Abstract
Wet distiller grains (WDG) are a corn by-product rich in protein and fiber that can be used in feedlot diets. This study evaluated F1 Angus-Nellore bulls fed on a control diet vs. WDG (n = 25/treatment). After a period of 129 days on these feeds, the animals were slaughtered and Longissimus thoracis samples were collected for both a meat quality evaluation and gel-based proteomic analyses. A greater ribeye area (99.47 cm²) and higher carcass weight (333.6 kg) (p < 0.05) were observed in the WDG-finished cattle compared to the control (80.7 cm²; 306.3 kg). Furthermore, there were differences (p < 0.05) in the intramuscular fat between the WDG and control animals (IMF = 2.77 vs. 4.19%), which led to a significant decrease (p < 0.05) in saturated fatty acids (FA). However, no differences (p > 0.10) were observed in terms of tenderness, evaluated using Warner–Bratzler shear force (WBSF). The proteomic and bioinformatic analyses revealed substantial changes in the biological processes, molecular functions, and cellular components of the WDG-finished cattle compared to the control. Proteins related to a myriad of interconnected pathways, such as contractile and structural pathways, energy metabolism, oxidative stress and cell redox homeostasis, and transport and signaling. In this experiment, the use of WDG supplementation influenced the protein expression of several proteins, some of which are known biomarkers of beef quality (tenderness and color), as well as the protein–protein interactions that can act as the origins of increases in muscle growth and reductions in IMF deposition. However, despite the effects on the proteome, the tenderness, evaluated by WBSF, and fatty acid profile were not compromised by WDG supplementation.
Keywords: beef quality, cattle, feedlot, proteome, mass spectrometry, nutrigenomics, skeletal muscle
1. Introduction
Wet corn distiller grains (WDG) have been extensively used for cattle feeding, especially in the USA, for more than 30 years [1]. The inclusion of WDG in feedlot diets is used strategically by feedlot owners who seek ingredients from the agroindustry in an attempt to reduce costs [2,3]. In the mid-west region of Brazil, where ethanol is produced from corn, the number of feedlot owners using this ingredient for feedlot cattle has been increasing.
Replacing corn/soybean meal ingredients with distiller grains can lead to a reduction in dietary starch levels, as well as changes in dietary protein degradability [4]. Corn WDG is an ingredient rich in rumen undegradable protein, and an earlier study [5] suggested that an increased protein supply to the intestine may stimulate pancreatic amylase secretion, hence increasing post-ruminal starch digestion. In this context, the use of distiller grains as an energy source in cattle feedlots represents a major paradigm shift, since these grains a protein feed [6].
Earlier studies evaluated the effects of high-concentrate (80%) diets containing increasing levels of WDG (0%, 30% or 60%) in Simmental-Angus steers [7]. The authors observed that traits such as the carcass yield, hot carcass weight, and subcutaneous fat thickness responded positively to the inclusion of 30% WDG, while the inclusion of 60% had a negative impact on these carcass traits. Similarly, a study of the effect of the inclusion of 15% dried distiller grains with solubles (DDGS) in the feedlot diet on the carcass finishing traits and beef lipid profile in Holstein steers was reported [8]. The study reported no difference in the quantity of subcutaneous fat between animals that received DDGS and the control. The fatty acid profile of the beef was also not affected. However, these authors did not describe the effects of distiller grains on the molecular changes at the muscle proteome level [7,8] and its association with the carcass and meat quality traits.
Studies have investigated the molecular alterations associated with beef quality traits such as tenderness and marbling [9,10]. However, few have evaluated the biochemical and molecular mechanisms that regulate the tenderness and marbling of meat from cattle fed a feedlot diet containing WDG. Such an evaluation of the bovine muscle tissue proteome is of great importance for the meat industry in order to add value to the end product [11,12]. Furthermore, there are a limited number of proteomic studies involving feedlot-finished, non-castrated crossbred males [13,14]. This biological type is increasingly being used in the tropical regions of Brazil, especially for F1 Angus-Nellore bulls, whose producers seek to satisfy more demanding consumer markets that require products with greater added value in terms of their sensory traits, especially meat tenderness and juiciness. Therefore, there is a growing expectation placed on the meatpacking industry regarding this genotype for the production of higher quality meat, as well as the use of by-products such as WDG by the producers of feedlot cattle. With the objective of better understanding the impacts of such supplementations and feeding methods on meat quality variation and muscle proteome changes, this study aimed to evaluate the meat quality and muscle tissue proteome of crossbred cattle fed on a feedlot diet containing WDG.
2. Materials and Methods
The animal study protocol was approved by Ethics Committee (CEUA) of the São Paulo State University “Júlio de Mesquita Filho”, UNESP, Campus of Botucatu (protocol number 0067/2017).
In this trial, F1 Angus-Nellore bulls (n = 50) from a commercial herd with an average initial body weight of 369.58 ± 49.17 kg, aged 20–24 months, were used. The animals were kept in collective pens (5 × 6 m, capacity of five animals per pen) with concrete a floor, equipped with a shell-type water trough. The pens were randomly allocated one of two treatments (25 animals/treatment), which consisted of a control (0%) vs. low-fat corn WDG (45%, DM basis). Feedlot diets (Table S1) include roughage (Tifton hay and sugarcane bagasse) and concentrate ingredients (ground dry corn grain, soybean meal, corn WDG, mineral core). The low-fat WDG used in the current study was produced by the same ethanol industry (SJC Bioenergia, Quirinópolis, Goiás, Brazil). The animals received the experimental diets ad libitum twice a day (10 a.m. and 4 p.m.) for 129 days and reached an average final body weight of 615.09 ± 57.53 kg.
2.1. Slaughtering, Sampling, and Carcass Quality Trait Evaluation
The animals were stunned by brain concussion with a captive dart pistol, followed by bleeding, hide removal, and evisceration. Subsequently, the carcasses were divided longitudinally, and samples (approximately 10 g) of the Longissimus thoracis (LT) muscle were collected from the left half of the carcass (approximately 10 min after slaughter) and stored using liquid nitrogen. These samples were then stored at −80 °C until molecular the biology and proteomic analysis.
The carcasses were weighed and cooled at 2 to 4 °C for 48 h. The hot carcass weight (HCW), cold carcass weight (CCW), backfat thickness (BFT, mm), and ribeye area (REA, cm2) at the 12th/13th rib interface were measured. Next, meat samples were collected from the LT muscle during deboning for the meat trait evaluation after three aging periods. All meat samples were collected between the 11th and 13th ribs (cranial direction).
2.2. Aging, Chemical Composition, and Marbling Score Determination of the Meat Samples
The steaks (2.54 cm thickness) were vacuum-packed in polyethylene bags and kept in a refrigerator for 3, 10, and 17 days post-mortem. Briefly, the samples were aged in a refrigerated BOD incubator (TE-371, TECNAL, Piracicaba, São Paulo, Brazil) at 0 to 2 °C in special plastic bags to ensure high vacuum conditions and a low oxygen permeability. The chemical composition was analyzed by infrared spectroscopy using a FoodScanTM (Foss NIRSystems, Laurel, MD, USA). The intramuscular fat (IMF) content was determined following previous procedures [15]. Additionally, the IMF was quantified chemically using a gravimetric method, as described previously [16]. Visual marbling scores were determined by two trained panelists for each slice of steak, using official USDA marbling photographs (prepared by the National Cattlemen Beef Association) as a reference [17]. The marbling categories were 1 = devoid, 2 = practically devoid, 3 = traces, 4 = slight, 5 = small, 6 = modest, 7 = moderate, 8 = slightly abundant, and 9 = moderately abundant. However, in the current study, this scale ranged from zero (devoid) to six (modest) because of the low degree of marbling.
2.3. Shear Force, Cooking Loss, and Myofibrillar Proteolysis
The Warner–Bratzler shear force (WBSF) and cooking loss (CL) were measured following each aging time (3, 10, and 17 days post-mortem). The recommendations of the American Meat Science Association were followed [18]. The samples were placed on a grid coupled with a glass refractory. A thermocouple connected to a digital thermometer (DT-612, ATP Instrumentation, Ashby-de-la-Zouch, UK) was used, which was inserted into the center of each sample to monitor the internal end-point temperature.
The steaks were cooked in an industrial electric oven (Feri90, Venâncio Aires, Rio Grande do Sul, Brazil) at 170 °C. Once the internal temperature of the steaks reached 40 °C, they were turned over and remained in the oven until the final temperature reached 71 °C. Subsequently, the beef samples were then kept at room temperature for 15 min, weighed, and refrigerated at 4 °C for 24 h.
The CL was divided into the evaporation loss (EL) and drip loss (DL), determined as percentages. The DL was obtained by weighing, using only the refractory, before and after cooking the sample. The EL was obtained by weighing the sample before and after cooking.
For the determination of the WBSF, cylinders with a diameter of 1.27 cm were applied parallel to the muscle fiber, with a hollow punch coupled with an industrial drill. Eight cylinders of each sample were sheared (perpendicular to the fiber direction) using a Brookfield CT-3 Texture Analyzer (AMETEK Brookfield, Middleborough, MA, USA), equipped with a stainless steel 3.07 mm-thick Warner–Bratzler blade with a V-shaped (60° angle) cutting edge [19]. The results were reported as the average of eight values per sample in kilograms (kg).
Post-mortem proteolysis was estimated by determining the myofibril fragmentation index (MFI), as previously described [20], and adapted for Bos indicus cattle [21].
2.4. Fatty Acid Profile
Additional LT samples were used to extract IMF and were subsequently used for the fatty acid (FA) profile analysis by gas chromatography. The IMF was extracted according to the procedures described in [22], and the samples were converted into fatty acid esters, as described in [23]. The transmethylated samples were analyzed using a gas chromatograph (Focus GC-Finnigan, Thermo Finnigan, Milan, Italy) equipped with a flame ionization detector and a CP-Sil 88 capillary column (Varian; 100 m long, 0.25 µm internal diameter, and film thickness of 0.20 µm). Hydrogen was used as the carrier gas at a flow rate of 1.8 mL/min. The initial oven temperature program was 70 °C—holding time 4 min, 175 °C (13 °C/min)—waiting time 27 min, and 215 °C (4 °C/min)—waiting time 9 min, followed by increments of 7 °C/min up to 230 °C, retained for 5 min and totaling 65 min. The temperatures of the vaporizer and detector were 250 °C and 300 °C, respectively.
Aliquot (1.0 μL) of the esterified extract was injected into the chromatograph device. All FAs were identified by comparing the retention times of the methyl esters of the samples with the FA standards using the SupelcoTM Component Mix (cat. 18919; Supelco, Bellefonte, PA, USA) and rumenic acid c9, t11 CLA (cat. 05632; Sigma-Aldrich Corporation, St. Louis, MO, USA). The percentages of fatty acids were obtained using the Chromquest 4.1 software (Thermo Electron, Milan, Italy). The FA results were expressed as area percentages (feeds) and as mg/100 g of meat.
2.5. Proteome
The muscle tissue proteome of the animals was investigated by two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and electrospray ionization mass spectrometry (ESI-MS/MS), following procedures described in the literature [24,25].
