Abstract
The genus Convallaria (Asparagaceae) comprises three herbaceous perennial species that are widely distributed in the understory of temperate deciduous forests in the Northern Hemisphere. Although Convallaria species have high medicinal and horticultural values, studies related to the phylogenetic analysis of this genus are few. In the present study, we assembled and reported five complete chloroplast (cp) sequences of three Convallaria species (two of C. keiskei Miq., two of C. majalis L., and one of C. montana Raf.) using Illumina paired-end sequencing data. The cp genomes were highly similar in overall size (161,365–162,972 bp), and all consisted of a pair of inverted repeats (IR) regions (29,140–29,486 bp) separated by a large single-copy (LSC) (85,183–85,521 bp) and a small single-copy (SSC) region (17,877–18,502 bp). Each cp genome contained the same 113 unique genes, including 78 protein-coding genes, 30 transfer RNA genes, and 4 ribosomal RNA genes. Gene content, gene order, AT content and IR/SC boundary structure were nearly identical among all of the Convallaria cp genomes. However, their lengths varied due to contraction/expansion at the IR/LSC borders. Simple sequence repeat (SSR) analyses indicated that the richest SSRs are A/T mononucleotides. Three highly variable regions (petA-psbJ, psbI-trnS and ccsA-ndhD) were identified as valuable molecular markers. Phylogenetic analysis of the family Asparagaceae using 48 cp genome sequences supported the monophyly of Convallaria, which formed a sister clade to the genus Rohdea. Our study provides a robust phylogeny of the Asparagaceae family. The complete cp genome sequences will contribute to further studies in the molecular identification, genetic diversity, and phylogeny of Convallaria.
Keywords: Convallaria, chloroplast genome, comparative analysis, phylogenomics, Asparagaceae
1. Introduction
The monocot genus Convallaria L. (Asparagaceae) are perennial herbs commonly found in the understory of temperate deciduous forests in the Northern Hemisphere [1,2]. Three morphologically similar but isolated species with different geographical distribution were recognized in the genus, namely C. keiskei, C. majalis, and C. montana [3,4]. Convallaria keiskei is widely distributed in Sakhalin, Korea, China, Japan and Eastern Siberia [5,6]. Convallaria majalis, commonly known as Lily of the Valley, is native to Europe and now widely ranged in the temperate regions of Eurasia and Eastern North America [7]. C. montana, the American Lily of the Valley, has a limited distribution endemic to the southern Appalachian Mountains in North America [5,6]. This genus is characterized by a 10–15 cm leaf length, two leaves and a raceme of about 10 white flowers on the stem apex, 4–10 mm bracts length, nearly globose seeds and a base chromosome number of 18 [8]. Like many perennial herbaceous plants, the Convallaria species reproduces asexually by rhizome and sexually by seed [9]. They always form extensive colonies by spreading underground stems [10,11]. The Convallaria species is widely cultivated as an ornamental plant for its white bell-shaped flowers and sweet fragrance [7]. However, potential poisonings are a concern in terms of the cardenolide glycosides found throughout these plants, such as convallatoxin and convallatoxol [12]. These components could have medicinal value. For example, steroidal glycosides extracted from C. majalis whole plants had the potential for use as an anti-lung cancer agent [13]. Convallaria keiskei plants was used in the treatment of salivary gland cancer as an efficient therapeutic alternative [14]. Although Convallaria species have high medicinal and horticultural values, studies on the phylogenetic relationships and evolution of these Convallaria species are few.
Asparagales is the largest order of monocots, though in APG III [15], the number of families recognized has fallen from 26 to 14 [16]. In previous research, the phylogeny of Asparagaceae was reconstructed using cp or nuclear loci, with low support value for the position of Convallaria [17,18]. In recent years, chloroplast genomes have been widely used in reconstructing the phylogenetic relationships among plant groups for their uniparental inheritance, lack of recombination, and conservativeness in gene content and gene order [19,20,21]. Many phylogenetic relationships that remained unresolved with few loci have been clarified by using whole cp genome sequences, especially in recently diverged plant groups [22,23,24,25]. Furthermore, the fast-evolving regions of the cp genome can be utilized as DNA barcodes to identify the morphological similar species, as well as molecular markers for systematic and phylogeographic analyses [26,27]. Thus, phylogenetic analyses with cp genomes offer an efficient method to obtain a robust phylogenetic relationship of the Asparagaceae for further evolutionary study.
In this study, we report and annotate five complete cp genomes of three Convallaria species. We aimed to (1) investigate the global structure patterns of Convallaria cp genomes; (2) analyze the repeat sequences and SSRs among the five cp genomes; (3) screen hotspot DNA regions; and (4) reconstruct the phylogenetic relationships within Asparagaceae to locate the phylogenetic position of Convallaria and confirm its monophyly.
2. Materials and Methods
2.1. Plant Samples, DNA Extraction, and Sequencing
Fresh leaves of five individual plants (two C. keiskei, two C. majalis and one C. montana) were collected in the field and dried with silica gel immediately. Voucher specimens were deposited in the Herbarium of Zhejiang Sci-Tech University. Total genomic DNA was extracted from ~10 mg leaf tissue using the CTAB extraction protocol with modification [28]. After purification, the extracted DNA was used to generate short-insert (<800 bp) paired-end sequencing libraries according to the Illumina standard protocol (Illumina, San Diego, CA, USA). The genomic DNA of each individual specimen was indexed by tags and pooled together in one lane of Hiseq 2500 (Illumina) for sequencing at Beijing Genomics Institute (BGI, Shenzhen, China). After sequencing and data treatment, 14,576,340–21,266,964 clean reads with pair-end 125 bp read length were retrieved for the five cp genomes.
