Abstract
Four immunoglobulin G1 monoclonal antibodies (MAbs) to the gp135 surface envelope glycoprotein (SU) of the 79–63 isolate of caprine arthritis-encephalitis virus (CAEV), referred to as CAEV-63, were characterized and evaluated for their ability to compete with antibody from CAEV-infected goats. Three murine MAbs (MAbs GPB16A, 29A, and 74A) and one caprine MAb (MAb F7-299) were examined. All MAbs reacted in nitrocellulose dot blots with native CAEV-63 SU purified by MAb F7-299 affinity chromatography, whereas none reacted with denatured and reduced SU. All MAbs reacted in Western blots with purified CAEV-63 SU or the SU component of whole-virus lysate following denaturation in the absence of reducing agent, indicating that intramolecular disulfide bonding was essential for epitope integrity. Peptide-N-glycosidase F digestion of SU abolished the reactivities of MAbs 74A and F7-299, whereas treatment of SU with N-acetylneuraminate glycohydrolase (sialidase A) under nonreducing conditions enhanced the reactivities of all MAbs as well as polyclonal goat sera. MAbs 29A and F7-299 were cross-reactive with the SU of an independent strain of CAEV (CAEV-Co). By enzyme-linked immunosorbent assay (ELISA), the reactivities of horseradish peroxidase (HRP)-conjugated MAbs 16A and 29A with homologous CAEV-63 SU were <10% of that of HRP-conjugated MAb 74A. The reactivity of HRP-conjugated MAb 74A was blocked by sera from goats immunized with CAEV-63 SU or infected with CAEV-63. The reactivity of MAb 74A was also blocked by sera from goats infected with a CAEV-Co molecular clone, although MAb 74A did not react with CAEV-Co SU in Western blots. Thus, goats infected with either CAEV-63 or CAEV-Co make antibodies that inhibit binding of MAb 74A to CAEV-63 SU. A competitive-inhibition ELISA based on displacement of MAb 74A reactivity has potential applicability for the serologic diagnosis of CAEV infection.
Caprine arthritis-encephalitis virus (CAEV), a monocyte/macrophage-tropic lentivirus, causes chronic progressive arthritis in up to 40% of domestic goats after a prolonged latent period 3, 14, 16, 17, 38. Transmission of CAEV occurs primarily by colostrum or milk from infected dams 2. The gp135 surface glycoprotein (SU), encoded by the CAEV env gene 29, 32, is the ligand for viral interaction with goat synovial membrane (GSM) cell receptors 21 and is a major target of goat humoral immune responses to CAEV 23, 24, 28. CAEV SU and the envelope transmembrane glycoprotein are immunodominant in most goats compared to gag-encoded virion core antigens 7, 28, 33, and the anti-SU antibody response is cross-reactive among independent isolates of CAEV as well as ovine maedi-visna virus 18. Therefore, CAEV SU is considered a potentially useful reagent for the serologic diagnosis of CAEV infection 1, 30 as well as vaccine development 26.
Envelope-specific antibody responses are commonly directed to conformational epitopes. In one study, anti-SU antibodies elicited by infection with human immunodeficiency virus type 1 (HIV-1) were directed to conformational epitopes independently of clinical status 34, and initial antibody responses to conformational epitopes of simian immunodeficiency virus SU have been reported 13. In equine infectious anemia virus infection, maturation of the antibody response to SU is associated with a shift from linear to conformational epitopes 19. Preliminary data indicate that CAEV infection is also associated with maturation of antibody responses toward increased recognition of conformational SU epitopes (J. D. Trujillo and W. P. Cheevers, unpublished data).
These observations suggest that a sensitive diagnostic test for CAEV infection could be based on strategies that enhance detection of antibodies to immunodominant conformational epitopes on SU that are cross-reactive among diverse CAEV strains. A broadly defined map of linear B-cell epitopes on CAEV SU and pepscan analysis of synthetic peptides have been completed 6, 49. However, the distribution of conformation-dependent B-cell epitopes is unknown. Accordingly, the present study reports (i) the derivation of four anti-CAEV SU monoclonal antibodies (MAbs) directed to conformational epitopes dependent on intramolecular disulfide bonding, (ii) evaluates the effects of glycosylation and sialylation on epitope exposure, and (iii) verifies that binding of one MAb is competitively blocked by sera of goats infected with an independent CAEV strain.
MATERIALS AND METHODS
Caprine MAb F7-299 and affinity purification of native CAEV-63 SU.
The derivation of anti-CAEV strain 79–63 (anti-CAEV-63) SU MAb F7-299 was described previously 39. Briefly, MAb F7-299 is secreted by a xenohybridoma formed by fusion of murine X63-Ag8.6.5.3 myeloma cells with splenocytes from a goat infected with the 79–63 isolate of CAEV 10, 14. The infected goat was immunized subcutaneously with adjuvant (RIBI, Hamilton, Mont.) containing recombinant SU derived from vaccinia virus rWR-63 expressing the CAEV-63 env gene 32 and was boosted intravenously with CAEV-63-infected GSM cells. MAb F7-299 from supernatants of triple cloned hybridoma cells was isotyped as immunoglobulin G1 (IgG1) by radial immunodiffusion, purified by chromatography on protein G agarose, and quantified with a bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.). Native CAEV-63 SU was purified as a soluble 135-kDa glycoprotein from the medium of CAEV-63-infected GSM cells by affinity chromatography on CNBr-activated Sepharose 4B coupled with MAb F7-299 as described previously 26, 33. SU concentrations were determined by the bicinchoninic acid assay.
Murine MAbs 74A, 16A, and 29A.
Balb/c mice were immunized subcutaneously with 50 μg of affinity-purified CAEV-63 SU in RIBI adjuvant and were boosted on days 14, 20, 134, and 158. The mice were then given two intravenous injections of 25 μg of affinity-purified SU in 200 μl of phosphate-buffered saline (PBS) on days 193 and 200. Three days later, two polyethylene glycol fusions were performed with splenocytes and X63-Ag8.6.5.3 myeloma cells at a ratio of 1:8. For the second fusion, splenocytes were prestimulated with 0.25 μg of purified SU per ml in the presence of 2.5 μg of pokeweed mitogen (Sigma, St. Louis, Mo.) per ml and 5 μg of Salmonella enterica serovar Typhimurium mitogen (RIBI). Hybridoma supernatants were screened by a nitrocellulose dot blot assay against affinity-purified native SU immobilized on nitrocellulose 39. Five SU-reactive hybridomas were obtained. Cloning by three terminal dilutions resulted in three stable hybridomas (hybridomas GPB74A, GPB16A, and GPB29A) that produced IgG1 MAb isotyped with a murine monoclonal sub: isotyping kit (Hyclone, Logan, Utah).
Irrelevant isotype control MAb and goat sera.
IgG1 MAb 79/17.18.5 (MAb 79/17) was used as an irrelevant murine isotype control. The derivation of MAb 79/17 against recombinant Babesia caballi RAP-1 protein has been described previously 25. CAEV-positive sera were from (i) goats 9302, 9304, 9305, and 9308 immunized with MAb F7-299 affinity-purified CAEV-63 SU 26; (ii) goats 8517 and 8528 infected orally with CAEV-63 10; (iii) goat 9111 infected intravenously with CAEV-63; (iv) goats 8935 and 8938 infected orally 32 with the Co strain of CAEV (CAEV-Co) 37; (v) goats 9128 and 9132 infected intravenously with CAEV-Co; and (vi) goats 9905, 9907, 9908, and 9909 infected intravenously with a molecular clone of CAEV-Co. Negative control sera comprised 8505, 9136, 9212, and 9213 and pooled G428 sera from a CAEV-free Saanen breeding herd maintained at Washington State University.