2.5.1. Protein Extraction and Precipitation
Individual biopsy samples of LT muscle collected early on post-mortem were used for protein extraction and separation on two-dimensional electrophoresis gels (2D-PAGE). For each treatment, ten individual samples (biological replicates) were used, with three technical replicates (three gels per sample). Approximately 0.2 g of each muscle sample was ground twice in 1.0 mL lysis buffer using an Ultra-Turrax high-shear mixer (Marconi—MA102/E, Piracicaba, São Paulo, Brazil) at 20,000 rpm for 30 s. The protein extracts were separated from the solid part by centrifugation at 10,000 rpm for 15 min at 4 °C. The protein contents of these extracts were stored in 80% (v/v) acetone solution in a refrigerator at 5 °C for 2–3 h to ensure that the procedure occurred for a sufficient period of time. The centrifugation process was then repeated for 25 min at 10,000 rpm to obtain protein pellets for quantification and 2D-PAGE.
2.5.2. Protein Separation by Two-Dimensional Polyacrylamide Gel Electrophoresis
The total protein concentration of the bovine muscle tissue samples was quantified using the Biuret method [26]. The protein concentrations obtained were used to calculate the volume of the samples and the solution needed for electrophoresis, considering a protein mass of 375 μg and a volume of 250 μL applied to the strip, resulting in a total protein concentration of 1.5 μg/μL. The samples were solubilized in a solution containing urea, thiourea, (3-[(3-cholaminopropyl)-dimethylammonium]-1-propanesulfonate), ampholytes, bromophenol blue, and dithiothreitol. An aliquot of each sample of 250 μL was added to a 13 cm isoelectric focusing strip containing an immobilized ampholyte with a pH gradient from 3 to 10, which was subjected to hydration for 12 h.
After this period, the strips were subjected to the first step (1D) of two-dimensional electrophoresis, using an Ettan IPGphor 3 device (GE Healthcare, Chicago, IL, USA), in which the proteins were fractionated by the isoelectric point (pI), the pH value when the net charge total protein is zero. The strips were placed in equilibrium solutions for reduction and alkylation and subjected to the second stage (2D) of electrophoresis for the fractionation of the protein spots according to the molecular weight on a 12.5% polyacrylamide gel. At the end of the 2D run, approximately 500 mL of colloidal Coomassie stain was used to stain the protein spots of the gels. The gels were decolorized with ultrapure water after 72 h.
2.5.3. Image Processing
The gels were scanned and the images were imported into the ImageMaster Platinum software for comparisons (contrasts) of the images between treatments and to obtain information such as the number of spots per gel, % matching (correspondence between the protein spots in the gels), isoelectric point (pI), molecular weight (MW), and spot volume. The gel correspondence (matching) of each sample (three technical replicates) was higher than 95%, i.e., 95% of the spots were present in the technical replicates, indicating a good reproducibility. For the image comparison, one reference gel per treatment was selected [27], which contained the largest number of most well-defined spots. The reference gel of the treatment was compared with each gel used for the other treatments.
2.5.4. Tryptic Digestion of Protein Spots and Identification of Proteins by ESI-MS/MS
Protein spots from the experimental groups (control vs. WDG) were selected based on their MW and pI obtained by image analysis, cut out (fragments of approximately 1 mm3), and prepared according to the method described in [28]. In brief, the sediments were transferred to microtubes and submitted to the following four steps: (1) removal of the dye; (2) reduction and alkylation; (3) trypsin digestion (Trypsin Gold Mass Spectrometry, Promega, Madison, WI, USA); and (4) elution of peptides extracted from the gel. Subsequently, mass spectra of the peptides were obtained by analyzing the aliquots of the solutions using a nanoACQUITY UPLC-Xevo TQ-MS System (Waters, Manchester, UK). The proteins of the Bos taurus genome were identified in the UniProt database (UniProtKB/Swiss-Prot, available online: www.uniprot.org, accessed on 1 August 2022).
2.5.5. Bioinformatics Procedures
Bioinformatic analyses were conducted for the classification of differentially expressed proteins in the muscle tissues from the animals of the control vs. WDG treatment group in terms of biological processes (BPs), the molecular function (MF), and cellular components (CC). For this purpose, the accession numbers of the proteins identified by ESI/MS/MS were entered into the UniProt database (available online: www.uniprot.org, accessed on 1 August 2022), and their FASTA sequences were extracted. After this step, the proteins were analyzed using the OMICSBOX v.2.0 (available online: https://www.biobam.com/omicsbox/, accessed on 1 August 2022) and Blast2GO tools [29].
Additionally, the interactions between the proteins identified in the treatments were analyzed using the open-source STRING 11.0 platform (available online: https://string-db.org/, accessed on 1 August 2022). The same list of proteins whose expression differed between the experimental groups were used in these analyses [30]. The minimum required interaction score was set at 0.900 (highest confidence), and no more than 20 interactions were allowed in the database search.
Subsequently, further bioinformatic analyses were performed, following the procedures described by Gagaoua et al. [31], using the Metascape® platform. Briefly, gene identifiers were converted using Uniprot Retrieve/ID mapping. Thus, key information regarding the proteins (gene names (GN)) and its relationships with the carcass and meat quality traits described in the current study were annotated for each treatment (control and WDG). These procedures aimed to compare the two protein lists so as to better understand the common and divergent molecular signatures. Hierarchical heatmap clustering was also carried out using enriched GO terms analyzed by Metascape® (available online: https://metascape.org/, accessed on 1 August 2022).
Additionally, the ProteQTL tool, included in ProteINSIDE (available online: http://www.proteinside.org/, accessed on 1 August 2022), was used for the rapid search of carcass and meat quality quantitative trait loci (QTL) among the list of putative biomarkers. ProteQTL interrogates a public library of published QTLs in Animal QTL Database (available online https://www.animalgenome.org/QTLdb, accessed on 1 August 2022) that contains cattle QTLs and association data collected from published scientific articles.
2.5.6. Data Analysis
The data were submitted to analysis of variance (ANOVA) by the F test using the GLM procedure of the Statistical Analysis System (SAS, version 9.1, Cary, NC, USA). Means were compared by the Tukey test and a p value < 0.05 was adopted as the critical probability. The design was completely randomized, and the following model was used:
| Yij = μ + ti + εij |
where Yij is the observed value of the experimental unit referring to treatment i in repetition j; μ is the general effect of the mean; t is the treatment effect (diet); and ε is the experimental error.
Regarding the proteome data, the protein spot volume was imported into ImageMaster Platinum software and the mean and standard deviation were calculated for the selected spots. The images were compared between treatments by matching the spots in terms of their distribution, volume, relative intensity, pI, and MW. The spot data were tested for homogeneity of variances and normality using the Levene and Shapiro–Wilk tests, respectively. Subsequently, the differences in the means between treatments were analyzed using Student’s t-test. Additionally, the Mann–Whitney test (Wilcoxon rank-sum test) was used when the normality criteria were breached in any of the treatments. For both tests (Student or Mann–Whitney), significance was detected at the 0.05 level. For all data, trends were considered at 0.05 < p ≤ 0.10.
3. Results
3.1. Carcass and Meat Quality Traits
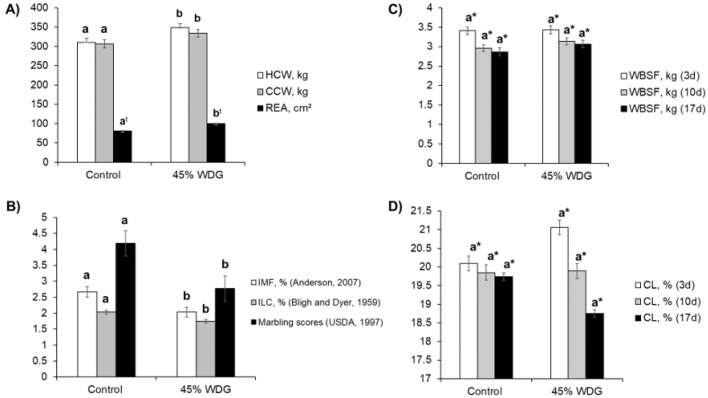
The feedlot-finished F1 Angus-Nellore bulls fed on diets with 45% WDG had a higher HCW and CCW (p < 0.05) compared to the control group (Figure 1A). The inclusion of WDG promoted an average increase of 10.5 and 10.25 kg in the HCW and CCW, respectively. In addition, there was a trend (p = 0.064) towards greater muscle growth, measured as REA, in the animals fed 45% WDG vs. the control diet (Figure 1A), complementing the HCW and CCW results. There were no differences (p > 0.10) in the BFT between the control vs. WDG diet (7.54 vs. 7.63 ± 0.56 mm).
Figure 1.
Carcass (A) and meat quality (B–D) traits of feedlot-finished F1 Angus-Nellore bulls fed on diets with 0% (control) and 45% corn wet distiller grains (WDG). HCW = hot carcass weight; CCW = cold carcass weight; REA = ribeye area; IMF = intramuscular fat; ILC = intramuscular lipid content; marbling score (0 = devoid, 2 = practically devoid, 3 = traces, 4 = slight, 5 = small, 6 = modest); WBSF = Warner–Bratzler shear force; CL = cooking loss. The bars indicate the standard deviation of the mean. a,b Different lowercase letters indicate differences at p < 0.05 except for REA (t), which has a p-value = 0.064 between feedlot diets. * Significant effects (p < 0.05) due to ageing time (3, 10, and 17 days post-mortem).
Differences (p < 0.05) in the IMF content, ILC, and visual marbling scores were observed between the control and WDG treatment groups (Figure 1B). The deposition of IMF was reduced in the feedlot-finished animals fed on the diet with 45% WDG compared to the control treatment. There were no differences (p > 0.10) in the WBSF or CL evaluated at the three aging times (Figure 1C and Figure 1D, respectively). However, as expected, differences in the WBSF were observed between the three time points (3, 10, and 17 days post-mortem), regardless of the treatment.
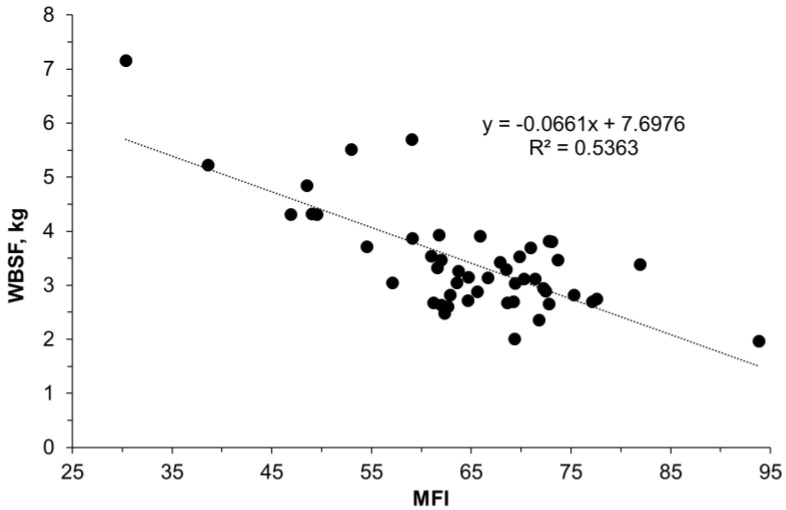
In the present study, the WBSF values were as expected, being inversely related to the MFI data (Figure 2). There were no differences between the control and WDG treatments (p > 0.05).
Figure 2.
Relationship between meat tenderness, measured by the myofibril fragmentation index (MFI), and Warner–Bratzler shear force (WBSF) in the Longissimus thoracis muscle of feedlot-finished F1 Angus-Nellore bulls fed on the control diet or a diet with 45% wet distiller grains.
3.2. Fatty Acid Profile
To better understand the effects of the diet containing WDG on the lipid profile of meat, we determined the fatty acid profile of the main lipid sources used in the diets (control vs. 45% WDG), including soybean meal, dry ground corn, and WDG (Table 1).
