Abstract
Ureaplasma urealyticum comprises 14 serotypes. The existing serotyping methods all use polyclonal antibodies. These methods are time-consuming and labor-intensive, and they cannot always be performed on primary isolates; in addition, the results are difficult to interpret. We developed a new enzyme-linked immunosorbent assay (ELISA) method using serotype-specific monoclonal antibodies (MAbs) to enable the serotyping of U. urealyticum isolates from primary broth cultures. Each of the 14 serotype reference strains was tested against 14 selected MAbs. Homologous reactions were very strong, while heterologous reactions were negligible. Three cross-reactions were observed: MAb 5 cross-reacted with serotype 2, MAb 14 cross-reacted with serotype 3, and MAb 8 cross-reacted with serotype 13. Despite the cross-reactions observed, all the serotype reference strains of U. urealyticum could be identified and differentiated using a combination of MAbs. Reproducibility was analyzed with a fractionated antigenic preparation and with several freshly prepared antigens of the same strain. No significant interrun variation was found with the fractionated antigen, but significant variations in optical density (OD) values were found when freshly prepared antigens were tested. However, the variation in OD values did not influence the overall interpretation of the ELISA: reactions with homologous MAbs were always prominent compared to those of the negative controls. This newly developed ELISA using MAbs seems promising for serotyping of U. urealyticum clinical isolates.
Ureaplasma urealyticum is a common inhabitant of the human lower genital tract. In some cases, a causal relationship between U. urealyticum and unfavorable fetal outcome was established 3, 8, 9, 14, 29, 31. Despite these case reports, its pathogenic role in diseases of reproduction remains controversial. Because of the difficulties in establishing the pathogenic role of U. urealyticum in diseases of the reproductive tract, it has been postulated that only certain subgroups of U. urealyticum are associated with disease. There are 14 serotypes of U. urealyticum which can be classified into two biovars according to differences in phenotypic and genotypic characteristics 6, 12, 20, 21, 23, 28. Serotyping of U. urealyticum strains is helpful for studying a possible link between serotypes and disease. Until now, advances made in this field were limited, since only a few serotyping studies have been performed. Moreover, these studies have shown conflicting results 10, 15, 17, 24, 26. The difficulty in interpretation of results for the available serotyping methods 15, 27 may be one explanation for these conflicting results. For serotyping of clinical strains, very often subculturing is a prerequisite. If one deals with mixed serotypes, subculturing may favor one of the serotypes, and this can influence the serotyping results. There is therefore a need for an easier and more reliable test for the serotyping of strains isolated from primary cultures which can detect mixed serotypes. For this purpose, we developed an enzyme-linked immunosorbent assay (ELISA) that could serve as a good alternative for the existing serotyping methods.
MATERIALS AND METHODS
Type strains.
Reference strains of U. urealyticum serotypes 1 to 10 were supplied by E. A. Freund (Institute of Medical Microbiology, University of Aarhus, Aarhus, Denmark), and those of serotypes 11 to 14 were supplied by J. A. Robertson (Department of Medical Microbiology and Infectious Diseases, University of Alberta, Edmonton, Canada).
Antigen preparation.
Antigens used in the ELISA were prepared by growing U. urealyticum reference strains in 10 ml of bromothymol blue broth 22. The cells were harvested by centrifugation at 25,000 × g for 30 min at 4°C. The pellet was then washed three times with phosphate-buffered saline (PBS), and the final pellet was resuspended in 100 μl of PBS and stored at −80°C until use.
MAb selection and purification.
Fourteen monoclonal antibodies (MAbs) able to differentiate the 14 serotypes 7 were selected for use in the ELISA. For serotypes 1, 2, 3, 4, 6, 7, 8, 9, 10, 11, and 12, serotype-specific MAbs were used. For serotyping of U. urealyticum serotypes 5, 13, and 14, we used cross-reactive MAbs as determined by indirect immunofluorescence assay (IFA). The MAb for serotype 5 cross-reacts with serotype 2, the MAb for serotype 14 cross-reacts with serotype 3, and the MAb for serotype 13 cross-reacts with serotype 8.