Nitrocellulose dot blot assay.
Nitrocellulose dot blot assay procedures were adapted from a method described previously 39. Affinity-purified native CAEV-63 SU was used directly or was denatured and reduced by heating at 100°C for 3 min in 0.03 M Tris (pH 6.8)–0.3 M 2-mercaptoethanol–2% sodium dodecyl sulfate (SDS). Nitrocellulose (Micron Separations, Westborough, Mass.) was spotted with native or denatured and reduced SU in a filtration manifold (Hybri-Dot; Life Technologies, Gaithersburg, Md.), removed from the manifold, and blocked for 1 h with PBS-Tween (PBS containing 0.2% Tween 20 and 5% nonfat dry milk). The membrane was returned to the manifold, and individual wells were incubated for 1 h with anti-SU MAbs or goat sera diluted in PBS-Tween. After aspiration of MAbs or sera, the membrane was removed from the manifold, cut into two strips, and incubated with goat anti-mouse or rabbit anti-goat horseradish peroxidase (HRP) conjugate (Kirkegaard & Perry, Gaithersburg, Md.) diluted in PBS-Tween. Binding of HRP conjugates was evaluated with an enhanced chemiluminescence (ECL) reagent (NEN Life Sciences, Boston, Mass.) developed on X-ray film (X-Omat; Kodak, Rochester, N.Y.).
Western blotting under reducing and nonreducing conditions.
Affinity-purified CAEV-63 SU and whole-virus lysates of CAEV-63 and CAEV-Co were evaluated for their reactivities with anti-CAEV-63 MAbs by Western blotting under reducing and nonreducing conditions. CAEV-63 and CAEV-Co were purified from the medium of infected GSM cells by differential centrifugation 27. Purified SU or virus was heated at 100°C for 3 min in 0.03 M Tris (pH 6.8) containing 2% SDS, 10% glycerol, and 0.01% bromphenol blue in the presence or absence of 100 mM dithiothreitol (DTT). After heating of SU or virus, some samples were incubated for 30 min at 37°C with 100 mM iodoacetamide (IA) to stabilize the SH groups. Reduced and nonreduced preparations were subjected to electrophoresis in 4 to 20% polyacrylamide gels (SDS-polyacrylamide gel electrophoresis [PAGE]) (Ready Gel; Bio-Rad, Hercules, Calif.) as described previously 10. The separated proteins were transferred to nitrocellulose membranes by electrophoresis at 100 V for 1 h at 4°C with a Mini TransBlot apparatus (Bio-Rad). Membranes were blocked for 1 h with PBS-Tween, reacted with MAbs or goat sera and secondary goat anti-mouse or rabbit anti-goat HRP conjugate, and developed with the ECL reagent as described above.
PNGase F digestion of SU.
Peptide-N-glycosidase F (PNGase F; GlycoPro; San Leandro, Calif.), which cleaves N-linked high-mannose hybrid and complex oligosaccharides in the context of N-X-S/T, was used to evaluate the effect of N-linked glycosylation on the reactivities of MAbs with nonreduced SU. Affinity-purified SU (35 μl of a stock preparation at 0.77 μg/μl) was mixed with 10 μl of 250 mM NaHPO4 (pH 7.5) and 2.5 μl of 2% SDS, and the mixture was heated at 100°C for 5 min. After cooling, 2.5 μl of 15% Triton X-100 was added to prevent inactivation of the PNGase F by SDS. Equal aliquots of this mixture were incubated for 2 or 16 h at 37°C with or without 2 μl of PNGase F (5 U/μl). Treated SU and untreated SU were analyzed for MAb reactivities by Western blotting. The activity of PNGase F was monitored independently with a DIG glycan detection kit (Roche, Mannheim, Germany). Transferrin glycoprotein and unglycosylated creatinase were used as positive and negative controls, respectively. This procedure is based on oxidation of carbohydrate hydroxyl groups to aldehydes, covalent binding of aldehyde groups with digoxigenin (DIG), and detection with an anti-DIG-alkaline phosphatase (AP) Fab conjugate. Following SDS-PAGE, the carbohydrate residues on proteins transferred to nitrocellulose were oxidized with 10 mM NaIO4 in 0.1 M sodium acetate buffer (pH 5.5) for 20 min and labeled with DIG-3-O-succinyl-ε-aminocaproic acid hydrazide followed by addition of the anti-DIG-AP conjugate. Binding of the anti-DIG-AP conjugate was detected by staining with nitroblue tetrazolium phosphate in 0.1 M Tris (pH 9.5)–0.05 M MgCl2–0.1 M NaCl.
Sialidase A digestion of SU.
N-Acetylneuraminate glycohydrolase (sialidase A; GlycoPro) cleaves all nonreducing terminal sialic acid residues as well as branched sialic acids. Digestion of SU with sialidase A under nonreducing conditions was used to evaluate the effect of sialic acids on MAb reactivities. Affinity-purified SU (13 μl of a stock preparation at 0.77 μg/μl) was mixed with 4 μl of 250 mM NaHPO4 (pH 6), and the mixture was incubated for 2 h at 37°C with or without 2 μl of sialidase A (0.005 U/μl). Digested SU and undigested SU were evaluated for binding with MAbs by Western blotting under nonreducing conditions. The densities of the bands were measured with an image analyzer (IS1000; Alpha Innotech, San Leandro, Calif.). Desialylation was monitored by DIG glycan detection as described above, except that sialic acid hydroxyl groups were oxidized specifically by incubation of the nitrocellulose membranes with 1 mM NaIO4 in 0.1 M sodium acetate buffer (pH 5.5) at 0°C for 20 min.
ELISA.
The reactivities of HRP-conjugated MAbs 74A, 16A, and 29A with native CAEV-63 SU were evaluated by enzyme-linked immunosorbent assay (ELISA). The MAbs were conjugated with HRP as described previously 36. MAb F7-299-affinity-purified SU in 100 μl of PBS was added to Immulon 2 plates (Dynatech, Chantilly, Va.), and the plates were incubated overnight. Following three washes with PBS, the plates were blocked for 2 h with 200 μl of PBS containing 0.1% Tween 20 and 20% nonfat dry milk. Blocking buffer was removed, and the plates were incubated for 15 min with 75 μl of HRP-conjugated MAbs diluted in blocking buffer. Following washes with PBS-Tween and PBS, 100 μl of peroxidase substrate was added (TMB Microwell; Kirkegaard & Perry). The reactions were allowed to develop for 30 min and were read at 620 nm with a Titertek Multiscan plate reader (MTX, McLean, Va.).