Table 1.
Dry matter (DM), ether extract (EE), and concentrations of the main fatty acids from the lipid sources used in the diets.
| Soybean Meal | Ground Corn | Corn Wet Distiller Grain | |
|---|---|---|---|
| DM (% as fed) | 89 | 88 | 32 |
| EE (% DM) | 2.0 | 4.3 | 4.0 |
| Fatty acids (% of total fatty acids) a | |||
| Myristic C14:0 | 0.13 | 0.06 | 1.48 |
| Palmitic C16:0 | 20.43 | 16.53 | 14.84 |
| Stearic C18:0 | 4.29 | 1.99 | 9.73 |
| Oleic C18:1 c9 | 14.85 | 28.30 | 23.1 |
| Linoleic C18:2 c9-c12 | 50.77 | 49.27 | 2.48 |
| Linolenic C18:3 n3 | 6.17 | 1.22 | 0.78 |
| ΣSFA | 26.07 | 19.55 | 27.35 |
| ΣUFA | 73.79 | 80.46 | 33.26 |
| ΣMUFA | 16.85 | 29.97 | 30.32 |
| ΣPUFA | 56.95 | 50.49 | 2.94 |
a SFA = saturated fatty acids; UFA = unsaturated fatty acids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; ΣSFA = sum of the saturated fatty acids; sum of the unsaturated fatty acids; ΣMUFA = sum of the monounsaturated fatty acids; ΣPUFA = sum of the polyunsaturated fatty acids.
The dietary inclusion of WDG reduced (p < 0.05) the IMF content of the meat compared to the control diet when the results were expressed as percentages (2.03 ± xx vs. 2.67 ± 0.16) or marbling scores (2.77 ± xx vs. 4.19 ± 0.4). The fatty acid composition of the LT muscle is presented in Table 2. The concentrations of C10:0, C11:0, lauric (C12:0), C13:0 iso, myristic (C14:0), pentadecanoic (C15:0), palmitoleic (C16:1 c9), margaric (C17:0), heptadecenoic acid (C17:1), stearic (C18:0), oleic (C18:1 c9), C18:1 c11, C18:12, C18:1 c15, α-linolenic (C18:3 n3), and C20:1 acid decreased in the meat of bulls fed on WDG. However, a decrease was observed in the total SFA (p < 0.01), UFA (p = 0.001) and MUFA (p = 0.001) concentrations of the LT muscle in the meat of bulls fed on WDG. In addition, there was a decrease in the MUFA:SFA (p = 0.001) and UFA:SFA (p = 0.004) ratios when WDG was included in the diet.
Table 2.
Effects of wet corn distiller grains (WDG) on the percentages of the main fatty acids in the intramuscular fat of longissimus muscle of F1 Angus-Nellore bulls.
| Control | 45% WDG | SEM | p-Value | |
|---|---|---|---|---|
| Intramuscular fat, % | 2.90 | 1.85 | 0.095 | <0.0001 |
| Fatty acids (mg/100 g of meat) a | ||||
| C6:0 | 1.849 | 1.075 | 0.383 | 0.077 |
| C10:0 | 1.829 | 1.149 | 0.219 | 0.037 |
| C11:0 | 0.099 | 0.033 | 0.012 | 0.003 |
| Lauric C12:0 | 2.343 | 1.127 | 0.240 | 0.009 |
| C13:0 iso | 0.341 | 0.103 | 0.055 | 0.001 |
| C14:0 iso | 0.717 | 0.503 | 0.185 | 0.861 |
| Myristic C14:0 | 87.829 | 51.924 | 7.358 | 0.009 |
| C15:0 iso | 1.897 | 1.550 | 0.283 | 0.410 |
| C15:0 anteiso | 4.375 | 3.454 | 0.694 | 0.487 |
| Myristoleic C14:1 c9 | 18.741 | 10.337 | 2.568 | 0.066 |
| Pentadecanoic C15:0 | 16.069 | 6.996 | 1.177 | <0.0001 |
| C16:0 iso | 1.795 | 1.278 | 0.367 | 0.492 |
| Palmitic C16:0 | 733.76 | 468.36 | 24.679 | 0.347 |
| C17:0 iso | 5.196 | 4.753 | 0.526 | 0.567 |
| Palmitoleic C16:1 c9 | 105.11 | 52.987 | 7.378 | 0.001 |
| Margaric C17:0 | 34.703 | 16.794 | 1.807 | 0.001 |
| Heptadecenoic C17:1 | 29.482 | 11.380 | 2.728 | <0.0001 |
| Stearic C18:0 | 378.11 | 262.61 | 17.397 | 0.002 |
| C18:1 trans | 75.933 | 75.957 | 11.334 | 0.998 |
| Oleic C18:1 c9 | 1062.82 | 645.70 | 61.888 | 0.001 |
| C18:1 c11 | 78.606 | 40.235 | 5.234 | 0.001 |
| C18:1 c12 | 8.163 | 5.821 | 0.391 | 0.003 |
| C18:1 c13 | 11.839 | 5.478 | 2.013 | 0.104 |
| C18:1 t16 | 1.855 | 2.101 | 0.427 | 0.694 |
| C18:1 c15 | 1.734 | 1.134 | 0.166 | 0.034 |
| Linoleic C18:2 c9c12 | 131.04 | 99.794 | 18.983 | 0.278 |
| C20:0 | 2.082 | 1.586 | 0.203 | 0.077 |
| C18:3 n6 | 0.851 | 0.532 | 0.205 | 0.303 |
| α-Linolenic C18:3 n3 | 10.389 | 6.348 | 0.882 | 0.012 |
| C20:1 | 3.017 | 1.002 | 0.413 | 0.009 |
| CLA C18:2 c9t11 | 3.877 | 4.772 | 0.826 | 0.465 |
| C18:2 t10c12 | 0.094 | 0.201 | 0.075 | 0.607 |
| C20:2 | 1.306 | 0.921 | 0.219 | 0.248 |
| C20:3 n6 | 5.702 | 4.974 | 1.185 | 0.676 |
| C22:0 | 0.0686 | 0.125 | 0.029 | 0.676 |
| Arachidonic C20:4 n6 | 32.616 | 24.869 | 6.922 | 0.452 |
| EPA C20:5 n3 | 7.576 | 5.204 | 1.434 | 0.276 |
| C24:0 | 0.076 | 0.0552 | 0.021 | 0.505 |
| C24:1 | 2.855 | 1.982 | 0.616 | 0.346 |
| C22:5 | 14.983 | 10.644 | 2.806 | 0.306 |
| C22:6 n3 | 1.500 | 1.273 | 0.325 | 0.634 |
| ΣSFA | 1271.29 | 822.41 | 34.943 | <0.0001 |
| ΣUFA | 1610.15 | 1013.69 | 83.816 | 0.001 |
| ΣMUFA | 1400.21 | 854.16 | 76.630 | 0.001 |
| ΣPUFA | 209.94 | 159.53 | 31.540 | 0.291 |
| ΣMUFA/ΣSFA | 32.418 | 19.179 | 2.519 | 0.001 |
| ΣPUFA/ΣSFA | 4.887 | 3.615 | 0.776 | 0.280 |
| ΣUFA/ΣSFA | 37.305 | 22.794 | 2.854 | 0.004 |
| Σn3 | 19.465 | 12.826 | 2.579 | 0.106 |
| Σn6 | 39.169 | 30.376 | 8.284 | 0.474 |
| ω-6/ω-3 | 54.802 | 42.263 | 6.873 | 0.233 |
a SFA = saturated fatty acids; UFA = unsaturated fatty acids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids. ΣSFA = sum of the saturated fatty acids; sum of the unsaturated fatty acids; ΣMUFA = sum of the monounsaturated fatty acids; ΣPUFA = sum of the polyunsaturated fatty acids.
3.3. Muscle Tissue Proteome
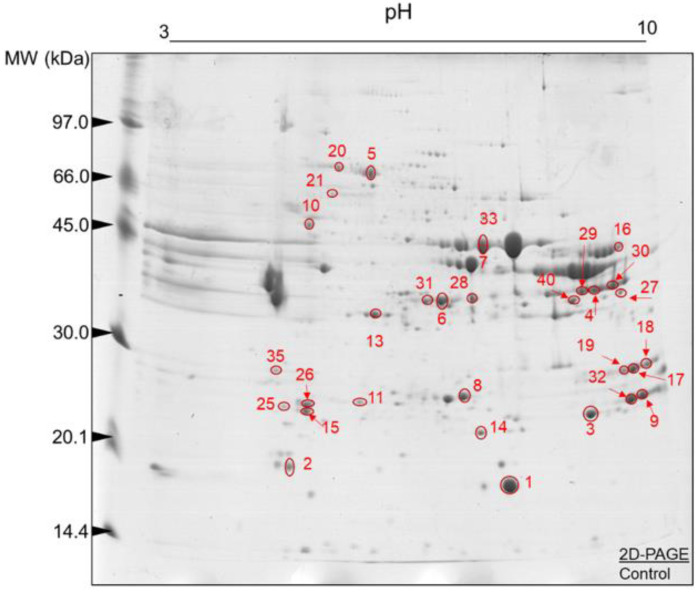
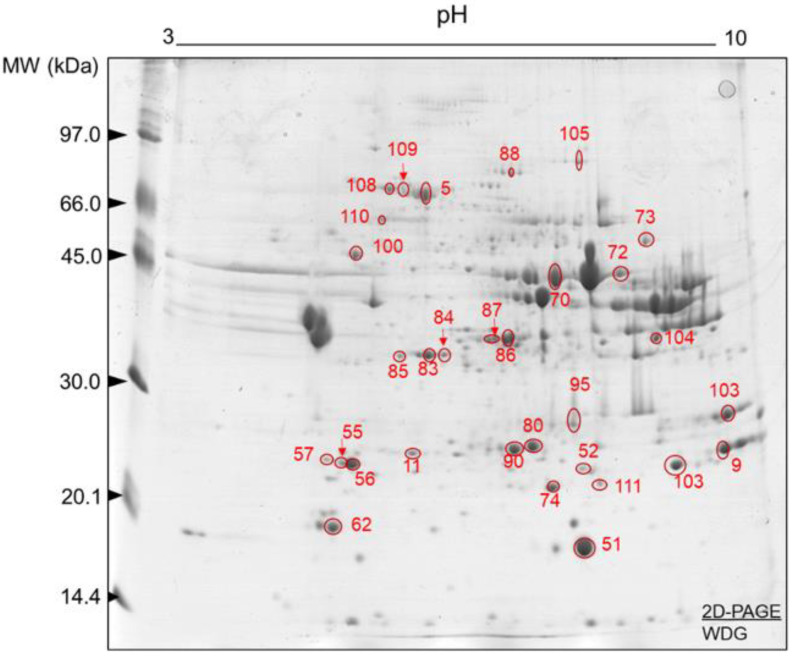
Representative gels obtained from LT muscle samples (sampled early post-mortem) of the control and WDG treatment groups are illustrated in Figure 3 and Figure 4, respectively. In both groups, the 2D-PAGE gels showed a good resolution (matching >50%), indicating efficient protein separation. A great array of protein spots was observed over the pH 3–10 gradient, promoting a good separation of the proteins in this pI range. Furthermore, most protein spots were found mainly in the MW range from 20 to 66 kDa, with most pIs in the range of 5.0 to 7.0, respectively. However, the presence of protein spots with a lower MW (<20 kDa) must be highlighted.
Figure 3.
