Abstract
Although productive progress has been made in colorectal cancer (CRC) researchs, CRC is the second most frequent type of malignancy and the major cause of cancer-related death among gastrointestinal cancers. As angiogenesis constitutes an important point in the control of CRC progression and metastasis, understanding the key signaling pathways that regulate CRC angiogenesis is critical in elucidating ways to inhibit CRC. Herein, we comprehensively summarized the angiogenesis-related pathways of CRC, including vascular endothelial growth factor (VEGF), nuclear factor-kappa B (NF-κB), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), Wingless and int-1 (Wnt), and Notch signaling pathways. We divided the factors influencing the specific pathway into promoters and inhibitors. Among these, some drugs or natural compounds that have antiangiogenic effects were emphasized. Furthermore, the interactions of these pathways in angiogenesis were discussed. The current review provides a comprehensive overview of the key signaling pathways that are involved in the angiogenesis of CRC and contributes to the new anti-angiogenic strategies for CRC.
Keywords: colorectal cancer, angiogenesis, VEGF, NF-κB, JAK-STAT, Wnt, Notch
1. Introduction
Colorectal cancer is the second most frequent type of malignancy and the major cause of cancer-related death among gastrointestinal cancers, despite significant advances over the past two decades in preventive screening and therapy aimed at improving patient survival [1]. The overall survival rate in patients is still low, particularly the patients who are diagnosed at an advanced stage. In terms of cancer etiology and CRC, the mechanism of cancer development in most patients remains unclear. Many signaling pathways in CRC are implicated in the regulation of several biological processes, including cell proliferation, differentiation, angiogenesis, apoptosis, and survival [2].
Judah Folkman’s early observations showed that fast-growing tumors have a large number of blood vessels compared to dormant tumors, leading to the concept that tumor progression requires the initiation of tumor angiogenesis and anti-angiogenesis [3]. Through further studies, Folkman isolated an inducer of tumor angiogenesis and speculated that inhibition of the angiogenesis might block the formation of new blood vessels and arrest solid tumors at a very small size [4]. This point of view set off a wave of research on tumor angiogenesis.
As a hallmark of cancer, angiogenesis is closely related to tumor growth, metastasis, invasion, prognosis, and recurrence [5,6,7,8]. Tumor cells need enough oxygen and nutrients to survive, and angiogenesis can provide them with these substances to promote cell growth and proliferation [9]. To promote angiogenesis, cancer cells often overexpress angiogenic factors, such as VEGFA [10]. However, in the process of tumor occurrence, the abnormalities and disorders of vessels structure and function often occur, which not only promote the metastasis of tumor cells but also hinder the delivery of chemotherapy drugs to tumor tissues, leading to the occurrence of chemotherapy drug resistance [11].
Angiogenesis is a crucial process in CRC development, in which VEGF signaling pathway is a classical regulatory pathway in CRC [12]. Thus, in previous reviews, treatment has mainly been targeted on the regulators of this pathway. In fact, many studies suggest that multiple pathways including VEGF signaling pathway have targets of high therapeutic value to regulate CRC angiogenesis. However, the mechanism of angiogenesis in CRC is not very clear at present. Therefore, understanding the mechanism of angiogenesis in CRC is of great significance for inhibiting the occurrence and development of tumors, resolving the resistance to various drugs, and even developing new antiangiogenic drugs. This review focuses on the role of the VEGF, NF-κB, JAK-STAT, Wnt, and Notch signaling pathways in angiogenesis in CRC.
2. VEGF Signaling Pathway in CRC
2.1. Introduction to the VEGF Signaling Pathway
The VEGF signaling pathway is established as one of the key regulators of tumor angiogenesis [13]. The VEGF/VEGF receptor axis is composed of multiple ligands and receptors, including ligands VEGFA, VEGFB, VEGFC, VEGFD, and placental growth factor (PLGF) and receptors VEGFR-1, VEGFR-2, and VEGFR-3 [13,14,15]. VEGFA is the predominant proangiogenic factor in CRC and is associated with metastases formation and poor prognosis in CRC patients [16]. More specifically, VEGF can regulate the formation of blood vessels and participate in the physiological activities of endothelial cells by binding to VEGFR to activate downstream signals such as PI3K/AKT and mitogen-activated protein kinase (MAPK) [17,18,19].
2.2. Factors Promoting CRC Angiogenesis by the VEGF Signaling Pathway
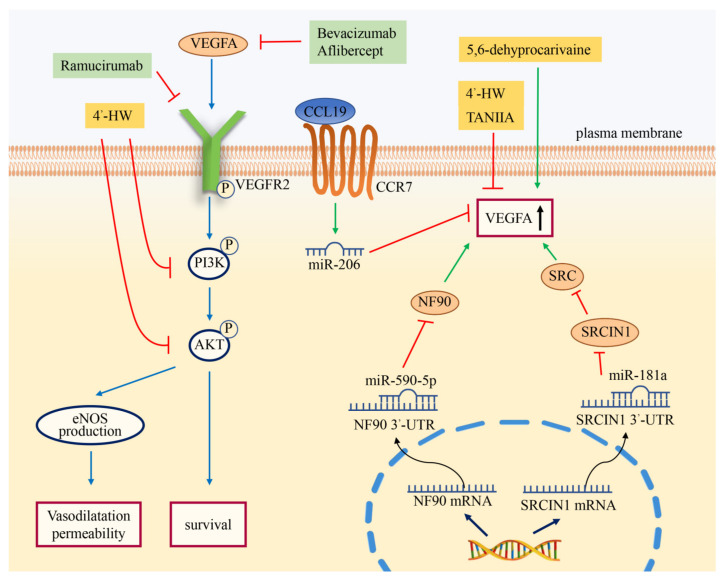
Many biologically active molecules promote angiogenesis by regulating the VEGF signaling pathway. It has been well established that the HIF-1 was a key mediator of hypoxic responses. Under hypoxia, HIF-1 could significantly upregulate the expression of VEGF [20,21]. As the core subunit of SWI/SNF (switch/sucrose nonfermentable) family complexes, brahma-related gene 1 (BRG1) was significantly overexpressed in CRC tissues compared with normal tissues. The study showed that BRG1 could promote VEGFA expression by interacting with HIF-1α to increase CRC angiogenesis [22]. As a key member of the Gab family, Grb2-associated binder 2 (Gab2) is a scaffolding protein that serves as a critical signaling amplifier downstream of tyrosine kinase receptors [23,24]. A study demonstrated that Gab2 expression was positively correlated with the levels of VEGF in CRC tissues. Gab2 could upregulate the expression of VEGF and activate the MEK/ERK/c-Myc pathway, thus promoting angiogenesis in CRC [25]. As a non-receptor protein tyrosine kinase, non-receptor protein tyrosine kinase (SRC) was activated following engagement of many different classes of cellular receptors, including receptor protein tyrosine kinases, G protein-coupled receptors, as well as cytokine receptors. A large amount of evidence indicated that SRC regulated angiogenesis via the SRC-STAT3-VEGF pathway in CRC [26]. It has been demonstrated that SRC could induce the expression of HIF-1α and then upregulate the expression of VEGFA [27]. In addition, SRC could stabilize the content of HIF-1α under normoxic conditions by inhibiting prolyl hydroxylation [28]. Previous studies have shown that miR-181a inhibited SRC kinase signaling inhibitor 1 (SRCIN1) to increase the activity of SRC (Figure 1) [26]. This process promoted the secretion of VEGF, which in turn induced angiogenesis in CRC. Serum response factor (SRF), a member of the MADS box superfamily of transcription factors, could promote tumor metastasis by enhancing the proliferation, invasion, and angiogenesis of tumor cells. As a key condition of VEGF-induced angiogenesis, SRF could act as an upstream regulator to affect the expression of VEGF. SRSF3 could bind to SRF pre-mRNA and participate in the splicing of SRF pre-mRNA, thereby promoting angiogenesis of CRC through this process [29]. CircRNAs can regulate various functions and gene expressions through sponging with microRNAs (miRNAs). Circ_0056618, a novel circRNA discovered in gastric cancer, has been reported to promote angiogenesis in CRC [30]. MiR-206 is a miRNA that can regulate invasion, proliferation, and angiogenesis by binding to the 3′ untranslated regions (UTR) of Met to inhibit Met/ERK/Elk-1/HIF-1α/VEGFA signaling in CRC [30,31]. Consistently, Zheng et al. demonstrated that circ_0056618 promoted the expression of VEGFA through sponging with miR-206 in CRC. Besides, it has also been shown that 5,6-dehydrocarvaine extracted from the rhizome of Galangal could induce the expression of VEGF in HT-29 cells, thereby promoting cancer angiogenesis [32]. As a high-production-volume industrial chemical, Bisphenol A (BPA) is widely distributed in air, soil, water, and sewage sludge. It has been reported that BPA exposure promoted the excessive production of reactive oxygen species (ROS), which in turn activated the HIF-1α/VEGF/PI3K/ AKT axis in CRC cells [33].
Figure 1.
Regulation of angiogenesis via the VEGF signaling pathway in CRC. As monoclonal antibodies to VEGFA or VEGFR2, bevacizumab, aflibercept, and ramucirumab can inhibit angiogenesis in CRC by binding to their corresponding molecules. The chemokine CCL19 can promote miR-206 to inhibit VEGFA in a CCR7-dependent manner. MiR-181a and miR-590-5p inhibit the expression of related target molecules by binding to the target 3’-UTR. These processes can affect the expression of VEGFA. 4’-HW can block PI3K and AKT phosphorylation and inhibit VEGFA expression. The compounds Tan IIA and 5,6-dehyprocarivaine can also affect the increase in VEGFA.
2.3. Factors Inhibiting CRC Angiogenesis by the VEGF Signaling Pathway
In addition, some molecules that inhibit angiogenesis through the VEGF signaling pathway have been described. These include chemokine CC ligand 19 (CCL19), also known as macrophage inflammatory protein 3-β (MIP-3B). The expression of CCL19 in CRC tissues was lower than that in normal tissues, and negatively correlated with cancer angiogenesis. CCL19 inhibited the Met/ERK/ELK-1/HIF-1α/VEGFA pathway by promoting the expression of miR-206 (Figure 1). CCL19 inhibited angiogenesis in CRC through this process [31]. Some microRNAs can also inhibit angiogenesis in CRC through the VEGF pathway. It was found that the expression of miR-590-5p was significantly downregulated in CRC compared with normal tissues. The direct target of miR-590-5p is nuclear factor 90 (NF90), which is a protein synthesis regulator of VEGF (Figure 1). MiR-590-5p could inhibit CRC angiogenesis and metastasis by inhibiting the NF90/VEGFA axis [34]. Besides, miR-148a has been shown to downregulate VEGF via the pERK/HIF-1α pathway [35].
2.4. Anti-Angiogenic Therapy of the VEGF Signaling Pathway
In CRC treatment, antiangiogenic therapy is an important method, and the VEGF signaling pathway plays a significant role. Bevacizumab is an FDA-approved antiangiogenic agent targeting VEGF for the treatment of metastatic colorectal cancer (MCRC) (Figure 1) (Table 1) [36]. Studies have shown that the overall response rate and disease control rate of bevacizumab combination therapy in 35 CRC patients were 3.2% and 51.6%, respectively [37]. Aflibercept is also an antiangiogenic drug already in use. Unlike bevacizumab, it can target not only VEGFA but also VEGFB and PLGF [38]. Ramuciumab is a monoclonal antagonist targeting VEGFR2. It inhibits angiogenesis by blocking VEGFA binding to VEGFR2 [39]. By inhibiting angiogenesis, other molecules can also play therapeutic roles. A compound called cantharidin (CTD) secreted by Blister Beetle inhibited angiogenesis by inhibiting the phosphorylation of JAK1, ERK, and AKT and the activation of STAT3 signaling induced by VEGF [40]. Scopoletin, the main bioactive component of the Erycibe obtusifolia Benth, inhibited angiogenesis by blocking the autophosphorylation and downstream signaling pathways of VEGFR2 [41]. In athymic nude mice bearing HCT-116 cells, scopoletin had a significant inhibitory effect on tumor vasculature. Tumors collected from nude mice showed a dramatic decrease in blood vessel density compared to the control group [42]. Toll-like receptor 4 (TLR4) could induce the PI3K/AKT signaling and play a central role in the progression of CRC [43]. Moreover, baicalein directly bound to TLR4 to inhibit TLR4/HIF-1α/VEGF signaling pathway and angiogenesis in CRC [44]. Ginkgetin and resveratrol could inhibit VEGF-mediated angiogenesis during tumorigenesis (Figure 1). These two molecules inhibited the phosphorylation of VEGFR2, AKT, endothelial nitric oxide synthases (eNOS), and ERK in human umbilical vein endothelial cells (HUVECs) [45]. Tanshinone IIA (Tan IIA) could reduce the expression of angiogenin, VEGF and basic fibroblast growth factor (bFGF) in CRC to inhibit angiogenesis [46]. The study showed that 4’-hydroxywogonin (4’-HW) could not only decrease the mRNA and protein expression of VEGFA but also inhibit the phosphorylation of PI3K and AKT in CRC cells. This suggested that 4’-HW was a promising anticancer drug targeting angiogenesis in CRC [47].
Table 1.