In some experiments, reactivity with HRP-conjugated MAb 74A was used to assess capture of purified SU by MAb F7-299, 16A, or 29A. Additional experiments assessed the ability of MAb F7-299 to capture soluble SU from the culture medium (CM) of CAEV-63-infected GSM cells. The wells were coated overnight with MAb F7-299, 16A, or 29A in 100 μl of 50 mM Na2CO3-NaHCO3 buffer (pH 9.6) (carbonate buffer). Following washes with PBS-Tween, the wells were incubated for 2 h with 200 μl of blocking buffer. The blocking buffer was removed, and the wells were incubated for 1 h with purified SU in 100 μl of blocking buffer or with 100 μl of CM and were processed for reactivity with HRP-conjugated MAb 74A as described above.
Competitive binding of CAEV-63 SU by anti-SU MAbs and goat sera.
The abilities of CAEV antibody-positive goat sera and control sera to block binding of MAbs to SU were evaluated by ELISA. These experiments used Immulon 2 plates coated with SU directly or SU captured with MAb F7-299. After incubation with blocking buffer, the wells were incubated for 15 min with 75 μl of undiluted goat sera. HRP-conjugated MAb diluted in 25 μl of blocking buffer was added, and incubation was continued for 30 min prior to washing and addition of tetramethylbenzidine substrate.
CI-ELISA.
On the basis of preliminary ELISA data, a competitive-inhibition ELISA (CI-ELISA) method was evaluated for detection and titration of anti-CAEV SU antibodies in goat sera by displacement of binding by HRP-conjugated MAb 74A. Each well of flat-bottom 96-well plates (Costar, Cambridge, Mass.) was coated overnight with 4.5 ng of MAb F7-299 in 50 μl of 100 mM carbonate buffer. The coated wells were blocked for 1 h with 100 mM NaH2PO4-Na2HPO4 buffer (pH 7.2) containing 0.75% glycine, 2% sucrose, 0.1% Tween 20, and 1% nonfat dry milk. After removal of blocking buffer, the wells were incubated overnight with 50 μl of CM to capture soluble SU. The plates were blocked again and were air dried for 48 h. The blocked wells were incubated for 1 h with 50 μl of undiluted goat serum or 50 μl of serum diluted in blocking buffer for end point titration. The wells were washed with PBS-Tween and were incubated for 30 min with HRP-conjugated MAb 74A and 20 min with tetramethylbenzidine substrate. Results were expressed as percent inhibition of MAb 74A binding calculated by 1 − [OD620 (for the sample)/OD620 (for the plate control)] × 100, where OD620 is the optical density at 620 nm. End points for serum antibody titrations were extrapolated by linear regression analysis of percent inhibition plotted against serum dilutions and were defined relative to the background reactivity of the control sera on the plate.
RESULTS
Nitrocellulose dot blotting of affinity-purified native or denatured and reduced SU.
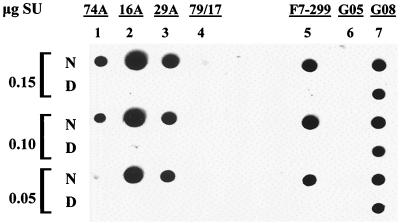
The initial binding properties of the MAbs were determined by immunoblot analysis of native or denatured and reduced SU bound to nitrocellulose. The results are shown in Fig. 1. Murine MAbs 74A, 16A, and 29A as well as caprine MAb F7-299 reacted with native SU (data 1N, 2N, 3N, and 5N, respectively) but did not bind to denatured and reduced SU (data 1D, 2D, 3D, and 5D). Positive control serum from SU-immunized goat 9308 reacted with both native and denatured and reduced SU (lane 7), whereas SU was not reactive with negative control serum from uninfected goat 8505 (lane 6) or irrelevant isotype control MAb 79/17 (lane 4).
FIG. 1.
Nitrocellulose dot blot of anti-SU MAbs with native or denatured and reduced SU. The indicated concentrations of affinity-purified native SU (N) or SU denatured and reduced in SDS with 2-mercaptoethanol (D) were immobilized on nitrocellulose membranes and reacted with murine anti-SU MAb 74A (25 μg/ml), 16A (3 μg/ml), or 29A (50 μg/ml), irrelevant isotype control MAb 79/17 (50 μg/ml), or caprine MAb F7-299 (50 μg/ml). Negative control serum from uninfected goat 8505 (G05) and positive control serum from CAEV SU-immunized goat 9308 (G08) were used at dilutions of 1:1,000.
Western blotting of reduced or nonreduced SU.
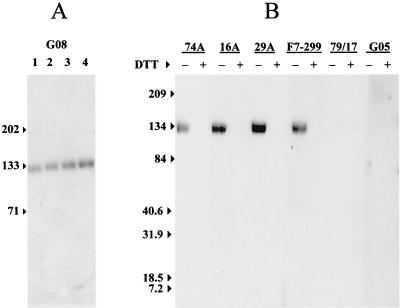
The data in Fig. 1 demonstrate that epitopes recognized by MAbs 74A, 16A, 29A, and F7-299 are dependent on the conformation of native SU. Western blot analysis of MAbs with reduced and nonreduced SUs was used to evaluate the dependence of epitope exposure on cysteine S-S bonding. An initial electrophoretic mobility assay with polyclonal goat serum 9308 was performed to confirm retention of S-S bonds following denaturation of SU glycoprotein with SDS without reduction by DTT. Compared to SU treated with 100 mM DTT in 2% SDS (Fig. 2A, lane 4), the mobility of SU progressively increased following denaturation in 2% SDS with 10 mM DTT (Fig. 2A, lane 3), 1 mM DTT (Fig. 2A, lane 2), or no DTT (Fig. 2A, lane 1).
FIG. 2.
Western blot of anti-SU MAbs with reduced or nonreduced SU. (A) Affinity-purified SU (0.1 μg) was analyzed by Western blotting after heating in 2% SDS buffer without DTT (lane 1) or with 1 mM DTT (lane 2), 10 mM DTT (lane 3), or 100 mM DTT (lane 4). The membrane was probed with a 1:1,000 dilution of serum from goat 9308 (G08). (B) Affinity-purified SU (0.1 μg) was heated in 2% SDS (lanes designated with a minus sign) or 2% SDS with 100 mM DTT (lanes designated with a plus sign). Following the addition of 100 mM IA, SU preparations were analyzed by Western blotting with anti-SU or irrelevant isotype control MAb. Negative control serum from goat 8505 (G05) was used at a dilution of 1:1,000. Numbers to the left of each panel are in kilodaltons.
Western blots with anti-SU MAb used SU denatured in 2% SDS with or without 100 mM DTT. In these experiments, 100 mM IA was also included to stabilize reduced SH groups. All four MAbs reacted with nonreduced SU (Fig. 2B, lanes designated with minus signs), whereas none of the MAbs reacted with reduced SU (Fig. 2B, lanes designated with plus signs). Similar results were obtained with nonreduced SU denatured in 0.2% SDS (data not shown). Thus, Western blot analysis of MAb binding to reduced or nonreduced SU denatured with SDS demonstrated that epitopes recognized by MAbs are dependent on S-S bonding of SU cysteine residues and are not dependent on other structural properties of SU sensitive to denaturation by SDS.
Western blotting of SU treated with PNGase F under nonreducing conditions.
