Abstract
Plasma cell-free DNA (cfDNA) originates from various tissues and cell types and can enable minimally invasive diagnosis, treatment and monitoring of cancer and other diseases. Proper extraction of cfDNA is critical to obtain optimal yields and purity. The goal of this study was to compare the performance of six commercial cfDNA kits to extract pure, high-quality cfDNA from human plasma samples and evaluate the quantity and size profiles of cfDNA extracts—among them, two spin-column based, three magnetic bead-based and two automatic magnetic bead-based methods. Significant differences were observed in the yield of DNA among the different extraction kits (up to 4.3 times), as measured by the Qubit Fluorometer and Bioanalyzer. All kits isolated mostly small fragments corresponding to mono-nucleosomal sizes. The highest yield and reproducibility were obtained by the manual QIAamp Circulating Nucleic Acid Kit and automated MagNA Pure Total NA Isolation Kit. The results highlight the importance of standardizing preanalytical conditions depending on the requirements of the downstream applications.
Keywords: liquid biopsy, cell-free DNA, plasma, cell-free DNA isolation
1. Introduction
Cell-free DNA (cfDNA) is a minimally invasive and real-time biomarker for the early detection, identification and monitoring of various diseases. Liquid biopsy has been investigated as a minimally invasive technique that can avoid the inherent shortcomings of tissue biopsy, such as sampling bias, tissue heterogeneity and difficulty in repetitive sample extraction [1,2]. However, due to the great variability and relatively low abundance (average 10–30 ng/mL, range 1.8–44 ng/mL) [3,4] of cfDNA in circulation and the high degree of fragmentation, it remains a highly challenging analyte.
Preanalytical factors can significantly affect the quality and quantity of cfDNA and have to be investigated thoroughly [5]. Such factors include the choice of matrix (plasma or serum), the sample collection tubes and processing (centrifugation regime), storage, thawing conditions (temperature and freeze–thaw cycles), DNA isolation method, storage of isolated DNA, method of quantification and the intended downstream analysis [6,7,8]. General requirements for efficient isolation techniques include fast, robust, simple and automatable methods that extract cfDNA with satisfactory purity and yield. This is crucial to ensure reliable results in downstream applications such as NGS or PCR. However, the method of choice also depends on the number and volume of samples.
There is a variety of commercially available DNA isolation methods based on different binding chemistries. Each of these chemistries can influence the efficiency and purity of the isolation, and each has a characteristic binding capacity. Ethanol precipitation, anion-exchange resin (coupled with ethanol precipitation), silica gel membrane binding and magnetic silica particle binding technologies can be used. Out of these, magnetic particle-based methods are cheaper, faster and easier to upscale and automate, while membrane binding methods can provide higher yields. The method of choice depends on the number of samples and their volume, required output, purity and downstream applications. Most commercial kits favor the isolation of DNA fragments from 50 to 800 bp, which encompasses the generally reported size of cfDNA (130–170 bp) [9]. However, it is increasingly recognized that the view of mono-nucleosomes as the most important fraction of cfDNA is biased. Recently, smaller fragment sizes have been shown to be of clinical interest [10,11,12], and there is a growing body of evidence that a significant portion of longer cfDNA fragments may not represent contaminants form lysed peripheral blood cells, such as extrachromosomal circular DNA [13,14]. Long cfDNA fragments (larger than 1 kb) from hepatocellular carcinoma patients have recently been shown to contain lower methylation levels than those from non-cancer patients and carry characteristic methylation patterns, opening new possibilities for cancer liquid biopsy [15].
The goal of this study was to compare six commercial cfDNA kits to extract pure, high-quality cfDNA from human plasma samples and evaluate the quantity and size profiles of the obtained extracts.
2. Materials and Methods
2.1. Plasma Samples
First, 11 mL plasma samples were collected in Sarstedt S-Monovettes 9 mL K3E (1,6 mg K3 EDTA/mL blood; Sarstedt Diagnostics GmbH; Nürmbrecht; Germany) from ten healthy individuals. These samples were collected as part of the quality control for biobanked blood samples. Blood samples were taken from patients of the Department of Cardiology during routine venipuncture or from healthy individuals after informed consent for blood collection for the Cardiovascular Biobank of the German Heart Centre Munich was obtained. Blood collection for biobanking was approved by the Ethics Commission of the Technical University Munich (Nr. 5943/13; 16.10.2013).
