ABSTRACT
Shigella continues to be a major contributor to diarrheal illness and dysentery in children younger than 5 years of age in low- and middle-income countries. Strategies for the prevention of shigellosis have focused on enhancing adaptive immunity. The interaction between Shigella and intrinsic host factors, such as the microbiome, remains unknown. We hypothesized that Shigella infection would impact the developing microbial community in infancy and, conversely, that changes in the gastrointestinal microbiome may predispose infections. To test this hypothesis, we characterized the gastrointestinal microbiota in a longitudinal birth cohort from Malawi that was monitored for Shigella infection using 16S rRNA amplicon sequencing. Children with at least one Shigella quantitative polymerase chain reaction (qPCR) positive sample during the first 2 years of life (cases) were compared to uninfected controls that were matched for sex and age. Overall, the microbial species diversity, as measured by the Shannon diversity index, increased over time, regardless of case status. At early time points, the microbial community was dominated by Bifidobacterium longum and Escherichia/Shigella. A greater abundance of Prevotella 9 and Bifidobacterium kashiwanohense was observed at 2 years of age. While no single species was associated with susceptibility to Shigella infection, significant increases in Lachnospiraceae NK4A136 and Fusicatenibacter saccharivorans were observed following Shigella infection. Both taxa are in the family Lachnospiraceae, which are known short-chain fatty acid producers that may improve gut health. Our findings identified temporal changes in the gastrointestinal microbiota associated with Shigella infection in Malawian children and highlight the need to further elucidate the microbial communities associated with disease susceptibility and resolution.
IMPORTANCE Shigella causes more than 180 million cases of diarrhea globally, mostly in children living in poor regions. Infection can lead to severe health impairments that reduce quality of life. There is increasing evidence that disruptions in the gut microbiome early in life can influence susceptibility to illnesses. A delayed or impaired reconstitution of the microbiota following infection can further impact overall health. Aiming to improve our understanding of the interaction between Shigella and the developing infant microbiome, we investigated changes in the gut microbiome of Shigella-infected and uninfected children over the course of their first 2 years of life. We identified species that may be involved in recovery from Shigella infection and in driving the microbiota back to homeostasis. These findings support future studies into the elucidation of the interaction between the microbiota and enteric pathogens in young children and into the identification of potential targets for prevention or treatment.
KEYWORDS: Shigella, gut microbiome, infant microbiome
INTRODUCTION
Shigella is one of the top three causes of moderate to severe diarrhea (MSD) in the first 5 years of life in children living in Asia and Sub-Saharan Africa (1–4). It is an invasive enteric pathogen that causes mucosal inflammation and the disruption of the intestinal barrier (5, 6), leading to watery diarrhea and dysentery (bloody diarrhea) (4). Frequent and repeated bouts of diarrheal disease in children result in debilitating sequelae, including impaired growth and stunting, deficits in cognitive development, and an increased risk of metabolic syndrome (7–9), which can result in lifelong health impairments (10, 11). The transmissibility and clinical severity of disease in this vulnerable group, along with the emergence of antibiotic-resistant strains, make Shigella prevention a public health priority. While efforts to determine the roles of innate and adaptive immunity in preventing and reducing susceptibility to Shigella infection in infants are ongoing (12–17), the interaction between Shigella and the gastrointestinal microbiome, as well as its subsequent consequences to the gastrointestinal and the overall host health, remain largely unexplored.
The colonization of the gastrointestinal tract in infants is dynamic and sensitive to many factors, such as gestational age (18–20), mode of delivery (vaginal or cesarean delivery) (18, 21), and nutritional status (e.g., breastfeeding status and malnutrition) (22–26), and these can vary greatly across different geographical settings (27, 28). There has been an increased interest in understanding the role of the gastrointestinal microbiota in promoting health, including its potential to reduce illnesses early in life (29–31). Conversely, disruptions in the gut microbiome early in life have been associated with gastrointestinal disorders, such as necrotizing enterocolitis (NEC), inflammatory bowel disease (IBD) (30), and infectious diarrhea (32, 33). However, the roles of commensal bacteria (presence or absence), either acting as a barrier to Shigella infection or facilitating its colonization, and the influence of Shigella infection in establishing the developing microbiota in early life have not been characterized.
Previous studies exploring the role of the microbiota in infectious disease in children have generally focused on diarrhea (32–34) or on other pathogens, (e.g., cholera [35–37] or diarrheagenic E. coli infection [38]). An epidemiological study on the impact of Shigella infection on the infant microbiome relied on data obtained from a single time point (cross-sectional samples, [39]), and therefore failed to capture the dynamic nature of the microbiome preceding and succeeding Shigella infection. We hypothesized that Shigella infection would impact the evolving microbial community in infancy and, conversely, that changes in the gastrointestinal microbiome may predispose future infections. To address this question, we conducted a longitudinal characterization of the gastrointestinal microbiota in children from birth to 2 years of age living in Malawi, where Shigella is endemic and is a major contributor to diarrheal disease (40). Using 16S rRNA amplicon sequencing, we examined the relationship between microbial communities and the incidence of Shigella infection. The dynamic composition of the microbiome postinfection was also examined. We identified temporal changes in the microbiota and an increased abundance of taxa consistent with health recovery following infection. The study highlights the dynamic nature of the microbiome in early life and the impact of Shigella infection on the developing microbiome.
RESULTS
Cohort and sample summary.
Participants were recruited as part of a mother-infant cohort in a malaria surveillance study (41). All of the infants were born between February and November of 2016. Rectal swab samples were collected every 6 months during routine well-child visits, as well as each time the child presented at the clinic with diarrhea. Of the 369 rectal swabs collected, 37 (10%) were positive for Shigella by (qPCR), which matches the overall Shigella prevalence over the first 2 years of life in children from 8 countries enrolled in the MAL-ED study (42). Most of the Shigella qPCR positive samples were detected in children older than 12 months of age (25 out of 37, 69%) (Fig. 1A), consistent with the results of previous studies (3, 43). In addition, most of the Shigella positive samples (29 out of 37, 81%) were collected between the months of November and April, which coincides with the rainy season in Malawi (Fig. 1A) (44), reflecting the previously described seasonality of Shigella infections (42). The earliest time point of a rectal swab collection was 2 months of age.
FIG 1.
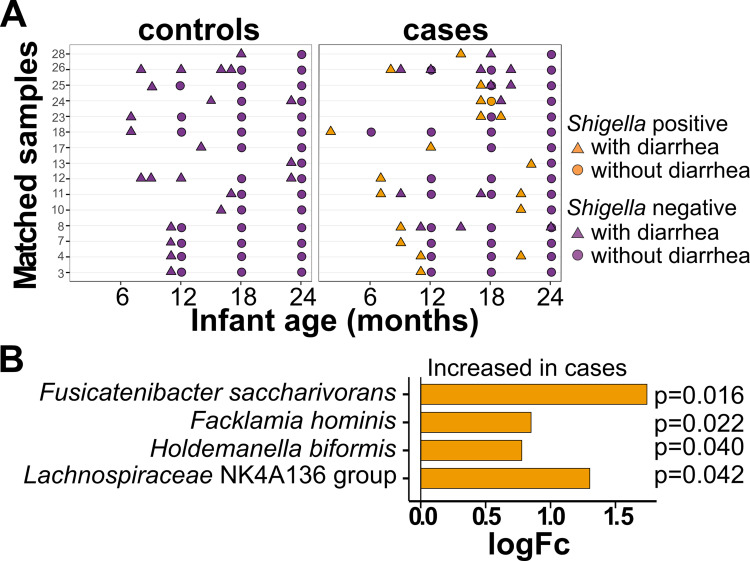
Cohort sample summary. (A) Number of samples collected (right y axis) and infant age at sample collection (left y axis) in each month of the year (x axis). The orange symbols represent samples that were Shigella quantitative polymerase chain reaction (qPCR) positive. (B) Sample matching strategy: samples from matched cases and controls from 0 to 24 months of age. Each row represents samples collected at each time point from each matched pair of cases (right panel) and controls (left panel). The triangles represent samples collected with diarrhea, and the circles represent samples with no diarrhea. The orange symbols represent samples that were Shigella qPCR positive.
Out of 90 children whose rectal swabs were tested for Shigella by qPCR, 33 (37%) had at least one Shigella qPCR positive sample during the 24-month study period. Of these, 30 children with a complete set of longitudinal swab samples from each of the 6-, 12-, 18-, and 24-month visits were included in our analysis and classified as cases. To reduce the effects of age and sex as potential confounders, 30 children (with no Shigella qPCR positive samples) matching the cases by sex and age (born within the same month or as close as possible), were included as matched controls (Fig. 1B). We also noted whether samples were collected on “diarrheal” visits (as defined by the health care provider). Diarrheal samples that were negative by Shigella qPCR were deemed to have diarrhea caused by a pathogen other than Shigella (Fig. 1B).
