Abstract
The hyperimmunoglobulinemia D syndrome (HIDS) is an autosomal recessive disorder characterized by recurrent febrile attacks with abdominal, articular, and skin manifestations. Apart from elevated immunoglobulin D (IgD) levels (>100 IU/ml), there are high IgA levels in the majority of cases. Mutations in the gene encoding mevalonate kinase constitute the molecular defect in HIDS. The cause of elevated IgA concentrations in HIDS patients remains to be elucidated. We studied the hyper-IgA response in serum of a group of HIDS patients. Elevated IgA concentrations result from increased IgA1 concentrations. IgA and IgA1 concentrations correlated significantly with IgD concentrations, and levels of IgA polymers were significantly higher than the levels in healthy donors. These results indicate a continuous, presumably systemic, stimulation of IgA in HIDS patients.
The hyperimmunoglobulinemia D and periodic fever syndrome (HIDS; MIM [Mendelian inheritance in man] 260920; http://www.hids.net) was originally described by van der Meer et al. in 1984 31 and was later more extensively described by Hiemstra et al. 16 and Drenth et al. 9. Clinical features of this autosomal recessive disorder consist of recurrent attacks of fever, which is frequently preceded by chills and accompanied by headaches, bilateral cervical lymphadenopathy, and sometimes also by abdominal pain and diarrhea. In addition, articular and skin manifestations are a prominent feature of the febrile attacks 1, 8–10, 20, 25. In most patients febrile attacks start in early life, and in some patients they start immediately after birth. The diagnosis of HIDS is based on clinical criteria and elevated serum immunoglobulin D (IgD) levels (>100 IU/ml).
HIDS is caused by a defect in the isoprenoid pathway. We, and others, detected mutations in the gene encoding mevalonate kinase (MVK), which is involved in cholesterol synthesis 7, 17, 30. How defects in MVK eventually lead to the clinical picture and to high levels of IgD is far from clear. Presumably, intermediary metabolites of the isoprenoid pathway (or a shortage of certain metabolites) influence the immune system in such a way that high levels of IgD are produced.
A minority of patients suffers from a variant form of HIDS. Clinically and by the IgD levels they are classified as HIDS patients, but no MVK mutations can be detected (J. P. H. Drenth, personal communication).
Although high serum IgD concentrations constitute a unique hallmark of this syndrome, the precise role of IgD in the pathogenesis, if any, has not yet been defined. Despite extensive study a specific role for serum IgD also has not been discovered yet 32. The level of serum IgD in HIDS does not correlate with disease severity or frequency of attacks, and attacks of HIDS antedate the rise of serum IgD levels above age-dependent reference values and/or 100 IU/ml 14, 15. On the other hand, IgD stimulates peripheral blood mononuclear cells to produce inflammatory cytokines 11, which does suggest a possible role in the pathogenesis of attacks.
The alterations of the immunoglobulin pattern in HIDS are not exclusively limited to IgD. In some HIDS patients we found elevated IgG and IgM values, but high IgA levels are found in the large majority of patients. In a series of 50 patients, IgA levels were found to be elevated beyond reference values in 83% of HIDS patients 9.
Human serum IgA consists of two subclasses, IgA1 and IgA2, which differ in their carbohydrate composition, sensitivity to bacterial proteases, and distribution between mucosal and nonmucosal compartments. Some 75 to 90% of the IgA in serum is composed of the IgA1 subclass 3, 22–24. Both subclasses can be present in mono- or polymeric form. Mucosal IgA is predominantly polymeric, while serum IgA consists of approximately 90% monomers 3, 22–24. Subclass distribution and the molecular size of IgA might shed insight into the immune response in HIDS. To this end we investigated the composition of IgA subclasses in a cohort of 18 HIDS patients, and we also present data on the molecular form (monomers and polymers) of IgA in 4 HIDS patients.
MATERIALS AND METHODS
Patients and sera.
The patient group presented in Table 1 consists of a part of the group described earlier by Drenth et al. 9. All the patients included here were from the Nijmegen, The Netherlands, HIDS registry, and the same patient numbering as used in the previous study 9 was applied to the present study. In total, 18 HIDS patients were included (10 male and 8 female), and the age (mean ± standard deviation) at the time of the study was 29.2 ± 11.3 years. Two patients donated sera on two separate occasions, while a third patient's sera were obtained on three separate occasions and a fourth patient's sera were obtained on four separate occasions.