Existing/potential drugs with anti-angiogenic effects in CRC.
| Signaling Pathways | Drugs | Target | Classification | Functions | References |
|---|---|---|---|---|---|
| VEGF signaling pathway | Bevacizumab | VEGFA | Clinical | Monoclonal antibody for VEGFA | [36] |
| Ramuciumab | VEGFR2 | Clinical | Monoclonal antibody for VEGFR2 | [39] | |
| Scopoletin | VEGFR2 | Under study | VEGFR2 inhibitor | [41] | |
| Ginkgetin and Resveratrol | VEGFR2 and AKT | Under study | VEGFR2 and AKT inhibitor | [45] | |
| Tan IIA | HIF-1α and TGF-β1 | Under study | HIF-1α inhibitor | [46] | |
| 4‘-HW | PI3K and AKT | Under study | VEGFA inhibitor | [47] | |
| NF-κB signaling pathway | Imatinib | VEGFR and ERK | Clinical | Tyrosine kinase inhibitor | [48] |
| Curcumin | NF-κB | Clinical | NF-κB inhibitor | [49,50] | |
| Andrographolide | NF-κB | Under study | NF-κB inhibitor | [51] | |
| Parthenolide | NF-κB | Under study | NF-κB inhibitor | [52] | |
| STAT3 signaling pathway | Aflibercept | IL-6 | Clinical | STAT3 inhibitor | [53] |
| Ponatinib | IL-6/1L-11/EGF | Clinical | STAT3 inhibitor | [54] | |
| Napabucasin | IL-6 | Clinical | ROS and STAT3 inhibitor | [55] | |
| Convallatoxin | JAK2 | Under study | STAT3 inhibitor | [56] | |
| Wnt signaling pathway | Vitamin D | VDR | Clinical | Mxd1/Mad1 inducer and c-Myc inhibitor | [57,58,59] |
| SAH-BCL9 | β-catenin/BCL-9 complex | Under study | β-catenin and Wnt inhibitor | [60] | |
| Notch signaling pathway | Berberine | Notch1 | Clinical | Notch1 inhibitor | [61] |
| ABL001 | DLL4 and Notch1 | Under study | DLL4/ NOTCH and VEGF/VEGFR inhibitor | [62] | |
| Quercetin | Notch1 and γ-secretase | Under study | Notch1 and γ-secretase inhibitor | [63] |
3. NF-κB Signaling Pathway in CRC
3.1. Introduction to the NF-κB Signaling Pathway
NF-κB subunits in mammals are divided into five molecules: p65/RelA, RelB, c-Rel/Rel, NF-κB1 (p50 and its precursor p105), and NF-κB2 (p52 and its precursor p100) [64,65]. The NF-κB pathway has two distinct but interacting branches: the canonical pathway, which is activated by tumor necrosis factor-α (TNF-α), Toll-like receptor ligands, interleukin-1 (IL-1) and angiotensin II, and the non-canonical pathway, which is activated by the TNF superfamily members B cell-activating factor (BAFF), CD40, receptor-activated NF-κB ligand (RANKL), lymphotoxin β, and RNA viruses [49,66]. The transcriptional activity of NF-κB requires the formation of homo- or heterodimeric protein complexes [64], such as p65:p50 heterodimers and p52:RelB heterodimers; the former acts on the canonical pathway, while the latter participates in the non-canonical pathway [67].
In general, the inhibitor IκB in the cytoplasm of most resting cells binds to NF-κB, covers the nuclear localization sequence (NLS) of NF-κB, blocks DNA binding and nuclear localization, and leads to its inactivation in the cytoplasm. When stimulated by extracellular stimuli such as bacteria, viruses, and cytokines, the IκB kinase (IKK) complex upregulates and phosphorylates IκB, contributing to the degradation of IκB in the proteasome [66,67,68]. NF-κB is subsequently exposed to the NLS, enters the nucleus and binds to a specific DNA sequence, triggering downstream gene expression [66]. However, for the non-canonical NF-κB signaling pathway, the key to its activation lies in the transformation of p100 to p52 and the formation of heterodimers with RelB, which depends on NF-κB-inducing kinase (NIK) and IKKα-mediated phosphorylation of p100 [65,67,68].
Activation of the NF-κB signaling pathway is closely associated with the progression of CRC, such as cell proliferation, apoptosis, angiogenesis, and metastasis. Therefore, NF-κB is usually considered a therapeutic target.
3.2. Factors Promoting CRC Angiogenesis by the NF-κB Signaling Pathway
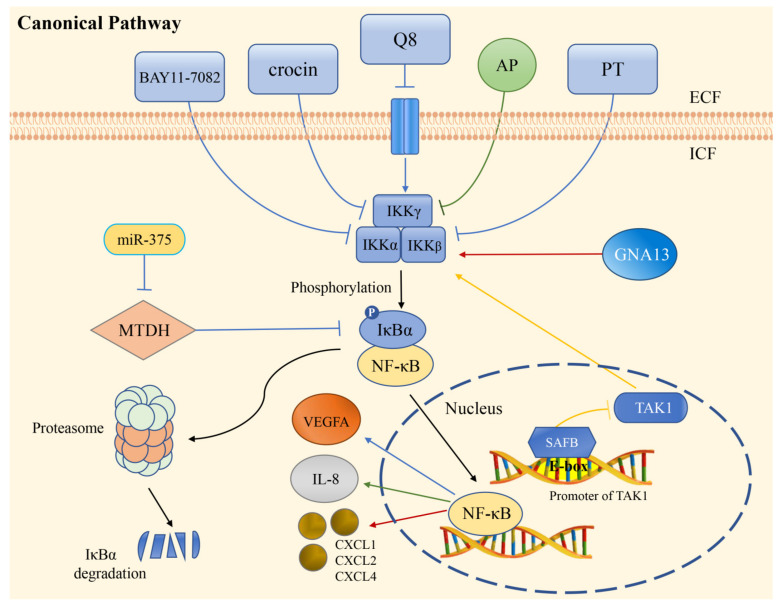
Angiogenesis plays an important role in the migration and invasion of solid tumors. The NF-κB pathway regulates the expression of many angiogenesis regulatory factors, such as VEGF, PDGF-BB, MMP-2, MMP-9, CXCL1, CXCL8, IL-8, and COX-2, ultimately regulating tumor angiogenesis [9,49]. A recent study validated that the overexpression of B7-H3 increased the activity of NF-κB through a luciferase reporter assay and found that the expression of VEGFA in B7-H3-induced CRC cells was regulated by the NF-κB pathway [9]. In summary, B7-H3 induced VEGFA expression by activating the NF-κB pathway, which ultimately promoted CRC angiogenesis. CXCL5 belongs to the CXC-type chemokine family, and studies showed that CXCL5 regulated the expression of FOXD1 and VEGFA and promoted tumor angiogenesis by activating the AKT/NF-κB pathway [69]. In addition, activation of the AKT/NF-κB pathway might be involved in CCR6-mediated tumor angiogenesis, thereby promoting the secretion of VEGFA [70]. Previous studies reported that protein phosphatase of regenerating liver-3 (PRL-3) might be associated with triggering angiogenesis and establishing the microvascular system [71]. In addition, PRL-3 in cancer cells could upregulate the secretion of IL-6 and IL-8 through MAPK signals acting on tumor-associated macrophages. Finally, the NF-κB pathway was activated to promote angiogenesis in CRC cells [72]. Overexpression of GNA13 has also been shown to upregulate the expression of the chemokines CXCL1, CXCL2, and CXCL4 by activating NF-κB/p65, thus playing an important role in promoting angiogenesis in CRC (Figure 2) [73].
Figure 2.
Regulation of angiogenesis via the canonical NF-κB pathway in CRC. The extracellular compounds BAY11-7082, crocin, Q8, and PT inhibit the expression of VEGFA by suppressing the phosphorylation of IκB. While GNA13 promotes angiogenesis through the regulation of chemokines production. AP restrains IL-8 production and angiogenesis by inhibiting the degradation of IκB, then prevents NF-κB from entering the nucleus. SAFB targets the promoter of TAK1 in the nucleus and inhibits angiogenesis. The expression of miR-375 can downregulate MTDH level, leading to the inhibitory effect of IκBα on NF-κB. Q8: IUPAC name (E)-2-(2-quinolin-2-yl-vinyl)-benzene-1,4-diol HCl; AP: Andrographolide; PT: Parthenolide; IKK: IκB kinase complex; SAFB: scaffold attachment factor B; MTDH: metadherin.
3.3. Factors Inhibiting CRC Angiogenesis by the NF-κB Signaling Pathway
It is common knowledge that members of the IκB family are closely related to the activity of NF-κB as inhibitory factors. As prototypical IκB proteins, IκBα and IκBβ could block the NF-κB pathway-mediated angiogenesis in CRC [51,52,74]. Kinases in the NF-κB signaling pathway, such as IKK and NIK, are essential for NF-κB to promote angiogenesis. Therefore, when the activity of these kinases is inhibited, the above process will be restrained. A study investigated that the specific inhibition of NF-κB activity by IKK1/2 siRNA could reduce the expression of c-Myc and further inhibit angiogenesis [75]. Similarly, NIK-targeting siRNA could inhibit NIK mediated non-canonical NF-κB pathway and play the role of inhibiting angiogenesis [76]. Besides, the NEMO-binding domain peptide, which is an amino-terminal α-helical region of NEMO, could block the association of NEMO (also known as IKKγ) with IKKβ and inhibit the activity of NF-κB involved in angiogenesis [77].
There are several factors that have effects against NF-κB-mediated angiogenesis in different ways. Scaffold attachment factor B (SAFB) is a transcriptional suppressor in CRC progression. It has been demonstrated in vitro and in vivo that SAFB can inhibit TAK1 activity by targeting the first E-box of its promoter, leading to the inhibition of the NF-κB signaling pathway involved in CRC invasion, metastasis, and angiogenesis (Figure 2) [74]. As a microRNA overexpressed in CRC, miR-375 could directly target metadherin (MTDH), upregulate IκBα expression, downregulate p65:p50 heterodimers levels, and thereby served to suppress angiogenesis [78].
In addition, some inhibitors of NF-κB have a similar effect of suppressing angiogenesis in CRC. PDTC could inhibit CXCL5-dependent induction of FOXD1 and VEGFA expression [69]. BAY11-7082 could significantly reduce p-IKKα and p-p65 levels, thereby inhibiting VEGFA expression [9,72]. SN50 served to downregulate the expression of MMP7 and suppress angiogenesis in CRC by blocking the NF-κB pathway [79].
3.4. Anti-Angiogenic Therapy of the NF-κB Signaling Pathway
It has been reported that imatinib could activate different intracellular signaling pathways, thereby breaking the feedback loop between proinflammatory cytokines and transcription factors (NF-κB, JAK3/STAT3). Imatinib also appeared to inhibit the coordination of proinflammatory cytokines by intracellular signaling, which was also involved in the upregulation of angiogenic factors in CRC (Table 1) [48]. As a natural dietary product, curcumin can be used as a chemosensitizer to inhibit angiogenesis in most cancer cells and is a promising approach for the treatment of CRC [80]. A study showed that curcumin inhibited tumors by influencing angiogenesis regulators and other molecular bases through NF-κB and then served as an antiangiogenic therapeutic pathway [50].
Prevention of angiogenesis by NF-κB-specific inhibitors has been the core of anticancer therapy. Andrographolide (AP), a natural phytochemical found in Andrographis paniculata, antagonized IL-8 induced by TNF-α by inhibiting NADPH oxidase/ROS/NF-κB and other signaling pathways, which led to the inhibition of angiogenesis in the tumor microenvironment (Table 1) [51]. Parthenolide (PT) was also found to be a NF-κB inhibitor that significantly inhibited hypoxia-dependent HIF-1α activity and angiogenesis by inhibiting NF-κB activation (Figure 2) [52]. In addition, piperine, a natural alkaloidal pungent product presented in pepper plants, has been demonstrated to modify enzymes and transcription factors activity to inhibit angiogenesis, invasion, and metastasis [81]. It could suppress the expression of IL-8 stimulated by lithocholic acid through inhibiting the transcriptional activity of NF-κB, then affecting the activity of CRC angiogenesis [82].
4. JAK-STAT Signaling Pathway in CRC
4.1. Introduction to the JAK-STAT Signaling Pathway
The JAK/STAT signaling pathway plays a critical role in various aspects of CRC, especially angiogenesis in CRC. This pathway consists of tyrosine kinase-associated receptors, JAK and STAT, which are coupled to activate. By binding with tyrosine kinase-associated receptors, over 40 different cytokines or growth factors can induce the STAT3 signaling pathway. The JAK family proteins include four members, JAK1, JAK2, JAK3, and TYK2, related to the cytoplasmic regions of tyrosine kinase-associated receptors [83]. STAT family proteins have seven members: STAT1, STAT2, STAT3, STAT4, STAT5, STAT6, and STAT7. STAT family proteins have dual functions of signal transduction and transcriptional activation [84].
The end of the active STAT signaling pathway enables a succession of gene expression changes, which then participate in biological processes, including proliferation, angiogenesis, and metastasis [85,86,87,88]. When cytokines (IL-6, IL-11, IFN, etc.) bind to their corresponding ligands, the receptors and JAK are aggregated, and the adjacent JAK is activated by mutual phosphorylation. After JAK is activated, STAT1/3/5 are activated through phosphorylation [89]. Then, STAT1/3/5 expose the NLS, enter the nucleus and in turn activate STAT1/3/5-mediated transcription of genes. When this pathway is upregulated, it can lead to angiogenesis in CRC [90,91]. It has been reported that activation of the IL-6/STAT3 pathway downregulates the expression of genes to promote tumor angiogenesis. A study suggested that the blockade of proangiogenic signaling significantly reduced colorectal tumor growth in mice with constitutive STAT3 activation in COLVI+ fibroblasts [92]. Activation of STAT3 in tumor-associated fibroblasts promotes angiogenesis in CRC. In addition, STAT2 activates the oncogenic STAT3 signaling pathway to promote CRC [93].
4.2. Factors Promoting CRC Angiogenesis by the JAK/STAT Signaling Pathway
Promoting factors in the JAK/STAT signaling pathway play a very important role in the regulation of CRC angiogenesis. These biologically active molecules include cytokines (chemokines), various proteins, and lncRNAs (Figure 3). The agonists IL-6/IL-6R, EGF/EGFR and IGF/IGFR are three major ligand/receptor systems that drive the JAK/STAT pathway in CRC [94,95,96]. It has been reported that there is increased production of IL-6 in tumor tissues and in the serum of patients with CRC. Several studies have shown that IL-11, which has similar cellular mechanism as IL-6, is also a central regulator of STAT3 activation and angiogenesis [92].