Both the case and the control groups had similar weights at birth (means of 3.2 kg and 3.1 kg, respectively), and there were no significant differences between the ages of the mothers (means of 27 years and 28 years, respectively) or the gestational ages (means of 38 and 39 weeks, respectively). Each group of children was composed of 50% males and females. Length for age z scores (LAZ), a measure for stunting which has been associated with Shigella infection in children (9, 45–48), did not significantly differ between the case and control groups at birth or at 24 months of age (Table 1).
TABLE 1.
Cohort characteristics
| Characteristics | Casesa (n = 30) | Controlsb (n = 30) | P valuec |
|---|---|---|---|
| Female Sex, No. (%) | 15 (50%) | 15 (50%) | |
| Birthweight, mean in kg (range) | 3.2 (1.5 to 4.3) | 3.1 (2.0 to 4.3) | 0.69 |
| Length-for-age z-scores | |||
| at birth, mean (range) | 0.04 (−6.25 to 6.40) | −0.21 (−1.96 to 2.60) | 0.62 |
| at 24 mo, mean (range) | −1.71 (−3.54 to −0.09) | −1.74 (−4.37 to 1.44) | 0.88 |
| Maternal age at birth, mean no. of years (range) | 27 (17 to 38) | 28 (17 to 45) | 0.36 |
| Gestational age at birth, mean no. of weeks (range) | 38 (33 to 45) | 39 (34 to 44) | 0.10 |
Cases: infants with at least one Shigella quantitative polymerase chain reaction (qPCR) positive sample.
Controls: infants with no Shigella qPCR positive samples.
P values are from unpaired t tests comparing the cases and the controls.
The total read counts did not differ significantly between the Shigella positive samples and the negative samples (Fig. S1A), nor did the Escherichia/Shigella read counts (Fig. S1B). However, for the Shigella qPCR positive samples, the mean Escherichia/Shigella read count in the diarrheal samples was significantly higher than that of the samples without diarrhea (Fig. S1C). For the Shigella qPCR positive samples, we did not observe an association between qPCR quantitation cycle (Cq) values and Escherichia/Shigella read counts (Fig. S1D).
Comparisons between read counts and Shigella qPCR status. (A) Boxplots showing the total read counts in the Shigella qPCR positive and negative samples. (B) Boxplots showing Escherichia/Shigella read counts in Shigella qPCR positive and negative samples. (C) Boxplots showing Escherichia/Shigella read counts in Shigella qPCR positive samples that were either diarrhea positive or negative. *, P < 0.05, from unpaired t tests comparing diarrhea positive and negative samples. (D) Correlation between Shigella qPCR quantitation cycle (Cq) values and Escherichia/Shigella read counts. Spearman’s r values are shown within the graph. Shigella qPCR Cq values that were greater than 35 (qPCR negative) were considered undetermined. The symbols represent individual samples. Download FIG S1, TIF file, 1.2 MB (1.2MB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Longitudinal variation in microbiome composition.
We first set out to identify temporal changes in the gastrointestinal microbiota by calculating the alpha diversity as measured by the Shannon diversity index (SDI). SDI was positively correlated with age (R2 = 0.25) and increased during the first 2 years for all infants (Fig. 2A). The increase in SDI did not differ between the cases and the controls (F = 2.78, P = 0.096) (Fig. 2B).
FIG 2.
Alpha diversity comparisons in infant microbiomes. (A) Shannon diversity indices at different ages at the time of sample collection, from 2 months to 24 months of age. The symbols represent individual values. The R2 and P values are from a simple linear regression model. (B) Shannon diversity indices of samples from case (orange) versus control (purple) individuals at the time of sample collection. The symbols represent individual values. The R2 values from simple linear regression models are shown separately for the cases and the controls within the graph.
For an overview of the taxonomic composition of the gastrointestinal microbiomes during the first 2 years of life, we focused on the top 10 identified taxa based on the relative abundance for all individuals in the cohort (Fig. 3A; Table S1). The infant gastrointestinal microbiota was enriched with species from the phyla Actinobacteria, Bacteriodetes, Firmicutes, and Proteobacteria. These included Bifidobacterium spp., which have been previously demonstrated to be enriched in the gastrointestinal microbiome of breastfed infants (49, 50), Prevotella spp., which are commonly isolated at higher frequencies in fecal samples isolated from African populations (32, 39, 51), as well as Escherichia/Shigella, (16S rRNA sequencing does not distinguish these separately), which would be expected in Shigella positive samples and are common in infant gastrointestinal microbiomes (52, 53).
FIG 3.
Taxonomic compositions of infant gastrointestinal microbiomes by age. (A) Mean relative abundance of the 10 most abundant taxa by infant age. n represents the number of samples at each time point (month). (B) Bar graphs showing the mean relative abundances (%) of the top 10 most abundant taxa at the infant ages of 6, 12, 18, and 24 months. The symbols represent individual values. The mean relative abundances at different ages were compared via a one-way analysis of variance (ANOVA). *, P < 0.05.
Mean relative abundance of the top 10 taxa. Download Table S1, DOCX file, 0.01 MB (13.9KB, docx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We identified shifts in the abundance of Bifidobacterium longum, which dominated the microbial community at early time points but decreased significantly (P < 0.05) by 24 months (13.3% of the sequenced reads at 6 months versus 0.7% at 24 months) (Fig. 3A and B; Table S1). Of interest, another member of the bifidobacterial species, Bifidobacterium kashiwanohense exhibited the opposite trend, increasing in relative abundance over time (0.3% of the sequenced reads at 6 months compared to 3.5% of the sequenced reads at 24 months). The mean relative abundance of the Escherichia/Shigella group decreased significantly from 4.4% of the sequencing reads at 6 months to 0.9% of the sequencing reads at 18 months (P < 0.05). The opposite was observed for Prevotella 9, which had a relative abundance of 2.4% of the sequenced reads at 6 months but dominated the microbiota at month 24 (9.7% of the sequenced reads). The mean relative abundance of each of the top 10 taxa at months 6, 12, 18, and 24 is shown in Fig. 3A and B and in Table S1.
Differences in microbiota dynamics in cases and controls at the time of, prior to, and following Shigella infection.
To identify changes in the gastrointestinal microbiota that are concomitant with Shigella infection, we compared the alpha diversity at the first Shigella qPCR positive visit in the cases (the index visit) and their matched controls. After adjusting for variables that could affect alpha diversity, including infant age (32, 33, 38), diarrhea (32, 54), and antibiotic use (55, 56), we determined that the SDI did not differ significantly by Shigella infection status (P = 0.52) (Table S2). Notably, alpha diversity was significantly associated with infant age (P < 0.001), diarrhea (P = 0.012), and recent antibiotic use (P = 0.017) (Table S2).
Cross-sectional comparison between case index visits and matched control index visits. Download Table S2, DOCX file, 0.01 MB (13.4KB, docx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Considering that the presence or absence of certain microbial community members may facilitate or inhibit Shigella infection, we examined whether any bacterial communities were differentially abundant between the cases and the matched controls at the index visit. While Escherichia/Shigella were among the taxa increased in the cases, no taxa were significantly associated with case status (Shigella infected versus controls) after controlling for multiple variables (Fig. S2).
Taxa differentially abundant between cases and controls at Shigella index visit. Estimated differences (log2-fold change, logFc) between the abundance of taxa in the cases compared to the controls at the time of Shigella qPCR positive sample collection. A positive logFc indicates a greater abundance in the cases than in the controls. Species are ordered by increasing P value (before controlling for multiple variables). Escherichia/Shigella is highlighted in red. None of the taxa were significantly associated with case status after controlling for multiple variables. Download FIG S2, TIF file, 1.1 MB (1.1MB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We then examined changes in the gastrointestinal microbiota that potentially predisposed infants to Shigella infection. Considering all samples immediately prior to the case or control index visits (Fig. S3), we identified that the alpha diversity prior to incident Shigella infection did not differ between the cases and the controls. (Table S3). In addition, no taxa were significantly associated with case status (Shigella-infected versus controls) during this time interval (Fig. S3).
Taxa differentially abundant between cases and controls before Shigella infection. Estimated differences (log2-fold change, logFc) between the abundance of taxa in the cases compared to the controls before Shigella infection. A positive logFc indicates a greater abundance in the cases than in the controls. Species are ordered by increasing P value (before controlling for multiple variables). None of the taxa were significantly associated with case status after controlling for multiple variables. Download FIG S3, TIF file, 0.4 MB (387.2KB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparisons of the Shannon diversity index (SDI) between cases and controls. Download Table S3, DOCX file, 0.01 MB (13.8KB, docx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To determine the effects of Shigella infection on subsequent microbial community composition, we compared the SDI from all samples after the case and control index visits (Fig. 4A). Shigella infection did not significantly alter the SDI of cases postinfection compared to their matched controls (Table S3). However, abundances of Fusicatenibacter saccharivorans and Lachnospiraceae NK4A136 group, both of which are members of the family Lachnospiraceae, were significantly more abundant in the cases than in the controls following Shigella infection (Fig. 4B). This was also observed when comparing the relative abundance of both species over time. There was an increase in the relative abundance of both species after the first year, which was significant for Fusicatenibacter saccharivorans (Fig. S5A). When comparing the cases versus the controls, there was a trend of greater abundance in the cases versus the controls for both species at months 18 and 24 (Fig. S5B).
