Abstract
Highly virulent strains of Mycoplasma mycoides subsp. mycoides SC belonging to the African cluster contain an operon with the genes gtsA, gtsB, and gtsC, encoding membrane ATP binding cassette transporter proteins GtsA, GtsB, and GtsC, which are involved in glycerol transport. Strain Afadé from the African cluster incorporated [U-14C]glycerol with a time-dependent increase. The less virulent strain L2 of the European cluster, which lacks gtsB and gtsC, failed to incorporate glycerol. Antibodies against GtsB noncompetitively inhibited glycerol uptake. l-α-Glycerophosphate was not transported by M. mycoides subsp. mycoides SC. It is postulated to be synthesized by phosphorylation of glycerol during transport and subsequently metabolized further to dihydroxyacetone phosphate accompanied by release of H2O2. Peroxide production in glycerol-containing growth medium was high for the African strain Afadé but very low for the European strain L2. Virtually no H2O2 was produced by both strains without glycerol. Hence, the efficient glycerol uptake system found in the virulent strain of the African cluster leads to a strong release of peroxide, a potential virulence factor which is lacking in the less virulent European strains. M. mycoides subsp. mycoides SC might have adopted, as a strategy for virulence, a highly efficient uptake system for glycerol which allows the production of an active metabolic intermediate that damages host cells.
Mycoplasma mycoides subsp. mycoides small-colony type (SC) is the etiological agent of contagious bovine pleuropneumonia (CBPP), a severe, highly contagious disease of cattle that has drastic economical and socioeconomic consequences 16, 39. The disease was eradicated in the middle of the 20th century in the industrialized continents, but it reemerged in Europe in the last two decades in a milder, more insidious form with a low mortality rate 30. Genetic typing methods using insertion elements IS1296 and IS1634 revealed two distinct clonal lineages of M. mycoides subsp. mycoides SC, one containing strains from the reemerging European outbreaks and the other containing isolates from the African and Australian continents 14, 18, 44. Differences between strains of the European and the African-Australian clusters of M. mycoides subsp. mycoides SC were also evidenced serologically by detection of lipoprotein LppB exclusively in strains of the African-Australian cluster 43. Controlled differential experimental infections of cattle showed that strain L2, a representative strain of the European cluster isolated from the recent reemerging outbreaks, was significantly less virulent than the African strain Afadé 1. This confirmed the observations from outbreaks of CBPP in Africa and in Europe by Nicholas et al. 30 and showed that the difference in virulence of CBPP was due to differences of the strains.
In spite of the high pathogenicity of M. mycoides subsp. mycoides SC and the enormous losses of livestock production caused by this mycoplasma worldwide, its virulence factors are virtually unknown. This is also true for other pathogenic mycoplasmas and is due to the difficulties encountered in microbiology and genetics of mycoplasmas. So far, no classical virulence factors such as toxins or invasins have been found in mycoplasmas, as revealed by the full genomic sequences of two species of this organism 17, 21. This might be due to their extremely small genome, leading mycoplasmas to a drastic economization in genetic resources, which are reduced to essential functions of life 34, 35. Mycoplasmas seem therefore to adopt endogenous structural and metabolic functions as virulence effectors to cause disease 42. Thus, membrane lipoproteins of several pathogenic mycoplasmas have been suggested as possible virulence factors due to their capability to induce blastogenesis and secretion of proinflammatory cytokines in vitro 11, 19, 33. Furthermore, the formation of active metabolic intermediates such as hydrogen peroxide (H2O2) 15, 23, 28, 29, 38, galactan 13, 25, adhesins 9, 24, and variable surface-located membrane antigens 4, 46 has been suggested as a potential virulence attribute of mycoplasmas. Despite these many proposals, the impact of H2O2 on virulence is not clear 27, and direct comparative genetic evidence explaining the basic differences between highly virulent and moderately virulent strains of M. mycoides subsp. mycoides SC is still lacking. Moreover, no tools for efficient gene transfer systems and genetic complementation experiments are available for this pathogen.
Because of their parasitic mode of life, mycoplasmas must acquire macromolecular precursors and high-energy compounds such as sugars from their environment in order to safeguard their life cycle and to produce active metabolic intermediates. For this reason, a significant number of mycoplasmal genes (about 30%) are devoted to adhesins and transporter proteins 34. Among the latter, ATP-binding cassette (ABC) transporters, which are membrane proteins ubiquitously present from bacteria to humans, are involved in the active ATP-dependent transport of a broad variety of compounds, ranging from inorganic ions to large polypeptides 20. These transporters are proteins that are built from combinations of conservative domains like the ATP-binding ABC units and membrane-bound regions. These membrane proteins normally function as transport ATPases, by hydrolyzing ATP in combination with transporting their substrate molecules through cellular or intracellular membranes. The recently discovered major genetic difference between highly virulent African strains and significantly less virulent European strains of M. mycoides subsp. mycoides SC, which consists of an 8.84-kb chromosome segment present uniquely in African-Australian strains 43, revealed several open reading frames (ORFs) for potential ABC transporters. We present the genetic basis for an efficient glycerol uptake system consisting of three ABC transporters encoded on the genes gtsA, gtsB, and gtsC and propose a biochemical pathway involved in the production of H2O2. Its potential role in virulence of M. mycoides subsp. mycoides SC is discussed.
MATERIALS AND METHODS
Strains, growth conditions, and DNA extraction.
The isolates of M. mycoides subsp. mycoides SC used in this study were strain L2 (isolated recently by our laboratory from pathological lung material of a cattle with CBPP, received from F. G. Santini, Teramo, Italy) as a representative of the European cluster and strain Afadé (isolated in Chad from a bovine from Northern Cameroon in 1969 and received from F. Thiaucourt, CIRAD-EMVT, Montpellier, France) as a representative of the African-Australian cluster 14. Mycoplasmal cultures were made in a standard medium 8 to a density of 108 to 109 cells/ml. Crude lysate preparations with GES buffer (5 M guanidium thiocyanate, 100 mM EDTA, 0.5% N-lauroylsarcosine) and DNA extraction were performed as previously described 14.
