Abstract
Jatropha is a small woody perennial biofuel-producing shrub. Stress-associated proteins (SAPs) are novel stress regulatory zinc-finger proteins and are mainly associated with tolerance against various environmental abiotic stresses in Jatropha. In the present study, the JcSAP gene family were analyzed comprehensively in Jatropha curcas and 11 JcSAP genes were identified. Phylogenetic analysis classified the JcSAP genes into four groups based on sequence similarity, similar gene structure features, conserved A20 and/or AN1 domains, and their responsive motifs. Moreover, the divergence analysis further evaluated the evolutionary aspects of the JcSAP genes with the predicted time of divergence from 9.1 to 40 MYA. Furthermore, a diverse range of cis-elements including light-responsive elements, hormone-responsive elements, and stress-responsive elements were detected in the promoter region of JcSAP genes while the miRNA target sites predicted the regulation of JcSAP genes via a candid miRNA mediated post-transcriptional regulatory network. In addition, the expression profiles of JcSAP genes in different tissues under stress treatment indicated that many JcSAP genes play functional developmental roles in different tissues, and exhibit significant differential expression under stress treatment. These results collectively laid a foundation for the functional diversification of JcSAP genes.
Keywords: abiotic stress, divergence, expression profile, jatropha, phylogeny, SAP genes
1. Introduction
Plant growth and productivity is severely affected by various biotic and abiotic stresses because of their sessile nature. These environmental stresses down- or upregulate a large pool of genes. To eliminate or reduce the harsh environmental effect, plants have evolved complex internal molecular mechanisms to modulate stress-responsive or regulatory genes [1,2]. Regulatory genes code for sensors that perceive stress signals, kinases that transmit the signals, and transcription factors that are down- or upregulated as a result of perceived stress. Thus, defence mechanisms start with the perception of stress, followed by signal transduction, synthesis of transcription factors, and finally down- or upregulation of genes that produce protective proteins and metabolites [2]. Hitherto, a large number of genes have been characterized that play an important role during different stresses and are involved at different levels of regulation, such as perception, signalling, transcription, and production of protective biomolecules [3,4,5,6,7]. The characterisation of genes belonging to the signal transduction and transcription factor categories is of great importance because of their effect on a wide range of stress-related genes. Zinc finger proteins are associated with this category and are considered the important proteins that contribute to protection against environmental stress [8,9,10,11].
Stress-associated proteins (SAP) are the newly identified stress regulatory zinc-finger proteins in plants with the A20 domain at N-terminal and AN1 domain at C-terminal, and are mainly associated with tolerance against various environmental abiotic stresses [1,12,13,14,15]. SAP genes in plants were first identified in Oryza sativa, and OsSAP1 was declared to be largely involved in abscisic acid, healing wounds, heavy metals, salt, drought, and cold [9]. Similarly, other OsSAP genes have also been expressed under several environmental stresses, indicating their role in stress-response [5]. Recently, the response of SAP genes has been identified in several plant species and has been studied against various environmental stresses. The evidence from the previous literature has shown the presence of the SAP gene family in many species, like 18 SAP gene members in rice [5], 14 in Arabidopsis [5], 17 in barley [13], 27 in soybean [12], 37 in cotton [16], 13 in tomato [2], 12 in cucumber [17], 21 in eggplant [1], and 9 in almond [18]. However, the identification of SAP genes in Jatropha genome is not systematically deliberated.
Jatropha (Jatropha curcas L.) is a small woody perennial shrub that belongs to the subfamily Crotonoideae of Euphorbiaceae. Jatropha is a biofuel-producing non-food crop mostly grown in subtropical as well as tropical regions, with 40 to 50% of oil content in its seeds. Particularly important is the fact that Jatropha can grow on degraded soils, making it an attractive crop for biodiesel feedstock as it can be widely planted on marginal land considered inappropriate for food crops [19]. In addition, Jatropha is adaptable to grow in a wide range of agro-climatic conditions and possesses several properties like easy propagation, short gestation period, rapid growth, high oil content, low seed cost, and tolerance to salt and drought, making it suitable for biodiesel production [19,20,21]. With the increasing costs, gradual depletion of fossil energy resources, and having a strong potential for biofuel production, Jatropha is recently attracting the widespread attention of researchers. The identification of the SAP gene family in Jatropha might encompass a novel understanding of the flow and appearance of the mechanism of stress regulatory genes. In the current study, we identified the SAP gene family genome-wide in Jatropha and comprehensively analysed their phylogeny, domain, motif, gene structure, chromosome location, gene duplication, cis-acting elements, miRNA target sites, and the expression analysis in various tissues against stress, which laid a foundation for further studies on the biological function of SAP genes in Jatropha.
2. Materials and Methods
2.1. Identification, Sequence Alignment, and Phylogenetic Analysis of SAP Genes in Jatropha Curcas
To identify SAP genes in Jatropha, Pfam server (http://pfam.xfam.org/) (accessed on 31 August 2022) was searched for the A20 domain (PF01754) and AN1 domain (PF01428), and then the HMMER (https://www.ebi.ac.uk/Tools/hmmer/search/hmmsearch) (accessed on 31 August 2022) Hidden Markov model was used as a probe to screen all the candidate proteins. To ensure the reliability of the sequences and to remove redundant sequences, the search results of all candidate SAP protein sequences were further searched for the presence of A20/AN1 domains using the Pfam database (http://pfam.janelia.org/) (accessed on 31 August 2022), MOTIF search (https://www.genome.jp/tools/motif/) (accessed on 31 August 2022), NCBI conserved domain database (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (accessed on 31 August 2022), SMART database (http://smart.embl-heidelberg.de/) (accessed on 31 August 2022), and Inter ProScan program (http://www.ebi.ac.uk/Tools/pfa/ iprscan5/) (accessed on 31 August 2022). The protein sequences of the SAP gene family of rice and Arabidopsis (Supplementary Table S1) were retrieved from the previously published report [5]. Multiple sequence alignments of all the sequences of Jatropha, Arabidopsis, and rice were performed using Muscle. Subsequently, to create a phylogenetic tree, the alignments were imported to MEGA7 software (https://www.megasoftware.net/home) (accessed on 1 September 2022) using the neighbor-joining (NJ) method with a bootstrap option of 1000 replications. The phylogenetic tree was further visualized and edited via MEGA7 software (https://www.megasoftware.net/home) (accessed on 1 September 2022) [22,23,24,25,26].
2.2. JcSAP Protein Physicochemical Characteristics
To find the physicochemical properties of JcSAP genes, the NCBI database (https://www.ncbi.nlm.nih.gov/) (accessed on 1 September 2022) was searched to find the amino acid (bp), CDS (bp) and location on Chromosome. The ProtParam tool of ExPASy server (http://web.expasy.org/protparam) (accessed on 1 September 2022) was searched to predict the isoelectric points (pI), molecular weight (MW), grand average of hydropathicity (GRAVY), and Formula of each SAP gene in Jatropha curcas. Softberry server for plant protein location (http://www.softberry.com/berry.phtml?topic=protcomppl&group=programs&subgroup=proloc) (accessed on 1 September 2022) was employed to predict the subcellular localization each SAP protein in Jatropha curcas [22].
