Abstract
For environmental applications, nanosized TiO2-based materials are known as the most important photocatalyst and are intensively studied for their advantages such as their higher activity, lower price, and chemical and photoresist properties. Zn or Cu doped TiO2 nanoparticles with anatase crystalline structure were synthesized by sol−gel process. Titanium (IV) butoxide was used as a TiO2 precursor, with parental alcohol as a solvent, and a hydrolysing agent (ammonia-containing water) was added to obtain a solution with pH 10. The gels were characterized by TG/DTA analysis, SEM, and XPS. Based on TG/DTA results, the temperature of 500 °C was chosen for processing the powders in air. The structure of the samples thermally treated at 500 °C was analysed by XRD and the patterns show crystallization in a single phase of TiO2 (anatase). The surface of the samples and the oxidation states was investigated by XPS, confirming the presence of Ti, O, Zn and Cu. The antibacterial activity of the nanoparticle powder samples was verified using the gram−positive bacterium Staphylococcus aureus. The photocatalytic efficiency of the doped TiO2 nanopowders for degradation of methyl orange (MO) is here examined in order to evaluate the potential applications of these materials for environmental remediation.
Keywords: copper/zinc doped TiO2 powders, sol−gel method, thermal behaviour, photocatalytic activity, antibacterial properties
1. Introduction
The intensive use of oxide nanoparticles in various applications is based on a number of exceptional properties of these oxides, among which we can mention good thermal and chemical stability, electrical and optical properties and high photocatalytic activity, low cost, etc. Based on potential technological applications, metal oxide nanoparticles are attracting considerable interest from researchers in the fields of materials chemistry, biomedical, optical, electronics, medicine, agriculture, information technology, catalysis, environment, energy, and sensing.
Among these metal oxides, TiO2 is one of the most studied materials for many potential applications, particularly for its photocatalytic activity.
The improvement of the photocatalytic activity (by increasing the photon absorption, increasing the recombination time of the electron–hole pairs and improving the separation efficiency of photocarriers) of titanium dioxide was done by self-doping, non-metal doping, transitional metal doping and rare-earth metal doping [1,2,3,4,5,6,7].
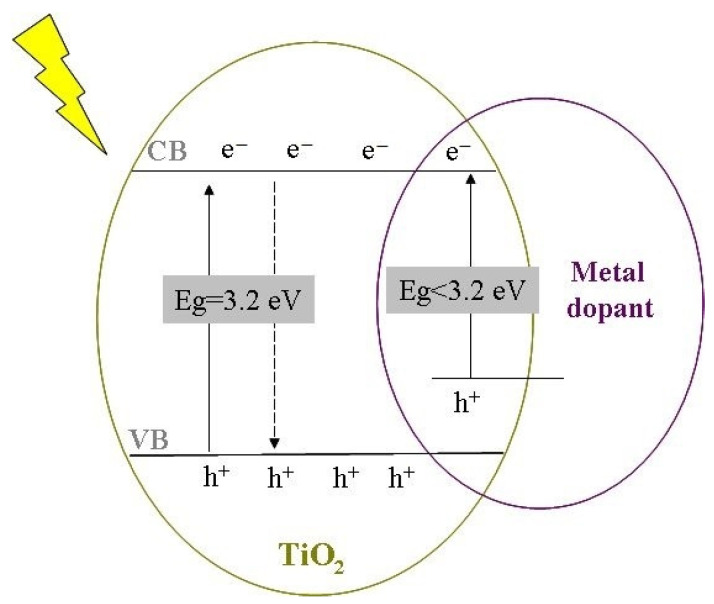
Some of these dopants, such as transitional metals (e.g., Fe, Cr, Ru, Cu, V etc) or non-metals (C, N, S etc.) [8,9,10,11,12,13,14,15] lead to an increase in photocatalytic activity due to the formation of a new electron energy levels inside the bandgap (Figure 1).
Figure 1.
Schematic representation of bandgap narrowing by TiO2 doping.
The synthesis methods, the doping techniques, and the dopant types influence the structural properties of TiO2. Many works reported that a co-catalyst bonded to a semiconductor’s surface can act as an electron trap to reduce the recombination of the photogenerated electron−hole pairs and thus improve the photocatalytic activity [16,17,18]. Furthermore, the loading of metal dopants on TiO2 was shown to lead to a red shift, moving the absorption maxima to the visible region, as the wide band of bare TiO2 is not suitable for absorbing visible light for practical applications [19,20,21]. However, by increasing thermal treatment the dopants diffuse on the particle’s surface, where they precipitate and create q-sites with plasmonic effects, improving the photocatalytic activity of the material [22].
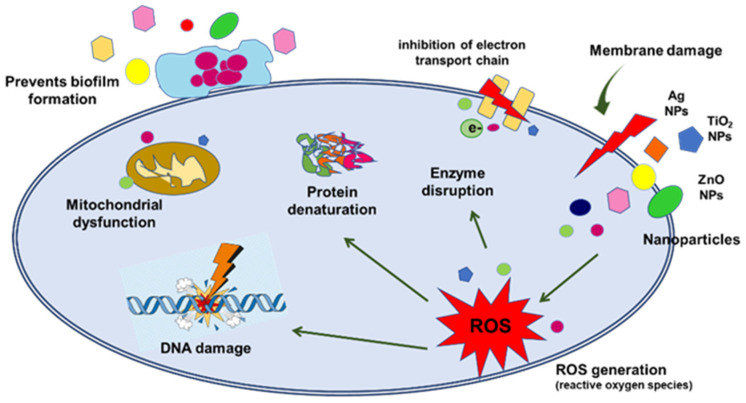
To improve the TiO2 antibacterial properties, Cu and Zn doping are an attractive approach because both are antibacterial elements [23,24]. The effect of dopants on the antibacterial properties of titanium dioxide materials was investigated by different groups [10,20,23] and showed that the doped TiO2 extend the light absorption spectrum toward the visible light domain and improves the bacteria inactivation efficiency of the nanoparticles. A schematic diagram of the various nanoparticles’ effects on bacteria cells is shown in Figure 2. NPs can attack and kill bacterial cells through multiple mechanisms. Thus, the formation of ROS causes membrane and DNA damage, protein denaturation, inhibiting the electron transport chain, enzyme disruption, and photocatalysis.
Figure 2.
Antimicrobial mechanisms of various nanoparticles (adapted from ref. [25]).
For the preparation of TiO2 or doped TiO2 materials, different methods are available [26,27,28,29,30]. Among them, the sol−gel method is often used to synthesize doped TiO2 because it has advantages such as high purity, relatively low processing temperature and the fact that it offers the possibility of controlling stoichiometry [31,32,33,34,35,36].
In accordance with one of the most exhaustive definitions [37], the sol−gel process takes place in two steps: the first step is the formation of an inorganic polymeric network by reaction in solution at low temperatures. During the second step, a conversion of the inorganic amorphous polymers to glass or into crystalline materials takes place. The process temperatures are significantly lower than the melting point of the corresponding oxides.
The advantages of the sol−gel method against other preparation methods are a good mixture of the reagents at the molecular level, leading to a homogeneous end−product; the ability to obtain pre−established structures and shapes (gels, films, fibres, powders, etc.) by varying the experimental conditions [38].
In the present paper, Cu− and Zn− doped TiO2 nanometre-sized powders were prepared by the sol−gel method in a basic medium. The aim of this work was the doping of TiO2 in order to improve the absorption capacity in a visible light domain to decrease the bandgap and to increase the recombination time by introducing additional energy levels. The influence of the dopant on the structure and the properties of these materials were evaluated. The photocatalytic activity of the doped TiO2 nanopowders for degradation of methyl orange (MO) is here examined in order to evaluate the potential of these materials for environmental applications. The antibacterial activity of the nanoparticle powder samples was tested using the gram−positive bacterium Staphylococcus aureus.
2. Results and Discussion
The samples were investigated following the thermal behaviour, morphology, structure and their properties (antimicrobial or photocatalytic). In the case of the TiO2−Cu 2.0% sample a white−green amorphous powder was obtained, while for the TiO2−Zn 2.0% sample the obtained powder was amorphous and had a white colour powder.