Southern blot analysis.
Genomic mycoplasmal DNA was digested with HindIII, separated electrophoretically on a 0.7% agarose gel, and transferred to a positively charged nylon membrane (Boehringer Mannheim, Rotkreuz, Switzerland). The membrane was briefly rinsed with 1× SSC (150 mM NaCl, 15 mM sodium citrate [pH 7.7]), and DNA was denatured at 80°C under vacuum for 30 min 5. The membrane was preincubated with 10 ml of hybridization buffer, consisting of 5× SSC, 0.02% sodium dodecyl sulfate (SDS), 0.1% N-lauroylsarcosine, and 1% blocking reagent (Boehringer Mannheim), at 68°C for 2 h and then hybridized with 5 ml of hybridization buffer, containing 3 μl of digoxigenin-11-dUTP (DIG)-labeled probe, for 15 h at 68°C. The membrane was then washed twice for 5 min at room temperature with 2× SSC containing 0.1% SDS and twice for 15 min at room temperature with 0.2× SSC containing 0.1% SDS. The hybridized DIG-labeled probes were detected using phosphatase-labeled anti-DIG antibodies (Boehringer Mannheim) as described in the producer's protocol.
Sequencing strategies and gene cloning.
DNA sequence analysis of segments adjacent to the locus involving the 8.84-kb deletion in European-cluster strains of M. mycoides subsp. mycoides SC was performed using the Vectorette II System (Genosys Biotechnologies, London, United Kingdom). This system is based on a unidirectional PCR approach. The previously determined DNA sequence of the 3,414-bp HindIII fragment (GenBank accession number AF165134) from strain L2 43 was used to design specific primers. Briefly, genomic DNA from Afadé was digested with BamHI and ligated to Vectorette units. Thereafter, unidirectional PCR amplification using one primer specific for the Vectorette unit and primer ORF0-S3 (5′-TTTGGCATCAAGTGCTGAAAATGGTTCATC-3′), matching bases 3149 to 3178 of AF165134, produced a fragment of about 8 kb, which was then sequenced with primer ORF0-S (5′-TCTTTGTTTTTGACCACCAG-3′), matching bases 3227 to 3246. From this new sequence, another oligonucleotide primer, ORF0-rev (5′-ATTCAAAATGAGTTAAACAAGC-3′), was designed and used with primer ORF0-S2 (5′-CCACCAGATAACTGGTTAAC-3′), matching bases 3240 to 3259 of AF165134, to produce a DIG-labeled ORF0-specific probe of 323 bp from the genomic DNA of Afadé (Fig. 1).
FIG. 1.
Genetic map of the locus containing the operon for the glycerol transport system in strain Afadé. The genetic map of the locus in M. mycoides subsp. mycoides SC which differentiates European strains (top) from African cluster strains (middle) is shown. A magnification of the operon for the glycerol transport system derived from sequence data (AF251037) is shown at the bottom. Open boxes indicate the copies of the two IS elements IS1296 and IS1634. Dotted boxes represent the 478-bp direct repeats created by the IS1634 insertion close to lppB in strain Afadé. Boxes with arrowheads indicate ORFs found in the three DNA sequences. HindIII sites are represented by vertical bars marked H. Thin dotted lines align analogous genomic sections between the two different strains. The arrow in the lower part indicates the site of the deletion in strain L2, −10 denotes the signal box of the transcription promoter, and the hairpin structure represents the potential transcription terminator signal. The scales are in kilobase pairs.
To complete the sequence of ORF0, genomic HindIII fragments of M. mycoides subsp. mycoides SC strain L2 were cloned into vector pBluescriptII SK(−) (Stratagene, La Jolla, Calif.) and reacted by colony screening using the ORF0-specific probe by standard methods 5. Plasmid DNA of the positive colonies was isolated using the QIAprep spin plasmid kit from Qiagen AG (Basle, Switzerland), digested with HindIII, and further verified by Southern blot hybridization with the ORF0-specific probe. Plasmid pJFFev1.5-L2(MP2.2), containing a 1.5-kb HindIII insert with the 5′ part of ORF0, was retained and sequenced. Double-stranded nested deletion, using exonuclease III (Pharmacia Biotech, Uppsala, Sweden), was carried out as specified by the manufacturer. The subclones obtained were sequenced with a DNA Sequenator AB310 and the Taq Dye Deoxy Terminator cycle-sequencing kit (Perkin-Elmer, Norwalk, Conn.), using primers matching the T3 and T7 promoters of the vector and primers progressively derived from sequenced segments.
Production of monospecific rabbit antibodies against the glycerol uptake system and immunoblot analysis.
To produce polyclonal antibodies against the peptides encoded on the 3′-terminal part of ORF2 or on ORF3, which are absent in the genome of European strains 43, their derived amino acid sequences were analyzed to deduce the best antigenic segment(s) for immunization. A peptide segment of 25 amino acids from the C terminus of the glycerol transport protein GtsB (ORF2) was calculated to have the best antigenicity and hydrophilicity score, using the software ProtScale (http://www.expasy.ch/cgi-bin/protscale.pl) 7 and TMpred (http://www.ch.embnet.org/software/TMPRED_form.html) 22. Monospecific, polyclonal antibodies directed against the 25-amino-acid GtsB-derived peptide 1002 (IKSIYKLIKKVILKNRGKYESKNNN; synthesized by E. Servi, Laboratoire des Peptides, Université de Lausanne, Lausanne, Switzerland) were obtained by immunizing rabbits with 500 μg of GtsB peptide 1002 in 500 μl of 0.85% (wt/vol) NaCl mixed with 500 μl of Freund's complete adjuvant (Difco Laboratories, Detroit, Mich.) followed by a booster immunization with the same amount of protein in Freund's incomplete adjuvant (Difco Laboratories) 3 weeks later. The animals were bled 10 days after the booster immunization. Antiserum 89-d32 was prepared from the blood samples and stored at −20°C. The production of monospecific polyclonal rabbit antibodies directed against lipoprotein LppB was described previously 43.
