Abstract
In the past two decades, studies of Y chromosomal single nucleotide polymorphisms (Y-SNPs) and short tandem repeats (Y-STRs) have shed light on the demographic history of Central Asia, the heartland of Eurasia. However, complex patterns of migration and admixture have complicated population genetic studies in Central Asia. Here, we sequenced and analyzed the Y-chromosomes of 187 male individuals from Kazakh, Kyrgyz, Uzbek, Karakalpak, Hazara, Karluk, Tajik, Uyghur, Dungan, and Turkmen populations. High diversity and admixture from peripheral areas of Eurasia were observed among the paternal gene pool of these populations. This general pattern can be largely attributed to the activities of ancient people in four periods, including the Neolithic farmers, Indo-Europeans, Turks, and Mongols. Most importantly, we detected the consistent expansion of many minor lineages over the past thousand years, which may correspond directly to the formation of modern populations in these regions. The newly discovered sub-lineages and variants provide a basis for further studies of the contributions of minor lineages to the formation of modern populations in Central Asia.
Keywords: Central Asia, Y-chromosome, paternal lineage, admixture
1. Introduction
The demographic history of Central Asia, the Eurasian heartland, has long fascinated linguists, archaeologists, and geneticists. The genomes of ancient human individuals from Ust’Ishim, Mal’ta, and Yana sites in northern Eurasia suggest that Central Asia is a route for the dispersal of Homo sapiens sapiens from the Middle East to northern Eurasia [1,2,3]. The Last Glacial Maximum (LGM) substantially reshaped the distribution of ancient human populations in Central Asia and adjacent regions [4,5,6,7,8]. Several studies of ancient DNA have focused on the genetic structure of ancient populations before and after the rise of Neolithic farmers and Indo-European populations in Central Asia [9,10,11,12,13,14,15,16]. The ancient Botai people [9] may be genetically related to populations in Ancient Northern Eurasia, as revealed by studies of a 24,000-year-old Mal’ta boy and related ancient remains [2]. Neolithic farmers diffused in all directions from the Middle East and established the initial Neolithic archaeological cultures in the Indus Valley and the southern part of Central Asia [11]. About 5000 years ago, Indo-European-speaking populations arose in the western part of the Eurasian steppe. The eastward diffusion of Indo-European-like populations led to the emergence of the Chalcolithic Age and Bronze Age across Central Asia and the eastern Eurasian steppe [9,10,11,12,13,14]. It is generally accepted that the Andronovo culture complex was created by the ancestors of Indo-Iranian-speaking populations [17]. The later rise of Scythians triggered large-scale migration and admixture across the Eurasian steppe, including Central Asia [12,15].
The trend towards the diffusion of human populations from west to east shifted about 1200 years ago. The Karasuk culture arose in South Siberia and expanded across a vast geographical area, giving rise to a series of successors [18]. In a later historical period, Yuezhi, Usuns, and Ephthalite people migrated from east to west and established a series of polities in Central Asia [19]. Since the beginning of the Christian era, the ancient Xiongnu/Huns moved westward on a large scale and significantly changed the ethnic pattern in Central Asia and eastern Europe [20]. Later, ancient Turkic and Mongolic tribes spread westward and scattered throughout Central Asia, the Middle East, and Eastern Europe [21]. The descendants of the above-mentioned ancient people mixed and formed the modern populations in Central Asia over a long historical period [22,23,24].
Previous studies of modern samples have shed light on the origin and genetic structure of populations from Central Asia [25]. Ancient DNA analyses of remains from various historical periods have revealed the genetic structure of ancient people and their contribution to the gene pool of modern populations as well as patterns of admixture [9,10,11,12,13,14]. In general, paternal, maternal, and autosomal gene pools of most populations from this region are characterized by high diversity and admixture with migration from the Middle East, South Asia, East Asia, Northern Asia, and Europe.
Previous research has also focused on the formation of modern populations in the recent millennium [23,24]. A strong tribe structure has been detected among populations from this region [26]. The tribal tradition leads to great differences in dominant paternal lineage between tribes [27,28,29,30,31,32,33,34,35,36]. By contrast, within a tribe, paternal Y-chromosomal short tandem repeat (Y-STR) diversity is generally low [34]. Although gene flow is common, the genetic relationships among populations are still closely related to the language category. For example, the Tajik people are genetically related to other Indo-European-speaking populations [28,30,37], while many genetic components from Siberia have been detected in the Kyrgyz people [38]. In addition, Y-chromosome sequences of specific tribes or family clans have been evaluated to explore the formation of related populations [27,32,33]. Nevertheless, the demographic histories of populations from Central Asia are complex, and more work is needed for a clearer understanding.
In this study, we analyzed 187 Y-chromosome sequences of populations from Central Asia. Our first objective was to reveal general patterns in the paternal gene pool based on full-length sequences. Our second objective was to identify paternal lineages that are nearly unique to a specific population, and to analyze the demographic history. Specifically, we evaluated the different distinct periods of genetic exchange and divergence among ancient populations in Central Asia.