2.1. As Prepared Samples
2.1.1. SEM Results
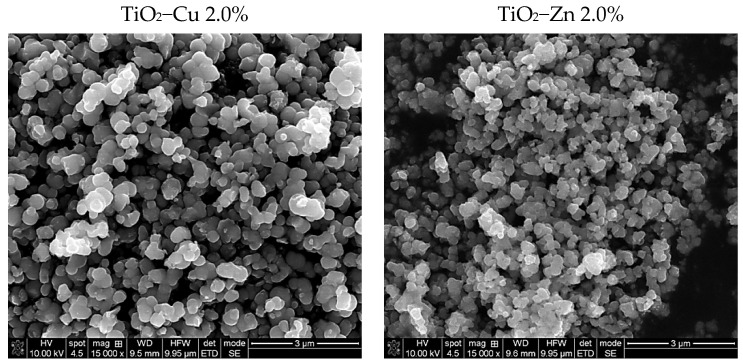
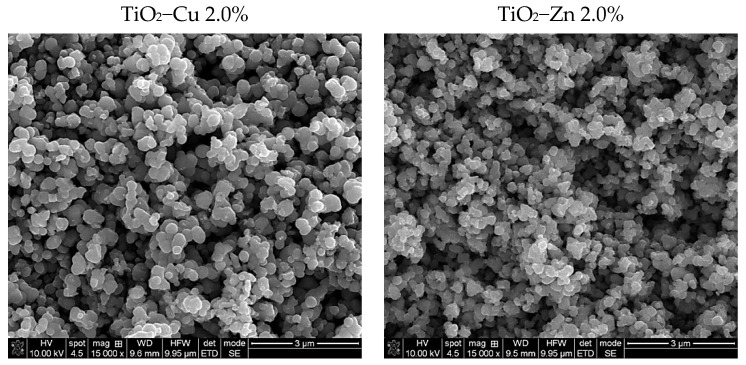
SEM analysis was performed to investigate the morphology of the as-prepared samples, and the micrographs are shown in Figure 3.
Figure 3.
SEM micrographs of the as−prepared samples.
According to SEM observations, for the as-prepared samples, the nanoparticles’ surfaces appear to be well-defined with quasi-spherical profiles. The mean diameter, found by Image J software, is 86 nm in TiO2−Cu 2.0% sample slightly larger than 75 nm for TiO2−Zn 2.0%, which also seems to be more agglomerated.
2.1.2. Thermal Behaviour
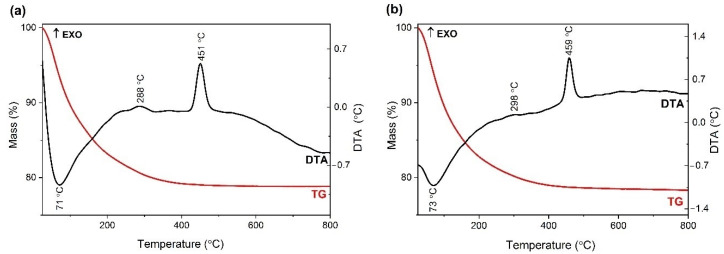
The thermogravimetric and differential thermal analysis (TG/DTA) were used to examine the thermal behaviour of the as-prepared samples and to set the subsequent thermal treatment. The mass loss and the thermal effects are shown in Figure 4. From Figure 4a the TG curves of the TiO2−Cu 2.0% sample show a total mass loss of 21%. Three important heat effects are observed on the DTA curve. The endothermic effect at 71 °C is assigned to the elimination of adsorbed water; the two exothermic effects are the one at 288 °C assigned to the decomposition of the samples, by the elimination of the organic residues and the structural hydroxyls (dehydroxylation), and the one at 451 °C, which can be attributed to the crystallization of anatase phase. In Figure 4b, the TG curves of the TiO2−Zn 2.0% sample show a total mass loss of 22.3%.
Figure 4.
The TG (red)/DTA (black) curves of (a) TiO2−Cu 2.0% sample and (b) TiO2−Zn 2.0% sample; heating rate was 10 °C/min and using air as carrier gas.
Three important heat effects are observed on the DTA curve. The endothermic effect at 73 °C is attributed to the elimination of adsorbed water; the two exothermic effects are the one at 298 °C assigned to the decomposition of the samples, by the elimination of the organic residues and the structural hydroxyls (dehydroxylation) and the one at 459 °C, which can be assigned to the crystallization of anatase phase. In both cases, no further weight loss could be observed at temperatures above 500 °C.
2.1.3. XPS on the As-Prepared Samples
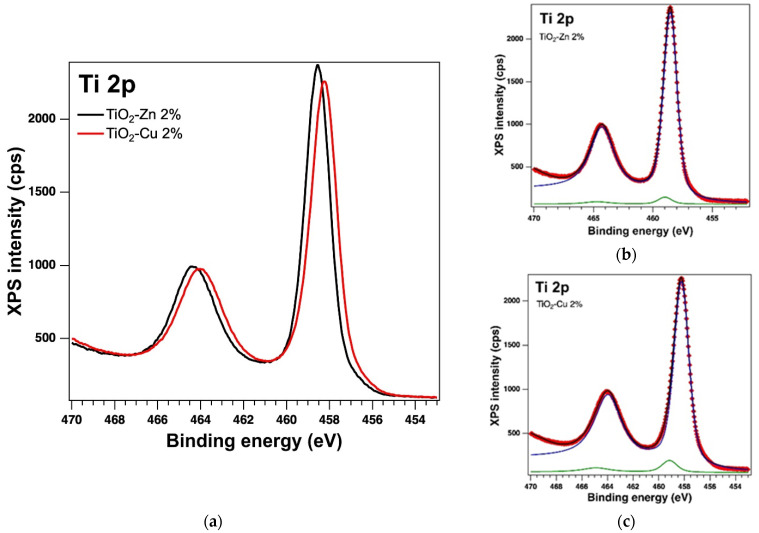
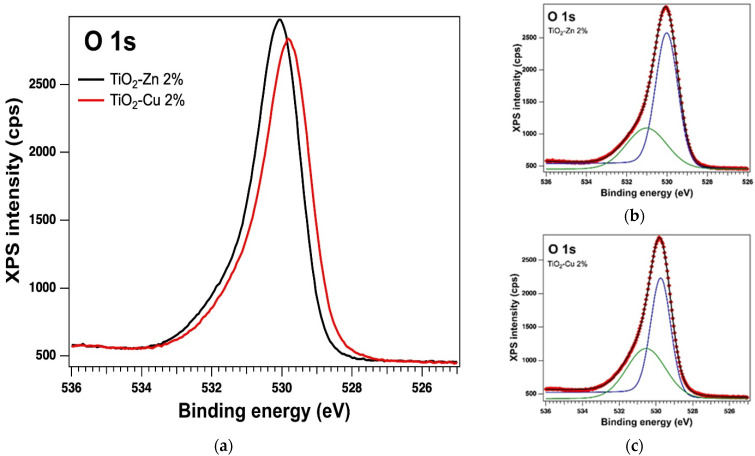
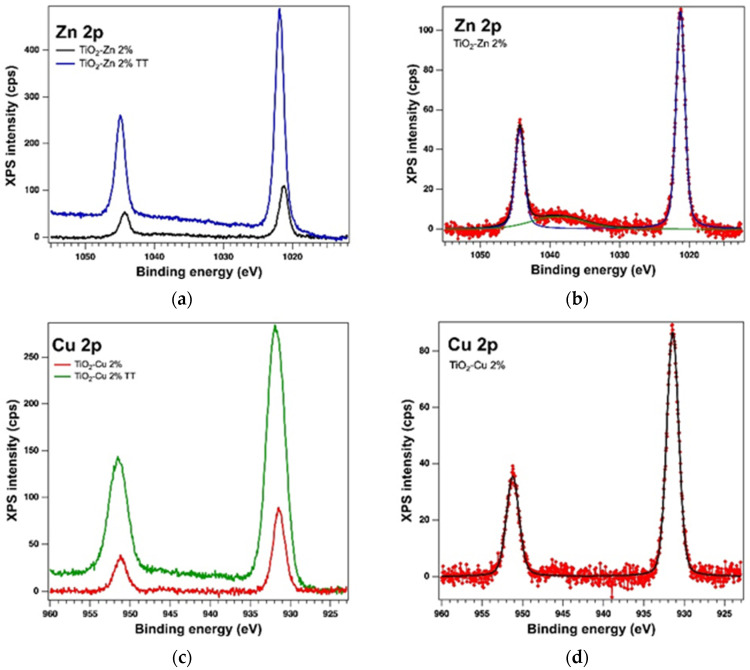
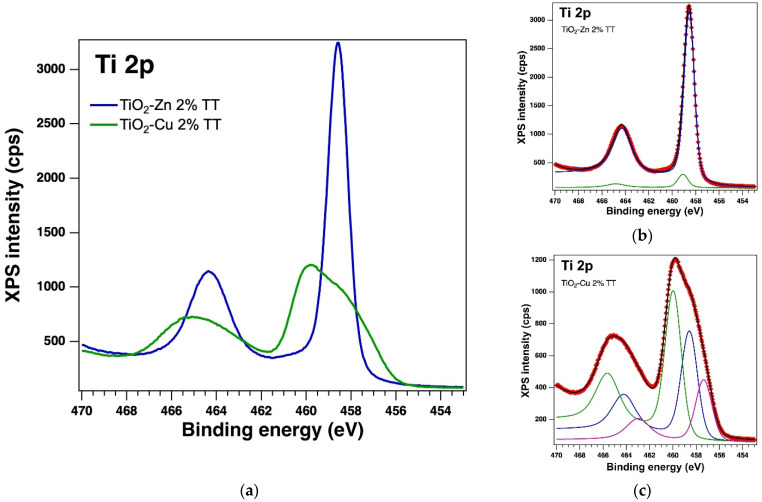
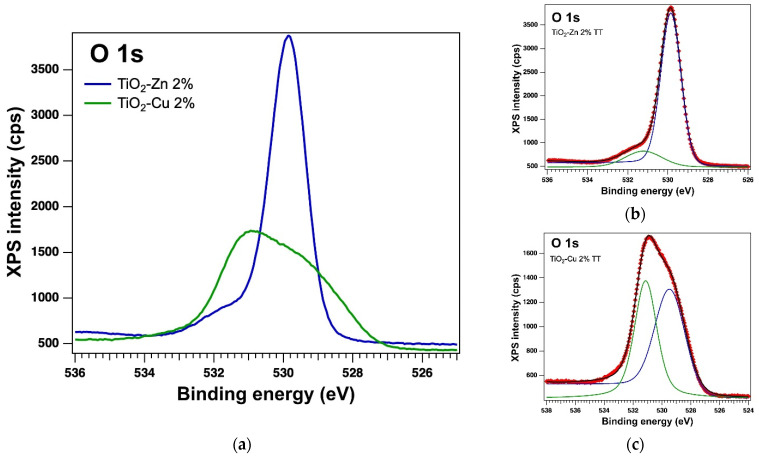
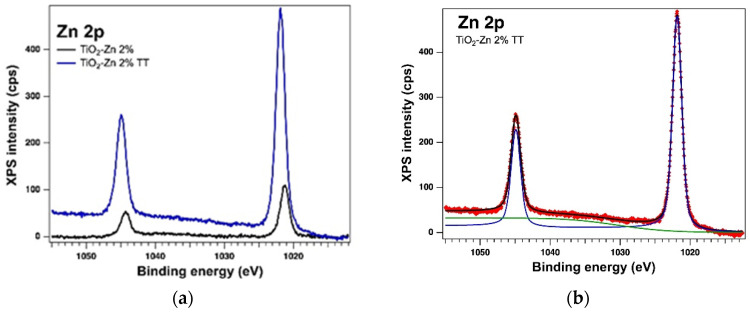
The oxidation states of the elements and the surface of the samples were analysed by X−ray Photoelectron Spectroscopy (XPS). The analysis showed the presence of the Ti 2p, O 1s, Zn 2p and Cu 2p core levels and indicates the presence of different species as follows: Ti(IV), Zn(II) and Cu(I). The binding energy (BE) for Ti 2p3/2 is around 458.53 eV with a spin-orbit splitting of 5.7 eV, consistent with the database [39]. The Zn 2p3/2 peak is centred at 1021.26 eV attributed to Zn(II) [40] and for Cu 2p3/2 we found the BE at 931.44 eV, which could be attributed to Cu(I), with a very weak specific satellite at about 945 eV [41]. Table 1 lists the essential parameters (binding energy and at. %) obtained as a result of the deconvolutions of the spectra for the core levels of interest, of Ti 2p, O 1s, Zn 2p, and Cu 2p for the as-prepared samples (see the Figure 5, Figure 6 and Figure 7). The values of the amplitude were normalized by the atomic sensitivity factors given by ref. [42].
Table 1.