TABLE 1.
Absolute and relative IgA subclass levels in sera of 18 HIDS patientsa
| Patient no. | Sex | Age (yr) | Absolute IgA level (g/liter)
|
Relative IgA level (%)
|
IgD level (IU/liter) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total IgA | IgA1 + IgA2 | IgA1 | IgA2 | IgA1 | IgA2 | ||||
| 24 | F | 11 | 1.56 | 1.04 | 0.97 | 0.07 | 93.3 | 6.7 | 154 |
| 51 | F | 31 | 2.76 | 1.40 | 1.07 | 0.33 | 76.4 | 23.6 | 80 |
| 11 | M | 49 | 1.75 | 1.63 | 1.51 | 0.12 | 92.6 | 7.4 | 165 |
| 54 | F | 18 | 4.20 | 4.29 | 4.12 | 0.17 | 96.0 | 4.0 | 318 |
| 10 | F | 42 | 4.37 | 4.58 | 4.42 | 0.16 | 96.5 | 3.5 | 106 |
| 3 | F | 43 | 4.79 | 4.65 | 4.04 | 0.61 | 86.9 | 13.1 | 946 |
| 45 | M | 22 | 3.97 | 4.72 | 4.64 | 0.08 | 98.3 | 1.7 | 286 |
| 17 | F | 24 | 4.64 | 5.24 | 4.97 | 0.27 | 94.8 | 5.2 | 467 |
| 35 | M | 32 | 6.56 | 5.32 | 5.08 | 0.24 | 95.5 | 4.5 | 266 |
| 55 | F | 27 | 5.26 | 5.76 | 5.61 | 0.15 | 97.4 | 2.6 | 476 |
| 55 | F | 27 | 5.21 | 5.88 | 5.76 | 0.12 | 98.0 | 2.0 | 400 |
| 13 | M | 45 | 6.26 | 6.13 | 5.76 | 0.37 | 94.0 | 6.0 | 457 |
| 55 | F | 27 | 5.72 | 6.15 | 6.02 | 0.13 | 97.9 | 2.1 | 613 |
| 52 | F | 24 | 6.44 | 6.27 | 5.86 | 0.41 | 93.5 | 6.5 | 96 |
| 35 | M | 32 | 7.55 | 6.32 | 6.07 | 0.25 | 96.0 | 4.0 | 316 |
| 55 | F | 27 | 6.47 | 6.53 | 6.44 | 0.09 | 98.6 | 1.4 | 616 |
| 15 | M | 34 | 8.91 | 7.53 | 7.41 | 0.12 | 98.4 | 1.6 | 492 |
| 16 | M | 35 | 10.44 | 8.16 | 7.96 | 0.20 | 97.5 | 2.5 | 472 |
| 2 | M | 32 | 10.30 | 8.38 | 7.53 | 0.85 | 89.9 | 10.1 | 1,008 |
| 44 | M | 27 | 8.32 | 8.40 | 8.13 | 0.27 | 96.8 | 3.2 | 960 |
| 27 | M | 21 | 10.18 | 9.07 | 8.98 | 0.09 | 99.0 | 1.0 | 492 |
| 27 | M | 21 | 10.25 | 9.30 | 9.21 | 0.09 | 99.0 | 1.0 | 450 |
| 30 | M | 9 | 11.61 | 10.04 | 9.94 | 0.10 | 99.0 | 1.0 | 2,140 |
| 30 | M | 8 | 12.20 | 10.28 | 10.18 | 0.10 | 99.0 | 1.0 | 2,290 |
| 30 | M | 8 | 20.71 | 18.06 | 17.90 | 0.16 | 99.1 | 0.9 | 2,510 |
Results are sorted by IgA1 plus IgA2 levels. Some patients are represented more than once. The patient numbers are the same as those used previously 9. M, male; F, female. Patient numbers in bold indicate that the serum was taken during a febrile attack. Values in italics for total IgA, IgA1 plus IgA2, and IgD indicate levels above reference values. The absolute IgA levels were determined by ELISA.
