Abstract
Objective
To assess whether supplementation with long chain n-3 fatty acids during pregnancy, lactation, or infancy reduces the risk of developing asthma or atopic disease during childhood.
Methods
Searches were performed in MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and Scopus up to 2021-09-20, for randomized controlled trials (RCTs) that investigated the effect of supplemental long chain n-3 fatty acids during pregnancy, lactation, or infancy for the prevention of childhood asthma or allergy. Article selection, data extraction, and risk of bias assessment (Cochrane’s Risk of Bias 2.0) were independently conducted by two assessors. The evidence was synthesized qualitatively according to the criteria of the World Cancer Research Fund and meta-analyzed.
Results
A total of nine RCTs met inclusion criteria; six were conducted during pregnancy, two during infancy, and one during both pregnancy and infancy. Meta-analysis showed that long chain n-3 fatty acid supplementation during pregnancy significantly reduced the risk of asthma/wheeze in the child (RR 0.62 [95% confidence interval 0.34–0.91], P = 0.005, I2 = 67.4%), but not other outcomes. Supplementation during lactation of infancy showed no effects on any outcome. The strength of evidence that long chain n-3 fatty acid supplementation during pregnancy reduces risk of asthma/wheeze in the offspring was considered limited – suggestive. No conclusion could be made for the effects of long chain n-3 fatty acid supplementation during pregnancy for other atopic diseases, or for supplementation during lactation or infancy for any outcome.
Conclusion
The intake of long chain n-3 fatty acid supplements during pregnancy may reduce the risk of asthma and/or wheeze in the offspring, but the strength of evidence is low. There is inconclusive evidence for the effects of long chain n-3 fatty acid supplements during pregnancy for other outcomes, as well as for supplementation during lactation or infancy.
Keywords: asthma, atopy, nutrition, fatty acids, omega 3
Popular scientific summary
This systematic review assessed whether the intake of omega three supplements during pregnancy, lactation, or infancy reduces atopic disease such as allergies or asthma in childhood.
The results show that omega three supplements during pregnancy may reduce the risk of asthma or asthma symptoms during childhood, but the evidence is limited.
No conclusions could be drawn for effects on other atopic diseases or for effects of supplementation during lactation of infancy.
More high-quality research is needed to clarify if and how omega three supplements during pregnancy or infancy reduce risk of asthma or allergies during childhood.
The prevalence of atopic disease, including both asthma and allergic asthma, has increased in recent decades in the Western world, and asthma prevalence is approximately 10% in adult Nordic populations (1, 2). Nordic countries also have some of the world’s highest disability-adjusted life-years lost from atopic dermatitis (3). The increase in prevalence of asthma and atopic diseases has been ascribed to changes in environmental exposures, including dietary intake (4, 5).
Early life nutrition, i.e. nutrition during pregnancy and infancy, is linked to chronic disease risk later in life (6) and may also impact the risk of atopic disease and asthma in children (7). Omega three (n-3) polyunsaturated fatty acids (PUFAs), especially the long-chain n-3 polyunsaturated fatty acids (LCn3PUFAs), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), are nutritional factors of specific interest due to their immunomodulatory properties, caused by altered synthesis of bioactive lipid mediators and cell membrane composition (8, 9).
Observational studies show disparate findings regarding association between fish consumption in pregnancy or childhood and risk of asthma or other atopic diseases during childhood (10–14). Both observational and interventional studies show unclear evidence for LCn3PUFA supplementation during pregnancy or infancy for the prevention of childhood atopic disease (15, 16). Use of n3-supplements differs between the Nordic countries and is reported by a majority of pregnant women in Norway (17) and Iceland (18) and less frequently in other countries (19–21). Based on the uncertainties regarding the available evidence and new data published, the Nordic Nutrition Recommendations (NNR) Committee recommended that the topic of LCn3PUFA supplementation during pregnancy, lactation, or infancy for the prevention of atopic disease in the offspring be shortlisted for a systematic review in preparation for the 2022 edition of NNR (22).
The aim of this systematic review was to assess whether supplementation with LCn3PUFA during pregnancy, lactation, or infancy reduces the risk of asthma or atopic disease during childhood.
Methods
This systematic review was conducted according to the guidelines for systematic reviews, developed for the 2022 revision of the NNR (23, 24) and Preferred reporting for systematic reviews (25). The NNR 2022 is funded by the Nordic Council of Ministers and governmental food and health authorities of Norway, Finland, Sweden, Denmark, and Iceland (26). A study protocol was published prior to article selection in the database PROSPERO (https://www.crd.york.ac.uk, CRD42021275309).
Eligibility criteria
The research question was specified by the NNR 2022 Committee and the NNR Systematic Review Center (i.e. the authors) by defining the population, intervention/exposure, control, timing, study design, and setting (PI/ECOTSS).
As outlined in the PI/ECOTSS in Table 1, the intervention included supplemental intake of LCn3PUFA during pregnancy, lactation, or infancy, with placebo or other oils as comparator. Outcomes should have been assessed at age 0–18 years and were the following: asthma and/or wheeze, allergy (either allergic rhinitis, allergic sensitization, or specific allergies), and atopic dermatitis or eczema. Only randomized control trials (RCTs), with a ≥4 weeks intervention duration, were eligible for inclusion.
Table 1.