Immunoblot analysis with antiserum 89-d32 (at a dilution of 1:1,000) was carried out by standard methods 5, using SDS-polyacrylamide gel electrophoresis (10% polyacrylamide) PAGE for separation of the proteins and phosphatase-labeled conjugate goat anti-rabbit immunoglobulin G (heavy plus light chains) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.; catalogue no. 075-1506) at a dilution of 1:5,000.
Time-dependent glycerol- and G3P uptake assays.
Measurements of time-dependent glycerol or l-α-glycerophosphate (G3P) incorporation into cellular material of M. mycoides subsp. mycoides SC strains L2 and Afadé were carried out with radioactive substrates as described elsewhere 12, 28, 40. Briefly, 70-ml mycoplasmal cultures of both L2 and Afadé, grown to a density of 108 to 109 cells/ml in standard medium 8, were centrifuged at 12,000 × g for 10 min and the cell pellets were washed and resuspended in 30 ml of isotonic HEPES buffer (67.6 mM HEPES [pH 7.3], 140 mM NaCl). Cell suspensions of 1.2 ml were then adjusted to an optical density at 550 nm (OD550) of 1.0. After starvation for 1 h at 37°C, the isotonic buffer was adjusted to 6 mM G3P, 570 nM [U-14C]G3P (from a stock of 147 mCi/mmol; Amersham International plc, Little Chalfont, United Kingdom), 9 mM ATP, and 7 mM MgCl2 for G3P uptake. For glycerol uptake, the isotonic buffer was adjusted to 20 μM glycerol, 280 nM [U-14C]glycerol (from a stock of 149 mCi/mmol, Amersham International plc), and 7 mM MgCl2. Aliquots of 200 μl were vacuum filtered at different time intervals (30 s, 1 min 45 s, 3 min, 4 min 15 s, and 5 min 30 s), and filters (pore size, 0.22 μm; Millipore, Bedford, Mass.) were washed immediately with HEPES buffer and finally counted in a scintillation counter. It has to be noted that a minor amount of incorporated glycerol is not measured in this assay since it is catabolized and excreted by the bacteria.
Kinetic studies for glycerol uptake in Afadé.
Concentration-dependent glycerol incorporation into cellular material of M. mycoides subsp. mycoides SC strain Afadé was measured as described above. After starvation of the cells for 1 h at 37°C in the isotonic buffer, the final glycerol concentration was adjusted to 0, 2, 5, 10, 20, 50, 100, or 200 μM in eight different samples, using a 14C-labeled/unlabeled glycerol molar ratio of 1:357, and 7 mM MgCl2 (final concentration) was added. Aliquots of 200 μl were vacuum filtered after 4 min, and incorporation of 14C-glycerol was measured as above. Inhibition assays with anti-GtsB serum 89-d32 (at a dilution of 1:100) were performed by preincubating cells with the antibodies for 15 min at 37°C prior to addition of the substrate. As a control, preimmunization serum from the same rabbit was used.
Quantification of H2O2 production.
Measurements of time-dependent H2O2 production in M. mycoides subsp. mycoides SC strains L2 and Afadé were carried out using the Merckoquant peroxide test (Merck KgaA, Darmstadt, Germany), which has a detection range of 0.5 to 25 μg of H2O2 per ml of solution. Briefly, 30-ml mycoplasmal cultures of both L2 and Afadé, grown to a density of approximately 108 cells/ml 8, were centrifuged at 12,000 × g for 10 min and the cell pellets were washed and resuspended in 10 ml of isotonic HEPES buffer containing 7 mM MgCl2. From each strain, two cell suspensions of 1.0 ml were adjusted to an OD550 of 1.0. After starvation for 1 h at 37°C, the isotonic buffer of one sample was adjusted to 100 μM glycerol while the buffer of the other sample was kept without glycerol. At different time intervals, ranging from 5 s to 20 min, the test strips were dipped into the suspensions for 1 s and subsequently read.
Assessment of hemolytic activity.
Mycoplasma strains L2 and Afadé were grown on PPLO agar plates containing 5% washed sheep erythrocytes 8. Cultures were incubated both aerobically and anaerobically at 37°C for 72 h. The incubations were performed in the absence or in the presence of 1 mM glycerol in the growth medium. Hemolysis was estimated as described below.
Nucleotide sequence accession number.
The EMBL/GenBank accession number for the nucleotide sequence of the operon of 3,169 bp for the glycerol transport system, consisting of gtsA, gtsB, and gtsC, from M. mycoides subsp. mycoides SC strain Afadé, is AF251037.
RESULTS
Sequence analysis of the operon for three ABC transporters in M. mycoides subsp. mycoides SC.
Cloning and DNA sequence analysis of a genetic locus, specific to the virulent African-cluster strains of M. mycoides subsp. mycoides SC, revealed an operon structure of 3,169 bp containing three ORFs, ORF0, ORF2, and ORF3, which are partially overlapping. These ORFs are preceded by a consensus sequence for a −10 signal box of a prokaryotic transcription promoter and are followed by a sequence which can form a hairpin structure with a ΔG of −9.1 kcal/mol, representing a potential rho-independent transcription termination signal (Fig. 1). ORF0 encodes a peptide of 406 amino acids (aa) with a calculated molecular mass of 47.5 kDa. It is preceded by a canonical ribosome binding sequence 16 bp upstream of the ATG start codon. ORF0 contains three mycoplasma-specific UGATrp codons. It is followed by ORF2, which encodes a 342-aa peptide with a calculated molecular mass of 39.8 kDa. ORF2 overlaps ORF0 by 23 bp and contains three UGATrp codons. It is followed by ORF3, encoding a peptide of 269 aa with a molecular mass of 31.6 kDa. ORF3 overlaps ORF2 by 23 bp and contains seven UGATrp codons. In contrast to the African strain Afadé, the European strain L2 has a truncated operon with only an intact ORF0 and a partial ORF2. The A+T content of the 3.17-kb segment is 80.5%, reflecting the A+T-rich genome characteristic for mycoplasmas.
