Abstract
Gamma interferon (IFN-γ) and the cellular responses induced by it are essential for controlling mycobacterial infections. Most patients bearing an IFN-γ receptor ligand-binding chain (IFN-γR1) deficiency present gross mutations that truncate the protein and prevent its expression, giving rise to severe mycobacterial infections and, frequently, a fatal outcome. In this report a new mutation that affects the IFN-γR1 ligand-binding domain in a Spanish patient with mycobacterial disseminated infection and multifocal osteomyelitis is characterized. The mutation generates an amino acid change that does not abrogate protein expression on the cellular surface but that severely impairs responses after the binding of IFN-γ (CD64 and HLA class II induction and tumor necrosis factor alpha and interleukin-12 production). A patient's younger brother, who was also probably homozygous for the mutation, died from meningitis due to Mycobacterium bovis. These findings suggest that a point mutation may be fatal when it affects functionally important domains of the receptor and that the severity is not directly related to a lack of IFN-γ receptor expression. Future research on these nontruncating mutations will make it possible to develop new therapeutical alternatives in this group of patients.
Gamma interferon (IFN-γ) is a widely studied cytokine and one of the most promising biological agents, with great therapeutic potential for several pathologies. This is mainly due to its immunomodulatory and antiproliferative effects and, probably, to its antiviral capacity 13. IFN-γ, after binding to its high-affinity receptor, regulates over 200 genes 7. IFN-γ upregulates major histocompatibility complex (MHC) class I protein expression and induces MHC class II proteins on a variety of leucocytes and epithelial cells. IFN-γ is also the major cytokine responsible for activating or regulating the phagocytic function of mononuclear cells. It also regulates the production of several immunomodulatory or proinflammatory cytokines such as interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α) 7.
The IFN-γ high-affinity receptor is composed of at least two subunits. IFN-γR1 (alpha chain or CD119) is the IFN-γ binding chain. It is encoded by a 30-kb gene located on the long arm of chromosome 6 23, and it is expressed at moderate levels on the surfaces of nearly all cells. IFN-γR2 (beta chain or accessory factor 1) is the signaling chain 27, and it is encoded by a gene located on chromosome 21q22.1 8.
The relationship between IFN-γ, IL-12, and TNF-α makes up a particularly important system, since it controls mycobacterial infections in humans 14. Recently, several mutations in some components of this system (ligands or receptors) have been described 4, 11, 12, 17–19, 21. Patients with these mutations have similar susceptibilities to infections by atypical and nontuberculous mycobacteria 5.
MATERIALS AND METHODS
Subjects.
A 5-year-old Spanish girl from consanguineous parents was referred to our hospital with disseminated infection and multifocal osteomyelitis due to Mycobacterium avium complex and Mycobacterium szulgai. A younger brother of this patient is healthy, and a second brother died before the birth of the patient due to meningitis by Mycobacterium bovis at 10 years of age (data collected from the necropsy). The patient did not present with any other important diseases or any secondary reaction to the diphtheria/tetanus/pertussis and oral polio immunizations. She has not been vaccinated with tuberculoid bacillus Calmette-Guérin. The histological study of the patient's samples revealed the absence of mature granulomes. Partial remission was obtained with antimycobacterial drugs, which were continued. Immunological investigation detected no classical immunodeficiency conditions that might predispose the patient to mycobacterial infections. Further immunologic studies revealed elevated levels of plasmatic complement proteins C3 and C4 and immunoglobulin G (IgG) (mostly due to high levels of IgG1 and IgG3 subclasses). The production of soluble CD25 in serum was elevated, while IL-5 and IL-1β were within normal limits. The level of TNF-α in serum was 0 pg/ml. The proliferative response of the patient's peripheral blood mononuclear cells (PBMCs) after in vitro challenges with different mitogens (IL-2, T and B antigens, lectins, calcium ionophores, and monoclonal antibodies [MAb] against CD2, CD3, and CD28) were within the range of control values (data not shown).
Cytofluorographic analysis.