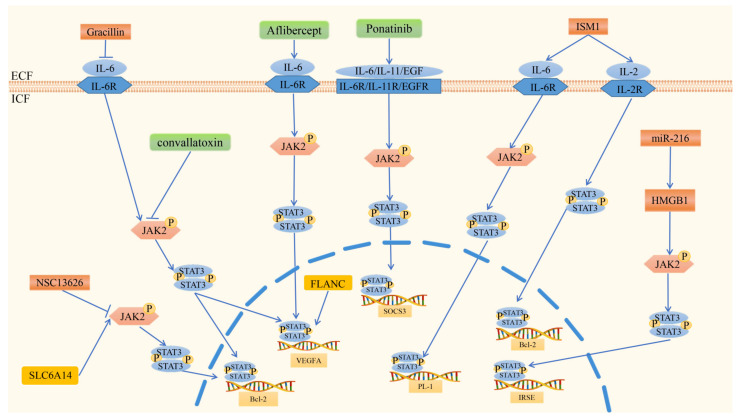
Figure 3.
Regulation of angiogenesis via the JAK2/STAT3 signaling pathway in CRC. In the JAK2/STAT3 signaling pathway, SLC6A14 and FLANC promote angiogenesis. SLC6A14 activates JAK2 through phosphorylation, after which STAT3 is activated by mutual phosphorylation, and STAT3 then enters the nucleus and activates the transcription of Bcl-2. FLANC enters the nucleus directly and activates the transcription of VEGFA. Factors inhibiting CRC angiogenesis via JAK2/STAT3 signaling include ISM1, NSC13626, Gracillin, and miR-216. ISM1 activates the transcription of Bcl-2 via the IL2/STAT5 signaling pathway and activates PL-1 via the IL6/JAK2/STAT3 signaling pathway. NSC13626 has a negative feedback mechanism with JAK2, and the downstream target of JAK2 is p-STAT3. Gracillin inhibits the transcription of Bcl-2 and VEGFA via the IL6/JAK2/STAT3 signaling pathway. MiR-216 inhibits angiogenesis by targeting HMGB1, which mediates the JAK2/STAT3 pathway. Aflibercept, convallatoxin, and ponatinib are antiangiogenic drugs in CRC. Aflibercept modulates inflammation-related angiogenesis via the IL-6/JAK2/STAT3 axis, ultimately regulating the transcription of VEGFA. CNT also shows efficacy in the inhibition of JAK2/STAT3 to inhibit the expression of Bcl-2 and VEGFA. Ponatinib inhibits JAK2/STAT3 activity driven by EGF/EGFR, IL-6/IL-6R, and IL-11/IL-11R, regulating the transcription of SOCS3.
Solute carrier family 6 member 14 (SLC6A14) expression, which is low in normal human cells, is subject to the change of the JAK2/STAT3 pathway in CRC [97,98]. SLC6A14 is overexpressed in CRC cells. The JAK2/STAT3 signaling pathway is substantially activated when SLC6A14 is overexpressed [98]. When SLC6A14 expression was blocked in vivo, researchers found that it protected against intestinal colitis-associated tumorigenesis, meaning that metastasis and angiogenesis of cancer were inhibited. Through further research, they concluded that SLC6A14 produces a manifest effect by the activation of the JAK2/STAT3 signaling pathway. In turn, it is feasible to inhibit JAK2/STAT3 signaling and reduce angiogenesis mediated by SLC6A14.
In terms of the lncRNA FLANC, there are connections with the JAK/STAT3 signaling pathway. FLANC is a primate-specific lncRNA residing within the first intron of Cadherin EGF LAG seven-pass G-type receptors 1 (CELSR1), which is weakly expressed in normal colon cells [99]. This gene encodes a protein that is one of the components of the cadherin superfamily [100]. In CRC cells, FLANC expression was much higher than that in normal colon cells [101]. Of note, FLANC is a significant promoting factor of angiogenesis in vitro and in vivo. Mechanistically, FLANC overexpression prolongs the half-life of phosphorylated STAT3 (pSTAT3 at Tyr705) and induces VEGFA transcription, which is a key regulator of angiogenesis [102,103,104].
4.3. Factors Inhibiting CRC Angiogenesis by the JAK/STAT Signaling Pathway
Various factors inhibiting angiogenesis via the JAK/STAT3 signaling pathway in CRC have been described. These include proteins, plant-based compounds, and RNAs (Figure 3).
Na+/H+ exchanger regulatory factor 2 (NHERF2) is an angiogenesis-inhibiting protein. The NHERF family of proteins are scaffolds that orchestrate the interaction of receptors and cellular proteins [105]. A study found that NHERF2 expression was increased in advanced-stage CRC to upregulate the phosphorylation of STAT3 through the IL-6-JAK-STAT3 pathway [106]. Notably, the absence of NHERF2 in vivo decreases STAT3 activation and tumor growth, which means that NHERF2 may be a potential target for cancer treatment. A relationship between the upregulation of Isthmin 1 (ISM1) in CRC and tumor angiogenesis was also described. A study by Yuhui Wu on the effect of ISM1 in CRC showed that ISM1 was highly associated with immune-related pathways, such as the IL-2/STAT5 and IL-6/JAK/STAT3 signaling pathways [107]. They also demonstrated that angiogenesis was significantly positively associated with ISM1 [108]. It was proven that compound NSC13626 inhibited CRC cell growth through a negative feedback mechanism with JAK and arrested the cell cycle in the S phase [109,110]. The downstream targets of JAK2 include STAT3 and STAT5, in which STAT3 signaling plays an important role in angiogenesis of CRC [111].
Certain plant-based compounds with an inhibitory influence on angiogenesis have also been described. Genistein, a chemopreventive phytochemical drug against CRC, has efficient interactions with STAT proteins. In vitro, significant suppression of cell proliferation and STAT3 protein expression has been shown after treatment with genistein [112]. The curcumin derivative 5Br-6b can inhibit the proliferation of CRC cells by blocking the activation of STAT3 and its target gene [113]. Gracillin exerts potent anticancer and antiangiogenic effects against CRC by inhibiting the IL-6/STAT3 pathway [114]. A study found that curcumin combined with (−)-epigallocatechin-3-gallate (EGCG) reduced tumor growth and angiogenesis by inhibiting the JAK/STAT3/IL-8 signaling pathway in CRC [115]. Noncoding RNAs are also responsible for modulating protein-coding gene expression related to this signaling. Upregulation of lncRNA RP11-468E2.5 interacts with STAT5 and STAT6 and inhibits the JAK/STAT signaling pathway to affect the progression of CRC [116]. MiR-216 is another tumor suppressor that inhibits angiogenesis by targeting high mobility group box 1 (HMGB1). In turn, HMGB1 is strongly expressed in CRC and mediates the JAK2/STAT3 pathway [117].
4.4. Anti-Angiogenic Therapy of the JAK/STAT Signaling Pathway
As previously stated, the STAT signaling molecule enters the nucleus to modulate the transcription of target genes, especially VEGFA. JAK/STAT signaling also offers potential sites for antiangiogenic therapy, such as JAK1, JAK2 [56], STAT1 [118], STAT3 [119], and STAT5. There are several antiangiogenic drugs that can act against these therapeutic targets. Aflibercept (Figure 3) (Table 1) modulates inflammation-related angiogenesis via the IL-6-STAT3 axis. A recent study showed that IL-6 expression generates a position feedback loop with VEGF [53]. Therefore, neutralization of VEGF with aflibercept decreases the activation of STAT3 and reduces IL-6 expression levels 24 h after treatment of HUVECs with the drug [120]. CNT also shows efficacy in the inhibition of the JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in CRC [56]. Interestingly, the current results reveal that crosstalk between the two signaling pathways can collaboratively regulate STAT3 activation and that CNT plays a role in this process. Ponatinib, as a lead candidate, inhibits STAT3 activity driven by EGF/EGFR, IL-6/IL-6R, and IL-11/IL-11R. Likewise, ponatinib inhibits CRC migration and tumor growth compared with control-treated mice [54]. Chemotherapy-based comprehensive treatment is the usual way to treat CRC [121]. Napabucasin is a chemoradio-sensitizer for CRC, but it inhibits angiogenesis through an ROS-mediated effect and alteration of STAT3 signaling [55].
5. Wnt Signaling Pathway in CRC
5.1. Introduction to the Wnt Signaling Pathway
The Wnt family is a group of proteins that act in many cellular functions including organ formation, stem cell renewal, and cell survival [122]. The gain or loss of function of the Wnt signaling pathway can result in angiogenesis and abnormal vascular development [123].
The Wnt signaling pathway is classified as canonical pathway and non-canonical pathways. In the canonical pathway, Wnt signaling is activated by binding to Wnt proteins to surface receptors composed of the seven transmembrane frizzled proteins and the low-density lipoprotein receptor-related protein 5/6 (LRP5/6). After binding, the cytoplasmic protein disheveled (Dvl) is activated. The activation of Dvl induces the dissociation of glycogen synthase kinase 3β (GSK-3β) from Axin and causes the inhibition of GSK-3β. In the Wnt signaling pathway, the level of β-catenin was controlled by the “destruction complex” composed of Axin, GSK3β, casein kinase 1α (CK1α), APC, etc. [124]. Because of the inactivation of the “destruction complex”, the phosphorylation and degradation of β-catenin was inhibited. Then, stabilized β-catenin was translocated into the nucleus and led to the transcription of target genes such as c-Myc and cyclin D1 [125]. Furthermore, the canonical Wnt signaling pathway is correlated with angiogenesis and vascular differentiation, which is important in vascular sprouting and network maturation.
The major non-canonical pathways contain Wnt/Ca2+ and Wnt/PCP pathways. In the Wnt/Ca2+ pathway, Wnt binds to Frizzled and activates Dvl, causes the release of Ca2+ from the endoplasmic reticulum, activates Ca2+ binding proteins including PKC and CamKII. Signal transduction activates the nuclear factor of activated T cells (NFAT) through Ca2+. The Wnt/PCP pathway is mediated by the GTPases RhoA and Ras and can exert effects on the cytoskeleton through the ROCK axis.
5.2. Factors Promoting CRC Angiogenesis by the Wnt Signaling Pathway
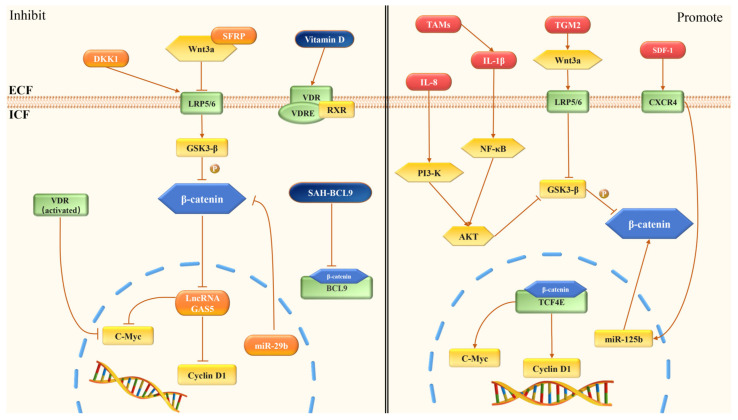
Many factors have been shown to promote CRC angiogenesis through the Wnt signaling pathway. Transglutaminase 2 (TGM2) is a novel molecular marker that is important for the therapy and prognosis of CRC. In CRC, the expression of TGM2 was higher than that in normal tissues [126]. TGM2 could promote angiogenesis and upregulate the expression of Wnt3a, β-catenin, and cyclin D1. When TGM2 was inhibited, the apoptosis of CRC cells was promoted and then inhibited the angiogenesis of cancer [127]. Transmembrane-4 L-six family member-1 (TM4SF1), the founding member of the TM4SF, is an antigen regulated by oncogenes [128]. TM4SF1 expression was higher in CRC tissues than in non-tumor tissues and was positively correlated with poor prognosis. TM4SF1 regulated SOX2 via the Wnt/β-catenin/c-Myc/SOX2 signaling pathway [129]. In addition, with the knockdown of TM4SF1, the expression of c-Myc and epithelial to mesenchymal transition (EMT) were suppressed [129].
Tumor associated macrophages (TAMs) had a leading position in the tumor microenvironment (TME), which was closely correlated with tumor initiation, progression, and metastasis [130]. TAMs could release plenty of cytokines, including IL-1β, CXCL-4, CXCL-8, and CXCL-12. These cytokines synergistically regulated endothelial cells, matrix remodeling, and vascularization in CRC angiogenesis [130]. IL-1β could inactivate GSK3β by inducing the phosphorylation of AKT and PDK1. This process enhanced TCF4/β-catenin transcriptional activity and activated Wnt target genes in CRC cells, such as c-Myc and c-Jun [131].
It has been shown that IL-8 (also known as CXCL-8) promotes the formation of the TME, which could facilitate the generation of tumor cells and the invasiveness of cancer by enhancing the level of arginase in myeloid-derived suppressor cells. Moreover, IL-8 could promote the AKT/GSK3β/β-catenin/MMP7 pathway in CRC by upregulating BCL-2 (Figure 4) [132]. CXCL-12 could activate CXC chemokine receptor 4 (CXCR-4), which was correlated with the invasion of CRC cells. The expression of CXCR-4 in CRC cells was much higher than that in normal tissues [133]. Further studies have shown that the activation of CXCL-12/CXCR-4 axis in vascular endothelial cells could stimulate the angiogenesis through the upregulation of the Wnt/β-catenin signaling pathway [134].
Figure 4.
Regulation of angiogenesis via the Wnt/β-catenin signaling pathway in CRC. DKK1 acts on LRP5/6. LRP5/6 causes the degradation of β-catenin. SFRPs combine with Wnt3a to inhibit the expression of β-catenin. LncRNA GAS5 reduces the expression of β-catenin, c-Myc, and cyclin D1. MiR-29b downregulates the expression of β-catenin. Vitamin D can activate VDR, and the activated VDR inhibits the expression of c-Myc. SAH-BCL9 dissociates native β-catenin/BCL9 complex and then suppresses Wnt transcription selectively. IL-8 acts on AKT/GSK3β/β-catenin, which promotes the Wnt/β-catenin pathway. TGM2 upregulates the expression of Wnt3a, β-catenin, and cyclin D1. TAMs release IL-1β, which acts on NF-κB/AKT/GSK3β and promotes Wnt signaling. SDF-1 activates CXCR4, which causes the expression of miR-125b and activates the Wnt/β-catenin signaling pathway.