FIG 4.
Taxa differentially abundant in cases after Shigella infection. (A) Chart representing samples that were included in the analysis. All samples after the collection of a Shigella qPCR positive sample in the case individuals (right column) and their matching controls (left column). Samples in orange represent those that were found to be Shigella qPCR positive. (B) Taxa identified to be significantly (adjusted P < 0.05) abundant in cases versus controls after Shigella infection. The x axis indicates the estimated difference (log2-fold change, logFc) between the abundance of taxa in the cases compared to the controls. These estimates were obtained from logistic regression models. A positive logFc indicates a greater abundance in the cases than in the controls. The adjusted P values were determined using a mixed effects linear regression model controlled for match, sample age, infant ID, diarrhea, and short-term antibiotic use.
Mean relative abundances of F. saccharivorans and Lachnospiraceae NK4A136 by age. (A) Bar graphs showing the mean relative abundances (%) of F. saccharivorans and Lachnospiraceae NK4A136 at the infant ages of 6, 12, 18, and 24 months and (B) comparing the cases and the controls. The symbols represent individual values. The mean relative abundances at the different ages were compared via a one-way analysis of variance (ANOVA). *, P < 0.05; **, P < 0.01. Download FIG S5, TIF file, 0.3 MB (365.3KB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Shigella-associated diarrhea results in increases in taxonomic groups that are distinct from infection with other pathogens.
Multiple studies in children younger than 5 years of age have identified shifts in microbiome composition associated with infectious diarrhea, as measured by the SDI (32, 33, 38). We examined the specific impact of Shigella-associated diarrhea on microbiota composition in our cohort in two ways. First, we compared the microbiota of the case individuals who were symptomatic (with diarrhea) with those who were asymptomatic (without diarrhea) before and after Shigella infection. The SDI values for the microbiomes of infants who had a symptomatic infection were not significantly different from those without diarrhea either before (P = 0.25) or after (P = 0.65) infection (Table S3). In addition, no taxa were significantly associated with symptomatic Shigella infection either before or after infection (Fig. S4).
Taxa differentially abundant in children with symptomatic versus asymptomatic Shigella. Estimated differences (log2-fold change, logFc) between the abundance of taxa in children with a symptomatic infection (with diarrhea) and those with an asymptomatic infection (without diarrhea) prior to (A) or following (B) Shigella infection. These estimates were obtained from logistic regression models. A positive logFc indicates a greater abundance in symptomatic infections than in asymptomatic infections. Species are ordered by increasing P value (before controlling for multiple variables). None of the taxa were significantly associated with symptomatic or asymptomatic Shigella infection after controlling for multiple variables. Download FIG S4, TIF file, 0.8 MB (829KB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
As diarrhea can result from colonization by multiple different pathogens, we next compared the microbiota from the cases with a diarrhea-positive, Shigella qPCR positive sample at the index visit (Fig. 5A, right) with matched control samples (diarrhea-positive and Shigella qPCR negative) collected within a month of the case sample (Fig. 5A, left). The alpha diversity did not significantly differ following the Shigella-associated diarrheal visits compared to the diarrheal visits not associated with Shigella infection (P = 0.42) (Table S3). However, Facklamia hominis and Holdemanella biformis, both of which are members of the Firmicutes phylum, and the two species that had been identified as increased in the cases after Shigella infection, namely, Fusicatenibacter saccharivorans and Lachnospiraceae NK4A136 group, were significantly increased in the cases after Shigella-associated diarrhea (Fig. 5B).
FIG 5.
Taxa differentially abundant in cases after Shigella-driven diarrhea versus diarrhea from other causes. (A) Chart representing samples that were included in the analysis. Case samples are those that were collected from case individuals after a Shigella qPCR positive and diarrhea positive sample (right column, orange triangles). Control samples are those from matched control individuals after a diarrhea-positive sample was collected within a month of the case sample (left column, purple triangles). (B) Taxa identified to be significantly (adjusted P < 0.05) abundant in cases after Shigella positive diarrheal infection versus the controls after a diarrhea positive sample was collected. The x axis indicates the estimated difference (log2-fold change, logFc) between the abundance of taxa in the cases compared to the controls. These estimates were obtained from logistic regression models. A positive logFc indicates a greater abundance in the cases than in the controls. The adjusted P values were determined using a mixed effects logistic regression model controlled for infant sex, birth month, sample age, and infant ID.
DISCUSSION
The first years of life are a critical period for the colonization, expansion, and maturity of the gastrointestinal microbiome, which in turn is essential for appropriate mucosal immune development (30, 31). Enteric infections during this time may alter the gut microbiome (32, 33) with long-lasting consequences. Here, we examined the dynamics of the infant gastrointestinal microbial communities in Shigella-infected and noninfected children over the first 2 years of life. Several large field studies of the etiology of diarrhea in developing countries have shown that Shigella infection peaks between the first and second years of life (3, 43). This was also observed in our Malawi cohort, in which Shigella cases progressively increased toward the second year of life (Table 1). Hence, the timing of our microbiome analysis is relevant, as it spans the critical age range when the risk of infection is highest. The analysis of this particular age group was important in attempting to identify perturbations that may be associated with repeated illness and long-lasting health impairments.
Overall, in all children, the gastrointestinal microbiota was dominated by the species Bifidobacterium longum at 6 months of age, and this was replaced by the abundance of various Prevotella spp. by the time the infants reached the 2-year mark. A previous study of microbiomes of children from Malawi at only 2 time points, 6 and 18 months of age, identified an increase of Prevotella that tracked with age, with a corresponding decrease of Bifidobacteriaceae and Enterobacteriaceae (57). Bifidobacterium spp. have been demonstrated to be enriched in the gastrointestinal microbiome of breastfed infants (49, 50). Therefore, the decrease of these species that we observed is consistent with that observed in children who are being weaned from breastfeeding.
We found that the alpha diversity increased during the first 2 years of life (Fig. 2A), which was consistent with previous reports (32, 54). Unlike previous studies that reported a decrease in alpha diversity after diarrheal infection (32, 33), we observed in our study that Shigella infection alone did not alter the trajectory of alpha diversity in infants over the first 2 years of life (Fig. 2B). This finding suggests that the competition between Shigella and the commensals in the gastrointestinal milieu may open a niche, allowing for the expansion of other bacteria to maintain microbiota diversity. Alternatively, the stability in alpha diversity may reflect the dynamic nature of the infant gastrointestinal microbiota that allows for recovery after the clearance of Shigella. This may be readily appreciated in a longitudinal, as opposed to a cross-sectional, study. It is also possible that our cohort was relatively healthy and that studies that have shown a decrease in alpha diversity compared healthy children with children who experienced more severe disease (32) than did those in our cohort.
The longitudinal design of our study also allowed us to infer a cause-and-effect relationship between the composition of the microbiota and Shigella infection. We had hypothesized that the presence of specific microbial communities could impact (i.e., increase susceptibility and help predict) infection status. Surprisingly, no single species, not even Escherichia/Shigella, was identified as significantly associated with susceptibility to Shigella infection. The latter is probably due to the inability to separate Escherichia species from Shigella and the presence of E. coli as a constituent of the healthy microbiome (58–60). This observation also suggests that Shigella infection is not only dependent on the presence of that organism but is determined by other host-related factors, such as maternal immunity (i.e., antibodies in breast milk) and the individual’s own local innate defenses. We and others have identified placentally acquired maternal antibodies to Shigella antibodies at birth (16, 17, 41). Shigella-specific antibodies have been found in breast milk (61). Host (infant) innate immunity also likely influences infection outcome. Further studies are needed to ascertain the role of maternal and infant immune components in preventing infection in early life.