A comparison of the amino acid sequence of ORF0, ORF2, and ORF3 with the complete EMBL/GenBank database for search of related sequences was performed using the National Center for Biotechnology Information BLASTP program 3. ORF0 was found to be very similar (28% identical and 50% similar amino acids) to a hypothetical ABC transporter from M. genitalium, MG187, assumed to be involved in the G3P transport system. ORF2 has 25% identical and 44% similar amino acids to the probable ABC transporter MG188 from M. genitalium, and ORF3 has 24% identical and 48% similar amino acids to MG189, another hypothetical ABC transporter of M. genitalium 17.
Using ScanProsite software (http://www.expasy.ch/tools/scnpsit1.html), the protein encoded by ORF0 revealed an ATP/GTP binding-site motif A (P-loop) at aa 48 to 55 and an ABC transporter family signature at aa 213 to 227. ORF2 and ORF3 did not show particular features for ABC transporters. However, TMpred software (http://www.ch.embnet.org/software/TMPRED_form.html) predicted that ORF2 and ORF3 consist each of six transmembrane domains.
Expression of the ABC transporter protein in M. mycoides subsp. mycoides SC.
To assess the ABC cassette protein encoded on ORF2, total antigen preparations of M. mycoides subsp. mycoides SC strain Afadé (African cluster) and strain L2 (European cluster) were analyzed on immunoblots using monospecific polyclonal antibodies from rabbit serum 89-d32 directed against synthetic peptide 1002 derived from a strong antigenic C-terminal domain of the ORF2 amino acid sequence designated GtsB. The analysis revealed that serum 89-d32 reacted with a 40-kDa peptide, which has the predicted molecular mass of the GtsB protein in strain Afadé. In addition, a slightly weaker reaction to a 32-kDa peptide, which might be a degradation product of the 40-kDa protein, was detected in strain Afadé (Fig. 2). In contrast, no protein reacting with serum 89-d32 was found in the European-cluster strain L2 (Fig. 2), as expected from the absence of GtsB in this strain (Fig. 1), providing further evidence that the 40-kDa protein corresponds to GtsB. As a control, immunoblots made with the same antigens, which were reacted with a serum from a cow experimentally infected with strain Afadé 1, showed the integrity of the antigen preparations. Moreover, further control blots were reacted with anti-LppB 43, which revealed the characteristic 70-kDa band only in strain Afadé (Fig. 2). These results proved that GtsB (ORF2) (and, by implication, ORF0 and ORF3) is expressed in the African strain Afadé of M. mycoides subsp. mycoides SC and further showed the absence of this transporter system in the European strain L2.
FIG. 2.
Distribution of GtsB expression in strains of M. mycoides subsp. mycoides SC. Immunoblots are shown which contain 10 μg of total antigen per lane of strain Afadé or strain L2, as indicated below the panels. The immunoblots were incubated with serum from a cow experimentally infected with African strain Afadé (lanes 1), antiserum against LppB (lanes 2), and rabbit serum 89-d32 directed against GtsB (lanes 3). Arrows indicate the positions of LppB and GtsB on the immunoblots.
Analysis of glycerol and G3P uptake.
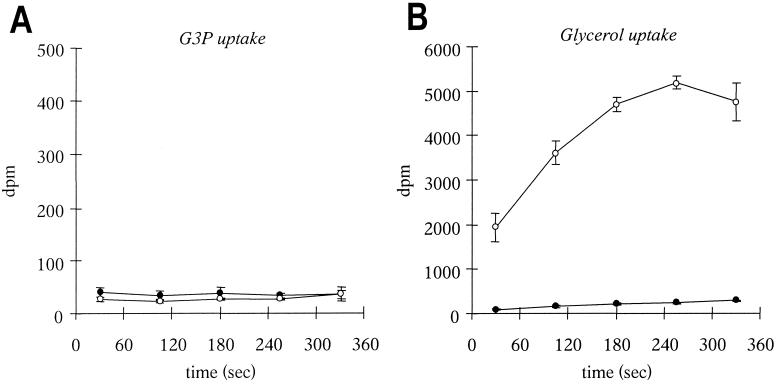
Due to the similarity of ORF0, ORF2, and ORF3 to the putative G3P uptake genes MG187, MG188, and MG189 of M. genitalium 17, we performed time-dependent measurements of both G3P and glycerol (a precursor of G3P in glycolysis and fatty acid and phospholipid metabolism) incorporation into M. mycoides subsp. mycoides SC strains L2 and Afadé (Fig. 3). The results clearly showed that both the African strain Afadé and the European strain L2 had no active G3P uptake (Fig. 3A) and hence the peptides encoded by ORF0, ORF2, and ORF3 seemed not to be involved in G3P transport. In contrast, the African strain Afadé showed a prominent active transport of glycerol (Fig. 3B). This uptake was absent in L2. The European strain showed only a weak linear incorporation of glycerol, probably due to passive diffusion into cells 26, 36. The absence of active glycerol transport in the European strain L2 (which lacks ORF2 and ORF3) gave the first evidence that the three ORFs ORF0, ORF2, and ORF3 might be involved in glycerol uptake. We have therefore designated the genes corresponding to these ORFs gtsA, gtsB, and gtsC (glycerol transport system) and have named their respective gene products GtsA, GtsB, and GtsC.
FIG. 3.
