Abstract
Introduction: The aim of this study was to determine whether preoperative nutritional status and inflammatory status, specifically polyunsaturated acids and the omega 6/3 ratio, would affect postoperative outcomes and complications in patients with lung cancer undergoing lung resection. Methods: This prospective observational study included 68 patients with early-stage non-small-cell lung cancer who were candidates for radical surgery. A complete nutritional assessment was performed. The primary study variable was postoperative complications and mortality in the first 30 days. Descriptive, bivariate, and logistic regression analyses were carried out. Results: A total of 50 men (73.53%) and 18 women (26.47%) underwent surgery, with a median age of 64.2 (±9.74) years. The mean omega 6/3 ratio was 17.39 (±9.45). A complication occurred in 39.7% of the study sample (n = 27), the most common being persistent air leak in 23.53% (n = 16). After performing the bivariate analysis, the only variable that remained significant was the omega 6/3 ratio; we observed that it had a prognostic value for persistent air leak (p = 0.001) independent of age, sex, comorbidity, preoperative respiratory function, and approach or type of surgery. The remaining nutritional and inflammatory markers did not have a statistically significant association (p > 0.05) with postoperative complications. However, this significance was not maintained in the multivariate analysis by a small margin (p = 0.052; 95% CI: 0.77–1.41). Conclusions: Omega 6/3 ratio may be a prognostic factor for air leak, independent of the patient’s clinical and pathological characteristics.
Keywords: fatty acids, ratio omega 6/3, lung cancer, thoracic surgery, prolonged air leak
1. Introduction
Despite recent advances in early diagnosis and the potential implementation of screening programs in the near future [1], lung cancer remains the second most commonly diagnosed tumor worldwide and the leading cause of oncological deaths [2,3], with an overall 5-year survival of 10.6% [4,5].
Nutritional status has been postulated as a determining factor in the postoperative outcomes of oncology patients undergoing pulmonary resection [6]. A good nutritional status helps ensure a good immunological response; it is, therefore, pertinent to carry out a complete nutritional assessment that includes, among other variables, the status of micronutrients such as omega fatty acids. Omega-3 fatty acids are polyunsaturated fatty acids characterized in their chemical structure by the presence of a double bond three atoms away from the methyl terminal [7].
The modern diet (influenced by Anglo-Saxon countries) has a great imbalance in this aspect, as it contains a high proportion of lipids, with an omega-6 (ω-6) polyunsaturated fatty acid content much higher than the omega 3 (ω-3) content. It is suggested to maintain the ratio of omega-6/omega-3 consumption in the range from 1/5 to 1/10 and, ideally, as in Japanese society, from 1/2 to 1/4. These fatty acids are reported as having proinflammatory and anti-inflammatory functions, respectively [7,8]. The right balance between omega-6 and omega-3 is essential for the correct function of cell membranes, enzyme activity, and genetic expression.
Previous studies on this subject in patients with lung cancer have mainly been conducted in patients with advanced-stage disease, and omega-3 fatty acid supplementation has been reported to have positive effects on quality of life and physical activity during multimodal treatments [9,10].
We, therefore, sought to study the preoperative omega 6/3 ratio, among other nutritional and inflammatory variables, and their effects on postoperative outcomes in patients with early-stage lung cancer.
2. Material and Methods
2.1. Study Design
This was a prospective observational study (case series) on preoperative nutritional status in patients with early-stage non-small-cell lung cancer who were candidates for radical surgery. The study was carried out in accordance with good clinical practice and was approved by the hospital’s ethics committee. All participants gave signed informed consent to participate in the study.
2.1.1. Inclusion Criteria
Adult patients (aged 18 years or older), male or female, of any race/ethnicity;
Patients who gave informed consent to participate;
Patients with early-stage non-small-cell lung cancer who were candidates for surgical treatment, diagnosed before or during surgery.
2.1.2. Exclusion Criteria
Patients who did not consent to participate in any of the study phases;
Aged younger than 18 years;
Locally advanced lung cancer on neoadjuvant treatment;
History of rheumatologic, systemic or hepatic disease, or immunodeficiency;
Infection prior to surgery requiring treatment with a specific antibiotic;
Patients who declined surgery.
2.1.3. Sample Size
It was intended to recruit 100 patients to make a model of logistic regression for the main analysis. Following the conservative rule of 10 events per independent variable and taking into account between 10 and 15% of patients with some postsurgical complication in the first 30 days, it would be a sufficient sample size to estimate the model. Data collection started in September 2017 and ceased in 2020 due to the COVID-19 situation, with a total of 71 patients recruited.
2.2. Data Collection and Analysis
Data were collected on demographics, past medical history, respiratory function tests, American Society of Anesthesiologists (ASA) classification, Charlson Index, tumor characteristics, type of surgery, and surgical approach. A preoperative nutritional assessment and preoperative blood tests, including nutritional and inflammatory markers, were performed.
The postoperative variables analyzed were drain duration, persistent (>5 days) air leak, drain output, and hospital length of stay. Also included were complications, mortality, and grade of complication according to the Clavien–Dindo classification (10).