For the study on the molecular form of the IgA, sera of four children and young adults suffering from HIDS were used. Patient 27 was a 23-year-old male, patient 30 was a 4-year-old male, patient 31 was a 13-year-old male, and patient 66 was a 4-year-old female. Reference values on the molecular form of IgA were obtained from a separate study using healthy Icelandic children (C. M. R. Weemaes, I. S. Klasen, J. H. C. Göertz, S. Jonasdottic, M. Belthuis-Valkis, and A. Haraldsson, unpublished data). Sera were stored for at least 2 years at −20°C before IgA subclass values or IgA polymer levels were determined.
IgD ELISA.
The procedure for the enzyme-linked immunosorbent assay (ELISA) for measurement of IgD has been published earlier 11. Briefly, microtiter plates (Nunc, Roskilde, Denmark) were coated with rabbit anti-human IgD (Dako, Copenhagen, Denmark). Serum samples or standard serum dilutions were added in two different dilutions. After overnight incubation, mouse monoclonal anti-human IgD recognizing the Fc part of the IgD molecule was added (Dako), followed by horseradish peroxidase-labeled rabbit anti-mouse immunoglobulins (Dako). The color that developed after substrate incubation (orthophenylenediamine; Sigma, St. Louis, Mo.) was read at 492 nm using a Titertek Multiskan ELISA reader (Eflab, Oy; Helsinki, Finland). As standard serum, OTRD 02/03 (Behring, Marburg, Germany) calibrated against British research standard 67/37 was used. This standard serum contains IgD Fc fragments. In this ELISA only Fc regions are measured, and standardization is also performed on Fc regions. Presumably, there is no influence of the notorious splitting 12, 27 of IgD molecules with our ELISA. Even after storage at room temperature for 24 h, the same values were obtained. The lower limit of detection of this ELISA was 1 IU/ml (1.4 mg/liter). The interassay coefficient of variation (CV) was calculated to be 10%.
ELISA for IgA subclasses.
In the IgA ELISA, which was essentially comparable to the IgD ELISA, we made use of mouse monoclonal anti-IgA1 (69-11.4) or anti-IgA2 (16-512-H5) 29 (Nordic, Tilburg, The Netherlands). After incubation of serum samples (in three different dilutions in duplicate) or standard serum dilutions, detection of IgA was performed by horseradish peroxidase-labeled goat anti-human IgA (Cappel, Organon Teknika, Turnhout, Belgium). As the standard serum, KIK-21 was used. This is a pool of normal human serum that was a kind gift of J. Radl (Department of Immunology and Infectious Diseases, Netherlands Organization for Applied Scientific Research [TNO], Leiden, The Netherlands). The serum pool was repeatedly tested by various techniques, always with the result of 2 g of IgA1 and 0.2 g of IgA2 per liter. A secondary standard calibrated against this standard was produced and was used in the study presented here. The lower limits of detection of IgA1 and IgA2 were 5 and 2.25 mg/liter, respectively. The interassay CVs were 5 and 6.5% for IgA1 and IgA2, respectively.
IgA ELISA.
The ELISA used to determine the total IgA concentration was essentially the same as that the ELISA used for IgA subclasses. Coating was performed with goat anti-human IgA (Cappel). Detection of IgA was performed as described for IgA subclasses. As standard serum, normal human serum was used that was calibrated against the international World Health Organization standard 67/86. The lower limit of detection was 0.1 mg/p liter. The interassay CV was 4.5%.
Gel filtration.
Sera were separated by gel filtration (fast protein liquid chromatography system; Pharmacia Biotech, Roosendaal, The Netherlands) using a 10/30 Superose 6 column 28 which was calibrated in a 0.05 M phosphate-buffered saline (PBS) buffer containing 0.005% sodium azide, pH 7.4, with a mixture of purified human secretory IgA and IgG (Nordic). Sera were diluted with PBS, and a maximum of 15 μg of IgA was applied to the column. Gel filtration was performed at room temperature at a flow rate of 0.2 ml/min. Fraction volumes of 500 μl were collected in polystyrene cups containing 50 μl of 0.05% Tween 20 in PBS. The fractions were then, within 1 h after elution, analyzed by ELISA as described above. The standard serum used in this assay was the same as that used in the IgA and IgA subclass ELISAs and itself contained 13% IgA polymers as determined by this gel filtration method. Secretory IgA was found in fraction 11 and IgG was found in fraction 17 (IgG determined by an ELISA, comparable to the IgA ELISA described above). The mean recovery of IgA was 108% ± 8.4% (26 separations).