2. Materials and Methods
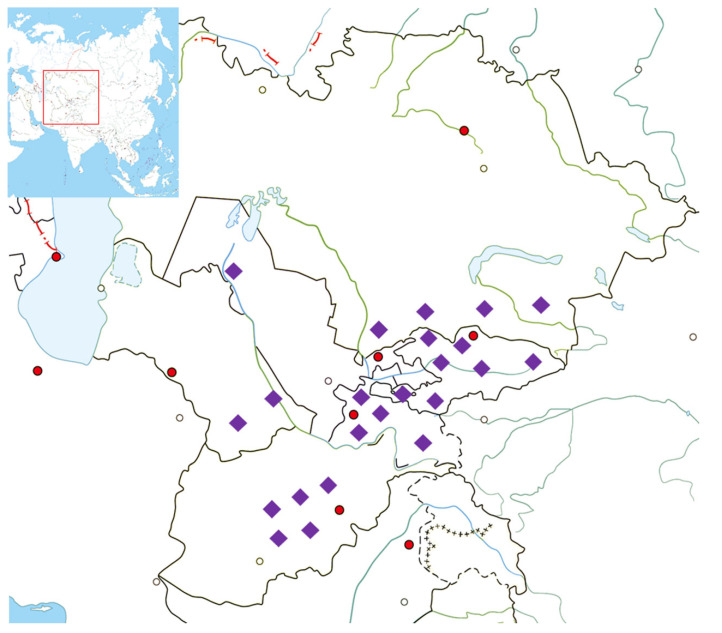
Saliva samples were collected from unrelated healthy males from populations in Central Asia. All participants provided signed consent prior to participating in the study. The present study complies with the ethical principles of the 2013 Helsinki Declaration of the World Medical Association. The Local Ethical Commission of at the National Center for Biotechnology and Nazarbayev University Institutional Research Ethics Committee approved the study. DNA specimens extracted from 187 samples were sent for next-generation sequencing using the Illumina HiSeq2000 platform (San Diego, CA, USA). The target depth was 10× and data were sufficient for analyses. The sample set included 20 Kyrgyz, 20 Uzbeks, 17 Karakalpaks, 20 Hazara, 9 Karluks, 20 Tajiks, 19 Uyghur, 20 Dungan, 20 Turkmen, and 22 Kazakhs from Kazakhstan. Figure 1 shows the places of origin of individuals’ paternal ancestors, which are the historical homelands of their grandfathers and fathers in Afghanistan, Uzbekistan, Kyrgyzstan, Turkmenistan and Tajikistan. Detailed sample information can be found in Supplementary Table S1.
Figure 1.
Approximate sampling locations. Detailed information is provided in Table S1. Purple rhombi indicate the sampling locations. Red and transparent circles indicate the capitals and major cities of countries, respectively.
Data analysis: Read mapping and SNP calling from next-generation sequencing data were conducted using standard procedures (BWA and SAMtools) and the human reference genome sequence hg38 [39,40]. Bayesian evolutionary analyses were conducted using BEAST (v.2.4.3) [41]. To calculate divergence times in the phylogenetic tree, a point mutation rate of 0.74 × 10−9 per site per year was applied [42], which was very similar to the rate of 0.78 × 10−9 per site per year reported in analyses of the ancient genome of the 45,000-year-old Ust’Ishim male [1]. We refer to the protocol used in our previous publications for data processing and Bayesian evolutionary analyses [7,43,44]. The details of data analysis can be found in the Supplementary Text.
3. Results
We used Y-chromosome sequences of 187 males to construct a revised phylogenetic tree (Figure 2; Table S1). The detailed tree can be found in Figure S1. The frequencies of Y-chromosome haplogroups in the studied populations are shown in Table 1. Haplogroup C2a1a1b1-F1756 was the predominant paternal lineage of the ancient Dong-Hu and Xian-Bei tribes [45,46,47]. The appearance of this lineage among Hazara, Kyrgyz, and Turkmen indicated that these populations originated in Eastern Eurasia. Haplogroup C2a1a2a-M48-F6379 had a high frequency in the Junior Juz of Kazakh and Oirat people, which are western Mongolic-speaking populations [32]. The appearance of this lineage among the Karakalpak suggested that this population is closely related to Kazakhs and ancient Mongols. It is thought that the expansion in Central Eurasia of C2a1a3-M504 is directly related to the activity of ancient Mongol tribes in the past millennium [26,48,49]. The high frequency of this lineage in the Hazara, Karakalpak, Kazakh, Kyrgyz, and Uyghur populations in this study is consistent with the proposed origin of these populations and previous research [24,48]. The downstream lineages of N-M231 among Kazakhs (Tables S1 and S2) are related to ancient Turkic tribes. D-M174 and O-M175 detected in populations from Central Asia resulted from admixture with populations from East Asia over the past 2000 years.
Figure 2.
Revised phylogenetic tree based on Y-chromosomes of populations in Central Asia.
Table 1.