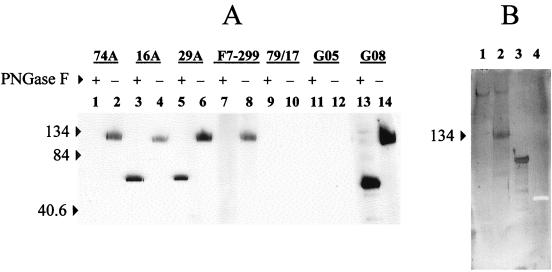
The 550-amino-acid SU of CAEV-63 contains 23 potential N-linked glycosylation sites as well as 22 cysteine residues 29. To determine if N-glycans contribute to MAb epitopes or epitope exposure, PNGase F-digested SU was analyzed by Western blotting under nonreducing conditions. The extent of deglycosylation of SU by PNGase F was monitored independently by glycan staining. As expected from the data in Fig. 2B, all anti-SU MAbs as well as polyclonal serum from goat 9308 recognized mock-treated SU under nonreducing conditions (Fig. 3A, lanes 2, 4, 6, 8, and 14). SU was not reactive with isotype control MAb 79/17 (Fig. 3A, lane 10) or serum from uninfected goat 8505 (Fig. 3A, lane 12). PNGase F digestion for 16 h reduced the apparent molecular mass of SU from ∼133 kDa (Fig. 3A, lane 14) to ∼60 kDa (Fig. 3A, lane 13). This reduction in apparent molecular mass, accompanied by the loss of glycan staining (Fig. 3B, lanes 1 and 2), was consistent with complete removal of glycans 29. Anti-SU MAbs 16A and 29A reacted with deglycosylated SU (Fig. 3A, lanes 3 and 5), whereas deglycosylated SU did not bind to MAb 74A or F7-299 (Fig. 3A, lanes 1 and 7).
FIG. 3.
Western blot of anti-SU MAbs with nonreduced SU digested with PNGase F. (A) Affinity-purified SU incubated for 16 h at 37°C without PNGase F (lanes designated with minus signs) or with PNGase F (lanes designated with plus signs) was analyzed by Western blotting with anti-SU MAb or an irrelevant isotype control MAb under nonreducing conditions. Negative control serum from goat 8505 (G05) and positive control serum from goat 9808 (G08) were used at dilutions of 1:1,000. (B) SU incubated with or without PNGase F (lanes 1 and 2), positive control glycoprotein (transferrin) (lane 3), and unglysosylated negative control protein (creatinase) (lane 4) were subjected to SDS-PAGE and transferred to nitrocellulose. Glycan residues were oxidized with 10 mM NaIO4 in 0.1 M acetate buffer (pH 5.5) for 20 min at room temperature and stained by using a DIG glycan detection kit. Numbers to the left of each panel are in kilodaltons.
Western blotting of SU treated with sialidase A under nonreducing conditions.
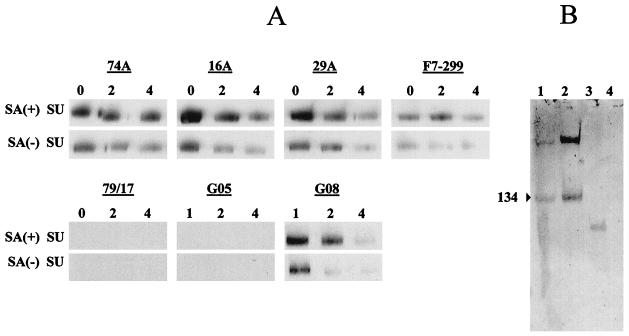
Sialic acid residues have been implicated in masking exposure of CAEV and HIV-1 SU epitopes 5, 22. To determine if sialic acids affect binding of anti-CAEV SU MAbs, SU was digested with sialidase A and analyzed by Western blotting under nonreducing conditions. Desialylation was monitored independently and confirmed that sialic acid residues were substantially digested by sialidase A (Fig. 4B, lanes 1 and 2). Desialylation of SU provided 1.8- to 4.7-fold enhanced reactivity of both MAbs and polyclonal antibodies with SU (Fig. 4A).
FIG. 4.
Western blot of anti-SU MAbs with nonreduced SU digested with sialidase A. (A) Affinity-purified SU was incubated for 2 h at 37°C with sialidase A [SA(+)] or without sialidase A [SA(−)] and analyzed by Western blotting with anti-SU MAbs or irrelevant isotype control MAbs under nonreducing conditions. Triplicate blots used the indicated dilutions of MAbs (lanes 0, undiluted; lanes 2, 1:2; lanes 4, 1:4), with initial MAb concentrations of 25 μg/ml (MAb 74A), 3 μg/ml (MAb 16A), or 50 μg/ml (MAbs 29A, F7-299, and 79/17). Negative control serum from goat 8505 (G05) and positive control serum from goat 9808 (G08) were used at dilutions of 1:1,000 (lanes 1), 1:2,000 (lanes 2), and 1:4,000 (lanes 4). (B) SU incubated with or without sialidase A (lanes 1 and 2), positive control glycoprotein (transferrin) (lane 3), and unglysosylated negative control protein (creatinase) (lane 4) were subjected to SDS-PAGE and transferred to nitrocellulose. Sialic acid residues were oxidized with 1 mM NaIO4 in 0.1 M acetate buffer (pH 5.5) for 20 min at 0°C and were stained with a DIG glycan detection kit.
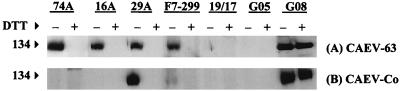
Cross-reactivity of anti-CAEV-63 SU MAbs with CAEV-Co SU.
Western blots of MAbs 74A, 16A, 29A, and F7-299 against whole-virus lysates of homologous CAEV-63 and heterologous CAEV-Co are shown in Fig. 5. All four MAbs reacted specifically with CAEV-63 SU under nonreducing conditions (Fig. 5A, lanes designated with minus signs). MAb 29A cross-reacted with CAEV-Co SU, and MAb F7-299 was also weakly cross-reactive with CAEV-Co SU (Fig. 5B). However, MAbs 74A and 16A did not detectably react with CAEV-Co SU (Fig. 5B).
FIG. 5.
Western blot of anti-CAEV-63 SU MAbs with CAEV-Co SU. CAEV-63 (A) and CAEV-Co (B) were recovered from the medium of infected GSM cells and disrupted in 2% SDS without DTT (lanes designated with a minus sign) or with 100 mM DTT (lanes designated with a plus sign). Following addition of 100 mM IA, lysates of CAEV-63 (7 μg) and CAEV-Co (5 μg) were analyzed by Western blotting with anti-CAEV-63 SU or an irrelevant isotype control MAb. Negative control serum from goat 8505 (G05) and positive control serum, from goat 9808 (G08) were used at dilutions of 1:1,000.
ELISA reactivity of HRP-conjugated MAbs with CAEV-63 SU.
MAbs were evaluated for use in the development of a CI-ELISA for detection of anti-CAEV SU antibodies in goat sera. On the basis of the use of MAb F7-299 for affinity purification of SU, the ability of MAb F7-299-coated plates to capture SU was tested. As expected from immunoblotting results, HRP-conjugated MAb 74A reacted with SU incubated with MAb F7-299-coated wells (Fig. 6, open bar). In contrast, HRP-conjugated MAb 74A did not react with SU incubated with wells coated with MAb 16A and 29A (data not shown). In addition, the reactivities of HRP-conjugated MAbs 16A and 29A with SU bound to plates was <10% of that of MAb 74A (data not shown).