2.3. Gene Duplication Events, Homology and Synteny Analysis of JcSAP Genes
For Gene conservation, duplication events, homology, and Synteny analysis, a comparative Synteny analysis was performed by using circoletto Tool (https://www.tools.bat.infspire.org/circoletto/) (accessed on 2 September 2022) to visualize genome conservation. Protein sequences of Arabidopsis SAP genes were used against the identified JcSAP sequences and were finally exhibited by circus by running the Circoletto tool [27].
2.4. Conserved Domains, Motifs, and Gene Structure Organization of JcSAP Genes
The identified JcSAP protein sequences were subjected to NCBI CDD online software (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (accessed on 2 September 2022) for domain analysis, and the obtained results were visualized via the TBtools software (https://github.com/CJ-Chen/TBtools) (accessed on 2 September 2022). Similarly, to analyze the JcSAP proteins for the conserved motifs, the protein sequences of JcSAP were submitted to MEME suite software 5.4.1 (https://meme-suite.org/meme/tools/meme) (accessed on 2 September 2022). Consistently, to display the gene structure organization of JcSAP genes, the gene structure display server (http://gsds.gao-lab.org/) (accessed on 2 September 2022) was used by submitting the CDS and genomic sequences of JcSAP genes [22,24].
2.5. Divergence Analysis
For divergence analysis of JcSAP genes, the server Ka/Ks calculation tool (http://services.cbu.uib.no/tools/kaks) (accessed on 2 September 2022) was used and the non-synonymous substitution per nonsynonymous site (Ka) and synonymous substitution per synonymous site (Ks) was determined by inputting the DNA sequences of JcSAP genes using default parameters. The divergence time was calculated by the given formula [24,28].
2.6. Protein Structure Analysis of JcSAP Genes
To identify the structural composition of 11 JcSAP genes, an online tool for the prediction of secondary structure SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/secpredsopma.pl) (accessed on 2 September 2022) were used. The tertiary structure of JcSAP proteins were visualized via uniprot (https://www.uniprot.org) (accessed on 2 September 2022) to further support the secondary structure of JcSAP proteins [29].
2.7. Cis-Elements Analysis and Predicted miRNA Target Sites
To analyse the cis-regulatory element, the upstream region of 1500 bp of each genomic sequence of the JcSAP genes was submitted to PlantCARE server (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (accessed on 5 September 2022) and was searched for the presence of cis-regulatory elements. The results were then visualized using TBtools software (https://github.com/CJ-Chen/TBtools) (accessed on 6 September 2022) [24].
For the prediction miRNA target sites for JcSAP genes, the published miRNAs of Jatropha curcas were downloaded from the plant miRNA encyclopaedia (https://pmiren.com/download) (accessed on 6 September 2022) and were then subjected to the psRNATarget database (https://www.zhaolab.org/psRNATarget/analysis?function=3) (accessed on 6 September 2022) along with the CDS of JcSAP genes to predict the miRNA target sites and interaction with JcSAP genes. Finally, the results were visualized using Cytoscape software (Cytoscape Consortium, San Francisco, CA, USA) (http://apps.cytoscape.org/apps/stringapp) (accessed on 7 September 2022) [24,30].
2.8. Gene Expression Profiling of JcSAP Genes
For JcSAP gene expression patterns in different plant tissues under abiotic stress, the raw expression data of leaf and root tissues subjected to drought stress was retrieved from the public database of NCBI (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE61109) (accessed on 11 September 2022) under the GEO accession number GSE61109. The expression profiles of all JcSAP genes were exhibited as reads per kilobase per million (RPKM) values and illustrated via heat map using TBtools [30,31].
3. Results
3.1. Identification and Phylogenetic Analysis of JcSAP Genes
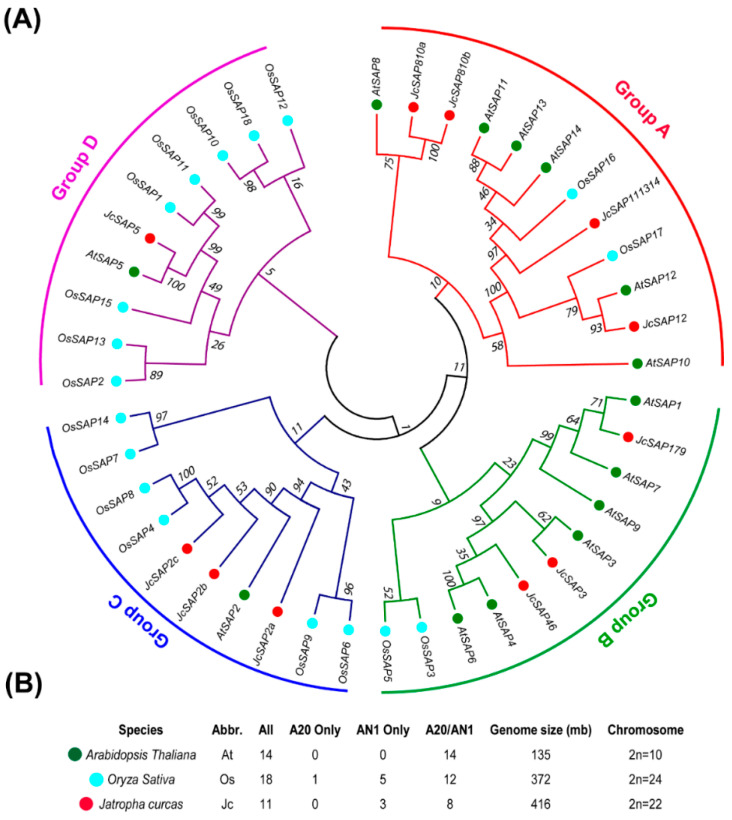
To gain insight into the identification and evolution of JcSAP genes, the Hidden Markov model profiles of the A20 domain and AN1 domain were used as a probe to screen all the candidate proteins and were then used with the protein sequences of Arabidopsis thaliana and Oryza sativa to construct a phylogenetic tree (see materials and methods). Results demonstrated that A. thaliana, having a genome size of 135 mb and 2n chromosome number of 10, had a total of 14 SAP genes following both the A20 and AN1 domain. Oryza sativa, having a genome size of 372 mb and 2n chromosome number of 24, had a total of 18 SAP genes in which 12 members were following both the A20 and AN1 domain, 5 members were following only the AN1 domain, while 1 member was following only the A20 domain. Jatropha curcas, having a genome size of 416 mb and 2n chromosome number of 22, had a total of 11 SAP genes in which 8 members were following both the A20 and AN1 domain, 3 members were following only the AN1 domain, while there were no single gene members following the A20 domain only (Figure 1B, Table S1). To further investigate the classification and the evolutionary characteristics of the JcSAP proteins, an unrooted phylogenetic tree was constructed based on the SAP protein sequences of Arabidopsis thaliana, Oryza sativa, and Jatropha (Figure 1A). All available 34 sequences, including 14 AtSAP, 18 OsSAP, and 11 JcSAP, were mainly clustered into four groups. Group A contained four JcSAP members (JcSAP810a, JcSAP810b, JcSAP111314, and JcSAP12), clustered to AtSAP8, AtSAP10, AtSAP11, AtSAP13, and AtSAP14. Group B consisted of three JcSAP members (JcSAP179, JcSAP3, JcSAP46), clustered to AtSAP1, AtSA3, AtSAP4, AtSAP6, AtSAP7, and AtSAP9. Group C consisted of three JcSAP members (JcSAP2a, JcSAP2b, and JcSAP2c), clustered to AtSAP2. Group D included only one JcSAP member (JcSAP5) clustered to AtSAP5. These results of the comparative phylogenetic relationship predicted that the SAP members clustered together or with other species may share similar biological functions against stresses.