The MAbs of the immunoglobulin G isotype were purified using the TrapGII kit (Amersham Pharmacia Biotechnologies, Uppsala, Sweden). The MAbs of the immunoglobulin M isotype were purified with the E-Z-SEP kit (Amersham Pharmacia Biotechnologies). The concentrations of the purified MAbs were determined by measuring the absorbancy at 280 nm.
ELISA method.
For coating of microtiter plates, 100 μl of the antigenic preparation diluted in ethanol was added to each well and incubated at 37°C until complete evaporation of the ethanol. The wells were saturated with bovine serum albumin (3% [wt/vol]) in PBS and then washed twice. Washing was performed with PBS containing 0.1% Tween 20. One hundred microliters of MAbs diluted in PBS was added to the wells and incubated for 1 h at 37°C. After a wash, 100 μl of horseradish peroxidase-labeled polyclonal anti-mouse immunoglobulins (called conjugate below) diluted in PBS containing 0.05% Tween 20 was added to the wells and the mixtures were incubated for 1 h at 37°C. After a wash, the peroxidase substrate (o-phenylenediamine) was added to the wells and the mixtures were incubated for 30 min in the dark at room temperature. The substrate reaction was stopped by adding H2SO4 (4 N), and the optical density (OD) was measured at 492 nm.
Method optimization.
The reference strains of serotypes 4 and 9 were used to study the optimal conditions for the test procedure. After optimization, each antibody was tested against the 14 different U. urealyticum serotypes. Negative controls were obtained by testing the conjugate without adding MAbs. The blank wells received ELISA reagents but no antigens and no MAbs.
RESULTS
Method optimization. (i) Coating conditions.
The coating was optimized by testing the following three coating conditions: (i) Maxisorp Nunc plate with the coating buffer Tris-buffered saline, pH 8.6; (ii) Maxisorp Nunc plates with the coating buffer carbonate-bicarbonate, pH 9.63; and (iii) Polysorp Nunc plates with ethanol as the coating buffer. The conditions were tested with whole-cell antigens as well as with sonicated antigens. The highest OD values, corresponding to the reaction of MAbs with homologous antigens, were obtained using the Polysorp Nunc plates with ethanol as the coating buffer (data not shown). The use of sonicated antigens did not increase the OD values compared to the use of whole-cell antigens. For further experiments we used whole-cell antigens diluted in ethanol to coat Polysorp Nunc plates.
(ii) Antigen and antibody concentrations.
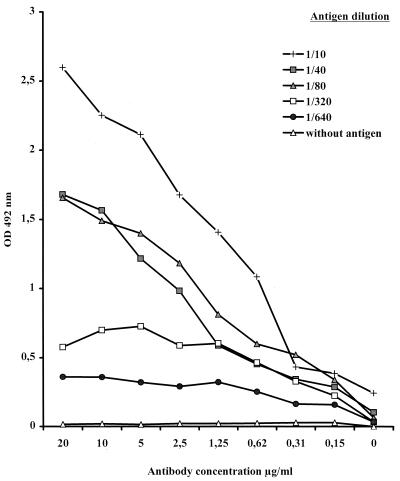
Results of determination of the optimal concentrations of antigen and antibody are shown in Fig. 1. MAb concentrations ranging between 2.5 and 5 μg/ml and antigen dilutions ranging between 1/40 and 1/80 yielded adequate OD values with minimal background.
FIG. 1.
Absorbancy curves for MAb 4 at different concentrations tested with U. urealyticum serotype 4 antigen at different dilutions.
(iii) Reduction of nonspecific reactivity.