Protein spots selected for characterization by mass spectrometry (ESI-MS) after image analysis. Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE): 12.5% (w/v) and pH gradient 3–10. Muscle tissue samples (Longissimus thoracis) from feedlot-finished F1 Angus-Nellore bulls fed on diets without the inclusion of wet corn distiller grains (control).
Figure 4.
Protein spots selected for characterization by mass spectrometry (ESI-MS) after image analysis. Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE): 12.5% (w/v) and pH gradient 3–10. Muscle tissue samples (Longissimus thoracis) from feedlot-finished F1 Angus-Nellore bulls fed on diets with the inclusion of wet corn distiller grains (WDG).
The analysis of the images of the 2D-PAGE runs of the control and WDG groups revealed correlations between the gels of each treatment (n = 10) of 63% and 65%, respectively. These results indicate that the protein spots were present in the biological replicates of these gels. The mean numbers of protein spots in the gel replicates were 167 ± 25 and 162.2 ± 15.5 in the control and WDG groups, respectively. Several protein spots were characterized as the most expressed in the control (Table 3) and WDG (Table 4) treatments and subsequently used to better understand the main molecular signatures and pathways.
Table 3.
Proteins identified by mass spectrometry in Longissimus thoracis (LT) muscle of F1 Angus-Nellore bulls fed on the control diet.
| Spot ID | Uniprot ID | Gene Symbol | Full Protein Names | Mascot Score | Protein Coverage (%) | pI/MW Experimental | pI/MW Theoretical |
|---|---|---|---|---|---|---|---|
| Contractile and associated proteins | |||||||
| 02 | Q0P571 | MYLPF | Myosin regulatory light chain 2, skeletal muscle isoform | 35,055.73 | 53.53 | 3.69/19,127.56 | 4.735/19,012.55 |
| 15 | A0JNJ5 | MYL1 | Myosin light chain 1/3, skeletal muscle isoform | 6287.59 | 63.54 | 3.78/21,045.95 | 4.82/20,931.84 |
| 25 | P85100 | MYL3 | Myosin light chain 3 | 1868.16 | 32.16 | 3.6/22,110.17 | 4.87/21,939.03 |
| 26 | P60661 | MYL6 | Myosin light polypeptide 6 | 354.34 | 10.6 | 4.44/17,101.18 | 4.41/16,930.05 |
| 06 | Q8MKH6 | TNNT1 | Troponin T, slow skeletal muscle | 208.97 | 4.56 | 3.79/31,284.30 | 5.67/31,284.25 |
| 04 | Q8MKI3 | TNNT3 | Troponin T, fast skeletal muscle | 22,402.47 | 19.93 | 9.59/321,259.78 | 6.07/32,125.94 |
| Energy metabolism | |||||||
| 40 | P19858 | LDHA | L-lactate dehydrogenase A chain | 69.01 | 5.12 | 9.97/36,939.87 | 7.85/36,597.64 |
| 28 | Q5E9B1 | LDHB | L-lactate dehydrogenase B chain | 51.97 | 6.59 | 6.56/37,009.55 | 6.13/36,723.64 |
| 29 | Q32LG3 | MDH2 | Malate dehydrogenase, mitochondrial | 257.13 | 18.05 | 9.73/36,124.77 | 8.45/35,668.5 |
| 30 | Q2KJE5 | GAPDHS | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | 969.47 | 6.08 | 8.29/43,687.24 | 7.98/43,287.96 |
| 31 | Q5EA88 | GPD1 | Glycerol-3-phosphate dehydrogenase [NAD(+)], cytoplasmic | 51,064.30 | 65.90 | 4.26/38,218.10 | 6.54/37,647.67 |
| 32 | Q02373 | NDUFB10 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 10 | 2416.24 | 27.27 | 6.95/21,250.06 | 8.41/20,964.84 |
| 10 | P00829 | ATP5F1B | ATP synthase subunit beta, mitochondrial | 125,429.60 | 56.44 | 5.32/56,284.23 | 5.04/56,283.53 |
| 08 | Q5E956 | TPI1 | Triosephosphate isomerase | 408,896.4 | 93.98 | 5.31/26,917.47 | 6.59/26,689.51 |
| 07 | Q3ZC09 | ENO3 | Beta-enolase | 250,468.30 | 69.59 | 4.26/47,438.48 | 7.55/47,096.01 |
| 33 | Q9XSJ4 | ENO1 | Alpha-enolase | 7368.94 | 34.56 | 4.26/47,668.70 | 6.48/47,326.13 |
| 16 | Q9XSC6 | CKM | Creatine kinase M-type | 81.84 | 7.87 | 9.73/4322.14 | 6.80/42,988.95 |
| Oxidative stress, cell redox homeostasis, chaperones and heat shock proteins | |||||||
| 14 | P41976 | SOD2 | Superoxide dismutase [Mn], mitochondrial | 4030.24 | 28.38 | 4.44/24,890.60 | 8.53/24,637.95 |
| 20 | Q27975 | HSPA1A | Heat shock 70 kDa protein 1A | 72,555.56 | 54.45 | 4.07/70,544.33 | 5.64/70,258.51 |
| 21 | P34933 | HSPA2 | Heat shock-related 70 kDa protein 2 | 504.82 | 5.19 | 4.07/70,024.93 | 5.32/69,739.7 |
| 09 | Q58DR2 | DNAJB12 | DnaJ homolog subfamily B member 12 | 58.07 | 8.65 | 4.26/41,568.48 | 8.69/41,340.28 |
| 14 | P02510 | CRYAB | Alpha-crystallin B chain | 16,025.71 | 73.14 | 5.32/20,036.81 | 7.05/20,036.79 |
| 06 | P04272 | ANXA2 | Annexin A2 | 136.22 | 12.68 | 5.23/38,897.28 | 7.03/38,612.07 |
| Protein binding | |||||||
| 17 | Q5E9D1 | SUMO1 | Small ubiquitin-related modifier 1 | 141.4561 | 30.69 | 3.93/11,614.06 | 5.27/17,949.28 |
| 27 | O97764 | CRYZ | Zeta-crystallin | 1171.379 | 37.88 | 9.26/35,553.94 | 8.15/35,382.8 |
| Transport and signaling | |||||||
| 18 | O97827 | ADGRL3 | Adhesion G protein-coupled receptor L3 | 58.17 | 3.04 | 6.56/67,847.39 | 7.82/67,329.78 |
| 35 | Q3SX00 | ANKRD46 | Ankyrin repeat domain-containing protein 46 | 73.30 | 13.16 | 4.17/25,557.93 | 5.44/25,329.79 |
| 05 | P02769 | ALB | Albumin | 14,091.32 | 78.25 | 4.26/71,289.43 | 5.87/69,293.41 |
| 19 | Q3SZ57 | AFP | Alpha-fetoprotein | 137.99 | 1.15 | 6.64/70,412.61 | 5.98/68,587.64 |
| 01 | P02192 | MB | Myoglobin | 373,870.70 | 87.66 | 9.98/170,776.21 | 7.19/17,077.59 |
Table 4.
Proteins identified by mass spectrometry in Longissimus thoracis (LT) muscle of F1 Angus-Nellore bulls fed on a diet with 45% corn wet distiller grains.
| Spot ID | Uniprot ID | Gene Symbol | Full Protein Names | Mascot Score | Protein Coverage (%) | pI/MW Experimental | pI/MW Theoretical |
|---|---|---|---|---|---|---|---|
| Contractile and associated proteins | |||||||
| 52 | A0JNJ5 | MYL1 | Myosin light chain 1/3, skeletal muscle isoform | 68,715.63 | 63.54 | 6.87/20,856.32 | 4.83/20,931.84 |
| 62 | Q0P571 | MYLPF | Myosin regulatory light chain 2, skeletal muscle isoform | 74,702.68 | 63.53 | 3.82/19,126.56 | 4.73/19,012.55 |
| 55 | A0JNJ5 | MYL1 | Myosin light chain 1/3, skeletal muscle isoform | 68,715.63 | 63.54 | 7.00/21,459.50 | 4.83/20,931.84 |
| 56 | P85100 | MYL3 | Myosin light chain 3 | 1554.92 | 12.56 | 7.6/22,110.17 | 4.87/21,939.03 |
| 57 | P60661 | MYL6 | Myosin light polypeptide 6 | 316.95 | 10.6 | 4.44/17,101.18 | 7.86/52,285.46 |
| 72 | Q8MKH6 | TNNT1 | Troponin T, slow skeletal muscle | 1464.83 | 14.45 | 9.77/31,284.03 | 5.67/31,284.25 |
| 73 | Q8MKI3 | TNNT3 | Troponin T, fast skeletal muscle | 13,253.61 | 19.93 | 9.59/32,126.78 | 6.07/32,125.94 |
| Energy metabolism | |||||||
| 83 | P19858 | LDHA | L-lactate dehydrogenase A chain | 4527.98 | 29.22 | 6.10/36,939.71 | 7.85/36,597.64 |
| 14 | Q5E9B1 | LDHB | L-lactate dehydrogenase B chain | 272.92 | 17.66 | 6.1/37,008.86 | 6.13/36,723.64 |
| 85 | Q3T145 | MDH1 | Malate dehydrogenase, cytoplasmic | 346.09 | 20.36 | 4.07/36,723.40 | 6.25/36,438.19 |
| 86 | P10096 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 59.52 | 8.71 | 6.1/36,096.27 | 8.29/35,868.09 |
| 87 | Q5EA88 | GPD1 | Glycerol-3-phosphate dehydrogenase [NAD(+)] | 239.72 | 23.78 | 4.26/38,218.01 | 6.54/37,647.67 |
| 100 | P00829 | ATP5F1B | ATP synthase subunit beta, mitochondrial | 42,545.85 | 39.77 | 6.78/56,283.25 | 5.04/56,283.53 |
| 70 | Q3ZC09 | ENO3 | Beta-enolase | 6853.10 | 48.62 | 6.29/47,438.25 | 7.55/47,096.01 |
| 70 | Q9XSJ4 | ENO1 | Alpha-enolase | 3591.51 | 17.74 | 6.29/47,668.37 | 6.48/47,326.13 |
| 80 | Q5E956 | TPI1 | Triosephosphate isomerase | 367,356.90 | 93.57 | 6.89/26,917.47 | 6.59/26,689.51 |
| 90 | Q5E956 | TPI1 | Triosephosphate isomerase | 1955.57 | 32.13 | 5.23/26,917.64 | 6.59/26,689.51 |
| 104 | A4IFD0 | Ak5 | Adenylate kinase isoenzyme 5 | 6634.23 | 1.60 | 6.78/63,843.41 | 4.91/63,272.94 |
| 103 | P00570 | AK1 | Adenylate kinase isoenzyme 1 | 8082.40 | 48.45 | 7.53/21,778.04 | 8.35/21,663.94 |
| Oxidative stress, cell redox homeostasis, chaperones and heat shock proteins | |||||||
| 95 | P41976 | SOD2 | Superoxide dismutase [Mn], mitochondrial | 4030.24 | 28.38 | 7.00/24,809.60 | 8.53/24,637.95 |
| 08 | Q27965 | HSPA1B | Heat shock 70 kDa protein 1B | 642.10 | 27.15 | 4.07/70,513.42 | 5.64/70,228.42 |
| 108 | P19120 | HSPA8 | Heat shock cognate 71 kDa protein | 107.21 | 3.69 | 4.07/71,468.73 | 5.25/71,240.51 |
| 109 | P0CB32 | HSPA1L | Heat shock 70 kDa protein 1-like | 188.49 | 9.83 | 3.96/70,788.37 | 5.91/70,389.07 |
| 110 | P34933 | HSPA2 | Heat shock-related 70 kDa protein 2 | 107.21 | 3.77 | 4.07/70,024.93 | 5.32/69,739.7 |
| 111 | Q3T149 | HSPB1 | Heat shock protein beta-1 | 158,366.90 | 86.57 | 6.78/22,450.99 | 6.11/22,393.06 |
| 74 | P02510 | CRYAB | Alpha-crystallin B chain | 90,018.13 | 70.86 | 5.32/20,036.06 | 7.05/20,036.79 |
| 88 | Q0VCX2 | HSPA5 | Endoplasmic reticulum chaperone BiP | 79.91 | 1.68 | 4.07/72,514.18 | 4.93/72,400.03 |
| Transport and signaling | |||||||
| 05 | P02769 | ALB | Albumin | 30,645.09 | 50.74 | 4.26/71,289.30 | 5.87/69,293.41 |
| 105 | Q3SZ57 | AFP | Alpha-fetoprotein | 42.61 | 1.15 | 6.64/70,410.61 | 5.98/68,587.64 |
| 51 | P02192 | MB | Myoglobin | 1483.74 | 16.88 | 4.42/17,077.21 | 7.20/17,077.59 |
The BPs, MF, and CC of the identified proteins differed between the control (Figure S1) and WDG treatment (Figure S2). Considering the distribution of the 20 top-level gene ontology terms, BPs were identified to a greater extent in the control treatment, particularly cellular (40), regulatory (27) and metabolic (22) processes. The same BPs were observed in smaller numbers in the WDG treatment (27, 21, and 22, respectively). The same trend was found for the number of MFs identified in the control treatment (45) vs. WDG (30), with a predominance of proteins whose MFs were related to molecule binding, catalytic activity, structural activity, and chaperones in both treatments. This is further in agreement with the classifications depicted in Table 3 and Table 4. On the other hand, the levels of CC identified in the two treatments were rather similar.