Whole-blood samples were stained by direct immunofluorescence with different MAb, their erythrocytes were lysed, and the remaining cells were fixed for flow cytofluorometric analysis by standard techniques (Qprep; Coulter, Hialeah, Fla.) 9. The results were recorded as the percentage of positive cells for each MAb (those displaying fluorescence intensities above the upper limit of a negative control). The MAb used were phycoerythrin (PE)-labeled anti-CD45 and anti-CD64 (Caltag, San Francisco, Calif.), PE- or fluorescein isothiocyanate (FITC)-labeled anti-CD14 (Ortho Pharmaceuticals), and FITC-labeled anti-CD119 (Hölzel Diagnostika, Cologne, Germany). Isotypic antibodies were used as a control. Samples were analyzed with an EPICs XL cytometer (Coulter).
Proliferation assays with PBMCs.
PBMCs were obtained from donors or immunodeficient patients by density gradient centrifugation using Ficoll-Hypaque (Lymphoprep; Nyegaard, Oslo, Norway) following standard procedures 24. Isolated cells (8 × 104) were placed in round-bottom microtiter plates (Nunc, Roskilde, Denmark) in 170 μl of AIM-V culture medium (Gibco BRL, Paisley, United Kingdom) supplemented with 1% penicillin-streptomycin (Difco) and 1% glutamine 20 mM (Whittaker, Walkersville, Md.). Stimuli were used as previously described 2, 25.
Cytokine quantification in serum samples.
Different cytokines were measured in duplicate in the sera of the patient, relatives, and controls by using an enzyme-linked immunosorbent assay system in accordance with the manufacturers' protocols: IL-5 and IL-1β, R&D Systems, Minneapolis, Minn.; IFN-γ and TNF-α, Bender MedSystems, Vienna, Austria; soluble IL-2 receptor, T Cell Diagnostics, Woburn, Mass.
Humoral and innate immunity.
Total serum Ig levels (IgG, IgA, and IgM) and levels of complement factors (C3 and C4) were determined by nephelometry (Array 360 system; Beckman Instruments, Brea, Calif.). Serum hemolytic capacity (CH100) was assessed using a radial-immunodiffusion (RID) kit (The Binding Site Limited, Birmingham, United Kingdom). Serum IgE from the patients and the controls was measured by using the RID kit (The Binding Site Limited).
Phagocytic activity.
Quantification of phagocytic activity of monocytes was performed with heparinized whole blood using the Phagotest (Orpegen Pharma, Heidelberg, Germany) test kit in accordance with the manufacturer's protocol.
RNA and DNA amplification.
Cytoplasmic RNA was extracted from cultured cells or PBMCs using the Nonidet P-40 lysis method with modifications 10. DNA was obtained from the nuclear pellet by standard methods 10. The RNA was used as a template for a one-step reverse transcriptase PCR (RT-PCR) (Gibco BRL) performed with specific primers RIFN-γUPA (5′-CCAGCGACCGTCGGTAGCAGC-3′) and RIFN-γLOA (5′-ATCCTCTTTACGCTTTCA-3′) 1, rendering a product of 1,641 bases that contains the complete coding region. Two additional primers were used to completely sequence the cDNA: RIFN-γUPG (5′-GCTGTATGCCGAGATGGAA-3′) and RIFN-γUPH (5′-GTTTCAGCAGAAGGAGTCTTA-3′) 1. For DNA or RNA amplification, reactions were carried out in 100 μl containing 5 U of Taq DNA polymerase (Perkin-Elmer, Foster City, Calif.) 200 μM deoxynucleoside triphosphates, 0.5 μM (each) primer, and 1/10 of the RT-PCR products or 1 μg of genomic DNA. RT-PCR conditions were as follows: one cycle of RT (20 min at 50°C followed by 2 min at 94°C) and 35 cycles of PCR (15 s at 94°C, 30 s at 58°C, and 2 min at 72°C), followed by 10 min at 72°C for the final elongation.
DNA sequencing.