5.3. Factors Inhibiting CRC Angiogenesis by the Wnt Signaling Pathway
Dickkopf-1 (DKK-1) is a secreted protein and an extracellular inhibitor of the Wnt signaling pathway [135]. DKK-1 bound to LRP5/6, and sequestered LRP5 away from the Frizzled/LRP6 complex, thereby inhibiting the transcription of TCF/LEF and the canonical Wnt signaling pathway (Figure 4) [135,136]. DKK-1 overexpression also downregulated the expression of VEGF and decreased the microvessel density [135]. Secreted frizzled-related proteins (SFRPs) are a series of extracellular inhibitors of the Wnt signaling pathway. SFRPs are bound to Wnt/β-catenin signaling by their cysteine-rich domains. For example, SFRP-1 bound to Wnt3a to block the Wnt signaling pathway and thereby inhibited the expression of β-catenin and c-Myc [137]. LncRNA GAS5 is considered to be effective in the inhibition of CRC by regulating the Wnt signaling pathway (Figure 4). Research on lncRNAs in CRC showed that oe-GAS5 expression could decrease the expression of β-catenin, c-Myc, and cyclin D1, which inhibited the angiogenesis of CRC [138].
Evidence suggests that the synthetic role of miR-29b was essential for the inhibition of CRC cells [139]. MiR-29b downregulated the expression of BCL9L by targeting the 3’UTR of BCL9L. BCL9L, TCF7L2, and Snail are coactivators of β-catenin. Thus, it was proposed that miR-29b inhibited the expression of many coactivators and downstream targets of CTNNB1/Wnt signaling in CRC cells. This caused the inhibition of the cell growth, tumor angiogenesis and EMT [140].
5.4. Anti-Angiogenic Therapy of the Wnt Signaling Pathway
Vitamin D (1,25(OH)2D3) has the potential as a therapy for CRC by inhibiting the angiogenesis through the Wnt pathway (Table 1). Vitamin D combines with vitamin D receptor (VDR), and VDR binds to retinoid X receptor (RXR). The VDR-RXR heterodimer bound to the vitamin D response element (VDRE) and participated in antineoplastic properties [57]. After being activated, VDR was associated with β-catenin and inhibited the expression of c-Myc, which was a downstream signal of the Wnt pathway [58]. With the lower expression of c-Myc, vitamin D suppressed the development of angiogenesis in CRC. In one research, for vitamin D vs. no vitamin D, the expression of β-catenin decreased by an estimated 3% (p = 0.41) in the full length of the colon crypts [141].
B-cell CLL/lymphoma 9 (BCL9) was regarded as a co-activator of the Wnt/β-catenin signaling pathway by participating in TCF-mediated transcription in CRC [142]. Stabilized Alpha-Helix of BCL9 (SAH-BCL9) was developed by Takada’s team in order to block the interaction with BCL9. It was reported that SAH-BCL9 had peptides from A to C and SAH-BCL9 peptide B (SAH-BCL9B) was the most effective. SAH-BCL9B could target β-catenin and dissociate native β-catenin/BCL9 complex selectively, then suppressed the transcription activity of Wnt (Table 1). By targeting the disruption of BCL9/β-catenin, it inhibited the proliferation, angiogenesis, and migration of CRC cells [60]. Due to the high binding of serum proteins, SAH-BCL9 peptides did not have pharmacokinetic properties conducive to clinical development. However, a novel β-catenin/BCL9 complex inhibitor E722-2648 is considered more effective than SAH-BCL9B [143]. In a previous study, E722-2648 could inhibit β-catenin/BCL9 complex formation and E722-2648 treatment could significantly reduce the tumor growth in the mice compared to the control group. It shows that E722-2648 may have more important implications for the development of novel therapies in CRC.
6. Notch Signaling Pathway in CRC
6.1. Introduction to the Notch Signaling Pathway
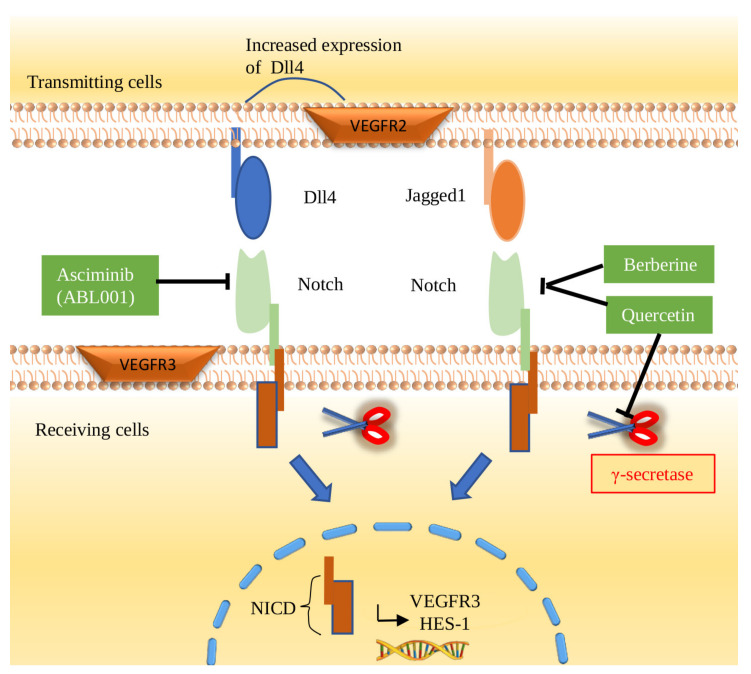
The Notch signaling pathway is one of the most important signaling modes that plays a part in physiological and tumor pathology. It is not only essential for differentiation, proliferation, and apoptosis, but also angiogenesis, tip/stalk cell selection, and arteriovenous specification [144,145]. There are a series of ligands and receptors in the Notch signaling pathway. Jagged-1, Jagged-2, Delta-like-1 (Dll1), Delta-like-3 (Dll3), and Delta-Like-4 (Dll4) are ligands of the Notch signaling pathway. Meanwhile, there are four receptors in this signaling pathway: Notch-1, -2, -3, and -4. Ligand receptor binding causes γ-secretase activation. As a result, the Notch intracellular domain (NICD) enters the nucleus and binds to the related transcription factors to affect downstream target genes (Figure 5). The abnormal function of the Notch signaling pathway in CRC depends on the overexpression of ligands and receptors compared with normal tissues [146,147].
Figure 5.
Regulation of angiogenesis via the Notch signaling pathway in CRC. Notch1 is a single transmembrane protein located on the cell surface that interacts with Dll4 and Jagged1 expressed on adjacent cells. As a result, γ-secretase is activated to split Notch to induce NICD into the nucleus. Then, VEGFR3 and HES-1 are upregulated and are involved in subsequent angiogenesis in CRC. As inhibitors of the Notch pathway, ABL001, berberine, and quercetin are considered potential therapeutic agents to inhibit angiogenesis in CRC.
6.2. Factors Promoting CRC Angiogenesis in the Notch Signaling Pathway
The Notch signaling pathway is regularly altered in many cancers, as well as CRC [148]. In the endothelial cells of CRC, the Notch signaling pathway can be activated by overexpression of associated ligands and receptors, such as Dll4 and Notch1 (Figure 5). In general, activation of the Notch signaling pathway has been shown to play a role in the development and angiogenesis of CRC [149]. Once the Notch signaling pathway is activated, NICD enters the nucleus, leading to upregulation of HECS-1 and VEGFR3, which promotes subsequent angiogenesis in CRC cells [150,151]. Several factors can promote angiogenesis through the Notch signaling pathway. HDAC5 is an important factor of the histone deacetylase (HDAC) family. HDAC5 activates the Notch signaling pathway and promotes angiogenesis of CRC cells by upregulating the expression of Dll4 [152]. Currently, it has been reported that leptin can induce the expression of Notch1-4/Jagged-1/Dll4, IL-1 and VEGF/VEGFR2. In CRC, leptin promotes angiogenesis through the Notch, IL-1, and leptin crosstalk outcome (NILCO) pathway [118].
6.3. Factors Inhibiting CRC Angiogenesis in the Notch Signaling Pathway
It is highly important to explore the factors that inhibit angiogenesis in CRC by inhibiting the Notch signaling pathway. γ-Secretase and Dll4 are the most common targets of small-molecule inhibitors or antibodies for the Notch signaling pathway blockade [153]. An increasing number of inhibitors and monoclonal antibodies against Dll4 have been discovered. α-Mangostin has been shown to have an antiproliferative effect on cancer cells. α-Mangostin-encapsulated PLGA nanoparticles (Mang-NPs) is formulated in order to enhance the biological effect of α-Mangostin. Current studies have shown that α-Mangostin and Mang-NPs could inhibit the Notch signaling pathway by inhibiting the expression of Notch1, Notch2 and their ligand Dll4 in CRC. As a result, α-Mangostin can be used for the treatment and prevention of CRC [154,155]. Selenium binding protein 1 (SELENBP1) is frequently downregulated in tumor vessels in CRC. A study found that SELENBP1 could inhibit angiogenesis by binding with Dll4 and antagonizing the Dll4/Notch1 signaling pathway in CRC, which made SELENBP1 a potential tumor suppressor [156]. In addition, Torin-1 is an inhibitor of mammalian target of rapamycin (mTOR), which has been considered an important regulator of cancer. As markers related to angiogenesis, Notch1 and Dll4 were significantly reduced in a Torin-1-treated group compared with a control group, suggesting that Torin-1 has an anti-angiogenesis effect by inhibiting the Notch signaling pathway. Therefore, Torin-1 is considered an effective candidate drug for metastatic CRC therapy [157]. Portulaca oleracea significantly downregulates the expression of the Notch1 and β-catenin genes in CRC. The results of this study showed that Portulaca oleracea extract inhibited the growth of CRC stem cells in a dose-dependent manner by inhibiting the Notch signaling pathway, thus playing an important role in preventing angiogenesis in CRC [158]. In addition, ethanol extracted from radix of Actinidia chinensis (EERAC) was also found to suppress the expression of Notch1 and Jagged1 to inhibit angiogenesis in CRC. Meanwhile, EERAC inhibits mastermind-like transcriptional coactivator 1 (MAML1), which can activate the Notch signaling pathway [159]. In general, more molecules blocking angiogenesis of CRC by inhibiting the Notch signaling pathway are expected.
6.4. Anti-Angiogenic Therapy of the Notch Signaling Pathway
Targeting the Notch signaling pathway to prevent angiogenesis in CRC is feasible (Figure 5). As a natural product, berberine (BBR) has been reported to treat diarrhea and gastroenteritis in the clinic. In recent years, BBR has been shown to have some anticancer effects [160]. For CRC, BBR inhibited the Notch signaling pathway by downregulating the expression of Notch1 in SW480 cells. Inhibition of Notch1 increased the expression of the tumor suppressor gene PTEN to inhibit CRC by regulating angiogenesis, transcription, translation, and cell cycle progression [61]. This suggested the significance of BBR as an existing drug in the treatment of CRC (Table 1) [161]. As a bispecific antibody, ABL001 has been reported to block the Dll4/Notch signaling pathway to play a superior anticancer role through an antiangiogenic effect [62]. Yana Li offered a therapeutic strategy for CRC treatment that combined quercetin (20 μM) and IR (5 Gy). Quercetin enhanced the radiosensitivity of CRC by regulating the related proteins. In CRC cells, this strategy reduced the expression of Notch1 and all five proteins of the γ-secretase complex to inhibit the Notch signaling pathway, thus inhibiting related angiogenesis [63]. Regarding the Notch signaling pathway, it is important to explore the antiangiogenic effects of drugs, and further explorations in the clinic are needed.
7. Crosstalk
Multiple pathways commonly interact during angiogenesis in CRC. Current studies found that the Notch signaling pathway was associated with the Wnt and VEGF signaling pathways in CRC angiogenesis. Many studies have suggested that the Wnt/β-catenin/TCF signaling pathway positively regulated Jagged1 expression to activate Notch expression in CRC [162]. As an angiogenesis-related gene, Jagged1 has been shown to be closely associated with poor prognosis of CRC by regulating blood supply and tumor growth [163]. In detail, Notch1-mediated control of phosphorylated β-catenin could negatively regulate the Wnt/β-catenin signaling pathway [164]. Dll4, an important ligand of the Notch signaling pathway, is a downstream molecule of the VEGF pathway [145]. It promoted the transformation of normal blood vessels into tumor vessels by upregulating VEGFR3 and downregulating VEGFR1. This also suggested the importance of Dll4 and Notch pathway-targeted therapy in CRC angiogenesis. Tan IIA could regulate CRC cells via the cyclooxygenase-2-Wnt/β-catenin signaling pathway. Tan IIA downregulated the level of cyclooxygenase-2 and activated the Wnt/β-catenin pathway, which could inhibit CRC and lower the expression of VEGF [165]. In addition, β-catenin could also combine with TCF/LEF and activate VEGF [166].
Several studies have confirmed that NF-κB could interact with VEGF to jointly promote angiogenesis. These interactions included the aforementioned B7-H3/NF-κB/ VEGFA axis and the AKT/NF-κB/FOXD1/VEGFA pathway [9,69]. Furthermore, IκBα could be degraded by calpain-2 as a calcium-activated cysteine endopeptidase, thereby translocating NF-κB to the nucleus and inducing VEGF [167]. In addition, the activation of the AKT/NF-κB pathway could promote the secretion of VEGFA [70]. The EGFR/AKT/NF-κB pathway was involved in promoting angiogenesis by stimulating the production of VEGFA and IL-8 [168]. Furthermore, CCR6 was a CCR chemokine receptor. The AKT/NF-κB pathway played an important role in CCR6-mediated tumor angiogenesis. The Wnt/β-catenin signaling pathway was also correlated with other signaling pathways in CRC cancer, especially active β-catenin. Cyclin-dependent kinase 8 module (CDK8) regulated several relevant signaling pathways, including the Wnt/β-catenin signaling pathway. The CDK8 module and its analog CDK19 affected downstream transcription factors such as NF-κB and transcribed target genes [169]. CRC angiogenesis could also be affected by the regulation of NF-κB and β-catenin through the PI3K/AKT/IKKα pathway [49].