Population, intervention/exposure, comparator, outcomes, timing, setting, and study designs (PI/ECOTSS) criteria for the papers to be included in the systematic review
| Population | Intervention or exposure | Comparators | Outcomes | Timing | Setting | Study design |
|---|---|---|---|---|---|---|
| Pregnant and lactating women and their offspring | Supplemental intake of long chain n-3 fatty acids (fish oil, tran and pure marine n-3) Intervention during: pregnancy, lactation, or infancy (0–12 months) |
Placebo or other oils | Asthma, wheeze, and allergies at 0–18 years of age To be included:
|
Minimum 4 week-intervention | Relevant for children and adolescents in the Nordic and Baltic countries | RCTs |
n-3: omega 3; RCTs: randomized controlled trials.
Search strategy
The literature searches were performed by research librarians from Karolinska Institute on 2021-09-20 in MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and Scopus. The search strategy (Supplement 1) was developed in collaboration with the authors and peer reviewed by university librarians from the University of Oslo. Reference lists of relevant retrieved articles were also screened to identify additional articles. The searches utilized no restrictions on publication dates or language. Grey literature and unpublished studies were not searched.
Article selection and data collection
Screening and selection of studies for inclusion/exclusion was performed by two authors (LB and CLA), working independently. The screening of titles and abstracts was performed in Rayyan (27). A pilot test was conducted using 10% of the titles and abstracts in order to harmonize the process. If at least one of the assessors voted for inclusion, a paper was selected for full text screening. Discrepancies were resolved by discussion with a third author (AÅ). Data from full-text papers included in the systematic review were extracted in standardized extraction forms by pairs of two authors working independently (LB, CLA, and FS).
Risk of bias assessment
Risk of bias of each included study was assessed by two authors (BN and JD) working independently. The assessment tool used was Cochrane’s Risk of bias 2 (28). The risk of bias in each individual study was classified as low, high, or ‘some concerns’ for risk of bias, per outcome and population.
Synthesis and strength of evidence
The evidence was synthesized qualitatively, based on study characteristics, context, strengths and limitations, heterogeneity, and relevance. In accordance with the guidelines for systematic reviews, meta-analyses were considered if deemed appropriate to combine the different studies, but only when more than three independent RCTs existed. We performed meta-analysis using a random-effects model to pool effect estimates from studies judged to be sufficiently homogeneous (regarding their clinical, methodological, and statistical aspects). Meta-analysis results are presented graphically in forest plots. Heterogeneity between effect sizes of included studies was assessed by visual inspection of forest plots and by using the Chi-square test for heterogeneity between studies, which was expressed as the percentage of the variability in effect estimates that is due to heterogeneity rather than chance (I2). In the meta-analysis, the effect estimates were included as originally reported. When results were reported at several time points, the last follow-up was chosen for the meta-analysis. When results were reported for outcomes both as ‘any’ (e.g. any asthma or any food reaction) and allergic or IgE associated (e.g. allergic asthma or IgE-associated food allergy), the outcomes associated with allergy or IgE associated were chosen. The meta-analysis was performed using Stata 14 (Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Strength of evidence was appraised based on risk of bias, consistency/heterogeneity and precision of the evidence, according to the World Cancer Research Fund’s grading: ‘Convincing’, ‘Probable’, ‘Limited – suggestive’, ‘Limited – no conclusion’, and ‘Substantial effects unlikely’ (26).
Results
A total of 1,127 unique articles were identified and 48 read in full text after screening of title and abstract (Figure 1). Excluded articles with reasons is shown in Supplement 2. A total of 18 articles reporting from nine RCTs were included in this review (Table 2). In six studies, the LCn3PUFA intervention was given during pregnancy (29–38). In two studies, LCn3PUFA intervention was given during infancy and early childhood (39–44). In one additional study, LCn3PUFA intervention was given both during pregnancy and infancy as maternal supplementation continued during lactation (45, 46). In five of the studies (31, 35, 39, 40, 45, 47), only infants or women carrying children with high risk of developing atopic disease due to heredity were included.
Fig. 1.
Prisma flow chart of the article selection process.
Table 2.
Description of the included studies
| Author, year (ref) Country | Intervention | N recruited (% lost to follow-up) | Intervention, daily intake | Control | Follow-up at age |
|---|---|---|---|---|---|
| Furuhjelm 2009 (45) Furuhjelm 2011 (46) Sweden |
Pregnancy and lactation (from GW 25) |
N = 145 (19%) N = 145 (18%) |
Capsules (4,500 mg) 35% EPA 25% DHA |
Soy oil | 1 years 2 years |
| Bisgaard 2016 (29) Denmark |
Pregnancy (from GW 22–26) |
N = 365 (5.6%) | Marine oil 2.4 g n-3 1.3 g EPA 0.9 g DHA |
Olive oil | 1–1.5, 3–5, 5 and 7 years |
| Olsen 2008 (30) Denmark | Pregnancy (from GW 30) |
N = 402 (<1%) | Fish oil capsules 2.7 g n-3 0.9 g EPA 0.6 g DHA |
Olive oil (3rd arm received nothing and are not included here) |
16 years |
| Dunstan 2003 (31) Australia | Pregnancy (from GW 20–30) |
N = 98 (15%) | Fish oil capsules 3.7 g n-3 1.0 g EPA 2.1 g DHA |
Olive oil | 1 years |
|
DOMInO study Palmer 2012 (32) Palmer 2013 (33) Best 2016 (34) Best 2018 (35) Australia |
Pregnancy (from GW 21) |
N = 706 (4%) N = 706 (10%) N = 706 (<1% in ITT) N = 706, cumulative analysis of previous studies |
Fish oil 0.9 g n-3 0.1 g EPA 0.8 g DHA |
Rapeseed, sunflower, and palm oil blend (1,500 mg/d) | 1 years 3 years 6 years 1–6 years |
| Imhoff-Kunsch 2011 (36)Escamilla-Nunez 2014 (37) Mexico | Pregnancy (from GW 18–22) |
N = 1,094 (22%) N = 1,094 (?%) |
Algae derived DHA 0.4 g DHA |
Corn and soy oil blend | 1–6 months 1.5 years |
| Berman 2016 (38) USA | Pregnancy (from GW 12–20) |
N = 126 (33%) | 1) EPA-rich fish oil (1.06 g EPA and 0.274 g DHA) 2) DHA rich fish oil (0.9 g DHA and 0.180 g EPA) |
Soy oil | 3 years |
|
CAPS study Mihrshahi 2003 (40) Peat 2004 (41) Marks 2006 (42) Toelle 2010 (43) Toelle 2013 (44) Australia |
Infancy (6 months–5 years) |
N = 616 (12%) N = 616 (15%) N = 616 (16%) N = 616 (27%) N = 616 (40%) |
Tuna fish oil (500 mg) plus canola-based oils and spreads) |
Sunola oil supplements plus oils and spreads low in n-3 fatty acids |
1.5 years 3 years 5 years 8 years 11.5 years |
| D’Vaz 2012 (39) Australia | Infancy (birth until 6 months) |
N = 420 (23%) | Fish oil (650 mg) 0.11 g EPA 0.28 g DHA |
Olive oil | 1 years |
GW: gestational week; n-3: omega 3; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid.