On the day of surgery, a blood sample was taken to analyze the inflammatory and nutritional parameters and the proportion of omega fatty acids to calculate the omega 6/3 ratio.
2.2.1. Assessment of Inflammatory Status
The neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR), and systemic immune-inflammation index (SII) were calculated. C-reactive protein (CRP), transferrin, and other inflammatory variables were measured.
2.2.2. Nutritional Assessment
All the patients were seen between 15 and 30 days before surgery by a dietician who performed a preoperative nutritional assessment that included the following: weight, height, bioelectrical impedance analysis, dynamometry, overall subjective assessment (OSA), body mass index (BMI), nutritional risk index (NRI), nutritional prognostic index (NPI), assessment of adherence to the Mediterranean diet, assessment of malnutrition status using the Chang method, International Physical Activity Questionnaire (IPAQ), fat-free mass (FFM), body cell mass index (BCMI), fat mass (FM), and malnutrition screening tool (MST).
2.2.3. Quantification of Blood Omega 6/3 Ratio
A 5 mL heparinized blood sample was taken after anesthetic induction and before starting surgery after an overnight fast. It was allowed to clot for at least 30 min, then centrifuged (10 min) at 700× g within 1 h of being taken. The samples were aliquoted and kept frozen at −70 to −80 °C until analyzed (maximum 1 year). Quantification was by gas chromatography and spectrometry performed by the Scientific Department of the University of Barcelona.
2.3. Statistical Analysis
All collected data were stored in a Microsoft Access database, with the coding of patients who were assigned a number at study inclusion (pseudonymization).
All the patient variables included were described for the whole sample. We used descriptive statistics according to the nature of the variable. Continuous variables were described using number of valid observations with measures of central tendency (mean or median) and dispersion (standard deviation, interquartile range) and certain other descriptors of interest (minimum, first quartile, third quartile, maximum). Categorical variables were described as the number of valid observations and percentage.
A cutoff was calculated for the variable omega 6/3 ratio using the Youden index, and its sensitivity and specificity were studied using the area under the curve (AUC).
The Kolmogorov–Smirnov test was used to determine normality of distribution of the quantitative variables. In the bivariate analysis, we used the Student’s t-test for quantitative variables that followed a normal distribution and the Mann–Whitney U test for those that did not. For qualitative variables, the Chi-squared test was used.
For the multivariate analysis, logistic regression was performed. Potential confounding factors considered for the multivariate model were age, sex, preoperative BMI, preoperative BCMI, history of COPD, drain duration, persistent air leak, and NPI.
The conditions of application of the models were validated, and the 95% confidence intervals (95% CI) of the estimator were calculated. Statistical significance was set at the <0.05 confidence level. The statistical package SPSS 25.0 (IBM Corp., Endicott, NY, USA) was used.
3. Results
A total of 71 patients were included during the recruitment period between September 2017 and January 2020. Three (4.2%) dropped out, so their data were removed, leaving 68 for the definitive analysis. The three dropouts were because these patients did not want to travel for a nutritional assessment (2) or blood test (1).
The analysis included 68 patients, 50 (73.35%) of whom were men, and the mean age was 64.25 ± 9.74 years. One-third of patients had type 2 diabetes (27.94%). Only nine patients were nonsmokers (13.24%). Most patients (72.1%; n = 49) had a high preoperative level of comorbidity, with a Charlson Index of 3 or more, implying a 3-year mortality of 52–58%.
The median preoperative FEV1 (maximal expiratory volume in the first second) was 100.44 ± 94.95 mL. Lobectomy was performed in 86.76% of the patients (n = 59). The most common histology was adenocarcinoma in 46 patients (63.24%), and most patients had pathological stage IB (n = 16; 23.52%). The remaining pathological, clinical, and surgical variables are reported in Table 1.
Table 1.
Clinicopathological and surgical characteristics of the patients.