Frequencies of Y-chromosome haplogroups in various populations. Gray shading indicates the data that had a frequency higher than 10%.
| Haplogroup | Dungan | Hazara | Karakalpak | Karluk | Kazakhs | Kyrgyz | Tajik | Turkmen | Uyghur | Uzbek | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C2a1a1b1-F1756 | 6 (0.3) | 2 (0.1) | 2 (0.1) | 10 | |||||||
| C2a1a2a-M48 | 2 (0.12) | 1 (0.05) | 1 (0.05) | 4 | |||||||
| C2a1a3-M504 | 3 (0.15) | 5 (0.29) | 10 (0.45) | 3 (0.15) | 3 (0.14) | 1 (0.05) | 25 | ||||
| C2b*-F1067xM407 | 1 (0.05) | 1 (0.05) | 2 | ||||||||
| C2b1a1a1a-M407 | 2 (0.09) | 1 (0.05) | 3 | ||||||||
| D1a1a2-N1 | 2 (0.1) | 1 (0.05) | 3 | ||||||||
| D1a2a-P47 | 1 (0.05) | 1 (0.05) | 2 | ||||||||
| E1b1b1-M35.1 | 1 (0.06) | 1 (0.05) | 1 (0.05) | 3 | |||||||
| G-M201 | 1 (0.05) | 1 (0.06) | 1 (0.45) | 1 (0.05) | 2 (0.09) | 6 | |||||
| H-L901 | 1 (0.05) | 1 (0.05) | 2 | ||||||||
| I-M170 | 1 (0.05) | 1 (0.45) | 1 (0.05) | 3 | |||||||
| J1a-CTS5368 | 1 (0.06) | 1 (0.45) | 2 (0.1) | 1 (0.05) | 1 (0.05) | 6 | |||||
| J2a-M410 | 4 (0.2) | 4 (0.2) | 2 (0.12) | 2 (0.1) | 3 (0.15) | 4 (0.2) | 1 (0.05) | 5 (0.25) | 25 | ||
| L-M20 | 1 (0.05) | 9 (1) | 1 (0.05) | 1 (0.05) | 1 (0.05) | 13 | |||||
| N-M231 | 1 (0.05) | 1 (0.06) | 4 (0.18) | 1 (0.05) | 1 (0.05) | 1 (0.05) | 1 (0.05) | 10 | |||
| O-M175 | 6 (0.3) | 3 (0.18) | 3 (0.14) | 4 (0.2) | 16 | ||||||
| Q-M242 | 4 (0.2) | 2 (0.09) | 2 (0.1) | 3 (0.15) | 2 (0.09) | 13 | |||||
| R1a1a-M17 | 1 (0.05) | 3 (0.15) | 1 (0.06) | 11 (0.55) | 7 (0.35) | 1 (0.05) | 4 (0.18) | 2 (0.1) | 30 | ||
| R1b-M343 | 1 (0.05) | 1 (0.45) | 1 (0.05) | 2 (0.1) | 1 (0.05) | 1 (0.05) | 7 | ||||
| R2a-M124 | 3 (0.15) | 1 (0.05) | 4 | ||||||||
| Sum | 20 | 20 | 17 | 9 | 22 | 20 | 20 | 20 | 19 | 20 | 187 |
Consistent with previous research, as shown in Table 1, haplogroups E-M96, G-M201, H-L901, I-M170, and L-M20 were widely distributed with low frequencies in populations from Central Asia. These lineages represent admixture from South Asia, the Middle East, and Europe. Nevertheless, we found a special lineage, L-M20, in the Karluks. Nine samples of this lineage from the Karluks were sequenced. We found a unique sub-branch, L1a2a-M2398-Y236528, in these samples; this lineage separated from the lineages found in India about 1500 years ago (Tables S1 and S2, Figure S1).
Previous studies have demonstrated that South Siberia is likely the center of diffusion of haplogroup Q-M242 beginning 30,000 thousand years ago [6,50,51]. Furthermore, many minor sub-lineages of Q-M242 have been detected in populations from Inner Eurasia, including Mongolic- and Turkic-speaking populations [6,23,50,51]. We detected diverse sub-lineages of Q-M242 (Table S1). As revealed by previous studies, R1a1a-M17 and R1b-M343 are two major paternal lineages of Indo-European-speaking populations [52,53]. Most modern populations in Central Asia still show a high frequency of R1a1a-M17 (Table 1). The haplogroup R2a-M124 was widely found in South Asia [25,54]. The diffusion of this lineage in Central Asia and the Mongolian Plateau has been unclear. The revised high-resolution phylogenetic tree with age estimation provides a detailed overview of paternal gene flow in Central Asia populations (Table 1, Figure 2, Figure 3 and Figure S1). The high diversity of macro-haplogroup lineages suggested that there was intense admixture from different peripheral areas of the Eurasian continent, like C/D/N/O from Eastern Eurasia, E/J from the Middle East, H/L from South Asia, I/R1 from Western Eurasia, and Q from South Siberia.
Figure 3.
Schematic tree of unique minor lineages in populations from Central Asia.
The most important finding of this project is the identification of unique minor lineages that experienced a recent expansion in Central Asia (Figure 3 and Figure S1, Table S2). We compared the results of these study with data from other sources, like ancient DNA [11,12,13,14,15,16] (Table S3) and a publicly available phylogenetic tree of global humans from https://www.yfull.com/tree (accessed on 1 September 2022) and https://www.23mofang.com/ancestry/ytree/root (accessed on 1 September 2022). We identified the closest branch to these minor lineages with a recent separation time among populations from peripheral areas of Eurasia. As shown in Figure 3, we identified tens of minor lineages (also see Figure S1), including 33 minor lineages with ages younger than 2000 years.