2.2. Chloroplast Genome Assembly and Annotation
The cp genomes were assembled with both de novo and reference guided approaches [29]. The cp genome sequence of Rohdea chinensis (MH356725; [30]) obtained from NCBI was used as a reference (http://www.ncbi.nlm.nih.gov/) (accessed on 5 February 2020). Firstly, we assembled the clean reads into contigs on the de novo assembler using CLC Genomics Workbench with the following optimized parameters: bubble size of 98, minimum contig length of 200, mismatch cost of 2, deletion and insertion costs of 3, length fraction of 0.9, and similarity fraction of 0.8. Next, all the contigs were aligned to the reference genome using local BLAST (http://blast.ncbi.nlm.nih.gov/) (accessed on 12 February 2020) with ≥90% similarity and query coverage. The contigs of each individual were aligned with R. chinensis genome and ordered in Geneious v11.1.2 (http://www.geneious.com) (accessed on 15 February 2020) to construct the draft chloroplast genomes. Then, the clean reads were mapped to the draft genome sequences again to check the contigs’ concatenation. Finally, the complete chloroplast genome sequences were obtained by connecting these overlapping contigs. The yielded cp genomes were annotated through the online program Dual Organellar Genome Annotator (DOGMA; [31]. The annotated organelle genomes of C. keiskei (A4, A118), C. majalis (A63, A69 and C. montana(A114) were deposited in GenBank (accession numbers ON645923, ON303653, ON303655, ON645922 and ON303654). The start and stop codons and the exon–intron boundaries of the encoded genes were accurately confirmed by comparison with homologous genes of R. chinensis using MAFFT v7 [32]. In addition, the tRNAscan-SE v1.21 was used to verify the tRNA genes with default parameters [33]. The graphic maps of the cp genomes of Convallaria were drawn in Organellar Genome DRAW [34].
2.3. Comparative Analysis of Convallaria cp Genomes and Hotspot Regions Identification
We used the five complete chloroplast genomes of Convallaria for comparative analyses. MAFFT v7 was used in the alignment of the plastid genome sequences [32]. Boundary regions between the LSC, IR and SSC and their lengths were compared and analyzed using cp genomes. The sequence identity of the five Convallaria cp genomes was plotted using the online software mVISTA (http://genome.lbl.gov/vista/mvista/submit.shtml) (accessed on 22 February 2020) with the Shuffle-LAGAN mode [35]. The cp DNA rearrangement analysis of the five chloroplast genomes were conducted using Mauve Alignment [36]. In order to screen the fast-evolving regions among the cp genomes, the sequence alignment was subjected to a sliding window analysis to evaluate the total number of mutations (Eta) and nucleotide diversity (Pi) for all protein-coding and intergenic spacer regions using the DNA Polymorphism calculation in DNASP v5.1 [37]. Regions that met the following two criteria were used: (1) an aligned length > 200 bp; and 2) mutation site > 0. We also calculated the Eta and Pi among the Asparagaceae species to obtain the hypervariable regions which could be used in future molecular evolutionary and systematic studies of this family.
2.4. Long Repeats and Simple Sequence Repeats
The position and size of three repeat sequence types, including direct (forward), inverted (palindromic) and reverse repeats, were identified in the five cp genomes of Convallaria using the online program REPuter [38] (accessed on 10 March 2020). For the above repeat types, we set the constraints in REPuter as the following: (1) repeat size longer than 30 bp; and (2) sequence identity more than 80%, with a hamming distance of 3 (i.e., the gap size between repeats larger than 3 bp). SSRs were detected using MIcroSAtellite (MISA) perl script [39] with a threshold for mono-, di-, tri-, tetra-, penta-, and hexanucleotide SSRs containing 10, 5, 5, 3, 3, and 3 repeat units, respectively.
2.5. Phylogenetic Analysis
The 5 cp genomes of Convallaria and other 43 cp genomes of Asparagaceae downloaded from NCBI were recovered to infer their phylogenetic relationships within this family (Table S1). Agapanthus coddii was used as the outgroup. Multiple alignment of coding sequences from 48 cp genomes were performed using MAFFT v7 [32]. The phylogenetic reconstructions were applied using two methods: maximum likelihood (ML) and Bayesian inference (BI). The best-fitting models (GTR + F + R3) of nucleotide substitutions were determined by the Bayesian Information Criterion (BIC) yielded using jModelTest v2.1.7 [40]. The ML analyses were performed in RAxML-HPC v8.2.20 [41] with 5000 bootstrap (BS) replicates. Bayesian inference (BI) analyses were run with MrBayes v.3.2.5 [42]. The Markov chain Monte Carlo (MCMC) algorithm was run for 1,000,000 generations. Trees were sampled every 500 generations. The first 25% trees were discarded as burn-in. A consensus tree was obtained from the remaining trees, and we estimated the posterior probabilities (PPs) and visualized them in FigTree v1.4.2.5.
3. Results
3.1. Genome Organization and Features
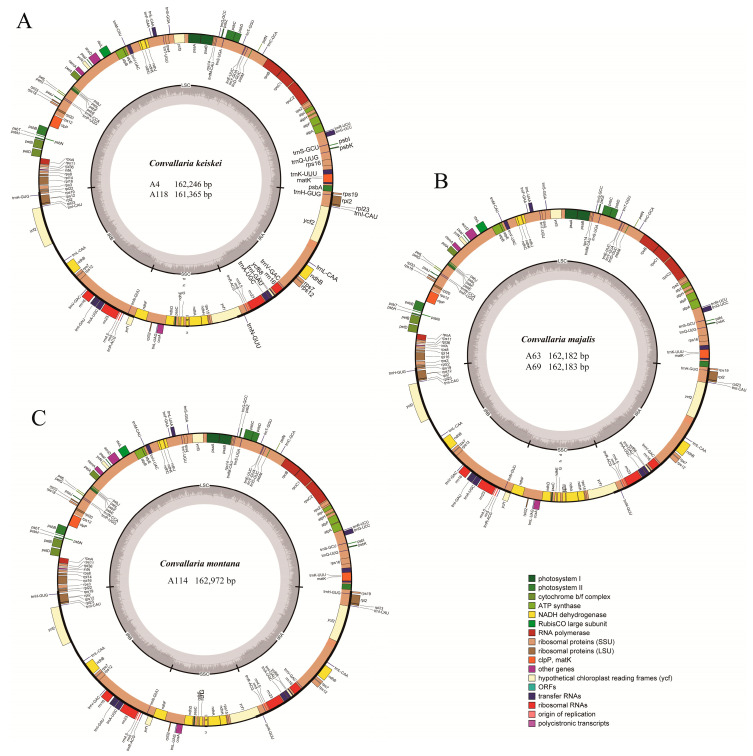
Five complete cp genomes of Convallaria were assembled with no gaps. The full length of five Convallaria chloroplast genomes ranged from 161,365 bp to 162,972 bp (Figure 1, Table 1). All these chloroplast genomes exhibited the typical quadripartite structure, consisting of a pair of IRs (58,280–58,950 bp) separated by the LSC (85,183–85,521 bp) and SSC (17,877–18,502 bp) regions. The total GC content of the five cp genomes were the same (37.9%), whereas the GC content varied in the LSC, SSC, and IR regions as 35.6–35.7%, 31.4–31.6%, and 43.2–43.3%, respectively (Table 1). All cp genomes identically contain 78 protein-coding genes, 30 tRNAs, and four rRNAs. Altogether, the five cp genomes of Convallaria were highly conserved in gene content, gene order, and GC content. Nine of the protein-coding genes (rps16, atpF, rpoC1, petB, petD, rpl16, rpl2, ndhB and ndhA) contained one single intron, whereas three genes (clpP, ycf3, and rps12) contained two introns. The ycf1 gene in IRa was partially duplicated and formed a pseudogene (Table 2).