| Variable | N (%) |
|---|---|
| Age | 64.25 (±9.74) * |
| Male sex | 50 (73.52%) |
| Active smoker | 18 (26.47%) |
| Never smoker | 9 (13.245) |
| Exsmoker | 22 (32.35%) |
| Year packages | 34.36 (±21.14) |
| Diabetes | 19 (27.94%) |
| COPD | 19 (27.94%) |
| Ischemic heart disease | 6 (8.82%) |
| With previous pulmonary neoplasia | 1 (1.47%) |
| Without previous pulmonary neoplasia | 21 (30.88%) |
| Cerebral vascular accident (CVA) | 3 (4.41%) |
| Peripheral vasculopathy | 6 (8.82%) |
| Previous cardiac surgery | 1 (1.47%) |
| Nephropathy | 4 (5.88%) |
| Dyslipidemia | 28 (41.18%) |
| Hypertension | 35 (51.47%) |
| Charlson Index | |
| 0 | 2 (2.94%) |
| 1 | 6 (8.82%) |
| 2 | 11 (16.18%) |
| 3 | 13 (19.12%) |
| 4 | 14 (29.59%) |
| 5 | 12 (17.65%) |
| 6 or more | 10 (14.71%) |
| Thoracoscore (predicted mortality) | 3.95 (±0.99) |
| Maximal expiratory volume in the first second (FEV1) | 100.44 (±94.95) * |
| Histology | |
| Adenocarcinoma | 43 (63.24%) |
| Squamous cell carcinoma | 12 (17.65%) |
| Carcinoide tumor | 2 (2.94%) |
| Large cell | 4 (5.88%) |
| Benign | 6 (8.82%) |
| Metastasis CCR | 3 (4.41%) |
| Pathological staging | |
| Ia1 | 3 (4.41%) |
| Ia2 | 11(16.17%) |
| Ia3 | 6 (8.82%) |
| Ib | 16 (23.52%) |
| IIa | 3 (4.41%) |
| IIb | 13 (19.12%) |
| IIIa | 7 (10.29%) |
| IIIb | 2 (2.94%) |
| Surgery | |
| Lobectomy | 59 (86.76%) |
| Wedge | 8 (11.76%) |
| Bilobectomy | 1 (1.47%) |
| Approach | |
| Thoracotomy | 35 (51.47%) |
| Thoracoscopy | 31 (45.59%) |
| RATS (robotic-assisted thoracic surgery) | 2 (2.94%) |
* IQR, median ± interquartile range.
The main parameters of preoperative nutritional status and inflammatory status are presented in Table 2. The mean (±SD) preoperative patient weight was 75.61 ± 14.22 kg, and the mean preoperative BMI was 27.06 ± 4.96. Preoperative adherence to a Mediterranean diet was reported in 58.82% of the participants. The mean value of the primary study variable, omega 6/3 ratio, was 17.39 ± 9.45, and the mean vitamin D level was 35.78 ± 21.05 ng/mL. The median preoperative phA was 6.46 ± 2.1, and mean BCMI was 11 ± 2.33.
Table 2.
Preoperative nutritional and inflammatory study.
| Variable | Mean or Median (SD or IQR) |
|---|---|
| Height | 1.66 (±0.09) |
| Weight | 75.61 (±14.22) |
| PhA grades | 6.46 (±2.10) * |
| BCMI (body cell mass index) | 11 (±2.33) |
| BCM (body composition monitor) (kg) | 30.29 (±8.01) |
| FFM (fat-free mass) (kg) | 54.6 (±9.92) |
| FM (fat mass) (kg) | 19.52 (±8.92) |
| MST (multistage testing) | 12 (±17.65) |
| Adherence to the Mediterranean diet test | 40 (±58.82) |
| BMI (body mass index) | 27.06 (±4.96) |
| IPN (prognostic nutritional index) | 47.1 (±5.85) |
| Preoperative Plasmatic Test | |
| Albumin | 39.63 (±3.75) * |
| Prealbumin | 225.05 (±67.69) * |
| Cholesterol | 34.64 (117.91) |
| Vitamin D | 35.78 (±21.05) * |
| Ácidos Grasos | |
| Ratio omega 6/3 | 17.39 (±9.45) |
| EPA | 0.36 (±0.29) |
| DHA | 1.23 (±0.71) |
| Linolenic acid | 0.41 (±0.27) |
| ARA | 6.33 (±1.90) |
| Linoleic acid | 24.07 (±7.40) |
| Inflammatory Parameters | |
| Neutrophils | 4720.44 (±2398.10) |
| Lymphocytes | 1561.03 (±779.70) |
| Platelets | 205,177.94 (±79,743.10) |
| Monocytes | 525.59 (±206.60) |
| Ratio neutrophils/lymphocytes | 3.91 (±3.64) |
| Ratio platelets/lymphocytes | 152.32 (±70.36) |
| Ratio lymphocytes/monocytes | 3.3 (±1.64) |
| Transferrin | 41.66 (±58.30) |
| PCR | 9.04 (±16.22) |
| Fibrinogen | 4.05 (±51.63) * |
| SII (systemic immune-inflammation index) | 12,162.21 (±581,087.45) |
* IQR, median ± interquartile range.
Postoperative complications occurred in 39.71% (n = 27) of the patients (Table 3), the most common being persistent air leak in 16 patients (23.53%), followed by respiratory failure (10.29%), surgical wound infection (7.35%), pneumonia (4.41%), and empyema (4.41%). All complications are reported in Table 3. None of the patients had deep vein thrombosis, pulmonary embolism, acute coronary syndrome, acute heart failure, stroke, vocal cord paralysis, bronchial fistula, or thoracic wall abscess, and none died during the study; there were no cases of postoperative hemothorax or chylothorax. The median hospital stay was 5.73 days (IQR = 4.68).
Table 3.