G3P and glycerol uptake. Logarithmic-phase cultures of M. mycoides subsp. mycoides SC strains L2 and Afadé were washed with appropriate isotonic buffers and adjusted to an OD550 of 1.0. After starvation of the cultures for 1 h at 37°C, [U-14C]G3P (147 mCi/mmol) was added to a final concentration of 570 nM together with 6 mM unlabeled G3P (A) or [U-14C]glycerol (149 mCi/mmol) was added to a final concentration of 280 nM together with 20 μM unlabeled glycerol (B). Aliquots of 200 μl were filtered at different time intervals, and the filters were put into vials containing scintillation cocktail. Disintegrations per minute (dpm) of 14C incorporated by mycoplasmas were counted. Symbols: ●, strain L2; ○, strain Afadé. The data are the mean of three independent measurements. Error bars, standard errors.
Inhibition of glycerol uptake upon preincubation with a specific antiserum.
To obtain direct evidence on the impact of GtsB (ORF2) on glycerol transport of strain Afadé of M. mycoides subsp. mycoides SC, kinetic studies were performed in the absence and in the presence of rabbit serum 89-d32 (diluted 1:100) directed against the C-terminal end of GtsB. The concentration-dependent glycerol uptake in strain Afadé followed a typical Michaelis-Menten model of enzyme kinetics (Fig. 4). The rate of the reaction was considered to be the number of picomoles of glycerol incorporated into 109 cells during the uptake. Untreated cells showed a glycerol uptake with a Km of 59 μM and a Vmax of 22,700 pmol. Preincubation of the cells with antiserum 89-d32 did not alter the Km value of glycerol uptake (57 μM) but reduced the Vmax by about 60% to 13,700 pmol. A control experiment using cells preincubated with serum taken from the same rabbit prior to immunization with peptide 1002 did not significantly alter the glycerol uptake (Vmax, 19,900 pmoles) (Fig. 4). Hence, GtsB and consequently GtsA and GtsC are ABC transporter proteins effecting active glycerol uptake in M. mycoides subsp. mycoides SC. Blocking GtsB with antibodies directed against its C-terminal domain results in a noncompetitive inhibition (unaffected Km but lowered Vmax) of the glycerol uptake.
FIG. 4.
Kinetic measurements for the glycerol uptake in strain Afadé. Strain Afadé was grown to the logarithmic phase, washed, and starved for 1 h at 37°C in isotonic buffer. The cells were then preincubated for 15 min at 37°C with no serum (control) (●), rabbit preimmune serum (○), or anti-GtsB serum 89-d32 (■). Serial concentrations (range, 0 to 200 μM) of glycerol with a labeled/unlabeled glycerol molar ratio of 1:357 were added, and incubation at 37°C was continued for another 4 min. Thereafter, the aliquots were immediately filtered and the filters were counted in vials containing scintillation cocktail. The graph depicts the picomoles of glycerol incorporated in 109 cells. A Lineweaver-Burke plot for the estimation of the kinetic parameters is shown in the inset. The data are the mean of three independent measurements.
Production of H2O2 as a consequence of glycerol uptake and metabolism.
In view of the highly efficient glycerol uptake system encoded by gtsABC, H2O2, a product of the metabolism of glycerol, was expected to be accumulated and then released by M. mycoides subsp. mycoides SC. We therefore measured H2O2 production in the presence of glycerol by the African strain Afadé of M. mycoides subsp. mycoides SC and compared it with that produced in the European strain L2, which is devoid of the glycerol uptake system. As shown in Fig. 5, strain Afadé released large amounts of H2O2 after addition of glycerol but not in the absence of glycerol, while, in contrast, strain L2 produced only small amounts of H2O2 after addition of glycerol. This minor production of H2O2 was attributed to passive diffusion of glycerol through the mycoplasma membrane, followed by its metabolization. These results indicate that the highly efficient glycerol uptake system encoded by gtsABC in strain Afadé and probably also in the other strains of the African cluster is responsible for the release of large amounts of H2O2. Our data suggest that this release is absent in European-cluster strains due to the lack of the gtsB and gtsC genes of the glycerol uptake system.
FIG. 5.
Hydrogen peroxide production upon incubation with glycerol. Logarithmic-phase cultures of M. mycoides subsp. mycoides SC strains L2 and Afadé were washed with isotonic buffer and adjusted to an OD550 of 1.0. After starvation for 1 h at 37°C, samples were kept without glycerol or supplemented with glycerol: ●, L2 without glycerol; ○, L2 with 100 μM glycerol; ■, Afadé without glycerol; □, Afadé with 100 μM glycerol. H2O2 production (micrograms per milliliter per 109 cells) was read at different times (0 to 20 min) after glycerol addition. The data are the mean of five independent measurements.
M. mycoides is known to be a hemolytic mycoplasma 15. Assessment of the hemolytic activity of strains Afadé and L2 of M. mycoides subsp. mycoides SC on solid medium containing sheep erythrocytes showed a significantly stronger hemolytic activity of strain Afadé when grown on medium supplemented with 1 mM glycerol under aerobic conditions than when grown on medium without glycerol supplementation or grown under anaerobic conditions (Table 1). Our data suggest that the hemolytic activity of the African strain is to a large extent due to H2O2 production. The European strain L2, in contrast, showed only weak hemolytic activity independent of glycerol supplementation and aerobic growth conditions.
TABLE 1.
Hemolytic assay
| Concn (mM) Glycerol | Hemolytic activity of strain:
|
|||
|---|---|---|---|---|
| L2
|
Afadé
|
|||
| Aerobic | Anaerobic | Aerobic | Anaerobic | |
| 0 | + | + | + | + |
| 1 | + | + | +++ | + |
DISCUSSION
Although it is over 100 years since the causative organism of CBPP was discovered 31, the effectors which are responsible for the high virulence of M. mycoides subsp. mycoides SC are still not well understood. The current knowledge of the significantly lower virulence of strain L2, representative of the European cluster of M. mycoides subsp. mycoides SC, compared to strain Afadé, representative of the African cluster 1, led us to analyze the biochemical functions encoded on the genetic locus which differentiates the two clusters 43. This locus represents a 8.84-kb segment which is present only in strains of the African-Australian cluster and is deleted in strains isolated in Europe from the reemerging outbreaks since 1980. We have recently reported that this segment contains a gene encoding lipoprotein LppB, which is found only in African-Australian-cluster strains, in addition to a copy of insertion element IS1634 43. Moreover, at one extremity this locus contains an operon encoding three potential ABC transporter proteins.