Figure 1.
Identification and phylogenetic Analysis of SAP members of Jatropha against Arabidopsis and rice. (A) Phylogenetic tree of 14 arabidopsis, 18 rice, and 11 Jatropha SAP members, (B) Identified SAP members of Arabisopsis, rice, and Jatropha with followed domains, genome size, and chromosome numbers.
3.2. Physicochemical Characteristics of JcSAP Genes
To further get into the physicochemical characteristic of the total 11 identified SAP genes in Jatropha in a comprehensive manner (Figure 1), the physicochemical parameters of JsSAP genes including gene code, location on chromosome, amino acids and CDS length, molecular weight (MW/kDa), isoelectric point (PI), GRAVY, Formula, and Predicted subcellular localization were investigated insilico and exhibited in Table 1 and Table S1. Results demonstrated that the amino acid length JcSAP genes were varied from 133 (JcSAP2a) to 288 (JcSAP111314). CDS was ranged from 402 (JcSAP2a) to (867) (JcSAP111314). Molecular weight (MW/kDa) JcSAP genes varied from 14686.65 kDA (JcSAP2a) to 31998.28 (JcSAP111314), while the isoelectric point (PI) ranged from 8 PI (JcSAP179) to 9.4 PI (JcSAP5). Moreover, the predicted subcellular localization revealed that all JcSAP genes are cytoplasmic expect JcSAP111314 and JcSAP12, which appeared to be extracellular. The presence of each JcSAP gene member on a specific chromosome was not predicted in silico, however the GRAVY and formula were given in Table 1. These results provide information about the basic known parameters of the JcSAP genes.
Table 1.
Physicochemical characteristics of JcSAP genes.
| Gene Name | Gene ID (Uniprot) | Chr | AA | CDS | MW/kDa | PI | GRAVY | Formula | Predicted Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|
| JcSAP179 | A0A067JZZ9_JATCU | Un | 178 | 537 | 18834.23 | 8 | −0.453 | C809H1279N231O261S13 | Cytoplasmic |
| JcSAP2a | A0A067KTX6_JATCU | Un | 133 | 402 | 14686.65 | 9.1 | −0.612 | C630H990N188O194S12 | Cytoplasmic |
| JcSAP2b | A0A067JE11_JATCU | Un | 171 | 516 | 18507.08 | 8.5 | −0.442 | C785H1266N236O249S16 | Cytoplasmic |
| JcSAP2c | A0A067L4U9_JATCU | Un | 170 | 513 | 18087.44 | 8.8 | −0.304 | C761H1233N229O251S15 | Cytoplasmic |
| JcSAP3 | A0A067KRF9_JATCU | Un | 170 | 513 | 18661.18 | 8.8 | −0.492 | C793H1276N236O254S15 | Cytoplasmic |
| JcSAP46 | A0A067L6B6_JATCU | Un | 159 | 480 | 17559.01 | 8.6 | −0.521 | C754H1197N225O231S14 | Cytoplasmic |
| JcSAP5 | A0A067L4Q5_JATCU | Un | 185 | 558 | 20311.78 | 9.4 | −0.665 | C849H1395N271O283S12 | Cytoplasmic |
| JcSAP810a | A0A067JER8_JATCU | Un | 135 | 408 | 14879.04 | 9.3 | −0.49 | C643H1038N186O199S10 | Cytoplasmic |
| JcSAP810b | A0A067JQU7_JATCU | Un | 204 | 615 | 22711.95 | 9.3 | −0.775 | C972H1602N282O314S14 | Cytoplasmic |
| JcSAP111314 | A0A067K2L3_JATCU | Un | 288 | 867 | 31998.28 | 8.6 | −0.665 | C1367H2182N416O426S23 | Extracellular |
| JcSAP12 | A0A067LAI2_JATCU | Un | 197 | 594 | 21773.79 | 9 | −0.639 | C924H1487N285O288S18 | Extracellular |
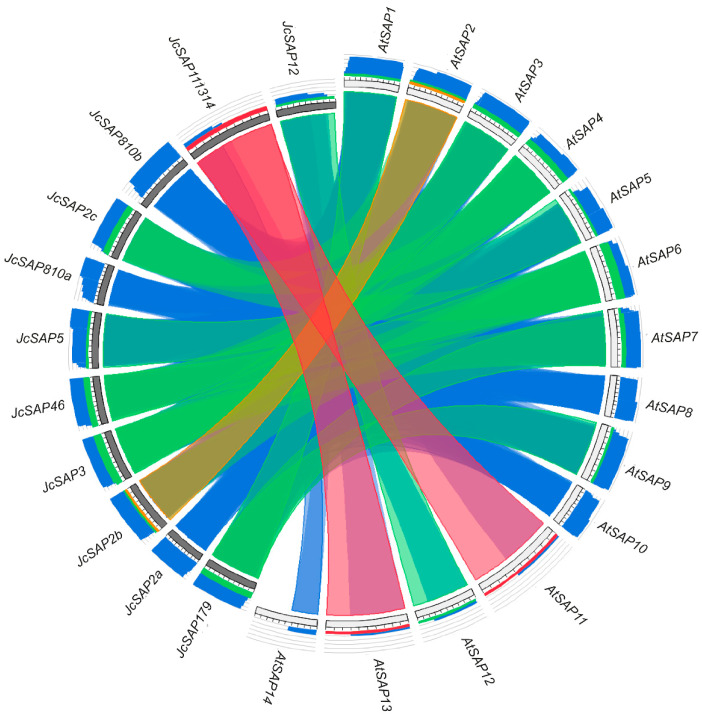
3.3. Synteny Analysis of JcSAP Genes
To further support the identification and homologous relationship among SAP genes, Synteny analysis of all the identified SAP genes of Jatropha and Arabidopsis was subjected to the circoletto tool to make a map of comparative synteny circos (see materials and methods). The Synteny map illustrated the relationship among the SAP genes of Jatropha and Arabidopsis species regarding their function, expression, duplication events, and evolution (Figure 2). The sequences were placed clockwise around a circle, starting at 12 o’clock, and the ideograms were placed in order to maximally untangle the ribbons; the queries and database entries were intertwined. Ribbon colors of the map diagram represent the alignment length, visualizing the sequence similarity and identity level, i.e., blue ≤ 0.25, green ≤ 0.50, orange ≤ 0.75, red > 0.75, providing an essential first glimpse at sequence relationships. The obtained results revealed that Arabidopsis AtSAP1, AtSAP7, and AtSAP9 showed Synteny with JcSAP179 of Jatropha. Similarly, AtSAP2 showed Synteny with JcSAP2a, JcSAP2b, and JcSAP2c. AtSAP3 showed Synteny with JcSAP3. AtSAP4 and AtSAP6 showed Synteny with JcSAP46. AtSAP5 showed Synteny with JcSAP5. AtSAP10 showed Synteny with JcSAP810a, JcSAP810b. AtSAP11, AtSAP13, and AtSAP14 showed Synteny with JcSAP111314. AtSAP12 showed Synteny with JcSAP12. Moreover, based on the color intensity, the inward and outward tangling of ribbons showed conservation and duplication events, respectively, suggesting that SAP genes were conserved in Jatropha during evolution.