4. Discussion
4.1. Multiple Layers of Deposition of Ancient Components
Central Asia is not a region of origin of agriculture in the Neolithic age or technological systems of the Bronze Age. Therefore, the first wave of population growth caused by these technological revolutions did not occur in Central Asia. Genetic studies generally support the results of studies in other disciplines. Available genetic data indicate that the sources of populations in Central Asia include all peripheral regions of Eurasia, including South Asia, the Middle East, Europe, North Asia, and East Asia. This general scenario is consistent with the geographical location of Central Asia as the heartland of Eurasia.
We also observed multiple layers of deposition of genetic components of ancient populations. The Neolithic evolution in the Middle East led to the global expansion of haplogroup J-M172 and related haplogroups [55]. Ancient DNA studies suggest that the paternal haplogroup J-M172 from the Middle East led to the appearance of the initial Neolithic settlements in the southern part of Central Asia [11]. In a later historical period, continuous migration from the Middle East also brought more sub-branches of J-M172. Many ancient DNA studies have shown that the expansion of paternal R1a1a-M17 and R1b-M269 is consistent with the spread of Indo-European populations [16,56,57,58], and this may be related to the diverse sub-branches of R1a1a-M17 in Central Asia. In addition, G-M201 is also likely derived from Indo-European populations. Based on previously reported ancient DNA and modern population data, we speculate that some lineages downstream of Q-M242, N1a1-M46, R1a1a-M17, and C2a1b1-F1756 in Central Asia could be attributed to migration by Turkic people. Finally, referring to previous literature, we speculate that the spread of Mongolian populations introduced C2a1a3-M504, C2a1b1-F1756, and sub-branches of C2a1a2a-M48 to Central Asia. During East-to-West migration over thousands of years, many paternal haplogroups in East Asia spread to Central Asia, including several sub-branches of D-M174, C2a-F1096, and O-M175. In short, due to its high mutation rate, the Y chromosome easily forms population-specific sub-branches. Therefore, the main haplogroups differ among populations from different regions in the periphery of Eurasia. Based on an analysis of the complete Y chromosome sequence, we can identify patterns of large-scale population diffusion in different periods from the complex genetic structure of the population across Central Asia.
4.2. Recent Expansion and the Formation of Modern Populations
We predicted that the recent consistent expansion of minor lineages contributed directly to the formation of modern populations in Central Asia. The main paternal haplogroups of populations in different regions of Eurasia include C-M217, D-M174, E-M96, G-M201, H-L901, I-M170, I-M304, L-L298, T-M184, N-M231, O-M175, Q-M242, and R-M207. These macro-haplogroups are common in Central Asian populations; however, the frequencies vary substantially. At the sub-branch level, Central Asian populations have specific dominant haplogroups. As shown in Figure 3, there were many ethnicity-specific minor lineages among populations in Central Asia, such as R1a-MM17-Y34292, and R1a-M17-Y111548 of the Tajiks. We also observed many sub-branches that expanded in the last 1000 years, such as C2a1a3-M504-F5481, L-M20-M2648, and R1a1a-M17-YP1460. Many minor lineages of R1a1a-M17 in the Tajik people are related to Indo-European ancestors. Additionally, many minor branches that expanded in the past 1000 years were observed in the Kazakh, Kyrgyz, Qarluk, Karakalpak, and Hazara. In conclusion, we believe that the expansion of these specific lineages is directly related to the formation of modern ethnic groups in Central Asia. The differentiation of different paternal lineages in different periods led to population increases and laid the foundation for modern Central Asian populations.
In summary, we constructed a high-resolution phylogeny of paternal lineages for populations from Central Asia. We detected high haplogroup diversity among populations. Admixture from all peripheral areas of the Eurasian continent was observed among the paternal gene pool of these populations. Further phylogeny analyses revealed many minor lineages specific to populations from Central Asia and expansion events in the past 3000 or 1000 years. We proposed that the multiple large-scale demographic events in different historical periods of Central Asia left clear layers of deposition of ancient components in the gene pool of modern populations. More importantly, the expansion of minor lineages observed in this study may correspond to the formation of modern populations in Central Asia in the past thousand years. More work is needed to explore the detailed demographic history in Central Asia.
Acknowledgments
We thank all donors for providing DNA samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13101776/s1. Table S1: Detailed information on the studied samples. Table S2: List of variants of Y-chromosome sequences used in the study. Figure S1: Detailed phylogenetic tree of the studied samples. Table S3: Y-chromosomal information of ancient individuals from Central Asia and adjacent regions. Supplementary Text: Details of data analysis. The references [59,60,61,62,63,64] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.Z., L.-H.W., Z.S., P.-C.M., J.S., Z.D., E.B., H.L. and Y.R.; literature search, M.Z., L.-H.W., Z.S., P.-C.M. and J.S.; writing—original draft preparation, M.Z., L.-H.W. and Y.R.; writing—review and editing, M.Z., L.-H.W., Z.S., P.-C.M., J.S., Z.D., E.B., H.L. and Y.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all participants in the study.
Data Availability Statement
Following the regulations of the Ethical Commission of at the National Center for Biotechnology, the data that support the findings of this study are available on request from the corresponding author. A detailed list of variants is provided in Supplementary Table S2; this list is sufficient for the replication of genetic analyses in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP09259560) and Faculty-development competitive research grants programs of Nazarbayev University (Grant No. SST2019012). This work was also supported by the the Russian Ministry of Science and Higher Education (State Assignments for the Research Centre for Medical Genetics). The study was also supported by the Scientific and Technology Committee of Shanghai Municipality (18490750300) and the Key projects of strategic international scientific and technological innovation cooperation of the Chinese Ministry of Science and Technology (2020YFE0201600). LHW was supported by National Natural Science Foundation of China (31900406). This study was also supported by the funders, who had no role in study design, data collection, or analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fu Q., Li H., Moorjani P., Jay F., Slepchenko S.M., Bondarev A.A., Johnson P.L.F., Aximu-Petri A., Prüfer K., De Filippo C., et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghavan M., Skoglund P., Graf K.E., Metspalu M., Albrechtsen A., Moltke I., Rasmussen S., Stafford T.W., Jr., Orlando L., Metspalu E., et al. Upper Paleolithic Siberian genome reveals dual ancestry of Native Americans. Nature. 2014;505:87–91. doi: 10.1038/nature12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sikora M., Pitulko V.V., Sousa V.C., Allentoft M.E., Vinner L., Rasmussen S., Margaryan A., Damgaard P.D.B., De La Fuente C., Renaud G., et al. The population history of northeastern Siberia since the Pleistocene. Nature. 2019;570:182–188. doi: 10.1038/s41586-019-1279-z. [DOI] [PubMed] [Google Scholar]
- 4.Derenko M., Malyarchuk B., Grzybowski T., Denisova G., Rogalla U., Perkova M., Dambueva I., Zakharov I. Origin and Post-Glacial Dispersal of Mitochondrial DNA Haplogroups C and D in Northern Asia. PLoS ONE. 2010;5:e15214. doi: 10.1371/journal.pone.0015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pugach I., Matveev R., Spitsyn V., Makarov S., Novgorodov I., Osakovsky V., Stoneking M., Pakendorf B. The Complex Admixture History and Recent Southern Origins of Siberian Populations. Mol. Biol. Evol. 2016;33:1777–1795. doi: 10.1093/molbev/msw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei L.-H., Wang L.-X., Wen S.-Q., Yan S., Canada R., Gurianov V., Huang Y.-Z., Mallick S., Biondo A., O’Leary A., et al. Paternal origin of Paleo-Indians in Siberia: Insights from Y-chromosome sequences. Eur. J. Hum. Genet. 2018;26:1687–1696. doi: 10.1038/s41431-018-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J., Ma P., Cheng H., Wang C., Li Y., Cui Y., Yao H., Wen S., Wei L. Post-last glacial maximum expansion of Y-chromosome haplogroup C2a-L1373 in northern Asia and its implications for the origin of Native Americans. Am. J. Phys. Anthr. 2020;174:363–374. doi: 10.1002/ajpa.24173. [DOI] [PubMed] [Google Scholar]
- 8.Yu H., Spyrou M.A., Karapetian M., Shnaider S., Radzevičiūtė R., Nägele K., Neumann G.U., Penske S., Zech J., Lucas M., et al. Paleolithic to Bronze Age Siberians Reveal Connections with First Americans and across Eurasia. Cell. 2020;181:1232–1245.e20. doi: 10.1016/j.cell.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Jeong C., Wilkin S., Amgalantugs T., Bouwman A.S., Taylor W.T.T., Hagan R.W., Bromage S., Tsolmon S., Trachsel C., Grossmann J., et al. Bronze Age population dynamics and the rise of dairy pastoralism on the eastern Eurasian steppe. Proc. Natl. Acad. Sci. USA. 2018;115:E11248–E11255. doi: 10.1073/pnas.1813608115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong C., Balanovsky O., Lukianova E., Kahbatkyzy N., Flegontov P., Zaporozhchenko V., Immel A., Wang C.-C., Ixan O., Khussainova E., et al. The genetic history of admixture across inner Eurasia. Nat. Ecol. Evol. 2019;3:966–976. doi: 10.1038/s41559-019-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narasimhan V.M., Patterson N., Moorjani P., Rohland N., Bernardos R., Mallick S., Lazaridis I., Nakatsuka N., Olalde I., Lipson M., et al. The formation of human populations in South and Central Asia. Science. 2019;365:eaat7487. doi: 10.1126/science.aat7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnecchi-Ruscone G.A., Khussainova E., Kahbatkyzy N., Musralina L., Spyrou M.A., Bianco R.A., Radzeviciute R., Martins N.F.G., Freund C., Iksan O., et al. Ancient genomic time transect from the Central Asian Steppe unravels the history of the Scythians. Sci. Adv. 2021;7:4414. doi: 10.1126/sciadv.abe4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damgaard P.D.B., Martiniano R., Kamm J., Moreno-Mayar J.V., Kroonen G., Peyrot M., Barjamovic G., Rasmussen S., Zacho C., Baimukhanov N., et al. The first horse herders and the impact of early Bronze Age steppe expansions into Asia. Science. 