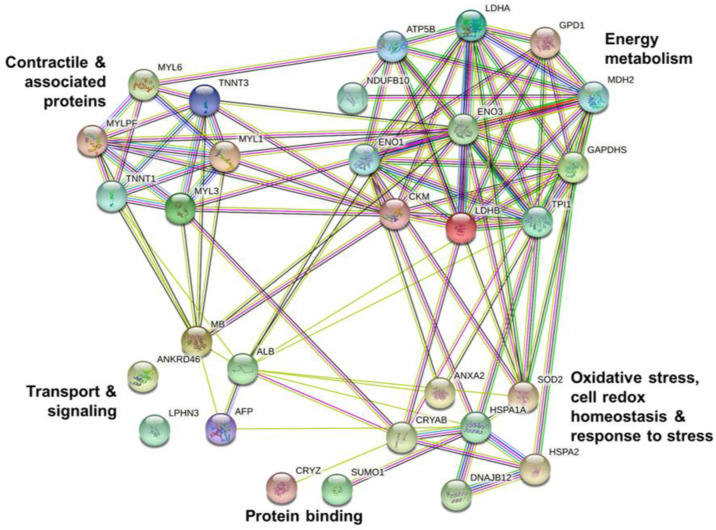
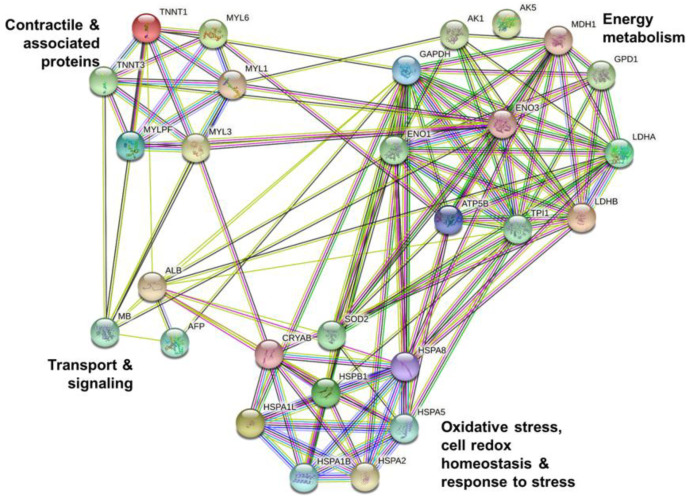
The protein–protein interactions were analyzed in the control (Figure 5) and WDG (Figure 6) treatments.
Figure 5.
Analysis of protein–protein interactions using the differentially expressed proteins in the muscle tissue (Longissimus thoracis) of feedlot-finished F1 Angus-Nellore bulls fed on diets without the inclusion of wet corn distiller grains (control).
Figure 6.
Analysis of protein–protein interactions using the differentially expressed proteins in the muscle tissue (Longissimus thoracis) of feedlot-finished F1 Angus-Nellore bulls fed on diets with the inclusion of 40% wet corn distiller grains (WDG).
These bioinformatic results demonstrated consistent interactions between the proteins characterized in the bovine muscle tissue samples. There were two main groups (clusters) of molecules in the control treatment: proteins related to energy metabolism (LDHA, LDHB, MDH2, GAPDHS, GPD1, NDUFB10, ATP5F1B, TPI1, ENO3, ENO1, and CKM) and proteins related to muscle contraction (MYLPF, MYL1, MYL3, MYL6, TNNT1, and TNNT3). Additionally, in the control treatment, a small interaction network involving proteins ATP5F1B (ATP synthase) and NDUFB10 (NADH dehydrogenase) was identified.
The same two groups (clusters) were also identified in the WDG treatment group, but these samples also included other proteins related to energy metabolism (LDHA LDHB, MDH1, GAPDH, GPD1, ATP5F1B, ENO3, ENO1, TPI1, TPI1, Ak5, and AK1). Protein AK5 (adenylate kinase isoenzyme 5) appears to have no interaction with any of the proteins identified in these animals. This finding may be related to the limited information available about the role of Ak5 in bovine muscle tissue.
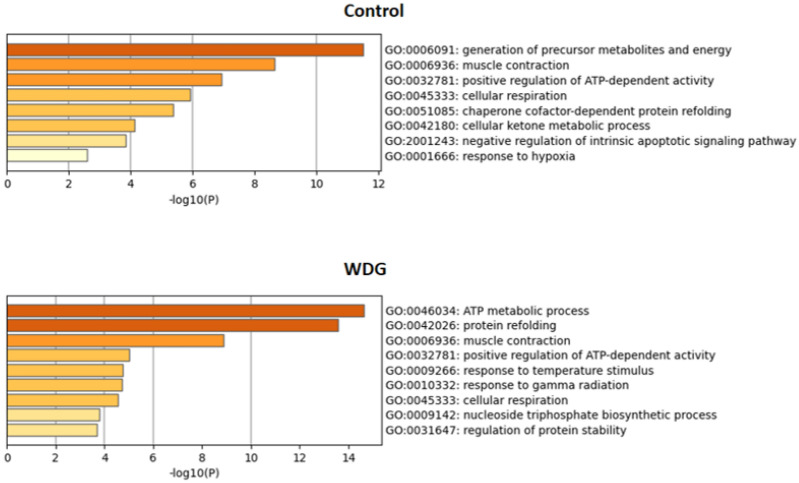
The main enriched terms and pathways identified in this study using the differentially expressed proteins for the control and WDG treatments are summarized in Figure 7, based on gene ontology terms. The energy metabolism pathway, through the “generation of precursor metabolites and energy”, was highly and significantly upregulated in the control compared to the WDG diet group. In animals fed on the WDG diet, cluster pathways related to the ATP metabolic process and protein refolding were more enriched, which helps us to explain the greater HCW and REA observed in these animals. Pathways of generation of metabolites and energy in the control diet group help us to explain the higher IMF found in the meat of these animals.
Figure 7.
Enriched ontology clusters based on significantly enriched gene ontology (GO), obtained using the protein lists of the control (n = 30) and corn wet distiller grain (WDG) diet groups (n = 28) identified in the muscle tissue (Longissimus thoracis) of feedlot-finished F1 Angus-Nellore bulls. The graphs highlight all the enriched terms (functional clusters = 8 (control) and 9 (WDG)) of the protein list early post-mortem, highlighting the importance of the energy metabolism (metabolic and ATP process), muscle contraction, and apoptosis processes according to the Log p-values.
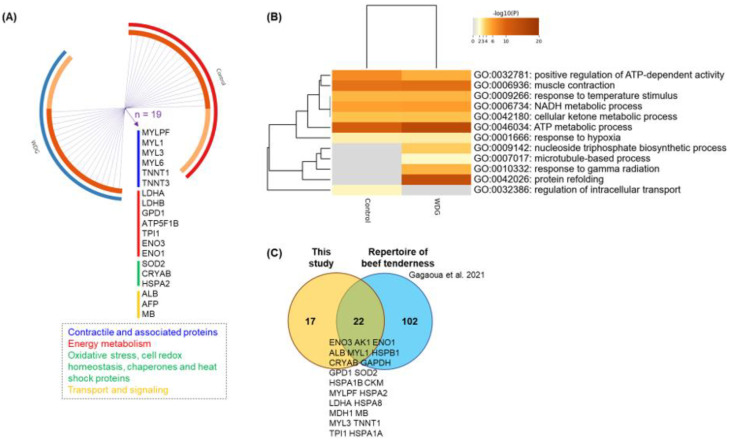
Further bioinformatic analyses allowed us to compare the protein overlap, using Circos plots, for the total number of 58 proteins between dietary treatments (control vs. WDG). Such an analysis displays the overlap and functional connections between genes and allowed us to compare the enriched ontology terms between the treatments in order to identify those that were common or specific to the treatments (Figure 8).
Figure 8.
Gene ontology (GO) pathway and clustering. (A) Protein overlap analysis using a Circos plot, illustrating the degree of overlap between experimental diets (control vs. corn wet distiller grains—WDG) based on the list of 58 proteins identified in the muscle tissue (Longissimus thoracis) of feedlot-finished F1 Angus-Nellore bulls. Each outer arch represents a feedlot diet with a different color. On the inside, the dark orange color represents the proteins that appear in multiple lists and the light orange color represents proteins that are unique to that protein list. Purple lines link the same proteins (gene names) that are shared by the input. Nineteen proteins were found that were common to both treatments. (B) Hierarchical heatmap clustering indicating the first 12 enriched GO terms analyzed by Metascape® (available online: https://metascape.org/, accessed on 1 August 2022), which were significant. The heatmap is colored, with the p-values indicated by colors, where grey cells indicate a lack of significant enrichment, pale brown indicates a low p-value, and dark brown indicates a high p-value. (C) Proteins described by Gagaoua et al. [31] using an integromic approach, in which 124 protein were biomarkers of beef tenderness. From this repertoire, 22 putative protein biomarkers of beef tenderness were also found to be impacted by the feeding regime in the current study (for protein details, see Table 3 and Table 4).
The current GO analysis (Figure 8B) suggests that the “nucleoside triphosphate biosynthetic process”, “response to gamma radiation”, “microtubule-based process”, and “protein refolding” are associated with, and specific to, the WDG treatment and can be associated with muscle growth (REA) and a greater hot carcass weight in the bulls fed on the WDG diet. Additionally, the “regulation of intracellular transport” was specifically associated with a greater IMF, found in animals fed on the control diet. Other GO terms were common to both protein lists, some of which were more significant for the WDG, such as “ATP metabolic process”.