Figure 1.
Gene maps of the five Convallaria chloroplast genomes. (A) Two C. keiskei individuals; (B) two C. majalis individuals; (C) one C. montana individual. Different color indicates different functional gene groups. The dark gray in the inner represents GC content, and the light gray represents AT content.
Table 1.
The sample information and characteristics of Convallaria chloroplast genomes.
| Characteristics | C. keiskei (A4) | C. keiskei(A118) | C. montana (A114) | C. majalis (A63) | C. majalis (A69) |
|---|---|---|---|---|---|
| Location | China: Hebei | Japan: Fukushima | USA: Georgia | USA: Iowa | USA: Washington |
| Latitude (N) | 30°12′1″ | 35°43′6″ | 29°43′55″ | 30°44′27″ | 28°48′21″ |
| Longitude (E) | 120°71′6″ | 139°44′47″ | 121°5′10″ | 116°27′9″ | 120°54′47″ |
| Total cp DNA Size (bp) | 162,246 | 161,365 | 162,972 | 162,183 | 162,182 |
| LSC length (bp) | 85,432 | 85,183 | 85,521 | 85,419 | 85,418 |
| SSC length (bp) | 18,495 | 17,877 | 18,502 | 18,485 | 18,485 |
| IR length (bp) | 29,160 | 29,153 | 29,475 | 29,140 | 29,140 |
| Total GC content (%) | 37.9 | 37.9 | 37.9 | 37.9 | 37.9 |
| LSC | 35.6 | 35.7 | 35.7 | 35.6 | 35.6 |
| SSC | 31.4 | 31.6 | 31.4 | 31.4 | 31.4 |
| IR | 43.2 | 43.2 | 43.3 | 43.2 | 43.2 |
| Total number of genes | 133 | 133 | 133 | 133 | 133 |
| Protein-coding genes | 85 | 85 | 85 | 85 | 85 |
| rRNAs genes | 8 | 8 | 8 | 8 | 8 |
| tRNAs genes | 38 | 38 | 38 | 38 | 38 |
| Duplicated genes | 20 | 20 | 20 | 20 | 20 |
IR, inverted repeat region; LSC, large single-copy region; SSC, small single-copy region.
Table 2.
Gene list of the Convallaria chloroplast genomes.
| Groups of Gene | Name of Genes |
|---|---|
| Ribosomal RNAs | rrn16(×2), rrn23(×2), rrn4.5(×2), rrn5(×2) |
| Transfer RNAs | trnA-UGC(×2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, trnG-UCC, trnH-CAU, trnH-GUG, trnI-CAU(×2), trnI-GAU(×2), trnK-UUU, trnL-CAA(×2), trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU(×2), trnP-UGG, trnQ-UUG, trnR-ACG(×2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(×2), trnV-UAC, trnW-CCA, trnY-GUA |
| Photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT |
| Cytochrome | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT |
| ATP synthase | atpA, atpF a, atpH, atpI, atpE, atpB |
| Rubisco | bri ck |
| NADH dehydrogenase | ndhJ, ndhK, ndhC, ndhB a (×2), n dhF, ndhD, ndhE, ndhG, ndhI, ndhA a, ndhH |
| ATP-dependent protease subunit P | clpP b |
| Chloroplast translational initiation factor | i nfA |
| Chloroplast envelope membrane protein | cemA |
| Large units | rpl33, rpl20, rpl36, rpl14, rpl16 a, rpl2, rpl2 a (×2), rpl23(×2), rpl32 |
| Small units | rps16 a, rps2, rps14, rps4, rps18, rps12 b (×2), rps11, rps8, rps19, rps3, rps7 (×2), rps15 |
| RNA polymerase | rpoC2, rpoC1 a, rpoB, rpoA |
| Miscellaneous proteins | matK, aacD, ccsA |
| Hypothetical proteins and conserved reading frame | ycf1, ycf2(×2), ycf3 b, ycf4 |
| Pseudogenes | Ψy cf 1, Ψ infA |
a Indicates the genes containing one single intron. b Indicates the genes containing two introns. (×2) indicates genes duplicated in the IR regions. Ψ indicates pseudogene.
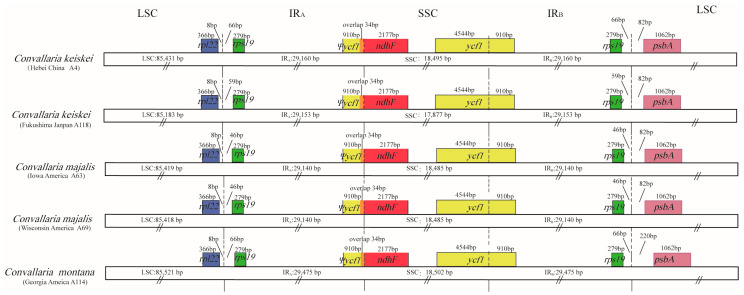
3.2. Variation at IR/SC Boundaries
Comparison of the 5 cp genomes of Convallaria revealed minor differences at the IR/LSC boundaries (Figure 2). At the IRA/LSC border, the space length from rpl19 to the border varied from 46 bp to 66 bp. At the IRB/LSC border, the space length from psbA to the border was all 82 bp, except in C. montana (220 bp). No variation was observed at the IR/LSC boundaries. All the IRb regions expanded 910 bp into ycf1 and formed a pseudogene Ψycf1 in IRa by duplication. All IRa regions expanded into ndhF, causing a 34 bp overlap with Ψycf1.
Figure 2.
Comparison of LSC, IRs, and SSC junctions among Convallaria plastomes.