Interventions during pregnancy
In the Swedish study conducted during both pregnancy and during lactation (45, 46), Furuhjelm et al. randomized pregnant women to LCn3PUFA supplementation or soy oil placebo that continued throughout lactation. Among the six studies where the LCn3PUFA intervention was performed only during pregnancy, two were conducted in Denmark (29, 30), two in Australia (31–34), one in Mexico (36, 37), and one in the US (38). Interventions started in mid-pregnancy and continued throughout pregnancy. Doses of LCn3PUFA supplemental intake (either total n-3 or calculated sum of EPA and DHA) ranged from 0.4 to 3.7 g/day, DHA from 0.4 to 2.1 g/day, and EPA from 0 to 1.5 g/day. Placebo controls were vegetable oils (olive, soy, or blends) (Table 2).
Long chain n-3 fatty acid supplementation for the prevention of asthma or wheeze
Seven studies included asthma and/or wheeze as an outcome. The two studies conducted in Denmark (29, 30) both found a significant, protective effect of LCn3PUFA supplementation during pregnancy for asthma and/or persistent wheeze (Table 3). Bisgaard et al. found a ~30% reduced risk of asthma and/or persistent wheeze at 3–5 years of age, which persisted at 5 years and 5–7 years (29). Subgroup analyses showed that the effect was mainly seen among women with low EPA and DHA levels in blood at baseline (29). Olsen et al. found a ~60% reduced risk for any asthma and ~90% reduced risk of allergic asthma at 16 years of follow-up. Olsen et al. also found a non-significant protective effect for any asthma in the third arm receiving no oil compared to olive oil (HR [95% CI] 0.29 [0.08–1.03]) (30). The other individual studies found no significant effects on asthma and/or wheeze (31, 35, 38, 46). Meta-analysis of all seven studies (29–31, 35, 36, 38, 46) showed a reduction in the incidence of asthma or wheeze in the child from LCn3PUFA supplementation during pregnancy corresponding to RR 0.62 (95% CI 0.34–0.91, P = 0.005, I2 = 67.4%) (Figure 2). No dose–response analysis was conducted due to the limited number of studies.
Table 3.
Results for long chain n-3 supplementation interventions conducted during pregnancy
| Author, year (ref)Country | Asthma and/or wheeze | Eczema | Food allergy | Sensitization | Other outcomes |
|---|---|---|---|---|---|
| Furuhjelm 2009 (45) Furuhjelm 2011 (46) Sweden |
IgE-associated asthma: RR (95% CI)*: 1 year: N/A 0–2 years: 0.59 (0.11–3.11) Any asthma: RR (95% CI)*: 1 year: N/A 0–2 years: 1.05 (0.41–2.72) |
OR (95% CI): 1 year: 0.22 (0.06–0.81) 0–2 years: 0.33 (0.1–1.1) |
OR (95% CI): 1 year: 0.09 (0.01–0.74) 0–2 years: 0.26 (0.07–0.99) |
Any skin prick test OR (95% CI): 1 year: 0.36 (0.14–0.95) 0–2 years: 0.43 (0.17–1.10) IgE RR (95% CI)*: 1 year: 0.53 (0.24–1.20) 0–2 years: 0.75 (0.39–1.45) |
Any allergic disease OR (95% CI): 1 year: N/A 0–2 years: 0.29 (0.1–0.86) |
| Bisgaard 2016 (29) Denmark |
Persistent wheeze or asthma: HR (95% CI): 0–3 or 5 years: 0.69 (0.49–0.97) 0–5 years: 0.68 (0.49–0.95) 0–7 years: 0.66 (0.47–0.91) |
HR (95% CI): 0–3 or 5 years: 1.19 (0.89–1.57) 0–5 years: 1.10 (0.83–1.44) |
N/A | Any skin prick test OR (95% CI): 0.5–1.5 years: 1.34 (0.76–2.37) IgE OR (95% CI): 0.5–1.5 years: 1.72 (0.96–3.15) |
Allergic rhinoconjunctivitis OR (95% CI): 0–5 years: 0.70 (0.43–1.12) Lung function tests 0–5 years: all NS |
| Olsen 2008 (30) Denmark |
Allergic asthma 0–16 years HR (95% CI): 0.13 (0.03–0.60) Any asthma 0–16 years HR (95% CI): 0.37 (0.15–0.92) |
N/A | N/A | N/A | Allergic asthma, atopic dermatitis, or allergic rhinitis 0–16 years HR (95% CI): 0.31 (0.11–0.84) |
| Dunstan 2003 (31) Australia |
Asthma 1 year RR (95% CI)*: 0.36 (0.08–1.67) |