Binding energies (BE) at. % and interpretation of the components of the core levels of interest for the samples prepared by sol-gel method.
| Sample | Core Levels | BE (eV) | at. % | Interpretation | |
|---|---|---|---|---|---|
| TiO2−Zn 2.0% | Ti 2p | C1 | 458.53 | 42.40 | Ti(IV) vol. |
| C2 | 459.00 | 1.60 | Ti(IV) surf. | ||
| O 1s | C1 | 530.02 | 35.61 | Ti(IV) (vol + surf) | |
| C2 | 531.02 | 19.89 | Ti(IV) + Zn(II) + cont | ||
| Zn 2p | C1 | 1021.26 | 0.50 | Zn(II) | |
| TiO2−Cu 2.0% | Ti 2p | C1 | 458.23 | 42.35 | Ti(IV) vol. |
| C2 | 459.10 | 2.71 | Ti(IV) surf. | ||
| O 1s | C1 | 529.74 | 29.93 | Ti(IV) | |
| C2 | 530.54 | 24.52 | Ti(IV) + Zn(II) + cont | ||
| Cu 2p | C1 | 931.44 | 0.48 | Cu(I) | |
Figure 5.
XPS spectra of the core level Ti 2p (a) overlayed XPS spectra of the samples prepared by the sol−gel method and the experimental data (red symbols) with the fit and deconvolutions given separately for (b) the sample with Zn and (c) the sample with Cu; deconvolutions: components C1–blue line and C2–green line.
Figure 6.
XPS spectra of the core level O 1s (a) overlayed XPS spectra of the samples prepared by the sol−gel method and the experimental data (red symbols) with the fit and deconvolutions given separately for (b) the sample with Zn and (c) the sample with Cu; deconvolutions: components C1–blue line and C2–green line.
Figure 7.
XPS spectra of the core levels Zn 2p (a) and Cu 2p (c) overlayed XPS spectra of both kinds of samples and the experimental data (red symbols) with the fit and deconvolutions for (b) the sol−gel sample with Zn (deconvolutions: components C1–blue line and C2–green line) and (d) the sol−gel sample with Cu (only one component–black line).
The as-prepared powders were thermally treated at 500 °C for 1 h. No mass loss was measured above this temperature and anatase phase crystallization is already completed.
2.2. Thermally Treated Samples (Powders)
2.2.1. SEM Results
The surface morphology of the thermally treated powders, performed by SEM, is presented in Figure 8, showing nanoparticles clusters with similar sizes compared with as−prepared samples. Following the thermal treatment, the samples’ surfaces are slightly aggregate, with more irregular shapes, related to untreated ones, as confirmed by BET measurements.
Figure 8.
SEM images of the thermally treated samples at 500 °C 1h.
2.2.2. X−ray Diffraction (XRD)
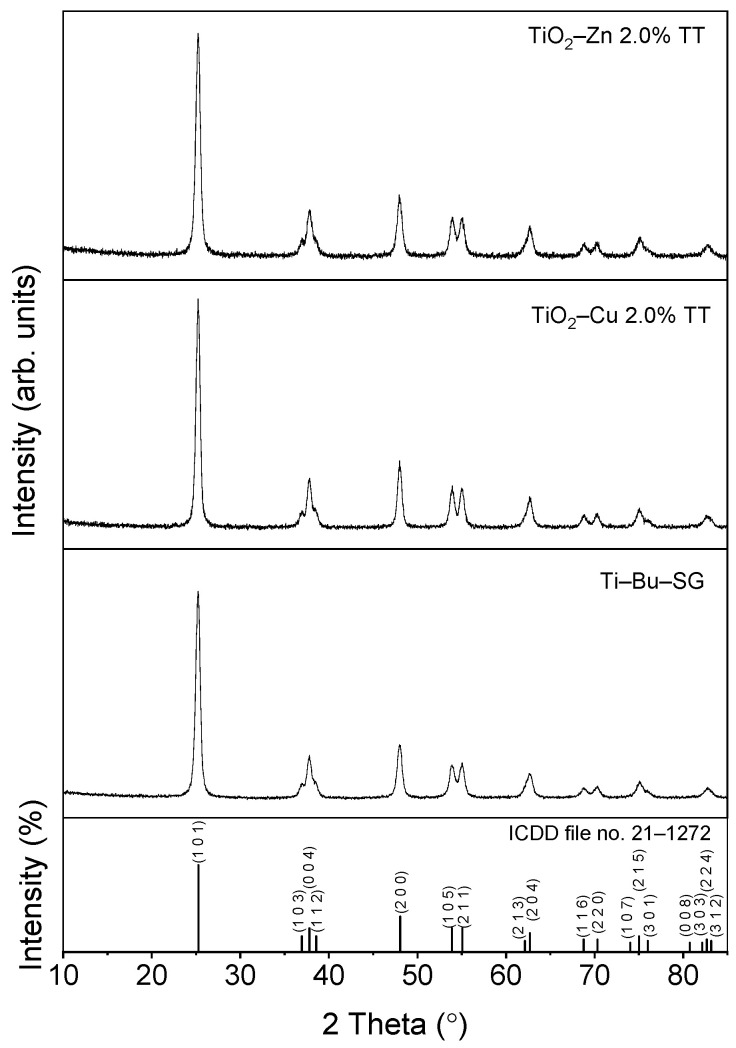
The crystallinity and structure of the thermally treated samples were assessed by X−ray diffraction (XRD). Figure 9 shows the patterns of thermally treated Cu− and Zn−doped TiO2 samples at 500 °C for 1 h. Moreover, the undoped TiO2 sample (Ti−Bu−SG) was measured as a reference. Single-phase anatase TiO2 was identified in all the samples, according to ICDD file no. 21-1272. No diffraction lines related to TiO2 polymorph phases or additional diffraction lines related to Zn or Cu compounds were detected in the XRD patterns, which may suggest that Cu and Zn were in a dispersed state or the ions were properly entered into the anatase lattice. The lattice parameters, as well as the mean crystalline domain sizes, calculated by the Williamson−Hall method, are shown in Table 2. No major differences in terms of unit cell parameters are noticed against the standard reference file (ICDD 21-1272), pointing out that Cu and Zn dopants probably substitute for Ti in the TiO2 host lattice. The mean crystalline domain sizes of the Ti−Bu−SG and TiO2−Cu 2.0% TT samples are similar (~14 nm), while the value for the TiO2−Zn 2.0% TT sample is 1 nm smaller (~13 nm).