In addition to the above, some compounds could influence interactions in multiple pathways. Enalapril could significantly enhance the sensitivity of CRC to 5-FU and its antitumor effect by inhibiting NF-κB/STAT3 regulatory protein, proliferation and angiogenesis [170]. Crocin could remarkably inhibit CRC cells metastasis and angiogenesis by blocking the TNF-α/NF-κB/VEGF pathway [171]. Moreover, curcumin and its analogs significantly inhibited VEGFA synthesis and secretion in cell lines, suggesting that the inhibition of NF-κB was associated with p-STAT3 expression [172].
8. Conclusions
In this review, we summarized the pathways that influence angiogenesis in CRC. The specific content includes the basic introduction of the pathway, mechanism, some promoting and inhibiting factors of the pathway, targeted therapy and crosstalk. This review introduced not only the interactions among signaling pathways and some drugs already in clinical use but also the natural compounds that affect the crosstalk of multiple signaling pathways, which have great value in the research of targeted therapy for signaling pathways. We anticipated that these natural compounds could lead to new directions in the treatment of CRC in the future. Although many studies have focused on the role of signaling pathways in CRC angiogenesis, further research is needed to find more effective therapeutic agents.
Abbreviations
| AP | Andrographolide |
| BAFF | B cell-activating factor |
| BBR | berberine |
| BCL9 | B-cell CLL/lymphoma 9 |
| bFGF | basic fibroblast growth factor |
| BPA | Bisphenol A |
| BRG1 | Brahma-related gene 1 |
| CCL19 | chemokine CC ligand 19 |
| CELSR1 | Cadherin EGF LAG seven-pass G-type receptors 1 |
| CK1α | casein kinase 1α |
| CRC | colorectal cancer |
| CTD | Cantharidin |
| CXCR4 | CXC chemokine receptor 4 |
| DKK-1 | Dickkopf-1 |
| Dll1 | Delta-like-1 |
| Dll3 | Delta-like-3 |
| Dll4 | Delta-Like-4 |
| Dvl | disheveled |
| EERAC | Ethanol extracted from radix of Actinidia chinensis |
| EMT | epithelial to mesenchymal transition |
| eNOS | endothelial nitric oxide synthases |
| Gab2 | Grb2-associated binder 2 |
| HMGB1 | High mobility group box 1 |
| HUVECs | human umbilical vein endothelial cells |
| IKK | IκB kinase complex |
| IL-1 | interleukin-1 |
| ISM1 | Isthmin 1 |
| JAK | janus kinase |
| LRP5/6 | low-density lipoprotein receptor-related protein 5/6 |
| MAML1 | mastermind like transcriptional coactivator 1 |
| Mang-NPs | α-Mangostin-encapsulated PLGA nanoparticles |
| MAPK | mitogen-activated protein kinase |
| MCRC | metastatic colorectal cancer |
| MIP-3B | macrophage inflammatory protein 3-β |
| MTDH | metadherin |
| NF-κB | Nuclear Factor-kappa B |
| NF90 | Nuclear Factor 90 |
| NHERF2 | Na+/H+ exchanger regulatory factor 2 |
| NICD | Notch intracellular domain |
| NIK | NF-κB-inducing kinase |
| NLS | nuclear localization sequence |
| PLGF | placental growth factor |
| PRL-3 | protein phosphatase of regenerating liver-3 |
| PT | Parthenolide |
| Q8 | (E)-2-(2-quinolin-2-yl-vinyl)-benzene-1, 4-diol HCl |
| RANKL | receptor-activated NF-κB ligand |
| ROS | reactive oxygen species |
| RXR | retinoid X receptor |
| SAFB | scaffold attachment factor B |
| SAH-BCL9 | Stabilized Alpha-Helix of BCL9 |
| SFRPs | Secreted frizzled-related proteins |
| SLC6A14 | Solute carrier family 6 member 14 |
| SRC | non-receptor protein tyrosine kinase |
| SRCIN1 | SRC kinase signaling inhibitor 1 |
| SRF | serum response factor |
| STAT | signal transducer and activator of transcription |
| Tan ⅡA | Tanshinone ⅡA |
| TAMs | Tumor associated macrophages |
| TGM2 | Transglutaminase 2 |
| TLR4 | Toll-like receptor 4 |
| TME | tumor microenvironment |
| TM4SF1 | Transmembrane-4 L-six family member-1 |
| TNF-α | tumor necrosis factor-α |
| VDR | vitamin D receptor |
| VDRE | vitamin D response element |
| VEGF | vascular endothelial growth factor |
| Wnt | Wingless and int-1 |
| 4’-HW | 4’-hydroxywogonin |
Author Contributions
Conceptualization, M.C., Y.W., G.L., H.Q., P.L. and J.L.; writing—original draft, M.C., Y.W., G.L., H.Q. and P.L.; writing—review and editing, M.C., X.D. and J.L.; supervision, J.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Natural Science Foundation of China (no. 81572972), the Supporting Plan of Scientific and Technological Innovation Team in Universities of Henan Province (no. 20IRTSTHN029), and the scientific and technological research project of Henan Province (no. 212102310250).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong A., Ma B.B. Personalizing Therapy for Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2014;12:139–144. doi: 10.1016/j.cgh.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Fearon E.R. Molecular Genetics of Colorectal Cancer. Annu. Rev. Pathol. Mech. Dis. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/nejm197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971;133:275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He L., Zhu W., Chen Q., Yuan Y., Wang Y., Wang J., Wu X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9:8206–8220. doi: 10.7150/thno.37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Kundu S., Feenstra T., Li X., Jin C., Laaniste L., Abu El Hassan T.E., Ohlin K.E., Yu D., Olofsson T., et al. Pleiotrophin promotes vascular abnormalization in gliomas and correlates with poor survival in patients with astrocytomas. Sci. Signal. 2015;8:ra125. doi: 10.1126/scisignal.aaa1690. [DOI] [PubMed] [Google Scholar]
- 7.Zhu P., Wu Y., Yang A., Fu X., Mao M., Liu Z. Catalpol suppressed proliferation, growth and invasion of CT26 colon cancer by inhibiting inflammation and tumor angiogenesis. Biomed. Pharmacother. 2017;95:68–76. doi: 10.1016/j.biopha.2017.08.049. [DOI] [PubMed] [Google Scholar]
- 8.Cao J., Liu X., Yang Y., Wei B., Li Q., Mao G., He Y., Li Y., Zheng L., Zhang Q., et al. Decylubiquinone suppresses breast cancer growth and metastasis by inhibiting angiogenesis via the ROS/p53/ BAI1 signaling pathway. Angiogenesis. 2020;23:325–338. doi: 10.1007/s10456-020-09707-z. [DOI] [PubMed] [Google Scholar]
- 9.Wang R., Ma Y., Zhan S., Zhang G., Cao L., Zhang X., Shi T., Chen W. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell Death Dis. 2020;11:55. doi: 10.1038/s41419-020-2252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W., Zhang X., Huang S., Chen J., Ding P., Wang Q., Li L., Lv X., Li L., Zhang P., et al. FOXM1D potentiates PKM2-mediated tumor glycolysis and angiogenesis. Mol. Oncol. 2021;15:1466–1485. doi: 10.1002/1878-0261.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garzon J.F.G., Pastrello C., Jurisica I., Hottiger M.O., Wenger R.H., Borsig L. Tumor cell endogenous HIF-1α activity induces aberrant angiogenesis and interacts with TRAF6 pathway required for colorectal cancer development. Neoplasia. 2020;22:745–758. doi: 10.1016/j.neo.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battaglin F., Puccini A., Intini R., Schirripa M., Ferro A., Bergamo F., Lonardi S., Zagonel V., Lenz H.-J., Loupakis F. The role of tumor angiogenesis as a therapeutic target in colorectal cancer. Expert Rev. Anticancer Ther. 2018;18:251–266. doi: 10.1080/14737140.2018.1428092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicklin D.J., Ellis L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology. 2005;69((Suppl. S3)):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 15.Claesson-Welsh L., Welsh M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013;273:114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 16.Canavese M., Ngo D.T., Maddern G.J., Hardingham J.E., Price T.J., Hauben E. Biology and therapeutic implications of VEGF-A splice isoforms and single-nucleotide polymorphisms in colorectal cancer. Int. J. Cancer. 2017;140:2183–2191. doi: 10.1002/ijc.30567. [DOI] [PubMed] [Google Scholar]
- 17.Stefani C., Miricescu D., Stanescu-Spinu I.-I., Nica R.I., Greabu M., Totan A.R., Jinga M. Growth Factors, PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021;22:10260. doi: 10.3390/ijms221910260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ntellas P., Mavroeidis L., Gkoura S., Gazouli I., Amylidi A.-L., Papadaki A., Zarkavelis G., Mauri D., Karpathiou G., Kolettas E., et al. Old Player-New Tricks: Non Angiogenic Effects of the VEGF/VEGFR Pathway in Cancer. Cancers. 2020;12:3145. doi: 10.3390/cancers12113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khodabakhsh F., Merikhian P., Eisavand M.R., Farahmand L. Crosstalk between MUC1 and VEGF in angiogenesis and metastasis: A review highlighting roles of the MUC1 with an emphasis on metastatic and angiogenic signaling. Cancer Cell Int. 2021;21:200. doi: 10.1186/s12935-021-01899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimna A., Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015;2015:549412. doi: 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masoud G.N., Li W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan J., Li H., Luo X., Hu J., Wang G. BRG1 promotes VEGF-A expression and angiogenesis in human colorectal cancer cells. Exp. Cell Res. 2017;360:236–242. doi: 10.1016/j.yexcr.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Ding C.-B., Yu W.-N., Feng J.-H., Luo J.-M. Structure and function of Gab2 and its role in cancer (Review) Mol. Med. Rep. 2015;12:4007–4014. doi: 10.3892/mmr.2015.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams S.J., Aydin I.T., Celebi J.T. GAB2—a Scaffolding Protein in Cancer. Mol. Cancer Res. 2012;10:1265–1270. doi: 10.1158/1541-7786.MCR-12-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding C., Luo J., Fan X., Li L., Li S., Wen K., Feng J., Wu G. Elevated Gab2 induces tumor growth and angiogenesis in colorectal cancer through upregulating VEGF levels. J. Exp. Clin. Cancer Res. 2017;36:56. doi: 10.1186/s13046-017-0524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun W., Wang X., Li J., You C., Lu P., Feng H., Kong Y., Zhang H., Liu Y., Jiao R., et al. MicroRNA-181a promotes angiogenesis in colorectal cancer by targeting SRCIN1 to promote the SRC/VEGF signaling pathway. Cell Death Dis. 2018;9:438. doi: 10.1038/s41419-018-0490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S.I., Shah A.N., Zhang J., Gallick G.E. Regulation of angiogenesis and vascular permeability by Src family kinases: Opportunities for therapeutic treatment of solid tumors. Expert Opin. Ther. Targets. 2007;11:1207–1217. doi: 10.1517/14728222.11.9.1207. [DOI] [PubMed] [Google Scholar]
- 28.Chan D.A., Sutphin P.D., Denko N.C., Giaccia A.J. Role of Prolyl Hydroxylation in Oncogenically Stabilized Hypoxia-inducible Factor-1α. J. Biol. Chem. 2002;277:40112–40117. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Yang M., Meng F., Zhang Y., Wang M., Guo X., Yang J., Zhang H., Zhang H., Sun J., et al. SRSF3 Promotes Angiogenesis in Colorectal Cancer by Splicing SRF. Front. Oncol. 2022;12:810610. doi: 10.3389/fonc.2022.810610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng X., Ma Y.-F., Zhang X.-R., Li Y., Zhao H.-H., Han S.-G. Circ_0056618 promoted cell proliferation, migration and angiogenesis through sponging with miR-206 and upregulating CXCR4 and VEGF-A in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4190–4202. doi: 10.26355/eurrev_202004_20999. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z., Zhu C., Chen C., Zong Y., Feng H., Liu D., Feng W., Zhao J., Lu A. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018;9:974. doi: 10.1038/s41419-018-1010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malami I., Muhammad A., Abubakar I.B., Etti I.C., Waziri P.M., Abubakar R.M., Mshelia H.E. 5,6-dehydrokawain from the rhizome of Alpinia mutica Roxb. induced proangiogenic tumour-derived VEGF of HT-29 colorectal cancer. Nat. Prod. Res. 2018;32:2964–2967. doi: 10.1080/14786419.2017.1392954. [DOI] [PubMed] [Google Scholar]