After purification of PCR products with QIAquick gel extraction kit (Qiagen, Hilden, Germany), they were cloned in the pMOS-Blue vector (Amersham, Buckinghamshire, United Kingdom). Double-stranded DNA templates were sequenced using the dideoxy chain terminator method of Sanger 6, and sequences were confirmed by analysis of three or more clones from two different amplifications.
PCR-SSCP in genomic DNA.
For PCR, a specific genomic primer of intron II, RIFN-γINTIILO (5′-CCTTAAAGGTTCCTGGATTTG-3′) 1, and a specific primer of exon II, RIFN-γUPE (5′-GTTACAATTGAATCCTATAACATG-3′) 1, were used on genomic DNA. The amplification rendered a 160-bp product. Single-strand conformation polymorphism (SSCP) was used in 27 unrelated controls in accordance with previous protocols 15.
Expression of CD64 on monocytes in response to IFN-γ.
Monocytes were isolated as previously published 20, prepared by adherence with a purity of 60 to 80% (evaluated by flow cytometry using anti-CD14 and anti-CD45 MAb), and placed in round-bottom microtiter plates (Nunc) in 170 μl of AIM-V culture medium (Gibco BRL) supplemented with 1% penicillin-streptomycin (Difco, Detroit, Mich.) and 1% (20 mM) glutamine (Whittaker). Monocytes were cultured for 24 h in the presence of 1,000 IU of rhIFN-γ (Bender MedSystems)/ml or medium alone. Detection of cell surface CD64 on fresh and cultured monocytes was performed by flow cytometry as described above.
TNF-α production by monocytes in response to LPS and IFN-γ.
Monocytes were isolated and placed as described above. TNF-α produced by monocytes after stimulation with Escherichia coli-derived lipopolysaccharide (LPS; 1 μg/ml;Sigma, St. Louis, Mo.) and IFN-γ (Bender MedSystems; 1,000 IU/ml) was assayed in duplicate by a previously reported procedure 20 with minor modifications.
IL-12 production by PBMC in response to OKT3 and IL2 stimulation.
PBMCs (8 × 104) were placed in round-bottom microtiter plates (Nunc) in 170 μl of AIM-V culture medium (Gibco BRL) supplemented with 1% penicillin-streptomycin (Difco) and 1% (20 mM) glutamine (Whittaker). PBMCs were stimulated with OKT3 plus IL-2 used as previously described 3, 25 or with medium alone. After 48 h of culture, supernatants were collected and the IL-12 was measured in duplicate by enzyme-linked immunosorbent assay (Bender MedSystems).
Cell lines.
T lymphoblastoid cell lines were established by transformation of PBMCs with herpesvirus saimiri (HVS) supernatant 25. Briefly, PBMCs from the patient, the patient's family, and from a healthy 5-year-old girl were resuspended at 0.5 × 106 cells/ml in RPMI 1640 (Biochrom, Berlin, Germany)–CG medium (Vitromex GmbH, Vilshofen, Germany) (1:1) supplemented with 10% fetal calf serum (Flow Laboratories, Beckenham, United Kingdom) and 1 μg of phytohemagglutinin-A (Difco)/ml. Three days after stimulation, cells were resuspended in medium containing human recombinant IL-2 (Boehringer Mannheim, Mannheim, Germany), seeded in 24-well plates (Costar, Cambridge, Mass.), and inoculated with 1 ml of supernatant from cultures of an owl monkey kidney cell line (ATCC CRL 1556) lytically infected with HVS strain C488. Cells were fed regularly as described previously 25.
IFN-γRI immunoprecipitation from the T HVS cell lines.
T HVS cells (4 × 107) from the patient and control were washed twice with phosphate-buffered saline and lysed as described previously 26 with minor modifications. Extracts were immunoprecipitated using an anti-IFN-γR1 (C-20) polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) as described previously 26. The immunoprecipitates were then analyzed by sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis followed by electrophoretic transfer to cellulose nitrate membrane (Schleicher & Schuell, Dassel, Germany) and Western blotted with a polyclonal antibody described previously 16.
Nucleotide sequence accession number.
The sequence determined in this paper has been deposited at the GenBank database under accession no. AF056979.