Figure 9.
X−ray patterns for powders thermally treated at 500 °C 1 h.
Table 2.
The lattice parameters and the mean crystalline domain sizes (Dm) of the samples.
| Sample | a = b (Å) | c (Å) | Dm (nm) |
|---|---|---|---|
| Ti−Bu−SG [43] | 3.7837 (6) | 9.4969 (15) | 14 |
| TiO2−Cu 2.0% TT | 3.7892 (2) | 9.5145 (6) | 14 |
| TiO2−Zn 2.0% TT | 3.7854 (10) | 9.496 (3) | 13 |
| Anatase, syn ICDD 21−1272 | 3.7850 | 9.5140 | - |
2.2.3. X−ray Fluorescence (XRF)
Concerning the presence of the dopant in the samples, X−ray fluorescence analysis was performed, which demonstrated the presence of dopant (copper or zinc) in both studied samples. The elemental composition of the samples is listed in Table 3. Other elements (C, S, Si) were detected as traces. Oxide composition by mass percentage was also calculated. More Cu was detected in the final mixture compared to Zn. A possible explanation can be that, during synthesis, a small amount of compound was lost by washing.
Table 3.
XRF results of the analysed samples.
| Sample | Composition | Values | U.M. | Line |
|---|---|---|---|---|
| TiO2−Zn 2.0% | Ti | 58.0482 | mass% | Ti−KA |
| Zn | 1.6157 | mass% | Zn−KA | |
| O | 39.2373 | mass% | O−KA | |
| C, S, Si (traces) | 1.0989 | mass% | ||
| TiO2 | 94.1104 | mass% | Ti−KA | |
| ZnO | 1.9373 | mass% | Zn−KA | |
| C, S, Si oxides (traces) | 3.9524 | mass% | ||
| Ti | 58.7187 | mass% | Ti−KA | |
| TiO2−Cu 2.0% | Cu | 1.9474 | mass% | Cu−KA |
| O | 38.3098 | mass% | O−KA | |
| C, Si, S (traces) | 1.0241 | mass% | ||
| TiO2 | 94.0071 | mass% | Ti−KA | |
| CuO | 2.3092 | mass% | Cu−KA | |
| C, S, Si oxides (traces) | 3.6837 | mass% |
2.2.4. XPS on the Thermally Treated Samples
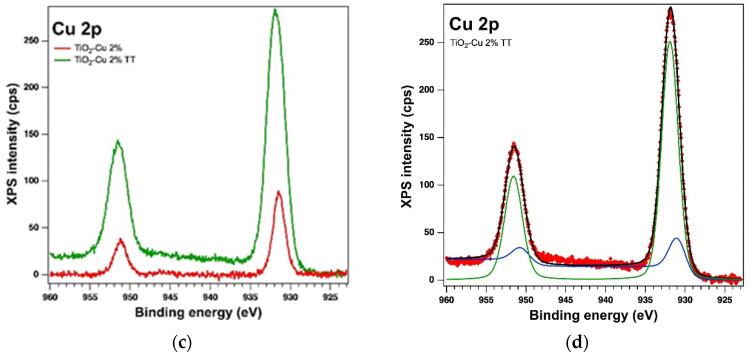
The surface of the thermally treated samples was also examined by X−Ray Photoelectron Spectroscopy (XPS). The core levels of interest were deconvoluted using the same method [44] as for the as-prepared samples. In the case of thermally treated samples, the XPS measurements also confirmed the presence of the Ti 2p, O 1s, Zn 2p and Cu 2p core levels and from the deconvolutions, we obtained: Ti(IV), which is the major component, Zn(II) and Cu(I); but in the case of the TiO2−Cu 2.0% TT sample we observed the presence of a small amount of Ti(II) and Cu(0), which can be assumed to be related to the thermal treatment. The integral area was used to determine the atomic composition. The integral area was computed using the Voigt profiles deconvolution procedure and scaled the atomic sensitivity factors provided by ref. [42]. The XPS spectra of the Ti 2p, O 1s, Zn 2p and Cu 2p core levels of the thermally treated samples are displayed in Figure 10, Figure 11 and Figure 12. Table 4 lists the binding energy and at. %, obtained as a result of the deconvolutions of the spectra for the core levels of interest.
Figure 10.
XPS spectra of the core level Ti 2p (a) overlayed XPS spectra of the thermally treated samples and the experimental data (red symbols) with the fit and deconvolutions for (b) the sample with Zn (deconvolutions: components C1 – blue line and C2–green line) and (c) the sample with Cu (deconvolutions: components C1–blue line, C2–green line and C3–magenta line).
Figure 11.
XPS spectra of the core level O 1s (a) overlayed XPS spectra of the thermally treated samples and the experimental data (red symbols) with the fit and deconvolutions for (b) the sample with Zn and (c) the sample with Cu; deconvolutions: components C1–blue line and C2–green line.
Figure 12.
XPS spectra of the core levels Zn 2p (a) and Cu 2p (c) overlayed XPS spectra of both kinds of samples and the experimental data (red symbols) with the fit and deconvolutions for (b) the thermally treated sample with Zn (deconvolution with component C1–blue line; the green line represents an Auger line, which is not taken into account in the chemical analysis) and (d) the thermally treated sample with Cu (deconvolutions: components C1–blue line and C2–green line).
Table 4.
Binding energies, at. % and interpretation of the components of the core levels of interest for the thermally treated samples.
| Sample | Core Levels | BE (eV) | at. (%) | Interpretation | |
|---|---|---|---|---|---|
| TiO2−Zn 2.0% TT | Ti 2p | C1 | 458.58 | 0.78 | Ti(IV) vol. |
| C2 | 459.78 | 39.04 | Ti(IV) surf. | ||
| O 1s | C1 | 529.85 | 48.77 | Ti(IV) (vol + surf) | |
| C2 | 531.22 | 9.21 | Ti(IV) + Zn(II) + cont | ||
| Zn 2p | C1 | 1021.85 | 2.2 | Zn(II) | |
| TiO2−Cu 2.0% TT | Ti 2p | C1 | 457.29 | 6.8 | Ti(II) |
| C2 | 458.56 | 12.42 | Ti(IV) vol. | ||
| C3 | 459.97 | 18.12 | Ti(IV) surf. | ||
| O 1s | C1 | 529.43 | 29.96 | Ti(IV) (vol + surf) | |
| C2 | 531.13 | 30.19 | Cu(I) + cont | ||
| Cu 2p | C1 | 930.95 | 0.32 | Cu(0) | |
| C2 | 931.91 | 2.19 | Cu(I) | ||
2.2.5. Antibacterial Activity
The antibacterial activity of the nanoparticle powder samples was investigated using the gram-positive bacterium Staphylococcus aureus. In previous experiments using the diffusion method on doped samples, the best results were obtained for S. aureus [45], therefore we continued with this bacterium for the experiments in a broth culture medium. For this experiment, the method of testing in a liquid culture medium was used. The evaluation of the antibacterial activity was performed by measuring the absorbance (see Table 5) after 24 h of incubation. The negative control was represented by the nanoparticle powders immersed separately in the culture medium without inoculating the S. aureus strain. The real OD (optical density) of the samples is obtained through the subtraction of the OD of the samples with S. aureus and the OD of the negative controls (samples containing the nanopowders but without bacteria) and, in the end, the results are compared with the biological positive control of S. aureus.
Table 5.
Absorbance values for nanopowders in broth medium.
| Sample | Concentration | OD of Sample with S. aureus |
Inhibition Rate % |
|---|---|---|---|
| Ti−Bu−SG | 200 µg mL−1 | 0.060 | 96.29 |
| TiO2−Zn 2.0% TT | 200 µg mL−1 | 0.235 | 85.47 |
| TiO2−Cu 2.0% TT | 200 µg mL−1 | 0.245 | 84.85 |
| Biological positive control of S. aureus | 3 × 105 CFU mL−1 | 1.618 | - |
Additionally, the bacterial growth inhibition rate was calculated according to the formula and can be seen in Table 5.
The antibacterial activity of nanopowders against S. aureus in Mueller Hinton broth was the highest for the Ti−Bu−SG sample with an inhibition rate of 96.29%, followed by TiO2−Cu 2.0% TT and TiO2−Zn 2.0% TT, with inhibition of 85.47% and 84.85%, respectively. Many studies have shown the good antimicrobial activity of TiO2 nanoparticles [46,47].
