Abstract
Background:
Cervical cancer is the leading cause of cancer among women in Tanzania, with approximately 10,000 new cases and 7,000 deaths annually. In April 2018, the Government of Tanzania introduced 2 doses of human papillomavirus (HPV) vaccine nationally to adolescent girls to prevent cervical cancer, following a successful 2-year pilot introduction of the vaccine in the Kilimanjaro Region.
Methods:
We interviewed key informants at the national level in Tanzania from February to November 2019, using a semi-structured tool to better understand national decision-making and program implementation. We conducted a comprehensive desk review of HPV vaccine introduction materials and reviewed administrative coverage data.
Results:
Ten key informants were interviewed from the Ministry of Health, Community Development, Gender, Elderly, and Children, the World Health Organization, and other partners, and HPV vaccine planning documents and administrative coverage data were reviewed during the desk review. Tanzania introduced HPV vaccine to a single-age cohort of 14-year-old girls, with the decision-making process involving the Tanzania Immunization Technical Advisory Group and the national Interagency Coordination Committee. HPV vaccine was integrated into the routine immunization delivery strategy, available at health facilities and through outreach services at community sites, community mobile sites (>10 km from the health facility), and primary and secondary schools. Pre-introduction activities included trainings and microplanning workshops for health workers and school personnel at the national, regional, council, and health facility levels. Over 6,000 health workers and 22,000 school personnel were trained nationwide. Stakeholder and primary health care committee meetings were also conducted at the national level and in each of the regions as part of the advocacy and communication strategy. Administrative coverage of the first dose of HPV vaccine at the end of 2019 was 78%, and second dose coverage was 49%. No adverse events following HPV vaccination were reported to the national level.
Discussion:
Tanzania successfully introduced HPV vaccine nationally targeting 14-year-old girls, using routine delivery strategies. Continued monitoring of vaccination coverage will be important to ensure full 2-dose vaccination of eligible girls. Tanzania can consider periodic intensified vaccination and targeted social mobilization efforts, as needed.
Keywords: Human papillomavirus, Human papillomavirus vaccine, Tanzania
1. Introduction
Cervical cancer is the fourth most common cancer among women worldwide [1]. It is estimated that each year, there are nearly 570,000 new cases and over 310,000 deaths from cervical cancer [2]. >87% of all new cases and deaths occur in low- and lower-middle-income countries, largely because routine cervical cancer screening and treatment are not widely available [3]. In Tanzania, cervical cancer is the leading cause of female cancer, with almost 10,000 new cervical cancer cases and 7,000 estimated deaths annually [1,4].
The World Health Organization (WHO) recommends HPV vaccination for girls aged 9–14-years old for primary prevention of HPV infection. Gavi, the Vaccine Alliance (Gavi) has offered support to eligible countries for the introduction of HPV vaccine since 2012—initially for pilot introduction programs to determine the best strategies for reaching this novel target age and subsequently for national introductions [5–8].
Tanzania first introduced HPV vaccine in 2014 via a two-year pilot program in the Kilimanjaro region with Gavi support targeting 9-year-old girls or older enrolled in Class 4 in school and 9-year-old girls out-of-school with two doses of the quadrivalent HPV vaccine administered six months apart [9–11]. To identify the best strategies to reach this novel target age, Kilimanjaro implemented a school-based campaign delivery strategy during the first year of the pilot followed by a routine delivery strategy during the second year, using both fixed and outreach sites [12]. Administrative coverage was 93% for both the first and the second doses of HPV vaccine during the first year of the pilot (2014), then 89% for the first dose and 78% for the second dose during the second year (2015) [12] (Fig. 1).
Fig. 1.

Human papillomavirus (HPV) vaccine administrative coverage1 in the Kilimanjaro Region, Tanzania, 2014–2015 (1The total number of doses administered to the target population/the total estimated number of people in the target population; administrative coverage provided by the Tanzania Ministry of Health, Community Development, Gender, Elderly, and Children).
Following a successful HPV vaccination pilot program, Tanzania decided to scale-up and introduce HPV vaccine into the national immunization program. Tanzania successfully applied for Gavi support for national introduction in 2017 [13]. In April 2018, HPV vaccine was introduced into Tanzania’s national immunization schedule for 14-year-old girls, using the routine immunization delivery strategy, and not via campaigns or other point-in-time delivery strategies. In Tanzania, HPV vaccine is offered at both fixed health facility and outreach sites (community, community mobile (>10 km from the health facility), and school sites). Tanzania was among the first Gavi-supported countries to introduce HPV vaccine into its national immunization program. Tanzania initially planned to vaccinate a multiple-age cohort of 9–14-year-old girls. However, due to limited global vaccine supply, Tanzania was only able to vaccinate a single-age cohort of girls. 14-year-old girls were chosen for this temporary single-age cohort vaccination in order to expand the reach of HPV vaccination as these girls would no longer be age eligible (≥15 years old) in the future [14]. Kilimanjaro region continued vaccinating 9-year-old girls following their demonstration project. Nationwide alignment of the HPV vaccine target age group to 9-year-old girls was expected following increased vaccine supply to vaccinate the multiple-age cohort of 9–14-year-old girls.
We aimed to document the HPV vaccine introduction process to understand national scale-up and program implementation. In this manuscript, we describe the decision-making, introduction, and implementation of HPV vaccine into the national immunization program in Tanzania.