Figure 2.
Synteny map of identified SAP genes in Arabidopsis thaliana and Jatropha curcas.
3.4. Determination of Non-Synonymous (Ka) and Synonymous (Ks) Substitution Rate
The divergence analysis was performed to gain insight into the evolutionary aspects of JcSAP genes by determining the non-synonymous substitution per non-synonymous site (Ka) and synonymous substitution per synonymous site (Ks) for each pair of paralogous JcSAP genes according to the phylogenetic tree (Figure S1) generated by Ka/Ks calculation server, in order to indicate the evolutionary discretion among JcSAP genes (Table 2). Results with a Ka/Ks value of <1 each pair of paralogous genes indicated the purifying selection pressure during the evolution. The divergence time for each pair of JcSAP genes ranged from 9.1 to 40 million years ago (MYA) (Table 3).
Table 2.
Secondary Structure of 11 JcSAP proteins.
| H (%) | T (%) | E (%) | RC (%) | |
|---|---|---|---|---|
| JcSAP11/13/14 | 22.22 | 1.04 | 11.11 | 65.62 |
| JcSAP12 | 13.2 | 2.54 | 13.71 | 70.56 |
| JcSAP1/7/9 | 21.35 | 2.81 | 11.8 | 64.04 |
| JcSAP3 | 27.65 | 3.53 | 11.76 | 57.06 |
| JcSAP2b | 25.15 | 4.68 | 10.53 | 59.65 |
| JcSAP4/6 | 30.19 | 4.4 | 12.58 | 52.83 |
| JcSAP2a | 26.32 | 4.51 | 13.53 | 55.64 |
| JcSAP5 | 37.84 | 2.7 | 10.27 | 49.19 |
| JcSAP8/10a | 14.07 | 3.7 | 13.33 | 68.89 |
| JcSAP2c | 28.82 | 4.12 | 11.76 | 55.29 |
| JcSAP8/10b | 21.08 | 2.94 | 13.24 | 62.75 |
Table 3.
Non-synonymous (Ka) and synonymous (Ks) substitution rate and divergence time of JcSAP genes.
| Paralogous Genes | Ka | Ks | Ka/Ks | T (MYA) | |
|---|---|---|---|---|---|
| JcSAP111314 | JcSAP12 | 0.2614 | 0.5275 | 0.495545 | 40.2 |
| JcSAP810b | JcSAP810a | 0.04609 | 0.11945 | 0.385851 | 9.1 |
| JcSAP2b | JcSAP2c | 0.12225 | 0.33925 | 0.360354 | 25.8 |
| JcSAP4/6 | JcSAP3 | 0.1221 | 0.4072 | 0.299853 | 31.0 |
| JcSAP5 | JcSAP179 | 0.26015 | 0.46745 | 0.55653 | 35.6 |
3.5. Gene Structure, Domain and Motif Analysis of JcSAP Genes
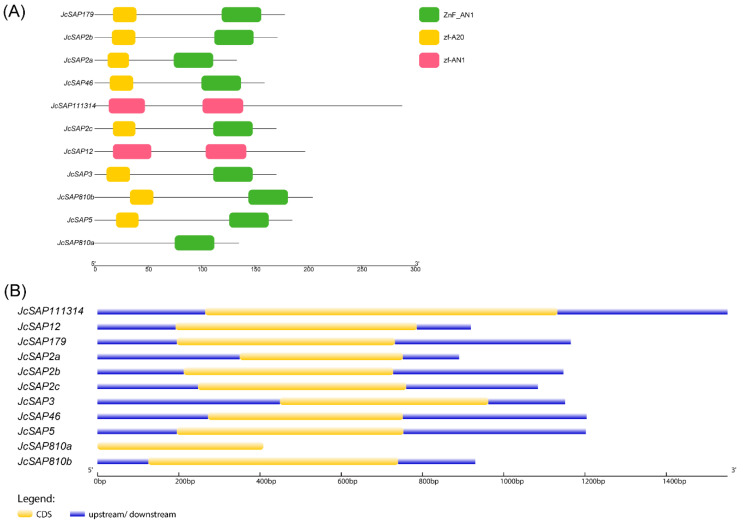
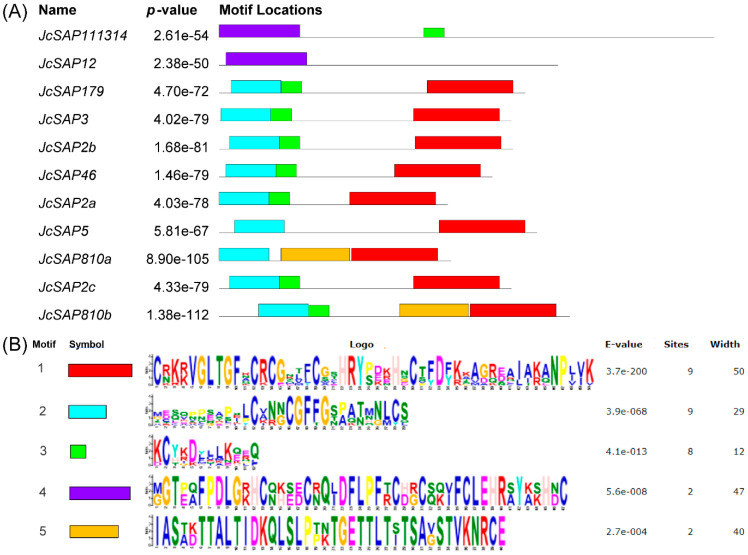
To further explore the critical fundamental function of the identified JcSAP genes, the JcSAP were further investigated for conserved domains, gene structure organization, and motifs. Eight JcSAP genes like JcSAP179, JcSAP2a, JcSAP2b, JcSAP2c, JcSAP3, JcSAP46, JcSAP5, and JcSAP810b followed both the A20 domain at the N terminal and AN1 domain at C terminal, except JcSAP111314, JcSAP12, and JcSAP810a, which followed only the AN1 domain and had no A20 domain (Figure 3A). Similarly, the motif analysis revealed that all the JcSAP genes followed five conserved motifs corresponding to the A20 and AN1 domains (Figure 4A). The logos of the five identified motifs are present in Figure 4B. The demonstration of the gene structure organization further revealed the coding region (CDS) distribution (Figure 3B), indicating that JcSAP179, JcSAP2a, JcSAP5, JcSAP810a, and JcSAP810b have one exon, 2 JcSAP3, JcSAP46, JcSAP111314, and JcSAP12 have two exons, while JcSAP2b and JcSAP2c have three exons. All these results collectively further support the conserved domain, motif, and structure and thus suggested to share similar biological functions in response to environmental abiotic stresses.