3.3. Comparative Analysis of Convallaria cp Genomes and Hotspot Regions Identification
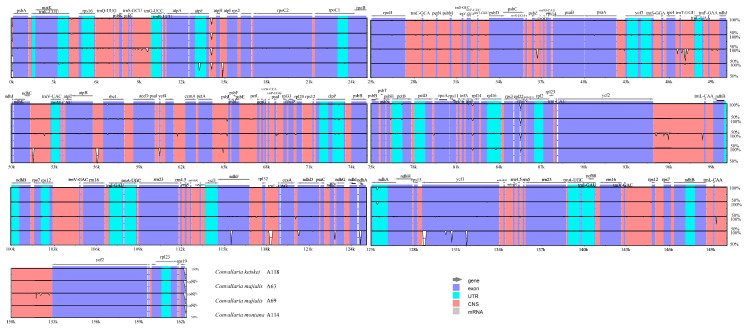
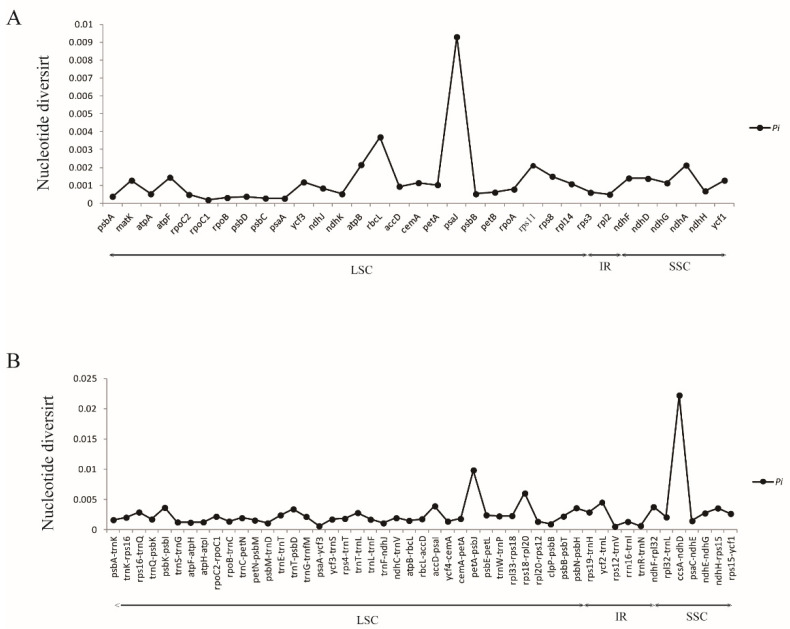
We analyzed the whole sequence divergence of the five Convallaria cp genomes using the mVISTA software with C. keiskei (A4) as reference. After alignment, the sequences showed high similarities with only a few regions’ sequence identities less than 90%, suggesting that the cp genomes of Convallaria species are conservative (Figure 3). The two IR regions showed a lower level of sequence divergence than LSC and SSC regions. The coding genes and non-coding regions were compared among 5 individuals to detect divergence hotspots. In total, 82 loci (33 coding genes and 49 intergenic spacers) were generated (Figure 4; Table S2), and the nucleotide diversity (Pi) value for each locus ranged from 0.00019 (rpoC1) to 0.02222 (ccsA-ndhD). Three regions, psaJ (0.0093), petA-psbJ (0.00985) and ccsA-ndhD (0.02222), showed a Pi > 0.009, which were recognized as hotspot regions that could be applied as molecular markers in species identification and phylogenetic analyses. For all chloroplast genome sequences of Asparagareae, we obtained three hypervariable regions, petA-psbJ (0.0929), psbI-trnS (0.08097), and ccsA-ndhD (0.07816), which could be used in future molecular evolutionary and systematic studies of this family (Table S3).
Figure 3.
Sequence identity plots among five Convallaria chloroplast genomes, with S. keiskei as a reference. CNS: conserved non-coding sequences; UTR: untranslated region.
Figure 4.
Comparison of nucleotide variability (Pi) values in Convallaria plastomes. (A) Pi values among protein-coding genes (CDS). (B) Pi values among intergenic spacer (IGS) regions.
3.4. Long Repeats and Simple Sequence Repeats
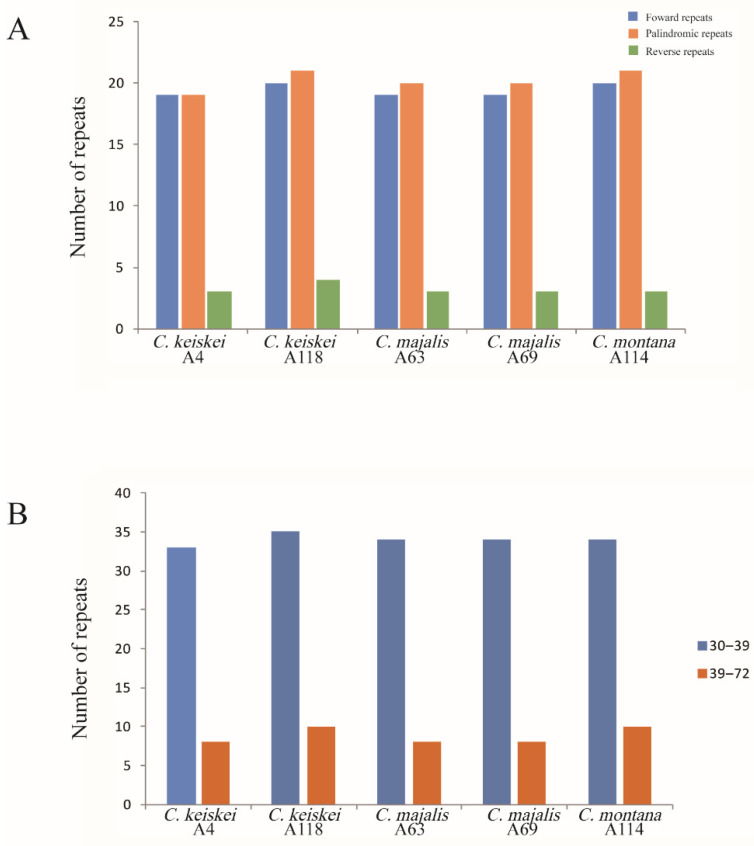
A total of 214 repeats, including 97 forward, 101 palindromic, and 16 reverse repeats, were detected in the five Convallaria cp genomes using REPuter (Figure 5A). Convallaria keiskei (A118) possessed the most repeats (45), while C. keiskei (A4) contained the fewest (41). In all the individuals of Convallaria, 79.4% of the repeats ranged from 30 to 39 bp in size (Figure 5B; Table S4). There were 37 repeats shared by the five Convallaria cp genomes. Additionally, 3 and 4 repeats were unique in C. keiskei (A118) and C. montana (A114), respectively (Table S5).
Figure 5.
Repeat sequences analysis in five Convallaria chloroplast genomes. (A) Frequency of repeat types. (B) Frequency of repeats by length.