Atopic dermatitis 1 year RR (95% CI)*: 1.49 (0.84–2.63) |
Food allergy 1 year RR (95% CI)*: 0.65 (0.17–2.53) |
Any skin prick test 1 year RR (95% CI)*: 0.68 (0.34–1.37) |
N/A |
|
DOMInO Palmer 2012 (32) Palmer 2013 (33) Best 2016 (34) Best 2018 (35) Australia |
Asthma/wheeze with sensitization RR (95% CI) 1 year: N/A 1–3 years*: 1.10 (0.34 to 3.58) 6 years: N/A 0–6 years: 0.85 (0.62–1.17) |
Eczema with sensitization RR (95% CI) 1 year: 0.64 (0.40 to 1.03) 1–3 years: 0.75 (0.53–1.05) 6 years: 0.95 (0.59–1.53) 0–6 years: 0.77 (0.53–1.13) |
Food allergy with sensitization RR (95% CI) 1 year: 0.96 (0.41 to 2.25) 1–3 years: 1.25 (0.63–2.49) 6 years: N/A 0–6 years: N/A |
Sensitization RR (95% CI) 1 year: 0.75 (0.53 to 1.04) 1–3 years: 0.85 (0.68–1.06) 6 years: 0–6 years: 0.97 (0.82–1.15) |
Allergic disease with sensitization RR (95% CI) 1 year: 0.70 (0.45 to 1.09) 1–3 years: 0.78 (0.58–1.06) 6 years: 1.04 (0.82–1.33) 0–6 years: 0.88 (0.69, 1.12) Allergic rhinitis with sensitization RR (95% CI) 1 year: N/A 1–3 years: 0.82 (0.43–1.53) 6 years: 0.98 (0.72–1.35) 0–6 years: 0.86 (0.63, 1.16) Rhino-conjunctivitis with sensitization: RR (95% CI) 1 year: N/A 1–3 years: N/A 6 years: 1.12 (0.72–1.73) 0–6 years: 0.81 (0.55, 1.21) |
| Imhoff-Kunsch 2011 (36) Escamilla-Nunez 2014 (37) Mexico |
Wheezing OR (95% CI) 0.5 year: 1.11 (0.72–1.70) IRR (95% CI) 1.5 years, maternal atopy: 0.88 (0.64–1.21) 1.5 years, non-maternal atopy: 1.03 (0.83–1.28) |
N/A | N/A | N/A | N/A |
| Berman (38) USA |
Asthma/wheeze 3 years RR (95% CI)* DHA: 0.59 (0.20–1.79) EPA: 1.12 (0.48–2.60) |
Eczema 3 years OR (95% CI) DHA: 9.5 (1.5–59.6) EPA: 8.1 (1.4–46.3) |
Food allergy 3 years RR (95% CI)* 1.73 (0.46–6.52) |
N/A | N/A |
Calculated from reported n of events.
IgE: immunoglobulin E; RR: risk ratio; CI: confidence interval; y: years; OR: odds ratio; HR: hazard ratio; n-3: omega 3; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid.
Fig. 2.
Random effects meta-analyses of long chain omega 3 supplementation during pregnancy on the risk of offspring asthma and/or wheeze.
Long chain n-3 supplementation for the prevention of eczema or atopic dermatitis
Five studies included eczema or atopic dermatitis as an outcome. Furuhjelm et al. found that LCn3PUFA supplementation resulted in reduced risk of IgE-associated eczema at both 1 year (45) and during the first 2 years of childhood (P = 0.06 in adjusted analyses) (46). In contrast, Berman et al. found a significantly higher prevalence of eczema from LCn3PUFA supplements rich in either EPA or DHA (38). No other study found any effect of LCn3PUFA supplementation on the risk of eczema in adjusted analyses (29, 31, 35). Meta-analysis of all five studies (29, 31, 35, 38, 46) showed no significant effect of LCn3PUFA supplementation during pregnancy for eczema/atopic dermatitis in the child (RR 0.86 [95% CI 0.50–1.22], P = 0.055, I2 = 56.9%) (Figure 3).
Fig. 3.
Random effects meta-analyses of long chain omega 3 supplementation during pregnancy on the risk of offspring eczema or atopic dermatitis.
Long chain n-3 fatty acid supplementation for the prevention of food allergy
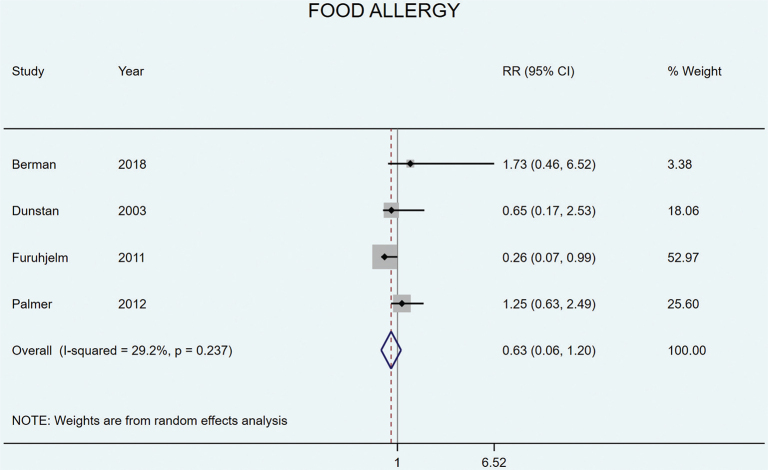
Four studies included food allergy as an outcome. Furuhjelm et al. found that LCn3PUFA supplementation resulted in reduced risk of food allergy at both 1 year (45) and during the first 2 years of childhood (46). None of the other studies found any effects from LCn3PUFA supplementation on food allergy (31, 33, 38). Meta-analysis of all four studies (31, 33, 38, 46) showed no significant effect from LCn3PUFA supplementation on food allergy (RR 0.63 [95% CI 0.06–1.20], P = 0.237, I2 = 29.2%) (Figure 4).