Statistics.
Correlation coefficients between IgD and IgA or IgA1 concentrations were determined by Pearson's linear regression analysis. IgA polymer levels were compared by the Student t test.
RESULTS
Measurement of IgD in sera of HIDS patients.
In our series of HIDS patients we detected very high IgD concentrations, of up to 2,500 IU/ml. However, in two patients we detected IgD values below 100 IU/ml. In patient 51, the IgD concentrations were 310 and 219 IU/ml on earlier occasions. After her first pregnancy, at the age of 31, the concentrations of IgD in her serum decreased to below 100 IU/ml. This patient also showed a reproducible discrepancy between the total IgA concentrations measured by ELISA and the sum of IgA1 and IgA2 concentrations. We presume that this is caused by the low reactivity of the monoclonal anti-IgA1 antibody used, since the relative IgA level is exceptionally low. Patient 52 had an IgD concentration of 96 IU/ml at the age of 28, but measurement at the ages of 19 and 21 showed concentrations of 304 and 166 IU/ml, respectively.
Although the phenotypes of patients 24, 51, and 54 were fully compatible with HIDS, we were unable to detect MVK mutations in these patients. This suggests that their clinical syndrome might be a variant of HIDS. In all other patients MVK mutations were demonstrable.
Measurement of IgA and IgA subclasses.
The distribution of IgA1 and IgA2 subclasses in serum of HIDS patients and their concentrations were determined by ELISA. In all but three patients, IgA concentrations were grossly elevated to beyond 4 g/liter, as can be seen in Table 1. Total IgA in HIDS is mainly composed of IgA1. A statistically significant correlation between IgA and IgD was found (r = 0.793), as was also the case between total IgA1 and IgD (r = 0.786) (P < 0.0001).
Size analysis of IgA in HIDS.
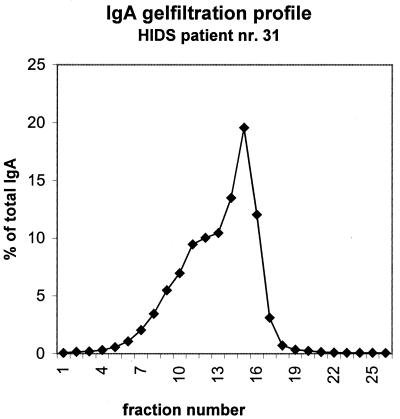
In four HIDS patients (with confirmed MVK mutations) we determined the molecular form (monomers and polymers) of IgA. IgA was measured by ELISA in each fraction after gel filtration. The elution profile on Sepharose for patient 31 (total IgA concentration of 5.6 g/liter) is presented in Fig. 1. Fractions 6 to 13 were considered to contain the IgA polymers. IgA monomers were present in fractions 14 to 19. A summary of IgA polymer percentages in four HIDS patients as determined by the IgA contents in fractions 6 to 13 compared to the total IgA recovered from the column is presented in Table 2, and the polymer levels in healthy children aged 0 to 6 months and 2 to 9 years are presented in Table 3.
FIG. 1.
Percentages of (totally recovered) IgA in the fractions after gel filtration on Superose 6. Secretory IgA (used as a calibrator) was found in fraction 11, and IgG was found in fraction 17.
TABLE 2.
Percentages of polymers in HIDS patientsa
| Patient no. | Total IgA (g/liter) | % Polymersa |
|---|---|---|
| 27 | 12.0 | 32 |
| 30 | 4.0 | 30 |
| 31 | 5.6 | 29 |
| 66 | 4.1 | 23 |
Percentage of IgA in fractions 6 to 13 compared to all IgA recovered after gel filtration. P < 0.0001 for comparison of the percent polymers of the HIDS patients to those of the healthy children aged 2 to 9 years.