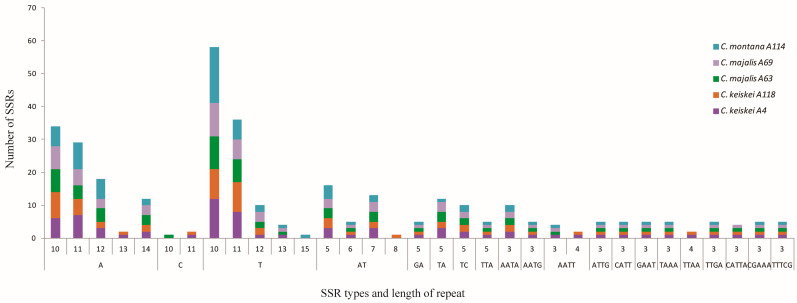
A total of 338 SSRs were identified by MISA analysis across the five Convallaria cp genomes. The number of SSRs ranged from 64 (C. majalis A69) to 74 (C. montana A114) SSRs, with 21 SSRs shared among all genomes (Table S6). Among these SSRs, more than one-half (60.35%) were composed of A/T bases (Figure 6). In general, the SSRs of these cp genomes showed abundant variation, which can be used in population genetics study of these Convallaria species.
Figure 6.
Distribution of simple sequence repeats (SSRs) in the five Convallaria chloroplast genomes.
3.5. Phylogenetic Analysis
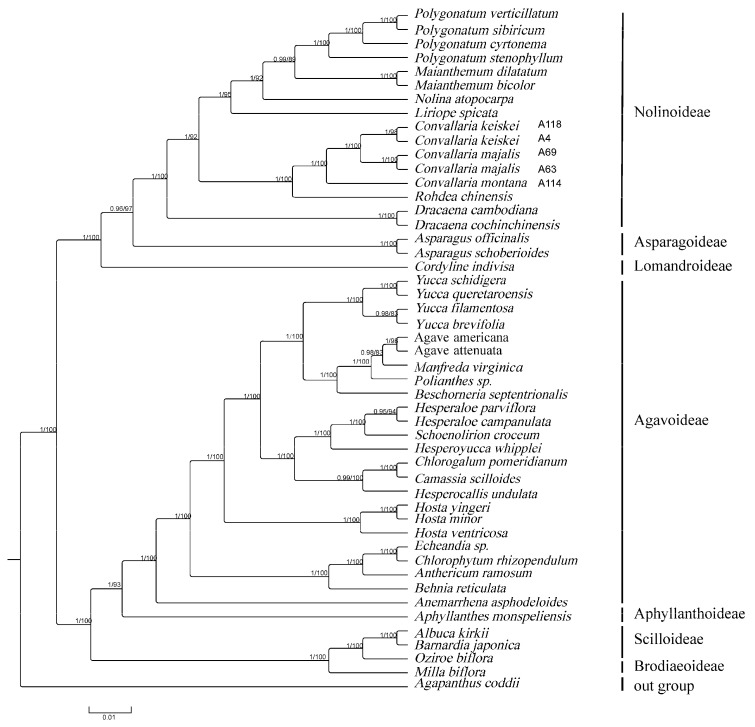
We used 48 cp genome sequences of Asparagareae and 1 outgroup of Agapanthoideae in total for phylogenetic analysis. The trees reconstructed with the 68 common CDSs shared between the plastomes displayed almost identical topologies with generally high support values, in both ML and BI analyses. The phylogenetic tree was almost fully supported (PP/BS = 1/100) at all nodes (Figure 7). The phylogenetic tree indicated that the subfamilies Scilloideae and Brodiaeoideae constituted the earliest diverging lineage in Asparagaceae. All these phylogenetic trees identically supported the monophyly of Convallaria, which in turn formed a sister clade to the Rohdea. Within Convallaria, C. keiskei was resolved as a monophyletic clade with a sister relationship to C. majalis, and C. montana was at the basal position.
Figure 7.
Phylogenetic relationships of Asparagaceae reconstructed using Bayesian inference (BI) and maximum likelihood (ML) based on 68 common CDS. Support values are indicated above the branches. PP, posterior probability; BS, bootstrap support.
4. Discussion
Comparative analysis results indicate that five cp genome sequences of Convallaria showed highly conserved genomic structures. No variation and rearrangement of the gene content were found between the five cp genomes of Convallaria. All plastomes had the same number of protein-coding genes, tRNAs and rRNAs. Genes of ycf1 and infA were found to be pseudogenes in five individuals. The pseudogenizations of ycf1 and locations of ψycf1 copies commonly found in other plants [43,44]. The pseudogene was firstly thought to have lost the ability of protein coding [45] but was now considered as an evolutionary relic of the functional gene [46]. Such conservatism revealed accords with the low substitution rate of chloroplast genomes and the presumed recent divergence within genus Convallaria. Similar findings were also found in other closely related species [47,48]. Within cp genomes, the results of comparative analysis indicated that CDS and IRs regions were more conserved than IGS and SCs, correspondingly. The IR regions are highly conserved, which is important for the stabilization of the chloroplast genome structure [49]. Comparison of the IR/SC boundary areas among species suggested expansions and contractions of the IR region. The expansion and contraction of the IR regions often results in the length change of cp genomes [50]. The mechanism of larger IRs expansion may be caused by the double-strand break repair (DSBR) [51]. Because of the conservatism, the IR regions showed a lower level of sequence divergence than LSC and SSC regions in Convallaria cp genomes, in accordance with other studies [52,53,54].
The polymorphic cp DNA non-coding regions have been widely used to investigate species identification and molecular phylogeny at the interspecific level [55,56]. We have detected three variable regions (petA-psbJ, psbI-trnS and ccsA-ndhD) that can be used in species identification and phylogeny. SSR is a repetitive sequence consisting of simple repeating units in tandem and has been widely used as molecular marker in genetic structure and genetic diversity analysis [57,58]. The SSRs detected in present study showed abundant variation, and can therefore be applied in the genetic diversity, population genetics analyses [59,60].