- 33.Xia T., Guo J., Zhang B., Song C., Zhao Q., Cui B., Liu Y. Bisphenol A Promotes the Progression of Colon Cancer Through Dual-Targeting of NADPH Oxidase and Mitochondrial Electron-Transport Chain to Produce ROS and Activating HIF-1α/VEGF/PI3K/AKT Axis. Front. Endocrinol. 2022;13:933051. doi: 10.3389/fendo.2022.933051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q., Zhu Y., Wei X., Zhou J., Chang L., Sui H., Han Y., Piao D., Sha R., Bai Y. MiR-590-5p inhibits colorectal cancer angiogenesis and metastasis by regulating nuclear factor 90/vascular endothelial growth factor A axis. Cell Death Dis. 2016;7:e2413. doi: 10.1038/cddis.2016.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai H., Miao Z., Chen Y., Huang C., Yeh Y., Yang I., Wang J. miR-148a inhibits early relapsed colorectal cancers and the secretion of VEGF by indirectly targeting HIF-1α under non-hypoxia/hypoxia conditions. J. Cell. Mol. Med. 2019;23:3572–3582. doi: 10.1111/jcmm.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karpuz T., Araz M., Korkmaz L., Kılınc I., Findik S., Karaagaç M., Eryilmaz M.K., Artac M. The Prognostic Value of Serum Semaphorin3A and VEGF Levels in Patients with Metastatic Colorectal Cancer. J. Gastrointest. Cancer. 2020;51:491–497. doi: 10.1007/s12029-019-00263-4. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-Lago N., Chucla T.C., De Castro B.A., Ponte R.V., Rendo C.R., Rodriguez M.I.G.-R., Diaz S.S., Suarez B.G., de la Camara Gomez J., Fernández F.B., et al. Efficacy, safety and prognostic factors in patients with refractory metastatic colorectal cancer treated with trifluridine/tipiracil plus bevacizumab in a real-world setting. Sci. Rep. 2022;12:14612. doi: 10.1038/s41598-022-18871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denda T., Sakai D., Hamaguchi T., Sugimoto N., Ura T., Yamazaki K., Fujii H., Kajiwara T., Nakajima T.E., Takahashi S., et al. Phase II trial of aflibercept with FOLFIRI as a second-line treatment for Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019;110:1032–1043. doi: 10.1111/cas.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ju M., Cheng H., Qu K., Lu X. Efficacy and safety of ramucirumab treatment in patients with advanced colorectal cancer: A protocol for systematic review and meta analysis. Medicine. 2020;99:e20618. doi: 10.1097/MD.0000000000020618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T., Liu J., Xiao X.-Q. Cantharidin inhibits angiogenesis by suppressing VEGF-induced JAK1/STAT3, ERK and AKT signaling pathways. Arch. Pharmacal Res. 2015;38:282–289. doi: 10.1007/s12272-014-0383-8. [DOI] [PubMed] [Google Scholar]
- 41.Pan R., Dai Y., Gao X.-H., Lu D., Xia Y.-F. Inhibition of vascular endothelial growth factor-induced angiogenesis by scopoletin through interrupting the autophosphorylation of VEGF receptor 2 and its downstream signaling pathways. Vasc. Pharmacol. 2011;54:18–28. doi: 10.1016/j.vph.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Tabana Y.M., Hassan L.E.A., Ahamed M.B.K., Dahham S.S., Iqbal M.A., Saeed M.A., Khan S.S., Sandai D., Majid A.S.A., Oon C.E., et al. Scopoletin, an active principle of tree tobacco (Nicotiana glauca) inhibits human tumor vascularization in xenograft models and modulates ERK1, VEGF-A, and FGF-2 in computer model. Microvasc. Res. 2016;107:17–33. doi: 10.1016/j.mvr.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Yesudhas D., Gosu V., Anwar M.A., Choi S. Multiple Roles of Toll-Like Receptor 4 in Colorectal Cancer. Front. Immunol. 2014;5:334. doi: 10.3389/fimmu.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen M., Zhong K., Tan J., Meng M., Liu C.M., Chen B., Huang C., Wong H.L.X., Bian Z., Su T., et al. Baicalein is a novel TLR4-targeting therapeutics agent that inhibits TLR4/HIF-1α/VEGF signaling pathway in colorectal cancer. Clin. Transl. Med. 2021;11:e564. doi: 10.1002/ctm2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu W.-H., Chan G.K.-L., Duan R., Wang H.-Y., Kong X.-P., Dong T.T.-X., Tsim K.W.-K. Synergy of Ginkgetin and Resveratrol in Suppressing VEGF-Induced Angiogenesis: A Therapy in Treating Colorectal Cancer. Cancers. 2019;11:1828. doi: 10.3390/cancers11121828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L., Sui H., Wang T., Jia R., Zhang Z., Fu J., Feng Y., Liu N., Ji Q., Wang Y., et al. Tanshinone IIA reduces secretion of pro-angiogenic factors and inhibits angiogenesis in human colorectal cancer. Oncol. Rep. 2020;43:1159–1168. doi: 10.3892/or.2020.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Z.-H., Peng J.-H., Zhang R.-X., Wang F., Sun H.-P., Fang Y.-J., Wan D.-S., Pan Z.-Z. Dihydroartemisinin inhibits colon cancer cell viability by inducing apoptosis through up-regulation of PPARγ expression. Saudi J. Biol. Sci. 2017;25:372–376. doi: 10.1016/j.sjbs.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhiman D.K., Sanyal S.N., Vaish V. Imatinib exhibit synergistic pleiotropy in the prevention of colorectal cancer by suppressing proinflammatory, cell survival and angiogenic signaling. Cell. Signal. 2020;76:109803. doi: 10.1016/j.cellsig.2020.109803. [DOI] [PubMed] [Google Scholar]
- 49.Patel M., Horgan P.G., McMillan D.C., Edwards J. NF-κB pathways in the development and progression of colorectal cancer. Transl. Res. 2018;197:43–56. doi: 10.1016/j.trsl.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Ahmed B., Mehta K., Kurzrock R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007;6:1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 51.Yuan M., Meng W., Liao W., Lian S. Andrographolide Antagonizes TNF-α-Induced IL-8 via Inhibition of NADPH Oxidase/ROS/NF-κB and Src/MAPKs/AP-1 Axis in Human Colorectal Cancer HCT116 Cells. J. Agric. Food Chem. 2018;66:5139–5148. doi: 10.1021/acs.jafc.8b00810. [DOI] [PubMed] [Google Scholar]
- 52.Kim S.L., Park Y.R., Lee S.T., Kim S.-W. Parthenolide suppresses hypoxia-inducible factor-1α signaling and hypoxia induced epithelial-mesenchymal transition in colorectal cancer. Int. J. Oncol. 2017;51:1809–1820. doi: 10.3892/ijo.2017.4166. [DOI] [PubMed] [Google Scholar]
- 53.Chang Q., Bournazou E., Sansone P., Berishaj M., Gao S.P., Daly L., Wels J., Theilen T., Granitto S., Zhang X., et al. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia. 2013;15:848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan F.H., Putoczki T.L., Lou J., Hinde E., Hollande F., Giraud J., Stylli S.S., Paradiso L., Zhu H.-J., Sieber O.M., et al. Ponatinib Inhibits Multiple Signaling Pathways Involved in STAT3 Signaling and Attenuates Colorectal Tumor Growth. Cancers. 2018;10:526. doi: 10.3390/cancers10120526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagaraju G.P., Farran B., Farren M., Chalikonda G., Wu C., Lesinski G.B., El-Rayes B.F. Napabucasin (BBI 608), a potent chemoradiosensitizer in rectal cancer. Cancer. 2020;126:3360–3371. doi: 10.1002/cncr.32954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z.H., Li M.Y., Wang Z., Zuo H.X., Wang J.Y., Xing Y., Jin C., Xu G., Piao L., Piao H., et al. Convallatoxin promotes apoptosis and inhibits proliferation and angiogenesis through crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in colorectal cancer. Phytomedicine. 2020;68:153172. doi: 10.1016/j.phymed.2020.153172. [DOI] [PubMed] [Google Scholar]
- 57.Klampfer L. Vitamin D and colon cancer. World J. Gastrointest. Oncol. 2014;6:430–437. doi: 10.4251/wjgo.v6.i11.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salehi-Tabar R., Nguyen-Yamamoto L., Tavera-Mendoza L.E., Quail T., Dimitrov V., An B.-S., Glass L., Goltzman D., White J.H. Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc. Natl. Acad. Sci. USA. 2012;109:18827–18832. doi: 10.1073/pnas.1210037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berger M.D., Stintzing S., Heinemann V., Cao S., Yang D., Sunakawa Y., Matsusaka S., Ning Y., Okazaki S., Miyamoto Y., et al. A Polymorphism within the Vitamin D Transporter Gene Predicts Outcome in Metastatic Colorectal Cancer Patients Treated with FOLFIRI/Bevacizumab or FOLFIRI/Cetuximab. Clin. Cancer Res. 2018;24:784–793. doi: 10.1158/1078-0432.CCR-17-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takada K., Zhu D., Bird G.H., Sukhdeo K., Zhao J.-J., Mani M., Lemieux M., Carrasco D.E., Ryan J., Horst D., et al. Targeted Disruption of the BCL9/β-Catenin Complex Inhibits Oncogenic Wnt Signaling. Sci. Transl. Med. 2012;4:148ra117. doi: 10.1126/scitranslmed.3003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luongo F., Colonna F., Calapà F., Vitale S., Fiori M.E., De Maria R. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers. 2019;11:1076. doi: 10.3390/cancers11081076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeom D.-H., Lee Y.-S., Ryu I., Lee S., Sung B., Lee H.-B., Kim D., Ahn J.-H., Ha E., Choi Y.-S., et al. ABL001, a Bispecific Antibody Targeting VEGF and DLL4, with Chemotherapy, Synergistically Inhibits Tumor Progression in Xenograft Models. Int. J. Mol. Sci. 2020;22:241. doi: 10.3390/ijms22010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y., Wang Z., Jin J., Zhu S.-X., He G.-Q., Li S.-H., Wang J., Cai Y. Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. Biochem. Biophys. Res. Commun. 2020;523:947–953. doi: 10.1016/j.bbrc.2020.01.048. [DOI] [PubMed] [Google Scholar]
- 64.Tago K., Funakoshi-Tago M., Ohta S., Kawata H., Saitoh H., Horie H., Aoki-Ohmura C., Yamauchi J., Tanaka A., Matsugi J., et al. Oncogenic Ras mutant causes the hyperactivation of NF-κB via acceleration of its transcriptional activation. Mol. Oncol. 2019;13:2493–2510. doi: 10.1002/1878-0261.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dejardin E., Droin N., Delhase M., Haas E., Cao Y., Makris C., Li Z.-W., Karin M., Ware C.F., Green D.R. The Lymphotoxin-β Receptor Induces Different Patterns of Gene Expression via Two NF-κB Pathways. Immunity. 2002;17:525–535. doi: 10.1016/S1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 66.Sakowicz A., Bralewska M., Pietrucha T., Habrowska-Górczyńska D.E., Piastowska-Ciesielska A.W., Gach A., Rybak-Krzyszkowska M., Witas P.J., Huras H., Grzesiak M., et al. Canonical, Non-Canonical and Atypical Pathways of Nuclear Factor кb Activation in Preeclampsia. Int. J. Mol. Sci. 2020;21:5574. doi: 10.3390/ijms21155574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji B., Zhang Y., Zhen C., Fagan M.J., Yang Q. Mathematical modeling of canonical and non-canonical NF-κB pathways in TNF stimulation. Comput. Methods Programs Biomed. 2020;196:105677. doi: 10.1016/j.cmpb.2020.105677. [DOI] [PubMed] [Google Scholar]
- 68.Vatsyayan J., Qing G., Xiao G., Hu J. SUMO1 modification of NF-κB2/p100 is essential for stimuli-induced p100 phosphorylation and processing. EMBO Rep. 2008;9:885–890. doi: 10.1038/embor.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen C., Xu Z.-Q., Zong Y.-P., Ou B.-C., Shen X.-H., Feng H., Zheng M.-H., Zhao J.-K., Lu A.-G. CXCL5 induces tumor angiogenesis via enhancing the expression of FOXD1 mediated by the AKT/NF-κB pathway in colorectal cancer. Cell Death Dis. 2019;10:178. doi: 10.1038/s41419-019-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu C.-C., Chen C., Xu Z.-Q., Zhao J.-K., Ou B.-C., Sun J., Zheng M.-H., Zong Y.-P., Lu A.-G. CCR6 promotes tumor angiogenesis via the AKT/NF-κB/VEGF pathway in colorectal cancer. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2018;1864:387–397. doi: 10.1016/j.bbadis.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 71.Guo K., Li J., Wang H., Osato M., Tang J.P., Quah S.Y., Gan B.Q., Zeng Q. PRL-3 Initiates Tumor Angiogenesis by Recruiting Endothelial Cells In vitro and In vivo. Cancer Res. 2006;66:9625–9635. doi: 10.1158/0008-5472.CAN-06-0726. [DOI] [PubMed] [Google Scholar]
- 72.Zhang T., Liu L., Lai W., Zeng Y., Xu H., Lan Q., Su P., Chu Z. Interaction with tumorassociated macrophages promotes PRL3induced invasion of colorectal cancer cells via MAPK pathwayinduced EMT and NFkappaB signalinginduced angiogenesis. Oncol. Rep. 2019;41:2790–2802. doi: 10.3892/or.2019.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z., Tan X., Luo J., Cui B., Lei S., Si Z., Shen L., Yao H. GNA13 promotes tumor growth and angiogenesis by upregulating CXC chemokines via the NF-κB signaling pathway in colorectal cancer cells. Cancer Med. 2018;7:5611–5620. doi: 10.1002/cam4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Jiao H.-L., Ye Y.-P., Yang R.-W., Sun H.-Y., Wang S.-Y., Wang Y.-X., Xiao Z.-Y., He L.-Q., Cai J.-J., Wei W.-T., et al. Downregulation of SAFB Sustains the NF-κB Pathway by Targeting TAK1 during the Progression of Colorectal Cancer. Clin. Cancer Res. 2017;23:7108–7118. doi: 10.1158/1078-0432.CCR-17-0747. [DOI] [PubMed] [Google Scholar]
- 75.Wu T., Wang G., Chen W., Zhu Z., Liu Y., Huang Z., Huang Y., Du P., Yang Y., Liu C.-Y., et al. Co-inhibition of BET proteins and NF-κB as a potential therapy for colorectal cancer through synergistic inhibiting MYC and FOXM1 expressions. Cell Death Dis. 2018;9:315. doi: 10.1038/s41419-018-0354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maracle C.X., Jeucken K.C., Helder B., Van Gulik T.M., Steins A., Van Laarhoven H.W., Tas S.W. Silencing NIK potentiates anti-VEGF therapy in a novel 3D model of colorectal cancer angiogenesis. Oncotarget. 2018;9:28445–28455. doi: 10.18632/oncotarget.25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakamoto K., Maeda S., Hikiba Y., Nakagawa H., Hayakawa Y., Shibata W., Yanai A., Ogura K., Omata M. Constitutive NF-κB Activation in Colorectal Carcinoma Plays a Key Role in Angiogenesis, Promoting Tumor Growth. Clin. Cancer Res. 2009;15:2248–2258. doi: 10.1158/1078-0432.CCR-08-1383. [DOI] [PubMed] [Google Scholar]