Recovery from a Shigella infection was linked with a distinct alteration in microbiota composition. The species Lachnospiraceae NK4A136 group and Fusicatenibacter saccharivorans were increased in children who had a Shigella infection compared to controls (Fig. 4B), and this was also observed in children with Shigella-associated diarrhea (Fig. 5B). In a study that compared the microbiota profiles of obese and lean adults in Spain, the Lachnospiraceae NK4A136 group was found to be negatively associated with cardiovascular risk factors, including body fat, LDL, and total cholesterol levels (62). F. saccharivorans has been associated with a reduction in intestinal inflammation: it was found to be depleted in patients with active ulcerative colitis, (63). In the same study, the authors showed that the daily administration of heat-inactivated F. saccharivorans counteracted colitis symptoms in a mouse model of colitis (63). Both species are part of the Family Lachnospiraceae, whose members are known for their production of beneficial metabolites and fermentation of fiber and plant carbohydrate to short-chain fatty acids (SCFA), specifically butyrate (reviewed in reference [64]). Multiple in vivo and in vitro studies have demonstrated butyrate to display multiple roles in the gut epithelium, including colonocyte proliferation and differentiation, and it has been proposed to have anti-inflammatory effects, all of which point to its capacity to promote gut health (reviewed in reference [65]). Species in the Family Lachnospiraceae have been associated with recovery from other enteric infections, including Vibrio cholerae in 2- and 3-year-old children (35) and traveler’s diarrhea (66) in adults. Two other taxa, Facklamia hominis and Holdemanella biformis, both part of the Firmicutes phylum, were also increased after Shigella-associated diarrheal infection. F. hominis is an uncommon pathogen that has been reported in a few cases of human infections (67). H. biformis was found to be reduced in the microbiota of patients with colorectal adenomas, and it also produces SCFAs that reduce tumor cell proliferation (68). In addition, H. biformis was shown to produce long-chain fatty acids (LCFAs) that reduced inflammation in a dextran sulfate sodium (DSS)-induced mouse colitis model (69). Shigella infection produces an extensive inflammatory local response (70). It is likely that an increase in species that reduce inflammation and promote broad gastrointestinal health improvements represents an attempt to stabilize the commensal repertoire following infection.
Several ways in which members of the family Lachnospiraceae would facilitate the recovery from Shigella infection can be envisioned. Another member of this family, Ruminococcus obeum (reclassified as Blautia obeum [71]), was correlated with gut microbiota recovery from cholera in Bangladeshi infants, and it was shown to restrict colonization by V. cholerae in a mouse model (36). Notably, B. obeum was increased among Shigella cases at the index visit in our study (Fig. S2), though this increase was not statistically significant after controlling for multiple variables. The “recovery species” may also interact with other bacteria, their metabolites, or the host immune system (29, 72). A study of 6- to 24-month-old malnourished children in Gambia implicated species belonging to Lachnospiraceae in observed shifts from acute malnutrition following nutritional interventions (26). While the infants’ diets were not recorded in our study, a similar recovery effect may be plausible in our study. Whether the changes we observed are a natural progression of the microbiota in response to Shigella infection or are caused by environmental factors (e.g., antibiotics, oral rehydration, diet) warrants investigation in future studies.
An unexpected finding from our study was the identification of the Bifidobacterium kashiwanohense taxa, which not only was one of the most abundant but also displayed the opposite trend expected of other Bifidobacterium spp., increasing significantly over the first 2 years of life (Fig. 3). To our knowledge, this species has not been reported as part of the developing early life gastrointestinal microbiome, although it had been reportedly cultured from feces from a healthy Japanese infant (73). B. kashiwanohense has also been transiently identified in formula-fed (compared to breastfed) infants in Thailand (74). Like others in the genus, isolates of B. kashiwanohense have been shown to utilize the human milk oligosaccharide component, fucosyllactose (75, 76), yet the significance of this process within the gastrointestinal environment is unknown. Interestingly, in a study of anemic (compared to healthy) 6-month-old Kenyan infants, B. kashiwanohense was enriched in the anemic infants (77), likely due to its ability to bind to iron. B. kashiwanohense’s iron-sequestration function was also associated with the inhibition of growth and the adhesion of the enteropathogens Salmonella Typhimurium and Enterohemorrhagic Escherichia coli (EHEC) in vitro (78). Further studies incorporating diverse populations would be needed to identify the prevalence of B. kashiwanohense in early life gastrointestinal microbiota and to elucidate its role in relation to microbiome composition, infant nutrition, and its potential to control enteric infections.
Our study is the first involving a longitudinal analysis of the microbiota associated with Shigella infection in a well-characterized clinical cohort from birth up to 2 years (the age range of the highest risk of infection) that compares cases to matched controls. One limitation is that the 16S rRNA gene amplicon only amplifies bacterial species and therefore did not allow us to analyze the effects of other microbial species (e.g., viruses, fungi, or protozoa) that could affect the incidence and outcomes of shigellosis. Additionally, we could not confirm that Shigella was the only etiological agent, as children could be colonized by multiple diarrheal pathogens (40, 79), all of which could have influenced the outcomes. The duration between the initiation of diarrheal symptoms and sample collection was also not recorded, which may have affected our ability to discern significant microbiome changes. This variable will need to be accounted for in future studies, possibly by increasing sampling density and metadata collection. Nutritional information, including data on breastfeeding patterns, weaning, and infant diets, were not captured in our study, and we were unable to infer their effects on the outcomes measured. Several Shigella control human infection models are being pursued with adult volunteers. Such studies offer an opportunity to interrogate Shigella-microbiome associations in a setting of controlled dosing, active monitoring (with extensive sampling), and reduced variability.
In summary, in this study, we have characterized the infant gastrointestinal microbiota in children from birth to 2 years of age living in Malawi, and the information generated adds to the limited studies of the gastrointestinal microbiomes of African populations (80). We also demonstrated that Shigella infection did not profoundly impact overall species diversity but led to the expansion of species known to improve gastrointestinal health and drive the microbiota back to homeostasis. The exploration of the impact and interaction of these species with Shigella during and after infection may be warranted to identify protective elements and therapeutic targets. Therefore, our work provides a foundation for the interrogation of the dynamic nature of the pediatric gastrointestinal microbiome in health and disease.
MATERIALS AND METHODS
Study participants and sample collection.
The study participants were a cohort of children from birth to 2 years of age enrolled in a longitudinal malaria surveillance study in Malawi (41) who were also monitored for diarrheal disease. Mothers and infants were recruited between January and November of 2016 at Mfera Health Clinic in Chikwawa, Malawi, and they were followed for 2 years. Healthy pregnant women who were HIV seronegative were enrolled either at their prenatal clinic visit or during their stay for delivery. The infants were enrolled at birth, and their ages, sexes, and baseline health information (e.g., birth weights and lengths) were recorded. Infant health information, including diarrhea status (as defined by the health care provider at the time of the sample) and any antibiotics prescribed, was also obtained at each subsequent visit to the health clinic. All but two of the infants (one case and one control) were prescribed antibiotics (amoxicillin, co-trimoxazole, metronidazole, or erythromycin) at least once during the 2 years. Short-term antibiotic treatment, defined as any antibiotic given within 2 weeks before sample collection, was therefore included as a covariate in our subsequent analyses. While breastfeeding was common up to 24 months of age, data on the duration of exclusive breastfeeding, weaning, the introduction of solid food, and the infants’ diets were not available. Therefore, these factors were not included as covariates in our analysis.
Rectal swab samples were collected every 6 months at each scheduled well-child visit as well as at every time that the infant presented at the clinic with diarrhea to diagnose Shigella infection. Samples were immediately placed in 350 μL of DNA/RNA Shield (Zymo Research, Irvine, CA), frozen, and stored at −80°C. Only infants with a complete set of samples obtained at scheduled clinic visits at 6, 12, 18, and 24 months were studied. Cases were defined as children with at least one Shigella qPCR positive sample during the 2-year period. Controls were selected as children without a Shigella qPCR positive sample during the 2-year period, and they were matched for sex and age (within 1 month of birth where possible) (Fig. 1A).
This study was approved by the Institutional Review Board of the University of Maryland School of Medicine (UMB IRB No: HP-00087456) and the College of Medicine Research and Ethics Committee (COMREC) at the College of Medicine in Malawi (COMREC Ref. No: P.01/16/1859). All of the participating mothers provided written informed consent for themselves and for their infants.
Rectal swab genomic DNA extraction and Shigella qPCR analysis.
DNA was isolated from 100 μL aliquots of the rectal swab samples using the QIAamp DNA Stool Extraction Kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s protocol. Extracted DNA was precipitated with ethanol in a final elution volume of 200 μL. Each DNA sample was tested for Shigella by qPCR using the previously described SYBR green-based fluorescent dye method to detect the ipaH gene (81). The primers for ipaH were originally created by Vu et al. (82). Quantitation cycle (Cq) values of <35 detected in duplicate wells were required to consider a sample qPCR positive. Controls for the qPCR assay included extraction-negative, qPCR negative, and a known qPCR positive control. While this method cannot distinguish between Shigella and enteroinvasive Escherichia coli (EIEC), EIEC prevalence is minimal compared with Shigella in similar geographic settings (43, 83). Therefore, all of the ipaH positive samples were assumed to be Shigella (79).
Rectal swab genomic DNA extraction, amplicon sequencing, and taxonomic assignment.