In recent years, chloroplast genomes have been widely used to evaluate the relationship of closely related species in taxonomic studies. For example, cp genomes of 35 species representing 31 genera from Ranunculaceae were sequenced and utilized to clarify the long-standing systematic controversies of this family [22]. Our phylogenetic analyses based on 48 cp genomes successfully resolved intergeneric relationships within Asparagaceae. We obtained a well-resolved and robust phylogenetic tree. Two main clades including seven subfamilies were confirmed: Lomandroideae + (Asparagoideae + Nolinoideae) and Brodiaeoideae + (Scilloideae + (Aphyllanthoideae + Agavoideae)). The first two diverged clades were Lomandroideae and Brodiaeoideae, respectively. This result was congruent with previous research [18,61]. Convallaria was confirmed as a monophyly most closely related to the genus Rohdea. The Convallaria clade consisted of two lineages: one contains the North American species C. montana, and another contains two Eurasian distributed species, C. keiskei and C. majalis. A similar phylogenetic relationship was revealed in other plant taxa that displayed an Eastern Asian–Eastern North American disjunct distribution, such as Croomia, Polygonatum, Maianthemum [62,63,64]. This prevalent phylogeographic pattern in plants was explained as the result of vicariance events after dispersal via the Bering or North Atlantic land bridge during the Late Tertiary [65,66,67,68].
5. Conclusions
In conclusion, our findings reveal the detailed characteristics of the complete cp genome of three Convallaria species. The gene content, gene order, and gene orientation are highly conserved. Comparative analyses revealed that no rearrangements occurred in Convallaria, and that intergenic regions were more variable than coding regions. Three hypervariable regions (petA-psbJ, psbI-trnS and ccsA-ndhD) were identified as valuable molecular markers. The cp genome data provided strong support for the relationships within Convallaria and among the subfamily clades within Asparagareae, which proved the cp genome to be useful genetic resources in dealing with phylogenetically difficult taxa. The complete cp genome sequences will contribute to further studies in molecular identification, genetic diversity, and phylogeny.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13101724/s1; Table S1: Accession numbers of chloroplast genomes used for phylogenetic analyses; Table S2: Nucleotide variability (Pi) values and total number of mutation (Eta) in Convallaria; Table S3: Nucleotide variability (Pi) values and total number of mutation (Eta) in Asparagareae; Table S4: Repeat types and lengths of Convallaria chloroplast genomes; Table S5: Analyses of repeat sequences in five Convallaria chloroplast genomes; Table S6: Simple sequence repeat (SSR) polymorphism in five Convallaria chloroplast genomes.
Author Contributions
Conceptualization, Z.-C.Q., X.-L.Y., R.-H.W. and P.L.; methodology, X.W. and X.C.; formal analysis, X.W., J.G. and Q.-X.L.; investigation and resources, Z.-C.Q. and P.L.; writing—original draft preparation, J.G. and J.W.; writing—review and editing, Q.-X.L., Z.-C.Q., X.-L.Y. and R.-H.W. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data which support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov, accessed on 2 June 2022, reference number (C. keiskei A4, ON645923; C. keiskei A118, ON303655; C. majalis A63, ON303653; C. majalis A69, ON645922 and C. montana A114, ON303654).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 31700321, 31970225; The Special Fund for Scientific Research of Shanghai Landscaping & City Appearance Administrative Bureau, grant numbers G212405, G222403; National Wild Plant Germplasm Resource Center, grant number ZWGX1902; Special Fundamental Work of the Ministry of Science and Technology, grant number 2014FY120400; the Natural Science Foundation of Zhejiang Province, grant number LY21C030008, the Open Fund of Shaoxing Academy of Biomedicine of Zhejiang Sci-Tech University, grant number SXAB202020, and the Science Foundation of Zhejiang Sci-Tech University, grant number 19042144-Y.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lei W.J., Ni D.P., Wang Y.J., Shao J.J., Wang X.C., Yang D., Wang J.S., Chen H.M., Liu C. Intraspecific and heteroplasmic variations, gene losses and inversions in the chloroplast genome of Astragalus membranaceus. Sci. Rep. 2016;6:21669. doi: 10.1038/srep21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki K., Ohara M. Reproductive demography of ramets and genets in a rhizomatous clonal plant Convallaria keiskei. J. Plant Res. 2008;121:147–154. doi: 10.1007/s10265-007-0141-9. [DOI] [PubMed] [Google Scholar]
- 3.Lu Q.X., Gao J., Wu J.J., Zhou X., Wu X., Li M.D., Wei Y.K., Wang R.H., Qi Z.C., Li P. Development of 19 novel microsatellite markers of lily-of-the-valley (Convallaria, asparagaceae) from transcriptome sequencing. Mol. Biol. Rep. 2020;47:3041–3047. doi: 10.1007/s11033-020-05376-9. [DOI] [PubMed] [Google Scholar]
- 4.Streveler B. Masters Thesis. University of Wisconsin; Madison, WI, USA: 1966. A taxonomic Study of the Genus Convallaria (Liliaceae) [Google Scholar]
- 5.Araki K., Shimatani K., Ohara M. Floral distribution, clonal structure, and their effects on pollination success in a self-incompatible Convallaria keiskei population in northern Japan. Plant Ecol. 2007;189:175–186. doi: 10.1007/s11258-006-9173-9. [DOI] [Google Scholar]
- 6.Utech F.H., Kawano S. Floral vascular anatomy of Convallaria majalis L. and C. keiskei Miq. (Liliaceae-Convallariinae) Bot. Mag. 1976;89:173–182. doi: 10.1007/BF02488340. [DOI] [Google Scholar]
- 7.Van Ruth S.M., De Visser R. Provenancing flower bulbs by analytical fingerprinting: Convallaria majalis. Agriculture. 2015;5:17–29. doi: 10.3390/agriculture5010017. [DOI] [Google Scholar]
- 8.Kanchi G.N., James R.L., James Z.L. Nomenclatural and taxonomic analysis of Convallaria majalis, C. majuscula, and C. montana (Ruscaceae/Liliaceae) Phytoneuron. 2012;17:1–4. [Google Scholar]
- 9.Katrien V., Tim D.M., Hans J., Isabel R.R., Olivier H. The impact of extensive clonal growth on fine-scale mating patterns: A full paternity analysis of a lily-of-the-valley population (Convallaria majalis) Ann. Bot. 2013;111:623–628. doi: 10.1093/aob/mct024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandepitte K., Roldan-Ruiz I., Jacquemyn H., Honnay O. Extremely low genotypic diversity and sexual reproduction in isolated populations of the self-incompatible lily-of-the-valley (Convallaria majalis) and the role of the local forest environment. Ann. Bot. 2010;105:769–776. doi: 10.1093/aob/mcq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chwedorzewska K.J., Galera H., Kosiński I. Plantations of Convallaria majalis L. as a threat to the natural stands of the species: Genetic variability of the cultivated plants and natural populations. Biol. Conserv. 2008;141:2619–2624. doi: 10.1016/j.biocon.2008.07.025. [DOI] [Google Scholar]