Postoperative complications.
| Variable | N (%) |
|---|---|
| Any complication | 27 (39.71%) |
| Atelectasis | 2 (2.94%) |
| Pneumonia | 3 (4.41%) |
| Respiratory insufficiency | 7 (10.29%) |
| Atrial fibrillation | 3 (4.41%) |
| Sepsis | 2 (2.94%) |
| Fever (without pneumonia) | 1 (1.47%) |
| Urinary infection | 1 (1.47%) |
| Surgical complication | 4 (5.885%) |
| Prolonged air leak (more than 5 days) | 16 (23.54%) |
| Empyema | 3 (4.41%) |
| Surgical wound infection | 5 (7.35%) |
| Drainage days | 4.70 (±8.16) |
| Length of stay | 5.73 (±4.68) * |
| Recurrence | 15 (21.15%) |
| Exitus | 5 (7.4%) |
| Clavien–Dindo Classification | |
| None | 41 (60.29%) |
| Grade I | 23 (33.82%) |
| Grade II | 2 (2.94%) |
| Grade IIIa | 2 (2.94%) |
| Grade IIIb or higher | 0 |
* IQR, median ± interquartile range.
Omega 6/3 Ratio
Analysis of the omega 6/3 ratio was performed in 59 patients (87%) due to losses in processing the samples. The median omega 6/3 ratio was 17.39 ± 9.45. The association between the omega 6/3 ratio and the rest of the variables was studied. For this, the variable omega 6/3 ratio was dichotomized, with a cutoff established at 21 based on a sensitivity study for air leak (AUC = 0.704; 95% CI: 0.53–0.84) that indicated this was the optimal cutoff, with a sensitivity of 83.8% and specificity of 70.2%.
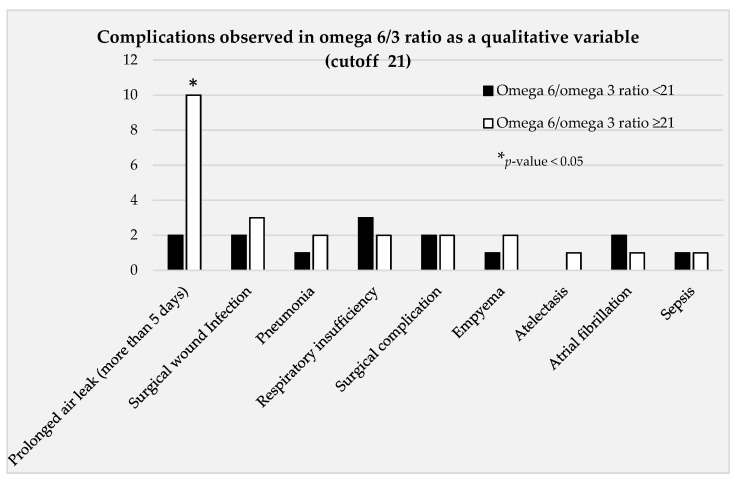
A bivariate analysis was performed, analyzing the relationship between the omega 6/3 ratio as a qualitative variable (with a cutoff of 21) and the different complications observed (Figure 1). This bivariate analysis showed a statistically significant association between omega 6/3 ratio and persistent (≥5 days) air leak (p = 0.001) (Table 4).
Figure 1.
Bivariate analysis taking the variable omega 6/3 as a qualitative variable and study of complications.
Table 4.
Bivariate analysis taking the variable omega 6/3 as a qualitative variable and study of complications.
| Ratio Omega 6/3 | <21 (n = 35) | ≥21 (n = 24) | p-Value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Complications | No | 27 | 77.1 | 10 | 41.7 | 0.006 |
| Yes | 8 | 22.9 | 14 | 58.3 | ||
| Atelectasis | No | 35 | 100 | 23 | 95.8 | 0.223 |
| Yes | 0 | 0 | 1 | 4.2 | ||
| Pneumonia | No | 34 | 97.1 | 22 | 91.7 | 0.347 |
| Yes | 1 | 2.9 | 2 | 8.3 | ||
| Respiratory insufficiency | No | 32 | 91.4 | 22 | 91.7 | 0.974 |
| Yes | 3 | 8.6 | 2 | 8.3 | ||
| Atrial fibrillation | No | 33 | 94.3 | 23 | 95.8 | 0.790 |
| Yes | 2 | 5.7 | 1 | 4.2 | ||
| Sepsis | No | 34 | 97.1 | 23 | 95.8 | 0.785 |
| Yes | 1 | 2.9 | 1 | 4.2 | ||
| Fever (without pneumonia) | No | 34 | 97.1 | 24 | 100 | 0.404 |
| Yes | 1 | 2.9 | 0 | 0 | ||
| Surgical complication | No | 33 | 94.3 | 22 | 91.7 | 0.694 |
| Yes | 2 | 5.7 | 2 | 8.3 | ||
| Prolonged air leak (more than 5 days) | No | 33 | 94.3 | 14 | 58.3 | 0.001 |
| Yes | 2 | 5.7 | 10 | 41.7 | ||
| Empyema | No | 34 | 97.1 | 22 | 91.7 | 0.347 |
| Yes | 1 | 2.9 | 2 | 8.3 | ||
| Surgical wound infection | No | 33 | 94.3 | 21 | 87.5 | 0.358 |
| Yes | 2 | 5.7 | 3 | 12.5 | ||
Statistically significant differences (p = 0.004) were observed between the different scores on the Charlson comorbidity scale regarding the omega 6/3 ratio as a qualitative variable, the ratio being lower in those with less comorbidity (score 0–6) and higher in those with more comorbidity (score 7–8). There was also a statistically significant association with the variables sex, never smoker, COPD (chronic obstructive pulmonary disease), IPAQ at 1 year, and complications (Table 5).