RESULTS
The patient was referred to our hospital with disseminated infection and multifocal osteomyelitis due to M. avium complex and M. szulgai, which suggested an IFN-γ receptor deficiency. Although the distribution of M. szulgai is worldwide and although the mycobacterium has been isolated in humans in other conditions (i.e., AIDS and others) 28, this is the first time that it has been isolated in an IFN-γ receptor-deficient patient.
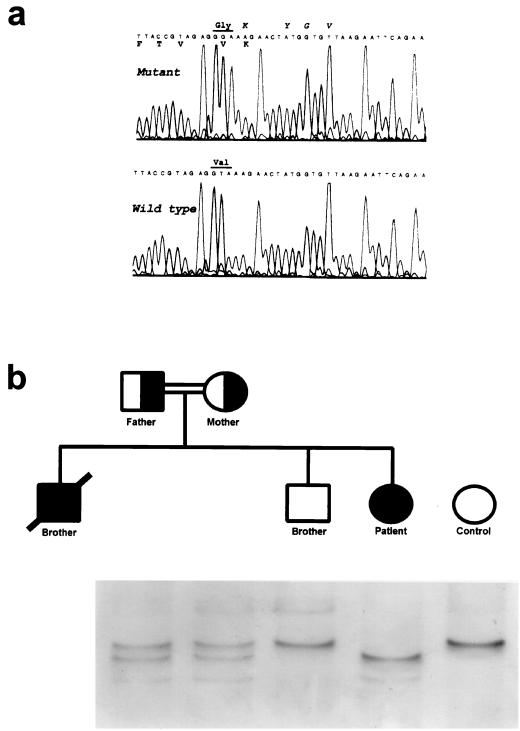
The patient presented a new mutation that affects exon 2 of IFN-γR1. The complete sequence of the patient's IFN-γR1 gene revealed the presence in homozygosis of a thymine-to-guanine mutation at position 188 (T188G), which generates a change of valine to glycine in position 63 of the protein (Fig. 1a). The patient's parents are consanguineous, and both are heterozygous for the mutation. A younger brother of the patient is healthy and homozygous for the wild-type sequence, and a second brother died before the birth of the patient due to meningitis by M. bovis when he was 10 (data collected from the necropsy) (Fig. 1b). Although samples of this brother are not available, it can be assumed that the cause of the mycobacterial infection was also the presence of the homozygous mutation. Thus, the relevant position of this change in the ligand-binding domain of IFN-γR1 may cause death in the first decade of life.
FIG. 1.
New mutation Val63Gly in the ligand-binding domain of IFN-γR1. (a) Sequence of the new mutation boundaries. Italics, amino acids implicated in the binding of IFN-γ to its receptor 28; roman amino acids conserved in murine IFN-γR1 4. (b) Pedigree and SSCP intrafamilial segregation of the Val63Gly mutation compared with a control.
The analysis of 27 unrelated individuals by SSCP showed the absence of the mutation, which indicates that the T188G change is not a polymorphism in the population (data not shown).
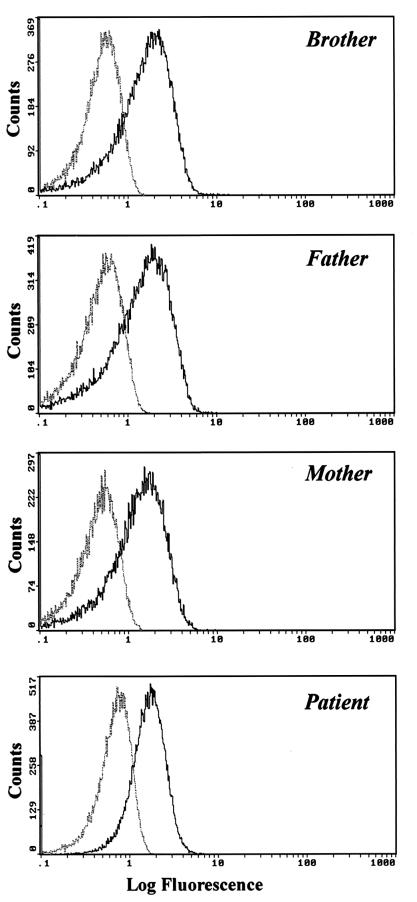
The results of the cytofluorographic analysis of IFN-γR1 with antibodies against CD119 demonstrate that the protein is correctly expressed in the patient's lymphocytes, monocytes, and granulocytes at levels similar to those in other members of her family (Fig. 2).