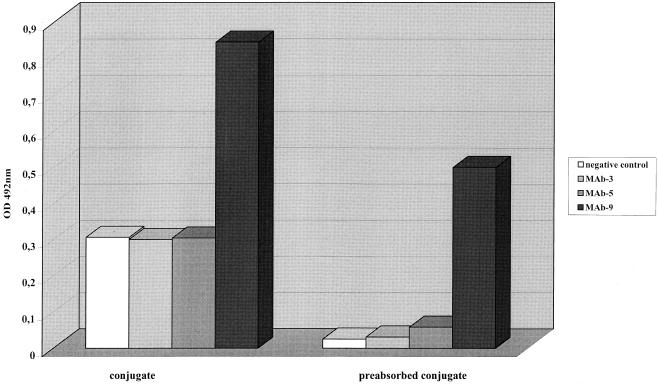
OD values for the blank wells were very low, while those for negative controls ranged between 0.25 and 0.3. In order to reduce the reactivity of the negative control as well as the reactivity of antigens to heterologous MAbs, the conjugate was preincubated with the culture medium of U. urealyticum. Preincubation for 1 h at room temperature allowed significant reduction of negative-control and heterologous-reaction values (Fig. 2).
FIG. 2.
Effect of preabsorption of the conjugate with the culture medium of U. urealyticum on the negative control and heterologous reaction mixture tested for serotype 9 antigen.
Reactivity of U. urealyticum reference strains by ELISA.
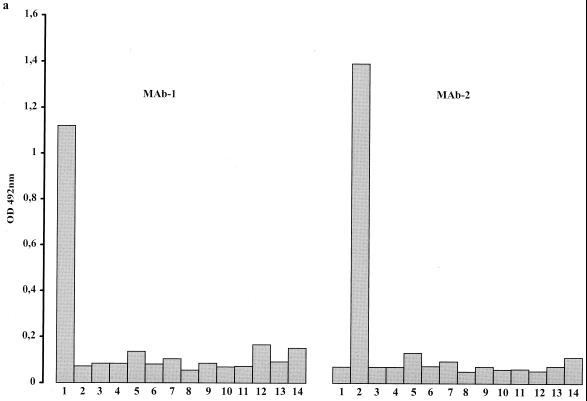
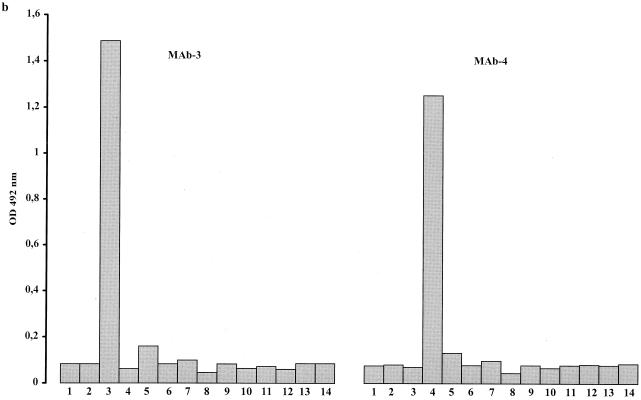
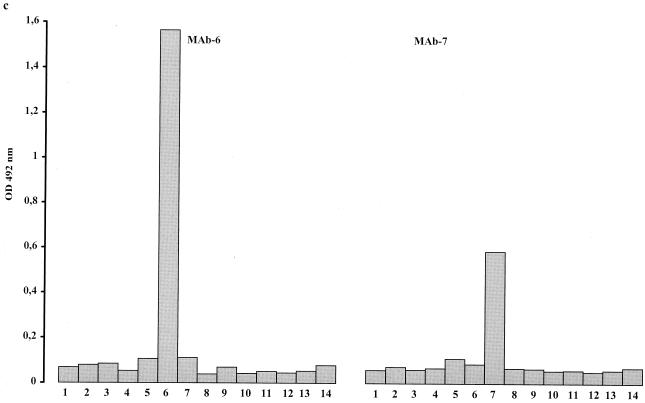
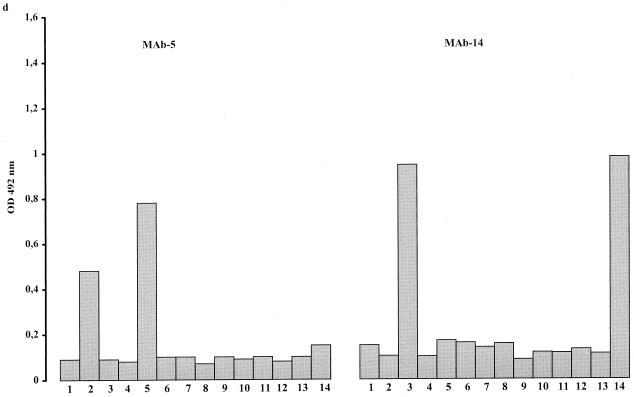
Each of the 14 reference strains was tested against all selected MAbs. Every serotype gave a strong reaction with its homologous MAb (Fig. 3). Reactions with heterologous MAbs were comparable to those of the negative controls, except for serotypes 2, 3, and 8: serotype 2 also reacted with MAb 5 (Fig. 3d), serotype 3 reacted with MAb 14 (Fig. 3d), and serotype 8 reacted with MAb 13.