2. Methods
We interviewed key informants in Tanzania to obtain information on the HPV vaccine introduction, programmatic decision-making, and implementation. Key informants were identified based on their involvement in the HPV vaccine introduction with the support of the Ministry of Health, Community Development, Gender, Elderly, and Children (MoHCDGEC) and the World Health Organization (WHO) Tanzania.
Key informants were interviewed by 1–2 study staff, using a semi-structured interview tool that consisted of two broad components: decision-making and program implementation. Decision-making included questions on drivers for decision-making and programmatic considerations; program implementation included questions on planning (Gavi application, coordination, and vaccine licensure), training, communications and social mobilization, and monitoring and evaluation. After each interview, all data were entered into a Microsoft Word table based on the structure of the interview guide.
We also conducted a comprehensive desk review of country planning documents, meeting reports, and implementation tools, and reviewed country administrative coverage data provided by the MoHCDGEC. Two study staff summarized the key themes from all interviews and the desk review, which were conducted between February and November 2019.
3. Results
A total of 10 key informants were interviewed, including the MoHCDGEC HPV Vaccine Officer, the WHO Tanzania EPI National Professional Officer, UNICEF health and communications officers, and core members of the Expanded Programme on Immunization (EPI) Technical Working Group (TWG). John Snow, Inc., JHPIEGO, the Clinton Health Access Initiative (CHAI), and other partners working in immunization in Tanzania comprise the core members of the EPI TWG. These key informants were chosen based on their involvement in the HPV vaccine introduction planning and implementation effort and represent the breadth of organizations and expertise within the EPI TWG for HPV vaccine. Members of the Tanzania Immunization Technical Advisory Group (TAITAG) and national Interagency Coordinating Committee (ICC) at the time of HPV vaccine introduction decision-making were not able to be reached for interviews. Desk review documents included the HPV vaccine introduction plans and operational guidelines, training materials, microplanning and monitoring documents, communication plans and social mobilization materials, and meeting minutes from the EPI TWG meetings. Documents that were reviewed from this group primarily originated between January 2018 and April 2018; earlier documents from the Kilimanjaro pilot project (2014) and post introduction coverage (2019) were also reviewed.
3.1. Decision to introduce HPV vaccine into the national immunization program
Tanzania’s national HPV vaccine introduction was largely informed by the high burden of cervical cancer in the country. Additional drivers for the national introduction of HPV vaccine were the successful HPV vaccine pilot program in the Kilimanjaro region in 2014–2015 and strong political commitment. In January 2016, the Tanzania Immunization Technical Advisory Group (TAITAG) requested data from the EPI TWG on HPV vaccine introduction (Fig. 2). After review, in November 2016, the TAITAG recommended HPV vaccine national introduction. The decision for national introduction was then endorsed by the national ICC during its 82nd meeting in January 2017. Tanzania applied for Gavi support for HPV vaccine national introduction to a multiple age cohort of 9–14-year-old girls in May 2017. However, due to the HPV vaccine supply constraint, Tanzania was informed that there was only sufficient supply for one age cohort. The TAITAG was required to provide guidance regarding the selection of a single age cohort for vaccination. During the TAITAG meeting later in May 2017, the TAITAG recommended HPV vaccine introduction to a single-age cohort of 14-year-old girls. Tanzania’s Gavi application for HPV vaccine national introduction was approved in January 2018 and the vaccine was introduced nationally in April 2018.
Fig. 2.
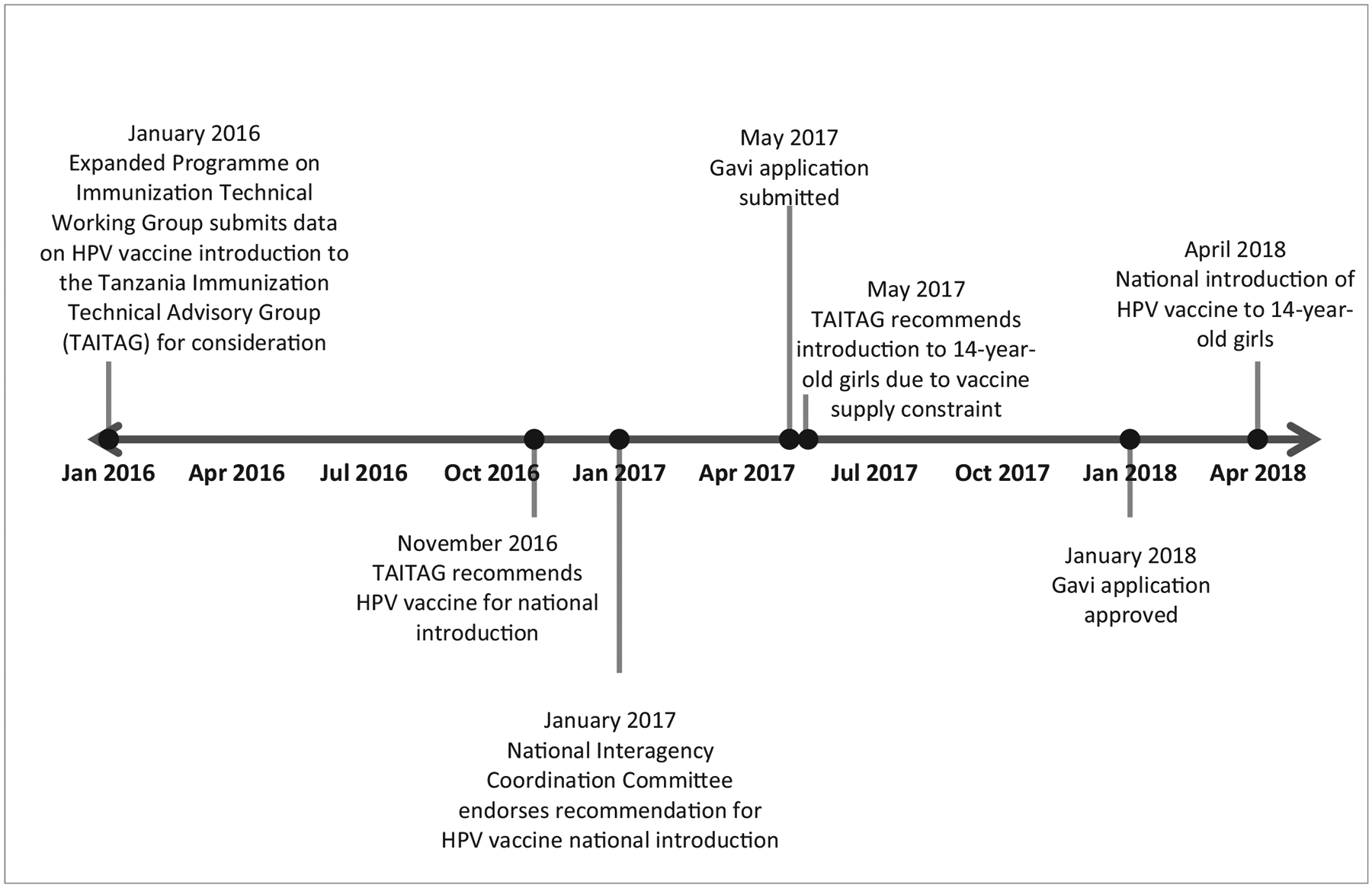
Tanzania national human papillomavirus (HPV) vaccine introduction decision-making timeline, 2016–2018.
3.2. Program considerations
3.2.1. Vaccine product choice