Table 5.
Bivariate analysis taking the variable omega 6/3 as a qualitative variable and baseline characteristics.
| Ratio Omega 6/3 | <21 (n = 35) | ≥21 (n = 24) | ||
|---|---|---|---|---|
| Mean or Median (SD or IQR) | Mean or Median (SD or IQR) | p-Value | ||
| Age | 65 (45) | 64.5 (34) | 0.877 | |
| Sex | Female | 13 (37.1) | 3 (12.5) | 0.036 |
| Male | 22 (62.9) | 21 (87.5) | ||
| Smoker | No | 28 (80) | 14 (58.3) | 0.071 |
| Yes | 7 (20) | 10 (41.7) | ||
| Never smoked | No | 27 (77.1) | 24 (100) | 0.012 |
| Yes | 8 (22.9) | 0.0 | ||
| Exsmoker | No | 28 (80) | 16 (66.7) | 0.248 |
| Yes | 7 (20) | 8 (33.3) | ||
| Year packages | 26 (21) | 45 (26) | 0.005 | |
| Diabetes | No | 24 (68.6) | 17 (70.8) | 0.853 |
| Yes | 11 (31.4) | 7 (29.2) | ||
| COPD | No | 30 (85.7) | 14 (58.3) | 0.018 |
| Yes | 5 (14.3) | 10 (41.7) | ||
| Ischemic heart disease | No | 32 (91.4) | 22 (91.7) | 0.974 |
| Yes | 3 (8.6) | 2 (8.3) | ||
| With previous pulmonary neoplasia | No | 34 (97.1) | 24 (100) | 0.404 |
| Yes | 1 (2.9) | 0.0 | ||
| Without previous pulmonary neoplasia | No | 22 (62.9) | 17 (70.8) | 0.525 |
| Yes | 13 (37.1) | 7 (29.2) | ||
| Cerebral vascular accident (CVA) | No | 34 (97.1) | 22 (91.7) | 0.347 |
| Yes | 1 (2.9) | 2 (8.3) | ||
| Peripheral vasculopathy | No | 33 (94.3) | 22 (91.7) | 0.694 |
| Yes | 2 (5.7) | 2 (8.3) | ||
| Previous cardiac surgery | No | 35 (100) | 23 (95.8) | 0.223 |
| Yes | 0.0 | 1 (4.2) | ||
| Nephropathy | No | 33 (94.3) | 23 (95.8) | 0.79 |
| Yes | 2 (5.7) | 1 (4.2) | ||
| Dyslipidemia | No | 18 (51.4) | 18 (75) | 0.068 |
| Yes | 17 (48.6) | 6 (25) | ||
| Hypertension | No | 15 (42.9) | 15 (62.5) | 0.138 |
| Yes | 20 (57.1) | 9 (37.5) | ||
| Charlson Index | 0 | 1 (2.9) | 1 (4.2) | 0.266 |
| 1 | 4 (11.4) | 1 (4.2) | ||
| 2 | 5 (14.3) | 4 (16.7) | ||
| 3 | 5 (14.3) | 5 (20.8) | ||
| 4 | 7 (20) | 6 (25) | ||
| 5 | 10 (28.6) | 1 (4.2) | ||
| 6 | 2 (5.7) | 2 (8.3) | ||
| 7 | 1 (2.9) | 2 (8.3) | ||
| 8 | 0.0 | 2 (8.3) | ||
| VEMS | 97 (501) | 91.25 (629) | 0.067 | |
We looked for differences in the association between omega 6/3 ratio and air leak according to the different surgical approaches used (thoracotomy vs. thoracoscopy) and found no significant differences. We also performed a breakdown of the different polyunsaturated fatty acids (eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), linolenic acid, arachidonic acid (ARA), and linoleic acid) and examined the association of these separately with the variable persistent air leak, finding no statistically significant results in the adjusted raw models.
In addition to its association with air leak, we found a statistically significant association between an omega 6/3 ratio of ≥21 and sex (p = 0.036), never smoker (p = 0.018), preoperative BCMI (p = 0.049), preoperative BMI (p = 0.015), IPAQ at 1 year (p = 0.047), hospital stay (p = 0.01), drain duration (p = 0.25), and overall postoperative complications (p = 0.006).
Before carrying out the multivariate analysis, a Bonferroni test was performed to identify potential interactions between the variables that had shown a statistically significant association with the omega 6/3 ratio in the bivariate analysis. This was conducted so that potential confounding variables could be considered when creating the models.