2.2.6. Textural Characterization
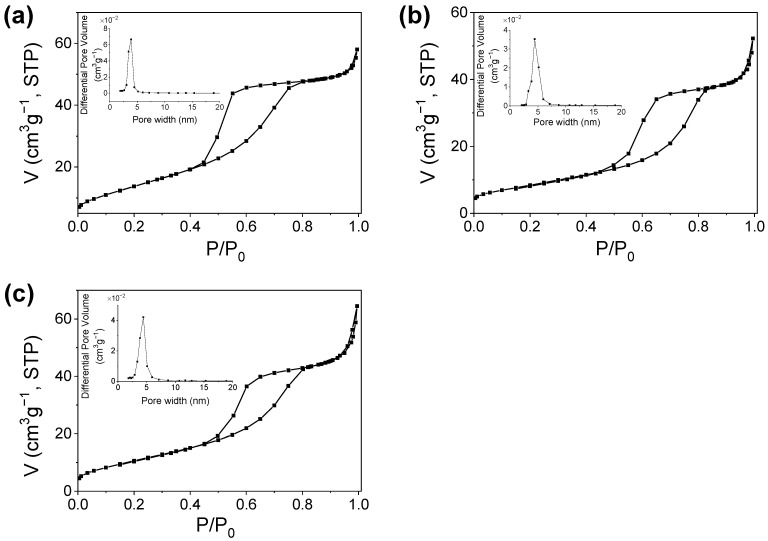
For the textural characterization of the thermally treated Cu and Zn doped and undoped TiO2, the adsorption−desorption isotherms N2 were registered and are shown in Figure 13. The specific surface area (SSA) was calculated by the Brunauer−Emmett−Teller (BET) method. The pore size distribution and average pore diameter were determined by Barrett−Joyner−Halenda (BJH) analysis. The textural parameters (BET surface area (SBET), total pore volume, and average pore size) are listed in Table 6. All three samples exhibit type IV isotherms accompanied by H3 hysteresis loops according to IUPAC classification [48], characteristic of mesoporous materials with slit−shape pores. The undoped TiO2 sample (Ti−Bu−SG) presents the largest surface area of 52.3 m2/g. Doping with Cu and Zn causes a decrease in surface area by 40% and 20%, respectively. At the same time, an increase in average pore diameter and a slight widening of pore size distribution could be observed, most probably due to the higher particle agglomeration. The total pore volume is influenced differently depending on the dopant ion. Compared to undoped TiO2, it shows a contraction of 10% upon doping with Cu and an increase of 10% upon doping with Zn, respectively.
Figure 13.
N2 adsorption–desorption isotherms and pore size distributions (inset of the figures) of Ti−Bu−SG (a), TiO2−Cu 2.0% TT (b), and TiO2−Zn 2.0% TT (c) samples.
Table 6.
The specific BET surface areas (SBET), total pore volume (Vtotal), average pore diameter (d) and band gap values of the samples.
| Sample | SBET (m2g−1) |
Vtotal (cm3g−1) |
dBJH (nm) |
Band Gap (eV) |
|---|---|---|---|---|
| Ti−Bu−SG | 52.3 | 0.089 | 4.4 | 3.15 |
| TiO2−Cu 2.0% TT | 31.3 | 0.080 | 6.2 | 1.5 |
| TiO2−Zn 2.0% TT | 41.5 | 0.099 | 6.1 | 2.85 |
2.2.7. Photocatalysis Investigation
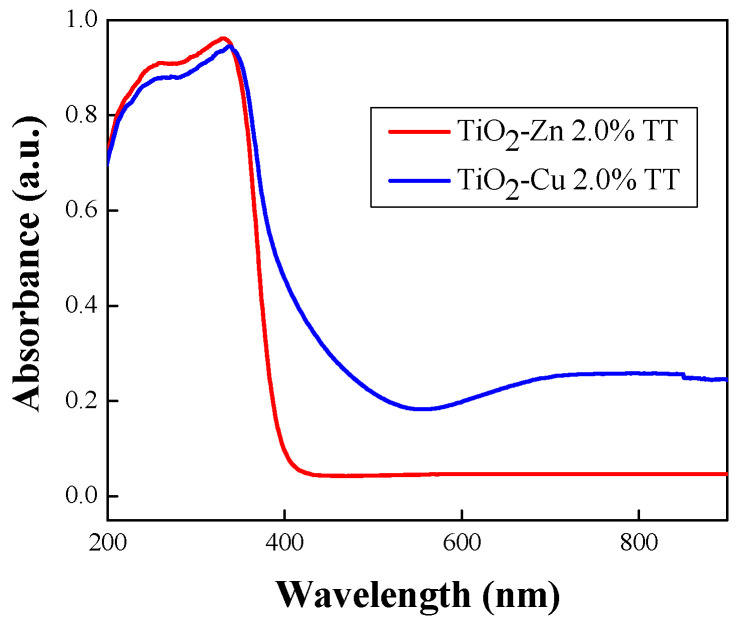
Figure 14 shows the absorbance spectra of TiO2−type materials, undoped and doped with 2.0% Zn or Cu, respectively. The spectrum of undoped TiO2 (Ti−Bu−SG sample) shows a band edge absorption located at ~370 nm, typically for the band gap of TiO2 nanoparticles in the anatase phase. In the case of sample TiO2−Zn 2.0% TT, no additional absorption band can be observed, ZnO exhibiting a band at ~369 nm [49]. Furthermore, a decrease in the absorption intensity after Zn addition can be observed, probably due to the displacement of titanium ions by zinc into the anatase phase, as XRD analysis suggested. By contrast, the absorption bands of TiO2−Cu 2.0% at around 250–550 nm can be observed due to the O2− (2p) → Ti4+ (3d) transitions in the tetrahedral symmetry. Additionally, a broad absorption band in the visible domain was evidenced, proving the presence of Cu species. According to Colon et al. [50], the absorption band between 400 nm and 600 nm, can be assigned to the presence of Cu1+ clusters, resulting after a partial reduction of Cu2+ species by a strong interaction between TiO2 support and copper nitrate. This strong interaction is also suggested by the obtaining of Ti(II) species in the case of the TiO2−Cu 2.0% sample, as XPS results revealed. The large absorption band located between 600–800 nm indicates the increase of CuO in octahedral symmetry [50]. Thus, the introduction of the dopants leads to a red shift of the absorption band, as was previously reported [51]. This behaviour can be attributed to the formation of impurity levels within the band gap states of TiO2. The band gap value of the bare TiO2 was 3.15 eV. When doping with a transitional metal (Cu or Zn), the band gap values decrease (see Table 6), these values being in agreement with the previously reported data [51,52]. It was suggested through theoretical calculations that the band gap narrows after Cu doping due to new electronic levels in the valence band resulting from the covalent interaction between Cu and O [53]. For the TiO2−Zn 2.0% TT sample, the band gap energy decreased due to the synergistic effect between the conduction band of TiO2 and that of ZnO. Both impurities and defects were introduced to the forbidden band of TiO2, leading to the formation of some sub-bands, thus, the value of the band gap energy was decreased [52].
Figure 14.
DR−UV−Vis absorption spectrum for TiO2−Zn 2.0% TT and TiO2−Cu 2.0% TT samples.
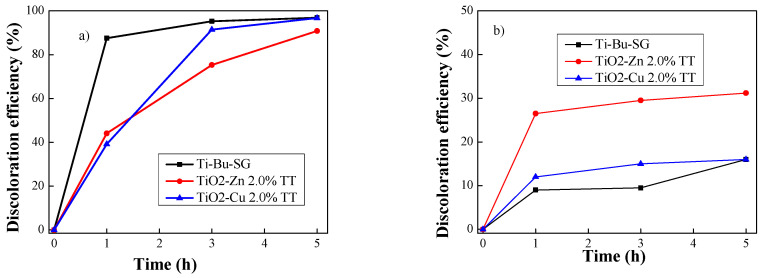
The photocatalytic degradation of methyl orange solution in the presence of undoped and doped TiO2 was carried out under UV and visible light irradiation. The results presented in Figure 15 showed that the introduction of a doping element in titanium dioxide leads to a higher photocatalytic activity.
Figure 15.
Discoloration of methyl orange under UV (a) and visible (b) light irradiation using synthesized materials.
It was observed that in the UV domain, the TiO2−Cu 2.0% TT sample, with crystalline domains size of 14 nm, presented the highest activity in photocatalytic degradation of MO, removing ~97% of the dye in 300 min (Figure 15), while the sample with a smaller value of crystalline domains size (13 nm), TiO2−Zn 2.0% TT, presented a lower degradation efficiency, about 90%. In the case of visible light irradiation, the most effective was the sample TiO2−Zn 2.0% TT, removing a total of 30% of MO in 300 min, while the sample doped with Cu presents activity comparable with the bare TiO2 (Ti−Bu−SG sample) (~ 16%). It can be observed that the presence of a doping metal (Cu, respectively, Zn) led to an increase of photocatalytic activity in the visible domain compared to bare TiO2. Furthermore, the higher photocatalytic activity of the TiO2−Zn 2.0% TT materials can be attributed to their increased absorption capacity due to a higher surface area compared to TiO2−Cu 2.0% TT (see Table 6) a process that ensures the presence of the dye molecules close to the active centres located on the surface, thus facilitating the photodegradation process.