Fig. 4.
Random effects meta-analyses of long chain omega 3 supplementation during pregnancy on the risk of offspring food allergy.
Long chain n-3 fatty acid supplementation for the prevention of allergic sensitization
Four studies included allergic sensitization or atopy (defined as positive skin prick test or IgE) as an outcome; none found any significant effects from LCn3PUFA supplementation in adjusted analyses (31, 35, 38, 46). Meta-analysis showed no significant effect on sensitization or atopy (RR 0.82 [95% CI 0.51–1.14], P = 0.091, I2 = 53.6%) (Figure 5).
Fig. 5.
Random effects meta-analyses of long chain omega 3 supplementation during pregnancy on the risk of offspring atopy/sensitization.
Long chain n-3 fatty acid supplementation for the prevention of other atopic outcomes
No study found any significant effect from LCn3PUFA supplementation on allergic rhinoconjunctivitis (29, 35, 46) or allergic rhinitis (35). As <3 studies included these outcomes, no meta-analyses were conducted.
Interventions during infancy
In two studies, the LCn3PUFA intervention was given during infancy; both conducted in Australia (39–44). D’Vaz et al. randomized 420 infants to receive either fish oil (650 mg/d, providing 280 mg DHA and 110 mg EPA) or olive oil control (650 mg/d) from birth to 6 months of age. When the children were 1 year old, LCn3PUFA supplementation showed no effect on incidence of allergic disease, eczema, food allergy, or sensitization (39) (Table 3). In the CAPS study, 616 infants were randomized to receive either tuna fish oil intervention (500 mg/d) or soy oil control from age 6 months (or at onset of bottle feeding) until 5 years. Half of the participants were also randomized to house dust mite avoidance. LCn3PUFA supplementation resulted in reduction incidence of wheeze at 1.5 years (40), but not at 3 years (41), 8 years (43), or 11.5 years (44). There were no reductions in incidence of asthma, eczema, atopy/sensitization, or rhinitis at any time point of follow-up (40–44).
Table 4.
Results for long chain n-3 supplementation interventions conducted during infancy
| Author, year (ref)Country | Asthma | Wheeze | Eczema | Food allergy | Sensitization | Other outcomes |
|---|---|---|---|---|---|---|
|
CAPS study Mihrshahi 2003 (40) Peat 2004 (41) Marks 2006 (42) Toelle 2010 (43) Toelle 2013 (44) Australia |
Abs RR (95% CI): 1.5 years: 3 years: NS 5 years: 0.9 (0.65–1.24) 8 years: –4.8 (–12.5–2.9) 11.5 years: 0.5 (–8.0–8.9) |
Abs RR (95% CI): 1.5 years: 9.8 (1.5–18.1)% difference in events 3 years: NS 5 years: 8 years: –8.6 (16.8–0.4) 11.5 years: –1.1 (–9.9–7.8) |
Abs RR (95% CI): 1.5 years: 0.6 (–5.7–6.9)% difference in events 3 years: NS 5 years: 1.39 (1.0–1.93) 8 years: –1.1 (–7.8–5.6) 11.5 years: 2.8 (–5.9–11.3) |
Abs RR (95% CI): 1.5 years: 3 years: 1.6% (NS) 5 years: 8 years: 11.5 years: |
Abs RR (95% CI): 1.5 years: 2.9 (–3.9–9.5)% difference in events 3 years: 5 years: 0.9 (0.74–1.1) 8 years: –0.2 (–9.9–9.6) 11.5 years: 12.4 (1.0–23.7) |
Rhinitis Abs RR (95% CI): 1.5 years: 3 years: 5 years: 1.08 (0.88–1.33) 8 years: –0.9 (–9.4–7.6) 11.5 years: 7.3 (–2.6–17.2) |
| D’Vaz 2012 (39) Australia | N/A | OR (95% CI): 1 year: 1.51 (0.75–3.04)* |
OR (95% CI): 1 year: 0.95 (0.6–1.5)* |
OR (95% CI): 1 year: 0.78 (0.41.5)* |
OR (95% CI): 1 year: 1.01 (0.62–1.65)* |
Any allergic disease OR (95% CI): 1 year: 0.94 (0.60–1.47)* |
Estimated from graph.
Abs RR: absolute risk ratio; CI: confidence interval; y: years.
Study quality and strength of evidence
For interventions performed during pregnancy, risk of bias was considered as low for two studies (30, 32, 33), while most studies were considered to have some concerns regarding risk of bias (29, 31, 34, 36–38, 45, 46) (Figures 6 and 7). Risk of bias assessment for Best et al. 2018 (35) was not conducted as it did not include any new follow-up, only a cumulative summary of the previous papers that were assessed for risk of bias (32–34). For interventions during infancy, risk of bias was considered high for the IFOS study, due to concerns regarding missing outcome data (39). For the CAPS study, risk of bias was considered as having some concerns for the earlier follow-ups (40–42) and high for the later follow-ups (43, 44).