- 12.Peng J., Cui J.M., Kang L.P., Ma B.P. Chemical constituents and pharmacological activities of Convallariaceae plants: Research advances. J. Int. Pharm. Res. 2013;40:726–735. [Google Scholar]
- 13.Matsuo Y., Shinoda D., Nakamaru A., Kamohara K., Sakagami H., Mimaki Y. Steroidal glycosides from Convallaria majalis whole plants and their cytotoxic activity. Int. J. Mol. Sci. 2017;18:2358. doi: 10.3390/ijms18112358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H.E., Nam J.S., Shin J.A., Hong I.S., Yang I.H., You M.J., Cho S.D. Convallaria keiskei as a novel therapeutic alternative for salivary gland cancer treatment by targeting myeloid cell leukemia-1. Head Neck. 2016;38:E761–E770. doi: 10.1002/hed.24096. [DOI] [PubMed] [Google Scholar]
- 15.The Angiosperm Phylogeny Group An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009;161:105–121. doi: 10.1111/j.1095-8339.2009.00996.x. [DOI] [Google Scholar]
- 16.Chase M.W., Reveal J.L., Fay M.F. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Bot. J. Linn. Soc. 2010;161:132–136. doi: 10.1111/j.1095-8339.2009.00999.x. [DOI] [Google Scholar]
- 17.Seberg O., Petersen G., Davis J.I., Pires J.C., Stevenson D.W., Chase M.W., Fay M.F., Devey D.S., Jorgensen T., Sytsma K.J., et al. Phylogeny of the asparagales based on three plastid and two mitochondrial genes. Am. J. Bot. 2012;99:875–889. doi: 10.3732/ajb.1100468. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.H., Kim D.K., Forest F., Fay M.F. Molecular phylogenetics of Ruscaceae sensu lato and related families (Asparagales) based on plastid and nuclear DNA sequences. Ann. Bot. 2010;106:775. doi: 10.1093/aob/mcq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruhsam M., Rai H.S., Mathews S., Ross T.G., Hollingsworth P.M. Does complete plastid genome sequencing improve species discrimination and phylogenetic resolution in Araucaria? Mol. Ecol. Resour. 2015;15:1067–1078. doi: 10.1111/1755-0998.12375. [DOI] [PubMed] [Google Scholar]
- 20.Jansen R.K., Ruhlman T.A. Genomics of Chloroplasts and Mitochondria. Springer; Berlin/Heidelberg, Germany: 2012. Plastid genomes of seed plants; pp. 103–126. [Google Scholar]
- 21.Shaw J., Lickey E.B., Beck J.T., Farmer S.B., Liu W., Miller J., Siripun K.C., Winder C.T., Schilling E.E., Small R.L. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005;92:142–166. doi: 10.3732/ajb.92.1.142. [DOI] [PubMed] [Google Scholar]
- 22.Zhai W., Duan X.S., Zhang R., Guo C.C., Li L. Chloroplast genomic data provide new and robust insights into the phylogeny and evolution of the Ranunculaceae. Mol. Phylogenetics Evol. 2019;135:12–21. doi: 10.1016/j.ympev.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Firetti F., Zuntini A.R., Gaiarsa J.W., Oliveira R.S., Lohmann L.G., Sluys M.A.V. Complete chloroplast genome sequences contribute to plant species delimitation: A case study of the Anemopaegma species complex. Am. J. Bot. 2017;104:1493–1509. doi: 10.3732/ajb.1700302. [DOI] [PubMed] [Google Scholar]
- 24.Attigala L., Wysocki W.P., Duvall M.R., Clark L.G. Phylogenetic estimation and morphological evolution of Arundinarieae (Bambusoideae: Poaceae) based on plastome phylogenomic analysis. Mol. Phylogenetics Evol. 2016;101:111–121. doi: 10.1016/j.ympev.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Jose C.C., Roberto A., Victoria I., Javier T., Manuel T., Joaquin D. A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus Citrus. Mol. Biol. Evol. 2015;32:2015–2035. doi: 10.1093/molbev/msv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R., Ma P.F., Wen J., Yi T.S. Complete sequencing of five Araliaceae chloroplast genomes and the phylogenetic implications. PLoS ONE. 2013;8:e78568. doi: 10.1371/journal.pone.0078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonie D., Barbara G., Youri L., Yavuz A., Thomas C., Klaas V. The complete chloroplast genome of 17 individuals of pest species Jacobaea vulgaris: SNPs, microsatellites and barcoding markers for population and phylogenetic studies. DNA Res. 2011;18:93–105. doi: 10.1093/dnares/dsr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd L.D., McLay T.G.B. Two micro-scale protocols for the isolation of DNA from polysaccharide-rich plant tissue. J. Plant Res. 2011;124:311–314. doi: 10.1007/s10265-010-0379-5. [DOI] [PubMed] [Google Scholar]
- 29.Cronn R., Liston A., Parks M., Gernandt D.S., Shen R., Mockler T. Multiplex sequencing of plant chloroplast genomes using Solexa sequencing-by-synthesis technology. Nucleic Acids Res. 2008;36:e122. doi: 10.1093/nar/gkn502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y.F., Wang Y.C., Shi X.W., Mao S.L. The complete chloroplast genome sequence of Campylandra chinensis (Liliaceae) Mitochondrial DNA B Resour. 2018;3:780–781. doi: 10.1080/23802359.2018.1491346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyman S.K., Jansen R.K., Boore J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20:3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- 32.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schattner P., Brooks A.N., Lowe T.M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33:W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohse M., Drechsel O., Bock R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007;52:267–274. doi: 10.1007/s00294-007-0161-y. [DOI] [PubMed] [Google Scholar]
- 35.Frazer K.A., Lior P., Alexander P., Rubin E.M., Inna D. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darling A., Mau B., Blattner F.R., Perna A. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Librado P., Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 38.Kurtz S., Choudhuri J.V., Ohlebusch E., Schleiermacher C., Stoye J., Giegerich R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29:4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiel T., Michalek W., Varshney R., Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare) Theor. Appl. Genet. 2003;106:411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- 40.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 42.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szczecińska M., Sawicki J. Genomic resources of three Pulsatilla species reveal evolutionary hotspots, species-specific sites and variable plastid structure in the family Ranunculaceae. Int. J. Mol. Sci. 2015;16:22258–22279. doi: 10.3390/ijms160922258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J., Yang B.X., Zhu W., Sun L.L., Tian J.K., Wang X.M. The complete chloroplast genome sequence of Mahonia bealei (Berberidaceae) reveals a significant expansion of the inverted repeat and phylogenetic relationship with other angiosperms. Gene. 2013;528:120–131. doi: 10.1016/j.gene.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 45.Vanin E.F. Processed pseudogenes: Characteristics and evolution. Annu. Rev. Genet. 1985;19:253–272. doi: 10.1146/annurev.ge.19.120185.001345. [DOI] [PubMed] [Google Scholar]