Figure 3.
Domain (A) and Gene structure organization (B) of JcSAP genes.
Figure 4.
Conserved motifs of JcSAP genes in Jatropha curcas. (A) Identified motifs; (B) logos of the identified motifs.
3.6. Protein Structure Analysis of JcSAP Genes
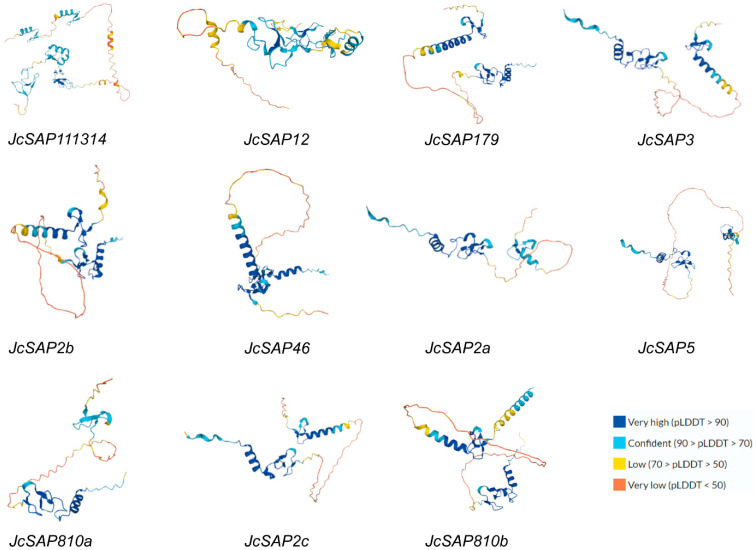
To delve further into the structure of the JcSAP genes, the secondary and tertiary structures of the JcSAP proteins were visualized (Table 2, Figure 5). The secondary structure of the JcSAP proteins explored the way these proteins fold and coil. The secondary structure of the JcSAP proteins consisted of four main elements including the α-helix (H%), β-turn (T%), extended chain (E%), and random coil (RC%). In the secondary structure of JcSAP proteins, the random coil (RC%) had the highest value ranging from 49.19% (JcSAP5) to 68.89% (JcSAP8/10a), followed by the α-helix (H%) ranging from 13.2% (JcSAP12) to 37.84% (JcSAP5), again followed by the extended chain (E%) ranging from 10.27% (JcSAP5) to 13.71% (JcSAP12), and β-turn (T%) ranging from 1.04% (JcSAP11/13/14) to 4.68% (JcSAP2b). Similarly, the tertiary structures of the JcSAP proteins visualized via uniprot (https://www.uniprot.org) (accessed on 2 September 2022) further supported the secondary structure of JcSAP proteins.
Figure 5.
Illustration of the predicted tertiary structure of 11 JcSAP proteins. The protein structures all have the same domain colour schemes, revealing the degree of homology. The structures reveal a high degree of structural homology in most gene members.
3.7. Promoter Region Analysis and Prediction of miRNA Target Sites of JcSAP Genes
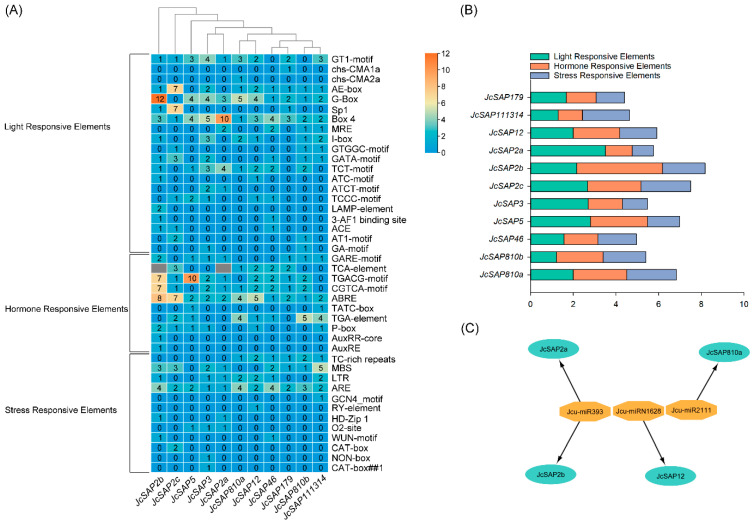
The promoter region analysis of the JcSAP genes resulted in the presence of a diverse range of cis-elements. The cis-elements identified during the promoter analysis of JcSAP genes were classified into three major groups/categories including light-responsive elements, hormone-responsive elements, and stress-responsive elements. The results revealed that there were 20 light responsive elements naming GT1-motif, chs-CMA1a, chs-CMA2a, AE-box, G-Box, Sp1, Box 4, MRE, I-box, GTGGC-motif, GATA-motif, TCT-motif, ATC-motif, TCT-motif, TCCC-motif, LAMP-element, 3-AF1 binding site, ACE, AT1-motif, GA-motif, 10 hormone responsive elements naming GARE-motif, TCA-element, TGACG-motif, CGTCA-motif, ABRE, TATC-box, TGA-element, P-box, AuxRR-core, AuxRE, and 12 Stress Responsive Elements naming TC-rich repeats, MBS, LTR, ARE, GCN4_motif, RY-element, HD-Zip 1, O2-site, WUN-motif, CAT-box, NON-box, CAT-box (Figure 6A). Moreover, the mean values of light-responsive elements ranged from 1.20 (JcSAP810b) to 3.50 (JcSAP2a), the mean values of hormone responsive elements ranged from 1.13 (JcSAP111314) to 4.00 (JcSAP2b), and the mean values of stress responsive elements ranged from 1.00 (JcSAP2a) to 2.33 (JcSAP810a and JcSAP2c) (Figure 6B).
Figure 6.
Cis regulatory elements and prediction of miRNA target sites of JcSAP genes. (A) The heat map of the Cis elements and their classification, present in the promoter region of JcSAP genes, (B) Graphical visualization of the groups of Cis elements in the promoter region of JcSAP genes, (C) Predicted miRNA target sites of JcSAP genes.
Similarly, to predict the miRNA target sites of the JcSAP genes, the coding sequences of the identified JcSAP genes were used against the miRNAs of Jatropha (see material and methods). The results revealed that only three miRNAs (Jcu-miR393, Jcu-miR1628, and Jcu-miR2111) showed interaction with only four JcSAP genes (JcSAP2a, JcSAP2b, JcSAP810a, and JcSAP12) (Figure 6C). Moreover, miRNA Jcu-miR393 showed interaction with two JcSAP genes, JcSAP2a and JcSAP2b, miRNA Jcu-miR1628 showed interaction with JcSAP12, while miRNA Jcu-miR2111 showed interaction with JcSAP810a. The Excel spreadsheet containing targeting sites predicted miRNAs ID, and the alignment with JcSAP gens are given in the Supplementary Table S2.
3.8. Gene Expression Profiling of JcSAP Genes
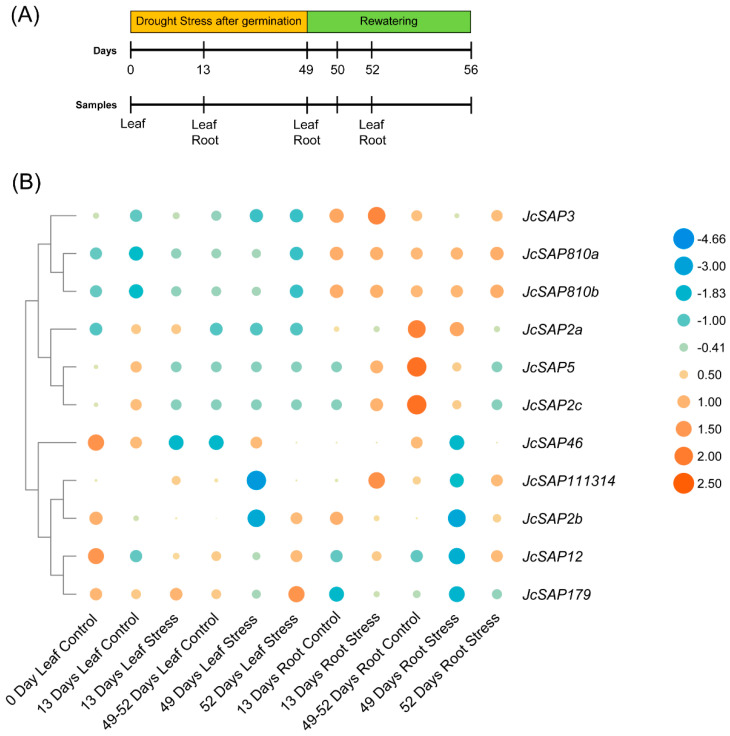
To investigate the possible function of JcSAP genes during environmental abiotic stress in Jatropha, the expression data were analysed from leaf and root tissues of Jatropha plants subjected to drought stress [32] (Figure 7A). The heat map showed the expression level of 11 JcSAP genes in different tissues of leaf and root during drought stress and after recovery (Figure 7B). The results further revealed that JcSAP179, JcSAP3, and JcSAP2b were highly expressed in leaf and in root, followed by JcSAP111314, JcSAP810a, and JcSAP810b as compared with the other JcSAP genes. Contrarily, JcSAP2a, JcSAP5, JcSAP2c were highly expressed in roots only. These results provide the possible involvement of JcSAP genes in abiotic stress tolerance in Jatropha.
Figure 7.
Expression levels of JcSAP genes in leaf and root tissues under abiotic stress. (A) Drought stress subjection and samples collected at different intervals during and after recovery of heat stress, (B) The expression levels the JcSAP genes at different intervals of leaf and root with their respective controls.
4. Discussion
Stress-associated proteins (SAP) are novel stress regulatory zinc-finger proteins and are strongly associated with tolerance against various abiotic stresses [1,12,13,14,15]. SAP gene family has previously identified and comprehensively studied in many plant species including Oryza sativa [5], Arabidopsis thaliana [5], Hordeum vulgare [13], Glycine max [12], Gossypium hirsutum [16], Solanum lycopersicum [2], Cucumis sativus [17], Solanum melongena [1], and Prunus dulcis [18], etc. However, there is no systematic study of SAP genes in the most important biofuel-producing shrub, Jatropha. In present study, 11 SAP genes were identified genome-wide in Jetropha and the phylogenetic divided the JcSAP genes into four groups (Figure 1); Synteny analysis showed that Jatropha SAP genes had a high homology with the Arabidopsis SAP genes (Figure 2). These results are highly in consistence with the previously reported results of [1,2], suggesting very little variation in SAP gene family members.
To obtain further insight into the similar features and biological functions of JcSAP genes, a search for the conserved domain, motif, and structure was conducted (Figure 3 and Figure 4), resulting in the presence of A20 or AN1 domains and their respective motifs, thus suggesting that they share a similar biological function in response to stresses. Zinc-finger A20 or AN1 domains are highly conserved in all plant species and these results are in agreement with the previous reports [15,33]. Moreover, the domain organization revealed some domain-wise grouping, illustrating the presence of solely the AN1 domain in three JcSAP genes, with the others having both the A20 and AN1 domains (Figure 3). Except for Arabidopsis and tomato, similar reports were also exhibited in some other plant species like Amborella trichopoda, soybean, rice, and eggplant [1]. Such results may be due to the existence of a homology structure beyond the domain sequences [2]. These cases may also indicate the ancient origin of the specific AN1 domain with respect to its characteristics as compared with the A20 domain, or may also be due to the loss of one domain during evolution, as such cases occur in prologue genes (Table 2) and were also in line with the previous report on Brassica napus [14]. Moreover, the presence of corresponding motifs of the A20 and AN1 domain and the diversity of exon in the gene structure organization further strengthen the understanding of the evolutionary mechanisms in the JcSAP gene members [34,35]. In the present study, no intron has been found in JcSAP genes (Figure 3B), and this intron-free characteristic of SAP genes is usually found in other plant species like eggplant, rice, and apple [1,5,12,36]. It may be attributed to the fact that intron-free gene families can reduce posttranscriptional processing and rapidly adjust transcript expression [37].
The differential expression pattern of JcSAP genes in leaf and root tissues against drought stress revealed a potential role of these genes in stress response (Figure 7). Various studies have declared the role of SAP genes in different biotic and abiotic stresses [12,13]. Previous studies indicated that SAP genes play a great role in mediating abiotic stresses including cold, salt, and drought [1,2]. Our results are also in line with the previous studies on other species [12,36]. Altogether, the present study provides a baseline for understanding the molecular role of JcSAP genes and for further study of these genes against different abiotic stresses.