The present work revealed the role of these three ABC transporters, named GtsA, GtsB, and GtsC, in the glycerol uptake of M. mycoides subsp. mycoides SC. This uptake mechanism seems to be specific to the highly pathogenic strains of the African-Australian cluster. The three genes gtsA, gtsB, and gtsC showed characteristics of a polycistronic prokaryotic operon. They were preceded by a single typical −10 box of prokaryotic promoter sequences, but no −35 box could be found, similarly to most other mycoplasmal genes. In addition, all three ORFs, gtsA, gtsB and gtsC, overlap each other and show only one putative ribosome binding site upstream of gtsA. However, we have demonstrated the expression of gtsB by the use of antibodies raised against a synthetic peptide derived from the C-terminal amino acid sequence of GtsB. This suggested that the three genes on the gtsABC operon are expressed by a mechanism that seems to be confined to mycoplasmas. It should be noted that the analogous locus found in M. genitalium is also composed of three overlapping ORFs, MG187, MG188 and MG189 17. Kinetic analysis and inhibition experiments with antibodies directed against GtsB, one of the components of this heterotrimeric ABC transporter system, revealed its function in glycerol uptake. However, it was functional only in African-Australian-cluster strains of M. mycoides subsp. mycoides SC, since European strains lack the entire gtsC gene and part of gtsB.
Glycerol may enter mycoplasmas by passive diffusion 26, 36 and eventually by a putative glycerol uptake facilitator gene (glpF) product, as is found in M. genitalium 17. However, the amount of glycerol that can be taken up by these two systems must be considerably smaller than that incorporated by the active uptake system specified by GtsABC. It is known that mycoplasmas lack hexokinase, which is essential for the first step in the glycolytic pathway 32. This absence is balanced by the presence of a sugar phosphotransferase, which catches sugars from the environment and phosphorylates them directly during import into the cell. Thus, the absence of G3P incorporation in M. mycoides subsp. mycoides SC, even in African strains which actively transport glycerol, was not striking. ABC transporters such as the newly discovered GtsA, GtsB, and GtsC function as transport ATPases. They hydrolyze ATP and sequester a phosphate group. Therefore, by analogy to the above-mentioned sugar phosphorylation, we assume that the phosphate group sequestered from the ABC transporters GtsA, GtsB, and GtsC can be transferred to glycerol during its transport in M. mycoides subsp. mycoides SC across the cellular membrane by ABC transporter itself, thus preventing the inability to import G3P. In our model (Fig. 6), G3P is obtained by concomitant phosphorylation of glycerol during active transport and is then metabolized further, accompanied by massive release of H2O2. A glycerol kinase activity has been reported for M. mycoides 45, but direct evidence for the existence of a gene analogous to the putative glycerol kinase gene glpK of M. genitalium 17 is still lacking.
FIG. 6.
Model for glycerol incorporation and H2O2 production in M. mycoides subsp. mycoides SC strain Afadé. This model is a modification of the model for sugar and central intermediate metabolism in M. pneumoniae reported by Himmelreich et al. 21. Glycerol is incorporated into M. mycoides subsp. mycoides SC (African-Australian-cluster strains) through the proteins GtsA, GtsB, and GtsC encoded by the gtsABC operon and phosphorylated during uptake. In the cytoplasm, G3P is used as substrate by α-GPO to produce H2O2 from O2. The other product, dihydroxyacetone phosphate (DHAP), is metabolized in the glycolytic pathway in combination with glyceraldehyde-3-phosphate (GA3P).
During active infections, mycoplasmas need to scavenge for macromolecular precursors from host cell membranes and intracellular pools. This is a possible explanation for the finding that mycoplasmas are rich in ABC transporters 10, 17, 21. These membrane proteins allow the organism to live in a drastic parasitic mode. M. mycoides subsp. mycoides SC does not have known genes coding for primary virulence factors such as toxins or invasins. Several reports suggested that H2O2, which is released by certain mycoplasmas, is a powerful mediator of cell injury 2, 23, 28, 41. The observed difference in O2 consumption in the presence of glycerol was the only biochemical difference known between African and European strains, and was suggested to play a role in their different degrees of virulence 23. We have directly measured H2O2 production in representative European and African strains of M. mycoides subsp. mycoides SC in the presence and absence of glycerol and were able to demonstrate a direct relation to hemolytic activity, which was found to be pronounced only in strains of the African-Australian cluster grown in glycerol-enriched medium but not in European-cluster strains. Correlation between hemolytic activity and H2O2 production in mycoplasmas was already proposed in the 1960s 15, 37. Our results showed that strains of M. mycoides subsp. mycoides SC that are impaired in glycerol uptake have lost nearly the entire capacity for H2O2 production and have lost almost all their hemolytic activity. H2O2 is assumed to damage the host by either directly impairing tissue cells or inducing gene expression in the host, e.g., proinflammatory genes via activation of NF-κB 6. At this point it should be noted that mycoplasmal infections generally cause immunopathological symptoms. Thus, the inability of European M. mycoides subsp. mycoides SC strains to actively transport glycerol may limit their pathogenic oxidative damage by H2O2 production and could explain the reduced morbidity and mortality of CBPP in Europe. Glycerol is metabolized via G3P oxidase (α-GPO) in a reaction which yields 1 mol of H2O2 per mol of G3P oxidized. This previously led to speculations that the lack of the H2O2-producing enzyme α-GPO was responsible for the reduced pathogenicity of some M. mycoides subsp. mycoides SC strains 28, 45. The present work shows, however, that the lack of H2O2 production by certain strains of M. mycoides subsp. mycoides SC such as the European-cluster strains was due to the lack of an active glycerol uptake system encoded on the gtsABC operon.
Summarizing, our results show that the Australian-African-cluster strains of M. mycoides subsp. mycoides SC are able to import glycerol, a precursor of H2O2 while the less virulent European cluster strains are devoid of this function, due to a deletion in the operon encoding the glycerol uptake system. Although the present results do not permit a direct comparison of isogenic strains and complementation experiments which are technically not possible in this mycoplasma species, we conclude that the lack of H2O2 production in the European cluster could be one of the reasons for its lower virulence. Consequently, the highly efficient glycerol uptake system allowing the production of H2O2, which damages host cells, might be a potential virulence attribute of M. mycoides subsp. mycoides SC.
ACKNOWLEDGMENTS
We are grateful to J. Nicolet for support and numerous discussions on this project, to M. Krawinkler for expert help with preparations of mycoplasma cultures, and to Y. Schlatter for technical assistance with DNA sequence analysis. We thank F. Santini (Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise, Teramo, Italy) and F. Thiaucourt (CIRAD-EMVT, Montpellier, France) for gifts of strains.
This study is part of the European COST action 826 on ruminant mycoplasmoses and was supported by grant C96.0073 from the Swiss Ministry of Education and Science and by the Swiss Federal Veterinary Office.
REFERENCES
- 1.Abdo E-M, Nicolet J, Miserez R, Gonçalves R, Regalla J, Griot C, Bensaide A, Krampe M, Frey J. Humoral and bronchial immune responses in cattle experimentally infected with Mycoplasma mycoides subsp. mycoides small colony type. Vet Microbiol. 1998;59:109–122. doi: 10.1016/s0378-1135(97)00184-3. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Groun E A, Taylor R R, Varsani H, Wadher B J, Leach R H, Miles R. Biochemical diversity within the “Mycoplasma mycoides” cluster. Microbiology. 1994;140:2033–2042. doi: 10.1099/13500872-140-8-2033. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Atkin C L, Wei S, Cole B C. The Mycoplasma arthritidis superantigen MAM: purification and identification of an active peptide. Infect Immun. 1994;62:5367–5375. doi: 10.1128/iai.62.12.5367-5375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 6.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 7.Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1995. Nucleic Acids Res. 1995;24:189–196. doi: 10.1093/nar/24.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannerman E S, Nicolet J. Isolation and identification of porcine Mycoplasma in Switzerland. Schweiz Arch Tierheilkd. 1971;113:697–710. [PubMed] [Google Scholar]
- 9.Baseman J B, Cole R M, Krause D C, Leith D K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982;151:1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bork P, Ouzounis C, Casari G, Schneider R, Sander C, Dolan M, Gilbert W, Gillevet P M. Exploring the Mycoplasma capricolum genome: a minimal cell reveals its physiology. Mol Microbiol. 1995;16:955–967. doi: 10.1111/j.1365-2958.1995.tb02321.x. [DOI] [PubMed] [Google Scholar]
- 11.Brenner C, Wroblewski H, Le Henaff M, Montagnier L, Blanchard A. Spiralin, a mycoplasmal membrane lipoprotein, induces T-cell-independent B-cell blastogenesis and secretion of proinflammatory cytokines. Infect Immun. 1997;65:4322–4329. doi: 10.1128/iai.65.10.4322-4329.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brzoska P, Rimmele M, Brzostek K, Boos W. The pho regulon-dependent Ugp uptake system for glycerol-3-phosphate in Escherichia coli is trans inhibited by P. J Bacteriol. 1994;176:15–20. doi: 10.1128/jb.176.1.15-20.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buttery S H, Cottew G S, Lloyd L C. Effect of soluble factors from Mycoplasma mycoides subsp. mycoides on the collagen content of bovine connective tissue. J Comp Pathol. 1980;90:303–314. doi: 10.1016/0021-9975(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 14.Cheng X, Nicolet J, Poumarat F, Regalla J, Thiaucourt F, Frey J. Insertion element IS1296 in Mycoplasma mycoides subsp. mycoides small colony identifies a European clonal line distinct from African and Australian strains. Microbiology. 1995;141:3221–3228. doi: 10.1099/13500872-141-12-3221. [DOI] [PubMed] [Google Scholar]
- 15.Cole B C, Ward J R, Martin C H. Hemolysin and peroxide activity of Mycoplasma species. J Bacteriol. 1968;95:2022–2030. doi: 10.1128/jb.95.6.2022-2030.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egwu G O, Nicholas R A J, Ameh J A, Bashiruddin J B. Contagious bovine pleuropneumonia: an update. Vet Bull. 1996;66:875–888. [Google Scholar]
- 17.Fraser C M, Gocayne J D, White O, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 18.Frey J, Cheng X, Kuhnert P, Nicolet J. Identification and characterization of IS1296 in Mycoplasma mycoides subsp. mycoides SC and presence in related mycoplasmas. Gene. 1995;160:95–100. doi: 10.1016/0378-1119(95)00195-c. [DOI] [PubMed] [Google Scholar]
- 19.Herbelin A, Ruuth E, Delorme D, Michel Herbelin C, Praz F. Mycoplasma arginini TUH-14 membrane lipoproteins induce production of interleukin-1, interleukin-6, and tumor necrosis factor alpha by human monocytes. Infect Immun. 1994;62:4690–4694. doi: 10.1128/iai.62.10.4690-4694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 21.Himmelreich R, Hilbert H, Plagens H, Pirkl E, Li B C, Herrmann R. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 1996;24:4420–4449. doi: 10.1093/nar/24.22.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann K, Stoffel W. TMbase—a database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 23.Houshaymi B M, Miles R J, Nicholas R A. Oxidation of glycerol differentiates African from European isolates of Mycoplasma mycoides subspecies mycoides SC (small colony) Vet Rec. 1997;140:182–183. doi: 10.1136/vr.140.7.182. [DOI] [PubMed] [Google Scholar]