- 46.Balakirev E.S., Ayala F.J. Pseudogenes: Are they “Junk” or functional DNA? Annu. Rev. Genet. 2003;37:123–151. doi: 10.1146/annurev.genet.37.040103.103949. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X., Zhou T., Yang J., Sun J.J., Ju M.M., Zhao Y.M., Zhao G.F. Comparative analyses of chloroplast genomes of Cucurbitaceae species: Lights into selective pressures and phylogenetic relationships. Molecules. 2018;23:2165. doi: 10.3390/molecules23092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng S.Y., Zhou T., Han K., Yang Y., Zhao J.H., Liu Z.L. The complete chloroplast genome sequences of six Rehmannia species. Genes. 2017;8:103. doi: 10.3390/genes8030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maréchal A., Brisson N. Recombination and the maintenance of plant organelle genome stability. New Phytol. 2010;186:299–317. doi: 10.1111/j.1469-8137.2010.03195.x. [DOI] [PubMed] [Google Scholar]
- 50.Kim K.J., Lee H.L. Complete chloroplast genome sequences from korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 2004;11:247–261. doi: 10.1093/dnares/11.4.247. [DOI] [PubMed] [Google Scholar]
- 51.Wang R.J., Cheng C.L., Chang C.C., Wu C.L., Su T.M., Chaw S.M. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol. Biol. 2008;8:36. doi: 10.1186/1471-2148-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao X.H., Tang P., Li Z.Z., Li D.W., Liu Y.F., Huang H.W. The first complete chloroplast genome sequences in Actinidiaceae: Genome structure and comparative analysis. PLoS ONE. 2015;10:e0129347. doi: 10.1371/journal.pone.0129347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nazareno A.G., Carlsen M., Lohmann L.G. Complete chloroplast genome of Tanaecium tetragonolobum: The first Bignoniaceae plastome. PLoS ONE. 2015;10:e0129930. doi: 10.1371/journal.pone.0129930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y.J., Du L.W., Liu A., Chen J.J., Wu L., Hu W.M., Zhang W., Kim K., Lee S.C., Yang T.J. The complete chloroplast genome sequences of five Epimedium species: Lights into phylogenetic and taxonomic analyses. Front. Plant Sci. 2016;7:306. doi: 10.3389/fpls.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldwin B.G. Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: An example from the compositae. Mol. Phylogenetics Evol. 1992;1:3–16. doi: 10.1016/1055-7903(92)90030-K. [DOI] [PubMed] [Google Scholar]
- 56.Taberlet P. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y.B., Yin J.L., Guo H.Y., Zhang Y.Y., Xiao W., Sun C., Wu J.Y., Qu X.B., Yu J., Wang X.M. The complete chloroplast genome provides insight into the evolution and polymorphism of Panax ginseng. Front. Plant Sci. 2015;5:696. doi: 10.3389/fpls.2014.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bodin S.S., Kim J.S., Kim J.H. Complete chloroplast genome of Chionographis japonica (Willd.) Maxim. (Melanthiaceae): Comparative genomics and evaluation of universal primers for Liliales. Plant Mol. Biol. Report. 2013;31:1407–1421. doi: 10.1007/s11105-013-0616-x. [DOI] [Google Scholar]
- 59.Perdereau A., Klaas M., Barth S., Hodkinson T.R. Plastid genome sequencing reveals biogeographical structure and extensive population genetic variation in wild populations of Phalaris arundinacea L. in north-western Europe. Gcb Bioenergy. 2017;9:46–56. doi: 10.1111/gcbb.12362. [DOI] [Google Scholar]
- 60.Tong W., Kim T.S., Park Y.J. Rice chloroplast genome variation architecture and phylogenetic dissection in diverse Oryza species assessed by whole-genome resequencing. Rice. 2016;9:57. doi: 10.1186/s12284-016-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raman G., Park S., Lee E.M., Park S. Evidence of mitochondrial DNA in the chloroplast genome of Convallaria keiskei and its subsequent evolution in the Asparagales. Sci. Rep. 2019;9:5028. doi: 10.1038/s41598-019-41377-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Q.X., Ye W.Q., Lu R.S., Xu W.Q., Qiu Y.X. Phylogenomic and comparative analyses of complete plastomes of Croomia and Stemona (Stemonaceae) Int. J. Mol. Sci. 2018;19:2383. doi: 10.3390/ijms19082383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim C., Cameron K.M., Kim J. Molecular systematics and historical biogeography of Maianthemum s.s. Am. J. Bot. 2017;104:939–952. doi: 10.3732/ajb.1600454. [DOI] [PubMed] [Google Scholar]
- 64.Wang J.J., Yang Y.P., Hang S., Wen J., Deng T., Nie Z.L., Meng Y., Berthold H. The biogeographic south-north divide of Polygonatum (Asparagaceae tribe Polygonateae) within Eastern Asia and its recent dispersals in the Northern Hemisphere. PLoS ONE. 2016;11:e0166134. doi: 10.1371/journal.pone.0166134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milne R.I., Abbott R.J. The origin and evolution of Tertiary relict floras. Adv. Bot. Res. 2002;38:281–314. [Google Scholar]
- 66.Xiang Q.J., Soltis D.E. Dispersal-vicariance analyses of intercontinental disjuncts: Historical biogeographical implications for Angiosperms in the Northern Hemisphere. Int. J. Plant Sci. 2001;162:S29–S39. doi: 10.1086/323332. [DOI] [Google Scholar]
- 67.Wen J. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Annu. Rev. Ecol. Syst. 1999;30:421–455. doi: 10.1146/annurev.ecolsys.30.1.421. [DOI] [Google Scholar]
- 68.Raman G., Lee E.M., Park S. Intracellular DNA transfer events restricted to the genus Convallaria within the Asparagaceae family: Possible mechanisms and potential as genetic markers for biographical studies. Genomics. 2021;113:2906–2918. doi: 10.1016/j.ygeno.2021.06.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data which support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov, accessed on 2 June 2022, reference number (C. keiskei A4, ON645923; C. keiskei A118, ON303655; C. majalis A63, ON303653; C. majalis A69, ON645922 and C. montana A114, ON303654).