2018;360:7711. doi: 10.1126/science.aar7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Järve M., Saag L., Scheib C.L., Pathak A.K., Montinaro F., Pagani L., Flores R., Guellil M., Saag L., Tambets K., et al. Shifts in the Genetic Landscape of the Western Eurasian Steppe Associated with the Beginning and End of the Scythian Dominance. Curr. Biol. 2019;29:2430–2441.e10. doi: 10.1016/j.cub.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Unterländer M., Palstra F., Lazaridis I., Pilipenko A., Hofmanová Z., Groß M., Sell C., Blöcher J., Kirsanow K., Rohland N., et al. Ancestry and demography and descendants of Iron Age nomads of the Eurasian Steppe. Nat. Commun. 2017;8:14615. doi: 10.1038/ncomms14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damgaard P.D.B., Marchi N., Rasmussen S., Peyrot M., Renaud G., Korneliussen T., Moreno-Mayar J.V., Pedersen M.W., Goldberg A., Usmanova E., et al. 137 ancient human genomes from across the Eurasian steppes. Nature. 2018;557:369–374. doi: 10.1038/s41586-018-0094-2. [DOI] [PubMed] [Google Scholar]
- 17.Dani A.H., Masson V.M. History of Civilizations of Central Asia, Vol. I: The Dawn of Civilization: Earliest Times to 700 B.C. History of Civilizations of Central Asia. Volume 1 UNESCO Publishing; Paris, France: 1999. [Google Scholar]
- 18.Jettmar K. The Karasuk Culture and its South-eastern Affinities. Bull. Mus. Far East. Antiq. 1950;22:83–126. [Google Scholar]
- 19.Harmatta J., Puri B.N., Etemadi G.F. History of Civilizations of Central Asia, Vol. II: The Development of Sedentary and Nomadic Civilizations: 700 B.C. to A.D. History of Civilizations of Central Asia. Volume 2 Motilal Banarsidass Publishers; Delhi, India: 1992. [Google Scholar]
- 20.Thompson E.A. The Huns. Wiley-Blackwell; Hoboken, NJ, USA: 1996. p. 340. [Google Scholar]
- 21.Asimov M.S., Bosworth C.E. History of Civilizations of Central Asia, Vol. IV: The Age of Achievement: A.D. 750 to the End of Fifteenth Century, Part One: The Historical, Social and Economic Setting. History of Civilizations of Central Asia. UNESCO Publishing; Paris, France: 1998. p. 485. [Google Scholar]
- 22.Adle C., Habib I., Baipakov K.M. History of Civilizations of Central Asia, Vol. V: Development in Contrast: From the Sixteenth to the Mid-Nineteenth Century. History of Civilizations of Central Asia. UNESCO Publishing; Paris, France: 1998. p. 485. [Google Scholar]
- 23.Zhabagin M., Balanovska E., Sabitov Z., Kuznetsova M., Agdzhoyan A., Balaganskaya O., Chukhryaeva M., Markina N., Romanov A., Skhalyakho R., et al. The Connection of the Genetic, Cultural and Geographic Landscapes of Transoxiana. Sci. Rep. 2017;7:3085. doi: 10.1038/s41598-017-03176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He G., Adnan A., Rakha A., Yeh I.H.-Y., Wang M., Zou X., Guo J., Rehman M., Fawad A., Chen P., et al. A comprehensive exploration of the genetic legacy and forensic features of Afghanistan and Pakistan Mongolian-descent Hazara. Forensic Sci. Int. Genet. 2019;42:e1–e12. doi: 10.1016/j.fsigen.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Wells R.S., Yuldasheva N., Ruzibakiev R., Underhill P.A., Evseeva I., Blue-Smith J., Jin L., Su B., Pitchappan R., Shanmugalakshmi S., et al. The Eurasian Heartland: A continental perspective on Y-chromosome diversity. Proc. Natl. Acad. Sci. USA. 2001;98:10244–10249. doi: 10.1073/pnas.171305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balaresque P., Poulet N., Cussat-Blanc S., Gerard P., Quintana-Murci L., Heyer E., Jobling M.A. Y-chromosome descent clusters and male differential reproductive success: Young lineage expansions dominate Asian pastoral nomadic populations. Eur. J. Hum. Genet. 2015;23:1413–1422. doi: 10.1038/ejhg.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balanovsky O., Zhabagin M., Agdzhoyan A., Chukhryaeva M., Zaporozhchenko V., Utevska O., Highnam G., Sabitov Z., Greenspan E., Dibirova K., et al. Deep Phylogenetic Analysis of Haplogroup G1 Provides Estimates of SNP and STR Mutation Rates on the Human Y-Chromosome and Reveals Migrations of Iranic Speakers. PLoS ONE. 2015;10:e0122968. doi: 10.1371/journal.pone.0122968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Cristofaro J., Pennarun E., Mazières S., Myres N.M., Lin A.A., Temori S.A., Metspalu M., Metspalu E., Witzel M., King R.J., et al. Afghan Hindu Kush: Where Eurasian Sub-Continent Gene Flows Converge. PLoS ONE. 2013;8:e76748. doi: 10.1371/journal.pone.0076748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abilev S., Malyarchuk B., Derenko M., Wozniak M., Grzybowski T., Zakharov I. The Y-chromosome C3* Star-Cluster Attributed to Genghis Khan’s Descendants is Present at High Frequency in the Kerey Clan from Kazakhstan. Hum. Biol. 2012;84:79–89. doi: 10.3378/027.084.0106. [DOI] [PubMed] [Google Scholar]