FIG. 2.
Cytofluorographic analysis of CD119 expression in the patient and family. Dotted lines, isotypic control fluorescence; solid lines, positive fluorescence. The graph shows expression in peripheral blood lymphocytes; similar results were obtained in peripheral monocytes and granulocytes (data not shown).
Immunoprecipitation of IFN-γR1 from an HVS T-cell line of the patient showed that the protein had a normal size (data not shown). This means that the translation and the subsequent processing of the protein are not altered by the presence of the mutation. However, the computer-generated model 22 revealed that the mutated protein had lost three bonds in comparison to the native one and that there were changes in the distances between atoms in the whole ligand-binding domain (data not shown). Taking all these data into account, it may be asserted that the mutation does not affect the transcription, translation, processing, and expression of the protein in the cellular surface.
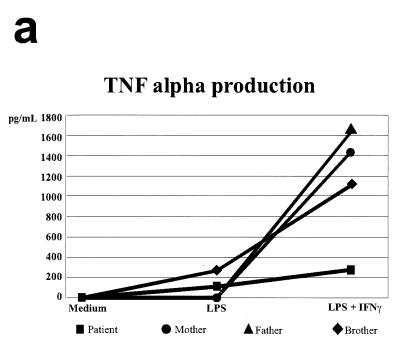
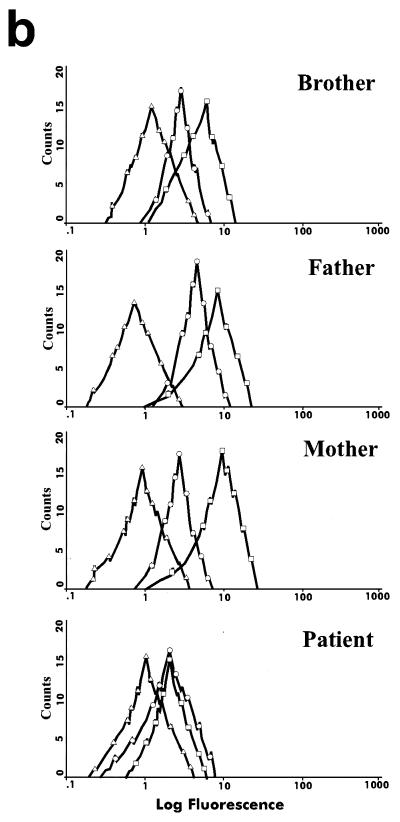
Several experiments were carried out in order to confirm the abnormal functionality of the receptor (Fig. 3). The monocytes of the patient presented a clear deficit in TNF-α production when stimulated with E. coli-derived LPS plus recombinant human IFN-γ (rhIFN-γ): 275.3 pg/ml in the patient versus 1,105.3 pg/ml in the brother, 1,627.6 pg/ml in the father, and 1,437.3 pg/ml in the mother (Fig. 3a), which means that the patient's TNF-α production is five times lower than those of other members of her family. Stimulation of the patient's PBMCs with OKT3 plus IL-2 revealed an absolute deficit in the production of IL-12 (data not shown). Finally, the patient's monocytes did not increase CD64 expression after stimulation with 1,000 IU of rhIFN-γ/ml (showing that unstimulated and stimulated patient monocytes produced similar mean fluorescence intensities [1.36 and 1.30, respectively]), and responses were normal in her parents and brother (Fig. 3b). Moreover, the measurement of the CD64-mediated monocyte phagocytic capacity gave poor results (data not shown). A similar pattern of HLA class II expression was also obtained after rhIFN-γ stimulation (data not shown).
FIG. 3.