Subsequently, a multivariate model was built that included the variables sex, age, smoker, preoperative BCMI, preoperative BMI, drain duration, history of COPD, NPI, and persistent air leak postoperatively. This model explained the 79.2% between what was expected and what was observed. Of the variables that were significantly associated with the omega 6/3 ratio in the bivariate analysis, we excluded from the multivariate model: IPAQ at 1 year due to excessive loss of data and hospital length of stay, never smoker, and overall postoperative complications due to finding interactions. NPI was included in the model, even when it was not found to be significant in the bivariate analysis because it is an important variable for the thoracic surgery team. In the multivariate analysis using the omega 6/3 ratio with a cutoff of 21, the only variable with a continued significant association was COPD (p = 0.03; 95% CI: 1.22–53.57). The association with persistent air leak did not remain significant by a small margin (p = 0.052; 95% CI: 0.77–1.41).
The study of the relationship between preoperative markers of inflammation (such as NPI, SII, NLR, PLR, and LMR) and air leak found no statistically significant differences.
4. Discussion
The relationship between nutritional status and lung cancer has been the subject of research in recent years [11,12,13]. In the study carried out by Thomas et al. [11], they observed in a sample of 19,635 patients that those with a BMI of less than 18.5 had more pulmonary, surgical, and infectious complications. Among the surgical complications, air leak and dehiscence of the bronchial stump were the most common. In another study carried out in a population of over-70-year-old patients undergoing lung resection surgery for cancer, life expectancy was lower in those patients with a BMI less than 18.5, and the authors discussed in that article the importance of nutritional support in the pre- and postoperative periods [14]; such rehabilitation and nutritional support programs have been demonstrated to have benefits in terms of postoperative complications [15]. In a study performed by our team, we found that patients with low weight and/or malnutrition, as stratified by the nutritional risk index (NRI), had a longer hospital stay and that NRI was an independent predictor of postoperative complications [6]. In the present study, we also analyzed the relationship between NRI and postoperative complications and did not find a significant association, possibly due to the smaller number of patients included.
However, until now, only Kaya et al. [10] had assessed the specific role played by micronutrients, in particular omega essential fatty acids, in patients’ nutritional status and their relationship with postoperative outcomes in lung cancer. They described how a specific preoperative nutritional program with supplementation with omega-3 and other micronutrients led to improved postoperative recovery and a lower grade of postoperative complications. In this study, we observed a possible association between the preoperative omega 6/3 ratio and persistent air leak (≥5 days). Based on the results from this sample in the bivariate analysis, a lower preoperative omega 6/3 ratio may mean a lower probability of persistent air leak. Therefore, an increase in omega-3 (through diet or targeted supplementation) could have a protective function against air leak. The healing process is directly related to inflammation, an area in which the omega 6/3 ratio may exert an effect, immunomodulating the secretion of cytokines and altering their function on fibroblasts at a cellular level [16]. The relationships between the omega 6/3 ratio, air leak, and markers of systemic inflammation (CRP, LMR, platelets, NRI) were studied, but in this study, no conclusive results could be drawn. A parallel immunohistochemistry study is currently underway to study markers of local inflammation in the surgical tissue specimen in this same group of patients.
Multiple studies have described how the incidence and prevalence of cardiovascular disease, diabetes, asthma, and other diseases can vary depending on the proportion of omega-3 acids in the diet [8,9,17]. In the present study, we observed significant differences in the Charlson Comorbidity Index according to the omega 6/3 ratio, with a lower ratio in those with less comorbidity (score 0–6) and higher in those with more comorbidity, which could be explained by the relationship between this ratio and the prevalence of various diseases. However, few patients in this study had a high level of comorbidity.
Omega-3 fatty acids have also been demonstrated to reduce coronary disease, as they reduce levels of triglycerides and platelets [18]. The omega- 3 fatty acids EPA and DHA have been shown to inhibit tumor growth induced by TPA and epidermal growth factor (EGF) [19]. In lung cancer, the few studies that exist have focused on advanced stages, describing a positive effect in response to chemotherapy [20]. In the study by Cheng et al., they gave supplements with EPA and DHA or a placebo to 60 patients with lung cancer for 12 weeks and observed that the experimental group had a higher weight and reduction in inflammatory markers such as TNF and CRP compared with the control group [21]. None of these patients underwent surgery, which is where the importance of our study comes in, as it is the first to relate baseline omega fatty acid values to postoperative outcomes in lung cancer.
Persistent air leak is directly associated with a longer hospital stay, as can be observed from our results and as has been described in the literature [22,23]. This means an increased financial expenditure, which is why there is great interest in determining the factors that cause a greater air leak. These factors are divided into two groups, the first being the patient’s baseline characteristics, such as female sex, COPD, low FEV1, and history of smoking [24,25]. These variables were included in a multivariate analysis of the effect of the omega 6/3 ratio and persistent air leak, and a statistically significant association was observed. The second group is external factors, such as nutrition, as discussed above, and surgical technique. Benefits have been described with some maneuvers, such as fissureless surgery [26], staple length, and type of resection [25,26]. In our database, all patients were operated on according to the routine clinical practice of the surgeons at this hospital, with the same surgical technique and via a fissureless approach. Regarding the different approaches, there were no differences in the association between the omega ratio and persistent air leak according to the type of approach.