Forty-one QTLs were identified for the carcass (n = 14) and meat quality traits (n = 27) on different chromosomes (Chr) (Table 5). The CRYAB on Chr.15 was common to two carcass traits, including the carcass weight and REA, while GPD1 on Chr.5 was related to the IMF content. Overall, fourteen chromosomes encompassed the 29 proteins (gene names), and among the major QTLs, most of the proteins were related to the “energy metabolism” pathway, followed by the “muscle contraction and structure” pathways and “apoptosis processes”. Several of the proteins found to change in this study were biomarkers of both beef tenderness and QTLs.
Table 5.
List of the quantitative trait loci (QTL) of the carcass and meat quality traits and their chromosomes (Chr.) obtained using the list of the 58 proteins from the LT muscle of F1 Angus-Nellore bulls fed on the control vs. corn wet distiller grain diet (WDG).
| QTL Linked to QTLdb a | Gene Name | Protein Name | UniProtID (Bovine) | Chr. |
|---|---|---|---|---|
| Carcass weight (n = 10) | MDH1 | Malate dehydrogenase, cytoplasmic | Q3T145 | Chr.11 |
| CRYAB | Alpha-crystallin B chain | P02510 V6F832 | Chr.15 | |
| ENO1 | Alpha-enolase | Q9XSJ4 | Chr.16 | |
| MYLPF | Myosin regulatory light chain 2 | Q0P571 | Chr.25 | |
| MDH2 | Malate dehydrogenase, mitochondrial | Q58DR9 Q32LG3 | Chr.25 | |
| HSPB1 | Heat shock protein beta-1 | Q3T149 E9RHW1 | Chr.25 | |
| LDHA | L-lactate dehydrogenase A chain | P19858 | Chr.29 | |
| AK5 | Adenylate kinase isoenzyme 5 | A4IFD0 | Chr.3 | |
| CRYZ | Zeta-crystallin | O97764 | Chr.3 | |
| MB | Myoglobin | A0A1K0FUF3 P02192 | Chr.5 | |
| Ribeye area (n = 3) | CRYAB | Alpha-crystallin B chain | P02510 V6F832 | Chr.15 |
| MB | Myoglobin | A0A1K0FUF3 P02192 | Chr.5 | |
| LDHB | L-lactate dehydrogenase B chain | Q5E9B1 | Chr.5 | |
| Marbling score (n = 6) | HSPA8 | Heat shock cognate 71 kDa protein | P19120 | Chr.15 |
| SUMO1 | Small ubiquitin-related modifier 1 | Q5E9D1 | Chr.2 | |
| HSPA1B HSPA1A | Heat shock 70 kDa protein 1B; Heat shock 70 kDa protein 1A | Q27965 Q27975 | Chr.23 | |
| HSPA1L | Heat shock 70 kDa protein 1-like | P0CB32 | Chr.23 | |
| Ak5 | Adenylate kinase isoenzyme 5 | A4IFD0 | Chr.3 | |
| CRYZ | Zeta-crystallin | O97764 | Chr.3 | |
| Intramuscular fat (n = 1) | GPD1 | Glycerol-3-phosphate dehydrogenase | Q5EA88 | Chr.5 |
| Oleic acid content (n = 1) | ENO3 | Beta-enolase | Q3ZC09 | Chr.19 |
| Omega-3 unsaturated fatty acid content (n = 1) | ANXA2 | Annexin A2 | P04272 | Chr.10 |
| Palmitic acid content (n = 2) | GAPDHS | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | Q2KJE5 | Chr.18 |
| CKM | Creatine kinase M-type | Q9XSC6 | Chr.18 | |
| Palmitoleic acid content (n = 2) | HSPA2 | Heat shock-related 70 kDa protein 2 | P34933 | Chr.10 |
| ENO3 | Beta-enolase | Q3ZC09 | Chr.19 | |
| Palmitoleic acid to palmitic acid ratio (n = 1) | DNAJB12 | DnaJ homolog subfamily B member 12 | Q58DR2 | Chr.28 |
| Stearic acid content (n = 4) | HSPA2 | Heat shock-related 70 kDa protein 2 | HSPA2 | Chr.10 |
| MDH1 | Malate dehydrogenase, cytoplasmic | MDH1 | Chr.11 | |
| HSPA5 | Endoplasmic reticulum chaperone BiP | HSPA5 | Chr.11 | |
| AK1 | Adenylate kinase isoenzyme 1 | AK1 | Chr.11 | |
| Trans-6/9-C18:1 fatty acid content (n = 1) | ATP5F1B | ATP synthase subunit beta, mitochondrial | ATP5F1B | Chr.2 |
| Shear force (n = 8) | HSPA8 | Heat shock cognate 71 kDa protein | P19120 | Chr.15 |
| NDUFB10 | NADH dehydrogenase | M5FHL5 Q02373 | Chr.25 | |
| MYLPF | Myosin regulatory light chain 2 | Q0P571 | Chr.25 | |
| ADGRL3 | Adhesion G protein-coupled receptor L3 | O97827 | Chr.6 | |
| ALB | Albumin | P02769 A0A140T897 | Chr.6 | |
| ADGRL3 | Adhesion G protein-coupled receptor L3 | O97827 | Chr.6 | |
| ALB | Albumin | P02769 A0A140T897 | Chr.6 | |
| AFP | Alpha-fetoprotein | Q3SZ57 | Chr.6 |
a ProteQTL tool included in ProteINSIDE (available online: http://www.proteinside.org/, accessed on 1 August 2022) interrogates a public library of published QTL in the Animal QTL Database (available online: https://www.animalgenome.org/QTLdb/, accessed on 1 August 2022) that contains cattle QTL and association data curated from published scientific articles.
4. Discussion
Tenderness and juiciness are among the most important meat quality attributes for consumers in some markets, who are willing to pay more for higher-quality products [32]. Meat quality research using proteomic approaches can help us to identify the markers of higher-quality products by enabling the overall analysis of cellular proteins using biotechnologies, such as two-dimensional electrophoresis, mass spectrometry, and bioinformatics [12,25,33]. To the best of our knowledge, this is the first study that used a proteomic approach to decipher the biochemical and molecular mechanisms that regulate meat tenderness, marbling, and the fatty acid profile in feedlot-finished cattle fed on a diet containing WDG.
4.1. Carcass and Meat Quality Traits
The trend towards higher muscle growth, measured as REA, could be due to the higher carcass weights of the animals fed on 45% WDG compared to the control group. The REA reflects carcass muscularity. Consequently, heavier carcasses with a greater REA can lead to an increased cut yield in beef. The yield implies the direct financial return for the producer and slaughterhouse, since it is directly related to the amount of commercializable meat [34]. However, a higher carcass yield also depends on the BFT, a carcass trait that was similar between the two treatments in the present trial. Divergent results for the same carcass traits have been reported in the literature for feedlot-finished Hereford steers fed on diets containing 45% sorghum distiller grains [35], suggesting that the divergences between studies are due to the type of feed tested and the genotype.
Beef tenderness is affected by different factors, such as the genotype, nutrition, and forms of carcass and meat cut processing by the industry [36]. In the present study, WBSF values were not influenced by the inclusion of 45% WDG in the feedlot diet of crossbred bulls, with a difference only being observed between aging times. Similar results have been reported in other studies that found no effect of distiller grains on the objective tenderness [37] or sensory attributes [38].
Intramuscular fat, also known as marbling, is the most important fraction of adipose tissue, from a nutritional point of view, because it is present within the edible portion. This fat is found in several meat cuts and is closely associated with the main organoleptic characteristics of meat, conferring a very specific final flavor [39]. The reduction in starch levels in the diet with 45% WDG is probably the reason for the lower IMF content in the LT muscle of these animals. According to the literature, this dietary condition can lead to changes in IMF deposition [40]. With less substrate for IMF synthesis, changes in the gene expression and protein–protein interactions related to nutrient transport and energy metabolism occurred in the LT muscle of the animals fed on feedlot diets with 45% WDG.
The development of post-slaughter rigor mortis is one of the essential conditions for the onset of a subsequent event. The proteolysis of the structural components of sarcomeres, fostering the binding reactions of proteolytic enzymes to their cellular substrates, is an event that strongly depends on the development of rigor mortis and related pathways, such as apoptosis, identified in this study as an impacted process. In fact, apoptosis is an early event that occurs in the post-mortem muscle and is suggested to play a pivotal role in meat quality determination [10]. Enzymatic proteolysis involves a relatively large set of proteins, with the reactions being catalyzed by enzymes such as caspases, calpains, or cathepsins with a low or high affinity for their substrates. According to the literature, the MFI, which is a proxy allowing for the evaluation of post-mortem proteolysis, can explain more than 50% of the variation in beef tenderness and shows a high positive (r = 0.75) correlation with sensory tenderness and a negative (r = −0.72) correlation with WBSF values [20]. In the present study, although an inverse relationship was observed between these quality variables, neither myofibrillar proteolysis nor the WBSF was affected by the treatments, as described in earlier studies on Zebu cattle [21,41].
4.2. Fatty Acid Profile
The reduction in the IMF content of meat produced with the inclusion of 45% WDG may explain, in part, the alteration in the fatty acid profile when compared to the control treatment. The proteome of animals fed WDG exhibited a lower expression of proteins such as SOD2, TPI1, MDH2, GPD1, ENO3, ENO1, ALB, LDHA, LDHB, and GAPDHS, which may be related not only to the lower IMF content but also to the reduced expression of glycerol-3-phosphate dehydrogenase, an enzyme associated with the differentiation of adipocytes [42]. Together with the other proteins that were less expressed when WDG was included in the diet, these findings may explain the lower IMF content.
The addition of distiller grains to ruminant diets has been shown to be associated with a reduction in the endogenous synthesis of MUFA and increased incorporation of PUFA in meat [43]. Our results only confirm the reduction in the MUFA concentrations, since the inclusion of WDG did not alter the PUFA concentrations. The reduced endogenous synthesis of MUFA might be related to the lower activity of ∆9 desaturase, an enzyme that converts saturated fatty acids, such as stearic acid (C18:0), into oleic acid (C18:1 c9).
In this respect, we previously reported and observed that the inclusion of WDG in cattle diets reduced the expression of the SDC-1 gene [44], which encodes this enzyme. The lower synthesis of MUFA as a result of the lower expression of the SDC-1 gene was associated with the dietary inclusion of WDG. Moreover, this result may be associated with a reduction in starch consumption by the animals subjected to this treatment, since the expression of this gene is higher in animals fed on high-starch diets [45]. However, it should be noted that the reduction in MUFA did not compromise the meat fatty acid profile of the animals on the WDG treatment.
4.3. Muscle Proteome Data and Changing Molecular Signatures
The experimental diets affected the muscle tissue proteome of the cattle without altering meat traits such as the tenderness or color. Animals fed on 45% WDG exhibited a higher expression of proteins related to interconnected pathways, such as energy metabolism (LDHA LDHB, MDH1, GAPDH, GPD1, ATP5F1B, ENO3, ENO1, TPI1, Ak5, and AK1) and muscle contraction (MYL1, MYLPF, MYL1, MYL3, MYL6, TNNT1, and TNNT3). Some of the proteins identified have been related to muscle growth in previous studies [46,47] and may explain the trend towards an increase in the REA and HCW in the animals treated with 45% WDG compared to the control. In a study on sheep fed on feedlot diets containing cottonseed co-products [48], the authors found that the experimental diets promoted an increase in TNNT3 and MYL1 in the LT muscle, proteins responsible for the regulation of myosin. Thus, the diets affected the main factors involved in muscle contraction, as observed in the present study.