Immune function parameters of the mutated IFN-γR1. (a) TNF-α production by monocytes stimulated with medium, LPS, and LPS plus rhIFN-γ; (b) induction of CD64 in monocytes after stimulation with IFN-γ. ▵, isotypic control fluorescence; ○, expression of CD64 after 24 h of stimulation with medium alone; □, expression of CD64 after 24 h of stimulation with 1,000 IU of rhIFN-γ/ml.
An elevated IFN-γ level in the patient's serum disproved the notion that the defects observed could be due to a deficient production of this cytokine (data not shown). All these data demonstrate that the mutation in homozygosis is severe enough to eliminate the functionality of the receptor although it does not abrogate its expression.
DISCUSSION
Several positions (Lys64, Tyr66, Gly67, and Val68) surrounding the mutation in IFN-γR1 found in the present study (Val63Gly) are fundamental in the interaction between the high-affinity receptor and its ligand 29. However, one could also hypothesize that IFN-γ may bind to another still-unknown receptor. Lysine 64, in particular, plays a key role since it directly interacts with IFN-γ. Furthermore, the conservation of residue 63 and other adjacent amino acids (Phe59, Tyr60, Val61, and Lys64) in the homologous murine IFN-γR1 27 reinforces the importance of this domain in the binding function (Fig. 1a). Additionally, it may be that more-subtle aspects on the mutation are present in the patient.
Reports on atypical mycobacterial infections have been published since almost 50 years ago 30, and in these cases there was probably an underlying defect in the IFN-γ signaling pathway. The first mutation in the alpha chain of the IFN-γ receptor was described in 1996 21. In 1997, Jouanguy et al. 19 proposed that the severity of the phenotype depends on the type of genetic defect and established a correlation among nonsense mutations 17, 18, 21 or deletions 17 and complete cellular phenotype defects, a histological phenotype of mycobacterial infection characterized by the inability to form mature granulomes and poor clinical outcome. On the other hand, “milder” mutations 19, which only produce an amino acid change, have been correlated with partial cellular phenotype defects, the ability to form mature granulomes, and a favorable prognosis 5. Our data suggest that the Val63Gly mutation profoundly affects the functionality of the receptor and contradict the thesis that milder mutations may be diagnosed a priori. The clinical severity (disseminated infection and multifocal osteomyelitis), the absence of mature granulomes in the histological study, and the family history suggest the possibility that missense IFN-γR1 mutations do not always correlate with a mild clinical phenotype. The present case describes one of the first cases of complete IFN-γR1 deficiency caused by mutations that disrupt the IFN-γ binding site without affecting surface expression.
It is feasible that the change of the amino acid generates a structural change in the receptor, giving rise to two possibilities: the reduction of the IFN-γR1 affinity for its ligand or the impairment of a correct interaction between the two complete receptor chains (IFN-γR1 and IFN-γR2) after the binding of the ligand. Our aim is to analyze these two possibilities. The results of this study will shed light on the importance of the three-dimensional structure and of the affinity by the ligand in the IFN-γ/IFN-γR1 system and the possibility of developing new therapeutic alternatives to current prevention with antibiotics.
Bone marrow transplantation has been proposed as likely the best treatment for these patients 5. The new, nontruncating mutation described here makes it possible to study new therapeutic strategies such as the use of alternative forms of IFN-γ that would efficiently bind to the mutant receptor or a hypothetical gene therapy with the wild-type alpha-chain gene. In addition, it may help to clarify whether hybrid receptors are generated in heterozygous individuals or if wild-type chain forms would displace the mutant chains at the cell surface. The pathways involved in host defense against mycobacteria and other intracellular pathogens will be clarified as more patients with IFN-γ receptor defects are recognized and studied.
ACKNOWLEDGMENTS
The contributions of A. López-Goyanes, L. M. Allende, and E. Paz-Artal are equal, and the order of authorship is arbitrary.
We thank S. Ferre-López for the preparation of the figures and B. J. Mayer, R. Geha, S. Katz, and N. Martínez-Quiles for their help in the immunoprecipitation of the protein.