3. Conclusions
In the present work, copper- and zinc-doped TiO2 materials prepared by the sol–gel method were investigated for their photocatalytic and antibacterial activities. The main results can be summarized as follows: by thermal analysis, the heat treatment temperature was set to 500 °C in order to obtain a crystalline phase without organic residues. By XRD, single-phase anatase with nanometre-sized crystallites was identified and, by XRF, the presence of the dopants was detected. The surface and the oxidation states of the samples prepared by the sol−gel method and thermally treatment method investigated by XPS confirmed, also, the presence of Ti 2p, O 1s, Zn 2p and Cu 2p core levels. The textural characterization of the TiO2 doped with Cu or Zn causes a decrease in surface area by 40% and 20%, respectively. Compared to undoped TiO2, it shows a contraction of 10% upon doping with Cu and an increase of 10% upon doping with Zn, respectively. The photocatalytic activity of doped TiO2 increased under visible light irradiation compared to the undoped TiO2 due to the newly formed sub-bands and to the active centres located on the surface, thus facilitating the photodegradation process. In the UV domain, a relation between the crystalline domain sizes and the photocatalytic discoloration of methyl orange was noticed. This paper examined the inhibitory effect determined by TiO2 nanopowders against S. aureus. The results of the antimicrobial test confirmed a considerable antimicrobial activity against gram-positive bacteria of the doped nanopowders.
4. Materials and Methods
4.1. Sample Preparation
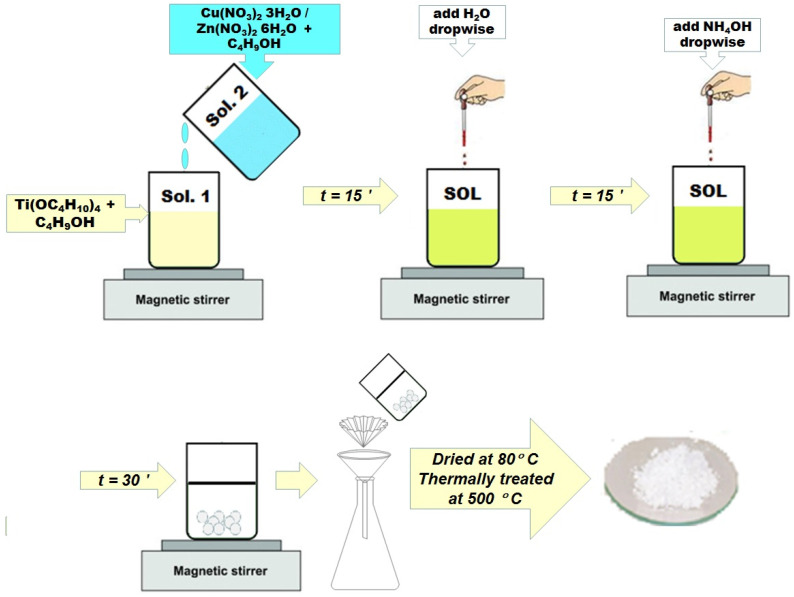
Nanopowders of Cu− or Zn−doped TiO2 were prepared by the sol−gel method. The initial calculated compositions corresponding to a TiO2:CuO or TiO2:ZnO molar percentage of 98:2 were chosen. The reaction started from titanium (IV) butoxide [TBOT = Ti(OC4H10)4] (Merck) as a precursor of TiO2 and copper nitrate [Cu(NO3)2·3H2O] (Merck) as a precursor of CuO or zinc nitrate [Zn(NO3)2·6H2O] (Merck) as a precursor of ZnO. Butanol [C4H9-OH] (J.T. Baker) was used as the solvent, H2O as the hydrolysis agent, and ammonium hydroxide [NH4OH] (Riedel-de Haën) as the catalyst. The synthesis took place at room temperature and the solution of reagents was homogenized for 60 min. The oxide powder was separated by filtration from the solution, washed with distilled water to remove adsorbed compounds, dried and then thermally treated at 500 °C, in air, with a plateau of 1 h and a heating rate of 1 °C/min, in order to eliminate the water and organic residues and to obtain crystallized nanometre-sized powders. A flowchart of the methodology used for the sample preparation is given in Figure 16. The thermal treatment was established based on the TG/DTG/DTA results.
Figure 16.
The flowchart of the sample preparation by sol-gel method.
The samples were denoted (TiO2−Cu 2.0%) and (TiO2−Zn 2.0%), and the thermally treated samples (TiO2−Cu 2.0% TT) and (TiO2−Zn 2.0% TT), respectively.
The composition of the solutions and the experimental conditions used are shown in Table 7.
Table 7.
The composition of the solution and the experimental parameters of the sol preparation.
| Sample | Precursors | Molar Ratio | pH Sol | Experimental Conditions | |||
|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | ||||||
| TiO2−Cu 2.0% | Ti(OC4H10)4 + Cu(NO3)2·3H2O | 36.5 | 3 | 0.003 | 10 | 25 | 60 |
| TiO2−Zn 2.0% | Ti(OC4H10)4 + Zn(NO3)2·6H2O | 36.5 | 3 | 0.003 | 10 | 25 | 60 |
ROH = C4H9-OH.
The synthesis procedure for the sample Ti−Bu−SG was described in our previous work (ref. [43]).
4.2. Methods of Characterization
The thermal behaviour of the as-prepared samples was determined by thermogravimetric and differential thermal analysis (TG/DTA) using Mettler Toledo TGA/SDTA 851e (Greifensee, Switzerland) equipment in open Al2O3 crucibles and in flowing air atmosphere. The maximum temperature was set at 800 °C and the heating rate was 10 °C/min.
SEM micrographics were recorded using FEI Quanta 3D FEG microscope (FEI, Brno, Czech Republic), operating at an accelerating voltage of 10 kV. The specimens were placed on conductive carbon tape and scanned in high vacuum mode in an uncoated state.
X−ray Photoelectron Spectroscopy (XPS) was performed in an AXIS Ultra DLD (Kratos Surface Analysis, Manchester, UK) system, using Al Kα1 (1486.74 eV) radiation produced by a monochromatized X−ray source at operating power of 240 W (12 kV × 20 mA) and a charge neutralizer operating at 1.7 A filament current, 2.8 V charge balance, 2.00 V filament bias. High-resolution core-level spectra were recorded using hybrid lens mode, 40 eV pass energy, and slot aperture. The binding energy scale was calibrated to the C 1s standard value of 284.6 eV. The core level spectra were deconvoluted using Voigt profiles, based on the methods described in ref. [44].
X−Ray Diffraction (XRD) patterns were recorded using a Rigaku Ultima IV X−ray diffractometer. The equipment was set in parallel beam geometry, with cross beam optics (CBO), operated at 40 kV and 30 mA, using CuKα radiation. The data were collected over the 2θ range 10−80° at a scanning rate of 2°/min. Rigaku’s PDXL software, connected to ICDD PDF−2 database, was used for phase identification. Lattice constants were refined using the diffraction line position. The mean crystalline domain size is calculated from the diffraction line width. The diffraction line position and width were corrected by the external standard method.
X−ray fluorescence (XRF) was used for elemental analysis. The measurements were performed using a Rigaku ZSX Primus II spectrometer (Rigaku Corp., Tokyo, Japan), equipped with 4.0 kW X−ray Rh tube. EZ−scan combined with Rigaku SQX fundamental parameters software (standard less) was used for data analysis.
Gram−positive bacteria Staphylococcus aureus ATCC 25,923, purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ) (Braunschweig, Germany), was used in the experiment. The working method for testing antibacterial activity of nanoparticle powders was as follows. First, the bacterial strain Staphylococcus aureus was replicated on the TSA (Tryptic Soy Agar) medium and incubated at 35 ± 2 °C for 24 h. The working inoculum was made by suspending 2–3 bacterial colonies in sterile physiological water (0.85%), obtaining turbidity of 3 × 105 CFU/mL, which was adjusted spectrophotometrically by measuring the absorbance. The study was carried out in 100-millilitre Erlenmeyer flasks with 30 mL culture medium. Doped TiO2 nanopowders were tested using a concentration of 200 µg mL−1. The nanoparticle powders were immersed in the Mueller Hinton broth (MHB) inoculated with 1 mL suspension of Staphylococcus aureus and incubated at 35 ± 2 °C for 24 h and 130 rpm. The composition of the culture media was Mueller Hinton broth from Scharlau, Sentmenat, Spain (1.5 g/L starch; 2 g/L meat extract; 17.5 g/L peptone). Tryptic Soy Agar from Scharlau, Sentmenat, Spain (2.5 g/L dextrose; 5 g/L sodium chloride; 20 g/L peptone; 2.5 g/L dipotassium phosphate; 15 g/L agar). Nanoparticle powders were also immersed in the culture medium without bacterial strain for the negative control. The positive biological control does not contain nanoparticle powders. The samples were inoculated in five replicates in 96-well plates, 200 μL in each well and incubated at 35 ± 2 °C for 24. After incubation, the absorbance was measured at 600 nm with the help of the Clariostar plate reader.
The inhibition rate (%) was determined using the following formula [54]:
| (1) |
Nitrogen adsorption−desorption isotherms at 77 K were recorded on a Micromeritics ASAP 2020 automated gas adsorption system (Norcross, GA, USA). The samples were degassed at 200 °C for 5 h under vacuum before analysis.