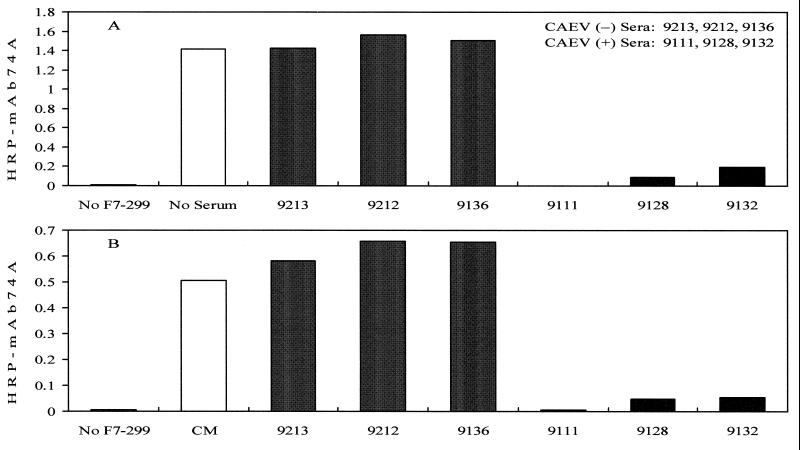
FIG. 6.
ELISA reactivity of HRP-conjugated MAb 74A with CAEV-63 SU captured by MAb F7-299. Affinity-purified SU (0.06 μg) or 100 μl of CM from CAEV-63-infected GSM cells was added to Immulon 2 wells coated with 100 ng of MAb F7-299. The wells were reacted with 0.06 μg of HRP-conjugated MAb 74A with or without preincubation with 75 μl of undiluted goat sera. (A) Purified SU captured with MAb F7-299. (B) Soluble SU from CM captured with MAb F7-299.
Competitive binding of CAEV-63 SU by MAb and goat sera.
The ability of goat sera to block the reactivity of MAb 74A with MAb F7-299-captured SU was evaluated by ELISA. Sera from uninfected goats (goats 9213, 9212, and 9136) did not compete with MAb 74A binding (Fig. 6A), whereas sera from CAEV-infected goats (goats 9111, 9128, and 9132) blocked MAb 74A reactivity with SU (Fig. 6A). Additional experiments demonstrated that MAb F7-299 was able to capture soluble SU from the CM of CAEV-63-infected GSM cells (Fig. 6B, open bar). MAb 74A reactivity with soluble SU was also blocked by sera from CAEV-infected goats (Fig. 6B, goats 9111, 9128, and 9132) but not by sera from uninfected goats (Fig. 6B, goats 9213, 9212, and 9136).
Titration of anti-CAEV SU antibodies in goat sera by CI-ELISA.
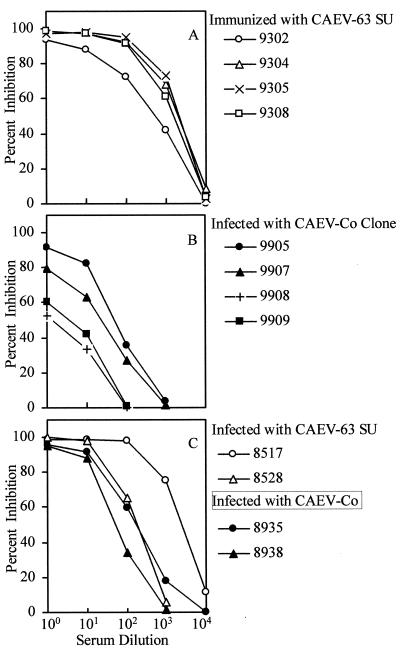
As expected, the results in Fig. 6 demonstrated that serum from CAEV-63-infected goat 9111 blocked binding of the MAb 74A conjugate to CAEV-63 SU. However, sera from goats 9128 and 9132 infected with CAEV-Co also inhibited binding of MAb 74A (Fig. 6), although MAb 74A did not react with CAEV-Co SU in Western blots (Fig. 5). Sera from goats 9128 and 9132 were drawn 4 years after experimental infection with CAEV-Co. Thus, one explanation for the data in Fig. 6 is that CAEV variants in goats 9128 and 9132 acquired the MAb 74A epitope. To test this possibility, sera from four goats immunized with CAEV-63 SU and four goats infected with a CAEV-Co molecular clone were compared by CI-ELISA. To minimize the effect of antigenic variation of SU, sera from goats infected with the CAEV-Co clone were evaluated at 4 weeks postinfection (corresponding to a maximum of 2 weeks after initial seroconversion). As expected, sera from CAEV-63 SU-immunized goats displaced MAb 74A binding to CAEV-63 SU with end point titers of 28,000 (goat 9302), 41,500 (goat 9304), 32,500 (goat 9305), and 23,500 (goat 9308) (Fig. 7A). However, sera from goats infected with the CAEV-Co clone also inhibited MAb 74A binding to CAEV-63 SU with end point titers of 3,250 (goat 9905), 1,900 (goat 9907), 190 (goat 9908), and 235 (goat 9909) (Fig. 7B). To confirm the results in Fig. 6, serum anti-SU antibody titers were determined for two additional goats chronically infected with CAEV-63 or CAEV-Co. On the basis of linear regression analysis of the data in Fig. 7C, serum anti-SU antibody titers were 37,000 and 2,800 for CAEV-63-infected goats 8517 and 8528 respectively, at 7 years postinfection, 5,500 for CAEV-Co-infected goat 8935 at 1 year postinfection, and 955 for CAEV-Co-infected goat 8938 at 3 years postinfection. These results suggest that the MAb 74A epitope on CAEV-63 SU was blocked by anti-CAEV-Co SU antibodies directed against an overlapping epitope conserved between CAEV-63 and CAEV-Co SU.
FIG. 7.
CI-ELISA reactivities of goat sera. Soluble SU released into the medium of CAEV-63-infected GSM cells was captured on Costar plates coated with MAb F7-299. The indicated dilutions of goat sera were reacted with captured SU and then with HRP-conjugated MAb 74A. Percent inhibition of MAb 74A binding was calculated with the equation 1 − [(OD620 for the sample/OD620 for the plate control)] × 100. (A) Immunization with CAEV-63 SU. Sera were assayed 2 weeks after the fifth immunization of goats with affinity-purified CAEV-63 SU in saponin adjuvant (plate control OD620 = 0.942). (B) Sera infected with CAEV-Co clone. Sera were assayed 4 weeks after intravenous inoculation with 105 50% tissue culture infectious doses of a CAEV-Co molecular clone (controls: plate control OD620 = 0.992; preinfection sera [four goats] OD620 = 1.028 ± 0.066). (C) Sera infected with CAEV-63. Sera were assayed 7 years after oral inoculation with 106 50% tissue culture infectious doses of CAEV-63 (plate controls were serum 8517 [OD620 = 0.942] and serum 8528 [OD620 = 1.125]). Sera were also infected with CAEV-Co. Sera were assayed 1 year (goat 8935) or 3 years (goat 8938) after oral inoculation with 106 50% tissue culture infectious doses of CAEV-Co (plate control OD620 = 0.942).