2.2. Sample Processing
After blood drawing, samples were kept at room temperature and were processed within 60 min. First, they were centrifuged at 1600× g for 10 min at 20 °C. Then, the upper layer was transferred into a 15 mL Falcon tube (BD, New Jersey, United States) and centrifuged again at 6000× g for 10 min at 20 °C. The plasma was aliquoted into 1.5 mL Eppendorf Safe-Lock tubes (Eppendorf, Hamburg, Germany) and stored at −80 °C within 30 min of the second centrifugation. Before further processing, all samples were thawed at room temperature. After isolation, all DNA samples were stored in 1.5 mL DNA-LoBind tubes (Eppendorf) at −20 °C.
2.3. cfDNA Isolation
Five manual magnetic bead or spin column methods and one automated method were used in a direct comparison. The isolation kits were: QIAamp Circulating Nucleic Acid Kit (QiaM, 55114 Qiagen GmbH, Hilden, Germany), NucleoSpin Plasma XS (Macherey-Nagel 740900.50, high-sensitivity protocol—MNaS, Macherey-Nagel GmbH, Düren, Germany), QIAmp MinElute ccfDNA Mini Kit (QiaS, 55204, Qiagen GmbH, Hilden, Germany), cfPure Cell-Free DNA Extraction Kit (BChM, K5011610-BC, BioChain Inc., Newark, CA, USA), MagMAX Cell-Free DNA Isolation Kit (TFiM, A29319, Thermo Fisher Scientific, Waltham, MA, USA) and the automated method MagNA Pure 24 Total NA Isolation Kit (RocA, 07658036001, Roche Diagnostics GmbH, Penzberg, Germany), using the cfNA ss 2000 protocol on the MagNA Pure 24 System (Roche Diagnostics). All kits and their code names for this study are summarized in Table 1. Isolations were performed in duplicate following the instructions provided in the manual of each kit. Moreover, 1 mL of plasma was used as input, with the exception of the MNaS kit, wherein 240 µL was used. For RocA, 1 mL of PBS was added to 1 mL of plasma to obtain the required 2 mL. The elution volume for QiaM and QiaS was 50 µL, for BChM and TFiM 30 µL, for MNaS 12 µL and for RocA 100 µL.
Table 1.
CfDNA extraction kits compared in this study.
| Product | Code | Type | Can Be Automated | Isolation Volume (mL) | Elution Volume (µL) |
|---|---|---|---|---|---|
| QIAamp Circulating Nucleic Acid Kit (Qiagen) | QiaS | spin column (vacuum manifold) | no | 1 | 50 |
| NucleoSpin Plasma XS (Macherey-Nagel) | MNaS | spin column | no | <0.24 | 5–30 |
| QIAmp MinElute ccfDNA Mini Kit (Qiagen) | QiaM | magnetic beads | yes | 1–4 | 20–80 |
| cfPure Cell-Free DNA Extraction Kit (BioChain) | BChM | magnetic beads | yes | 1–10 | 15–50 |
| MagMAX Cell-Free DNA Isolation Kit (Thermo Fisher Scientific) | TFiM | magnetic beads | yes | 0.5–10 | 15–50 |
| MagNA Pure 24 Total NA Isolation Kit (Roche) | RocA | magnetic beads (automated) | - | 2 | 50/100 |
2.4. cfDNA Quantification and DNA Sizing
The concentration of the isolated cfDNA was analyzed in duplicate by fluorometric quantification using the Qubit Fluorometer 3.0 (Thermo Fisher, Waltham, MA, USA) and the dsDNA HS Assay (quantification range: 10 pg/µL–100 ng/µL; Thermo Fisher). For four of the donors, we determined the representative quantity and fragment size profiles of cfDNA using a 2100 Bioanalyzer (Agilent Inc., Santa Clara, CA, USA) with the Agilent High-Sensitivity DNA Kit.
2.5. Statistics
Statistical analysis of the data was performed using the GraphPad Prism software version 8 and Excel. Differences between group means were calculated using one-way analysis of variance (ANOVA), followed by pairwise comparison using a post-hoc Tukey test. Differences were considered statistically significant if the p values were smaller than 5% (p < 0.05).