The TAITAG recommended the quadrivalent HPV vaccine for national introduction because it protects against the most prevalent HPV serotypes in Tanzania [15]. The quadrivalent HPV vaccine was also used successfully during the pilot program in Kilimanjaro region and had previously been licensed by the Tanzania Food and Drug Authority [16].
3.2.2. Target age for HPV vaccination
Following the 2017 WHO recommendations to vaccinate multiple cohorts in the first year of introduction, Tanzania applied for Gavi support for a multiple-age cohort introduction to 9–14-year-old girls [17]. Due to limited global vaccine supply since 2017, Tanzania was only able to receive enough vaccine supplies for a single-age cohort of girls. This older age cohort of 14-year-old girls was selected as the temporary age-eligibility to ensure protection to the greatest number of girls because those girls would not be age-eligible in subsequent years (≥15 years old); high enrolment in schools gave confidence in the ability to reach 14-year-old girls. The multiple-age cohort of 9–14-year-old girls would be vaccinated when sufficient vaccine supplies were available, which was expected 1–2 years from introduction, and then Tanzania would continue vaccinating 9-year-old girls each year thereafter. Of note, the Kilimanjaro Region continued vaccinating 9-year-old girls following the conclusion of the pilot program at the end of 2015 to maintain continuity of their program. Establishing 9-year-old girls as the routine age cohort following the multiple-age cohort vaccination would ensure nationwide alignment of age-eligibility in Tanzania.
3.2.3. Delivery strategy
Tanzania introduced HPV vaccine using their routine immunization delivery strategy with HPV vaccine available at health facilities and during outreaches at community sites, community mobile sites, and primary and secondary schools. The frequency of outreaches was determined by each individual health facility. With the introduction of HPV vaccine, health facilities were encouraged to add primary and secondary schools as outreach locations, in conjunction with other community and community mobile outreach activities to reach 14-year-old girls. Some health facilities were already visiting schools as part of their routine outreach to provide health education (sexual and reproductive health, nutrition, and hygiene). Health facilities were also encouraged to make special efforts to identify out-of-school girls through community health workers and community leaders.
Tanzania used an ‘opt-out’ approach to consent, in line with other routine vaccinations, as per WHO guidelines for HPV vaccination [18,19].
3.3. HPV vaccine introduction planning activities
3.3.1. Planning committee and sub-committees
The EPI TWG oversaw the planning and implementation of the HPV vaccine national introduction. The EPI TWG is comprised of the core immunization stakeholders in Tanzania (MoHCDGEC, WHO, UNICEF, and other partners). However, during the HPV vaccine introduction planning period, it was expanded to include other ministries outside of the MoHCDGEC. These ministries included the President’s Office of Regional Administration and Local Government (PORALG) to support coordination at the sub-national regional and council levels and the Ministry of Education (MoE) because of the eligible age group and in-school vaccination as part of the delivery strategy. The EPI TWG was also expanded to include community and religious organizations and professional associations (e.g., cervical cancer prevention stakeholders) relevant to the HPV vaccine introduction. There were three main sub-committees within the EPI TWG for HPV vaccine national introduction: 1) Logistics; 2) Advocacy, communications and social mobilization (ACSM); 3) Data and monitoring and evaluation. Each TWG sub-committee was comprised of 3–4 individuals from the expanded EPI TWG for HPV vaccine introduction who were identified based on their expertise and to ensure a range of organizations were represented. The main tasks of the sub-committees included outlining specific implementation plans and contributing to the training guidelines and microplanning plans for their focus areas. One representative from each TWG sub-committee reported back to the main EPI TWG during each meeting.