Despite these results, the need for supplementation with omega-3 in patients who are to undergo lung cancer surgery still requires further research, as there are some controversies: in the most recent meta-analysis by Lam et al. [27] on omega-3 supplementation in patients with cancer, they concluded that this supplementation did not improve patients’ quality of life, muscle mass, or weight, in contrast to what had been suggested in previous studies, although that analysis included all types of patients with cancer, while our group of patients had early-stage lung cancer and were candidates for radical surgical treatment.
It should be noted that the present study has some limitations, as it is an observational study and not a randomized controlled trial, as well as the obvious limitations due to the sample size; however, the findings from this sample show the importance of micronutrient analysis, particularly omega fatty acids. We recommend a preoperative assessment of omega fatty acid status with the goal of optimizing nutritional status, which could reduce the overall incidence of complications.
5. Conclusions
According to our findings, the omega 6/3 ratio in our population is high and could be correlated with immediate postsurgical complications.
Author Contributions
All the authors have contributed to this manuscript and approved the final version submitted. C.D. and R.R. participated in the study design, data collection, database creation, specific treatment, manuscript writing, and patient follow-up. C.R.-P. and C.M.-A. performed the statistical analysis. E.G.-R. was involved in the preoperative nutrition evaluation of patients. I.M., F.R., A.U., A.M., I.E., C.M. and I.S. participated in the specific treatment of the patients. None of the authors have any conflict of interest to disclose. None of the work included in this manuscript has been previously published in a peer-reviewed journal and is not under consideration for publication elsewhere. This project was approved by the institutional review board and was carried out with appropriate ethical standards. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the the hospital ethics committee (Comité Ètic d’investigació Clínica “CEIC”) (protocol code: RD 223/04 and date of 12 January 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The following grants were received to perform this study: SEPAR’s Grant (Spanish Society of Pulmonology and Thoracic Surgery), Investigation Project (2018); SECT‘s Grant (Spanish Society of Thoracic Surgery) (2017).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao Y., Xie X., De Koning H., Mali W., Vliegenthart R., Oudkerk M. NELSON lung cancer screening study. Cancer Imaging. 2011;11:S79–S84. doi: 10.1102/1470-7330.2011.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer. 2022. [(accessed on 1 August 2022)]. Available online: https://www.who.int/news-room/factsheets/detail/cancer#:~:text=Cancer%20is%20a%20leading%20cause,and%20rectum%20and%20prostate%20cancers.
- 3.Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J., Rosso S., Coebergh J.W., Comber H., Forman D., Bray F. Cancer inci-dence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Chirlaque M.D., Salmerón D., Galceran J., Ameijide A., Mateos A., Torrella A., Jiménez R., Larrañaga N., Marcos-Gragera R., REDECAN Working Group et al. Cancer survival in adult patients in Spain. Results from nine population-based cancer registries. Clin. Transl. Oncol. 2017;20:201–211. doi: 10.1007/s12094-017-1710-6. [DOI] [PubMed] [Google Scholar]
- 5.Green A., Hauge J., Iachina M., Jakobsen E. The mortality after surgery in primary lung cancer: Results from the Danish Lung Cancer Registry. Eur. J. Cardio-Thorac. Surg. 2015;49:589–594. doi: 10.1093/ejcts/ezv107. [DOI] [PubMed] [Google Scholar]
- 6.Ramos R., Nadal E., Peiró I., Masuet-Aumatell C., Macia I., Rivas F., Rosado G., Rodriguez P., Ureña A., Padrones S., et al. Preoperative nutritional status assessment predicts postoperative outcomes in patients with surgically resected non-small cell lung cancer. Eur. J. Surg. Oncol. (EJSO) 2018;44:1419–1424. doi: 10.1016/j.ejso.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Cholewski M., Tomczykowa M., Tomczyk M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients. 2018;10:1662. doi: 10.3390/nu10111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang H.O., Dyerberg J. Plasma Lipids and Lipoproteins in Greenlandic West Coast Eskimos. Acta Med. Scand. 1972;192:85–94. doi: 10.1111/j.0954-6820.1972.tb04782.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsuyama W., Mitsuyama H., Watanabe M., Oonakahara K., Higashimoto I., Osame M., Arimura K. Effects of omega-3 pol-yunsaturated fatty acids on inflammatory markers in COPD. Chest. 2005;128:3817–3827. doi: 10.1378/chest.128.6.3817. [DOI] [PubMed] [Google Scholar]