3. Results and Discussion
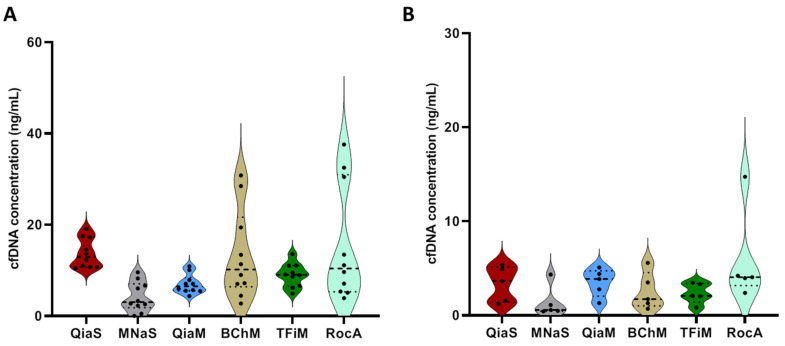
CfDNA is a source of clinical biomarkers in blood with applications ranging from cancer detection and monitoring to prenatal diagnostics. However, with concentrations ranging from a few ng/mL to several thousand ng/mL [3,16] and various fragment sizes, cfDNA is challenging to analyze. In this study, we compared six different extraction kits, including two spin column-based methods and four magnetic bead-based methods, to one that is automated. We isolated cfDNA from 1 mL plasma for each healthy volunteer (for Nucleospin Plasma XS (MNaS), only 240 µL plasma was used) and the yield and fragment sizes were assessed with the Qubit Fluorometer and Bioanalyzer (BA), respectively. Based on the Qubit and BA measurements, all methods were able to recover cfDNA from all plasma samples (Figure 1).
Figure 1.
Comparison of cell-free DNA (cfDNA) yields delivered by different DNA extraction kits. CfDNA was isolated from the plasma of 10 healthy donors using different extraction kits and then quantified by (A) the Qubit HS DNA kit and (B) the Agilent HS DNA Kit on the Bioanalyzer. Quantitative measurements of cfDNA are expressed as the total mass of cfDNA (ng) present in 1 mL of plasma.
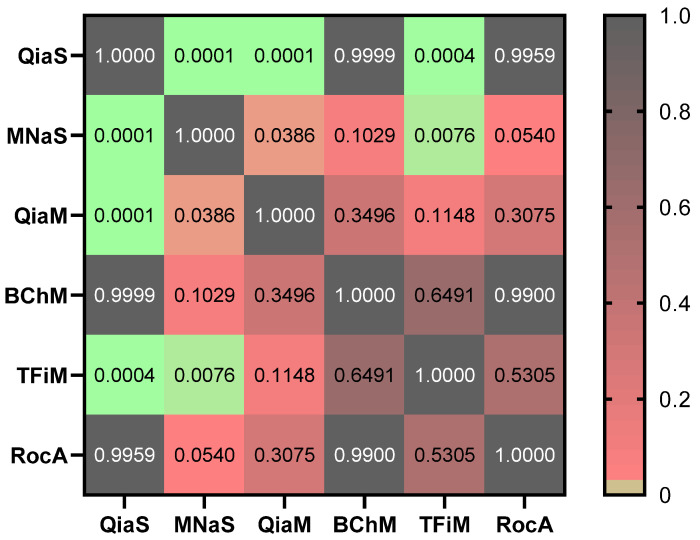
Overall, QiaS showed significantly greater recovery in comparison to MNaS (p = 0.0001), QiaM (p < 0.0001) or TFiM (p = 0.0004) when assessed by Qubit. No statistically significant difference was observed between QiaS and BChM or RocA. In addition, MNaS produced significantly lower yields compared to QiaM (p = 0.0386), TFiM (p = 0.0076) or QiaS. No other statistically significant difference was observed (Figure 2). Although spin column-based methods are typically more costly and time-consuming than magnetic approaches, they typically produce higher yields. In our study, QiaS resulted in the highest recovery, which is consistent with a number of comparative studies [5,17,18,19,20,21,22].
Figure 2.
A heat map representation of the post-hoc multiple comparison of cfDNA recovery isolated using different extraction kits and measured with the Qubit HS DNA assay. Significant differences (p < 0.05) are shown in green to orange.