Other studies evaluated the meat quality of Nellore (Bos indicus) and Aberdeen Angus (Bos taurus) cattle using phosphoproteomics [49]. The authors described the presence of proteins involved in muscle fiber types (MYL1, MYL3, and MYL6) and energy metabolism (ENO3, ENO1, LDHA, LDHB, and GAPDHS) in these animals. These proteins were identified as potential biomarkers of muscle growth, meat tenderness, and IMF [31,33] (ref). In the present study, proteins such as SOD2, TPI, MDH1, GPD1, ENO3, ENO1, ALB, LDHA, LDHB, and GAPDHS were more expressed in the LT muscle of animals fed on the control diet. This finding may be related to the higher IMF content of the meat of these animals.
Among the several proteins that were more expressed in the control treatment, those related to energy metabolism (LDHA LDHB, MDH1, GAPDHS, GPD1, ATP5F1B, ENO3, ENO1, TPI1, TPI1, Ak5, and AK1) should be highlighted. The genes encoding these molecules have been described in other studies on beef cattle as biomarkers of carcass and meat quality traits such as the REA [50], IMF [51], tenderness [31], and color [52]. For example, HSPs possess anti-apoptotic properties and may contribute to delaying the onset of post-mortem apoptosis, thus influencing the development of tenderness and the conversion rate of muscle to meat [25,52]. However, there were no differences in meat tenderness between the control and 45% WDG groups. The protein biomarkers of beef tenderness identified in both treatments may help us to rule out possible differences in WBSF. Further investigations are needed in this context.
On the other hand, the greater abundance of GAPDHS in the control group may be related to lower insulin sensitivity, which helps to explain the lower IMF content observed in these animals. The GAPDHS is a key molecule in energy metabolism, including glucose transport, glycolysis, Krebs cycle, mitochondrial respiratory chain, and the ß-oxidation of fatty acids [11].
Some important factors must be considered when the protein expression in muscle tissue is correlated with meat quality traits, such as the end-point cooking temperature. Within this context, the study by Gagaoua et al. [53] analyzed meat samples from three breeds (Aberdeen Angus, Limousin and Blond d’Aquitaine) cooked at two end-point cooking temperatures (55 and 74 °C), and the sensory tenderness was evaluated by trained panelists in two different countries (France and the UK). The authors reported six proteins related to muscle structure and contraction (MYHC7, MYH2 and MYH1), oxidative stress (PARK7 and PRDX6), and proteolysis (CAPN1) that were affected regardless of the cooking temperature, country of origin of the panelists, or animal breed. These proteins can be considered as predictors of sensory meat tenderness. Thus, when proteins are used as predictors of meat quality, it is important to consider factors that modify the muscle tissue proteome [54,55], especially during the finishing period (initial live weight, duration, dietary concentrate level, consumption, among others), as well as carcass-associated factors (REA, BFT, and marbling), as recently discussed in several studies [12,56].
The inclusion of WDG (and consequent reduction in starch content) in the feedlot diet possibly resulted in changes in the ruminal metabolism that caused alterations in the protein expression in the LT muscle and, consequently, in the IMF deposition of these animals. Additionally, the reduction in starch content associated with the dietary inclusion of WDG preserved post-mortem proteolysis and, thus, did not compromise the meat tenderness.
Less IMF deposition was observed in the present study, which seems to reflect changes in the energy and lipid metabolism, indirectly measured by the analysis of the animals’ proteome levels. The replacement of corn/soybean meal ingredients with WDG alters dietary protein degradability. WDG is an ingredient rich in rumen undegradable protein, and researchers [5] have suggested that a greater protein supply to the intestine may stimulate the secretion of pancreatic amylase (increasing post-ruminal starch digestion). In the present study, the assessment of the physical, chemical, and molecular characteristics of the LT muscle permitted us to understand the alterations in IMF deposition in the animals. These results could be associated with the greater supply of rumen undegradable protein and glutamic acid due to the inclusion of WDG in the diet of these animals.
5. Conclusions
The inclusion of WDG in the feedlot diet of F1 Angus-Nellore bulls affected the protein expression, the protein–protein interactions in the LT muscle, and important metabolic pathways, including the ATP production, growth, and IMF deposition in these animals. However, there was no negative impact on post-mortem proteolysis, meat tenderness, or the fatty acid profile of the animals. This is the first study to describe proteome data for the muscle tissue of feedlot-finished beef cattle fed on high-WDG diets.
Acknowledgments
We thank Cargill Animal Nutrition for their support regarding the study design and acquisition of feed resources from the experimental feedlot at São Paulo State University (UNESP). We also thank Maria Antonia Ladalardo (USP/ESALQ) for her supervision of the fatty acid profile assays. Mohammed Gagaoua further acknowledges the support of the Marie Sklodowska-Curie grant agreement No. 713654 under the project number MF20180029.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11203233/s1. Table S1: Composition of the experimental diets. Figure S1: Classification of proteins identified in the muscle tissue (Longissimus thoracis) of feedlot-finished F1 Angus-Nellore bulls fed on diets without the inclusion of wet corn distiller grains (control). Proteins were separated by 2D-PAGE and identified by mass spectrometry (ESI-MS/MS). The OMICSBOX software was used to classify the proteins according to the biological process (BP), molecular function (MF), and cellular component (CC). Figure S2: Classification of proteins identified in the muscle tissue (Longissimus thoracis) of feedlot-finished F1 Angus-Nellore bulls fed on diets with the inclusion of wet corn distiller grains (WDG). Proteins were separated by 2D-PAGE and identified by mass spectrometry (ESI-MS/MS). The OMICSBOX software was used to classify the proteins according to the biological process (BP), molecular function (MF), and cellular component (CC).
Author Contributions
Conceptualization, W.B. and O.M.N.; methodology, M.G., B.S., L.R., J.T., F.S. and R.T.; software, M.G., W.B. and R.T.; validation, W.B., M.G., P.P., F.S. and L.A.C.; formal analysis, W.B., M.G., B.S., L.R. and J.T.; investigation, W.B., M.G., B.S., F.S. and L.R.; resources, L.A.C., O.M.N. and D.P.L.; data curation, W.B. and M.G.; writing—original draft preparation, W.B. and M.G.; writing—review and editing, M.G., R.C., R.T. and J.T.; visualization, W.B. and M.G.; supervision, L.A.C.; project administration, O.M.N.; funding acquisition, L.A.C., O.M.N. and D.P.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All relevant data are within the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by São Paulo Research Foundation (FAPESP), grant numbers 2018/00981-5 and 2016/04478-0. This study was also financed in part by CAPES, finance code 001.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ott S.L., Rask N. Importance of by-products on the economics of alcohol production from corn. Energy Agric. 1983;2:257–266. doi: 10.1016/0167-5826(83)90022-0. [DOI] [Google Scholar]
- 2.Ponce C.H., Cole N.A., Sawyer J., Da Silva J.C.B., Smith D.R., Maxwell C., Brown M.S. Effects of wet corn distiller’s grains with solubles and nonprotein nitrogen on feeding efficiency, growth performance, carcass characteristics, and nutrient losses of yearling steers12. J. Anim. Sci. 2019;97:2609–2630. doi: 10.1093/jas/skz133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keomanivong F.E., Ruch M.C., Liu J.-H., Kirsch J.D., Bauer M.L., Dahlen C.R., Kapphahn M., Borhan S., Rahman S., Swanson K.C. Influence of dry-rolled corn processing and distiller’s grain inclusion rate on ruminal pH, ammonia and volatile fatty acid concentration, in vitro methane production and enzyme activity. Anim. Feed Sci. Technol. 2017;228:132–139. doi: 10.1016/j.anifeedsci.2017.04.016. [DOI] [Google Scholar]
- 4.Böttger C., Südekum K.-H. Review: Protein value of distillers dried grains with solubles (DDGS) in animal nutrition as affected by the ethanol production process. Anim. Feed Sci. Technol. 2018;244:11–17. doi: 10.1016/j.anifeedsci.2018.07.018. [DOI] [Google Scholar]
- 5.Blom E.J., Anderson D., Brake D.W. Increases in duodenal glutamic acid supply linearly increase small intestinal starch digestion but not nitrogen balance in cattle1. J. Anim. Sci. 2016;94:5332–5340. doi: 10.2527/jas.2016-0783. [DOI] [PubMed] [Google Scholar]
- 6.Klopfenstein T.J., Erickson G.E., Bremer V.R. BOARD-INVITED REVIEW: Use of distillers by-products in the beef cattle feeding industry1. J. Anim. Sci. 2008;86:1223–1231. doi: 10.2527/jas.2007-0550. [DOI] [PubMed] [Google Scholar]
- 7.Schoonmaker J.P., Claeys M.C., LeMenager R.P. Effect of increasing distillers grains inclusion on performance and carcass characteristics of early-weaned steers1. J. Anim. Sci. 2013;91:1784–1790. doi: 10.2527/jas.2011-5075. [DOI] [PubMed] [Google Scholar]
- 8.Nade T., Uchida K., Omori K., Matsubayashi K., Kimura N. Effects of feeding dried distillers grains with solubles (DDGS) on meat quality at the late stage of the fattening period of Holstein steers. Anim. Sci. J. 2012;83:310–317. doi: 10.1111/j.1740-0929.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y.N., Kim S.H., Yoon D.H., Lee H.G., Kang H.S., Seo K.S. Proteome Analysis of Bovine Longissimus dorsi Muscle Associated with the Marbling Score. Asian-Australasian J. Anim. Sci. 2012;25:1083–1088. doi: 10.5713/ajas.2012.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouali A., Gagaoua M., Boudida Y., Becila S., Boudjellal A., Herrera-Mendez C.H., Sentandreu M.A. Biomarkers of meat tenderness: Present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013;95:854–870. doi: 10.1016/j.meatsci.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Picard B., Gagaoua M., Al Jammas M., Bonnet M. Beef tenderness and intramuscular fat proteomic biomarkers: Effect of gender and rearing practices. J. Proteom. 2019;200:1–10. doi: 10.1016/j.jprot.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Gagaoua M., Zhu Y. Food Proteomics. Academic Press; Cambridge, MA, USA: 2022. Proteomics advances in beef production; pp. 151–182. [DOI] [Google Scholar]
- 13.Zhao Y.M., Basu U., Dodson M.V., Basarb J.A., Guan L.L. Proteome differences associated with fat accumulation in bovine subcutaneous adipose tissues. Proteome Sci. 2010;8:14. doi: 10.1186/1477-5956-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonelo D.S., Gómez J.F., Silva S.L., Beline M., Zhang X., Wang Y., Pavan B., Koulicoff L.A., Rosa A.F., Goulart R.S., et al. Proteome basis for the biological variations in color and tenderness of longissimus thoracis muscle from beef cattle differing in growth rate and feeding regime. Food Res. Int. 2022;153:110947. doi: 10.1016/j.foodres.2022.110947. [DOI] [PubMed] [Google Scholar]