- 10.Kaya S.O., Akcam T.I., Ceylan K.C., Samancılar O., Ozturk O., Usluer O. Is preoperative protein-rich nutrition effective on post-operative outcome in non-small cell lung cancer surgery? A prospective randomized study. J. Cardiothorac Surg. 2016;11:14. doi: 10.1186/s13019-016-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas P., Berbis J., Falcoz P.-E., Le Pimpec-Barthes F., Bernard A., Jougon J., Porte H., Alifano M., Dahan M., Alauzen M., et al. National perioperative outcomes of pulmonary lobectomy for cancer: The influence of nutritional status. Eur. J. Cardio-Thorac. Surg. 2013;45:652–659. doi: 10.1093/ejcts/ezt452. [DOI] [PubMed] [Google Scholar]
- 12.Jagoe R., Goodship T.H., Gibson G. The influence of nutritional status on complications after operations for lung cancer. Ann. Thorac. Surg. 2001;71:936–943. doi: 10.1016/S0003-4975(00)02006-3. [DOI] [PubMed] [Google Scholar]
- 13.Bagan P., Berna P., De Dominicis F., Pereira J.C.D.N., Mordant P., De La Tour B., Le Pimpec-Barthes F., Riquet M. Nutritional Status and Postoperative Outcome After Pneumonectomy for Lung Cancer. Ann. Thorac. Surg. 2013;95:392–396. doi: 10.1016/j.athoracsur.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Fiorelli A., Vicidomini G., Mazzella A., Messina G., Milione R., Di Crescenzo V.G., Santini M. The Influence of Body Mass Index and Weight Loss on Outcome of Elderly Patients Undergoing Lung Cancer Resection. Thorac. Cardiovasc. Surg. 2014;62:578–587. doi: 10.1055/s-0034-1373733. [DOI] [PubMed] [Google Scholar]
- 15.Harada H., Yamashita Y., Misumi K., Tsubokawa N., Nakao J., Matsutani J., Yamasaki M., Ohkawachi T., Taniyama K. Multidis-ciplinary team-based approach for comprehensive preoperative pulmonary rehabilitation including intensive nutri-tional support for lung cancer patients. PLoS ONE. 2013;8:e59566. doi: 10.1371/journal.pone.0059566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniel J.C., Belury M., Ahijevych K., Blakely W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16:337–345. doi: 10.1111/j.1524-475X.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Da Rocha C.M., Kac G. High dietary ratio of omega-6 to omega-3 polyunsaturated acids during pregnancy and prevalence of post-partum depression. Matern. Child Nutr. 2010;8:36–48. doi: 10.1111/j.1740-8709.2010.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris W.S., Miller M., Tighe A.P., Davidson M.H., Schaefer E.J. Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Liu G., Bibus D.M., Bode A.M., Ma W.-Y., Holman R.T., Dong Z. Omega 3 but not omega 6 fatty acids inhibit AP-1 activity and cell transformation in JB6 cells. Proc. Natl. Acad. Sci. USA. 2001;98:7510–7515. doi: 10.1073/pnas.131195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vega O.M., Abkenari S., Tong Z., Tedman A., Huerta-Yepez S. Omega-3 Polyunsaturated Fatty Acids and Lung Cancer: Nutrition or Pharmacology? Nutr. Cancer. 2021;73:541–561. doi: 10.1080/01635581.2020.1761408. [DOI] [PubMed] [Google Scholar]
- 21.Cheng M., Zhang S., Ning C., Huo Q. Omega-3 Fatty Acids Supplementation Improve Nutritional Status and Inflammatory Response in Patients with Lung Cancer: A Randomized Clinical Trial. Front. Nutr. 2021;8:686752. doi: 10.3389/fnut.2021.686752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardell T., Petsikas D. What keeps postpulmonary resection patients in hospital? Can. Respir. J. 2003;10:86–89. doi: 10.1155/2003/610570. [DOI] [PubMed] [Google Scholar]
- 23.Varela G., Jimenez M.F., Novoa N., Aranda J.L. Estimating hospital costs attributable to prolonged air leak in pulmonary lobec-tomy. Eur. J. Cardiothorac Surg. 2005;27:329–333. doi: 10.1016/j.ejcts.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Pan H., Chang R., Zhou Y., Gao Y., Cheng Y., Zhang C. Risk factors associated with prolonged air leak after video-assisted thoracic surgery pulmonary resection: A predictive model and meta-analysis. Ann. Transl. Med. 2019;7:103. doi: 10.21037/atm.2019.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugan K.C., Laxmanan B., Murgu S., Hogarth D.K. Incidence of persistent air leaks. Chest. 2017;152:417–423. doi: 10.1016/j.chest.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami K., Maehara S., Shimada Y., Makino Y., Hagiwara M., Kakihana M., Kajiwara N., Ohira T., Ikeda N. The Correlation Between Fissureless Technique and Prolonged Air Leak for Patients Undergoing Video-Assisted Right Upper Lobectomy. World J. Surg. 2021;45:1569–1574. doi: 10.1007/s00268-020-05935-y. [DOI] [PubMed] [Google Scholar]
- 27.Lam C.N., Watt A.E., Isenring E.A., de van der Schueren M.A.E., van der Meij B.S. The effect of oral omega-3 polyunsa-turated fatty acid supplementation on muscle maintenance and quality of life in patients with cancer: A systematic review and me-ta-analysis. Clin. Nutr. 2021;40:3815–3826. doi: 10.1016/j.clnu.2021.04.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.