The UV−Vis diffuse reflectance spectra were recorded on a JASCO V570 spectrophotometer (Tokyo, Japan). Photocatalytic experiments were carried out in batches with 5 mg photocatalyst in 10 mL methyl orange (MO) dye (1 × 10−5 M). The suspension was stirred during the experiment. The reaction mixture was first stirred in dark for 30 min in order to establish the adsorption of MO dye on the photocatalyst surface. Then it was irradiated in a closed box with an UV−Vis lamp at specific wavelengths. Photocatalytic experiments were performed for 300 min. There were taken the same aliquots of MO solution at regular time intervals and filtered using a 0.45 mm Millipore film in order to evaluate the progress of the reaction. The discoloration efficiency was evaluated as reported before [55].
Acknowledgments
This work was supported by the research programme “Materials Science and Advanced Methods for Characterization” of the Institute of Physical Chemistry “Ilie Murgulescu” of the Romanian Academy.
Author Contributions
Conceptualization, L.P.; methodology, L.P.; investigation, S.P., J.P.-C., S.V.P., E.M.C., G.P., D.C.C., N.G.A., R.M.C., I.R., M.C. and L.P.; resources, E.M.C., I.R. and M.C.; writing—original draft preparation, S.P., E.M.C., G.P., S.V.P., D.C.C., N.G.A., R.M.C., I.R., M.C. and L.P.; writing—review and editing, S.P., E.M.C., R.M.C., M.C. and L.P.; visualization, S.P. and L.P.; project administration, L.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Romanian Ministry of Research and Innovation grant PCCDI-UEFISCDI project number PN-III-P1-1.2-PCCDI-2017-0476/51PCCDI/2018.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao G. Nanostructures and Nanomaterials: Synthesis, Properties and Applications. Imperial College Press; London, UK: 2004. [DOI] [Google Scholar]
- 2.Alagarasi A. Chapter 1—Introduction to nanomaterials. In: Viswanathan B., editor. Nanomaterials. Narosa Publishing House; Mumbay, India: 2009. pp. 2–25. [Google Scholar]
- 3.Canas-Carrell J.E., Li S., Parra A.M., Shrestha B. Chapter 10—Metal oxide nanomaterials: Health and environmental effects. In: Njuguna J., Pielichowski K., Zhu H., editors. Health and Environmental Safety of Nanomaterials. Woodhead Publishing; Kidlington, Oxford, UK: 2014. pp. 200–221. [DOI] [Google Scholar]
- 4.Athar T. Chapter 14—Metal oxide nanopowder. In: Ahmed W., Jackson M.J., editors. Micro and Nano Technologies, Emerging Nanotechnologies for Manufacturing. 2nd ed. William Andrew Publishing; San Diego, CA, USA: 2015. pp. 343–401. [DOI] [Google Scholar]
- 5.Athar T. Chapter 17—Smart precursors for smart nanoparticles. In: Ahmed W., Jackson M.J., editors. Micro and Nano Technologies, Emerging Nanotechnologies for Manufacturing. 2nd ed. William Andrew Publishing; San Diego, CA, USA: 2015. pp. 444–538. [DOI] [Google Scholar]
- 6.Banerjee A.N. The design, fabrication, and photocatalytic utility of nanostructured semiconductors: Focus on TiO2-based nanostructures. Nanotechnol. Sci. Appl. 2011;4:35–65. doi: 10.2147/NSA.S9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang F., Yan A., Zhao H. Chapter 2—Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst. In: Cao W., editor. Semiconductor Photocatalysis—Materials, Mechanisms and Applications. IntechOpen; London, UK: 2016. pp. 31–80. [Google Scholar]
- 8.Pandele-Cușu J., Atkinson I., Rusu A., Apostol N., Teodorescu V., Predoană L., Szilágyi I.M., Pokol G., Zaharescu M. Thermal behavior of Cu-doped TiO2 gels synthesized by the sol-gel method. Rev. Roum. Chim. 2021;66:221–229. doi: 10.33224/rrch.2021.66.3.01. [DOI] [Google Scholar]
- 9.Stanciu I., Predoană L., Pandele-Cușu J., Preda S., Anastasescu M., Vojisavljević K., Malič B., Zaharescu M. Thermal behaviour of the TiO2 based gels obtained by microwaves assisted sol-gel method. J. Therm. Anal. Calorim. 2017;130:639–651. doi: 10.1007/s10973-017-6478-y. [DOI] [Google Scholar]
- 10.Gomez-Polo C., Larumbe S., Gil A., Muñoz D., Fernández L.R., Barquín L.F., García-Prieto A., Fdez-Gubieda M.L., Muela A. Improved photocatalytic and antibacterial performance of Cr doped TiO2 nanoparticles. Surf. Interfaces. 2021;22:100867. doi: 10.1016/j.surfin.2020.100867. [DOI] [Google Scholar]
- 11.Shen J.-H., Chuang H.-Y., Jiang Z.-W., Liu X.-Z., Horng J.-J. Novel quantification of formation trend and reaction efficiency of hydroxyl radicals for investigating photocatalytic mechanism of Fe-doped TiO2 during UV and visible light-induced degradation of acid orange. Chemosphere. 2020;251:126380. doi: 10.1016/j.chemosphere.2020.126380. [DOI] [PubMed] [Google Scholar]
- 12.Matias M.L., Pimentel A., Reis-Machado A.S., Rodrigues J., Deuermeier J., Fortunato E., Martins R., Nunes D. Enhanced Fe-TiO2 Solar Photocatalysts on Porous Platforms for Water Purification. Nanomaterials. 2022;12:1005. doi: 10.3390/nano12061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y., Zhang Q., Shi X., Song Q., Zhou C., Jiang D. Photocatalytic reduction of CO2 into CH4 over Ru-doped TiO2: Synergy of Ru and oxygen vacancies. J. Colloid Interf. Sci. 2022;608:2809–2819. doi: 10.1016/j.jcis.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Mancuso A., Morante N., De Carluccio M., Sacco O., Rizzo L., Fontana M., Esposito S., Vaiano V., Sannino D. Solar driven photocatalysis using iron and chromium doped TiO2 coupled to moving bed biofilm process for olive mill wastewater treatment. Chem. Eng. J. 2022;45015:138107. doi: 10.1016/j.cej.2022.138107. [DOI] [Google Scholar]
- 15.Piątkowska A., Janus M., Szymański K., Mozia S. C-,N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts. 2021;11:144. doi: 10.3390/catal11010144. [DOI] [Google Scholar]
- 16.Li X., Wen J., Low J., Fang Y., Yu J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014;57:70–100. doi: 10.1007/s40843-014-0003-1. [DOI] [Google Scholar]
- 17.Ran J., Jaroniec M., Qiao S.-Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018;30:1704649. doi: 10.1002/adma.201704649. [DOI] [PubMed] [Google Scholar]
- 18.Qin Q., Wang J., Xia Y., Yang D., Zhou Q., Zhu X., Feng W. Synthesis and Characterization of Sn/Ni Single Doped and Co–Doped Anatase/Rutile Mixed–Crystal Nanomaterials and Their Photocatalytic Performance under UV–Visible Light. Catalysts. 2021;11:1341. doi: 10.3390/catal11111341. [DOI] [Google Scholar]
- 19.Bachvarova-Nedelcheva A., Yordanov S., Iordanova R., Stambolova I., Stoyanova A., Georgieva N., Nemska V. The Influence of Nd and Sm on the Structure and Properties of Sol-Gel-Derived TiO2 Powders. Molecules. 2021;26:3824. doi: 10.3390/molecules26133824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahmasebizad N., Hamedani M.T., Shaban Ghazani M., Pazhuhanfar Y. Photocatalytic activity and antibacterial behavior of TiO2 coatings co-doped with copper and nitrogen via sol–gel method. J. Sol-Gel. Sci. Technol. 2020;93:570–578. doi: 10.1007/s10971-019-05085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura M., Ono A., Bae E., Murakami N., Ohno N. Improvement of visible light responsivity of rutile TiO2 nanorods by site-selective modification of iron (III) ion on newly exposed faces formed by chemical etching treatment. Appl. Catal. B. 2013;130:264–269. doi: 10.1016/j.apcatb.2012.11.012. [DOI] [Google Scholar]
- 22.Savio A.K.P.D., Fletcher J., Hernandez F.R. Sonosynthesis of nanostructured TiO2 doped with transition metals having variable bandgap. Ceram. Int. 2013;39:2753–2765. doi: 10.1016/j.ceramint.2012.09.042. [DOI] [Google Scholar]
- 23.Suzuki K., Yokoi T., Iwatsu M., Furuya M., Yokota K., Mokudai T., Kanetaka H., Kawashita M. Antibacterial properties of Cu-doped TiO2 prepared by chemical and heat treatment of Ti metal. J. Asian Ceram. Soc. 2021;9:1448–1456. doi: 10.1080/21870764.2021.1979287. [DOI] [Google Scholar]