Fig. 6.
Traffic plot of the risk of bias assessment for each study, per domain and overall.
Fig. 7.
Summary plot of the risk of bias assessment, per domain and overall.
Strength of evidence that LCn3PUFA supplementation during pregnancy reduces risk of asthma and/or wheeze in the offspring was considered Limited-suggestive (low), due to risk of bias, some unexplained heterogeneity in results, definitions of outcomes, and given interventions. Evidence that the LCn3PUFA supplementation during pregnancy reduces risk of eczema, food allergy, allergic sensitization, or other atopic outcomes in the offspring was considered Limited-inconclusive (low) due to large unexplained heterogeneity in results and low precision in the effect estimates. Strength of evidence that LCn3PUFA supplementation during infancy (either given directly to the infant or to the lactating mother) reduces risk of asthma/wheeze, eczema, allergy, or atopy was considered Limited – no conclusion (insufficient).
Discussion
The results of this systematic review of RCTs show that LCn3PUFA supplementation during pregnancy might reduce the risk of asthma and/or wheeze in the offspring, but evidence for other atopic conditions was insufficient. Supplementation during infancy did not yield any clear effects.
The current results are comparable to those of previous systematic reviews, concluding that fish oil or LCn3PUFA supplementation given during pregnancy, lactation, or in infancy may not reduce allergy or atopic disease (48–50). We did, however, find low-quality evidence for a protective effect for childhood asthma and/or wheeze, in line with findings from other recent systematic reviews (16, 51, 52). Earlier reviews finding no such effects were published in 2014–2016 after which new data have been published (29, 34, 38). Still, the strength of evidence is considered low, due to study heterogeneity and risk of bias.
The results of this systematic review show that the suggestive protective effects of LCn3PUFA supplementation during pregnancy are limited to asthma and/or wheeze and not other atopic conditions. In the COPSAC study, a significantly reduced risk of asthma and/or recurrent wheeze from LCn3PUFA supplementation was found, but no effect on lung function or asthma exacerbations. However, LCn3PUFA supplementation reduced the risk of lower respiratory tract infections (29), an outcome outside the scope of this systematic review. The here included study conducted in Mexico also found lower risk of symptoms of common cold at 1 month and until 18 months of age (36, 37). It is possible that LCn3PUFA supplementation reduces risk of asthma/wheeze through a reduction in risk of respiratory infections that may both induce and exacerbate asthma (48). It is noteworthy that most studies were conducted among children with a high risk of developing atopic disease, and studies in the general population may result in different findings. In addition, definitions of the outcome asthma and/or wheeze differed between studies and included a combination of asthma, allergic asthma, and wheeze. Classification of asthma and/or wheeze was performed using different assessments such as clinical diagnosis, questionnaires and symptoms diaries, and at different ages. Initially, we planned to only include outcomes based on doctors’ diagnosis, but since very few studies applied this criterion, and diagnosis of certain outcomes (e.g. asthma) in small children is difficult, this was discarded. Taken together, more RCT studies are required that use clearly defined endpoints and valid assessments to clarify the role of LCn3PUFA supplementation during pregnancy for the prevention of childhood asthma.
This systematic review has both strengths and limitations. Strengths include the methodology with two separate co-authors doing article selection, data extraction, and risk of bias assessment. There was also a fair amount of data for both meta-analyses and evidence synthesis. However, the number of included studies were too few to conduct analyses of publication bias and for subgroup or sensitivity analyses. Limitations include heterogeneity in both interventions, follow-up, and in reporting of data in the original studies and, therefore, also in the current paper (e.g. doses, follow-up duration, and classification of outcomes).
In conclusion, the intake of LCn3PUFA supplements during pregnancy may reduce the risk of asthma and/or wheeze in the offspring, but the strength of evidence is low. There is inconclusive evidence for the effects of LCn3PUFA supplements during pregnancy for other atopic outcomes as well as for supplementation during lactation or infancy.
Supplementary Material
Acknowledgments
The authors would like to thank university librarians Sabina Gillsund and Narcisa Hannerz at Karolinska Institutet for their invaluable assistance with the literature searches, and the university librarians at the University of Oslo for peer reviewing the search strategy.
Conflict of interest and funding
Funding was received from the Nordic Council of Ministers and governmental food and health authorities of Norway, Finland, Sweden, Denmark, and Iceland. The authors declare no potential conflicts of interest.