This work was supported in part by grants from the Spanish Ministry of Education and Science (PM 95-57 and PM 96-21) and the Autonomous Community of Madrid (06-70-97 and 8.3-14-98).
REFERENCES
- 1.Aguet M, Dembic Z, Merlin G. Molecular cloning and expression of the human interferon-γ receptor. Cell. 1988;55:273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 2.Allende L M, Corell A, Madroño A, Góngora R, Rodriguez-Gallego C, López-Goyanes A, Rosal M, Arnaiz-Villena A. Retinol (vitamin A) is a cofactor in CD3-induced human T-lymphocyte activation. Immunology. 1997;90:388–396. doi: 10.1111/j.1365-2567.1997.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allende L M, Corell A, Manzanares J, Madruga D, Marcos A, Madroño A, López-Goyanes A, García-Pérez M A, Moreno J M, Rodrigo M, Sanz F, Arnaiz-Villena A. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94:543–551. doi: 10.1046/j.1365-2567.1998.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altare F, Durandy A, Lammas D, Emile J F, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob J A, Meinl E, Segal A W, Fischer A, Kumararatne D, Casanova J L. Impairment of mycobacterial immunity in human interleukin 12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 5.Altare F, Jouanguy E, Lamhamedi S, Döffinger R, Fischer A, Casanova J L. Mendelian susceptibility to mycobacterial infection in man. Curr Opin Immunol. 1998;10:413–417. doi: 10.1016/s0952-7915(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 6.Arnaiz-Villena A, Timón M, Corell A, Pérez-Aciego P, Martín-Villa J M, Regueiro J R. Primary immunodeficiency caused by mutations in the gene encoding the CD3-γ subunit of the T-lymphocyte receptor. N Engl J Med. 1992;327:529–533. doi: 10.1056/NEJM199208203270805. [DOI] [PubMed] [Google Scholar]
- 7.Bach E A, Aguet M, Schreiber R D. The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 8.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell C W, Taylor H M. A rapid, no-wash method for immunophenotypic analysis by flow cytometry. Am J Clin Pathol. 1986;86:600–607. doi: 10.1093/ajcp/86.5.600. [DOI] [PubMed] [Google Scholar]
- 10.Corell A, Martín-Villa J M, Morales P, De Juan M D, Varela P, Vicario J L, Martinez-Laso J, Arnaiz-Villena A. Exon-2 nucleotide sequences, polymorphism and haplotype distribution of a new HLA-DRB gene: HLA-DRB sigma. Mol Immunol. 1991;28:533–543. doi: 10.1016/0161-5890(91)90168-j. [DOI] [PubMed] [Google Scholar]
- 11.De Jong R, Altare F, Haagen I-A, Elferink D G, De Boer T, van Breda Vriesman P J C, Kabel P J, Draaisma J M T, van Dissel J T, Kroon F P, Casanova J L, Ottenhoff T H M. Severe mycobacterial and salmonella infections in interleukin-12-receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 12.Dorman S E, Holland S M. Mutation in the signalling chain of the interferon gamma receptor is associated with severe mycobacterial infection. J Clin Investig. 1998;101:2364–2369. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrar M A, Schreiber R D. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 14.Flesch I E, Hess J H, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann S H. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon γ and tumor necrosis factor α. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glavac D, Dean M. Optimization of the single-strand conformational polymorphism (SSCP) technique for detection of point mutations. Hum Mutat. 1993;2:404–414. doi: 10.1002/humu.1380020513. [DOI] [PubMed] [Google Scholar]
- 16.Greenlund A C, Farrar M A, Viviano B L, Schreiber R D. Ligand-induced IFN γ receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91) EMBO J. 1994;7:1591–1600. doi: 10.1002/j.1460-2075.1994.tb06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland S M, Dorman S E, Kwon A, Pitha-Rowe I F, Frucht D M, Gerstberger S M, Noel G J, Vesterhus P, Brown M R, Fleisher T A. Abnormal regulation of interferon-γ, interleukin-12, and tumor necrosis factor-α in human interferon-γ receptor 1 deficiency. J Infect Dis. 1998;178:1095–1104. doi: 10.1086/515670. [DOI] [PubMed] [Google Scholar]