The method of quantification can also significantly affect the measured quantity of DNA. For example, Maas et al. 2021 compared the Qubit, PicoGreen assay, BA and TapeStation and found that the Qubit, BA and TapeStation provide excellent estimations of the sample concentrations, especially within the ranges specified by the manufacturer [23]. A few years prior, though, Solassol et al. demonstrated that the Qubit was superior to the TapeStation, especially for estimating higher concentrations [24]. In our analysis, cfDNA concentrations were lower when quantified by the BA as compared with the Qubit, and there were only minor significant differences between the various extraction methods when concentrations were assessed by the BA. MNaS resulted in significantly lower concentrations than QiaM (p = 0.0052) and TFiM (p = 0.03496). Additionally, BChM resulted in significantly lower concentrations than QiaM (p = 0.0335) and TFiM (p = 0.0377). This may be related to the fact that TFiM consistently isolated longer DNA fragments (>1 kb), which are excluded from the cfDNA estimation in the BA. A similar finding was reported by Cédile et al. in 2021, when they observed an underestimation of cfDNA plasma concentrations by BA (2.86–14.12 ng/mL) as compared with Qubit measurements (7.5–23.31 ng/mL) [25].
To estimate the reproducibility of each extraction method, the coefficient of variance for each sample was calculated and each CV% value was used to calculate the median CV% of each method, thereby avoiding a direct influence of sample variations. Median CV% and ranges are shown in Table 2. The smallest variation was found for QiaS (11.8%), followed by RocA (16.0%), which agrees with the observations of van Ginkel et al., 2017 [26]. The two methods also displayed comparable yields of cfDNA. BChM was found to have the lowest reproducibility, with a CV% of 34.6%, which is reported to show excellent reproducibility in the cell culture context [22]. Here, it is noteworthy that, in the context of plasma, there is often higher variability between replicate isolations, and although CV% values are not commonly reported (e.g., when each sample is isolated only once), we often encounter large standard deviations, especially for low amounts of cfDNA [19,23]. Variability seems to be lower for spike-in controls [17].
Table 2.
Reproducibility of cfDNA recovery of different kits.
| Kit | Code | Median Conc. (ng/mL) | CV% Range | Median CV% |
|---|---|---|---|---|
| QIAamp Circulating Nucleic Acid Kit | QiaS | 13.0 | 8.3–21.9 | 11.8 |
| NucleoSpin Plasma XS | MNaS | 3.0 | 3.1–64.8 | 16.2 |
| QIAmp MinElute ccfDNA Mini Kit | QiaM | 6.5 | 5.3–29.6 | 20.0 |
| cfPure Cell-Free DNA Extraction Kit | BChM | 10.2 | 3.5–52.4 | 34.6 |
| MagMAX Cell-Free DNA Isolation Kit | TFiM | 9.0 | 2.9–75.2 | 21.5 |
| MagNA Pure 24 Total NA Isolation Kit | RocA | 10.4 | 3.2–44.4 | 16.0 |
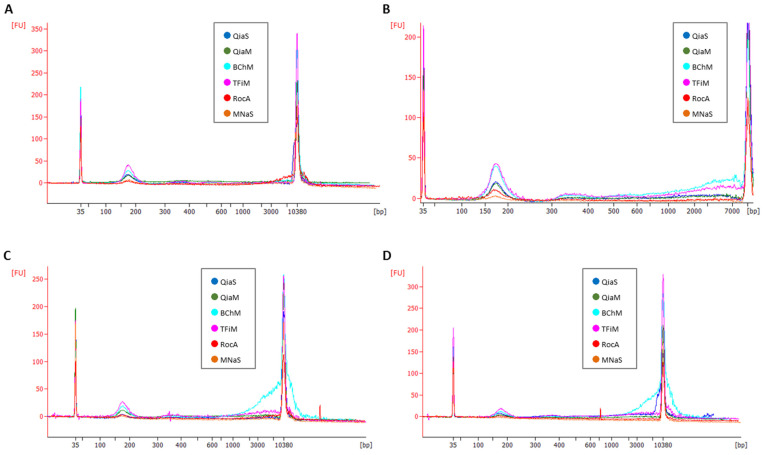
To evaluate the size distribution of the isolated cfDNA fragments extracted using the different kits, four of the ten isolated samples (samples from 4 of the 10 donors) were run on the Bioanalyzer using the Agilent High-Sensitivity DNA Kit (Figure 3).