- 78.Han S.-H., Mo J.-S., Park W.-C., Chae S.-C. Reduced microRNA 375 in colorectal cancer upregulates metadherin-mediated signaling. World J. Gastroenterol. 2019;25:6495–6507. doi: 10.3748/wjg.v25.i44.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao Y., Nan X., Shi X., Mu X., Liu B., Zhu H., Yao B., Liu X., Yang T., Hu Y., et al. SREBP1 promotes the invasion of colorectal cancer accompanied upregulation of MMP7 expression and NF-κB pathway activation. BMC Cancer. 2019;19:685. doi: 10.1186/s12885-019-5904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karthika C., Hari B., Rahman H., Akter R., Najda A., Albadrani G.M., Sayed A.A., Akhtar M.F., Abdel-Daim M.M. Multiple strategies with the synergistic approach for addressing colorectal cancer. Biomed. Pharmacother. 2021;140:111704. doi: 10.1016/j.biopha.2021.111704. [DOI] [PubMed] [Google Scholar]
- 81.Manayi A., Nabavi S.M., Setzer W.N., Jafari S. Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies. Curr. Med. Chem. 2019;25:4918–4928. doi: 10.2174/0929867324666170523120656. [DOI] [PubMed] [Google Scholar]
- 82.Li S., Nguyen T.T., Ung T.T., Sah D.K., Park S.Y., Lakshmanan V.-K., Jung Y.D. Piperine Attenuates Lithocholic Acid-Stimulated Interleukin-8 by Suppressing Src/EGFR and Reactive Oxygen Species in Human Colorectal Cancer Cells. Antioxidants. 2022;11:530. doi: 10.3390/antiox11030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haan C., Kreis S., Margue C., Behrmann I. Jaks and cytokine receptors—An intimate relationship. Biochem. Pharmacol. 2006;72:1538–1546. doi: 10.1016/j.bcp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 84.Fan Y., Mao R., Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du W., Hong J., Wang Y.-C., Zhang Y.-J., Wang P., Su W.-Y., Lin Y.-W., Lu R., Zou W.-P., Xiong H., et al. Inhibition of JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via mitochondrial pathway. J. Cell. Mol. Med. 2012;16:1878–1888. doi: 10.1111/j.1582-4934.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei C., Yang C., Wang S., Shi D., Zhang C., Lin X., Liu Q., Dou R., Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang L., Zhang R., Yang J., Bi T., Zhou S. FKBP14 Promotes The Proliferation And Migration Of Colon Carcinoma Cells Through Targeting IL-6/STAT3 Signaling Pathway. OncoTargets Ther. 2019;12:9069–9076. doi: 10.2147/OTT.S222555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kortylewski M., Kujawski M., Wang T., Wei S., Zhang S., Pilon-Thomas S., Niu G., Kay H., Mulé J., Kerr W., et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 89.Heinrich P.C., Behrmann I., Haan S., Hermanns H.M., Müller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen S.-H., Murphy D., Lassoued W., Thurston G., Feldman M.D., Lee W.M. Activated STAT3 is a mediator and biomarker of VEGF endothelial activation. Cancer Biol. Ther. 2008;7:1994–2003. doi: 10.4161/cbt.7.12.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aoki Y., Feldman G.M., Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 92.Heichler C., Scheibe K., Schmied A., Geppert C.I., Schmid B., Wirtz S., Thoma O.-M., Kramer V., Waldner M.J., Büttner C., et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69:1269–1282. doi: 10.1136/gutjnl-2019-319200. [DOI] [PubMed] [Google Scholar]
- 93.Gamero A.M., Young M.R., Mentor-Marcel R., Bobe G., Scarzello A.J., Wise J., Colburn N.H. STAT2 Contributes to Promotion of Colorectal and Skin Carcinogenesis. Cancer Prev. Res. 2010;3:495–504. doi: 10.1158/1940-6207.CAPR-09-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu L.-Q., Zhang L., Zhang J., Chang G.-L., Liu G., Yu D.-D., Yu X.-M., Zhao M.-S., Ye B. Evodiamine inhibits high-fat diet-induced colitis-associated cancer in mice through regulating the gut microbiota. J. Integr. Med. 2021;19:56–65. doi: 10.1016/j.joim.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Zhao F.-L., Qin C.-F. EGF promotes HIF-1α expression in colorectal cancer cells and tumor metastasis by regulating phosphorylation of STAT3. Eur. Rev. Med. Pharmacol. Sci. 2019;23:1055–1062. doi: 10.26355/eurrev_201902_16993. [DOI] [PubMed] [Google Scholar]
- 96.Yao C., Su L., Shan J., Zhu C., Liu L., Liu C., Xu Y., Yang Z., Bian X., Shao J., et al. IGF/STAT3/NANOG/Slug Signaling Axis Simultaneously Controls Epithelial-Mesenchymal Transition and Stemness Maintenance in Colorectal Cancer. Stem Cells. 2016;34:820–831. doi: 10.1002/stem.2320. [DOI] [PubMed] [Google Scholar]
- 97.Gupta N., Miyauchi S., Martindale R.G., Herdman A.V., Podolsky R., Miyake K., Mager S., Prasad P.D., Ganapathy M.E., Ganapathy V. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2005;1741:215–223. doi: 10.1016/j.bbadis.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 98.Mao H., Sheng J., Jia J., Wang C., Zhang S., Li H., He F. Aberrant SLC6A14 Expression Promotes Proliferation and Metastasis of Colorectal Cancer via Enhancing the JAK2/STAT3 Pathway. OncoTargets Ther. 2021;14:379–392. doi: 10.2147/OTT.S288709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Awan H.M., Shah A., Rashid F., Shan G. Primate-specific Long Non-coding RNAs and MicroRNAs. Genom. Proteom. Bioinform. 2017;15:187–195. doi: 10.1016/j.gpb.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tatin F., Taddei A., Weston A., Fuchs E., Devenport D., Tissir F., Makinen T. Planar Cell Polarity Protein Celsr1 Regulates Endothelial Adherens Junctions and Directed Cell Rearrangements during Valve Morphogenesis. Dev. Cell. 2013;26:31–44. doi: 10.1016/j.devcel.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pichler M., Rodriguez-Aguayo C., Nam S.Y., Dragomir M.P., Bayraktar R., Anfossi S., Knutsen E., Ivan C., Fuentes-Mattei E., Kil Lee S., et al. Therapeutic potential of FLANC, a novel primate-specific long non-coding RNA in colorectal cancer. Gut. 2020;69:1818–1831. doi: 10.1136/gutjnl-2019-318903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niu G., Wright K.L., Huang M., Song L., Haura E., Turkson J., Zhang S., Wang T., Sinibaldi D., Coppola D., et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 103.Apte R.S., Chen D.S., Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shah M.Y., Ferracin M., Pileczki V., Chen B., Redis R., Fabris L., Zhang X., Ivan C., Shimizu M., Rodriguez-Aguayo C., et al. Cancer-associated rs6983267 SNP and its accompanying long noncoding RNA CCAT2 induce myeloid malignancies via unique SNP-specific RNA mutations. Genome Res. 2018;28:432–447. doi: 10.1101/gr.225128.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ardura J.A., Friedman P.A. Regulation of G Protein-Coupled Receptor Function by Na+/H+ Exchange Regulatory Factors. Pharmacol. Rev. 2011;63:882–900. doi: 10.1124/pr.110.004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoshida M., Zhao L., Grigoryan G., Shim H., He P., Yun C.C. Deletion of Na+/H+ exchanger regulatory factor 2 represses colon cancer progress by suppression of Stat3 and CD24. Am. J. Physiol. Gastrointest Liver Physiol. 2016;310:G586–G598. doi: 10.1152/ajpgi.00419.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi H., Liu C., Tan H., Li Y., Nguyen T.-L.M., Dhungana Y., Guy C., Vogel P., Neale G., Rankin S., et al. Hippo Kinases Mst1 and Mst2 Sense and Amplify IL-2R-STAT5 Signaling in Regulatory T Cells to Establish Stable Regulatory Activity. Immunity. 2018;49:899–914.e6. doi: 10.1016/j.immuni.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Y., Liang X., Ni J., Zhao R., Shao S., Lu S., Han W., Yu L. Effect of ISM1 on the Immune Microenvironment and Epithelial-Mesenchymal Transition in Colorectal Cancer. Front. Cell Dev. Biol. 2021;9:681240. doi: 10.3389/fcell.2021.681240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Santos F.P., Verstovsek S. JAK2 inhibitors: What’s the true therapeutic potential? Blood Rev. 2011;25:53–63. doi: 10.1016/j.blre.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin T.E., Huangfu W.-C., Chao M.-W., Sung T.-Y., Chang C.-D., Chen Y.-Y., Hsieh J.-H., Tu H.-J., Huang H.-L., Pan S.-L., et al. A Novel Selective JAK2 Inhibitor Identified Using Pharmacological Interactions. Front. Pharmacol. 2018;9:1379. doi: 10.3389/fphar.2018.01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X., Hu F., Li G., Li G., Yang X., Liu L., Zhang R., Zhang B., Feng Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018;9:25. doi: 10.1038/s41419-017-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dariya B., Muppala S., Srivani G., Momin S., Alam A., Saddala M.S. Targeting STAT proteins via computational analysis in colorectal cancer. Mol. Cell. Biochem. 2020;476:165–174. doi: 10.1007/s11010-020-03893-6. [DOI] [PubMed] [Google Scholar]
- 113.Liu Z., Wang H., Guan L., Chen S., Lai M. A novel small molecular STAT3 inhibitor, 5Br-6b, induces apoptosis and inhibits migration in colorectal cancer cells. Anti-Cancer Drugs. 2018;29:402–410. doi: 10.1097/CAD.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 114.Yang L., Zhu T., Ye H., Shen Y., Li Z., Chen L., Wang C., Chen X., Zhao H., Xiang Y., et al. Gracillin shows potent efficacy against colorectal cancer through inhibiting the STAT3 pathway. J. Cell. Mol. Med. 2020;25:801–812. doi: 10.1111/jcmm.16134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jin G., Yang Y., Liu K., Zhao J., Chen X., Liu H., Bai R., Li X., Jiang Y., Zhang X., et al. Combination curcumin and (−)-epigallocatechin-3-gallate inhibits colorectal carcinoma microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway. Oncogenesis. 2017;6:e384. doi: 10.1038/oncsis.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang L., Zhao X.-H., Mao Y.-L., Wang J.-F., Zheng H.-J., You Q.-S. Long non-coding RNA RP11-468E2.5 curtails colorectal cancer cell proliferation and stimulates apoptosis via the JAK/STAT signaling pathway by targeting STAT5 and STAT6. J. Exp. Clin. Cancer Res. 2019;38:465. doi: 10.1186/s13046-019-1428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen X., Liu X., He B., Pan Y., Sun H., Xu T., Hu X., Wang S. MiR-216b functions as a tumor suppressor by targeting HMGB1-mediated JAK2/STAT3 signaling way in colorectal cancer. Am. J. Cancer Res. 2017;7:2051–2069. [PMC free article] [PubMed] [Google Scholar]
- 118.Erkasap N., Ozyurt R., Ozkurt M., Erkasap S., Yasar F., Ihtiyar E., Ciftci E., Canaz F., Colak E. Role of Notch, IL-1 and leptin expression in colorectal cancer. Exp. Ther. Med. 2021;21:600. doi: 10.3892/etm.2021.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu F., Sun X., Li G., Wu Q., Chen Y., Yang X., Luo X., Hu J., Wang G. Inhibition of SIRT2 limits tumour angiogenesis via inactivation of the STAT3/VEGFA signalling pathway. Cell Death Dis. 2018;10:9. doi: 10.1038/s41419-018-1260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Latifi-Navid H., Soheili Z., Samiei S., Sadeghi M., Taghizadeh S., Pirmardan E.R., Ahmadieh H. Network analysis and the impact of Aflibercept on specific mediators of angiogenesis in HUVEC cells. J. Cell. Mol. Med. 2021;25:8285–8299. doi: 10.1111/jcmm.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peeters M., Price T., Cervantes A., Sobrero A.F., Ducreux M., Hotko Y., André T., Chan E., Lordick F., Punt C.J., et al. Randomized Phase III Study of Panitumumab With Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) Compared With FOLFIRI Alone As Second-Line Treatment in Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2010;28:4706–4713. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 122.Croce J.C., McClay D.R. Evolution of the Wnt Pathways. Methods Mol. Biol. 2008;469:3–18. doi: 10.1007/978-1-60327-469-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bian J., Dannappel M., Wan C., Firestein R. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer. Cells. 2020;9:2125. doi: 10.3390/cells9092125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nong J., Kang K., Shi Q., Zhu X., Tao Q., Chen Y.-G. Phase separation of Axin organizes the β-catenin destruction complex. J. Cell Biol. 2021;220:e202012112. doi: 10.1083/jcb.202012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Krishnamurthy N., Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miyoshi N., Ishii H., Mimori K., Tanaka F., Hitora T., Tei M., Sekimoto M., Doki Y., Mori M. TGM2 Is a Novel Marker for Prognosis and Therapeutic Target in Colorectal Cancer. Ann. Surg. Oncol. 2009;17:967–972. doi: 10.1245/s10434-009-0865-y. [DOI] [PubMed] [Google Scholar]