5. Conclusions
In conclusion, a total 11 SAP genes were identified during this study of Jatropha and were divided into four groups based on the phylogenetic analysis and amino acid sequence similarity; they may share similar biological functions against stresses. The physicochemical properties of JcSAP genes uncovered the basic gene parameters like amino acids and CDS length, molecular weight (MW/kDa), isoelectric point (PI), GRAVY, and molecular formula, and further revealed that most of the JcSAP genes are cytoplasmic, however, no detailed information was found regarding the chromosomal localization of JcSAP genes. The Synteny analysis showed that most of the JcSAP proteins were highly homologous to the Arabidopsis SAP proteins, indicating that SAP genes are conserved in Jatropha during evolution. Further domains and motifs analysis revealed that the A20 and AN1 domains are conserved in JcSAP genes and their similar gene structure features may be due to the duplication events during evolution. The divergence analysis further provided insight into the evolutionary aspects of JcSAP genes revealing the time of divergence from 9.1 to 40 MYA. The promoter region analysis of JcSAP genes resulted in a diverse range of cis-elements including light-responsive elements, hormone-responsive elements, and stress-responsive elements. The predicted miRNA target sites revealed that JcSAP genes may be regulated by a complicated miRNA mediated posttranscriptional regulatory network. In addition, the expression profiles of JcSAP genes in different tissues and stress treatments indicated that many JcSAP genes play functional developmental roles in different tissues, and exhibit significant differential expression under different stress treatments. All these results collectively suggested that JcSAP genes share similar biological functions in response to stresses and provide valuable clues for further investigation of JcSAP genes’ function and diversity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13101766/s1, Figure S1: Phylogenetic tree for Ka/Ks paralogous genes; Table S1: Basic gene information; Table S2: miRNA target sites.
Author Contributions
Conceptualization, A.J.; methodology, A.J.; software, A.J.; validation, A.J. and Q.A. and H.M.; formal analysis, A.J.; investigation, A.J.; resources, A.J.; data curation, Q.A. and H.M.; writing—original draft preparation, A.J.; writing—review and editing, D.Z.; visualization, A.J.; supervision, D.Z.; project administration, D.Z.; funding acquisition, D.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the results are already mentioned in the main text and in supplementary files.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Key Research and Development Program of Jiangsu Province (Grant No. BE2021691), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wan F., Xu Y., Wang S., Gao J., Lu D., Zhou C., Liao Y., Ma Y., Zheng Y. Identification and Expression Analysis of Zinc Finger A20/AN1 Stress-Associated Genes SmSAP Responding to Abiotic Stress in Eggplant. Horticulturae. 2022;8:108. doi: 10.3390/horticulturae8020108. [DOI] [Google Scholar]
- 2.Solanke A.U., Sharma M.K., Tyagi A.K., Sharma A.K. Characterization and Phylogenetic Analysis of Environmental Stress-Responsive SAP Gene Family Encoding A20/AN1 Zinc Finger Proteins in Tomato. Mol. Genet. Genom. 2009;282:153–164. doi: 10.1007/s00438-009-0455-5. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional Regulatory Networks in Cellular Responses and Tolerance to Dehydration and Cold Stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 4.Chinnusamy V., Zhu J., Zhu J.-K. Cold Stress Regulation of Gene Expression in Plants. Trends Plant Sci. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Vij S., Tyagi A.K. Emerging Trends in the Functional Genomics of the Abiotic Stress Response in Crop Plants. Plant Biotechnol. J. 2007;5:361–380. doi: 10.1111/j.1467-7652.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar-Mathur P., Vadez V., Sharma K.K. Transgenic Approaches for Abiotic Stress Tolerance in Plants: Retrospect and Prospects. Plant Cell Rep. 2008;27:411–424. doi: 10.1007/s00299-007-0474-9. [DOI] [PubMed] [Google Scholar]
- 7.Solanke A.U., Sharma A.K. Signal Transduction during Cold Stress in Plants. Physiol. Mol. Biol. Plants. 2008;14:69–79. doi: 10.1007/s12298-008-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davletova S., Schlauch K., Coutu J., Mittler R. The Zinc-Finger Protein Zat12 Plays a Central Role in Reactive Oxygen and Abiotic Stress Signaling in Arabidopsis. Plant Physiol. 2005;139:847–856. doi: 10.1104/pp.105.068254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhopadhyay A., Vij S., Tyagi A.K. Overexpression of a Zinc-Finger Protein Gene from Rice Confers Tolerance to Cold, Dehydration, and Salt Stress in Transgenic Tobacco. Proc. Natl. Acad. Sci. USA. 2004;101:6309–6314. doi: 10.1073/pnas.0401572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciftci-Yilmaz S., Mittler R. The Zinc Finger Network of Plants. Cell. Mol. Life Sci. 2008;65:1150–1160. doi: 10.1007/s00018-007-7473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu D.-Q., Huang J., Guo S.-Q., Yang X., Bao Y.-M., Tang H.-J., Zhang H.-S. Overexpression of a TFIIIA-Type Zinc Finger Protein Gene ZFP252 Enhances Drought and Salt Tolerance in Rice (Oryza Sativa L.) FEBS Lett. 2008;582:1037–1043. doi: 10.1016/j.febslet.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X.-Z., Zheng W.-J., Cao X.-Y., Cui X.-Y., Zhao S.-P., Yu T.-F., Chen J., Zhou Y.-B., Chen M., Chai S.-C. Genomic Analysis of Stress Associated Proteins in Soybean and the Role of GmSAP16 in Abiotic Stress Responses in Arabidopsis and Soybean. Front. Plant Sci. 2019;10:1453. doi: 10.3389/fpls.2019.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baidyussen A., Aldammas M., Kurishbayev A., Myrzabaeva M., Zhubatkanov A., Sereda G., Porkhun R., Sereda S., Jatayev S., Langridge P. Identification, Gene Expression and Genetic Polymorphism of Zinc Finger A20/AN1 Stress-Associated Genes, HvSAP, in Salt Stressed Barley from Kazakhstan. BMC Plant Biol. 2020;20:156. doi: 10.1186/s12870-020-02332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X., Xie S., Xie P., Yao M., Liu W., Qin L., Liu Z., Zheng M., Liu H., Guan M. Genome-Wide Identification of Stress-Associated Proteins (SAP) with A20/AN1 Zinc Finger Domains Associated with Abiotic Stresses Responses in Brassica Napus. Environ. Exp. Bot. 2019;165:108–119. doi: 10.1016/j.envexpbot.2019.05.007. [DOI] [Google Scholar]