DISCUSSION
CAEV SU is a complex virion surface glycoprotein that comprises 546 to 550 amino acids with 22 cysteine residues and 20 to 23 potential N-linked glycosylation sites 29, 43, 49–51. An apparent molecular mass of ∼135 kDa based on SDS-PAGE under denaturing and reducing conditions indicates that most if not all of the potential N-linked sites are glycosylated. Extensive glycosylation of SU is also indicated by the pronounced reduction of the molecular mass following metabolic inhibition of glycosylation 11 or enzymatic deglycosylation of purified SU, as reported here. S-S bonds decrease the accessibility of SDS, resulting in increased SDS-PAGE mobility of denatured, nonreduced proteins according to the number of cysteines and the sizes of the cysteine loops 8, 15. However, we noted minimal increases in SDS-PAGE mobility of SU denatured with SDS in the presence of 1 to 100 mM DTT. This effect is attributed to heavy glycosylation of CAEV SU 40. Similar results were noted for HIV-1 gp120 12, which contains nine S-S bonds and 18 to 24 glycosylation sites that comprise ∼50% of the molecular mass 31.
On the basis of env gene sequencing, all 22 cysteines and >80% of the N-linked glycosylation sites are conserved among three North American isolates and four French isolates of CAEV 49. SU cysteines and potential N-linked glycosylation sites are also highly conserved between CAEV and ovine maedi-visna virus 4, 9, 29, 41–44, 46, 47, 49, 50. Knowledge of the antigenic structure of CAEV SU is essential for understanding mechanisms of disease pathogenesis and development of vaccines and diagnostic assays. The present study examined selected properties of three murine MAbs (IgG1 MAbs 16A, 29A, and 74A) and one caprine MAb (IgG1 MAb F7-299) to the SU of CAEV-63.
To obtain an initial assessment of the SU epitopes recognized by these MAbs, binding studies were performed with native SU and denatured SU with or without reduction of S-S bonds. These results demonstrated that all MAbs recognized conformation-dependent epitopes maintained by cysteine S-S bonding and that MAb binding was not dependent on the structural features of SU sensitive to SDS denaturation. In this regard, the CAEV SU MAbs are similar to a class of neutralizing MAbs directed to conformational epitopes of HIV-1 gp120 52, 53. However, in a preliminary experiment, none of the MAbs detectably neutralized 2 × 103 tissue culture infectious doses of homologous CAEV-63 (Trujillo and Cheevers, unpublished data).
To further characterize the S-S bond-dependent epitopes identified by MAbs, we evaluated the effect of SU glycosylation on MAb binding. The results demonstrated that MAb 74A and F7-299 epitopes are glycan dependent, whereas the MAb 16A and 29A epitopes are glycan-independent epitopes. Carbohydrate-dependent MAb 74A and F7-299 epitopes are spatially distinct, as shown by a lack of competitive inhibition of MAb 74A binding by MAb F7-299. In addition, MAb F7-299 differentially cross-reacts with the SU of an independent CAEV isolate (CAEV-Co). Glycan-independent epitopes recognized by MAbs 16A and 29A were distinguished by differential binding of MAb 29A to CAEV-Co SU. Thus, anti-CAEV SU MAbs identified four distinct conformational epitopes maintained by cysteine S-S bonds, two of which require glycosylation for MAb binding.
Binding of MAbs 74A and F7-299 was completely sensitive to deglycosylation of SU by PNGase F, whereas MAb 74A binding was differentially retained following partial deglycosylation of SU. Thus, different carbohydrate residues are involved in binding of MAbs 74A and F7-299. However, these studies do not establish the nature of these epitopes with regard to the role of glycans in MAb binding. Carbohydrates may be epitope components. Alternatively, MAb binding sites may be discontinuous peptidic epitopes, and conformational features of the SU imposed by nearby glycan residues are required for their exposure to antibody.
At least two HIV-1 gp120 epitopes, defined by MAbs CRA3 and 2G12, are similar to CAEV SU epitopes 74A and F7-299 35, 48. A third HIV-1 MAb (MAb G3-4) was originally reported to have a dual requirement for intact S-S bonds and N-linked glycans on the basis of digestion of gp120 with endo-β-N-acetylglucosaminidase H 20; however, in subsequent studies, MAb G3-4 did not bind to an endo-β-N-acetylglucosaminidase H-digested V1-V2 fusion protein 52. In any case, studies based on removal of specific N-linked glycans by site-directed mutagenesis have localized the glycan components of CRA3 and 2G12 epitopes to glycosylation sites adjacent to cysteine residues 48, 52. CAEV epitopes 74A and F7-299 may be comparable to HIV-1 epitopes CRA3 and 2G12, since 7 of 22 CAEV-63 SU cysteine residues have adjacent N-linked glycosylation sites 29. It has been suggested that high-mannose carbohydrate is an integral component of the HIV-1 2G12 epitope 52 because MAb 2G12 is broadly cross-reactive with primary and T-cell-adapted viruses, despite the variability of the underlying protein surface 48. By this criterion, it seems unlikely that CAEV MAb 74A binds directly to carbohydrate, since it is not cross-reactive with heterologous CAEV-Co SU, which shares 22 of 23 potential N-linked glycosylation sites with CAEV-63 SU 29, 43. Thus, present data indicate that 74A is a discontinuous S-S bond-dependent peptidic epitope whose exposure is influenced by adjacent or nearby glycans. However, the cross-reactivity of MAb F7-299 with CAEV-Co SU may indicate that this epitope has an integral carbohydrate component, in addition to a requirement for intact S-S bonds.
The negative charge of sialic acid as a terminal component of glycans inhibits intermolecular interactions 45 and shields exposure of CAEV SU neutralization epitopes 22 as well as cross-reactive epitopes on HIV-1 and HIV-2 5. The present study confirmed that desialylation of CAEV SU under nonreducing conditions enhances the exposure of MAb epitopes evaluated by Western blotting and also demonstrated enhancement of polyclonal antibody reactivity with desialylated SU. On the basis of these findings, we suggest that desialylated antigen will improve the sensitivity of Western blot assays for detection of antibodies to lentiviral envelope proteins.
Sera from infected goats were evaluated for their ability to block binding of MAbs to SU for possible use in a CI-ELISA for detection of anti-CAEV SU antibodies. On the basis of the MAb binding to SU bound directly or captured on microtiter plates with MAb F7-299, HRP-conjugated MAb 74A was selected for detailed studies. Inhibition of MAb 74A binding to CAEV-63 SU by sera from goats infected with molecularly cloned CAEV-Co demonstrated the potential utility of this assay for evaluation of field sera. Thus, the main outcome of this study with respect to diagnostics is that a CI-ELISA with MAb 74A may have diagnostic potential, especially when supplemented with a Western blot assay with desialylated nonreduced SU.
ACKNOWLEDGMENTS
We thank W. Harwood, L. Kappmeyer, E. Karel, N. Kumpula-McWhirter, K. Pretty On Top, and L. C. Wilson for technical assistance. I. Hötzel prepared the infectious molecular clone of CAEV-Co provirus, and K. Snekvik and J. Trujillo infected goats with the CAEV-Co clone. D. S. Adams provided help in the CI-ELISA design.