- 127.Yang P., Yu D., Zhou J., Zhuang S., Jiang T. TGM2 interference regulates the angiogenesis and apoptosis of colorectal cancer via Wnt/β-catenin pathway. Cell Cycle. 2019;18:1122–1134. doi: 10.1080/15384101.2019.1609831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ye L., Pu C., Tang J., Wang Y., Wang C., Qiu Z., Xiang T., Zhang Y., Peng W. Transmembrane-4 L-six family member-1 (TM4SF1) promotes non-small cell lung cancer proliferation, invasion and chemo-resistance through regulating the DDR1/Akt/ERK-mTOR axis. Respir. Res. 2019;20:106. doi: 10.1186/s12931-019-1071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang Q., Chen J., Di Z., Yuan W., Zhou Z., Liu Z., Han S., Liu Y., Ying G., Shu X., et al. TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer. J. Exp. Clin. Cancer Res. 2020;39:232. doi: 10.1186/s13046-020-01690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang H., Tian T., Zhang J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021;22:8470. doi: 10.3390/ijms22168470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaler P., Godasi B.N., Augenlicht L., Klampfer L. The NF-κB/AKT-dependent Induction of Wnt Signaling in Colon Cancer Cells by Macrophages and IL-1β. Cancer Microenviron. 2009;2:69–80. doi: 10.1007/s12307-009-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumar A., Cherukumilli M., Mahmoudpour S.H., Brand K., Bandapalli O.R. ShRNA-mediated knock-down of CXCL8 inhibits tumor growth in colorectal liver metastasis. Biochem. Biophys. Res. Commun. 2018;500:731–737. doi: 10.1016/j.bbrc.2018.04.144. [DOI] [PubMed] [Google Scholar]
- 133.Hu T.-H., Yao Y., Yu S., Han L.-L., Wang W.-J., Guo H., Tian T., Ruan Z.-P., Kang X.-M., Wang J., et al. SDF-1/CXCR4 promotes epithelial–mesenchymal transition and progression of colorectal cancer by activation of the Wnt/β-catenin signaling pathway. Cancer Lett. 2014;354:417–426. doi: 10.1016/j.canlet.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 134.Song Z.-Y., Wang F., Cui S.-X., Qu X.-J. Knockdown of CXCR4 Inhibits CXCL12-Induced Angiogenesis in HUVECs through Downregulation of the MAPK/ERK and PI3K/AKT and the Wnt/β-Catenin Pathways. Cancer Investig. 2018;36:10–18. doi: 10.1080/07357907.2017.1422512. [DOI] [PubMed] [Google Scholar]
- 135.Liu Z., Sun B., Qi L., Li Y., Zhao X., Zhang D., Zhang Y. Dickkopf-1 expression is down-regulated during the colorectal adenoma-carcinoma sequence and correlates with reduced microvessel density and VEGF expression. Histopathology. 2015;67:158–166. doi: 10.1111/his.12474. [DOI] [PubMed] [Google Scholar]
- 136.Sadeghi S., Poorebrahim M., Rahimi H., Karimipoor M., Azadmanesh K., Khorramizadeh M.R., Teimoori-Toolabi L. In silico studying of the whole protein structure and dynamics of Dickkopf family members showed that N-terminal domain of Dickkopf 2 in contrary to other Dickkopfs facilitates its interaction with low density lipoprotein receptor related protein 5/6. J. Biomol. Struct. Dyn. 2019;37:2564–2580. doi: 10.1080/07391102.2018.1491891. [DOI] [PubMed] [Google Scholar]
- 137.Lavergne E., Hendaoui I., Coulouarn C., Ribault C., Leseur J., Eliat P.-A., Mebarki S., Corlu A., Clément B., Musso O. Blocking Wnt signaling by SFRP-like molecules inhibits in vivo cell proliferation and tumor growth in cells carrying active β-catenin. Oncogene. 2011;30:423–433. doi: 10.1038/onc.2010.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song J., Shu H., Zhang L., Xiong J. Long noncoding RNA GAS5 inhibits angiogenesis and metastasis of colorectal cancer through the Wnt/β-catenin signaling pathway. J. Cell. Biochem. 2019;120:6937–6951. doi: 10.1002/jcb.27743. [DOI] [PubMed] [Google Scholar]
- 139.Amin A.R.M.R., Poudyal D., Cui X., Le P.M., Hofseth A.B., Windust A., Nagarkatti M., Nagarkatti P.S., Schetter A.J., Harris C.C., et al. A Key Role of microRNA-29b for the Suppression of Colon Cancer Cell Migration by American Ginseng. PLoS ONE. 2013;8:e75034. doi: 10.1371/journal.pone.0075034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Subramanian M., Rao S.R., Thacker P., Chatterjee S., Karunagaran D. MiR-29b downregulates canonical Wnt signaling by targeting BCL9L and other coactivators of β-catenin in human colorectal cancer cells. J. Cell. Biochem. 2014;115:1974–1984. doi: 10.1002/jcb.24869. [DOI] [PubMed] [Google Scholar]
- 141.Liu S., Barry E.L., Baron J.A., Rutherford R.E., Seabrook M.E., Bostick R.M. Effects of supplemental calcium and vitamin D on the APC/β-catenin pathway in the normal colorectal mucosa of colorectal adenoma patients. Mol. Carcinog. 2016;56:412–424. doi: 10.1002/mc.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.De la Roche M., Worm J., Bienz M. The function of BCL9 in Wnt/β-catenin signaling and colorectal cancer cells. BMC Cancer. 2008;8:199. doi: 10.1186/1471-2407-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tanton H., Sewastianik T., Seo H.-S., Remillard D., Pierre R.S., Bala P., Aitymbayev D., Dennis P., Adler K., Geffken E., et al. A novel β-catenin/BCL9 complex inhibitor blocks oncogenic Wnt signaling and disrupts cholesterol homeostasis in colorectal cancer. Sci. Adv. 2022;8:eabm3108. doi: 10.1126/sciadv.abm3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Previs R.A., Coleman R.L., Harris A.L., Sood A.K. Molecular Pathways: Translational and Therapeutic Implications of the Notch Signaling Pathway in Cancer. Clin. Cancer Res. 2015;21:955–961. doi: 10.1158/1078-0432.CCR-14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Caporarello N., Lupo G., Olivieri M., Cristaldi M., Cambria M.T., Salmeri M., Anfuso C.D. Classical VEGF, Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions. Mol. Med. Rep. 2017;16:4393–4402. doi: 10.3892/mmr.2017.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jubb A.M., Browning L., Campo L., Turley H., Steers G., Thurston G., Harris A.L., Ansorge O. Expression of vascular Notch ligands Delta-like 4 and Jagged-1 in glioblastoma. Histopathology. 2012;60:740–747. doi: 10.1111/j.1365-2559.2011.04138.x. [DOI] [PubMed] [Google Scholar]
- 147.Tiwari A., Saraf S., Verma A., Panda P., Jain S.K. Novel targeting approaches and signaling pathways of colorectal cancer: An insight. World J. Gastroenterol. 2018;24:4428–4435. doi: 10.3748/wjg.v24.i39.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanchez-Vega F., Mina M., Armenia J., Chatila W.K., Luna A., La K.C., Dimitriadoy S., Liu D.L., Kantheti H.S., Saghafinia S., et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. 2018;173:321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li G., Zhou Z., Zhou H., Zhao L., Chen N., Chen H., Zou H., Qi Y., Jia W., Pang L. The expression profile and clinicopathological significance of Notch1 in patients with colorectal cancer: A meta-analysis. Futur. Oncol. 2017;13:2103–2118. doi: 10.2217/fon-2017-0178. [DOI] [PubMed] [Google Scholar]
- 150.Ranganathan P., Weaver K.L., Capobianco A.J. Notch signalling in solid tumours: A little bit of everything but not all the time. Nat. Cancer. 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 151.Benedito R., Rocha S.F., Woeste M., Zamykal M., Radtke F., Casanovas O., Duarte A., Pytowski B., Adams R.H. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF–VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 152.He P., Liang J., Shao T., Guo Y., Hou Y., Li Y. HDAC5 promotes colorectal cancer cell proliferation by up-regulating DLL4 expression. Int. J. Clin. Exp. Med. 2015;8:6510–6516. [PMC free article] [PubMed] [Google Scholar]
- 153.Perez-Fidalgo J.A., Ortega B., Simon S., Samartzis E.P., Boussios S. NOTCH signalling in ovarian cancer angiogenesis. Ann. Transl. Med. 2020;8:1705. doi: 10.21037/atm-20-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boinpelly V.C., Verma R.K., Srivastav S., Srivastava R.K., Shankar S. α-Mangostin-encapsulated PLGA nanoparticles inhibit colorectal cancer growth by inhibiting Notch pathway. J. Cell. Mol. Med. 2020;24:11343–11354. doi: 10.1111/jcmm.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jo M.K., Moon C.M., Kim E.J., Kwon J.-H., Fei X., Kim S.-E., Jung S.-A., Kim M., Mun Y.-C., Ahn Y.-H., et al. Suppressive effect of α-mangostin for cancer stem cells in colorectal cancer via the Notch pathway. BMC Cancer. 2022;22:341. doi: 10.1186/s12885-022-09414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang X., Hong R., Bei L., Yang J., Zhao X., Hu Z., Chen L., Meng H., Zhang Q., Niu G., et al. Selenium binding protein 1 inhibits tumor angiogenesis in colorectal cancers by blocking the Delta-like ligand 4/Notch1 signaling pathway. Transl. Oncol. 2022;18:101365. doi: 10.1016/j.tranon.2022.101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Francipane M.G., Lagasse E. Selective targeting of human colon cancer stem-like cells by the mTOR inhibitor Torin-1. Oncotarget. 2013;4:1948–1962. doi: 10.18632/oncotarget.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jin H., Chen L., Wang S., Chao D. Portulaca oleracea extract can inhibit nodule formation of colon cancer stem cells by regulating gene expression of the Notch signal transduction pathway. Tumor Biol. 2017;39:1010428317708699. doi: 10.1177/1010428317708699. [DOI] [PubMed] [Google Scholar]
- 159.Hu W., Wu C., Yuan C., Chen M., Jin C., Zheng C. Ethanol Extracted from Radix of Actinidia Chinensis Inhibits Human Colon Tumor Through Inhibiting Notch-signaling Pathway. J. Cancer. 2021;12:622–629. doi: 10.7150/jca.51275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Farooqi A.A., Qureshi M.Z., Khalid S., Attar R., Martinelli C., Sabitaliyevich U.Y., Nurmurzayevich S.B., Taverna S., Poltronieri P., Xu B. Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy. Cancers. 2019;11:478. doi: 10.3390/cancers11040478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li G., Zhang C., Liang W., Zhang Y., Shen Y., Tian X. Berberine regulates the Notch1/PTEN/PI3K/AKT/mTOR pathway and acts synergistically with 17-AAG and SAHA in SW480 colon cancer cells. Pharm. Biol. 2021;59:21–30. doi: 10.1080/13880209.2020.1865407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sugiyama M., Oki E., Nakaji Y., Tsutsumi S., Ono N., Nakanishi R., Sugiyama M., Nakashima Y., Sonoda H., Ohgaki K., et al. High expression of the Notch ligand Jagged-1 is associated with poor prognosis after surgery for colorectal cancer. Cancer Sci. 2016;107:1705–1716. doi: 10.1111/cas.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Scherer D., Deutelmoser H., Balavarca Y., Toth R., Habermann N., Buck K., Kap E.J., Botma A., Seibold P., Jansen L., et al. Polymorphisms in the Angiogenesis-Related Genes EFNB2, MMP2 and JAG1 are Associated with Survival of Colorectal Cancer Patients. Int. J. Mol. Sci. 2020;21:5395. doi: 10.3390/ijms21155395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kwon C., Qian L., Cheng P., Nigam V., Arnold J., Srivastava D. A regulatory pathway involving Notch1/β-catenin/Isl1 determines cardiac progenitor cell fate. Nat. Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma S., Lei Y., Zhang L., Wang J. Research on the inhibiting effect of tanshinone IIA on colon cancer cell growth via COX-2-Wnt/beta-catenin signaling pathway. J. BUON. 2018;23:1337–1342. [PubMed] [Google Scholar]
- 166.Wu C., Chen J., Chen C., Wang W., Wen L., Gao K., Chen X., Xiong S., Zhao H., Li S. Wnt/β-catenin coupled with HIF-1α/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Sci. Rep. 2015;5:16151. doi: 10.1038/srep16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Butler C.T., Reynolds A.L., Tosetto M., Dillon E.T., Guiry P.J., Cagney G., O’Sullivan J., Kennedy B.N. A Quininib Analogue and Cysteinyl Leukotriene Receptor Antagonist Inhibits Vascular Endothelial Growth Factor (VEGF)-independent Angiogenesis and Exerts an Additive Antiangiogenic Response with Bevacizumab. J. Biol. Chem. 2017;292:3552–3567. doi: 10.1074/jbc.M116.747766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chung S.-Y., Chao T.-C., Su Y. The Stemness-High Human Colorectal Cancer Cells Promote Angiogenesis by Producing Higher Amounts of Angiogenic Cytokines via Activation of the Egfr/Akt/Nf-κB Pathway. Int. J. Mol. Sci. 2021;22:1355. doi: 10.3390/ijms22031355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen M., Liang J., Ji H., Yang Z., Altilia S., Hu B., Schronce A., McDermott M.S.J., Schools G.P., Lim C.-U., et al. CDK8/19 Mediator kinases potentiate induction of transcription by NFκB. Proc. Natl. Acad. Sci. USA. 2017;114:10208–10213. doi: 10.1073/pnas.1710467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang Y., Ma L., Xu Y., Liu Y., Li W., Cai J., Zhang Y. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins. Cell Death Dis. 2020;11:477. doi: 10.1038/s41419-020-2675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bakshi H.A., Quinn G.A., Nasef M.M., Mishra V., Aljabali A.A.A., El-Tanani M., Serrano-Aroca A., Da Silva M.W., McCarron P.A., Tambuwala M.M. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways. Cells. 2022;11:1502. doi: 10.3390/cells11091502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rajitha B., Nagaraju G.P., Shaib W.L., Alese O.B., Snyder J.P., Shoji M., Pattnaik S., Alam A., El-Rayes B.F. Novel synthetic curcumin analogs as potent antiangiogenic agents in colorectal cancer. Mol. Carcinog. 2016;56:288–299. doi: 10.1002/mc.22492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.