FIG. 3.
Histograms showing the reaction of selected MAbs with antigens of the 14 serotypes of U. urealyticum (columns 1 to 14). The height of each column corresponds to OD values of homologous and heterologous reaction mixtures.
Reproducibility of the test.
The reproducibility of the test was verified by analyzing a fractionated antigenic preparation in five independent assays and by testing five different antigenic preparations of serotype 4 in five independent assays. No significant interrun variation of a fractionated antigenic preparation was found (Table 1). However, interrun variations of different antigenic preparations of serotype 4 were considerable (Table 2). Nevertheless, reactions with homologous MAbs were always very prominent compared to those of negative controls.
TABLE 1.
Interassay variation of a single antigenic preparation of serotype 1
| Preparation tested | OD for indicated test
|
SD | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Negative control | 0.11 | 0.10 | 0.07 | 0.13 | 0.10 | 0.02 |
| Antigenic | 0.62 | 0.60 | 0.50 | 0.69 | 0.63 | 0.07 |
TABLE 2.
Interassay variation of different antigenic preparations of serotype 4
| Preparation tested | OD for indicated test
|
SD | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Negative control | 0.08 | 0.10 | 0.10 | 0.07 | 0.08 | 0.01 |
| Antigenic | 1.20 | 1.30 | 0.90 | 0.62 | 0.73 | 0.29 |
DISCUSSION
U. urealyticum comprises 14 serotypes currently distributed between two biovars: the Parvo biovar (serotypes 1, 3, 6, and 14) and the T960 biovar (serotypes 2, 5, and 7 through 13). Strong evidence has suggested recently that these two biovars should be classified as distinct species: Ureaplasma parvum and U. urealyticum 11. Different methods for serotyping, all using polyclonal antibodies (PAbs), have been described previously 1, 2, 13, 18, 19, 25, 30. Until now, IFA has been the test most commonly performed. Serotyping of U. urealyticum with PAbs in IFA is subject to many problems that make it difficult to interpret the serotyping results. Polyreactivity of some clinical strains is the major problem observed. Another problem is the lack of reproducibility: repeated testing of some strains with PAbs was sometimes associated with the disappearance of a positive reaction when more than one specificity was found or by a shift from a negative to a positive reaction 15. The comparison of serotyping results obtained by two laboratories using nonidentical immunoreagents showed discrepancies and clearly demonstrated the difficulty in the interpretation of serotyping results 16. To overcome some of the problems with serotyping U. urealyticum using PAbs, we produced MAbs to U. urealyticum serotypes and tested them by IFA with selected clinical isolates 4, 5, 7, 16. Serotyping of clinical isolates with MAbs seems better than serotyping with PAbs, since repeated tests were always reproducible and polyreactivity was not observed within clinical isolates. However, IFA is difficult to perform. Indeed, IFA requires well-grown colonies on agar plates. This is time-consuming, and once enough colonies have grown, the plates cannot be kept for a long time before being tested. In addition, the need for microscopic observation makes the technique very fastidious when large numbers of strains are to be serotyped. In this study, we developed an indirect ELISA method to serotype U. urealyticum strains using serotype-specific MAbs. Two different microtiter plates were tested: the Maxisorp Nunc plate and the Polysorp Nunc plate. The first plate is suitable for coating hydrophilic proteins, whereas the latter plate is suitable for coating hydrophobic as well as amphiphilic proteins. Significantly higher OD values were obtained when using the Polysorp plate, suggesting that serotype-specific antigens of U. urealyticum are probably amphiphilic and not hydrophilic.