- 30.Haber M., Platt D.E., Bonab M.A., Youhanna S.C., Soria-Hernanz D.F., Martínez-Cruz B., Douaihy B., Ghassibe-Sabbagh M., Rafatpanah H., Ghanbari M., et al. Afghanistan’s Ethnic Groups Share a Y-Chromosomal Heritage Structured by Historical Events. PLoS ONE. 2012;7:e34288. doi: 10.1371/journal.pone.0034288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhabagin M., Sabitov Z., Tarlykov P., Tazhigulova I., Junissova Z., Yerezhepov D., Akilzhanov R., Zholdybayeva E., Wei L.-H., Akilzhanova A., et al. The medieval Mongolian roots of Y-chromosomal lineages from South Kazakhstan. BMC Genet. 2020;21:1–8. doi: 10.1186/s12863-020-00897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhabagin M., Sabitov Z., Tazhigulova I., Alborova I., Agdzhoyan A., Wei L.-H., Urasin V., Koshel S., Mustafin K., Akilzhanova A., et al. Medieval Super-Grandfather founder of Western Kazakh Clans from Haplogroup C2a1a2-M48. J. Hum. Genet. 2021;66:707–716. doi: 10.1038/s10038-021-00901-5. [DOI] [PubMed] [Google Scholar]
- 33.Balanovsky O., Gurianov V., Zaporozhchenko V., Balaganskaya O., Urasin V., Zhabagin M., Grugni V., Canada R., Al-Zahery N., Raveane A., et al. Phylogeography of human Y-chromosome haplogroup Q3-L275 from an academic/citizen science collaboration. BMC Evol. Biol. 2017;17:18. doi: 10.1186/s12862-016-0870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhabagin M.K., Dibirova H.D., Frolova S.A., Sabitov Z. The Relation between the Y-Chromosomal Variation and the Clan Structure: The Gene Pool of the Steppe Aristocracy and the Steppe Clergy of the Kazakhs. Moscow University Anthropology Bulletin; Moscow, Russia: 2014. pp. 96–101. [Google Scholar]
- 35.Dulik M.C., Osipova L.P., Schurr T.G. Y-Chromosome Variation in Altaian Kazakhs Reveals a Common Paternal Gene Pool for Kazakhs and the Influence of Mongolian Expansions. PLoS ONE. 2011;6:e17548. doi: 10.1371/journal.pone.0017548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turuspekov Y., Sabitov Z., Daulet B., Sadykov M. The Kazakhstan DNA project hits first hundred Y-profiles for ethnic Kazakhs. Russ. J. Genet. Geneal. 2011;2:69–84. [Google Scholar]
- 37.Malyarchuk B., Derenko M., Wozniak M., Grzybowski T. Y-chromosome variation in Tajiks and Iranians. Ann. Hum. Biol. 2012;40:48–54. doi: 10.3109/03014460.2012.747628. [DOI] [PubMed] [Google Scholar]
- 38.Wen S.-Q., Du P.-X., Sun C., Cui W., Xu Y.-R., Meng H.-L., Shi M.-S., Zhu B.-F., Li H. Dual origins of the Northwest Chinese Kyrgyz: The admixture of Bronze age Siberian and Medieval Niru’un Mongolian Y chromosomes. J. Hum. Genet. 2021;67:175–180. doi: 10.1038/s10038-021-00979-x. [DOI] [PubMed] [Google Scholar]
- 39.Li H., Durbin R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H., Durbin R. Fast and accurate short read alignment with Burrows—Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouckaert R., Heled J., Kühnert D., Vaughan T., Wu C.-H., Xie D., Suchard M.A., Rambaut A., Drummond A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karmin M., Saag L., Vicente M., Sayres M.A.W., Järve M., Talas U.G., Rootsi S., Ilumäe A.-M., Mägi R., Mitt M., et al. A recent bottleneck of Y chromosome diversity coincides with a global change in culture. Genome Res. 2015;25:459–466. doi: 10.1101/gr.186684.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J., Li Y.X., Ma P.-C., Yan S., Cheng H.-Z., Fan Z.-Q., Deng X.-H., Ru K., Wang C.-C., Chen G., et al. Shared paternal ancestry of Han, Tai-Kadai-speaking, and Austronesian-speaking populations as revealed by the high resolution phylogeny of O1a-M119 and distribution of its sub-lineages within China. Am. J. Phys. Anthropol. 2021;174:686–700. doi: 10.1002/ajpa.24240. [DOI] [PubMed] [Google Scholar]
- 44.Sun J., Wei L., Wang L., Huang Y., Yan S., Cheng H., Ong R.T., Saw W., Fan Z., Deng X., et al. Paternal gene pool of Malays in Southeast Asia and its applications for the early expansion of Austronesians. Am. J. Hum. Biol. 2020;33:23486. doi: 10.1002/ajhb.23486. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Wu X., Li J., Li H., Zhao Y., Zhou H. The Y-chromosome haplogroup C3*-F3918, likely attributed to the Mongol Empire, can be traced to a 2500-year-old nomadic group. J. Hum. Genet. 2017;63:231–238. doi: 10.1038/s10038-017-0357-z. [DOI] [PubMed] [Google Scholar]
- 46.Li J., Zhang Y., Zhao Y., Chen Y., Ochir A., Sarenbilige, Zhu H., Zhou H. The genome of an ancient Rouran individual reveals an important paternal lineage in the Donghu population. Am. J. Phys. Anthr. 2018;166:895–905. doi: 10.1002/ajpa.23491. [DOI] [PubMed] [Google Scholar]