- 15.Anderson S., Aldana S., Beggs M., Birkey J., Conquest A., Conway R., Hemminger T., Herrick J., Hurley C., Ionita C., et al. Determination of Fat, Moisture, and Protein in Meat and Meat Products by Using the FOSS FoodScan Near-Infrared Spectrophotometer with FOSS Artificial Neural Network Calibration Model and Associated Database: Collaborative Study. J. AOAC Int. 2007;90:1073–1083. doi: 10.1093/jaoac/90.4.1073. [DOI] [PubMed] [Google Scholar]
- 16.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 17.USDA . United States Standards for Grades of Carcass Beef. Agricultural Marketing Service; Des Moines, ID, USA: 1997. [(accessed on 18 December 2017)]. Official United States Standards for Grades of Carcass Beef; pp. 1–20. Available online: https://www.ams.usda.gov/sites/default/files/media/CarcassBeefStandard.pdf. [Google Scholar]
- 18.AMSA . Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat. American Meat Science Association Educational Foundation; Des Moines, ID, USA: 2015. [Google Scholar]
- 19.Baldassini W.A., Neto O.R.M., Fernandes T.T., Ament H.d.P., Luz M.G., Santiago B.M., Curi R.A., Chardulo L.A.L. Testing different devices to assess the meat tenderness: Preliminary results. J. Food Sci. Technol. 2021;58:2441–2446. doi: 10.1007/s13197-020-04941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culler R.D., Parrish F.C., Jr., Smith G.C., Cross H.R. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. J. Food Sci. 1978;43:1177–1180. doi: 10.1111/j.1365-2621.1978.tb15263.x. [DOI] [Google Scholar]
- 21.Borges B.O., Curi R., Baldi F., Feitosa F.L.B., De Andrade W.B.F., Albuquerque L., Oliveira H., Chardulo L.A. Polymorphisms in candidate genes and their association with carcass traits and meat quality in Nellore cattle. Pesqui. Agropecuária Bras. 2014;49:364–371. doi: 10.1590/S0100-204X2014000500006. [DOI] [Google Scholar]
- 22.Hara A., Radin N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 23.Christie W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982;23:1072–1075. doi: 10.1016/S0022-2275(20)38081-0. [DOI] [PubMed] [Google Scholar]
- 24.Baldassini W.A., Braga C.P., Chardulo L.A.L., Silva J.A.I.V., Malheiros J.M., de Albuquerque L.G., Fernandes T.T., Padilha P.D.M. Bioanalytical methods for the metalloproteomics study of bovine longissimus thoracis muscle tissue with different grades of meat tenderness in the Nellore breed (Bos indicus) Food Chem. 2015;169:65–72. doi: 10.1016/j.foodchem.2014.07.131. [DOI] [PubMed] [Google Scholar]
- 25.Picard B., Gagaoua M. Meta-proteomics for the discovery of protein biomarkers of beef tenderness: An overview of integrated studies. Food Res. Int. 2020;127:108739. doi: 10.1016/j.foodres.2019.108739. [DOI] [PubMed] [Google Scholar]
- 26.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. doi: 10.1016/S0021-9258(18)57021-6. [DOI] [PubMed] [Google Scholar]
- 27.Paredi G., Mori F., de Marino M.G., Raboni S., Marchi L., Galati S., Buschini A., Fiego D.P.L., Mozzarelli A. Is the protein profile of pig Longissimus dorsi affected by gender and diet? J. Proteom. 2019;206:103437. doi: 10.1016/j.jprot.2019.103437. [DOI] [PubMed] [Google Scholar]
- 28.Shevchenko A., Tomas H., Havlis J., Olsen J.V., Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 29.Götz S., Garcia-Gomez J.M., Terol J., Williams T.D., Nagaraj S.H., Nueda M.J., Robles M., Talón M., Dopazo J., Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., Lin J., Minguez P., Bork P., von Mering C., et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagaoua M., Terlouw E.C., Mullen A.M., Franco D., Warner R.D., Lorenzo J.M., Purslow P.P., Gerrard D., Hopkins D.L., Troy D., et al. Molecular signatures of beef tenderness: Underlying mechanisms based on integromics of protein biomarkers from multi-platform proteomics studies. Meat Sci. 2021;172:108311. doi: 10.1016/j.meatsci.2020.108311. [DOI] [PubMed] [Google Scholar]
- 32.Delgado E.F., Aguiar A.P., Ortega E.M.M., Spoto M.H.F., Castillo C.J.C. Brazilian consumers’ perception of tenderness of beef steaks classified by shear force and taste. Sci. Agricola. 2006;63:232–239. doi: 10.1590/S0103-90162006000300004. [DOI] [Google Scholar]
- 33.Gagaoua M., Picard B. New Aspects of Meat Quality. Woodhead Publishing; Cambridge, UK: 2022. Proteomics to explain and predict meat quality; pp. 393–431. [DOI] [Google Scholar]
- 34.Delgado-Pando G., Allen P., Troy D.J., McDonnell C.K. Objective carcass measurement technologies: Latest developments and future trends. Trends Food Sci. Technol. 2021;111:771–782. doi: 10.1016/j.tifs.2020.12.016. [DOI] [Google Scholar]
- 35.Beretta V., Simeone A., Franco J., Bentancur O., Novac M., Panizza V., Rodríguez M.V. Using sorghum dry distillers’ grains plus solubles in sorghum-based finishing diets: Feed utilization, cattle performance and carcass traits. Anim. Feed Sci. Technol. 2021;271:114731. doi: 10.1016/j.anifeedsci.2020.114731. [DOI] [Google Scholar]
- 36.Gagaoua M., Duffy G., Alvarez C., Burgess C., Hamill R., Crofton E., Botinestean C., Ferragina A., Cafferky J., Mullen A., et al. Current research and emerging tools to improve fresh red meat quality. Ir. J. Agric. Food Res. 2022;141:1–23. doi: 10.15212/ijafr-2020-0141. [DOI] [Google Scholar]
- 37.Hart K.B., Ribeiro F.A., Henriott M.L., Herrera N.J., Calkins C.R. Quality effects on beef strip steaks from cattle fed high-protein corn distillers grains and other ethanol by-products. J. Anim. Sci. 2019;97:2087–2098. doi: 10.1093/jas/skz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Mello A., Jenschke B., Senaratne L., Carr T., Erickson G., Calkins C. Effects of finishing diets containing wet distillers grains plus solubles on beef quality attributes and fatty acid profile. Meat Sci. 2018;136:16–22. doi: 10.1016/j.meatsci.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Motoyama M., Sasaki K., Watanabe A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat Sci. 2016;120:10–18. doi: 10.1016/j.meatsci.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Teixeira P.D., Oliveira D.M., Chizzotti M.L., Chalfun-Junior A., Coelho T.C., Gionbelli M., Paiva L.V., Carvalho J.R.R., Ladeira M.M. Subspecies and diet affect the expression of genes involved in lipid metabolism and chemical composition of muscle in beef cattle. Meat Sci. 2017;133:110–118. doi: 10.1016/j.meatsci.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Haddad G.D.B.S., Gomes H.B., Buchili A.F.M., Rodrigues L.M., Fontes P.R., Ramos A.D.L.S., Ramos E.M. Accelerating the dry aging of bone-in beef from Nellore cattle by the freeze/thaw process. J. Food Process. Preserv. 2022;46:e16573. doi: 10.1111/jfpp.16573. [DOI] [Google Scholar]
- 42.Hossner K.L., editor. Development of Muscle, Skeletal System and Adipose Tissue. CABI Publishing; Wallingford, UK: 2005. pp. 55–93. [DOI] [Google Scholar]
- 43.Smith S.B., Gill C.A., Lunt D.K., Brooks M.A. Regulation of Fat and Fatty Acid Composition in Beef Cattle. Asian-Australas. J. Anim. Sci. 2009;22:1225–1233. doi: 10.5713/ajas.2009.r.10. [DOI] [Google Scholar]
- 44.Ferreira M.S., Tomaz L.D.A., Niehues M.B., Ladeira M., Curi R., Chardulo L.A., Baldassini W., Martins C.L., Arrigoni M.B., Neto O.M. The inclusion of de-oiled wet distillers grains in feedlot diets reduces the expression of lipogenic genes and fat content in Longissimus muscle from F1 Angus-Nellore cattle. PeerJ. 2019;7:e7699. doi: 10.7717/peerj.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graugnard D.E., Piantoni P., Bionaz M., Berger L.L., Faulkner D.B., Loor J.J. Adipogenic and energy metabolism gene networks in longissimus lumborum during rapid post-weaning growth in Angus and Angus × Simmental cattle fed high-starch or low-starch diets. BMC Genom. 2009;10:142. doi: 10.1186/1471-2164-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Alessandro A., Zolla L. Meat science: From proteomics to integrated omics towards system biology. J. Proteom. 2013;78:558–577. doi: 10.1016/j.jprot.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Lefaucheur L., Lebret B., Ecolan P., Louveau I., Damon M., Prunier A., Billon Y., Sellier P., Gilbert H. Muscle characteristics and meat quality traits are affected by divergent selection on residual feed intake in pigs1. J. Anim. Sci. 2011;89:996–1010. doi: 10.2527/jas.2010-3493. [DOI] [PubMed] [Google Scholar]
- 48.Paim T.D.P., Viana P., Van Tilburg M.F., Moura A.D.A., De Souza J.R., McManus C., Abdalla A.L., Louvandini H. Feeding effects of cottonseed and its co-products on the meat proteome from ram lambs. Sci. Agricola. 2019;76:463–472. doi: 10.1590/1678-992x-2018-0072. [DOI] [Google Scholar]
- 49.Rodrigues R.T.D.S., Chizzotti M.L., Vital C., Baracat-Pereira M.C., Barros E., Busato K., Gomes R.A., Ladeira M., Martins T. Differences in Beef Quality between Angus (Bos taurus taurus) and Nellore (Bos taurus indicus) Cattle through a Proteomic and Phosphoproteomic Approach. PLoS ONE. 2017;12:e0170294. doi: 10.1371/journal.pone.0170294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva-Vignato B., Coutinho L.L., Cesar A.S.M., Poleti M.D., Regitano L.C.A., Balieiro J.C.C. Comparative muscle transcriptome associated with carcass traits of Nellore cattle. BMC Genom. 2017;18:1–13. doi: 10.1186/s12864-017-3897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gagaoua M., Bonnet M., Picard B. Protein Array-Based Approach to Evaluate Biomarkers of Beef Tenderness and Marbling in Cows: Understanding of the Underlying Mechanisms and Prediction. Foods. 2020;9:1180. doi: 10.3390/foods9091180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picard B., Gagaoua M. Proteomics in Food Science: From Farm to Fork. Elsevier Inc.; Amsterdam, The Netherlands: 2017. Proteomic Investigations of Beef Tenderness. [DOI] [Google Scholar]
- 53.Gagaoua M., Terlouw C., Richardson I., Hocquette J.-F., Picard B. The associations between proteomic biomarkers and beef tenderness depend on the end-point cooking temperature, the country origin of the panelists and breed. Meat Sci. 2019;157:107871. doi: 10.1016/j.meatsci.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Purslow P.P., Gagaoua M., Warner R.D. Insights on meat quality from combining traditional studies and proteomics. Meat Sci. 2021;174:108423. doi: 10.1016/j.meatsci.2020.108423. [DOI] [PubMed] [Google Scholar]
- 55.Gagaoua M., Monteils V., Picard B. Data from the Farmgate-to-Meat Continuum Including Omics-Based Biomarkers to Better Understand the Variability of Beef Tenderness: An Integromics Approach. J. Agric. Food Chem. 2018;66:13552–13563. doi: 10.1021/acs.jafc.8b05744. [DOI] [PubMed] [Google Scholar]
- 56.Gagaoua M., Picard B., Monteils V. Assessment of cattle inter-individual cluster variability: The potential of continuum data from the farm-to-fork for ultimate beef tenderness management. J. Sci. Food Agric. 2019;99:4129–4141. doi: 10.1002/jsfa.9643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper.