Abstract
The sugar will eventually be exported transporters (SWEET) family is an important group of transport carriers for carbon partitioning in plants and has important functions in growth, development, and abiotic stress tolerance. Although the SWEET family is an important sugar transporter, little is known of the functions of the SWEET family in maize (Zea mays), especially in response to abiotic stresses. To further explore the response pattern of maize SWEET to abiotic stress, a bioinformatics-based approach was used to predict and identify the maize SWEET gene (ZmSWEET) family. Twenty-four ZmSWEET genes were identified using the MaizeGDB database. Phylogenetic analysis resolved these twenty-four genes into four clades. One tandem and five segmental duplication events were identified, which played a major role in ZmSWEET family expansion. Synteny analysis provided insight into the evolutionary characteristics of the ZmSWEET genes with those of three graminaceous crop species. A heatmap showed that most ZmSWEET genes responded to at least one type of abiotic stress. By an abscisic acid signaling pathway, among which five genes were significantly induced under NaCl treatment, eight were obviously up-regulated under PEG treatment and five were up-regulated under Cd stress, revealing their potential functions in response to abiotic stress. These findings will help to explain the evolutionary links of the ZmSWEET family and contribute to future studies on the functional characteristics of ZmSWEET genes, and then improve abiotic stress tolerance in maize through molecular breeding.
Keywords: maize, SWEET gene family, abiotic stress, ABA, expression pattern
1. Introduction
Sugar is an important and basic source of carbon to support plant morphogenesis and growth [1]. As a substrate for the production of primary and secondary metabolites, sugar participates in many physiological processes, including energy metabolism and signal transduction, to maintain plant growth and development and respond to stresses [2,3]. Therefore, the transport and distribution of sugar is crucial in plants [4]. Sugar requires carriers to mediate their transport through biofilms, and the transporters include monosaccharide transporters, sucrose transporters, and sugar will eventually be exported transporters (SWEETs) [5]. The SWEET protein family is an important intracellular and intercellular transporter of sugar, and is responsible for the transmembrane transport of sugar for various physiological functions such as providing sucrose for phloem loading, a response to abiotic stress and regulating seed filling, etc. [5,6,7].
SWEET proteins are distinguished by the presence of the MtN3/saliva (MtN3_slv) domain, also termed the PQ-loop repeat. The MtN3_slv domain is made up of three α-helical transmembrane domains (3-TMs). Eukaryotic SWEETs are normally composed of seven TM domains, comprising two tandemly repeated 3-TMs and a single linking TM domain [8]. The MtN3_slv domain was first identified in the MtN3 nodulation-specific gene of Medicago truncatula from constructed cDNA libraries [9]. A homolog of MtN3 was discovered in the saliva of Drosophila as a gene expressed specifically in the salivary glands during embryonic development [10]. Plant SWEET family members can be divided into four groups based on evolutionary relationships [5]. The Clade I and II members have a functional preference for hexose transport, the Clade III members are sucrose transporters, and the Clade IV members are localized to the tonoplast to transport fructose [5,11]. SWEET family genes play a pivotal role in plant growth and development [4,12]. The first identified SWEET gene, named AtSWEET1, was reported to have the ability to transport glucose in Arabidopsis thaliana [12]. In addition, SWEET8 and SWEET13 function in transporting sugar to develop pollen grains in Arabidopsis, and SWEET9 plays a crucial role as a sucrose transporter for nectar production [5]. SWEET15 mediates the export of sugar from the endosperm for embryo development [13]. SWEET17 was the first member of the SWEET gene family identified in plants for the transport of vesicular fructose and was highly expressed in plant roots, playing a specific role in fructose transport across the tonoplast [14]. In rice, the SWEET family genes OsSWEET11 and OsSWEET14 are crucial for rice reproductive organ development. The ossweet11 and ossweet14 mutants are defective in seed development, exhibiting a small grain and semidwarf phenotype [15]. In maize, ZmSWEET13 is the major family member responsible for loading sugar in the phloem [16]. In the zmsweet13a, b, and c mutants, the translocation of photosynthetic products is reduced, and soluble sugar and starch accumulate in the leaves, resulting in severe stunting [17]. The ZmSWEET4c protein is specifically expressed during seed germination and mediates entry into the scutellum of glucose resulting from starch hydrolysis [18]. ZmSWEET4 also plays an important role in the development and maturation of maize kernels, and the zmsweet4c mutation causes severe crumpling of the kernel, thereby reducing yield [7].
Members of the SWEET family are also extensively involved in biotic and abiotic stress responses [19,20]. Xanthomonas oryzae pv. oryzae (Xoo) infection may induce the expression of a transcription activator-like (TAL) effector, which binds to promoter region-specific regulatory elements of OsSWEET11/14 to activate their expression [21,22]. When the binding site is mutated, the infection capacity of Xoo is lost [22,23]. Abiotic stresses seriously influence the plant growth and development and photosynthetic product transport [14]. Under abiotic stresses, plants monitor carbohydrate redistribution by regulating SWEET expression [14]. Overexpression of AtSWEET16 and AtSWEET17 in Arabidopsisimproved the cold tolerance of transgenic plants [24]. Transgenic Arabidopsis overexpressing AtSWEET15 was more sensitive to salt stress and the atsweet15 mutant shows greater salt tolerance [25]. Heat stress negatively affected the SWEET function of loading, unloading, and long-range transport of sugar in the phloem, ultimately leading to abnormal growth and development of the plant [26]. Under drought stress, AtSWEET11 and AtSWEET12 expression was induced and the transport of sucrose from the leaves to the roots was enhanced [27]. Under Cd stress, PvSWEET24 was significantly induced, while the expressions of PvSWEET5 and PvSWEET20 were severely inhibited in the common bean (Phaseolus vulgaris L.) [28]. In addition, ZmSWEET13a/13b/13c were sucrose transporters responsive to abiotic stresses and their transcription levels were enhanced by heavy metal cadmium stress in maize [16]. SWEET genes have been shown to be involved in the response to abiotic stresses in other plants, such as rice, watermelon and pomegranate [11,23,29,30].
Abscisic acid (ABA) is a plant hormone widely involved in plant response to abiotic stresses [31,32]. Exogenous ABA treatment may increase the accumulation of sugar and proline in plants and thus enhance the resistance of plants to drought and cold [32]. In addition, ABA affects the sucrose distribution under abiotic stress by influencing the expression of genes involved in sugar synthesis, metabolism, and transport [33]. The expressions of OsSWEET13 and OsSWEET15 were increased under the regulation of the ABA-responsive transcription factor OsbZIP72, thereby affecting sugar transport in rice for maintenance of homeostasis under abiotic stresses [34].
Maize is one of the most important crops in the world. In addition to being used as food, maize also plays important roles in feed, industry, medical treatment and other aspects [35]. Studies of the gene family are important for the analysis of the origin and prediction of genes functions, and then predict the function of genes [5,29,30]. The SWEET gene family has been identified in many plants [29,30]. However, the functions of SWEET proteins are currently poorly understood in maize, especially in response to abiotic stresses. In the present study, twenty-four SWEET genes were identified by genome-wide analysis in maize and were classified into four clades. Comprehensive analysis of the SWEET genes was performed, including chromosomal distribution, phylogenetic relationships, exon–intron structure, motif composition, gene duplication, and collinearity analysis. The expression patterns of the SWEET genes were analyzed in response to polyethylene glycol (PEG), salt (NaCl), cadmium (Cd) stresses and exogenous ABA treatment. The results provide insights for functional characterization of the proteins and important information for further study of the regulatory mechanism of SWEET family members under abiotic stresses in maize.
2. Results
2.1. Identification, Phylogenetic Analysis, and Chromosomal Localization of Maize SWEET Genes
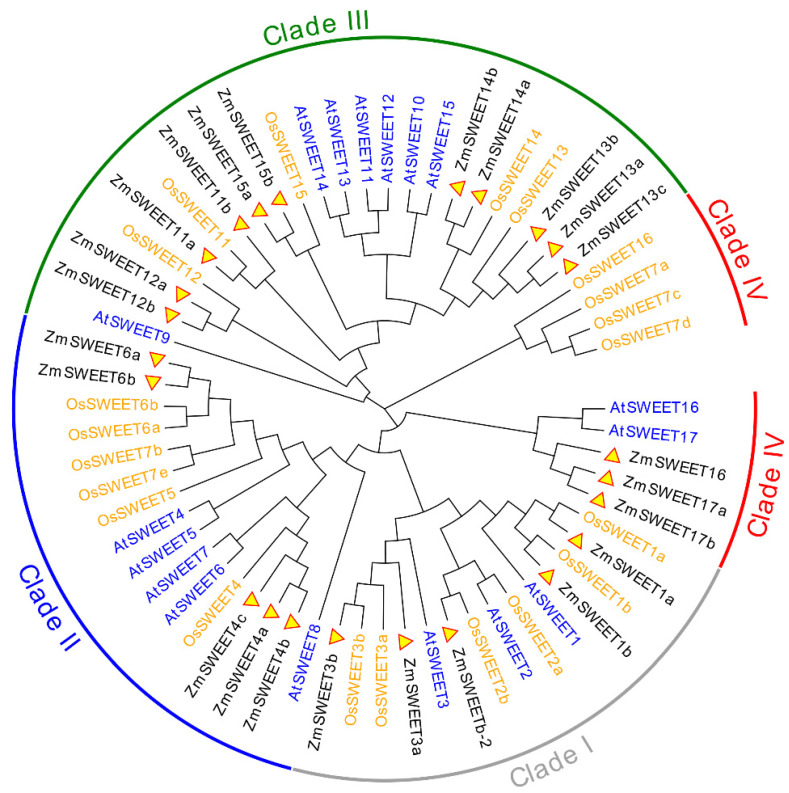
Maize SWEETs were identified using a hidden Markov model and BLASTP searches. Twenty-four predicted whole-length ZmSWEET genes were identified with Pfam 03083 in the maizegenome. In addition to SWEET proteins predicted with a Pfam MtN3_slv, ZmSWEET11b/13a/13b/13c/14a/14b/15a/15b were predicted with a Pfam PQ-loop (PF04193). The MaizeGDB website currently lists and names 23 SWEET genes; Zm00001d043735 on the NCBI website was named bidirectional sugar transporter SWEET2, and thus was named ZmSWEETb-2 in this study for convenience. The MW of the ZmSWEET proteins ranged from 24.71 kDa (ZmSWEET17a) to 37.27 kDa (ZmSWEET14a), and the proteins comprised 230 (ZmSWEETb-2) to 344 (ZmSWEET14a) amino acid residues. The pI ranged from 5.1 (ZmSWEET15b) to 9.64 (ZmSWEET13c) (Table 1). The amino acid sequences for 24 ZmSWEETs, 17 AtSWEETs, and 23 OsSWEETs were aligned, and a phylogenetic analysis performed to investigate evolutionary relationships. According to the evolutionary tree, SWEET genes can be classified into four clades (I to IV). Clade I included five maize SWEET genes (ZmSWEET1a/1b/b-2/3a/3b), Clade II included five genes (ZmSWEET4a/4b/6a/6b/6c), Clade III contained 13 genes (ZmSWEET11a/11b/12a/12b/13a/13b/13c/14a/14b/15a/15b), and Clade IV contained three genes (ZmSWEET16/17a/17b) (Figure 1). Most ZmSWEET genes (15 of 24) were located on chromosomes 1, 3, 5, and 8 (Figure S1). Chromosomes 2, 6, 7, and 9 each carried a single ZmSWEET gene (Figure S1).
Table 1.
The information of Sugar Will Eventually be Exported Transporter gene family in Maize.
| Name | GeneID | Chrom | Transcript | No. of Amino Acids | TMHs | Theoretical pI | Molecular Weight (Average) |
|---|---|---|---|---|---|---|---|
| ZmSWEET1a | Zm00001d000222 | B73V4_ctg26 | 1 | 250 | 6 | 9.26 | 28,631.97 |
| ZmSWEET1b | Zm00001d038226 | Chrom06 | 1 | 267 | 7 | 8.9 | 26,773.47 |
| ZmSWEET3a | Zm00001d010440 | Chrom08 | 2 | 239 | 7 | 9.02 | 26,104 |
| ZmSWEET3b | Zm00001d039347 | Chrom03 | 1 | 265 | 7 | 9.43 | 28,672.58 |
| ZmSWEET4a | Zm00001d015905 | Chrom05 | 1 | 252 | 6 | 9.44 | 27,278.59 |
| ZmSWEET4b | Zm00001d015914 | Chrom05 | 6 | 255 | 6 | 9.48 | 24,756.56 |
| ZmSWEET4c | Zm00001d015912 | Chrom05 | 1 | 261 | 7 | 9.54 | 28,279.61 |
| ZmSWEET6a | Zm00001d044421 | Chrom03 | 1 | 244 | 6 | 9.03 | 27,133.44 |
| ZmSWEET6b | Zm00001d011299 | Chrom08 | 1 | 243 | 7 | 8.87 | 26,912.16 |
| ZmSWEET11a | Zm00001d031647 | Chrom01 | 1 | 289 | 7 | 9.47 | 33,490.68 |
| ZmSWEET11b | Zm00001d021064 | Chrom07 | 1 | 310 | 7 | 9.2 | 31,314.46 |
| ZmSWEET12a | Zm00001d029135 | Chrom01 | 1 | 306 | 7 | 6.82 | 32,686.56 |
| ZmSWEET12b | Zm00001d047487 | Chrom09 | 1 | 282 | 7 | 9.2 | 30,837.73 |
| ZmSWEET13a | Zm00001d023677 | Chrom10 | 1 | 302 | 7 | 9.57 | 32,950.4 |
| ZmSWEET13b | Zm00001d023673 | Chrom10 | 1 | 301 | 7 | 9.57 | 32,929.51 |
| ZmSWEET13c | Zm00001d041067 | Chrom03 | 1 | 295 | 7 | 9.64 | 32,280.66 |
| ZmSWEET14a | Zm00001d007365 | Chrom02 | 1 | 344 | 7 | 9.48 | 37,267.29 |
| ZmSWEET14b | Zm00001d049252 | Chrom04 | 1 | 293 | 7 | 9.28 | 31,679.58 |
| ZmSWEET15a | Zm00001d050577 | Chrom04 | 1 | 304 | 7 | 5.67 | 32,944.15 |
| ZmSWEET15b | Zm00001d016590 | Chrom05 | 1 | 333 | 6 | 5.1 | 34,340.3 |
| ZmSWEET16 | Zm00001d029098 | Chrom01 | 1 | 317 | 7 | 6.6 | 33,998.45 |
| ZmSWEET17a | Zm00001d040656 | Chrom03 | 3 | 238 | 6 | 6.71 | 17,560.88 |
| ZmSWEET17b | Zm00001d009071 | Chrom08 | 2 | 239 | 7 | 6.1 | 25,906.62 |
| ZmSWEETb-2 | Zm00001d043735 | Chrom03 | 1 | 230 | 7 | 8.75 | 25,165.85 |
Figure 1.
Phylogenetic tree for SWEET proteins of maize, rice, and Arabidopsis. Multiple sequence alignment of the SWEET domains was performed using MUSCLE, and the phylogenetic tree was constructed using MEGA 7.0 with the maximum likelihood method with 1000 bootstrap replicates. The tree was divided into four clades, designated I, II, III, and IV. Proteins of maize, rice, and Arabidopsis are highlighted in black, orange, and blue, respectively.
2.2. Gene Structure and Conserved Motif Analysis of ZmSWEET Gene Family
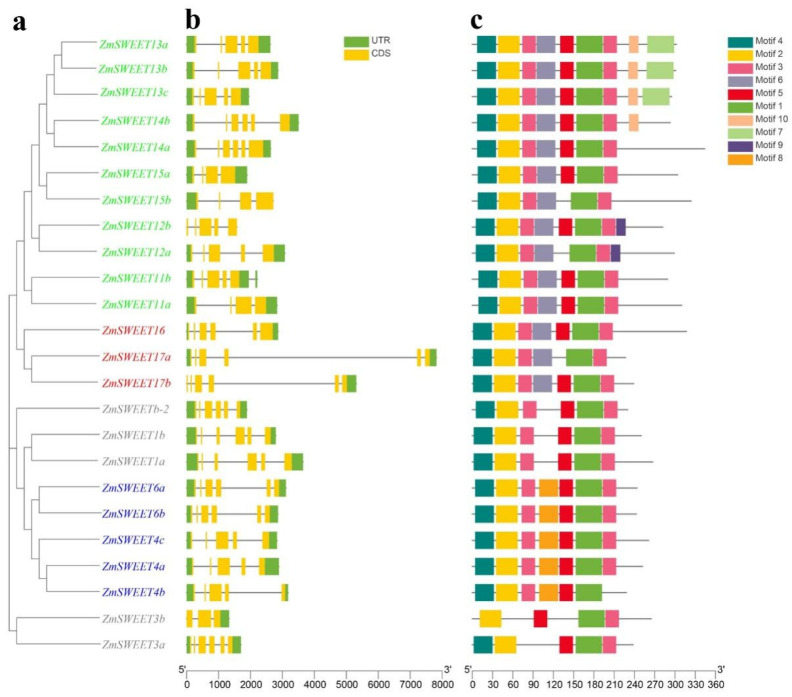
Structural features of the ZmSWEET genes were analyzed and conserved motifs were detected using the MEME Suite. The number of exons and introns was similar within a clade. Most of the ZmSWEET genes harbored 4–6 exons, but ZmSWEET3b had three exons (Figure 2). Similar motif compositions in the same clade were indicative of functional similarity among the members of each clade (Figure 2). The ZmSWEETs in Clades I and II harbored motifs 1, 2, 3, and 5. Clade III ZmSWEETs contained motifs 1, 2, 3, 4, 5, 6, 7, 9, and 10. Cluster IV ZmSWEETs featured motifs 1, 2, 3, 4, 5, and 6. All ZmSWEETs contained motifs 1 and 2, which are typical motifs of the MtN3_slv domain, and each clade shared similar motif features, further supporting the phylogenetic classification of the SWEET family.
Figure 2.
Conserved motifs and gene structure analyses of maize SWEETs based on phylogenetic relationships. All motifs were identified with the MEME Suite using the complete amino acid sequences. Exon–intron structure analyses were performed with TBtools. (a) Neighbor-joining tree indicating evolutionary relationships. Clades I, II, III, and IV are indicated in gray, green, blue, and red, respectively. (b) Exon–intron structure. Green boxes, yellow boxes, and black lines indicate the untranslated region, coding sequence, and gene length, respectively. (c) Conserved motifs.
2.3. Evolutionary Differentiation and Collinearity Analysis of ZmSWEET Genes
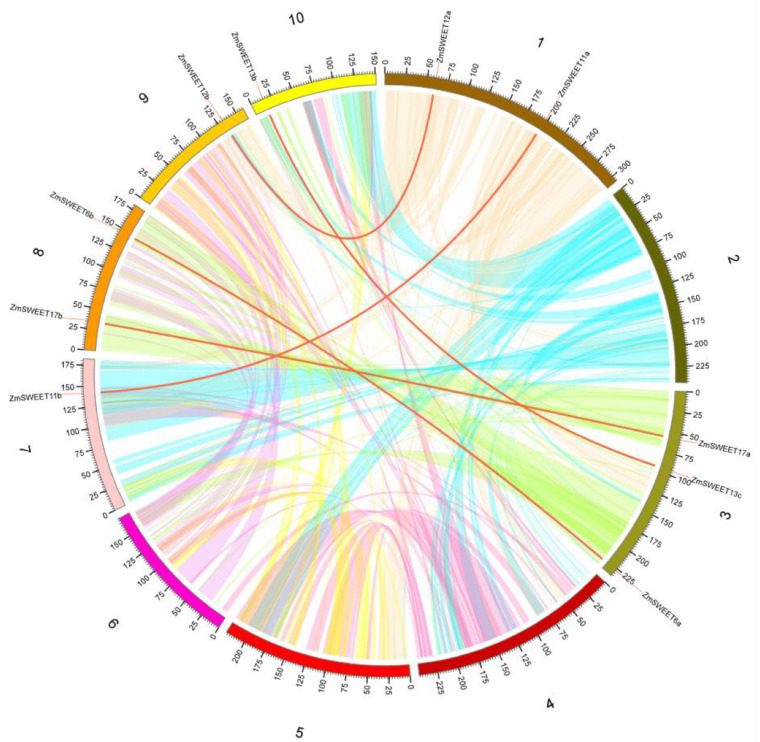
In the present study, only one pair of ZmSWEET genes (ZmSWEET4c/4b) was identified as a tandem duplication and were located on chromosome 5 (Figure S1). Five pairs of segmental duplication events were identified, comprising ZmSWEET6a/6b, ZmSWEET11a/11b, ZmSWEET12a/12b, ZmSWEET13b/13c, and ZmSWEET17a/17b (Figure 3 and Table S1). In comparison, seven, four, and four pairs of segmental duplication events were identified among SWEET genes in foxtail millet, rice, and sorghum, respectively (Table S1).
Figure 3.
Synteny analysis of the SWEET family in maize. Red curves linking ZmSWEET genes indicate duplicated gene pairs in the maize SWEET family.
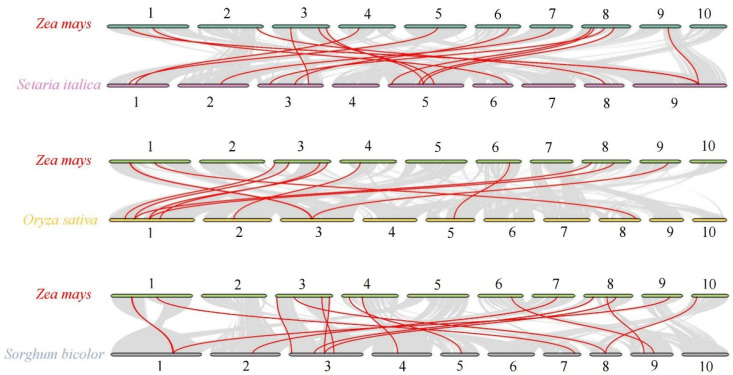
The syntenic relationships of the ZmSWEET family were analyzed by constructing six intergenomic collinear maps with three dicotyledons (Arabidopsis, Medicago sativa, and Brassica rapa) and three monocotyledons (Setaria italica, Oryza sativa, and Sorghum bicolor). A syntenic relationship was not detected between the SWEET genes of maize and dicotyledons. In contrast, 17, 15, and 16 pairs of SWEET collinear genes were identified between maize and foxtail millet, rice, and sorghum, respectively (Figure 4 and Table S2).
Figure 4.
Synteny analysis of SWEET genes between maize and three representative graminaceous species. Gray lines in the background show collinear blocks in the genomes of maize and foxtail millet, rice, and sorghum, and red lines highlight the collinear SWEET gene pairs.
The evolutionary constraints acting on the duplicated genes were estimated by calculating the rates of synonymous substitution (Ks) and non-synonymous substitution (Ka) and Ka/Ks ratio for duplicated gene pairs in maize, foxtail millet, rice, and sorghum (Table S1). Values of Ka/Ks = 1 indicate neutral selection, Ka/Ks < 1 indicates purifying selection, and Ka/Ks > 1 indicates positive selection to accelerate evolution. In the four graminaceous crops, KQL05781 and KQL14056 were the only gene pair detected with Ka/Ks close to 1. Other SWEET gene pairs had undergone purifying selection and the estimated divergence time ranged from 16 to 86 Mya (Table S1).
2.4. Promoter Region Analysis of ZmSWEET Gene Family
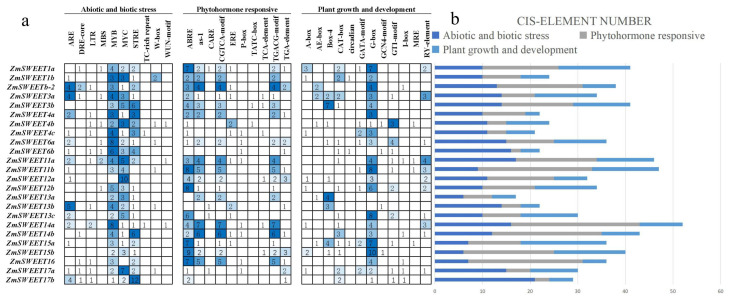
To analyze the transcriptional regulatory mechanism of the ZmSWEET genes, the cis-acting elements in the promoter region of the ZmSWEET genes were identified using the PlantCARE database. The cis-acting elements were identified and classified into three categories, comprising abiotic and biotic stresses, plant hormone response, and plant growth and development (Figure 5 and Table S3). Among the abiotic and biotic stress elements, two general stress response motifs were detected, namely MYB (CCAAT box) and MYC (CAC ATG box) binding sites, accounting for 29% and 25% of the total number of cis-acting elements identified, respectively. Other stress-specific cis-acting elements identified were responsive to injury and pathogens, including W-box, TC-rich repeats, and WUN-motif. Among the plant hormone response elements, the ABRE element involved in ABA response was identified in the promoter regions of 21 ZmSWEET members, the TCA-element involved in salicylic acid response was detected in six ZmSWEETs, and the TGACG-motif involved in methyl jasmonate response was identified in 17 ZmSWEETs. Other elements, such as as-1, which is involved in salicylic acid and oxidative stress response, the CGTCA-motif, which is involved in response to methyl jasmonate, and EREs, which are involved in ethylene reactions, accounted for 18%, 18%, and 4%, respectively, of the total number of cis-acting elements in this category. Twelve cis-acting elements associated with plant growth and development were identified in the ZmSWEET gene promoter regions (Figure 5). These results suggested that the ZmSWEET genes might be broadly involved in growth and development, and response to various abiotic stresses in maize.
Figure 5.
Cis-acting elements in the ZmSWEET gene family. (a) Numbers of different elements in the promoter region of the ZmSWEET genes, as indicated by different color intensities and numbers in the grid. (b) Total number of cis-acting elements in each response category. Dark blue indicates abiotic and biotic stresses, gray indicates phytohormones, and light blue represents plant growth and development.
2.5. Expression Profiles of ZmSWEET Genes in Different Tissues and Expression Analysis of ZmSWEET Genes in Response to Abiotic Stresses and Exogenous ABA
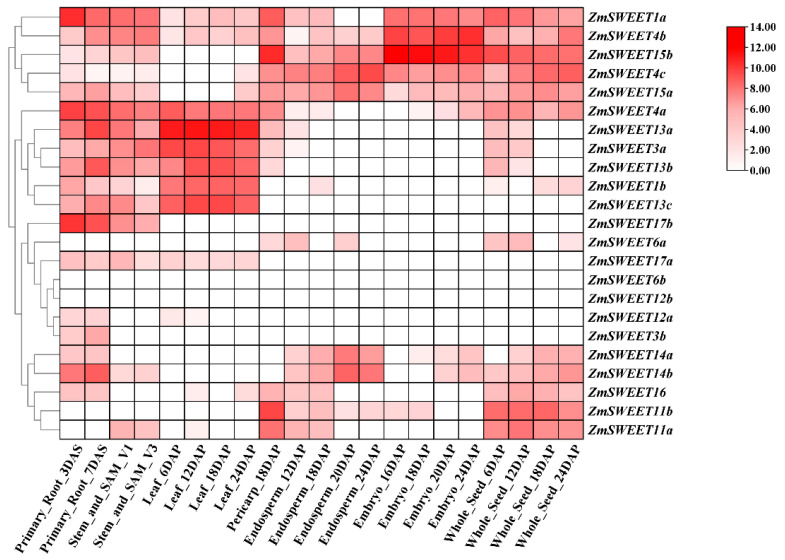
To explore the functions of ZmSWEET genes, transcriptome analysis was performed to investigate the expression of ZmSWEET genes in different tissues at different developmental stages of maize (Figure 6). ZmSWEET1a/4b/15b/4c/15a were expressed in almost all tissues analyzed. ZmSWEET4a/13a/3a/13b/1b/13c/17b were highly expressed in vegetative organs. ZmSWEET14a/14b/16/11a/11b were significantly expressed in the seed. ZmSWEET6b/12b were weakly expressed in all tissues. These findings suggested that the ZmSWEET genes play diverse roles in the growth and development of maize.
Figure 6.
Expression profiles of maize SWEETs in different tissues. Log2-based fold change data were used to create the heatmap. Fold changes in gene expression are indicated by the color scale. DAS: Days of growth after sprouting. DAP: Days after pollination.
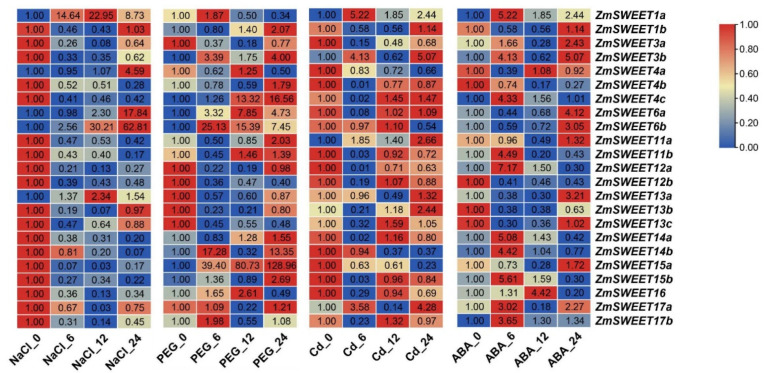
To investigate ZmSWEET gene expression in response to abiotic stresses, the transcript abundance of the ZmSWEET genes was analyzed by qRT-PCR (Figure 7). Under salt stress, the transcription of five ZmSWEET genes (ZmSWEET1a/4a/6a/6b/13a) was significantly induced, whereas seven genes (ZmSWEET4b/11b/12a/14a/14b/15a/15b) were significantly inhibited. Under drought stress, the transcription of ZmSWEET4c/14b/15a was significantly increased. Under Cd stress, the transcript abundance of ZmSWEET1a was initially increased and thereafter decreased, and the transcript abundance of ZmSWEET3b/11a/13b/17a was distinctly increased. Abscisic acid is an important phytohormone involved in plant response to abiotic stresses. Under exogenous ABA treatment, the transcription of 16 ZmSWEET genes was induced, of which five genes (ZmSWEET1a/3b/12a/14a/15b) were significantly induced. Overall, the expression of 23 ZmSWEET genes showed different alterations under the abiotic stresses and exogenous ABA treatment, and some genes were affected on multiple treatments (Figure S2).
Figure 7.
Expression analysis of ZmSWEET in response to abiotic stresses and exogenous ABA. Red represents induced expression; blue represents repressed expression. The 0 h relative expression level of each treatment was taken as 1.
3. Discussion
Crop growth and development are hampered by abiotic stresses, which affect crop yields [36,37]. Sugar metabolism is an important physiological activity and is involved in the plant response to diverse environmental factors [3]. SWEET proteins are among the carriers of sugars that influence sugar transport and distribution in the plant [5]. In the current study, 24 ZmSWEET genes were identified in the maize genome, and one new gene (ZmSWEETb-2) was identified on chromosome 3 from the results of a previous study [18]. However, Zm00001d009365 was not identified in the present study, probably because of the use of different versions of maize genomic data. In addition, 17, 21, 23, and 24 SWEETs were identified in Arabidopsis, rice, sorghum, and foxtail millet, respectively, which was consistent with the results of previous studies [12,29,36]. All evidence suggested that graminaceous species contain a similar number of SWEET genes.
The present phylogenetic analysis revealed that SWEET family genes (comprising ZmSWEETs, AtSWEETs, and OsSWEETs) could be divided into four clades (Figure 1). The SWEET proteins from maize and rice tended to cluster together, whereas proteins from Arabidopsis clustered separately in the phylogenetic tree, suggesting that SWEET genes may have mainly evolved after monocotyledon–dicotyledon divergence and before the differentiation of graminaceous species. The distinct clades within the gene families may adopt distinct roles during evolution to maximize the flexibility of environmental adaptation [5]. The SWEET proteins of Clades I and II preferentially transport hexoses (such as glucose and fructose), those of Clade III are effective sucrose transporters, according to studies of Arabidopsis and rice, and the proteins of Clade IV are localized to the vesicular membrane and regulate fructose inflow and efflux [5]. The MtN3_slv domain is the signature domain of the SWEET family of proteins and is represented by two predicted motifs, motif 1 and motif 2. The ZmSWEET family members frequently contained motifs 1, 2, 3, 4, and 5, indicating that these motifs are conserved within the ZmSWEET family and may be associated with the basic function of the protein. Motif 6 was detected in Clades III and IV, motifs 7, 9, and 10 were present in Clade III, whereas motif 8 was detected only in Clade II. The motif analysis showed that conserved domains, such as MtN3_slv, were widely present in all members, and unique motifs were detected in each clade (Figure 2). These results implied that, while SWEET genes transport sugar, the substrates may differ between each clade.
The evolution of gene function in higher plants suggests that each isoform is physiologically differentiated with regard to expression sites and regulatory modalities, thus helping the organism to adapt to different environments. SemiSWEETs, the bacterial homologs of SWEETs, are among the smallest transporter proteins known. In both SWEETs and SemiSWEETs, a collection of three TMHs combine to produce a single MtN3 unit [38,39], and the doubling of TMHs enhances the ability of the SWEET protein to transport sucrose. Currently, there are two hypothesized mechanisms to explain the evolution of SWEETs. The first hypothesis suggests that SWEET proteins arose through the replication and fusion of hemiglycan chains (containing three TMHs) [38]. The second hypothesis suggests that SWEET proteins were derived from the fusion of archaeal and bacterial hemiglycan chains [40]. Internal replication of 3-TMH genes must have occurred early in evolution to produce new proteins with 7-TMHs able to translocate greater amounts of sucrose [38]. Therefore, the current ZmSWEETs in the maize genome with 6–7 TMHs imply that the replication and fusion of SWEETs may have occurred in the early genome.
For the gene family expansion, three predominant mechanisms are whole-genome duplication (WGD) events, tandem duplication (TD), and chromosomal fragment duplication (SD) [41]. Among these mechanisms, WGD is an important mechanism that provides potential for the evolution of novel gene functions to enable new adaptations for physiological processes, such as plant tolerance of biotic and abiotic stresses, and generation of gene functional diversity, which in turn affects the evolutionary process of species. As a graminaceous crop, maize has undergone four WGD events (i.e., τ-, σ-, ρ-, and mWGD) of which ρ-WGD was graminaceous-specific and occurred ~95–115 Mya [42]. Maize underwent an additional species-specific WGD event (mWGD, ~26 Mya) [42]. In addition to WGD events, tandem and segmental duplications have played a critical role in the expansion of gene families [43]. In the present study, several gene duplication events were identified, including one tandem duplication and five segmental duplication events. The segmental duplication gene pairs ZmSWEET6a/6b, ZmSWEET11a/11b, and ZmSWEET13b/13c contained similar motif architectures. The tandem repeat gene pair ZmSWEET4b/4c differed somewhat with ZmSWEET4c missing motif 3. The motif structures of the remaining two segmental duplication gene pairs (ZmSWEET12a/12b and ZmSWEET17a/17b) differed slightly from one another, suggesting that the motif structures may have changed during gene duplication. SWEET genes with different structural domains may have diverged in function during replication. Thus, tandem duplication and segmental duplication are the major forms of SWEET family expansion in maize. The Ka/Ks ratio of 10 pairs of ZmSWEET genes indicated that all had undergone purifying selection. In addition, 17, 15, and 16 maize genes showed collinearity with the foxtail millet, rice, and sorghum genomes, respectively. In contrast, no gene collinearity was observed between maize and Arabidopsis. The results of collinearity analysis suggested that the expansion of the SWEET gene family may have mainly occurred after the divergence of monocotyledons and dicotyledons, and before the divergence of graminaceous species. This conclusion is consistent with previous evolutionary analyses.
The expression data showed different expression patterns of ZmSWEET genes in different tissues. ZmSWEET1a/4b/4c/15a/15b were significantly expressed in almost all tissues analyzed, ZmSWEET1b/3a/4a/13a/13b/13c were substantially expressed in vegetative organs, and ZmSWEET11a/11b/14a/14b/16 were mainly expressed in the endosperm of the seed, indicating that the genes play important roles in sugar transport in different maize tissues (Figure 6). The changes in expression during organ development also suggested that different ZmSWEET genes function at different stages of organ development. The expression of ZmSWEET genes under several abiotic stresses was analyzed by qRT-PCR. Some ZmSWEET genes were significantly induced by high salinity, PEG, or Cd stress. The results implied that certain ZmSWEET genes were induced to modify sugar distribution in response to abiotic stresses in maize. A number of ZmSWEET genes were induced by exogenous ABA treatment and abiotic stress, such as ZmSWEET1a/4c/14b/15b/16/17a, suggesting that these genes might be under the regulation of the ABA signaling pathway to respond to abiotic stresses. However, several genes were induced by abiotic stress but not by exogenous ABA treatment, such as ZmSWEET4a/6a/6b/15a, which implied that these genes were independent of the ABA signaling pathway.
Overall, 24 ZmSWEET genes have been identified in the maize genome. These genes function as sugar transporters and play essential roles in maize growth and development as well as in response to biotic and abiotic stresses. In this study, we analyzed the evolutionary relationships and expression patterns of the ZmSWEET genes. The results provide insight into the potential functions and characteristics of the ZmSWEET genes. The information will contribute to future studies on the biological roles of the ZmSWEET genes in maize.
4. Materials and Methods
4.1. Plant Materials and Treatments
The autogamous maize cultivar “inbred line” was used in the study. Seeds were preserved in our laboratory and incubated in the seedling culture room of the Laboratory of Plant Physiology and Germplasm, Shenyang Agricultural University, from August to December 2020. Seeds of uniform size and full grains were selected. The seeds were disinfected with 75% ethanol and washed with distilled water after 1 min to remove the residual ethanol. The cleaned seeds were evenly sown in seedling pots, covered with vermiculite to a depth of 2 cm, and irrigated with distilled water to allow the vermiculite to absorb sufficient water. The seeds germinated after approximately 3 days and the seedling roots protruded from the bottom grid of the pots. Hoagland’s nutrient solution (pH 6.0) was added to the basal tray in which the seedling pots were placed to ensure that the roots could access the nutrient solution. The nutrient solution was replaced every 3 days until the seedlings attained the three-leaf stage [44]. The three-leaf seedlings were then treated with drought, salt, and Cd stresses and exogenous ABA by application of half-strength Hoagland’s nutrient solution supplemented with 20% PEG for drought stress treatment, 200 mol/L NaCl for salt stress treatment, 40 mg/L CdCl2 for Cd stress treatment, or 100 µmol/L ABA. The uppermost mature leaves were collected at 0, 6, 12, and 24 h after initiation of the stress treatment, with three biological replicates at each time point. The sampled leaves were snap-frozen in liquid nitrogen and stored in an ultra-low-temperature refrigerator at −80 °C.
4.2. Identification and Evolutionary Analysis
Protein sequences of AtSWEETs of Arabidopsis and OsSWEETs of rice were downloaded from Ensembl (https://asia.ensembl.org/index.html accessed on 1 December 2021). The complete amino acid and nucleotide sequences of Zea mays B73 RefGen_v4 were downloaded from MaizeGDB (https://maizegdb.org/ accessed on 1 December 2021). In addition, transcriptomic data were downloaded from MaizeGDB (https://maizegdb.org/ accessed on 27 July 2022). The RNA-seq gene map of maize inbred line B73 included 79 different samples [45]. Expression heat maps were performed using TBtools (https://github.com/CJ-Chen/TBtools accessed on 19 March 2022), and transcriptome data were selected from 21 different maize tissues and developmental periods [46]. Row-scale and log-scale normalization calculations and row clustering were performed on the heat maps. Known SWEET protein sequences were used to construct the hidden Markov model archive and to query the maize dataset using HMMER (http://hmmer.org/ accessed on 5 December 2021) [47]. SWEETs from maize were validated by conducting a BLAST search using SWEETs from rice and Arabidopsis as queries. The conserved domains of identified maize SWEETs were predicted with the PFAM (http://pfam.xfam.org/ accessed on 5 December 2021) and CDD (https://www.ncbi.nlm.nih.gov/cdd/ accessed on 5 December 2021) databases [48,49]. Evolutionary trees were constructed (with 1000 bootstrap replicates) for the SWEETs proteins from Arabidopsis, rice, and maize using MEGA 7.0 (https://www.megasoftware.net/ accessed on 19 March 2022) and ClustalW software (https://www.genome.jp/tools-bin/clustalw accessed on 19 March 2022) [50,51]. Chromosomal localization of the maize SWEET genes was performed using a MapChart (http://mg2c.iask.in/mg2c_v2.0/ accessed on 3 April 2022) based on the chromosomal start and termination information downloaded from MaizeGDB (https://maizegdb.org/ accessed on 3 April 2022) [52]. The multiple covariance scanning toolkit (MCScanX, http://chibba.pgml.uga.edu/mcscan2/MCScanX.zip accessed on 5 April 2022) was used to locate tandem duplicated genes [53].
4.3. Sequence Analysis
The ExPASy proteomics server (http://web.expasy.org/protparam/ accessed on 5 December 2021) was used to predict the molecular weight (MW) and isoelectric points (pI) of the maize SWEET proteins (Table 1 and Table S4) [54]. The MEME Suite (http://meme-suite.org/ accessed on 7 December 2021) was employed to identify the conserved protein motifs of ZmSWEETs, which were further annotated with TBtools (https://github.com/CJ-Chen/TBtools accessed on 7 December 2021) [55]. The domain structure comprised ten motifs, with the motif length ranging from 6 to 50 bp (Table S5). The gene structure was assessed with GSDS (http://gsds.gao-lab.org/ accessed on 10 December 2021) [56]. The cis-acting elements in the 1500 bp sequence upstream of the coding sequences were predicted with the PlantCARE database (Table S3) (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ accessed on 13 December 2021). Elements (ABRE, DRE, LTRE, ERE, and MBS) associated with abiotic stress response were subjected to further analysis [57,58].
4.4. Replication Events and Ka/Ks Analysis of SWEET Genes
Maize SWEET genes were examined for relationships within genomes and between single or multiple intergenomic covariates using MCScanX (http://chibba.pgml.uga.edu/mcscan2/MCScanX.zip accessed on 6 May 2022) [59]. Multiple mechanisms may lead to gene family expansion and doubling, including whole-genome duplication or polyploidization, tandem duplication, segmental duplication, transposon-mediated transposon duplication, and retroposition.
To explore the selection pressure on the maize SWEET family, the ratio of Ka (non-synonymous substitution rate) and Ks (synonymous substitution rate) was calculated using ClustalW (https://www.genome.jp/tools-bin/clustalw accessed on 7 May 2022). The time of occurrence of segmental duplication events for homologous genes was calculated as
| T = Ks/2λ × 10−6 | (1) |
where λ is the rate of molecular substitution in grasses (6.5 × 10−9), and expressed as a million years ago (Mya) [60].
4.5. Quantitative Real-Time PCR Analysis
Total RNA isolation and quantitative real-time PCR (qRT-PCR) analysis were performed to analyze the expression of maize genes under salt, drought, and Cd stress and exogenous ABA treatment [61]. A total of 23 maize SWEET homologous genes were used for the analysis. Total RNA from plant leaves was extracted using TRIzol Reagent (CW Biotech) and subjected to DNase I treatment to remove genomic DNA contamination. The RNA concentration was determined by means of a BioDrop ultra-micro ultraviolet nucleic acid assay. First-strand cDNA was synthesized from 1 µg total RNA using the UEIris II RT-PCR system. The qRT-PCR assays were performed using a real-time PCR analyzer (Bio-Rad, Applied Biosystems PCR, SCILOGEX Gradient Thermal Cycler PCR Instrument TC1000-G). Each reaction mixture contained 10 µL of 2× SYBR® Green Pro Taq HS Premix, 1.0 µL cDNA sample, 0.4 µL forward primer (final concentration 10 µM), and 0.4 µL reverse primer (final concentration 10 µM) in a final volume of 20 µL. The thermal-cycling protocol was as follows: 95 °C for 5 min, then 45 cycles of 95 °C for 15 s and 60 °C for 1 min. Melting curve analysis was used to verify the specificity of the reaction. Three technical replicates of each cDNA sample were analyzed. The Zm00001d013367 genes were selected as an internal control to normalize the transcript levels of ZmSWEET genes. The relative gene expression levels were calculated using the 2−∆∆Ct method (Table S6). The normalized data were processed with TBtools and plotted as a heatmap to visualize the changes in SWEET gene expression (https://github.com/CJ-Chen/TBtools accessed on 19 March 2022) [62]. The number of genes with relative gene expression greater than 2 and relative gene expression less than 0.5 under the four treatments were shown by using the online site Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/ accessed on 22 August 2022) to create venny diagrams. All primer pairs were designed with Primer (v5.0) software (http://www.broadinstitute.org/ftp/pub/software/Primer5.0/ accessed on 1 December 2021) and are listed in Table S7.
Acknowledgments
We thank Robert McKenzie, from Liwen Bianji (Edanz) (www.liwenbianji.cn, accessed on 27 July 2022), for editing the English text of a draft of this manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13101682/s1, Figure S1: Chromosomal distribution of ZmSWEET genes. Tandem repeat genes are labeled in green. Figure S2: Venny diagram of the ZmSWEET relative expression under four treatments. a The number of ZmSWEET gene is induced under four treatments. b The number of ZmSWEET gene is inhibited under four treatments. Blue oval-shaped, yellow oval-shaped, green oval-shaped and red oval-shaped indicate NaCl treatment, PEG treatment, Cd treatment and ABA treatment, respectively; Table S1: Interchromosomal relationships of SWEET genes in maize, foxtail millet, rice, and sorghum. Table S2: Syntenic gene pairs between maize and foxtail millet, rice, and sorghum. Table S3: Distribution of cis-acting elements in the promoter region of maize SWEET genes. Table S4: The identification of SWEET members in rice, foxtail millet and sorghum. Table S5: Analysis and distribution of conserved motifs in maize SWEET proteins. Table S6: Quantitative results of the ZmSWEET under PEG, salt (NaCl), cadmium (Cd) stresses and exogenous ABA treatment. Table S7: Primers of sequences.
Author Contributions
Conceptualization, J.F. and C.L.; methodology, Y.R.; validation, J.Z., L.Z. and T.L.; formal analysis, J.Z.; data curation, J.Z.; writing—original draft preparation, J.Z.; writing—review and editing, C.L.; visualization, A.Z. and X.D.; supervision, Y.Z.; project administration, J.F.; funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
National Foreign Experts Program of China (DL2022006001); the scientific research projects of the Liaoning Provincial Department of Education, China (No. LSNZD201608).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Walmsley A.R., Barrett M.P., Bringaud F., Gould G.W. Sugar transporters from bacteria, parasites and mammals: Structure–activity relationships. Trends Biochem. Sci. 1998;23:476–481. doi: 10.1016/S0968-0004(98)01326-7. [DOI] [PubMed] [Google Scholar]
- 2.Yamada K., Osakabe Y. Sugar compartmentation as an environmental stress adaptation strategy in plants. Semin. Cell Dev. Biol. 2017;83:106–114. doi: 10.1016/j.semcdb.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Pommerrenig B., Ludewig F., Cvetkovic J., Trentmann O., Klemens P.A.W., Neuhaus H.E. In Concert: Orchestrated Changes in Carbohydrate Homeostasis Are Critical for Plant Abiotic Stress Tolerance. Plant Cell Physiol. 2018;59:1290–1299. doi: 10.1093/pcp/pcy037. [DOI] [PubMed] [Google Scholar]
- 4.Chen L.Q., Cheung L.S., Feng L., Tanner W., Frommer W.B. Transport of sugars. Annu. Rev. Biochem. 2015;84:865–894. doi: 10.1146/annurev-biochem-060614-033904. [DOI] [PubMed] [Google Scholar]
- 5.Eom J.S., Chen L.Q., Sosso D., Julius B.T., Lin I.W., Qu X.Q., Braun D.M., Frommer W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015;25:53–62. doi: 10.1016/j.pbi.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Li C., Liu Y., Tian J., Zhu Y.S., Fan J.J. Changes in sucrose metabolism in maize varieties with different cadmium sensitivities under cadmium stress. PLoS ONE. 2020;15:e0243835. doi: 10.1371/journal.pone.0243835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosso D., Luo D., Li Q.B., Sasse J., Yang J., Gendrot G., Suzuki M., Koch K.E., McCarty D.R., Chourey P.S., et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015;47:1489–1493. doi: 10.1038/ng.3422. [DOI] [PubMed] [Google Scholar]
- 8.Xie H., Wang D., Qin Y., Ma A., Fu J., Qin Y., Hu G., Zhao J. Genome-wide identification and expression analysis of SWEET gene family in Litchi chinensis reveal the involvement of LcSWEET2a/3b in early seed development. BMC Plant Biol. 2019;19:499. doi: 10.1186/s12870-019-2120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamas P., Fde C.N., Lescure N., Cullimore J. Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Mol. Plant Microbe Interact. 1996;9:233–242. doi: 10.1094/MPMI-9-0233. [DOI] [PubMed] [Google Scholar]
- 10.Artero R.D., Terol-Alcayde J., Paricio N., Ring J., Bargues M., Torres A., Perez-Alonso M. Saliva, a new Drosophila gene expressed in the embryonic salivary glands with homologues in plants and vertebrates. Mech. Dev. 1998;75:159–162. doi: 10.1016/S0925-4773(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen L.Q., Qu X.Q., Hou B.H., Sosso D., Osorio S., Fernie A.R., Frommer W.B. Sucrose Efflux Mediated by SWEET Proteins as a Key Step for Phloem Transport. Science. 2012;335:207–211. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
- 12.Chen L.Q., Hou B.H., Lalonde S., Takanaga H., Hartung M.L., Qu X.Q., Guo W.J., Kim J.G., Underwood W., Chaudhuri B., et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468:527–532. doi: 10.1038/nature09606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L.Q., Lin I.W., Qu X.Q., Sosso D., McFarlane H.E., Londono A., Samuels A.L., Frommer W.B. A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell. 2015;27:607–619. doi: 10.1105/tpc.114.134585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W.J., Nagy R., Chen H.Y., Martinoia E. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 2014;164:777–789. doi: 10.1104/pp.113.232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y.F., Peng W.M.H., Xiong F. Sucrose transport involves in disease response to Xanthomonas oryzae pathovar oryzae. Plant Signal. Behav. 2019;14:1656949. doi: 10.1080/15592324.2019.1656949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bezrutczyk M., Yang J., Eom J.S., Prior M., Sosso D., Hartwig T., Szurek B., Oliva R., Vera-Cruz C., White F.F., et al. Sugar flux and signaling in plant-microbe interactions. Plant J. 2018;93:675–685. doi: 10.1111/tpj.13775. [DOI] [PubMed] [Google Scholar]
- 17.Bezrutczyk M., Hartwig T., Horschman M., Char S.N., Yang J., Yang B., Frommer W.B., Sosso D. Impaired phloem loading in zmsweet13a,b,c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 2018;218:594–603. doi: 10.1111/nph.15021. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Coria M., Sanchez-Sanchez T., Martinez-Marcelo V.H., Aguilera-Alvarado G.P., Flores-Barrera M., King-Diaz B., Sanchez-Nieto S. SWEET Transporters for the Nourishment of Embryonic Tissues during Maize Germination. Genes. 2019;10:780. doi: 10.3390/genes10100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan M., Wang S. Rice MtN3/Saliva/SWEET Family Genes and Their Homologs in Cellular Organisms. Mol. Plant. 2013;6:665–674. doi: 10.1093/mp/sst035. [DOI] [PubMed] [Google Scholar]
- 20.Chen L.Q. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014;201:1150–1155. doi: 10.1111/nph.12445. [DOI] [PubMed] [Google Scholar]
- 21.Chu Z., Yuan M., Yao J., Ge X., Yuan B., Xu C., Li X., Fu B., Li Z., Bennetzen J.L., et al. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006;20:1250–1255. doi: 10.1101/gad.1416306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streubel J., Pesce C., Hutin M., Koebnik R., Boch J., Szurek B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 2013;200:808–819. doi: 10.1111/nph.12411. [DOI] [PubMed] [Google Scholar]
- 23.Romer P., Recht S., Strauss T., Elsaesser J., Schornack S., Boch J., Wang S., Lahaye T. Promoter elements of rice susceptibility genes are bound and activated by specific TAL effectors from the bacterial blight pathogen, Xanthomonas oryzae pv. oryzae. New Phytol. 2010;187:1048–1057. doi: 10.1111/j.1469-8137.2010.03217.x. [DOI] [PubMed] [Google Scholar]
- 24.Klemens P.A.W., Patzke K., Krapp A., Chardon F., Neuhaus H.E. SWEET16 and SWEET17, two novel vacuolar sugar carriers with impact on cellular sugar homeostasis and plant traits. Biochem. Cell Biol. 2014;92:589. [Google Scholar]
- 25.Seo P.J., Park J.M., Kang S.K., Kim S.G., Park C.M. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta. 2011;233:189–200. doi: 10.1007/s00425-010-1293-8. [DOI] [PubMed] [Google Scholar]
- 26.Julius B.T., Leach K.A., Tran T.M., Mertz R.A., Braun D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017;58:1442–1460. doi: 10.1093/pcp/pcx090. [DOI] [PubMed] [Google Scholar]
- 27.Mickaël D., Benoît P., Nils H., Laurence M., Rémi L., Nathalie P. Water Deficit Enhances C Export to the Roots in Arabidopsis thaliana Plants with Contribution of Sucrose Transporters in Both Shoot and Roots. Plant Physiol. 2016;170:1460–1479. doi: 10.1104/pp.15.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y.L., Li W.J., Geng J., Li S.Q., Zhang W.J., Liu X.X., Hu M.H., Zhang Z.N., Fan Y.R., Yuan X.K., et al. Genome-wide identification of the SWEET gene family in Phaseolus vulgaris L. and their patterns of expression under abiotic stress. J. Plant Interact. 2022;17:390–403. doi: 10.1080/17429145.2022.2044079. [DOI] [Google Scholar]
- 29.Xuan C.Q., Lan G.P., Si F.F., Zeng Z.L., Wang C.X., Yadav V., Wei C.H., Zhang X. Systematic Genome-Wide Study and Expression Analysis of SWEET Gene Family: Sugar Transporter Family Contributes to Biotic and Abiotic Stimuli in Watermelon. Int. J. Mol. Sci. 2021;22:8407. doi: 10.3390/ijms22168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X.H., Wang S., Ren Y., Gan C.Y., Li B.B., Fan Y.Y.W., Zhao X.Q., Yuan Z.H. Identification, Analysis and Gene Cloning of the SWEET Gene Family Provide Insights into Sugar Transport in Pomegranate (Punica granatum) Int. J. Mol. Sci. 2022;23:2471. doi: 10.3390/ijms23052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nobuhiro S., Elias B., Hamilton J.S., Inupakutika M.A., Izquierdo Z.S., Deesha T., Yuting L., Erin D., Ginga F., Ayana K., et al. ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress. PLoS ONE. 2016;11:e0147625. doi: 10.1371/journal.pone.0147625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs Z., Simon-Sarkadi L., Sovany C., Kirsch K., Galiba G., Kocsy G. Differential effects of cold acclimation and abscisic acid on free amino acid composition in wheat. Plant Sci. 2011;180:61–68. doi: 10.1016/j.plantsci.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Chen T.T., Li G.Y., Islam M.R., Fu G.F. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biol. 2019;19:525. doi: 10.1186/s12870-019-2126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathan J., Singh A., Ranjan A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol. Plant. 2021;171:620–637. doi: 10.1111/ppl.13210. [DOI] [PubMed] [Google Scholar]
- 35.Tetlow I.J., Emes M.J. Starch biosynthesis in the developing endosperms of grasses and cereals. Agronomy. 2017;7:81. doi: 10.3390/agronomy7040081. [DOI] [Google Scholar]
- 36.Liu Z., Fan H., Ma Z.Y. Comparison of SWEET gene family between maize and foxtail millet through genomic, transcriptomic, and proteomic analyses. Plant Genome. 2022;15:e20226. doi: 10.1002/tpg2.20226. [DOI] [PubMed] [Google Scholar]
- 37.Ayaz A., Huang H.D., Zheng M.L., Zaman W., Li D.H., Saqib S., Zhao H.Y., Lü S. Molecular cloning and functional analysis of GmLACS2-3 reveals its involvement in cutin and suberin biosynthesis along with abiotic stress tolerance. Int. J. Mol. Sci. 2021;22:9175. doi: 10.3390/ijms22179175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xuan Y.H., Hu Y.B., Chen L.Q., Sosso D., Ducat D.C., Hou B.H., Frommer W.B. Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. Proc. Natl. Acad. Sci. USA. 2013;110:E3685–E3694. doi: 10.1073/pnas.1311244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng L., Frommer W.B. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 2015;40:480–486. doi: 10.1016/j.tibs.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y.B., Sosso D., Qu X.Q., Chen L.Q., Ma L., Chermak D., Zhang D.C., Frommer W.B. Phylogenetic evidence for a fusion of archaeal and bacterial SemiSWEETs to form eukaryotic SWEETs and identification of SWEET hexose transporters in the amphibian chytrid pathogen Batrachochytrium dendrobatidis. FASEB J. 2016;30:3644–3654. doi: 10.1096/fj.201600576R. [DOI] [PubMed] [Google Scholar]
- 41.Song J., Ma D., Yin J., Yang L., He Y., Zhu Z., Tong H., Chen L., Zhu G., Liu Y., et al. Genome-Wide Characterization and Expression Profiling of Squamosa Promoter Binding Protein-Like (SBP) Transcription Factors in Wheat (Triticum aestivum L.) Agronomy. 2019;9:527. doi: 10.3390/agronomy9090527. [DOI] [Google Scholar]
- 42.Wang X., Wang J., Jin D., Guo H., Lee T.H., Liu T., Paterson A.H. Genome Alignment Spanning Major Poaceae Lineages Reveals Heterogeneous Evolutionary Rates and Alters Inferred Dates for Key Evolutionary Events. Mol. Plant. 2015;8:885–898. doi: 10.1016/j.molp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Li Q., Yu H., Cao P.B., Fawal N., Mathé C., Azar S., Cassan-Wang H., Myburg A.A., Grima-Pettenati J., Marque C., et al. Explosive tandem and segmental duplications of multigenic families in Eucalyptus grandis. Genome Biol. Evol. 2015;7:1068–1081. doi: 10.1093/gbe/evv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hothem S.D., Marley K.A., Larson R.A. Photochemistry in Hoagland’s Nutrient Solution. J. Plant Nutr. 2003;26:845–854. doi: 10.1081/PLN-120018569. [DOI] [Google Scholar]
- 45.Sekhon R.S., Lin H., Childs K.L., Hansey C.N., Buell C.R., de Leon N., Kaeppler S.M. Genome-wide atlas of transcription during maize development. Plant J. Cell Mol. Biol. 2011;66:553–563. doi: 10.1111/j.1365-313X.2011.04527.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Finn R.D., Clements J., Eddy S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011;39((Suppl. 2)):W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchler-Bauer A., Derbyshire M.K., Gonzales N.R., Lu S., Chitsaz F., Geer L.Y., Geer R.C., He J., Gwadz M., Hurwitz D.I., et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finn R.D., Coggill P., Eberhardt R.Y., Eddy S.R., Mistry J., Mitchell A.L., Potter S.C., Punta M., Qureshi M., Sangrador-Vegas A., et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 51.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voorrips R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y.P., Tang H.B., Debarry J.D., Tan X., Li J.P., Wang X.Y., Lee T., Jin H.Z., Marler B., Guo H., et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R.D., Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown P., Baxter L., Hickman R., Beynon J., Moore J.D., Ott S. MEME-LaB: Motif analysis in clusters. Bioinformatics. 2013;29:1696–1697. doi: 10.1093/bioinformatics/btt248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu B., Jin J., Guo A.Y., Zhang H., Luo J., Gao G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narusaka Y., Nakashima K., Shinwari Zabta K., Sakuma Y., Furihata T., Abe H., Narusaka M., Shinozaki K., Yamaguchi-Shinozaki K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003;34:137–148. doi: 10.1046/j.1365-313X.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 58.Yun K.Y., Park M., Mohanty B., Herath V., Xu F.Y., Mauleon R., Wijaya E., Bajic V., Bruskiewich R., de Los Reyes B.G. Transcriptional regulatory network triggered by oxidative signals configures the early response mechanisms of japonica rice to chilling stress. BMC Plant Biol. 2010;10:16. doi: 10.1186/1471-2229-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., Deng D., Shi Y., Miao N., Bian Y., Yin Z. Diversification, phylogeny and evolution of auxin response factor (ARF) family: Insights gained from analyzing maize ARF genes. Mol. Biol. Rep. 2012;39:2401–2415. doi: 10.1007/s11033-011-0991-z. [DOI] [PubMed] [Google Scholar]
- 60.Gaut B.S., Morton B.R., McCaig B.C., Clegg M.T. Substitution rate comparisons between grasses and palms: Synonymous rate differences at the nuclear gene Adh parallel rate differences at the plastid gene rbcL. Proc. Natl. Acad. Sci. USA. 1996;93:10274–10279. doi: 10.1073/pnas.93.19.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naeem M., Shahzad K., Saqib S., Asim S., Nasrullah M.Y., Muhammad I.A. The Solanum melongena COP1LIKE manipulates fruit ripening and flowering time in tomato (Solanum lycopersicum) Plant Growth Regul. 2022;96:369–382. doi: 10.1007/s10725-021-00785-7. [DOI] [Google Scholar]
- 62.Trick A.Y., Chen F.E., Schares J.A., Freml B.E., Lor P., Yun Y., Wang T.H. High resolution estimates of relative gene abundance with quantitative ratiometric regression PCR (qRR-PCR) Analyst. 2021;146:6463–6469. doi: 10.1039/D1AN01397A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.