- 47.Wang H., Chen L., Ge B., Zhang Y., Zhu H., Zhou H. Genetic data suggests that the Jinggouzi people are associated with the Donghu, an ancient nomadic group of North China. Hum. Biol. 2012;84:365–378. doi: 10.3378/027.084.0402. [DOI] [PubMed] [Google Scholar]
- 48.Zerjal T., Xue Y., Bertorelle G., Wells R.S., Bao W., Zhu S., Qamar R., Ayub Q., Mohyuddin A., Fu S., et al. The Genetic Legacy of the Mongols. Am. J. Hum. Genet. 2003;72:717–721. doi: 10.1086/367774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei L.-H., Yan S., Lu Y., Wen S.-Q., Huang Y.-Z., Wang L.-X., Li S.-L., Yang Y.-J., Wang X.-F., Zhang C., et al. Whole-sequence analysis indicates that the Y chromosome C2*-Star Cluster traces back to ordinary Mongols, rather than Genghis Khan. Eur. J. Hum. Genet. 2018;26:230–237. doi: 10.1038/s41431-017-0012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malyarchuk B., Derenko M., Denisova G., Maksimov A., Woźniak M., Grzybowski T., Dambueva I., Zakharov I. Ancient links between Siberians and Native Americans revealed by subtyping the Y chromosome haplogroup Q1a. J. Hum. Genet. 2011;56:583–588. doi: 10.1038/jhg.2011.64. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y.-Z., Pamjav H., Flegontov P., Stenzl V., Wen S.-Q., Tong X.-Z., Wang C.-C., Wang L.-X., Wei L.-H., Gao J.-Y., et al. Dispersals of the Siberian Y-chromosome haplogroup Q in Eurasia. Mol. Genet. Genom. 2018;293:107–117. doi: 10.1007/s00438-017-1363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Underhill P.A., Poznik G.D., Rootsi S., Järve M., Lin A.A., Wang J., Passarelli B., Kanbar J., Myres N.M., King R.J., et al. The phylogenetic and geographic structure of Y-chromosome haplogroup R1a. Eur. J. Hum. Genet. 2014;23:124–131. doi: 10.1038/ejhg.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myres N.M., Rootsi S., Lin A.A., Järve M., King R.J., Kutuev I., Cabrera V.M., Khusnutdinova E., Pshenichnov A., Yunusbayev B., et al. A major Y-chromosome haplogroup R1b Holocene era founder effect in Central and Western Europe. Eur. J. Hum. Genet. 2010;19:95–101. doi: 10.1038/ejhg.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sengupta S., Zhivotovsky L.A., King R., Mehdi S., Edmonds C.A., Chow C.-E.T., Lin A.A., Mitra M., Sil S.K., Ramesh A., et al. Polarity and Temporality of High-Resolution Y-Chromosome Distributions in India Identify Both Indigenous and Exogenous Expansions and Reveal Minor Genetic Influence of Central Asian Pastoralists. Am. J. Hum. Genet. 2006;78:202–221. doi: 10.1086/499411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh S., Singh A., Rajkumar R., Kumar K.S., Samy S.K., Nizamuddin S., Singh A., Sheikh S.A., Peddada V., Khanna V., et al. Dissecting the influence of Neolithic demic diffusion on Indian Y-chromosome pool through J2-M172 haplogroup. Sci. Rep. 2016;6:19157. doi: 10.1038/srep19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu Q., Posth C., Hajdinjak M., Petr M., Mallick S., Fernandes D., Furtwängler A., Haak W., Meyer M., Mittnik A., et al. The genetic history of Ice Age Europe. Nature. 2016;534:200–205. doi: 10.1038/nature17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haak W., Lazaridis I., Patterson N., Rohland N., Mallick S., Llamas B., Brandt G., Nordenfelt S., Harney E., Stewardson K., et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015;522:207–211. doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allentoft M., Sikora M., Sjögren K.-G., Rasmussen S., Rasmussen M., Stenderup J., Damgaard P.D.B., Schroeder H., Ahlström T., Vinner L., et al. Population genomics of Bronze Age Eurasia. Nature. 2015;522:167–172. doi: 10.1038/nature14507. [DOI] [PubMed] [Google Scholar]
- 59.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Y Chromosome Consortium A nomenclature system for the tree of human Y-chromosomal binary haplogroups. Genome Res. 2002;12:339–348. doi: 10.1101/gr.217602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouckaert R.R., Drummond A.J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017;17:42. doi: 10.1186/s12862-017-0890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasmussen M., Anzick S.L., Waters M.R., Skoglund P., DeGiorgio M., Stafford T.W., Rasmussen S., Moltke I., Albrechtsen A., Doyle S.M., et al. The genome of a Late Pleistocene human from a Clovis burial site in western Montana. Nature. 2014;506:225–229. doi: 10.1038/nature13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rambaut A. FigTree Download Page. 2018. [(accessed on 1 September 2022)]. Available online: http://tree.bio.ed.ac.uk/software/figtree/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Following the regulations of the Ethical Commission of at the National Center for Biotechnology, the data that support the findings of this study are available on request from the corresponding author. A detailed list of variants is provided in Supplementary Table S2; this list is sufficient for the replication of genetic analyses in this study.