The EPI TWG developed HPV vaccine introduction guidelines as one of its first activities. Two guidelines were produced: one set of guidelines for regional and district personnel in English and a pared-down version for service providers in Kiswahili (Supplemental file 1). The HPV vaccine introduction guidelines were comprehensive handbooks to guide effective national program implementation across all sub-committees, regions, and councils.
3.3.2. Trainings for regional and council-level stakeholders and health workers, and school personnel
A national three-day HPV vaccine and inactivated polio vaccine (IPV) Orientation and Macroplanning Workshop was conducted in January 2018 with representatives from all regions of Tanzania. The HPV vaccine training was combined with IPV vaccine in an effort to share training costs and capitalize on having all health workers gathered together because both vaccines were being introduced simultaneously into the national immunization schedule. Training for HPV vaccine included background information on HPV infection and cervical cancer, HPV vaccine administration and safety, advocacy, communications, and social mobilization, monitoring and evaluation, and microplanning.
Over the following two months, trainings were held in a cascaded manner: regions held council-level trainings, and councils conducted trainings for health workers and primary and secondary school personnel. At the national-level training, regional representatives received the tools necessary to conduct cascaded trainings, including all PowerPoint presentations from the national-level training, a tailored and simplified PowerPoint presentation for one-day lower-level trainings in Kiswahili, and the HPV vaccine introduction guidelines for distribution. A total of 6,131 health workers and 22,067 school personnel were trained nationwide between January – April 2018.
3.3.3. Microplanning and estimation of the target population
Microplanning was essential for identifying the targeted girls and the resources needed. It commenced following the national-level workshop in January 2018 once all levels were trained.
Microplanning for HPV vaccine introduction required identification and registration of all 14-year-old girls (both in-school and out-of-school), as well as enumeration of schools and health facilities, their locations, and a contact person. At the health facility level, target populations were enumerated by physical count and eligible girls were line-listed. This joint effort between health workers, community health volunteers, and school personnel required 1–2 days at each health facility. Table 1 shows the target numbers for the first year of introduction (2018). During this exercise, health facilities also assessed their cold chain capacity.
Table 1.
Human papillomavirus (HPV) vaccine microplanning registration figures, 2018 Tanzania HPV vaccine introduction.
| Total number of villages | Total number of health facilities | Total number of schools (primary and secondary) | Total number of 14-year-old in-school girls | Total number of 14-year-old out-of-school girls | Total number of 14-year-old girls |
|---|---|---|---|---|---|
| 16,935 | 6,131 | 21,957 | 552,805 | 182,054 | 734,859 |
Since the first year of introduction, Tanzania has used data provided by the National Bureau of Statistics on the number of age-eligible girls for planning and reporting purposes.
3.3.4. Advocacy, communications, and social mobilization
The ACSM sub-committee of the TWG developed a communication plan for HPV vaccine introduction that guided all advocacy, communications, and social mobilization activities. The communication plan outlined guidelines for advocacy and sensitization meetings, mass media involvement, launching ceremonies, and identification of audiences as well as the activities and materials meant for each audience. The sub-committee developed key messages to be communicated at each level (Supplemental file 2). Printed products (posters, brochures, fliers, tire wheel covers, and banners) were developed, produced, and distributed to health facilities, schools, and communities (Supplemental file 3). Mass media TV and radio spots were also developed and broadcasted, and messages were distributed through social media (WhatsApp).
The ACSM sub-committee also developed a crisis communication plan to address potential misinformation and resistance to HPV vaccine. This plan outlined the response strategy and procedures for all stakeholders including the flow of information (Supplemental file 4).
Overall, the ACSM sub-committee engaged several professional bodies working on HPV and cervical cancer in Tanzania for input on the development of materials and to build awareness. There were also several national and subnational stakeholder meetings involving political and religious leaders, as well as journalists prior to the launch.
National Stakeholder Advocacy Meetings
Tanzania held a high-level stakeholder advocacy meeting in February 2018 involving influential political, community, and religious leaders. They held another national-level religious leader meeting in April 2018. The goal of these meetings was to create awareness and support for the HPV vaccine introduction.
Subnational Stakeholder Meetings
One-day regional stakeholder meetings with political appointees, medical personnel, public health officials, representatives from education, and religious leaders were conducted in every region to build advocacy and sensitize stakeholders. In total, 26 regional stakeholder meetings were held with over 2,000 attendees nationwide. Regional and council primary health care committee (PHC) meetings were also held as part of the advocacy activities for the introduction of HPV vaccine. The PHC meetings for HPV vaccine were meant to educate the influential community leaders and members on the importance of HPV vaccination, the burden of cervical cancer in Tanzania, and to build awareness for this vaccine introduction. Overall, 196 PHC committee meetings were held nationwide prior to the HPV vaccine introduction.
Media Seminars
Media seminars were conducted at the national level and in all 30 regions. The national-level media seminar in Dar es Salaam was held one month prior to vaccination launch to inform the media of the HPV vaccine introduction and allow them the opportunity to ask questions. The seminar was chaired by the Minister of Health, and participants included media resource personnel from newspapers, radio and TV stations, as well as MoHCDGEC officials. A total of 20 personnel attended this seminar. One-day media seminars were held in each region with approximately 30 participants from various media outlets including TV, radio, and newspaper. Standardized messages were shared with all media personnel during these seminars.