Figure 3.
Cell-free DNA (cfDNA) size profiles yielded by different extraction kits. (A–D) Representative electropherograms from 4 of the 10 donors, as measured with the Agilent HS DNA Kit on the 2100 Bioanalyzer. The curves in these figures are not representative of the total cfDNA yield by the respective kits, as different elution volumes were used for the different extraction kits (100 µL for RocA, 50 µL for QiaS and QiaM and 30 µL for TFiM and BChM).
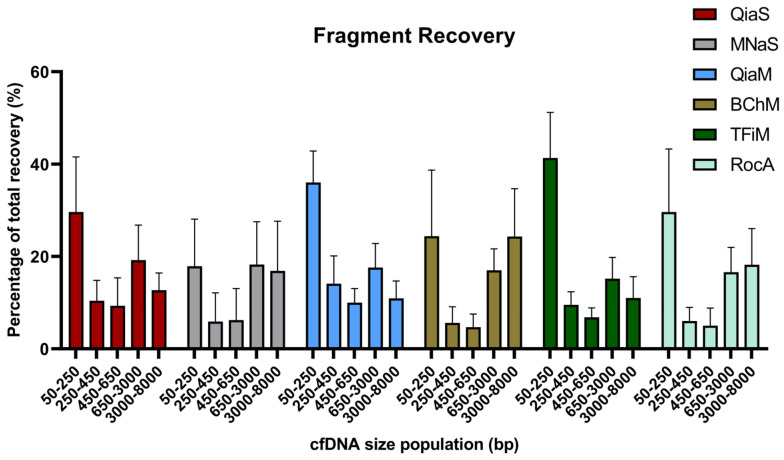
The on-board software of the BA was then used to calculate the relative proportions of different cfDNA size populations recovered by each method (Figure 4). Taken together, these size analyses showed that all kits recovered a high proportion of mono-nucleosomes, while all kits also recovered a small percentage of larger cfDNA fragments, where the BChM kit delivered the highest relative proportion of longer cfDNA fragments. The relative proportion of mono-nucleosomes in the eluates appears to be lower than what is commonly reported in plasma. This is most likely due to the low amounts of DNA extracted, which leads to an overestimation of the longer fragments by the BA as the background noise becomes higher. Thereby, all kits resulted in similar percentages of mono-nucleosomes, without significant differences, as measured with the dsDNA High-Sensitivity Kit on the Bioanalyzer. The median percentage for MNaS was 21%; 22% for BChM and RocA, 26.5% for QiaS, 34% for TFiM and 34.5% for QiaM. The same effect was seen in Maas et al. 2022, when they assessed the sensitivity and reproducibility of the BA and other methods in low concentration ranges [23]. Therefore, in our case, the Qubit is the preferred method for quantification, while the BA still provides valuable information about shorter fragment sizes, such as those of mono- and di-nucleosomes.
Figure 4.
Size bias of different cell-free DNA (cfDNA) extraction kits. The cfDNA size profiles delivered by the six different DNA extraction kits, as measured by the Agilent HS DNA Kit on the 2100 Bioanalyzer, were analyzed to determine the relative proportions of different size populations recovered by each kit. Each bar represents that average of four donor samples. Error bars indicate standard deviation.
4. Conclusions
Based on our results, we selected QiaS as the kit most suited to our needs. This kit produces cfDNA at a desirable concentration for downstream applications such as use with PCR and DNA sequencing. RocA delivers comparable cfDNA yields and it has some benefits over manual methods (better standardization), and it may be more suitable for high-throughput laboratories. BChM can result in higher yields of longer DNA fragments. The choice of kit will depend on the individual needs of the user. In the clinical setting, it is important to obtain the highest DNA quantity from as little sample volume as possible, in order to enable multiple tests even with precious and spare samples. In a future comparison, it would be interesting to see how different isolation methods show bias towards the capturing of cfDNA fragments that are both shorter and longer than mono-nucleosomes in the context of human plasma.
