated with systemic chemotherapy. Int. J. Clin. Oncol. 2018;23:338–346. doi: 10.1007/s10147-017-1207-x. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu R., Honda M., Teraoka S., Yumioka T., Yamaguchi N., Kawamoto B., Iwamoto H., Morizane S., Hikita K., Takenaka A. Sarcopenia is associated with survival in patients with urothelial carcinoma treated with systemic chemotherapy. Int. J. Clin. Oncol. 2022;27:175–183. doi: 10.1007/s10147-021-02032-5. [DOI] [PubMed] [Google Scholar]
- 34.Mitsiopoulos N., Baumgartner R.N., Heymsfield S.B., Lyons W., Gallagher D., Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 35.Shen W., Punyanitya M., Wang Z., Gallagher D., St-Onge M.P., Albu J., Heymsfield S.B., Heshka S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 36.Buccheri G., Ferrigno D., Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur. J. Cancer. 1996;32A:1135–1141. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 37.Chow R., Chiu N., Bruera E., Krishnan M., Chiu L., Lam H., DeAngelis C., Pulenzas N., Vuong S., Chow E. Inter-rater reliability in performance status assessment among health care professionals: A systematic review. Ann. Palliat. Med. 2016;5:83–92. doi: 10.21037/apm.2016.03.02. [DOI] [PubMed] [Google Scholar]
- 38.da Silva S.P., Santos J.M.O., Costa E Silva M.P., da Costa R.M.G., Medeiros R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle. 2020;11:619–635. doi: 10.1002/jcsm.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pin F., Barreto R., Couch M.E., Bonetto A., O’Connell T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle. 2019;10:140–154. doi: 10.1002/jcsm.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siddiqui J.A., Pothuraju R., Jain M., Batra S.K., Nasser M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim. Biophys. Acta Rev. Cancer. 2020;1873:188359. doi: 10.1016/j.bbcan.2020.188359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vermaete N., Wolter P., Verhoef G., Gosselink R. Physical activity and physical fitness in lymphoma patients before, during, and after chemotherapy: A prospective longitudinal study. Ann. Hematol. 2014;93:411–424. doi: 10.1007/s00277-013-1881-3. [DOI] [PubMed] [Google Scholar]
- 42.Cassie J., Lan-Juan Z., Kehao W. Race and socioeconomic effect on sarcopenia and sarcopenic obesity in the Louisiana Osteoporosis Study (LOS) JCSM Clin. Rep. 2018;3:1–8. doi: 10.17987/jcsm-cr.v3i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taguchi S., Nakagawa T., Fukuhara H. Inconsistencies in currently used definitions of sarcopenia in oncology. Ann. Oncol. 2020;31:318–319. doi: 10.1016/j.annonc.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Taguchi S., Nakagawa T., Uemura Y., Akamatsu N., Gonoi W., Naito A., Kawai T., Kume H., Fukuhara H. Comparison of major definitions of sarcopenia based on the skeletal muscle index in patients with urothelial carcinoma. Future Oncol. 2021;17:197–203. doi: 10.2217/fon-2020-0570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data can be shared up on request.