3.4. National launching ceremony
A national launching ceremony was held on April 10, 2018 in an open playground of Temeke District of Dar es Salaam, officiated by her Excellence Vice President of The United Republic of Tanzania, Mama Samia Suluhu Hassan, the Minister of Health, and other high-level religious and community leaders (Supplemental file 5). A total of 300 girls were vaccinated during the event; additionally, cervical cancer and HIV screening services were provided to eligible women. Following the national launch, all regions of mainland Tanzania and Zanzibar, and some councils conducted launching events. Launching events were felt to be critical to the success of the HPV vaccine introduction. Sub-national launches were held by the Regional Commissioner or the District Commissioner and were important for showing political commitment to the HPV vaccination program and creating awareness in the community.
3.5. HPV vaccine administrative coverage
Administrative coverage of the first dose of HPV vaccine (HPV1) at the end of 2019 was 78%, and the second dose of HPV vaccine (HPV2) coverage was 49%. Fig. 3 illustrates the trends in HPV1 and HPV2 vaccination coverage (number of doses administered/estimated number of eligible girls per month) from April 2018 to December 2019, by month. Increases in coverage of HPV1 were noted during periods of re-sensitization (increased community advocacy and redistribution of materials including flyers and posters) or supportive supervision (May 2018 and May 2019).
Fig. 3.
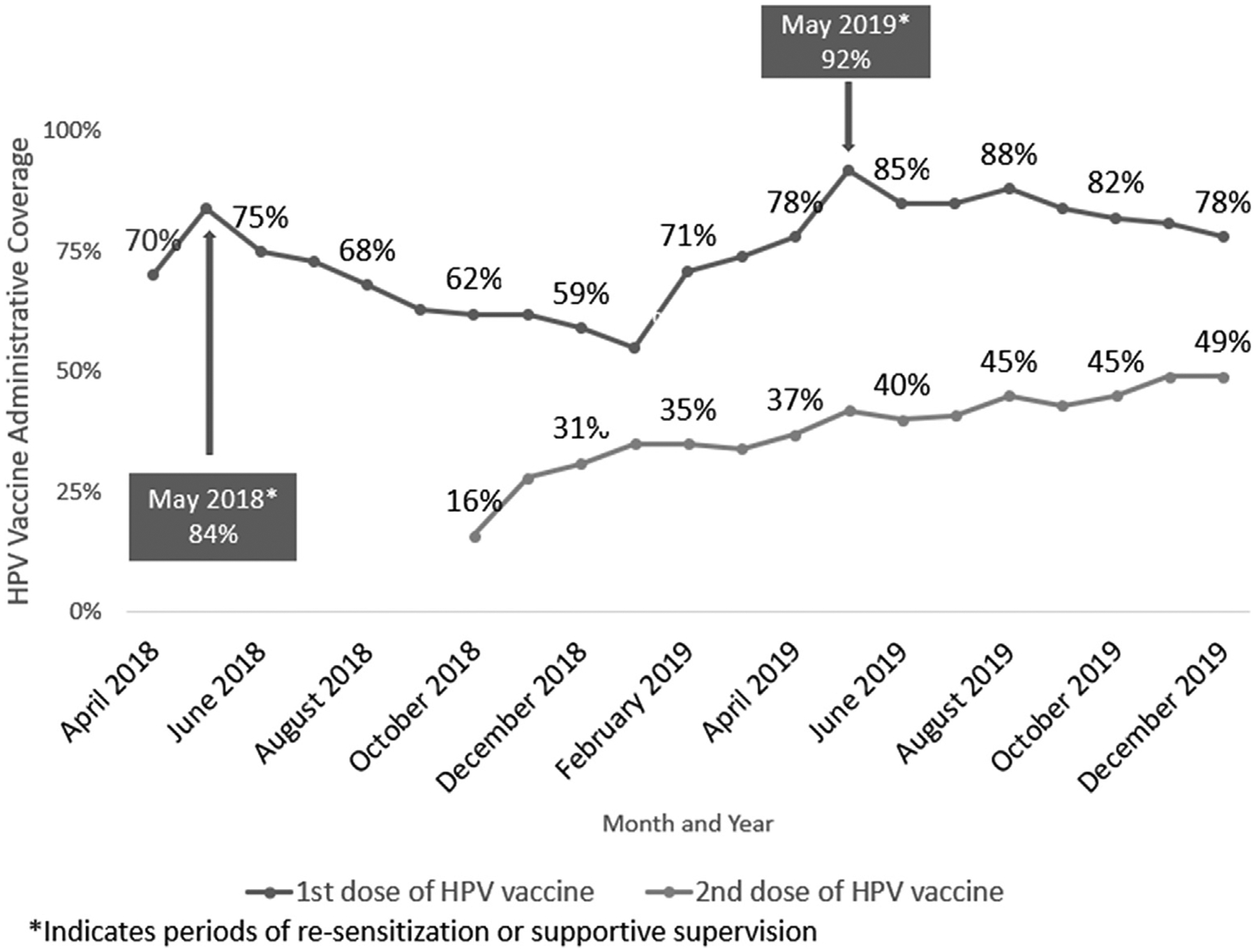
Tanzania human papillomavirus (HPV) vaccine administrative coverage1, April 2018–December 2019 (1The total number of doses administered to the target population/the total estimated number of people in the target population; administrative coverage provided by the Tanzania Ministry of Health, Community Development, Gender, Elderly, and Children).