The remainder of the frozen rectal swab samples were transported to the University of Maryland School of Medicine in Baltimore, and sequencing and sequence processing were performed at the Institute for Genome Sciences Microbiome Service Laboratory (https://msl.igs.umaryland.edu/). Total nucleic acids were extracted from the remaining (~200 μL) aliquot of the rectal swab sample using the Qiagen Microbiome RNA/DNA isolation kit (Qiagen, Hilden, Germany) using a semiautomated protocol for the Hamilton Star platform (Hamilton Company, Reno, NV), following the manufacturer’s protocol (84). Cells were lysed via physical disruption on the TissueLyser (Qiagen, Hilden, Germany) at 20 Hz for 20 min, and the final elution volume was 110 μL. 10 negative controls of water were extracted in the same manner (extraction negatives). For the rectal swab samples, as well as for five positive controls (Zymobiomics Microbial Community Standard) and five negative controls (PCR negatives), amplicon sequencing of the 16S rRNA gene V3-V4 variable regions was performed using the 2-Step PCR method and sequences processed as described in Holm et al. (85). Briefly, sequences were demultiplexed using the dual-barcode strategy, a mapping file linking the barcode to the samples, and split_libraries.py, a QIIME-dependent script (86). The resulting forward and reverse fastq files were split by sample using the QIIME-dependent script split_sequence_file_on_sample_ids.py, and the primer sequences were removed using TagCleaner (version 0.16) (87). Further processing followed the DADA2 Workflow for Big Data and dada2 (v. 1.5.2) (https://benjjneb.github.io/dada2/bigdata.html) (88). The forward and reverse reads were each trimmed using lengths of 255 and 225 bp, respectively, filtered to contain no ambiguous bases with a minimum quality score of two, and required to contain less than two expected errors based on their quality scores. The relationship between quality scores and error rates was estimated on the combined sequencing runs to reduce batch effects arising from run-to-run variability. The reads were assembled into amplicon sequence variants (ASV) and chimeras for the combined runs removed as per the dada2 protocol. Taxonomy was assigned using the RDP Classifier (89) and the SILVA database (v138) (90). For each amplicon sequence variant, we applied the lowest (finest) level of taxonomy possible and then combined the counts of the ASVs with the same taxonomic assignment. Thus, some ASVs were assigned at the level of species, of genus, etc.
The maximum number of reads in the PCR negative controls was 1,233. The genus Sphingomonas was observed in most samples as well as in the extraction negative and the PCR negative controls. Therefore, it was removed from all samples prior to the downstream analyses. Taxa identified as d_Bacteria were also removed, as these contained counts for unidentified reads. Additionally, taxa observed in <5% of samples study-wide were removed (n = 1,120 taxa). The mean number of reads per sample for taxa prevalent in >5% of the samples remained close to the original number (41201.36). Samples with fewer than 1,000 reads were culled (n = 10 samples). Following the filtering steps, the 16S rRNA gene amplicon libraries yielded an average of 23,720 reads per sample with a total of 8,729,027 reads (Data Set S4). A total of 325 taxa were identified after quality filtering. All data are provided in Data Set S5.
Sample metadata. Download DATA SET S4, XLSX file, 0.04 MB (43.1KB, xlsx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundance. Download DATA SET S5, XLSX file, 0.8 MB (787.2KB, xlsx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Statistical analyses.
The length for age z scores (LAZ) were calculated based on the World Health Organization child growth standards (91) and using the zscorer package (v0.3.1) in R. Population characteristics (birthweight, LAZ, maternal age at birth, and gestational age at birth) between the cases and the controls were compared using unpaired t tests in GraphPad Prism v9 (San Diego, CA). P values of <0.05 were considered significant.
The alpha diversity was calculated using the Shannon diversity index (SDI) for each sample via the diversity function from the vegan package (v. 2.5-7) in R (92). Correlations between SDI and infant age were determined via linear regression (lm function in R) (93). Here, infant age was the predictor variable, and SDI was the response. To test for an association between case status and changes in alpha diversity over time (age), the data were fit to a mixed effects linear regression model which accounted for match and for an interaction term between age and case status. P values of <0.05 were considered to be indicative of a statistically significant result.
Index visits were defined as the first visit at which a Shigella qPCR positive test was observed. The first visit was used for individuals who had more than one Shigella qPCR-positive sample. For the matched controls, the index visit was the sample from the same age (within 1 month). Alpha diversity at index visits was compared using a two-way analysis of variance (ANOVA) (Type III) analysis in which SDI was the response variable, case status was the predictor, and the following covariates were adjusted for: infant age, infant sex, month of sample collection, diarrhea status, and short-term antibiotic use. An adjusted P value of <0.05 was considered to be indicative of a statistically significant result.
The effect of case status on alpha diversity prior to incident infection was compared using a generalized logistic regression model where the response was case status and the predictor was SDI. Comparisons after infection were made using a mixed effects linear regression model in which the response was SDI and the predictor was case status. Covariates included in these models were matched sample (as per Fig. 1A), sample age, infant ID (to account for multiple samples collected from the same individual), diarrhea status, and short-term antibiotic use. An adjusted P value of <0.05 was considered to be indicative of a statistically significant result. Relationships were visualized using ggplot2 (v3.3.5) (94).
Regarding differential abundance, for each of the top 10 taxa, the mean relative abundances at months 6, 12, 18, and 24 were compared using a one-way ANOVA. The mean relative abundances at months 6, 12, 18, and 24 between the cases and the controls were compared using unpaired t tests. P values of <0.05 were considered to be indicative of a statistically significant result. The statistical analysis was conducted using GraphPad Prism v9.
To identify associations between taxa abundance and case status before and after Shigella infection, mixed effects logistic or linear regression models were used, respectively, adjusting for the following covariates: matched sample (as per Fig. 1A), sample age, infant ID, diarrhea status, and short-term antibiotic use. An adjusted P value of <0.05 was considered to be indicative of a statistically significant result.
To compare taxa that were differentially abundant before or after Shigella with or without diarrhea infection, a mixed effects logistic regression model was used, accounting for infant sex, sample age, birth month, infant ID, and short-term antibiotic use. A similar model was used to compare taxa after obtaining diarrheal samples with Shigella infections as opposed to infections of other pathogens. An adjusted P value of <0.05 was considered to be indicative of a statistically significant result.
Count data were normalized for differences in coverage using the “poscounts” estimator (deals with a gene with some zeros by calculating a modified geometric mean by taking the nth root of the product of the nonzero counts) in the DESeq2 package in R (95). A local regression of log dispersions was fit over the log base mean. P values were obtained using Wald’s test (uses the estimated standard error of the log2-fold change between conditions to test whether it is equal to zero). The P values for the multiple comparisons were adjusted using the false discovery rate (FDR). An adjusted P value of <0.05 was considered to be indicative of a statistically significant result.
Data availability.
All of the raw sequencing data were deposited into NCBI SRA under BioProject ID PRJNA834726.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health awards R01AI117734 and R01AI125841, as well as by Research Supplement to Promote Diversity in Health-Related Research awards 3R01AI117734-04S1 to M.F.P., U19AI110820 to D.A.R., and T32DK067872 to E.N. The sequencing costs were subsidized by the University of Maryland, Baltimore, Institute for Clinical & Translational Research and by the National Center for Advancing Translational Sciences (NCATS) Clinical Translational Science Award (CTSA), grant number 1UL1TR003098.
We thank Mike Humphrys and Justin Grant at the Microbiome Service Laboratory for sequencing support. We are also grateful to the support team at the Mfera Health Center in Malawi and to the mothers, infants, and families who volunteered to participate. We thank members of the Laufer and Pasetti labs for providing technical and logistic expertise during the sample collection process and for discussion.
Contributor Information
Marcela F. Pasetti, Email: mpasetti@som.umaryland.edu.
David A. Rasko, Email: drasko@som.umaryland.edu.
Ryan McClure, Pacific Northwest National Laboratory.