- 24.Wang Y., Yang H., Xue X. Synergistic antibacterial activity of TiO2 co-doped with zinc and yttrium. Vacuum. 2014;107:28–32. doi: 10.1016/j.vacuum.2014.03.026. [DOI] [Google Scholar]
- 25.Shaikh S., Nazam N., Mohd S., Rizvi D., Ahmad K., Baig M.H., Lee E.J., Choi I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019;20:2468. doi: 10.3390/ijms20102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S.G., Rao K.S.R.K. Polymorphic phase transition among the titania crystal structures in solution based approach: From precursor chemistry to nucleation process. Nanoscale. 2014;6:11574–11632. doi: 10.1039/C4NR01657B. [DOI] [PubMed] [Google Scholar]
- 27.Lu C.W., Cao Y., Li H., Webb C., Pan W.P. Synthesis of TiO2 based on hydrothermal methods using elevated pressures and microwave conditions. J. Therm. Anal. Calorim. 2014;116:1241–1248. doi: 10.1007/s10973-014-3740-4. [DOI] [Google Scholar]
- 28.Bregadiolli B.A., Fernandes S.L., Graef C.F.O. Easy and Fast Preparation of TiO2—Based Nanostructures Using Microwave Assisted Hydrothermal Synthesis. Mater. Res. 2017;20:912–919. doi: 10.1590/1980-5373-mr-2016-0684. [DOI] [Google Scholar]
- 29.Falk G.S., Borlaf M., López-Muñoz M.J., Farinas J.C., Rodrigues Neto J.B., Moreno R. Microwave-assisted synthesis of TiO2 nanoparticles: Photocatalytic activity of powders and thin films. J. Nanopart. Res. 2018;20:23. doi: 10.1007/s11051-018-4140-7. [DOI] [Google Scholar]
- 30.Kéri O., Kocsis E., Karajz D.A., Nagy Z.K., Parditka B., Erdélyi Z., Szabó A., Hernádi K., Szilágyi I.M. Photocatalytic Crystalline and Amorphous TiO2 Nanotubes Prepared by Electrospinning and Atomic Layer Deposition. Molecules. 2021;26:5917. doi: 10.3390/molecules26195917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinogradov A.V., Vinogradov V.V. Low-temperature sol–gel synthesis of crystalline materials. RSC Adv. 2014;4:45903–45919. doi: 10.1039/C4RA04454A. [DOI] [Google Scholar]
- 32.Nachit W., Ait Ahsaine H., Ramzi Z., Touhtouh S., Goncharova I., Benkhouja K. Photocatalytic activity of anatase-brookite TiO2 nanoparticles synthesized by sol gel method at low temperature. Opt. Mater. 2022;129:112256. doi: 10.1016/j.optmat.2022.112256. [DOI] [Google Scholar]
- 33.Sadek O., Touhtouh S., Rkhis M., Anoua R., El Jouad M., Belhora F., Hajjaji A. Synthesis by sol-gel method and characterization of nano-TiO2 powders. Mater. Today Proc. 2022;66:456–458. [Google Scholar]
- 34.Szołdra P., Frąc M., Pichór W. Effect of sol composition on the properties of TiO2 powders obtained by the sol–gel method. Powder Technol. 2021;387:261–269. doi: 10.1016/j.powtec.2021.04.037. [DOI] [Google Scholar]
- 35.Keshari A.K., Choudhary P., Shukla V.K. Precursor induced evolution in single anatase phase synthesis of TiO2 nanoparticles for water treatment and dye-sensitized solar cell. Phys. B Condens. Matter. 2022;631:413716. doi: 10.1016/j.physb.2022.413716. [DOI] [Google Scholar]
- 36.Rathore N., Kulshreshtha A., Shukla R.K., Sharma D. Study on morphological, structural and dielectric properties of sol–gel derived TiO2 nanocrystals annealed at different temperatures. Phys. B Condens. Matter. 2020;582:411969. doi: 10.1016/j.physb.2019.411969. [DOI] [Google Scholar]
- 37.Schmidt H. Chemistry of material preparation by the sol–gel process. J. Non-Cryst. Solids. 1998;100:51–64. doi: 10.1016/0022-3093(88)90006-3. [DOI] [Google Scholar]
- 38.Sakka S., Kamya K. The Sol–Gel Transition in the Hydrolysis of Metal Alkoxides in Relation to Formation of Glass Fibers and Films. J. Non-Cryst. Solids. 1982;48:31–46. doi: 10.1016/0022-3093(82)90244-7. [DOI] [Google Scholar]
- 39.ThermoFisher Scientific XPS Database. [(accessed on 26 September 2022)]. Available online: https://www.thermofisher.com/ro/en/home/materials-science/learning-center/periodic-table/transition-metal/titanium.html.
- 40.NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20. National Institute of Standards and Technology; Gaithersburg, MD, USA: 2000. Version 4.1, Last Update to Data Content: 2012, (retrieved 26 September 2022), 20899. [DOI] [Google Scholar]
- 41.ThermoFisher Scientific XPS Database. [(accessed on 26 September 2022)]. Available online: https://www.thermofisher.com/ro/en/home/materials-science/learning-center/periodic-table/transition-metal/copper.html.
- 42.Wagner C.D., Davis L.E., Zeller M.V., Taylor J.A., Raymond R.M., Gale L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal. 1981;3:211–225. doi: 10.1002/sia.740030506. [DOI] [Google Scholar]
- 43.Pandele-Cușu J., Petrescu S., Preda S., Petcu G., Ciobanu M., Predoană L. Comparative study of the TiO2 nanopowders prepared from diferent precursors and chemical methods for heterogeneous photocatalysis application. J. Therm. Anal. Calorim. 2022 doi: 10.1007/s10973-022-11544-9. [DOI] [Google Scholar]
- 44.Teodorescu C.M., Esteva J.M., Karnatak R.C., El Afif A. An approximation of the Voigt I profile for the fitting of experimental X-ray absorption data. Nucl. Instrum. Meth. Phys. Res. A. 1994;345:141–147. doi: 10.1016/0168-9002(94)90983-0. [DOI] [Google Scholar]
- 45.Fruth V., Todan L., Codrea C.I., Poenaru I., Petrescu S., Aricov L., Ciobanu M., Jecu L., Ion R.M., Predoana L. Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation. Nanomaterials. 2021;11:2586. doi: 10.3390/nano11102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khashan K.S., Sulaiman G.M., Abdulameer F.A., Albukhaty S., Ibrahem M.A., Al-Muhimeed T., AlObaid A.A. Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Appl. Sci. 2021;11:4623. doi: 10.3390/app11104623. [DOI] [Google Scholar]
- 47.Azizi-Lalabadi M., Ehsani A., Divband B., Alizadeh-Sani M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019;9:17439. doi: 10.1038/s41598-019-54025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sing K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984) Pure Appl. Chem. 1985;57:603–619. doi: 10.1351/pac198557040603. [DOI] [Google Scholar]
- 49.Fageria P., Gangopadhyay S., Pande S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014;4:24962–24972. doi: 10.1039/C4RA03158J. [DOI] [Google Scholar]
- 50.Colon G., Maicu M., Hidalgo M.C., Navıo J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006;67:41–51. doi: 10.1016/j.apcatb.2006.03.019. [DOI] [Google Scholar]
- 51.Augilar T., Novas J., Alcantara R. A route for the synthesis of Cu-doped TiO2 nanoparticles with a very low band gap. Chem. Phys. Lett. 2013;571:49–53. doi: 10.1016/j.cplett.2013.04.007. [DOI] [Google Scholar]
- 52.Perkgoz N.K., Toru R.S., Unal E., Sefunc M.A., Tek S., Mutlugun E., Soganci I.M., Celiker H., Celiker G., Demir H.V. Photocatalytic Hybrid Nanocomposites of Metal Oxide Nanoparticles Enhanced Towards the Visible Spectral Range. Appl. Catal. B. 2011;105:77–85. doi: 10.1016/j.apcatb.2011.03.037. [DOI] [Google Scholar]
- 53.Navas J., Sánchez-Coronilla A., Aguilar T., Hernández N.C., de los Santos D.M., Sánchez-Márquez J., Zorrilla D., Fernández-Lorenzo C., Alcántara R., Martín-Calleja J. Experimental and theoretical study of the electronic properties of Cu-doped anatase TiO2. Phys. Chem. Chem. Phys. 2014;16:3835–3845. doi: 10.1039/c3cp54273d. [DOI] [PubMed] [Google Scholar]
- 54.Mahdy S.A., Raheed Q.J., Kalaichelvan P. Antimicrobial activity of zero-valent iron nanoparticles. IJMER. 2012;2:578–581. [Google Scholar]
- 55.Gingașu D., Mîndru I., Culiță D.C., Predoană L., Petcu G., Ciobanu M., Preda S., Pandele-Cușu J., Petrescu S. MgO obtained by chemical and green synthesis methods and applied in photocatalytic degradation of methyl orange. Rev. Roum. Chim. 2021;66:463–473. doi: 10.33224/rrch.2021.66.5.10. [DOI] [Google Scholar]