3.6. Misinformation
At the start of the national HPV vaccine introduction, one religious radio station aired programming stating HPV vaccine causes infertility, and there was similar misinformation regarding infertility in one region on Tanzania’s southern border. Health communications specialists mitigated this misinformation by implementing their crisis communication plan and speaking to the public on the radio. Since then, misinformation has only circulated in small areas of Tanzania and has been addressed through the communication plan and community re-sensitization efforts including supplementary orientations to health workers, school personnel, and community leaders, redistribution of print materials, and media/social media advocacy.
3.7. Monitoring for adverse events following immunization
A monitoring system for adverse events following immunization (AEFI) coordinated by the Tanzania Food and Medical Authority, including a protocol and committee to respond to reports and conduct causality assessments, existed at the national level prior to HPV vaccine introduction [20]. The committee receives and discusses AEFIs after receiving reports from the subnational level. AEFIs are reported to the Vaccine Information Management System as an aggregate number for all vaccines. HPV vaccine was integrated into this existing system. In 2019, a total of 17 AEFIs were reported, of which 1 was a serious AEFI, unrelated to HPV vaccine.
4. Discussion
Tanzania successfully introduced HPV vaccine in 2018, targeting 14-year-old girls, using a routine immunization delivery strategy, which involved both facility-based and school-based delivery. Tanzania developed multisectoral collaborations during the preparatory phase; interviewees reported that the involvement of various ministries, partners, and community-based, religious, and professional associations and organizations created widespread awareness and support for the vaccine. These strong partnerships and high-level political commitment were thought to be critical to introducing the vaccine, the success of the HPV vaccine introduction, and the sustainability of the program.
Interviewees also reported successful coordination with the education sector to provide HPV vaccine to girls in school, as part of routine outreach. Continuing to partner with schools and the MoE will be essential to reaching eligible girls [21–23]. However, only primary school is compulsory in Tanzania (14-years-old is typically the oldest age in primary school) and as a result, some age-eligible girls are not in school. Engaging community health workers and community leaders will support efforts in identifying potentially missed girls [24–26]. Additionally, students might move to another area as they transition from primary to secondary school, making tracking for the second dose very difficult. Overall systematic registration of eligible girls and defaulter tracking will be necessary to achieve high coverage of both the first and second doses of HPV vaccine; Tanzania may consider incorporating HPV vaccine into its electronic registry used for other routine vaccines.
Results indicate that Tanzania’s HPV vaccine introduction required large-scale effort for training and social mobilization. As with other HPV vaccine introductions, it was necessary to train both health workers and school personnel, which increased the training needs compared to other vaccine introductions [21,22,27,28]. Sensitizing stakeholders across various fields (religious and political organizations, media/journalists, community leaders) was also thought to be a necessary component of the social mobilization strategy. Continued provision of on-the-job training or supportive supervision to health workers and school personnel, particularly regarding identifying eligible girls and recording and reporting, will be necessary to sustain a strong program.
Additionally, recognizing the sensitive nature of the HPV vaccine and learning from other countries’ experiences, Tanzania conducted hundreds of stakeholder meetings, community orientation sessions, and media seminars with a broad spectrum of individuals [6,25,29]. Nevertheless, despite these efforts, there was misinformation which led to vaccine hesitancy (vaccine refusal and skepticism) identified in-country during introduction. These were quickly mitigated by health communications specialists communicating to the public via the radio. The early preparation for these events and the involvement of multiple stakeholders in community sensitization (media, religious, and political leaders) was critical in preventing more damage from this misinformation. These issues illustrate that continuous and targeted social mobilization for HPV vaccine and engagement of community members is mandatory for the continued success of the program and demonstrates the value of a detailed communications and risk communications plan [25,28–32]. Health workers, school personnel, and community leaders need to be equipped with tools to address potential misinformation in their communities, not only at the beginning of HPV vaccine introduction but continuously. In conjunction, additional training for health workers on AEFIs is needed to ensure accurate reporting and combat fears of professional repercussions in reporting.
Since HPV vaccine introduction in April 2018, Tanzania has made efforts to address many of the issues raised during HPV vaccine introduction, including the misunderstandings of the delivery strategy, the difficulties in integrating school outreach into health facility workplans, and identifying eligible girls. In 2019, to increase coverage of HPV, Tanzania conducted additional regional-level supportive supervision visits in every region, beginning with the lowest-performing regions. Tanzania also utilized regular immunization performance review meetings to address performance issues and resolve them in a timely fashion. Tanzania began implementing a 2020 HPV vaccination coverage improvement plan aimed at increasing the uptake of HPV vaccine and achieving and maintaining 2nd dose coverage of at least 80% by December 2020. However, HPV vaccination was interrupted due to the COVID-19 pandemic and resulting school closures for several months. Tanzania has since pivoted to implement an HPV vaccine recovery effort which includes increased communication and collaboration with the MoE, printing and dissemination of HPV vaccine social mobilization and educational materials to communities and schools, and additional TV and radio spots.
Based on this review of HPV introduction, Tanzania should continue follow-up supportive supervision and expand the use of existing mechanisms to monitor HPV vaccination. This will enable Tanzania to target performance issues and resolve them appropriately, particularly regarding the second dose of HPV vaccine. In areas where coverage may be low, Tanzania may consider conducting periodic intensification of routine immunization for HPV vaccine. The adoption of other community engagement platforms using social media strategies, as well as health worker and caregiver engagement through SMS reminders or other new techniques may also help to raise awareness and ensure full coverage of girls.