This work was supported by grants from the National Institutes of Health (grants R01 AR43710 and R21 AI42690) and the Agricultural Research Service, U.S. Department of Agriculture (grant CWU S348-32000-015-00D). F. Özyörük thanks GDAR of the Turkish Ministry of Agricultural and Rural Affairs for scholarship support.
REFERENCES
- 1.Adams D S, Gorham J R. The gp135 of caprine arthritis encephalitis virus affords greater sensitivity than the p28 in immunodiffusion serology. Res Vet Sci. 1986;40:157–160. [PubMed] [Google Scholar]
- 2.Adams D S, Klevjer-Anderson P, Carlson J L, McGuire T C, Gorham J R. Transmission and control of caprine arthritis-encephalitis virus. Am J Vet Res. 1983;44:1670–1675. [PubMed] [Google Scholar]
- 3.Adams D S, Oliver R E, Ameghino E, DeMartini J C, Verwoerd D W, Houwers D J, Waghela S, Gorham J R, Hyllseth B, Dawson M, Trigo F J, McGuire T C. Global survey of serological evidence of caprine arthritis-encephalitis virus infection. Vet Rec. 1984;115:493–495. doi: 10.1136/vr.115.19.493. [DOI] [PubMed] [Google Scholar]
- 4.Andresson O S, Elser J E, Tobin G J, Greenwood J D, Gonda M A, Georgsson G, Andresdottir V, Benediktsdottir E, Carlsdottir H M, Mantyla E O. Nucleotide sequence and biological properties of a pathogenic proviral molecular clone of neurovirulent visna virus. Virology. 1993;193:89–105. doi: 10.1006/viro.1993.1106. [DOI] [PubMed] [Google Scholar]
- 5.Benjouad A, Mabrouk K, Gluckman J C, Fenouillet E. Effect of sialic acid removal on the antibody response to the third variable domain of human immunodeficiency virus type-1 envelope glycoprotein. FEBS Lett. 1994;341:244–250. doi: 10.1016/0014-5793(94)80465-6. [DOI] [PubMed] [Google Scholar]
- 6.Bertoni, G., C. Hertig, M.-L. Zahno, H.-R. Vogt, S. Dufour, P. Cordano, E. Peterhans, W. P. Cheevers, P. Sonigo, and G. Pancino. B cell epitopes of the envelope glycoprotein of the caprine arthritis-encephalitis virus and antibody response in infected goats. J. Gen. Virol., in press. [DOI] [PubMed]
- 7.Bertoni G, Zahno M-L, Zanoni R, Vogt H-R, Peterhans E, Ruff G, Cheevers W P, Sonigo P, Pancino G. Antibody reactivity to the immunodominant epitopes of the caprine arthritis-encephalitis virus gp38 transmembrane protein associates with the development of arthritis. J Virol. 1994;68:7139–7147. doi: 10.1128/jvi.68.11.7139-7147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braakman I, Hoover-Litty H, Wagner K R, Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991;114:401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun M J, Clements J E, Gonda M A. The visna virus genome: evidence for a hypervariable site in the env gene and sequence homology among lentivirus envelope proteins. J Virol. 1987;61:4046–4054. doi: 10.1128/jvi.61.12.4046-4054.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheevers W P, Knowles D P, Jr, Norton L K. Neutralization-resistant antigenic variants of caprine arthritis-encephalitis lentivirus associated with progressive arthritis. J Infect Dis. 1991;164:679–685. doi: 10.1093/infdis/164.4.679. [DOI] [PubMed] [Google Scholar]
- 11.Cheevers W P, Stem T A, Knowles D P, McGuire T C. Precursor polypeptides of caprine arthritis-encephalitis lentivirus structural proteins. J Gen Virol. 1988;69:675–681. doi: 10.1099/0022-1317-69-3-675. [DOI] [PubMed] [Google Scholar]
- 12.Chou M J, Lee T H, Hatzakis A, Mandalaki T, McLane M F, Essex M. Antibody responses in early human immunodeficiency virus type 1 infection in hemophiliacs. J Infect Dis. 1988;157:805–811. doi: 10.1093/infdis/157.4.805. [DOI] [PubMed] [Google Scholar]
- 13.Cole K S, Murphey-Corb M, Narayan O, Joag S V, Shaw G M, Montelaro R C. Common themes of antibody maturation to simian immunodeficiency virus, simian-human immunodeficiency virus, and human immunodeficiency virus type 1 infections. J Virol. 1998;72:7852–7859. doi: 10.1128/jvi.72.10.7852-7859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford T B, Adams D S, Cheevers W P, Cork L C. Chronic arthritis in goats caused by a retrovirus. Science. 1980;207:997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- 15.Darby N, Creighton T E. Probing protein folding and stability using disulfide bonds. Mol Biotechnol. 1997;7:57–77. doi: 10.1007/BF02821544. [DOI] [PubMed] [Google Scholar]
- 16.East N E, Rowe J D, Madewell B R, Floyd K. Serologic prevalence of caprine arthritis-encephalitis virus in California goat dairies. J Am Vet Med Assoc. 1987;190:182–186. [PubMed] [Google Scholar]
- 17.Garry R F, Krieg A M, Cheevers W P, Montelaro R C, Golding H, Fermin C D, Gallaher W R. Retroviruses and their roles in chronic inflammatory diseases and autoimmunity. In: Levy J A, editor. The retroviridae. New York, N.Y: Plenum Press; 1995. pp. 491–603. [Google Scholar]
- 18.Gogolewski R P, Adams D S, McGuire T C, Banks K L, Cheevers W P. Antigenic cross-reactivity between caprine arthritis-encephalitis, visna and progressive pneumonia viruses involves all virion-associated proteins and glycoproteins. J Gen Virol. 1985;66:1233–1240. doi: 10.1099/0022-1317-66-6-1233. [DOI] [PubMed] [Google Scholar]
- 19.Hammond S A, Cook S J, Lichtenstein D L, Issel C J, Montelaro R C. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J Virol. 1997;71:3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho D D, Fung M S, Cao Y Z, Li X L, Sun C, Chang T W, Sun N C. Another discontinuous epitope on glycoprotein gp120 that is important in human immunodeficiency virus type 1 neutralization is identified by a monoclonal antibody. Proc Natl Acad Sci USA. 1991;88:8949–8952. doi: 10.1073/pnas.88.20.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hullinger G A, Knowles D P, McGuire T C, Cheevers W P. Caprine arthritis-encephalitis lentivirus SU is the ligand for infection of caprine synovial membrane cells. Virology. 1993;192:328–331. doi: 10.1006/viro.1993.1037. [DOI] [PubMed] [Google Scholar]
- 22.Huso D L, Narayan O, Hart G W. Sialic acids on the surface of caprine arthritis-encephalitis virus define the biological properties of the virus. J Virol. 1988;62:1974–1980. doi: 10.1128/jvi.62.6.1974-1980.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson G C, Adams D S, McGuire T C. Pronounced production of polyclonal immunoglobulin G1 in the synovial fluid of goats with caprine arthritis-encephalitis virus infection. Infect Immun. 1983;41:805–815. doi: 10.1128/iai.41.2.805-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson G C, Barbet A F, Klevjer-Anderson P, McGuire T C. Preferential immune response to virion surface glycoproteins by caprine arthritis-encephalitis virus-infected goats. Infect Immun. 1983;41:657–665. doi: 10.1128/iai.41.2.657-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappmeyer L S, Perryman L E, Hines S A, Baszler T V, Katz J B, Hennager S G, Knowles D P. Detection of equine antibodies to Babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1999;37:2285–2290. doi: 10.1128/jcm.37.7.2285-2290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp R K, Knowles D P, Perry L L, McGuire T C, Besser T E, Cheevers W P. Crossreactive neutralizing antibodies induced by immunization with caprine arthritis-encephalitis virus surface glycoprotein. Vaccine. 2000;18:1282–1287. doi: 10.1016/s0264-410x(99)00181-4. [DOI] [PubMed] [Google Scholar]