- 18.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J F, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova J L. Interferon γ receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 19.Jouanguy E, Lamhamedi S, Altare F, Fondanèche M C, Tuerlinckx D, Blanche S, Emile J F, Gaillard J L, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova J L. Partial IFNγ R1 deficiency in a child with tuberculoid bacille Calmette-Guérin infection and a sibling with clinical tuberculosis. J Clin Investig. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin M, Newport M J, D'Souza S, Kalabalikis P, Brown I N, Lenicker H M, Agius P V, Davies E G, Thrasher A, Klein N, Blackwell J M. Familial disseminated atypical mycobacterial infection in chilhood: a human mycobacterial susceptibility gene? Lancet. 1995;345:79–83. doi: 10.1016/s0140-6736(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 21.Newport M, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. A mutation in the interferon-gamma receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 22.Peitsch M C. ProMod and Swiss-Model: internet-based tools for automated comparative protein modelling. Biochem Soc Trans. 1996;24:274–279. doi: 10.1042/bst0240274. [DOI] [PubMed] [Google Scholar]
- 23.Pfizenmaier K, Wiegmann K, Scheurich P, Krönke M, Merlin G, Aguet M, Knowles B B, Ucer U. High affinity human IFN-gamma-binding capacity is encoded by a single receptor gene located in proximity to c-ras on human chromosome region 6q16 to 6q22. J Immunol. 1998;141:856–860. [PubMed] [Google Scholar]
- 24.Regueiro J R, López-Botet M, Landázuri M O, Alcami J, Corell A, Martín-Villa J M, Vicario J L, Arnaiz-Villena A. An in vivo functional immune system lacking polyclonal T cell surface expression of the CD3/Ti (WT31) complex. Scand J Immunol. 1987;26:699–708. doi: 10.1111/j.1365-3083.1987.tb02306.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Gallego C, Corell A, Pacheco A, Timón M, Regueiro J R, Allende L M, Madroño A, Arnaiz-Villena A. Herpes virus saimiri transformation of T cells in CD3 γ immunodeficiency: phenotypic and functional characterization. J Immunol Methods. 1996;198:177–186. doi: 10.1016/s0022-1759(96)00156-1. [DOI] [PubMed] [Google Scholar]
- 26.Sakatsume M, Igarashi K, Winestock K D, Garotta G, Larner A C, Finblood D S. The Jak kinases differentially associate with the α and β (accessory factor) chains of the interferon γ receptor to form a functional receptor unit capable of activating STAT transcription factors. J Biol Chem. 1995;270:17528–17534. doi: 10.1074/jbc.270.29.17528. [DOI] [PubMed] [Google Scholar]
- 27.Soh J, Donnelly R J, Kotenko S, Mariano T M, Cook J R, Wang N, Emanuel S, Schwartz B, Miki T, Petska S. Identification and sequence of an accessory factor required for activation of the human interferon γ receptor. Cell. 1994;76:793–802. doi: 10.1016/0092-8674(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 28.Tortoli E, Besozzi G, Lacchini C, Penati V, Simonetti M T, Emler S. Pulmonary infection due to Mycobacterium szulgai, case report and review of the literature. Eur Respir J. 1998;11:975–977. doi: 10.1183/09031936.98.11040975. [DOI] [PubMed] [Google Scholar]
- 29.Walter M R, Windsor W T, Nagabhushan T L, Lundell D J, Lunn C A, Zauodny P J, Narula S K. Crystal structure of a complex between interferon-γ and its soluble high-affinity receptor. Nature. 1995;376:230–235. doi: 10.1038/376230a0. [DOI] [PubMed] [Google Scholar]
- 30.Yakovac W C, Baker R, Sweigert C, Hope J W. Fatal disseminated osteomyelitis due to an anonymous mycobacterium. J Pediatr. 1961;6:909–914. doi: 10.1016/s0022-3476(61)80322-3. [DOI] [PubMed] [Google Scholar]