Limitations
This study was designed to be a national-level evaluation. As such, our results are limited to the national-level perspective and do not include the sub-national or public perspective. We did not collect information on additional sub-national activities and the effectiveness of those activities and strategies implemented including communication and delivery strategies. Additionally, this evaluation focuses on the experience of the national introduction of HPV vaccine in all regions of Tanzania in 2018, excluding Kilimanjaro region as the region has been providing HPV vaccine since 2014.
Tanzania’s HPV vaccine introduction included many partners from various sectors. We were unable to reach some key players in the decision-making and introduction process and may have missed some important perspectives. For instance, we were unable to obtain the TAITAG perspective to better understand the data that was submitted that informed their recommendation for national HPV vaccine introduction.
Additionally, interviews began one year after national HPV vaccine introduction and there may have been some information loss due to the length of time from introduction to interview.
5. Conclusion
As Tanzania moves away from the initial introduction phase, HPV1 coverage is expected to slowly increase as the vaccine and necessary school outreach continues to be integrated into existing health facility workplans. Tanzania should continue efforts to improve HPV2 coverage through identifying barriers to vaccination and implementing mitigating strategies, particularly among girls who are in secondary school or now out-of-school. Tanzania plans to switch their routine cohort to 9-year-old girls, after vaccinating a multiple-age cohort and when the vaccine supply is adequate, while continuing to vaccinate this older age cohort of 14-year-old girls for the near future. It will be particularly important to strengthen the HPV vaccination program through innovative approaches for reaching special vulnerable populations to ensure equity in this older age group. There are opportunities to develop platforms to better reach out-of-school girls, ethnic minorities, and HIV + girls, and integrate with new school health programs. Continued multi-sectoral partner collaboration and political commitment to the HPV vaccination program are essential for its sustainability.
Supplementary Material
Acknowledgements
Timothy Brennan, Ngwegwe Christopher Bulula, Lotalis Norbert Gadau, Julie Garon, Anna Hidle, Joseline Ishengoma, Christian Maembe, Yusuf Makame, Bonaventura Nestory Muhindi, Green Sadru.
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
Funding
This work was supported by the Gavi, the Vaccine Alliance [“Evaluation of Human Papilloma Virus (HPV) Vaccine National Introduction in Low-and-Lower-Middle Income Countries” - Contract No. ME 9422 12 20].
Footnotes
CRediT authorship contribution statement
Alex Mphuru: Methodology, Formal analysis, Investigation, Data curation, Writing - original draft. Anyie J. Li: Methodology, Formal analysis, Investigation, Data curation, Writing - original draft. Furaha Kyesi: Methodology, Investigation, Writing - review & editing. William Mwengee: Methodology, Investigation, Writing - review & editing. Fikiri Mazige: Investigation, Writing - review & editing. Raphael Nshunju: Investigation, Writing - review & editing. Berrington Shayo: Investigation, Writing - review & editing. Mary Rose Giattas: Investigation, Writing - review & editing. Anagha Loharikar: Conceptualization, Methodology, Writing - review & editing, Supervision. Dafrossa Lyimo: Investigation, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The authors alone are responsible for the views expressed in this article, which do not necessarily represent the views, decisions, or policies of the institutions with which the authors are affiliated.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.04.025.
References
- [1].Bruni L, Albero G, Serrano B, Mena M, Gomez D, Munoz J, et al. Human Papillomavirus and Related Diseases in Tanzania. Summary Report. <http://www.hpvcentre.net/statistics/reports/TZA.pdf?t=1544735268334 >; (2018) [accessed 2018 Dec 10].
- [2].International Agency for Research on Cancer. Cervix uteri. <https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf >; (2018) [accessed 20 Nov 2019].
- [3].International Agency for Research on Cancer. Cancer Fact Sheets: Cervical Cancer. <http://gco.iarc.fr/today/data/pdf/fact-sheets/cancers/cancer-fact-sheets-16.pdf >; (2012) [accessed 20 Nov 2019].
- [4].International Agency for Research on Cancer. Tanzania, United Republic of Fact Sheet. <https://gco.iarc.fr/today/data/factsheets/populations/834-tanzania-united-republic-of-fact-sheets.pdf >; (2018) [accessed 2018 Dec 14].
- [5].Hanson CM, Eckert L, Bloem P, Cernuschi T. Gavi HPV Programs: Application to Implementation. Vaccines. 2015;3:408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Howard N, Gallagher KE, Mounier-Jack S, Burchett HED, Kabakama S, LaMontagne DS, et al. What works for human papillomavirus vaccine introduction in low and middle-income countries?. Papillomavirus research (Amsterdam, Netherlands). 2017;4:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Howard N, Mounier-Jack S, Gallagher KE, Kabakama S, Griffiths UK, Feletto M, et al. The value of demonstration projects for new interventions: The case of human papillomavirus vaccine introduction in low- and middle-income countries. Human vaccines & immunotherapeutics. 2016;12:2475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gallagher KE, Howard N, Kabakama S, Mounier-Jack S, Burchett HED, LaMontagne DS, et al. Human papillomavirus (HPV) vaccine coverage achievements in low and middle-income countries 2007–2016. Papillomavirus research (Amsterdam, Netherlands). 2017;4:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].World Health Organization. Stakeholders’ Meeting on the introduction of Human Papilloma Virus (HPV) Vaccine kicks off in the United Republic of Tanzania. <https://www.afro.who.int/news/stakeholders-meeting-introduction-human-papiilloma-virus-hpv-vaccine-kicks-united-republic >; [accessed 13 Apr 2020].