REFERENCES
- 1.Kotloff KL. 2017. The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am 64:799–814. doi: 10.1016/j.pcl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GBDCoD. 2018. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque AS, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder GN, Hilbi H. 2008. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev 21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattock E, Blocker AJ. 2017. How do the virulence factors of Shigella work together to cause disease? Front Cell Infect Microbiol 7:64. doi: 10.3389/fcimb.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil I, Troeger CE, Blacker BF, Reiner RC, Jr. 2019. Capturing the true burden of Shigella and ETEC: the way forward. Vaccine 37:4784–4786. doi: 10.1016/j.vaccine.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. 2013. The impoverished gut–a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrant RL, Bolick DT, Swann JR. 2021. Modeling enteropathy or diarrhea with the top bacterial and protozoal pathogens: differential determinants of outcomes. ACS Infect Dis 7:1020–1031. doi: 10.1021/acsinfecdis.0c00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troeger C, Colombara DV, Rao PC, Khalil IA, Brown A, Brewer TG, Guerrant RL, Houpt ER, Kotloff KL, Misra K, Petri WA, Jr, Platts-Mills J, Riddle MS, Swartz SJ, Forouzanfar MH, Reiner RC, Jr, Hay SI, Mokdad AH. 2018. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health 6:e255–e269. doi: 10.1016/S2214-109X(18)30045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DALYs GBD, Collaborators H. 2018. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oaks EV, Hale TL, Formal SB. 1986. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun 53:57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberhelman RA, Kopecko DJ, Salazar-Lindo E, Gotuzzo E, Buysse JM, Venkatesan MM, Yi A, Fernandez-Prada C, Guzman M, Leon-Barua R. 1991. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun 59:2341–2350. doi: 10.1128/iai.59.7.2341-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van de Verg LL, Herrington DA, Boslego J, Lindberg AA, Levine MM. 1992. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J Infect Dis 166:158–161. doi: 10.1093/infdis/166.1.158. [DOI] [PubMed] [Google Scholar]
- 15.Raqib R, Qadri F, SarkEr P, Mia SM, Sansonnetti PJ, Albert MJ, Andersson J. 2002. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand J Immunol 55:414–423. doi: 10.1046/j.1365-3083.2002.01079.x. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CN, Le TP, Anders KL, Nguyen TH, Lu LV, Nguyen VV, Vu TD, Nguyen NM, Tran TH, Ha TT, Tran VT, Pham VM, Tran do HN, Le TQ, Saul A, Martin LB, Podda A, Gerke C, Thwaites G, Simmons CP, Baker S. 2016. The transfer and decay of maternal antibody against Shigella sonnei in a longitudinal cohort of Vietnamese infants. Vaccine 34:783–790. doi: 10.1016/j.vaccine.2015.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisenga CC, Bosomprah S, Simuyandi M, Mwila-Kazimbaya K, Chilyabanyama ON, Laban NM, Bialik A, Asato V, Meron-Sudai S, Frankel G, Cohen D, Chilengi R. 2021. Shigella-specific antibodies in the first year of life among Zambian infants: a longitudinal cohort study. PLoS One 16:e0252222. doi: 10.1371/journal.pone.0252222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 19.La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, Stevens HJ, Bennett WE, Jr, Shaikh N, Linneman LA, Hoffmann JA, Hamvas A, Deych E, Shands BA, Shannon WD, Tarr PI. 2014. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci USA 111:12522–12527. doi: 10.1073/pnas.1409497111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korpela K, Blakstad EW, Moltu SJ, Strømmen K, Nakstad B, Rønnestad AE, Brække K, Iversen PO, Drevon CA, de Vos W. 2018. Intestinal microbiota development and gestational age in preterm neonates. Sci Rep 8:2453. doi: 10.1038/s41598-018-20827-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan K, Zhang C, Tian J. 2021. The effects of different modes of delivery on the structure and predicted function of intestinal microbiota in neonates and early infants. Pol J Microbiol 70:45–55. doi: 10.33073/pjm-2021-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J, Jun W. 2015. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Guo C, Zhou Q, Li M, Zhou L, Xu L, Zhang Y, Li D, Wang Y, Dai W, Li S, Zhang L. 2020. Breastfeeding restored the gut microbiota in caesarean section infants and lowered the infection risk in early life. BMC Pediatr 20:532. doi: 10.1186/s12887-020-02433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA, Jr, Ahmed T, Gordon JI. 2014. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouhani S, Griffin NW, Yori PP, Gehrig JL, Olortegui MP, Salas MS, Trigoso DR, Moulton LH, Houpt ER, Barratt MJ, Kosek MN, Gordon JI. 2020. Diarrhea as a potential cause and consequence of reduced gut microbial diversity among undernourished children in Peru. Clin Infect Dis 71:989–999. doi: 10.1093/cid/ciz905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nabwera HM, Espinoza JL, Worwui A, Betts M, Okoi C, Sesay AK, Bancroft R, Agbla SC, Jarju S, Bradbury RS, Colley M, Jallow AT, Liu J, Houpt ER, Prentice AM, Antonio M, Bernstein RM, Dupont CL, Kwambana-Adams BA. 2021. Interactions between fecal gut microbiome, enteric pathogens, and energy regulating hormones among acutely malnourished rural Gambian children. EBioMedicine 73:103644. doi: 10.1016/j.ebiom.2021.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lackey KA, Williams JE, Meehan CL, Zachek JA, Benda ED, Price WJ, Foster JA, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, Moore SE, Prentice AM, K DG, Kvist LJ, Otoo GE, Garcia-Carral C, Jimenez E, Ruiz L, Rodriguez JM, Pareja RG, Bode L, McGuire MA, McGuire MK. 2019. What's normal? Microbiomes in human milk and infant feces are related to each other but vary geographically: the INSPIRE study. Front Nutr 6:45. doi: 10.3389/fnut.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng D, Liwinski T, Elinav E. 2020. Interaction between microbiota and immunity in health and disease. Cell Res 30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. 2014. The intestinal microbiome in early life: health and disease. Front Immunol 5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. 2016. How colonization by microbiota in early life shapes the immune system. Science 352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pop M, Walker AW, Paulson J, Lindsay B, Antonio M, Hossain MA, Oundo J, Tamboura B, Mai V, Astrovskaya I, Corrada Bravo H, Rance R, Stares M, Levine MM, Panchalingam S, Kotloff K, Ikumapayi UN, Ebruke C, Adeyemi M, Ahmed D, Ahmed F, Alam MT, Amin R, Siddiqui S, Ochieng JB, Ouma E, Juma J, Mailu E, Omore R, Morris JG, Breiman RF, Saha D, Parkhill J, Nataro JP, Stine OC. 2014. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol 15:R76. doi: 10.1186/gb-2014-15-6-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The HC, Florez de Sessions P, Jie S, Pham Thanh D, Thompson CN, Nguyen Ngoc Minh C, Chu CW, Tran TA, Thomson NR, Thwaites GE, Rabaa MA, Hibberd M, Baker S. 2018. Assessing gut microbiota perturbations during the early phase of infectious diarrhea in Vietnamese children. Gut Microbes 9:38–54. doi: 10.1080/19490976.2017.1361093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker-Dreps S, Allali I, Monteagudo A, Vilchez S, Hudgens MG, Rogawski ET, Carroll IM, Zambrana LE, Espinoza F, Azcarate-Peril MA. 2015. Gut microbiome composition in young Nicaraguan children during diarrhea episodes and recovery. Am J Trop Med Hyg 93:1187–1193. doi: 10.4269/ajtmh.15-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monira S, Nakamura S, Gotoh K, Izutsu K, Watanabe H, Alam NH, Nakaya T, Horii T, Ali SI, Iida T, Alam M. 2013. Metagenomic profile of gut microbiota in children during cholera and recovery. Gut Pathog 5:1. doi: 10.1186/1757-4749-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsiao A, Ahmed AM, Subramanian S, Griffin NW, Drewry LL, Petri WA, Jr, Haque R, Ahmed T, Gordon JI. 2014. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 515:423–426. doi: 10.1038/nature13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.David LA, Weil A, Ryan ET, Calderwood SB, Harris JB, Chowdhury F, Begum Y, Qadri F, LaRocque RC, Turnbaugh PJ. 2015. Gut microbial succession follows acute secretory diarrhea in humans. mBio 6:e00381-15–e00315. doi: 10.1128/mBio.00381-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallardo P, Izquierdo M, Vidal RM, Chamorro-Veloso N, Rossello-Mora R, O'Ryan M, Farfan MJ. 2017. Distinctive gut microbiota is associated with diarrheagenic Escherichia coli infections in Chilean children. Front Cell Infect Microbiol 7:424. doi: 10.3389/fcimb.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsay B, Oundo J, Hossain MA, Antonio M, Tamboura B, Walker AW, Paulson JN, Parkhill J, Omore R, Faruque AS, Das SK, Ikumapayi UN, Adeyemi M, Sanogo D, Saha D, Sow S, Farag TH, Nasrin D, Li S, Panchalingam S, Levine MM, Kotloff K, Magder LS, Hungerford L, Sommerfelt H, Pop M, Nataro JP, Stine OC. 2015. Microbiota that affect risk for shigellosis in children in low-income countries. Emerg Infect Dis 21:242–250. doi: 10.3201/eid2101.140795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iturriza-Gomara M, Jere KC, Hungerford D, Bar-Zeev N, Shioda K, Kanjerwa O, Houpt ER, Operario DJ, Wachepa R, Pollock L, Bennett A, Pitzer VE, Cunliffe NA. 2019. Etiology of diarrhea among hospitalized children in Blantyre, Malawi, following rotavirus vaccine introduction: a case-control study. J Infect Dis 220:213–218. doi: 10.1093/infdis/jiz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ndungo E, Andronescu LR, Buchwald AG, Lemme-Dumit JM, Mawindo P, Kapoor N, Fairman J, Laufer MK, Pasetti MF. 2021. Repertoire of naturally acquired maternal antibodies transferred to infants for protection against shigellosis. Front Immunol 12:725129. doi: 10.3389/fimmu.2021.725129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogawski McQuade ET, Shaheen F, Kabir F, Rizvi A, Platts-Mills JA, Aziz F, Kalam A, Qureshi S, Elwood S, Liu J, Lima AAM, Kang G, Bessong P, Samie A, Haque R, Mduma ER, Kosek MN, Shrestha S, Leite JP, Bodhidatta L, Page N, Kiwelu I, Shakoor S, Turab A, Soofi SB, Ahmed T, Houpt ER, Bhutta Z, Iqbal NT. 2020. Epidemiology of Shigella infections and diarrhea in the first two years of life using culture-independent diagnostics in 8 low-resource settings. PLoS Negl Trop Dis 14:e0008536. doi: 10.1371/journal.pntd.0008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, Investigators M-EN, MAL-ED Network Investigators . 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564-75–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Data sources—Climate-Data.org. Available: https://en.climate-data.org/info/sources/. Accessed 23 April, 2022.