- 15.Giri J., Dansana P.K., Kothari K.S., Sharma G., Vij S., Tyagi A.K. SAPs as Novel Regulators of Abiotic Stress Response in Plants. Bioessays. 2013;35:639–648. doi: 10.1002/bies.201200181. [DOI] [PubMed] [Google Scholar]
- 16.Gao W., Long L., Tian X., Jin J., Liu H., Zhang H., Xu F., Song C. Genome-Wide Identification and Expression Analysis of Stress-Associated Proteins (SAPs) Containing A20/AN1 Zinc Finger in Cotton. Mol. Genet. Genom. 2016;291:2199–2213. doi: 10.1007/s00438-016-1252-6. [DOI] [PubMed] [Google Scholar]
- 17.Lai W., Zhou Y., Pan R., Liao L., He J., Liu H., Yang Y., Liu S. Identification and Expression Analysis of Stress-Associated Proteins (SAPs) Containing A20/AN1 Zinc Finger in Cucumber. Plants. 2020;9:400. doi: 10.3390/plants9030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatima S., Zafar Z., Gul A., Bhatti M.F. Genome-Wide Identification of Stress-Associated Proteins (SAPs) Encoding A20/AN1 Zinc Finger in Almond (Prunus Dulcis) and Their Differential Expression during Fruit Development. Plants. 2021;11:117. doi: 10.3390/plants11010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairless D. The Little Shrub That Could–Maybe: India, like Many Countries, Has High Hopes for Jatropha as a Biofuel Source, but Little Is Known about How to Make It a Successful Crop. Daemon Fairless Digs for the Roots of a New Enthusiasm. Nature. 2007;449:652–656. doi: 10.1038/449652a. [DOI] [PubMed] [Google Scholar]
- 20.Bhasanutra R., Sutiponpeibun S. Jatropha Curcas Oil as a Substitute for Diesel Engine Oil. Int. Energy J. 2017;4:56–70. [Google Scholar]
- 21.Openshaw K. A Review of Jatropha Curcas: An Oil Plant of Unfulfilled Promise. Biomass Bioenergy. 2000;19:1–15. doi: 10.1016/S0961-9534(00)00019-2. [DOI] [Google Scholar]
- 22.Dong Y., Lu J., Liu J., Jalal A., Wang C. Genome-Wide Identification and Functional Analysis of JmjC Domain-Containing Genes in Flower Development of Rosa Chinensis. Plant Mol. Biol. 2020;102:417–430. doi: 10.1007/s11103-019-00955-2. [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Wu S., Sun J., Sun J., Wang H., Cao X., Lu J., Jalal A., Wang C. Genome-Wide Analysis Reveals Widespread Roles for RcREM Genes in Floral Organ Development in Rosa Chinensis. Genomics. 2021;113:3881–3894. doi: 10.1016/j.ygeno.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Jalal A., Sun J., Chen Y., Fan C., Liu J., Wang C. Evolutionary Analysis and Functional Identification of Clock-Associated PSEUDO-RESPONSE REGULATOR (PRRs) Genes in the Flowering Regulation of Roses. Int. J. Mol. Sci. 2022;23:7335. doi: 10.3390/ijms23137335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., Ren M., Chen H., Wu S., Yan H., Jalal A., Wang C. Evolution of SHORT VEGETATIVE PHASE (SVP) Genes in Rosaceae: Implications of Lineage-Specific Gene Duplication Events and Function Diversifications with Respect to Their Roles in Processes Other than Bud Dormancy. Plant Genome. 2020;13:e20053. doi: 10.1002/tpg2.20053. [DOI] [PubMed] [Google Scholar]
- 26.Ayaz A., Saqib S., Huang H., Zaman W., Lü S., Zhao H. Genome-Wide Comparative Analysis of Long-Chain Acyl-CoA Synthetases (LACSs) Gene Family: A Focus on Identification, Evolution and Expression Profiling Related to Lipid Synthesis. Plant Physiol. Biochem. 2021;161:1–11. doi: 10.1016/j.plaphy.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 27.Errum A., Rehman N., Khan M.R., Ali G.M. Genome-Wide Characterization and Expression Analysis of Pseudo-Response Regulator Gene Family in Wheat. Mol. Biol. Rep. 2021;48:2411–2427. doi: 10.1007/s11033-021-06276-2. [DOI] [PubMed] [Google Scholar]
- 28.Gaut B.S., Morton B.R., McCaig B.C., Clegg M.T. Substitution Rate Comparisons between Grasses and Palms: Synonymous Rate Differences at the Nuclear Gene Adh Parallel Rate Differences at the Plastid Gene RbcL. Proc. Natl. Acad. Sci. USA. 1996;93:10274–10279. doi: 10.1073/pnas.93.19.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge M., Zhong R., Sadeghnezhad E., Hakeem A., Xiao X., Wang P., Fang J. Genome-Wide Identification and Expression Analysis of Magnesium Transporter Gene Family in Grape (Vitis Vinifera) BMC Plant Biol. 2022;22:217. doi: 10.1186/s12870-022-03599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., Jiang X., Bai H., Liu C. Genome-Wide Identification, Classification and Expression Analysis of the JmjC Domain-Containing Histone Demethylase Gene Family in Jatropha Curcas L. Sci. Rep. 2022;12:6543. doi: 10.1038/s41598-022-10584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali Q., Ayaz M., Mu G., Hussain A., Yuanyuan Q., Yu C., Xu Y., Manghwar H., Gu Q., Wu H. Revealing Plant Growth-Promoting Mechanisms of Bacillus Strains in Elevating Rice Growth and Its Interaction with Salt Stress. Front. Plant Sci. 2022;13:3190. doi: 10.3389/fpls.2022.994902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapeta H., Lourenço T., Lorenz S., Grumaz C., Kirstahler P., Barros P.M., Costa J.M., Sohn K., Oliveira M.M. Transcriptomics and Physiological Analyses Reveal Co-Ordinated Alteration of Metabolic Pathways in Jatropha Curcas Drought Tolerance. J. Exp. Bot. 2016;67:845–860. doi: 10.1093/jxb/erv499. [DOI] [PubMed] [Google Scholar]
- 33.Wei Q., Wang J., Wang W., Hu T., Hu H., Bao C. A High-Quality Chromosome-Level Genome Assembly Reveals Genetics for Important Traits in Eggplant. Hortic. Res. 2020;7:153. doi: 10.1038/s41438-020-00391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogozin I.B., Sverdlov A.V., Babenko V.N., Koonin E.V. Analysis of Evolution of Exon-Intron Structure of Eukaryotic Genes. Brief. Bioinform. 2005;6:118–134. doi: 10.1093/bib/6.2.118. [DOI] [PubMed] [Google Scholar]
- 35.Rose A.B. Intron-Mediated Regulation of Gene Expression. Nucl. pre-mRNA Process. Plants. 2008;326:277–290. doi: 10.1007/978-3-540-76776-3_15. [DOI] [PubMed] [Google Scholar]
- 36.Dong Q., Duan D., Zhao S., Xu B., Luo J., Wang Q., Huang D., Liu C., Li C., Gong X., et al. Genome-Wide Analysis and Cloning of the Apple Stress-Associated Protein Gene Family Reveals MdSAP15, Which Confers Tolerance to Drought and Osmotic Stresses in Transgenic Arabidopsis. Int. J. Mol. Sci. 2018;19:2478. doi: 10.3390/ijms19092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grzybowska E.A. Human Intronless Genes: Functional Groups, Associated Diseases, Evolution, and MRNA Processing in Absence of Splicing. Biochem. Biophys. Res. Commun. 2012;424:1–6. doi: 10.1016/j.bbrc.2012.06.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the results are already mentioned in the main text and in supplementary files.