- [10].Gallagher KE, Erio T, Baisley K, Lees S, Watson-Jones D. The impact of a human papillomavirus (HPV) vaccination campaign on routine primary health service provision and health workers in Tanzania: a controlled before and after study. BMC health services research. 2018;18:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Human Papillomavirus vaccination demonstration programme. Post-introduction evaluation report. Kilimanjaro region, Tanzania: 2014. [Google Scholar]
- [12].United Republic of Tanzania. Application for Gavi new vaccine support. <https://www.gavi.org/country/tanzania/documents/ >; (2017) [accessed 2018 Dec 13].
- [13].Gavi. Decision Letter NVS Tanzania 2018. <https://www.gavi.org/country/tanzania/documents/dlpas/decision-letter-nvs-tanzania-2018-(6)/ >; (2018) [accessed 20 Nov 2019].
- [14].Li AJ, Kyesi F, Mwengee W, Mphuru A, Giattas MR, Shayo B, et al. Impact of the human papillomavirus (HPV) vaccine supply shortage on Tanzania’s national HPV vaccine introduction. Vaccine 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dartell M, Rasch V, Kahesa C, Mwaiselage J, Ngoma T, Junge J, et al. Human papillomavirus prevalence and type distribution in 3603 HIV-positive and HIV-negative women in the general population of Tanzania: the PROTECT study. Sex Transm Dis 2012;39:201–8. [DOI] [PubMed] [Google Scholar]
- [16].Watson-Jones D, Baisley K, Ponsiano R, Lemme F, Remes P, Ross D, et al. Human papillomavirus vaccination in Tanzanian schoolgirls: cluster-randomized trial comparing 2 vaccine-delivery strategies. J Infect Dis 2012;206:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Human papillomavirus vaccines: WHO position paper, May 2017. Releve epidemiologique hebdomadaire. 2017;92:241–68. [PubMed] [Google Scholar]
- [18].World Health Organization. Considerations regarding consent in vaccinating children and adolescents between 6 and 17 years old. <https://apps.who.int/iris/bitstream/handle/10665/259418/WHO-IVB-14.04-eng.pdf?sequence=1 >; (2014) [accessed 13 Apr 2020].
- [19].World Health Organization. Guide to introducing HPV vaccine into national immunization programmes. <https://apps.who.int/iris/bitstream/handle/10665/253123/9789241549769-eng.pdf; jsessionid=C37D82864B0CA09FCACCEB26EE6EAE01?sequence=1 >; (2016) [accessed 13 Apr 2020].
- [20].Welfare TMoHaS. Guidelines for Surveillance of Adverse Events Following Immunization <https://www.tmda.go.tz/uploads/publications/en1554376427-TANZANIA%20AEFI%20GUIDELINES%20%20.pdf >; (2014) [accessed 15 May 2020].
- [21].Gallagher KE, Howard N, Kabakama S, Mounier-Jack S, Griffiths UK, Feletto M, et al. Lessons learnt from human papillomavirus (HPV) vaccination in 45 low-and middle-income countries. PLoS ONE 2017;12:e0177773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ladner J, Besson MH, Hampshire R, Tapert L, Chirenje M, Saba J. Assessment of eight HPV vaccination programs implemented in lowest income countries. BMC public health. 2012;12:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low-and middle-income countries. Bull World Health Organ 2011;89:821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ladner J, Besson MH, Audureau E, Rodrigues M, Saba J. Experiences and lessons learned from 29 HPV vaccination programs implemented in 19 low and middle-income countries, 2009–2014. BMC health services research. 2016;16:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Watson-Jones D, Tomlin K, Remes P, Baisley K, Ponsiano R, Soteli S, et al. Reasons for receiving or not receiving HPV vaccination in primary schoolgirls in Tanzania: a case control study. PLoS ONE 2012;7:e45231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wigle J, Fontenot HB, Zimet GD. Global Delivery of Human Papillomavirus Vaccines. Pediatr Clin North Am 2016;63:81–95. [DOI] [PubMed] [Google Scholar]
- [27].Mugisha E, LaMontagne DS, Katahoire AR, Murokora D, Kumakech E, Seruyange R, et al. Feasibility of delivering HPV vaccine to girls aged 10 to 15 years in Uganda. African health sciences. 2015;15:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Remes P, Selestine V, Changalucha J, Ross DA, Wight D, de Sanjose S, et al. A qualitative study of HPV vaccine acceptability among health workers, teachers, parents, female pupils, and religious leaders in northwest Tanzania. Vaccine. 2012;30:5363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kabakama S, Gallagher KE, Howard N, Mounier-Jack S, Burchett HE, Griffiths UK, et al. Social mobilisation, consent and acceptability: a review of human papillomavirus vaccination procedures in low and middle-income countries. BMC public health. 2016;16:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bonanni P, Zanella B, Santomauro F, Lorini C, Bechini A, Boccalini S. Safety and perception: What are the greatest enemies of HPV vaccination programmes?. Vaccine. 2018;36:5424–9. [DOI] [PubMed] [Google Scholar]
- [31].Perez S, Zimet GD, Tatar O, Stupiansky NW, Fisher WA, Rosberger Z. Human Papillomavirus Vaccines: Successes and Future Challenges. Drugs. 2018;78:1385–96. [DOI] [PubMed] [Google Scholar]
- [32].Perlman S, Wamai RG, Bain PA, Welty T, Welty E, Ogembo JG. Knowledge and awareness of HPV vaccine and acceptability to vaccinate in sub-Saharan Africa: a systematic review. PLoS ONE 2014;9:e90912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