With the new ELISA method, high OD values were obtained for the reference strains when tested against their homologous MAbs. Significant cross-reactivities were observed for serotypes 2, 3, and 13. Since identical cross-reactivities have been observed by IFA 7, it is likely that the cross-reactivities observed in the ELISA are due to the selected MAbs rather than to the methodology of the ELISA. Despite the cross-reactivities observed, all the serotypes of U. urealyticum could be identified and differentiated by the ELISA using a combination of the MAbs described.
Since reproducibility is very important in serotyping assays of U. urealyticum, we tested the reproducibility with a single fractioned antigenic preparation as well as with several newly prepared antigenic preparations. For the new ELISA, no significant variation was found between repeated tests of a fractionated antigenic preparation. However, for different antigenic preparations of the same strain, variation between repeated tests was important. This variation is probably due to the nonstandardized preparation of the antigens. Strains can be harvested at different phases of growth, leading to different antigenic concentrations. This could be overcome by introducing a supplementary step for exact determination of the concentration of each antigenic preparation and adjustment of the test to a standardized concentration. However, adjustment to a standardized concentration is very difficult with U. urealyticum. Concentration adjustment by measuring the concentration of the antigen in the pellet can lead to a false estimate, since the pellet issued from culture centrifugation contains a lot of medium components. Concentration adjustment before harvesting cannot be performed by measuring the turbidity, since U. urealyticum does not produce visible growth in broth medium. The only way that one could standardize the antigenic concentration is by measuring the CFU of U. urealyticum from the broth culture. This method, however, is very laborious and, if introduced in the serotyping procedure, would undo the handy aspect of the present serotyping method. Since the fluctuation in OD values had no influence on the final results of the ELISA reactivity—reactions with homologous MAbs were always very prominent compared to those of negative controls—we decided to omit this supplementary standardization. Nevertheless, the use of nonstandardized antigen should be taken into account when interpreting the ELISA results. For example, a minimal cutoff value is needed. We arbitrarily determined a cutoff OD value of 0.4 (after negative control subtraction). A strain with at least one positive reaction can be considered serotyped. However, if no positive reactions are observed with the 14 MAbs, the whole serotyping procedure has to be repeated with a more concentrated antigenic preparation. Indeed, a negative reaction can occur in the case of a nontypeable strain or in a serotyping procedure using insufficient antigenic preparation. Simply repeating the test with a higher antigenic concentration enables us to discriminate between nontypeable strains and false-negative reactions. The difficult growth characteristics of U. urealyticum are the reason for the inconsistent concentrations. These characteristics interfere with the antigenic concentration not only with the ELISA method but also with the other serotyping methods described before now. For IFA, serotyping can be performed only when enough growth is obtained, often after subculturing.
For the new ELISA method, antigens can be prepared from primary broth cultures. Serotyping before subculture constitutes an important advantage, because selection can occur in case of mixed serotypes. Antigens can be stored for a long time before performing the test, while for IFA the agar plates can be kept only for a short time. The ELISA offers the possibility of automation, and unlike in the IFA, where microscopic observation is necessary, reading of the final reaction in the ELISA is performed by a spectrophotometer. These characteristics make the test easy to perform and applicable for serotyping large numbers of strains for epidemiological purposes.
Since promising results were obtained with the new indirect ELISA using the reference strains of U. urealyticum, the method will be used to serotype a large number of clinical isolates in order to evaluate the test under clinical conditions in comparison with the existing IFA method.
ACKNOWLEDGMENT
This work was supported by a grant from the Steunfonds Marguerite-Marie Delacroix.
REFERENCES
- 1.Black F T. Fifth International Congress on Infectious Diseases. Vol. 1. Vienna, Austria: Weiner Medizinische Akademie; 1970. Serological methods for classification of human T-mycoplasmas; pp. 407–411. [Google Scholar]
- 2.Black F T. Modifications of the growth inhibition test and its application to human T-mycoplasmas. Appl Microbiol. 1973;25:528–533. doi: 10.1128/am.25.4.528-533.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassell G H, Waites K B, Watson H L, Crouse D T, Harasawa R. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns. Clin Microbiol Rev. 1993;6:69–87. doi: 10.1128/cmr.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng X, Naessens A, Lauwers S. Identification and characterization of serotype 4-specific antigens of Ureaplasma urealyticum by use of monoclonal antibodies. Infect Immun. 1993;61:2253–2256. doi: 10.1128/iai.61.5.2253-2256.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng X, Naessens A, Lauwers S. Identification of serotype 1-, 3-, and 6-specific antigens of Ureaplasma urealyticum by using monoclonal antibodies. J Clin Microbiol. 1994;32:1060–1062. doi: 10.1128/jcm.32.4.1060-1062.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiansen C, Black F T, Freundt E A. Hybridization experiments with deoxyribonucleic acid from Ureaplasma urealyticum serovars I to VIII. Int J Syst Bacteriol. 1981;31:259–262. [Google Scholar]
- 7.Echahidi F, Muyldermans G, Lauwers S, Naessens A. Development of monoclonal antibodies against Ureaplasma urealyticum serotypes and their use for serotyping clinical isolates. Clin Diagn Lab Immunol. 2000;7:563–567. doi: 10.1128/cdli.7.4.563-567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulon W, Naessens A, Dewaele M, Lauwers S, Amy J J. Chronic Ureaplasma urealyticum amnionitis associated with abruptio placentae. Obstet Gynecol. 1986;68:280–282. [PubMed] [Google Scholar]
- 9.Foulon W, Van Liedekerke D, Demanet C, Decatte L, Dewaele M, Naessens A. Markers of infection and their relationship to preterm delivery. Am J Perinatol. 1995;12:208–311. doi: 10.1055/s-2007-994454. [DOI] [PubMed] [Google Scholar]
- 10.Hewish M J, Birch D F, Fairley K F. Ureaplasma urealyticum serotypes in urinary tract disease. J Clin Microbiol. 1986;23:149–154. doi: 10.1128/jcm.23.1.149-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong F, James G, Ma Z, Gordon S, Wang B, Gilbert G L. Phylogenetic analysis of Ureaplasma urealyticum—support for the establishment of a new species, Ureaplasma parvum. Int J Syst Bacteriol. 1999;49:1879–1889. doi: 10.1099/00207713-49-4-1879. [DOI] [PubMed] [Google Scholar]
- 12.Mouches C, Taylor-Robinson D, Stipkovits L, Bove J M. Comparison of human and animal Ureaplasmas by one- and two-dimensional protein analysis on polyacrylamide slab gel. Ann Microbiol (Paris) 1981;132B:171–196. [PubMed] [Google Scholar]
- 13.Naessens A, Lauwers S. Modified indirect immunofluorescence test for serotyping large numbers of Ureaplasma urealyticum clinical isolates. J Clin Microbiol. 1987;25:191–192. doi: 10.1128/jcm.25.1.191-192.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naessens A, Foulon W, Cammu H, Goossens A, Lauwers S. Epidemiology and pathogenesis of Ureaplasma urealyticum in spontaneous abortion and early preterm labor. Acta Obstet Gynecol Scand. 1987;66:513–516. doi: 10.3109/00016348709015726. [DOI] [PubMed] [Google Scholar]
- 15.Naessens A, Foulon W, Breynaert J, Lauwers S. Serotypes of Ureaplasma urealyticum isolated from normal pregnant women and patients with pregnancy complications. J Clin Microbiol. 1988;26:319–322. doi: 10.1128/jcm.26.2.319-322.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naessens A, Cheng X, Lauwers S, Robertson J A. Development of a monoclonal antibody to a Ureaplasma urealyticum serotype 9 antigen. J Clin Microbiol. 1998;36:1125–1127. doi: 10.1128/jcm.36.4.1125-1127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piot P. Distribution of eight serotypes of Ureaplasma urealyticum in cases of non-gonococcal urethritis and in healthy persons. Br J Vener Dis. 1976;52:266–268. doi: 10.1136/sti.52.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purcell R H, Taylor-Robinson D, Wong D, Chanock R M. Color test for the measurement of antibody to T-strain mycoplasmas. J Bacteriol. 1966;92:6–12. doi: 10.1128/jb.92.1.6-12.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn P A, Arshoff L U, Li H C S. Serotyping of Ureaplasma urealyticum by immunoperoxidase assay. J Clin Microbiol. 1981;13:670–676. doi: 10.1128/jcm.13.4.670-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razin S, Harasawa R, Barile M F. Cleavage patterns of the mycoplasma chromosome, obtained by using restriction endonucleases as indicators of genetic relatedness among strains. Int J Syst Bacteriol. 1983;33:201–206. [Google Scholar]
- 21.Razin S, Yogev D. Genetic relatedness among Ureaplasma urealyticum serotypes (serovars) Pediatr Infect Dis. 1986;5(Suppl 6):S300–S304. doi: 10.1097/00006454-198611010-00022. [DOI] [PubMed] [Google Scholar]
- 22.Robertson J A. Bromothymol blue broth: improved medium for detection of Ureaplasma urealyticum (T-strain mycoplasma) J Clin Microbiol. 1978;7:127–132. doi: 10.1128/jcm.7.2.127-132.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson J A, Chen M H. Effects of manganese on the growth and morphology of Ureaplasma urealyticum. J Clin Microbiol. 1984;19:857–864. doi: 10.1128/jcm.19.6.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson J A, Honore L H, Stemke G W. Serotypes of Ureaplasma urealyticum in spontaneous abortion. Pediatr Infect Dis. 1986;5(Suppl. 6):S270–S272. doi: 10.1097/00006454-198611010-00014. [DOI] [PubMed] [Google Scholar]
- 25.Rosendal S, Black F T. Direct and indirect immunofluorescence of unfixed and fixed Mycoplasma colonies. Acta Pathol Microbiol Scand. 1972;80:615. doi: 10.1111/j.1699-0463.1972.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 26.Shepard M C, Lunceford C D. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol. 1978;8:566–574. doi: 10.1128/jcm.8.5.566-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stemke G W, Robertson J A. Problems associated with serotyping strains of Ureaplasma urealyticum. Diagn Microbiol Infect Dis. 1985;3:311–320. doi: 10.1016/0732-8893(85)90005-7. [DOI] [PubMed] [Google Scholar]
- 28.Swenson C E, Van Hamont J, Dunbar B S. Specific protein differences among strains of Ureaplasma urealyticum as determined by two-dimensional gel electrophoresis and a sensitive silver stain. Int J Syst Bacteriol. 1983;33:417–421. [Google Scholar]
- 29.Taylor-Robinson D, Csonka G W, Prentice M J. Human intra-urethral inoculation of ureaplasmas. Q J Med. 1977;46:309–326. [PubMed] [Google Scholar]
- 30.Turunen H, Leinikki P, Jansson E. Serological characterisation of Ureaplasma urealyticum strains by enzyme-linked immunosorbent assay (ELISA) J Clin Pathol. 1982;35:439–443. doi: 10.1136/jcp.35.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waites K B, Crouse D T, Philips J B, Canupp K C, Cassell G H. Ureaplasmal pneumonia and sepsis associated with persistent pulmonary hypertension of the newborn. Pediatrics. 1989;83:79–85. [PubMed] [Google Scholar]