- 45.Black RE, Brown KH, Becker S. 1984. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics 73:799–805. doi: 10.1542/peds.73.6.799. [DOI] [PubMed] [Google Scholar]
- 46.Lee G, Paredes Olortegui M, Penataro Yori P, Black RE, Caulfield L, Banda Chavez C, Hall E, Pan WK, Meza R, Kosek M. 2014. Effects of Shigella-, Campylobacter- and ETEC-associated diarrhea on childhood growth. Pediatr Infect Dis J 33:1004–1009. doi: 10.1097/INF.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 47.Schnee AE, Haque R, Taniuchi M, Uddin MJ, Alam MM, Liu J, Rogawski ET, Kirkpatrick B, Houpt ER, Petri WA, Jr, Platts-Mills JA. 2018. Identification of etiology-specific diarrhea associated with linear growth faltering in Bangladeshi infants. Am J Epidemiol 187:2210–2218. doi: 10.1093/aje/kwy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, Houpt ER, Investigators M-EN, MAL-ED Network Investigators . 2018. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bottacini F, van Sinderen D, Ventura M. 2017. Omics of bifidobacteria: research and insights into their health-promoting activities. Biochem J 474:4137–4152. doi: 10.1042/BCJ20160756. [DOI] [PubMed] [Google Scholar]
- 50.Di Gioia D, Aloisio I, Mazzola G, Biavati B. 2014. Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol 98:563–577. doi: 10.1007/s00253-013-5405-9. [DOI] [PubMed] [Google Scholar]
- 51.De Filippo C, Di Paola M, Ramazzotti M, Albanese D, Pieraccini G, Banci E, Miglietta F, Cavalieri D, Lionetti P. 2017. Diet, environments, and gut microbiota. a preliminary investigation in children living in rural and urban Burkina Faso and Italy. Front Microbiol 8:1979. doi: 10.3389/fmicb.2017.01979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GA, Muzny D, Gibbs RA, Vatanen T, Huttenhower C, Xavier RJ, Rewers M, Hagopian W, Toppari J, Ziegler AG, She JX, Akolkar B, Lernmark A, Hyoty H, Vehik K, Krischer JP, Petrosino JF. 2018. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562:583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amenyogbe N, Adu-Gyasi D, Enuameh Y, Asante KP, Konadu DG, Kaali S, Dosoo D, Panigrahi P, Kollmann TR, Mohn WW, Owusu-Agyei S. 2021. Bacterial and fungal gut community dynamics over the first 5 years of life in predominantly rural communities in Ghana. Front Microbiol 12:664407. doi: 10.3389/fmicb.2021.664407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kortekangas E, Fan YM, Chaima D, Lehto KM, Malamba-Banda C, Matchado A, Chingwanda C, Liu Z, Ashorn U, Cheung YB, Dewey KG, Maleta K, Ashorn P. 2022. Associations between gut microbiota and intestinal inflammation, permeability and damage in young Malawian children. J Trop Pediatr 68. doi: 10.1093/tropej/fmac012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibson MK, Crofts TS, Dantas G. 2015. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol 27:51–56. doi: 10.1016/j.mib.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korpela K, Salonen A, Saxen H, Nikkonen A, Peltola V, Jaakkola T, de Vos W, Kolho KL. 2020. Antibiotics in early life associate with specific gut microbiota signatures in a prospective longitudinal infant cohort. Pediatr Res 88:438–443. doi: 10.1038/s41390-020-0761-5. [DOI] [PubMed] [Google Scholar]
- 57.Cheung YB, Xu Y, Mangani C, Fan YM, Dewey KG, Salminen SJ, Maleta K, Ashorn P. 2016. Gut microbiota in Malawian infants in a nutritional supplementation trial. Trop Med Int Health 21:283–290. doi: 10.1111/tmi.12650. [DOI] [PubMed] [Google Scholar]
- 58.Human Microbiome Project C. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lloyd-Price J, Abu-Ali G, Huttenhower C. 2016. The healthy human microbiome. Genome Med 8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, Brady A, Creasy HH, McCracken C, Giglio MG, McDonald D, Franzosa EA, Knight R, White O, Huttenhower C. 2017. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 550:61–66. doi: 10.1038/nature23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGuire MK, Randall AZ, Seppo AE, Jarvinen KM, Meehan CL, Gindola D, Williams JE, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, Moore SE, Prentice AM, Foster JA, Otoo GE, Rodriguez JM, Pareja RG, Bode L, McGuire MA, Campo JJ. 2020. Multipathogen analysis of IgA and IgG antigen specificity for selected pathogens in milk produced by women from diverse geographical regions: the INSPIRE study. Front Immunol 11:614372. doi: 10.3389/fimmu.2020.614372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Companys J, Gosalbes MJ, Pla-Paga L, Calderon-Perez L, Llaurado E, Pedret A, Valls RM, Jimenez-Hernandez N, Sandoval-Ramirez BA, Del Bas JM, Caimari A, Rubio L, Sola R. 2021. Gut microbiota profile and its association with clinical variables and dietary intake in overweight/obese and lean subjects: a cross-sectional study. Nutrients 13:2032. doi: 10.3390/nu13062032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeshita K, Mizuno S, Mikami Y, Sujino T, Saigusa K, Matsuoka K, Naganuma M, Sato T, Takada T, Tsuji H, Kushiro A, Nomoto K, Kanai T. 2016. A single species of Clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflamm Bowel Dis 22:2802–2810. doi: 10.1097/MIB.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 64.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. 2020. The controversial role of human gut Lachnospiraceae. Microorganisms 8:573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. 2006. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243. [DOI] [PubMed] [Google Scholar]
- 66.Youmans BP, Ajami NJ, Jiang ZD, Campbell F, Wadsworth WD, Petrosino JF, DuPont HL, Highlander SK. 2015. Characterization of the human gut microbiome during travelers' diarrhea. Gut Microbes 6:110–119. doi: 10.1080/19490976.2015.1019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perez-Cavazos S, Cisneros-Saldana D, Espinosa-Villasenor F, Castillo-Bejarano JI, Vaquera-Aparicio DN, Sanchez-Alanis H, Mascarenas-De Los Santos A. 2022. Facklamia hominis pyelonephritis in a pediatric patient: first case report and review of the literature. Ann Clin Microbiol Antimicrob 21:4. doi: 10.1186/s12941-022-00497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, Fosso B, Melocchi L, Nizzoli G, Troisi J, Marzano M, Oresta B, Spadoni I, Atarashi K, Carloni S, Arioli S, Fornasa G, Asnicar F, Segata N, Guglielmetti S, Honda K, Pesole G, Vermi W, Penna G, Rescigno M. 2020. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol 5:511–524. doi: 10.1038/s41564-019-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pujo J, Petitfils C, Le Faouder P, Eeckhaut V, Payros G, Maurel S, Perez-Berezo T, Van Hul M, Barreau F, Blanpied C, Chavanas S, Van Immerseel F, Bertrand-Michel J, Oswald E, Knauf C, Dietrich G, Cani PD, Cenac N. 2021. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 70:1088–1097. doi: 10.1136/gutjnl-2020-321173. [DOI] [PubMed] [Google Scholar]
- 70.Philpott DJ, Edgeworth JD, Sansonetti PJ. 2000. The pathogenesis of Shigella flexneri infection: lessons from in vitro and in vivo studies. Philos Trans R Soc Lond B Biol Sci 355:575–586. doi: 10.1098/rstb.2000.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lawson PA, Finegold SM. 2015. Reclassification of Ruminococcus obeum as Blautia obeum comb. nov. Int J Syst Evol Microbiol 65:789–793. doi: 10.1099/ijs.0.000015. [DOI] [PubMed] [Google Scholar]
- 72.Huus KE, Petersen C, Finlay BB. 2021. Diversity and dynamism of IgA-microbiota interactions. Nat Rev Immunol 21:514–525. doi: 10.1038/s41577-021-00506-1. [DOI] [PubMed] [Google Scholar]
- 73.Morita H, Nakano A, Onoda H, Toh H, Oshima K, Takami H, Murakami M, Fukuda S, Takizawa T, Kuwahara T, Ohno H, Tanabe S, Hattori M. 2011. Bifidobacterium kashiwanohense sp. nov., isolated from healthy infant faeces. Int J Syst Evol Microbiol 61:2610–2615. doi: 10.1099/ijs.0.024521-0. [DOI] [PubMed] [Google Scholar]
- 74.Kongnum K, Taweerodjanakarn S, Hongpattarakere T. 2020. Longitudinal characterization of bifidobacterial abundance and diversity profile developed in Thai healthy infants. Arch Microbiol 202:1425–1438. doi: 10.1007/s00203-020-01856-5. [DOI] [PubMed] [Google Scholar]