- 27.Klevjer-Anderson P, Cheevers W P. Characterization of the infection of caprine synovial membrane cells by the retrovirus caprine arthritis-encephalitis virus. Virology. 1981;110:113–119. doi: 10.1016/0042-6822(81)90012-x. [DOI] [PubMed] [Google Scholar]
- 28.Knowles D P, Cheevers W P, McGuire T C, Stem T A, Gorham J R. Severity of arthritis is predicted by antibody response to gp135 in chronic infection with caprine arthritis-encephalitis virus. J Virol. 1990;64:2396–2398. doi: 10.1128/jvi.64.5.2396-2398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowles D P, Cheevers W P, McGuire T C, Brassfield A L, Harwood W G, Stem T A. Structure and genetic variability of envelope glycoproteins of two antigenic variants of caprine arthritis-encephalitis lentivirus. J Virol. 1991;65:5744–5750. doi: 10.1128/jvi.65.11.5744-5750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowles D P, Evermann J F, Shropshire C, VanderSchalie J, Bradway D, Gezon H M, Cheevers W P. Evaluation of agar gel immunodiffusion serology using caprine and ovine lentiviral antigens for detection of antibody to caprine arthritis-encephalitis virus. J Clin Microbiol. 1994;32:243–245. doi: 10.1128/jcm.32.1.243-245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leonard C K, Spellman M W, Riddle L, Harris R J, Thomas J N, Gregory T J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 32.Lichtensteiger C A, Knowles D P, McGuire T C, Cheevers W P. Recombinant gp135 envelope glycoproteins of caprine arthritis-encephalitis lentivirus variants inhibit homologous and heterologous variant-specific neutralizing antibodies. Virology. 1991;185:2–9. doi: 10.1016/0042-6822(91)90747-y. [DOI] [PubMed] [Google Scholar]
- 33.McGuire T C, Knowles D P, Davis W C, Brassfield A L, Stem T A, Cheevers W P. Transmembrane protein oligomers of caprine arthritis-encephalitis lentivirus are immunodominant in goats with progressive arthritis. J Virol. 1992;66:3247–3250. doi: 10.1128/jvi.66.5.3247-3250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J P, Sattentau Q J, Yoshiyama H, Thali M, Charles M, Sullivan N, Poon S W, Fung M S, Traincard F, Pinkus M, Robey G, Robinson J E, Ho D D, Sodroski J. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: human immune response to the V1 and V2 domains. J Virol. 1993;67:6136–6151. doi: 10.1128/jvi.67.10.6136-6151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakane P K, Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974;22:1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- 37.Narayan O, Clements J E, Strandberg J D, Cork L C, Griffin D E. Biological characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980;50:69–79. doi: 10.1099/0022-1317-50-1-69. [DOI] [PubMed] [Google Scholar]
- 38.Narayan O, Zink M, Gorrell M, Crane S, Huso D, Jolly P, Saltarelli M, Adams R, Clements J E. The lentiviruses of sheep and goats. In: Levy J A, editor. The retroviridae. New York, N.Y: Plenum Press; 1993. pp. 229–256. [Google Scholar]
- 39.Perry L L, Wilkerson M J, Hullinger G A, Cheevers W P. Depressed CD4+ T lymphocyte proliferative response and enhanced antibody response to viral antigen in chronic lentivirus-induced arthritis. J Infect Dis. 1995;171:328–334. doi: 10.1093/infdis/171.2.328. [DOI] [PubMed] [Google Scholar]
- 40.Pitt-Rivers R, Impiombato F S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968;109:825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pyper J M, Clements J E, Gonda M A, Narayan O. Sequence homology between cloned caprine arthritis encephalitis virus and visna virus, two neurotropic lentiviruses. J Virol. 1986;58:665–670. doi: 10.1128/jvi.58.2.665-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Querat G, Audoly G, Sonigo P, Vigne R. Nucleotide sequence analysis of SA-OMVV, a visna-related ovine lentivirus: phylogenetic history of lentiviruses. Virology. 1990;175:434–447. doi: 10.1016/0042-6822(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 43.Saltarelli M, Querat G, Konings D A, Vigne R, Clements J E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology. 1990;179:347–364. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- 44.Sargan D R, Bennet I D, Cousens C, Roy D J, Blacklaws B A, Dalziel R G, Watt N J, McConnell I. Nucleotide sequence of EV1, a British isolate of maedi-visna virus. J Gen Virol. 1991;72:1893–1903. doi: 10.1099/0022-1317-72-8-1893. [DOI] [PubMed] [Google Scholar]
- 45.Schauer R. Sialic acids and their role as biological masks. Trends Biochem Sci. 1985;10:357–360. [Google Scholar]
- 46.Sonigo P, Alizon M, Staskus K, Klatzmann D, Cole S, Danos O, Retzel E, Tiollais P, Haase A, Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985;42:369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- 47.Staskus K A, Retzel E F, Lewis E D, Silsby J L, St. Cyr S, Rank J M, Wietgrefe S W, Haase A T, Cook R, Fast D, Geiser P T, Harty J T, Kong S H, Lahti C J, Neufeld T P, Porter T E, Shoop E, Zachow K R. Isolation of replication-competent molecular clones of visna virus. Virology. 1991;181:228–240. doi: 10.1016/0042-6822(91)90488-w. [DOI] [PubMed] [Google Scholar]
- 48.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valas S, Benoit C, Baudry C, Perrin G, Mamoun R Z. Variability and immunogenicity of caprine arthritis-encephalitis virus surface glycoprotein. J Virol. 2000;74:6178–6185. doi: 10.1128/jvi.74.13.6178-6185.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valas S, Benoit C, Guionaud C, Perrin G, Mamoun R Z. North American and French caprine arthritis-encephalitis viruses emerge from ovine maedi-visna viruses. Virology. 1997;237:307–318. doi: 10.1006/viro.1997.8800. [DOI] [PubMed] [Google Scholar]
- 51.Wain-Hobson S, Sonigo P, Guyader M, Gazit A, Henry M. Erratic G-A hypermutation within a complete caprine arthritis-encephalitis virus (CAEV) provirus. Virology. 1995;209:297–303. doi: 10.1006/viro.1995.1261. [DOI] [PubMed] [Google Scholar]
- 52.Wu Z, Kayman S C, Honnen W, Revesz K, Chen H, Vijh-Warrier S, Tilley S A, McKeating J, Shotton C, Pinter A. Characterization of neutralization epitopes in the V2 region of human immunodeficiency virus type 1 gp120: role of glycosylation in the correct folding of the V1/V2 domain. J Virol. 1995;69:2271–2278. doi: 10.1128/jvi.69.4.2271-2278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]