TABLE 3.
Percentages of polymers in healthy children
| Age (no. of children) | Mean total IgA ± SD (g/liter) | Mean % polymers ± SDa | Range of % polymersa |
|---|---|---|---|
| 0–6 mo (8) | 0.15 ± 0.14 | 33.3 ± 11.7 | 15–54 |
| 2–9 yr (9) | 0.85 ± 0.26 | 13.4 ± 3.7 | 9–20 |
Percentage of IgA in fractions 6 to 13 compared to all IgA recovered after gel filtration.
DISCUSSION
HIDS is one of the rare disorders associated with elevated IgD levels. Indeed, immunoglobulin studies on HIDS have mainly focused on IgD. Little attention has been paid to the concomitant high levels of IgA found in HIDS, which is surprising given the fact that many HIDS patients have very high IgA levels 9. IgA and IgD do not fluctuate in parallel, which might suggest a different way of stimulation or different kinetics. Absolute IgA and IgD levels in the present study did correlate significantly. Levan-Petit et al. found that IgD production by human B cells is dependent on Th2 cytokines 19. Th2 cytokines, such as transforming growth factor β and interleukin-5, are also involved in IgA production, which might explain the concomitant elevation of IgD and IgA levels in HIDS.
IgA is composed of two subclasses, IgA1 and IgA2, and both subclasses can be present in serum as monomers or as polymers. Normally about 90% of serum IgA consists of IgA1 3, 22–24. In the study presented here, we found very high IgA1 concentrations, compared to normal IgA2 concentrations. This suggests that the high serum IgA levels in HIDS mainly originate from bone marrow. We cannot entirely exclude the mucosa as a source of IgA1, as certain protein antigens primarily elicit an IgA1 response from the human mucosa. For example, gluten exposure in patients suffering from dermatitis herpetiformis induces significantly increased IgA1 concentrations 13.
Relatively high levels of IgA polymers were found in the four HIDS patients studied, as reported in Table 2. Normally about 12% of serum IgA has been reported to consist of polymers 6, 26. In our hands, pooled serum of 500 adult healthy donors contained 13% polymers. For young children the IgA dimer levels were reported to be relatively high 5. In another study, using the same gel filtration technique, it was found that polymeric IgA levels were about 33% in children aged 0 to 6 months, and they decreased to a mean level of 13.4% in children aged 2 to 9 years, a level that can be considered an adult level (Weemaes et al., unpublished). Although only small numbers were compared, the polymer levels of the four HIDS patients were significantly higher than the levels for the age group of 2 to 9 years (P < 0.0001).
The main site of production of polymers and their biological function are still unclear. IgA polymers specific for several microbial antigens have been reported to predominate in sera shortly after systemic immunization or infection 2, 18, 21.
Taken together, our results provide evidence that elevated IgA levels constitute a feature of HIDS, which indicates a continuous stimulation of the IgA system. Our results seem to be in favor of a systemic stimulation of the immune system because of the following points. (i) Hyper-IgA in HIDS represents hyper-IgA1, suggesting bone marrow as the main production site of IgA. (ii) Hyper-IgA in HIDS contains a relatively high polymer IgA level, which can also be explained as a consequence of a continuous systemic stimulation 2, 18, 21. (iii) Hyper-IgD is a hallmark of HIDS. It is generally accepted that IgD is assembled in bone marrow. The significant correlation between IgA or IgA1 and IgD suggests a collective stimulatory site.
However, in the literature the relationship between serum IgA and mucosally produced IgA is still under debate, and there are no unambiguous rules as to where production of IgA subclasses or mono- or dimeric forms of IgA takes place. The specificity of the stimulating antigen also influences the outcome of the immune response with respect to IgA subclass concentration and appearance of molecular form. Therefore, our further studies will focus on the synthetic and catabolic rates of IgA and on the local stimulation of the immune system.
REFERENCES
- 1.Boom B W, Daha M R, Vermeer B J, van der Meer J W M. IgD immune complex vasculitis in a patient with hyperimmunoglobulinemia D and periodic fever. Arch Dermatol. 1990;126:1621–1624. [PubMed] [Google Scholar]
- 2.Conley M E, Delacroix D L. Systemic immunization can result in polymeric IgA production. Pediatr Res. 1986;20:293A. [Google Scholar]
- 3.Conley M E, Delacroix D L. Intravascular and mucosal immunoglobulin A: two separated but related systems of immune defense? Ann Intern Med. 1987;106:892–899. doi: 10.7326/0003-4819-106-6-892. [DOI] [PubMed] [Google Scholar]
- 4.Cooper E H, Turner R, Johns E A, Crockson R A. Identification and measurement of paraprotein polymers by high performance gel filtration chromatography. Biomed Pharmacother. 1985;39:78–82. [PubMed] [Google Scholar]
- 5.Delacroix D L, Liroux E, Vaerman J P. High proportion of polymeric IgA in young infants' sera and independence between IgA size and IgA subclass distributions. J Clin Immunol. 1983;3:51–56. doi: 10.1007/BF00919138. [DOI] [PubMed] [Google Scholar]
- 6.Delacroix D L, Elkom K B, Geubel A P, Hodgson H F, Drive C, Vaerman J P. Changes in size, subclass and metabolic properties of serum immunoglobulin A in liver diseases and in other diseases with high serum immunoglobulin A. J Clin Investig. 1983;71:358–367. doi: 10.1172/JCI110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drenth J P, Cuisset L, Grateau G, Vasseur C, Van de Velde-Visser S D, De Jong J G, Beckmann J S, Van der Meer J W, Delpech M. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat Genet. 1999;22:178–181. doi: 10.1038/9696. [DOI] [PubMed] [Google Scholar]
- 8.Drenth J P H, Prieur A M. Occurrence of arthritis in hyperimmunoglobulinemia D. Ann Rheum Dis. 1993;52:765–766. doi: 10.1136/ard.52.10.765-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drenth J P H, Haagsma C J, Van der Meer J W M the International Hyper-IgD Study Group. Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum of a series of 50 patients. Medicine. 1994;73:133–144. [PubMed] [Google Scholar]
- 10.Drenth J P H, Boom B W, Toonstra J, Van der Meer J W M. Cutaneous manifestations and histological findings in the hyperimmunoglobulinemia D syndrome. Arch Dermatol. 1994;130:59–65. [PubMed] [Google Scholar]
- 11.Drenth J P H, Goërtz J, Daha M R, Van der Meer J W M. Immunoglobulin D enhances the release of tumor necrosis factor-α and interleukin-1β as well as interleukin-1 receptor antagonist from human mononuclear cells. Immunology. 1996;88:355–362. doi: 10.1046/j.1365-2567.1996.d01-672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drenth J P H, Klasen I S, Van der Meer J P H. Recognition of IgD and periodic fever. Ann Intern Med. 1996;125:518. doi: 10.7326/0003-4819-125-6-199609150-00028. [DOI] [PubMed] [Google Scholar]
- 13.Hall R P, McKenzie K D. Comparison of the intestinal and serum antibody response in patients with dermatitis herpetiformis. Clin Immunol Immunopathol. 1992;62:33–41. doi: 10.1016/0090-1229(92)90020-o. [DOI] [PubMed] [Google Scholar]
- 14.Haraldsson A, Weemaes C M R, de Boer A W, Bakkeren J A J M, Stoelinga G B A. Immunological studies in the hyper-IgD syndrome. J Clin Immunol. 1992;12:424–428. doi: 10.1007/BF00918854. [DOI] [PubMed] [Google Scholar]
- 15.Haraldsson A, Weemaes C M R, Jonasdottir S, Olafsson O, Van de Wiel G A S, Göertz J H C, Klasen I S. Serum IgD in infants and children. Scand., J. Immunol. 2000;51:415–419. doi: 10.1046/j.1365-3083.2000.00702.x. [DOI] [PubMed] [Google Scholar]
- 16.Hiemstra I, Vossen J M, Van der Meer J W M, Weemaes C M R, Out T A, Zegers B J M. Clinical and immunological studies in patients with increased serum IgD level. J Clin Immunol. 1989;9:393–400. doi: 10.1007/BF00917104. [DOI] [PubMed] [Google Scholar]
- 17.Houten S M, Kuis W, Duran M, de Koning T J, van Royen-Kerkhof A, Romeijn G J, Frenkel J, Dorland L, de Barse M M, Huijbers W A, Rijkers G T, Waterham H R, Wanders R J, Poll-The B T. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome, Nat. Genet. 1999;22:175–177. doi: 10.1038/9691. [DOI] [PubMed] [Google Scholar]
- 18.Layward L, Allen A C, Harper S J, Hattesley J M, Feehally J. Increased and prolonged production of specific polymeric IgA after systemic immunization with tetanus toxoid in IgA nephropathy. Clin Exp Immunol. 1992;88:394–398. doi: 10.1111/j.1365-2249.1992.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levan-Petit I, Lelievre E, Barra A, Limosin A, Gombert B, Preud'homme J L, Lecron J C. T(h)2 cytokine dependence of IgD production by normal human B cells. Int Immunol. 1999;11:1819–1828. doi: 10.1093/intimm/11.11.1819. [DOI] [PubMed] [Google Scholar]
- 20.Loeliger A E, Kruize A, Bijlsma J W J, Derksen R H W M. Arthritis in hyperimmuno-globulinemia D. Ann Rheum Dis. 1993;52:81. doi: 10.1136/ard.52.1.81-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascart-Lemone F O, Duchateau J R, Oosterom J, Butzler J-P, Delacroix D L. Kinetics of anti-Campylobacter jejuni monomeric and polymeric immunoglobulin A1 and A2 responses in serum during acute enteritis. J Clin Microbiol. 1987;25:1253–1257. doi: 10.1128/jcm.25.7.1253-1257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mestecky J. Immunologic considerations of IgA and IgA containing immune complexes. Am J Kidney Dis. 1988;XII:378–383. [Google Scholar]
- 23.Mestecky J, Lue C, Russell M W. Selective transport of IgA. Cellular and molecular aspects. Gastroenterol Clin N Am. 1991;20:441–471. [PubMed] [Google Scholar]
- 24.Mestecky J, McGhee J R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 25.Miyagawa S, Kitamura W, Morita K, Saishin M, Shirai T. Association of hyperimmunoglobulinemia-D with erythema elevatum diutinum. Br J Dermatol. 1993;128:572–574. doi: 10.1111/j.1365-2133.1993.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 26.Newkirk M N, Klein M H, Katz A, Fisher M M, Underdown B J. Estimation of polymeric IgA in human serum: an assay based on binding of radiolabeled human secretory component with applications in the study of IgA nephropathy, IgA monoclonal gammopathy, and liver disease. J Immunol. 1983;130:1176–1181. [PubMed] [Google Scholar]
- 27.Skvaril F, Radl J. The fragmentation of IgD during storage. Clin Chim Acta. 1967;15:544–546. doi: 10.1016/0009-8981(67)90023-x. [DOI] [PubMed] [Google Scholar]
- 28.Smith D J, King W F, Taubman M A. Isotype, subclass and molecular size of immunoglobulins in salivas from young children. Clin Exp Immunol. 1989;76:97–102. [PMC free article] [PubMed] [Google Scholar]
- 29.Valentijn R M, Radl J, Haaijman J J, Vermeer B J, Weening J J, Kauffmann R H, Daha M R, Van Es L A. Circulating and mesangial secretory component-binding IgA1 in primary IgA nephropathy. Kidney Int. 1984;26:760–766. doi: 10.1038/ki.1984.213. [DOI] [PubMed] [Google Scholar]
- 30.Valle D. You give me fever. Nat Genet. 1999;22:121–122. doi: 10.1038/9624. [DOI] [PubMed] [Google Scholar]
- 31.van der Meer J W M, Vossen J M, Radl J, Van Nieuwkoop J A, Meyer C J, Lobatto S, Van Furth R. Hyperimmunoglobulinemia D and fever: a new syndrome. Lancet. 1984;i:1087–1090. doi: 10.1016/s0140-6736(84)92505-4. [DOI] [PubMed] [Google Scholar]
- 32.Vladutiu A O. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7:131–140. doi: 10.1128/cdli.7.2.131-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]