- 75.Bunesova V, Lacroix C, Schwab C. 2016. Fucosyllactose and L-fucose utilization of infant Bifidobacterium longum and Bifidobacterium kashiwanohense. BMC Microbiol 16:248. doi: 10.1186/s12866-016-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.James K, Bottacini F, Contreras JIS, Vigoureux M, Egan M, Motherway MO, Holmes E, van Sinderen D. 2019. Metabolism of the predominant human milk oligosaccharide fucosyllactose by an infant gut commensal. Sci Rep 9:15427. doi: 10.1038/s41598-019-51901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vazquez-Gutierrez P, Lacroix C, Jaeggi T, Zeder C, Zimmerman MB, Chassard C. 2015. Bifidobacteria strains isolated from stools of iron deficient infants can efficiently sequester iron. BMC Microbiol 15:3. doi: 10.1186/s12866-014-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vazquez-Gutierrez P, de Wouters T, Werder J, Chassard C, Lacroix C. 2016. High iron-sequestrating Bifidobacteria inhibit Enteropathogen growth and adhesion to intestinal epithelial cells in vitro. Front Microbiol 7:1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J, Almeida M, Kabir F, Shakoor S, Qureshi S, Zaidi A, Li S, Tamboura B, Sow SO, Mandomando I, Alonso PL, Ramamurthy T, Sur D, Kotloff K, Nataro J, Levine MM, Stine OC, Houpt E. 2018. Direct detection of Shigella in stool specimens by use of a metagenomic approach. J Clin Microbiol 56. doi: 10.1128/JCM.01374-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allali I, Abotsi RE, Tow LA, Thabane L, Zar HJ, Mulder NM, Nicol MP. 2021. Human microbiota research in Africa: a systematic review reveals gaps and priorities for future research. Microbiome 9:241. doi: 10.1186/s40168-021-01195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindsay B, Ochieng JB, Ikumapayi UN, Toure A, Ahmed D, Li S, Panchalingam S, Levine MM, Kotloff K, Rasko DA, Morris CR, Juma J, Fields BS, Dione M, Malle D, Becker SM, Houpt ER, Nataro JP, Sommerfelt H, Pop M, Oundo J, Antonio M, Hossain A, Tamboura B, Stine OC. 2013. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J Clin Microbiol 51:1740–1746. doi: 10.1128/JCM.02713-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vu DT, Sethabutr O, Von Seidlein L, Tran VT, Do GC, Bui TC, Le HT, Lee H, Houng HS, Hale TL, Clemens JD, Mason C, Dang DT. 2004. Detection of Shigella by a PCR assay targeting the ipaH gene suggests increased prevalence of shigellosis in Nha Trang, Vietnam. J Clin Microbiol 42:2031–2035. doi: 10.1128/JCM.42.5.2031-2035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garrine M, Matambisso G, Nobela N, Vubil D, Massora S, Acacio S, Nhampossa T, Alonso P, Mandomando I. 2020. Low frequency of enterohemorrhagic, enteroinvasive and diffusely adherent Escherichia coli in children under 5 years in rural Mozambique: a case-control study. BMC Infect Dis 20:659. doi: 10.1186/s12879-020-05380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tuddenham S, Ghanem KG, Caulfield LE, Rovner AJ, Robinson C, Shivakoti R, Miller R, Burke A, Murphy C, Ravel J, Brotman RM. 2019. Associations between dietary micronutrient intake and molecular-bacterial vaginosis. Reprod Health 16:151. doi: 10.1186/s12978-019-0814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holm JB, Humphrys MS, Robinson CK, Settles ML, Ott S, Fu L, Yang H, Gajer P, He X, McComb E, Gravitt PE, Ghanem KG, Brotman RM, Ravel J. 2019. Ultrahigh-throughput multiplexing and sequencing of >500-base-pair amplicon regions on the Illumina HiSeq 2500 platform. mSystems 4. doi: 10.1128/mSystems.00029-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmieder R, Lim YW, Rohwer F, Edwards R. 2010. TagCleaner: identification and removal of tag sequences from genomic and metagenomic datasets. BMC Bioinformatics 11:341. doi: 10.1186/1471-2105-11-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glockner FO. 2014. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res 42:D643–D648. doi: 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.WHO. 2006. World Health Organization child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight -for-height and body mass index-for-age: methods and development. World Health Organization. https://apps.who.int/iris/handle/10665/43413. [Google Scholar]
- 92.Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests M. 2007. The vegan package. Community Ecology Package 10:631–637. [Google Scholar]
- 93.Pinheiro J, Bates D, DebRoy S, Sarkar D, {R Core Team} . 2021. nlme: linear and nonlinear mixed effects models. R package version 3.1–153.
- 94.Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag; New York. [Google Scholar]
- 95.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparisons between read counts and Shigella qPCR status. (A) Boxplots showing the total read counts in the Shigella qPCR positive and negative samples. (B) Boxplots showing Escherichia/Shigella read counts in Shigella qPCR positive and negative samples. (C) Boxplots showing Escherichia/Shigella read counts in Shigella qPCR positive samples that were either diarrhea positive or negative. *, P < 0.05, from unpaired t tests comparing diarrhea positive and negative samples. (D) Correlation between Shigella qPCR quantitation cycle (Cq) values and Escherichia/Shigella read counts. Spearman’s r values are shown within the graph. Shigella qPCR Cq values that were greater than 35 (qPCR negative) were considered undetermined. The symbols represent individual samples. Download FIG S1, TIF file, 1.2 MB (1.2MB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mean relative abundance of the top 10 taxa. Download Table S1, DOCX file, 0.01 MB (13.9KB, docx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cross-sectional comparison between case index visits and matched control index visits. Download Table S2, DOCX file, 0.01 MB (13.4KB, docx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Taxa differentially abundant between cases and controls at Shigella index visit. Estimated differences (log2-fold change, logFc) between the abundance of taxa in the cases compared to the controls at the time of Shigella qPCR positive sample collection. A positive logFc indicates a greater abundance in the cases than in the controls. Species are ordered by increasing P value (before controlling for multiple variables). Escherichia/Shigella is highlighted in red. None of the taxa were significantly associated with case status after controlling for multiple variables. Download FIG S2, TIF file, 1.1 MB (1.1MB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Taxa differentially abundant between cases and controls before Shigella infection. Estimated differences (log2-fold change, logFc) between the abundance of taxa in the cases compared to the controls before Shigella infection. A positive logFc indicates a greater abundance in the cases than in the controls. Species are ordered by increasing P value (before controlling for multiple variables). None of the taxa were significantly associated with case status after controlling for multiple variables. Download FIG S3, TIF file, 0.4 MB (387.2KB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparisons of the Shannon diversity index (SDI) between cases and controls. Download Table S3, DOCX file, 0.01 MB (13.8KB, docx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mean relative abundances of F. saccharivorans and Lachnospiraceae NK4A136 by age. (A) Bar graphs showing the mean relative abundances (%) of F. saccharivorans and Lachnospiraceae NK4A136 at the infant ages of 6, 12, 18, and 24 months and (B) comparing the cases and the controls. The symbols represent individual values. The mean relative abundances at the different ages were compared via a one-way analysis of variance (ANOVA). *, P < 0.05; **, P < 0.01. Download FIG S5, TIF file, 0.3 MB (365.3KB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Taxa differentially abundant in children with symptomatic versus asymptomatic Shigella. Estimated differences (log2-fold change, logFc) between the abundance of taxa in children with a symptomatic infection (with diarrhea) and those with an asymptomatic infection (without diarrhea) prior to (A) or following (B) Shigella infection. These estimates were obtained from logistic regression models. A positive logFc indicates a greater abundance in symptomatic infections than in asymptomatic infections. Species are ordered by increasing P value (before controlling for multiple variables). None of the taxa were significantly associated with symptomatic or asymptomatic Shigella infection after controlling for multiple variables. Download FIG S4, TIF file, 0.8 MB (829KB, tif) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sample metadata. Download DATA SET S4, XLSX file, 0.04 MB (43.1KB, xlsx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundance. Download DATA SET S5, XLSX file, 0.8 MB (787.2KB, xlsx) .
Copyright © 2022 Ndungo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
All of the raw sequencing data were deposited into NCBI SRA under BioProject ID PRJNA834726.