Author Contributions
Conceptualization, S.H. and Z.M.; methodology, Z.M.; validation, E.P., Z.M. and A.J.B.; formal analysis, V.U. and E.P.; investigation, Z.M., E.P. and V.U.; resources, S.H.; writing—original draft preparation, E.P.; writing—review and editing, A.J.B. and S.H.; visualization, A.J.B. and S.H.; supervision, Z.M.; project administration, S.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Patients had given their informed consent for blood collection to the Cardiovascular Biobank of the German Heart Centre Munich (Nr. 5943/13; 16.10.2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. The blood collection for biobanking was approved by the Ethics Commission of the Technical University Munich (Nr. 5943/13; 16.10.2013).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee H., Park C., Na W., Park K.H., Shin S. Precision cell-free DNA extraction for liquid biopsy by integrated microfluidics. NPJ Precis. Oncol. 2020;4:3. doi: 10.1038/s41698-019-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu D., Park B.H. Liquid biopsy: Unlocking the potentials of cell-free DNA. Virchows Arch. 2017;471:147–154. doi: 10.1007/s00428-017-2137-8. [DOI] [PubMed] [Google Scholar]
- 3.Fleischhacker M., Schmidt B. Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys Acta. 2007;1775:181–232. doi: 10.1016/j.bbcan.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Perkins G., Yap T.A., Pope L., Cassidy A.M., Dukes J.P., Riisnaes R., Massard C., Cassier P.A., Miranda S., Clark J., et al. Multi-Purpose Utility of Circulating Plasma DNA Testing in Patients with Advanced Cancers. PLoS ONE. 2012;7:e47020. doi: 10.1371/journal.pone.0047020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markus H., Contente-Cuomo T., Farooq M., Liang W.S., Borad M.J., Sivakumar S., Gollins S., Tran N.L., Dhruv H.D., Berens M.E., et al. Evaluation of pre-analytical factors affecting plasma DNA analysis. Sci. Rep. 2018;8:7375. doi: 10.1038/s41598-018-25810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronkhorst A.J., Aucamp J., Pretorius P.J. Cell-free DNA: Preanalytical variables. Clin. Chim. Acta. 2015;450:243–253. doi: 10.1016/j.cca.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Bronkhorst A.J., Ungerer V., Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol. Detect. Quantif. 2019;17:100087. doi: 10.1016/j.bdq.2019.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heitzer E., van den Broek D., Denis M.G., Hofman P., Hubank M., Mouliere F., Paz-Ares L., Schuuring E., Sültmann H., Vainer G., et al. Recommendations for a practical implementation of circulating tumor DNA mutation testing in metastatic non-small-cell lung cancer. ESMO Open. 2022;7:100399. doi: 10.1016/j.esmoop.2022.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Underhill H.R., Kitzman J.O., Hellwig S., Welker N.C., Daza R., Baker D.N., Gligorich K.M., Rostomily R.C., Bronner M.P., Shendure J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016;12:e1006162. doi: 10.1371/journal.pgen.1006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peneder P., Stütz A.M., Surdez D., Krumbholz M., Semper S., Chicard M., Sheffield N.C., Pierron G., Lapouble E., Tötzl M., et al. Multimodal analysis of cell-free DNA whole-genome sequencing for pediatric cancers with low mutational burden. Nat. Commun. 2021;12:3230. doi: 10.1038/s41467-021-23445-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristiano S., Leal A., Phallen J., Fiksel J., Adleff V., Bruhm D.C., Jensen S.Ø., Medina J.E., Hruban C., White J.R., et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570:385–389. doi: 10.1038/s41586-019-1272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zvereva M., Roberti G., Durand G., Voegele C., Nguyen M.D., Delhomme T.M., Chopard P., Fabianova E., Adamcakova Z., Holcatova I., et al. Circulating tumour-derived KRAS mutations in pancreatic cancer cases are predominantly carried by very short fragments of cell-free DNA. EBioMedicine. 2020;55:102462. doi: 10.1016/j.ebiom.2019.