References
- 1.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012; 12: 204. doi: 10.1186/1471-2458-12-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backman H, Räisänen P, Hedman L, Stridsman C, Andersson M, Lindberg A, et al. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin Exp Allergy 2017; 47(11): 1426–35. doi: 10.1111/cea.12963 [DOI] [PubMed] [Google Scholar]
- 3.Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol 2021; 184(2): 304–9. doi: 10.1111/bjd.19580 [DOI] [PubMed] [Google Scholar]
- 4.Allan K, Devereux G. Diet and asthma: nutrition implications from prevention to treatment. J Am Diet Assoc 2011; 111(2): 258–68. doi: 10.1016/j.jada.2010.10.048 [DOI] [PubMed] [Google Scholar]
- 5.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol 2006; 6(11): 869–74. doi: 10.1038/nri1958 [DOI] [PubMed] [Google Scholar]
- 6.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 2001; 60: 5–20. doi: 10.1093/bmb/60.1.5 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Larsen V, Ierodiakonou D, Jarrold K, Cunha S, Chivinge J, Robinson Z, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med 2018; 15(2): e1002507. doi: 10.1371/journal.pmed.1002507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles EA, Calder PC. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients 2017; 9(7): 784. doi: 10.3390/nu9070784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miles EA, Childs CE, Calder PC. Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: a narrative review. Nutrients 2021; 13(1): 247. doi: 10.3390/nu13010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ. Consumption of oily fish and childhood asthma risk. Med J Aust 1996; 164(3): 137–40. doi: 10.5694/j.1326-5377.1996.tb122010.x [DOI] [PubMed] [Google Scholar]
- 11.Stratakis N, Roumeliotaki T, Oken E, Ballester F, Barros H, Basterrechea M, et al. Fish and seafood consumption during pregnancy and the risk of asthma and allergic rhinitis in childhood: a pooled analysis of 18 European and US birth cohorts. Int J Epidemiol 2017; 46(5): 1465–77. doi: 10.1093/ije/dyx007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salam MT, Li YF, Langholz B, Gilliland FD. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma 2005; 42(6): 513–18. doi: 10.1081/JAS-200067619 [DOI] [PubMed] [Google Scholar]
- 13.Papamichael MM, Shrestha SK, Itsiopoulos C, Erbas B. The role of fish intake on asthma in children: a meta-analysis of observational studies. Pediatr Allergy Immunol 2018; 29(4): 350–60. doi: 10.1111/pai.12889 [DOI] [PubMed] [Google Scholar]
- 14.Maslova E, Strøm M, Oken E, Campos H, Lange C, Gold D, et al. Fish intake during pregnancy and the risk of child asthma and allergic rhinitis – longitudinal evidence from the Danish National Birth Cohort. Br J Nutr 2013; 110(7): 1313–25. doi: 10.1017/S000711451300038X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venter C, Agostoni C, Arshad SH, Ben-Abdallah M, Du Toit G, Fleischer DM, et al. Dietary factors during pregnancy and atopic outcomes in childhood: a systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr Allergy Immunol 2020; 31(8): 889–912. doi: 10.1111/pai.13303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Zhang Y, Zhu X, Wang D, Dai J. Effects of supplementation with omega-3 fatty acids during pregnancy on asthma or wheeze of children: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2020; 33(10): 1792–801. doi: 10.1080/14767058.2018.1529161 [DOI] [PubMed] [Google Scholar]
- 17.Brantsæter AL, Englund-Ögge L, Haugen M, Birgisdottir BE, Knutsen HK, Sengpiel V, et al. Maternal intake of seafood and supplementary long chain n-3 poly-unsaturated fatty acids and preterm delivery. BMC Pregnancy Childbirth 2017; 17(1): 41. doi: 10.1186/s12884-017-1225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tryggvadottir EA, Halldorsson TI, Birgisdottir BE, Hrolfsdottir L, Landberg R, Hreidarsdottir IT, et al. [Correlation between intake of fish or supplements containing omega-3 fatty acids and early pregnancy plasma concentrations.]. Laeknabladid 2022; 108(5): 238–43. [DOI] [PubMed] [Google Scholar]
- 19.Bärebring L, Mullally D, Glantz A, Elllis J, Hulthen L, Jagner A, et al. Sociodemographic factors associated with dietary supplement use in early pregnancy in a Swedish cohort. Br J Nutr 2018; 119(1): 90–5. doi: 10.1017/S0007114517003270 [DOI] [PubMed] [Google Scholar]
- 20.Arvizu M, Afeiche MC, Hansen S, Halldorsson TF, Olsen SF, Chavarro JE. Fat intake during pregnancy and risk of preeclampsia: a prospective cohort study in Denmark. Eur J Clin Nutr 2019; 73(7): 1040–8. doi: 10.1038/s41430-018-0290-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nwaru BI, Erkkola M, Lumia M, Kronberg-Kippilä C, Ahonen S, Kaila M, et al. Maternal intake of fatty acids during pregnancy and allergies in the offspring. Br J Nutr 2012; 108(4): 720–32. doi: 10.1017/S0007114511005940 [DOI] [PubMed] [Google Scholar]
- 22.Høyer A, Christensen JJ, Arnesen EK, Andersen R, Eneroth H, Erkkola M, et al. The Nordic nutrition recommendations 2022 – prioritisation of topics for de novo systematic reviews. Food Nutr Res. 2021; 65: 7828. doi: 10.29219/fnr.v65.7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnesen EK, Christensen JJ, Andersen R, Eneroth H, Erkkola M, Høyer A, et al. The Nordic nutrition recommendations 2022 – structure and rationale of qualified systematic reviews. Food Nutr Res. 2020; 64: 4403; doi: 10.29219/fnr.v64.4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnesen EK, Christensen JJ, Andersen R, Eneroth H, Erkkola M, Høyer A, et al. The Nordic nutrition recommendations 2022 – handbook for qualified systematic reviews. Food Nutr Res. 2020; 64: 4404 doi: 10.29219/fnr.v64.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen JJ, Arnesen EK, Andersen R, Eneroth H, Erkkola M, Høyer A, et al. The Nordic nutrition recommendations 2022 – principles and methodologies. Food Nutr Res 2020; 64: 4402; doi: 10.29219/fnr.v64.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan – a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: 14898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 29.Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos AM, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med 2016; 375(26): 2530–9. doi: 10.1056/NEJMoa1503734 [DOI] [PubMed] [Google Scholar]