- 24.Krause D C, Baseman J B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983;39:1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd L C, Buttery S H, Hudson J R. The effect of the galactan and other antigens of Mycoplasma mycoides var. Mycoides on experimental infection with that organism in cattle. J Med Microbiol. 1971;4:425–439. doi: 10.1099/00222615-4-4-425. [DOI] [PubMed] [Google Scholar]
- 26.McElhaney R N, de Gier J, van der Neut-Kok E C. The effect of alterations in fatty acid composition and cholesterol content on the nonelectrolyte permeability of Acholeplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1973;298:500–512. doi: 10.1016/0005-2736(73)90376-3. [DOI] [PubMed] [Google Scholar]
- 27.Meier B, Habermehl G G. Evidence for superoxide dismutase and catalase in mollicutes and release of reactive oxygen species. Arch Biochem Biophys. 1990;277:74–79. doi: 10.1016/0003-9861(90)90552-a. [DOI] [PubMed] [Google Scholar]
- 28.Miles R J, Taylor R R, Varsani H. Oxygen uptake and H2O2 production by fermentative Mycoplasma spp. J Med Microbiol. 1991;34:219–223. doi: 10.1099/00222615-34-4-219. [DOI] [PubMed] [Google Scholar]
- 29.Niang M, Rosenbusch R F, Debey M C, Niyo Y, Andrews J J, Kaeberle M L. Field isolates of Mycoplasma ovipneumoniae exhibit distinct cytopathic effects in ovine tracheal organ cultures. J Vet Med Ser A. 1998;45:29–40. doi: 10.1111/j.1439-0442.1998.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 30.Nicholas R A J, Santini F G, Clark K M, Palmer N M A, DeSantis P, Bashiruddin J B. A comparison of serological tests and gross lung pathology for detecting contagious bovine pleuropneumonia in two groups of Italian cattle. Vet Rec. 1996;139:89–93. doi: 10.1136/vr.139.4.89. [DOI] [PubMed] [Google Scholar]
- 31.Nocard E I E, Roux E. Le microbe de la péripneumonie. Ann Inst Pasteur. 1898;12:240–262. [Google Scholar]
- 32.Pollack J D. Mycoplasma genes: a case for reflective annotation. Trends Microbiol. 1997;5:413–419. doi: 10.1016/s0966-842x(97)01113-x. [DOI] [PubMed] [Google Scholar]
- 33.Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induce proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun. 1996;64:637–643. doi: 10.1128/iai.64.2.637-643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Razin S. The minimal cellular genome of Mycoplasma. Indian J Biochem Biophys. 1997;34:124–130. [PubMed] [Google Scholar]
- 35.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romijn J C, van Golde L M, McElhaney R N, van Deenen L L. Some studies on the fatty acid composition of total lipids and phosphatidylglycerol from Acholeplasma laidlawii B and their relation to the permeability of intact cells of this organism. Biochim Biophys Acta. 1972;280:22–32. doi: 10.1016/0005-2760(72)90209-3. [DOI] [PubMed] [Google Scholar]
- 37.Somerson N L, Walls B E, Chanock R M. Hemolysin of Mycoplasma pneumoniae: tentative identification as a peroxide. Science. 1965;150:226–228. doi: 10.1126/science.150.3693.226. [DOI] [PubMed] [Google Scholar]
- 38.Taylor R R, Mohan K, Miles R J. Diversity of energy-yielding substrates and metabolism in avian mycoplasmas. Vet Microbiol. 1996;51:291–304. doi: 10.1016/0378-1135(96)00039-9. [DOI] [PubMed] [Google Scholar]
- 39.ter Laak E A. Contagious bovine pleuropneumonia. A review. Vet Q. 1992;14:104–110. doi: 10.1080/01652176.1992.9694343. [DOI] [PubMed] [Google Scholar]
- 40.Truniger V, Boos W. Glycerol uptake in Escherichia coli is sensitive to membrane lipid composition. Res Microbiol. 1993;144:565–574. doi: 10.1016/0923-2508(93)90006-n. [DOI] [PubMed] [Google Scholar]
- 41.Tryon V V, Baseman J B. Pathogenic determinants and mechanisms. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 457–471. [Google Scholar]
- 42.Valdivieso Garcia A, Rosendal S, Allen O B, Thompson C M, Watson S. Cytotoxicity of Mycoplasma mycoides subspecies mycoides for cultured endothelial cells. Int J Med Microbiol. 1989;272:202–209. doi: 10.1016/s0934-8840(89)80008-8. [DOI] [PubMed] [Google Scholar]
- 43.Vilei E M, Abdo E-M, Nicolet J, Botelho A, Gonçalves R, Frey J. Genomic and antigenic differences between the European and African/Australian clusters of Mycoplasma mycoides subsp. mycoides SC. Microbiology. 2000;146:477–486. doi: 10.1099/00221287-146-2-477. [DOI] [PubMed] [Google Scholar]
- 44.Vilei E M, Nicolet J, Frey J. IS1634, a novel insertion element creating long, variable-length direct repeats which is specific for Mycoplasma mycoides subsp. mycoides small-colony type. J Bacteriol. 1999;181:1319–1323. doi: 10.1128/jb.181.4.1319-1323.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadher B J, Henderson C L, Miles R J, Varsani H. A mutant of Mycoplasma mycoides subsp. mycoides lacking the H2O2-producing enzyme l-α-glycerophosphate oxidase. FEMS Microbiol Lett. 1990;72:127–130. doi: 10.1016/0378-1097(90)90358-w. [DOI] [PubMed] [Google Scholar]
- 46.Wise K S, Yogev D, Rosengarten R. Antigenic variation. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 473–489. [Google Scholar]