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu S.C.Y., Jiang P., Peng W., Cheng S.H., Cheung Y.T.T., Tse O.Y.O., Shang H., Poon L.C., Leung T.Y., Chan K.C.A., et al. Single-molecule sequencing reveals a large population of long cell-free DNA molecules in maternal plasma. Proc. Natl. Acad. Sci. USA. 2021;118:e2114937118. doi: 10.1073/pnas.2114937118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo S., Yi Y., Wang C., Li X., Zhou M., Peng Q., Zhou J., Yang Y., He Q. Extrachromosomal Circular DNA (eccDNA): From Chaos to Function. Front. Cell Dev. Biol. 2021;9:792555. doi: 10.3389/fcell.2021.792555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choy L.Y.L., Peng W., Jiang P., Cheng S.H., Yu S.C.Y., Shang H., Tse O.Y.O., Wong J., Wong V.W., Wong G.L.H., et al. Single-molecule Sequencing Enables Long Cell-free DNA Detection and Direct Methylation Analysis for Cancer Patients. Clin. Chem. 2022;68:1151–1163. doi: 10.1093/clinchem/hvac086. [DOI] [PubMed] [Google Scholar]
- 16.van der Vaart M., Pretorius P.J. Is the role of circulating DNA as a biomarker of cancer being prematurely overrated? Clin. Biochem. 2010;43:26–36. doi: 10.1016/j.clinbiochem.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Diefenbach R.J., Lee J.H., Kefford R.F., Rizos H. Evaluation of commercial kits for purification of circulating free DNA. Cancer Genet. 2018;228–229:21–27. doi: 10.1016/j.cancergen.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Jain M., Balatsky A.V., Revina D.B., Samokhodskaya L.M. Direct comparison of QIAamp DSP Virus Kit and QIAamp Circulating Nucleic Acid Kit regarding cell-free fetal DNA isolation from maternal peripheral blood. Mol. Cell Probes. 2019;43:13–19. doi: 10.1016/j.mcp.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Mauger F., Dulary C., Daviaud C., Deleuze J.F., Tost J. Comprehensive evaluation of methods to isolate, quantify, and characterize circulating cell-free DNA from small volumes of plasma. Anal. Bioanal. Chem. 2015;407:6873–6878. doi: 10.1007/s00216-015-8846-4. [DOI] [PubMed] [Google Scholar]
- 20.Devonshire A.S., Whale A.S., Gutteridge A., Jones G., Cowen S., Foy C.A., Huggett J.F. Towards standardisation of cell-free DNA measurement in plasma: Controls for extraction efficiency, fragment size bias and quantification. Anal. Bioanal. Chem. 2014;406:6499–6512. doi: 10.1007/s00216-014-7835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett A.N., Thadani H.A., Laureano-Asibal C., Ponnusamy S., Choolani M. Stability of cell-free DNA from maternal plasma isolated following a single centrifugation step. Prenat. Diagn. 2014;34:1283–1288. doi: 10.1002/pd.4468. [DOI] [PubMed] [Google Scholar]
- 22.Bronkhorst A.J., Ungerer V., Holdenrieder S. Comparison of methods for the isolation of cell-free DNA from cell culture supernatant. Tumour Biol. 2020;42:1010428320916314. doi: 10.1177/1010428320916314. [DOI] [PubMed] [Google Scholar]
- 23.Maass K.K., Schad P.S., Finster A.M.E., Puranachot P., Rosing F., Wedig T., Schwarz N., Stumpf N., Pfister S.M., Pajtler K.W. From Sampling to Sequencing: A Liquid Biopsy Pre-Analytic Workflow to Maximize Multi-Layer Genomic Information from a Single Tube. Cancers. 2021;13:3002. doi: 10.3390/cancers13123002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solassol J., Quantin X., Larrieux M., Senal R., Audran P., Grand D., Mangé A., Diamandis E.P., Vendrell J.A. Comparison of five cell-free DNA isolation methods to detect the EGFR T790M mutation in plasma samples of patients with lung cancer. Clin. Chem. Lab. Med. (CCLM) 2018;56:e243–e246. doi: 10.1515/cclm-2017-0967. [DOI] [PubMed] [Google Scholar]
- 25.Cédile O., Veyhe S.R., Hansen M.H., Titlestad K., Nyvold C.G. Investigation of circulating DNA integrity after blood collection. BioTechniques. 2021;71:550–555. doi: 10.2144/btn-2020-0167. [DOI] [PubMed] [Google Scholar]
- 26.van Ginkel J.H., van den Broek D.A., van Kuik J., Linders D., de Weger R., Willems S.M., Huibers M.M.H. Preanalytical blood sample workup for cell-free DNA analysis using Droplet Digital PCR for future molecular cancer diagnostics. Cancer Med. 2017;6:2297–2307. doi: 10.1002/cam4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.