- 30.Olsen SF, Østerdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr 2008; 88(1): 167–75. doi: 10.1093/ajcn/88.1.167 [DOI] [PubMed] [Google Scholar]
- 31.Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 2003; 112(6): 1178–84. doi: 10.1016/j.jaci.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 32.Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. BMJ 2012; 344: e184. doi: 10.1136/bmj.e184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy 2013; 68(11): 1370–6. doi: 10.1111/all.12233 [DOI] [PubMed] [Google Scholar]
- 34.Best KP, Sullivan T, Palmer D, Gold M, Kennedy DJ, Martin J, et al. Prenatal fish oil supplementation and allergy: 6-year follow-up of a randomized controlled trial. Pediatrics 2016; 137(6): e20154443. doi: 10.1542/peds.2015-4443 [DOI] [PubMed] [Google Scholar]
- 35.Best KP, Sullivan TR, Palmer DJ, Gold M, Martin J, Kennedy D, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood – a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J 2018; 11(1): 10. doi: 10.1186/s40413-018-0190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imhoff-Kunsch B, Stein AD, Martorell R, Parra-Cabrera S, Romieu I, Ramakrishnan U. Prenatal docosahexaenoic acid supplementation and infant morbidity: randomized controlled trial. Pediatrics 2011; 128(3): e505–12. doi: 10.1542/peds.2010-1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Escamilla-Nuñez MC, Barraza-Villarreal A, Hernández-Cadena L, Navarro-Olivos E, Sly PD, Romieu I. Omega-3 fatty acid supplementation during pregnancy and respiratory symptoms in children. Chest 2014; 146(2): 373–82. doi: 10.1378/chest.13-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman D, Clinton C, Limb R, Somers EC, Romero V, Mozurkewich E. Prenatal omega-3 supplementation and eczema risk among offspring at age 36 months. Insights Allergy Asthma Bronchitis 2016; 2(1): 1. doi: 10.21767/2471-304X.100014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Vaz N, Meldrum SJ, Dunstan JA, Martino D, McCarthy S, Metcalfe J, et al. Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics 2012; 130(4): 674–82. doi: 10.1542/peds.2011-3104 [DOI] [PubMed] [Google Scholar]
- 40.Mihrshahi S, Peat JK, Marks GB, Mellis CM, Tovey ER, Webb K, et al. Eighteen-month outcomes of house dust mite avoidance and dietary fatty acid modification in the childhood asthma prevention study (CAPS). J Allergy Clin Immunol 2003; 111(1): 162–8. doi: 10.1067/mai.2003.36 [DOI] [PubMed] [Google Scholar]
- 41.Peat JK, Mihrshahi S, Kemp AS, Marks GB, Tovey ER, Webb K, et al. Three-year outcomes of dietary fatty acid modification and house dust mite reduction in the childhood asthma prevention study. J Allergy Clin Immunol 2004; 114(4): 807–13. doi: 10.1016/j.jaci.2004.06.057 [DOI] [PubMed] [Google Scholar]
- 42.Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol 2006; 118(1): 53–61. doi: 10.1016/j.jaci.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 43.Toelle BG, Ng KK, Crisafulli D, Belousova EG, Almqvist C, Webb K, et al. Eight-year outcomes of the childhood asthma prevention study. J Allergy Clin Immunol 2010; 126(2): 388–9, 9 e1–3. doi: 10.1016/j.jaci.2010.04.031 [DOI] [PubMed] [Google Scholar]
- 44.Toelle BG, Garden FL, Ng KK, Belousova EG, Almqvist C, Cowell CT, et al. Outcomes of the childhood asthma prevention study at 11.5 years. J Allergy Clin Immunol 2013; 132(5): 1220–2.e3. doi: 10.1016/j.jaci.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 45.Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Böttcher MF, Fälth-Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr 2009; 98(9): 1461–7. doi: 10.1111/j.1651-2227.2009.01355.x [DOI] [PubMed] [Google Scholar]
- 46.Furuhjelm C, Warstedt K, Fagerås M, Fälth-Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol 2011; 22(5): 505–14. doi: 10.1111/j.1399-3038.2010.01096.x [DOI] [PubMed] [Google Scholar]
- 47.Mihrshahi S, Peat JK, Webb K, Tovey ER, Marks GB, Mellis CM, et al. The childhood asthma prevention study (CAPS): design and research protocol of a randomized trial for the primary prevention of asthma. Control Clin Trials 2001; 22(3): 333–54. doi: 10.1016/S0197-2456(01)00112-X [DOI] [PubMed] [Google Scholar]
- 48.Darveaux JI, Lemanske RF, Jr. Infection-related asthma. J Allergy Clin Immunol Pract 2014; 2(6): 658–63. doi: 10.1016/j.jaip.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newberry SJ, Chung M, Booth M, Maglione MA, Tang AM, O’Hanlon CE, et al. Omega-3 fatty acids and maternal and child health: an updated systematic review. Evid Rep Technol Assess 2016; (224): 1–826. doi: 10.23970/AHRQEPCERTA224 [DOI] [PubMed] [Google Scholar]
- 50.Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev 2015; 2015(7): CD010085. doi: 10.1002/14651858.CD010085.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu S, Li C. Influence of maternal fish oil supplementation on the risk of asthma or wheeze in children: a meta-analysis of randomized controlled trials. Front Pediatr 2022; 10: 817110. doi: 10.3389/fped.2022.817110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia Y, Huang Y, Wang H, Jiang H. A dose-response meta-analysis of the association between the maternal omega-3 long-chain polyunsaturated fatty acids supplement and risk of asthma/wheeze in offspring. BMC Pediatr 2022; 22(1): 422. doi: 10.1186/s12887-022-03421-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.