Abstract
A total of 100 untreated new leprosy patients were recruited prospectively and examined for the presence of phenolic glycolipid I (PGL-I) antigen in their serum specimens by dot enzyme-linked immunosorbent assay (ELISA) using rabbit anti-PGL-I antiserum. The presence of circulating PGL-I antigen was closely related to the bacterial indices (BI) of the patients. The PGL-I antigen was detectable in 27 (93.1%) of 29 patients with a BI of 4.0 or above and in 15 (68.2%) of 22 patients with a BI of 3.0 to 3.9. However, none of the 37 patients with a BI of less than 1.9 had detectable PGL-I antigen by the methods used in this study. The level of PGL-I in serum declined rapidly by about 90% 1 month after the start of multidrug therapy. This study showed clearly that anti-PGL-I IgM antibodies and circulating PGL-I antigen levels reflect the bacterial loads in untreated leprosy patients. The serological parameters based on the PGL-I antigen may therefore be useful in the assessment of leprosy patients at the time of diagnosis and possibly in monitoring patients following chemotherapy.
Despite the rapid reduction of registered leprosy cases in the last decade, leprosy is still a major public health problem in several countries 16. The finding of no substantial decrease in the new-case detection rate (684,998 new cases reported in 1997 [16] during the same period undermines the successful multidrug therapy (MDT) programs directed by the World Health Organization (WHO). Despite the WHO efforts to eliminate leprosy by the year 2000, areas of hyperendemic infection remain in many countries. In such areas, sensitive and specific laboratory diagnostic tests will be of great value in detecting leprosy patients at the early stages.
There have been tremendous efforts to develop sensitive and specific serodiagnostic tests, and these have been reported in the literature. Among the antigens evaluated for immunoassays, phenolic glycolipid I (PGL-I) is still the only Mycobacterium leprae-specific antigen 8, and it has been widely used for the serodiagnosis of leprosy. The presence of the M. leprae-specific antigen(s) in clinical samples would be indicative of current M. leprae infection. PGL-I was thus the target antigen of choice because of its specificity to M. leprae and its abundance. As well, there have been several studies of the detection of PGL-I in various clinical specimens such as serum 1, 4, 12, 17, urine 4, 9, 10, 13, nasal washes 13, and biopsy specimens 14 for diagnosis and determination of the prognosis following chemotherapy for leprosy.
In general, the PGL-I antigen is detectable in clinical specimens mostly from multibacillary (MB) patients, mainly due to the limited sensitivity of the current detection methods and to the rapid decline of its level in sera soon after starting chemotherapy against leprosy 1, 10, 12. However, it has not been well established what proportion of MB patients are positive for the PGL-I antigen in their sera. The present study was therefore designed to compare the bacterial indices with PGL-I detection in sera from untreated leprosy patients. In addition, the PGL-I antigen level was measured semiquantitatively in sera obtained serially from leprosy patients after starting MDT. The results were then compared with bacterial indices (BI) and immunoglobulin M (IgM) antibodies to the antigen to determine which parameter was the better indicator to monitor the effectiveness of chemotherapy against leprosy.
MATERIALS AND METHODS
Study patients and serum samples.
A total of 100 untreated patients were recruited prospectively among the leprosy patients who presented at the Skin Clinics of the Leonard Wood Memorial Center for Leprosy Research in Cebu City, Philippines. All patients were classified based on clinical findings, histopathological examination, and BI on the Ridley and Jopling scale 11. Of 100 patients enrolled for the study, 28 patients were classified as lepromatous (LL), 32 as borderline lepromatous (BL), 27 as borderline tuberculoid (BT), 12 as tuberculoid (TT), and one as indeterminate; 89 patients were bacteriologically positive (MB), and 11 were acid-fast bacillus negative (paucibacillary). Serum samples were obtained from the study patients before starting treatment and serially at regular intervals of 1, 2, 4, 6, 9 and 12 months after starting WHO MDT.
Detection of PGL-I antigen in serum specimens.
The procedure was a modification of that described previously 4, and it was faster 3, 5. To facilitate the extraction of total lipids from serum specimens, a single 100-μl specimen was added to a filter paper disk (0.5 in. in diameter) (Schleicher & Schuell, Inc., Keene, N.H.) and dried completely. The lipids were then extracted using 2 to 3 ml of CHCl3-CH3OH (2:1) solution and dried under N2. Serum lipids were dissolved in CHCl3, applied to a Pasteur pipette packed with Florisil 60–100 mesh (Sigma Chemical Co., St. Louis, Mo.), and eluted with CHCl3 followed by 5% CH3OH in CHCl3. The lipid fraction eluted with 5% CH3OH was saved and dried under N2 and examined for the presence of PGL-I.
The dot enzyme-linked immunosorbent assay (ELISA) described by Hawkes et al. 7 was used with minor modification as reported previously 3, 5. The purified lipid was dissolved in 100 μl of hexane, and a 5-μl portion was applied to a Tuffryn (polysulfone) membrane (HT-200; Gelman Sciences, Inc., Ann Arbor, Mich.), as originally used by Young et al. 17. High-titer rabbit anti-PGL-I antiserum, prepared as described previously 4, was used as the primary antibody, and peroxidase-conjugated goat anti-rabbit IgG (Cappel, Organon Teknika Co., Durham, N.C.) was used as the secondary antibody. For color development, 4-chloro-1-naphthol (Bio-Rad Laboratories, Inc., Richmond, Calif.) was used and the results were read visually.
For comparison of the PGL-I antigen level in serum samples, purified lipid from 100 μl of serum was twofold serially diluted to 1:256 in hexane, and 5 μl of diluted lipid was applied to the Tuffryn membrane and subjected to the dot ELISA procedures described above. The highest dilution showing evidence of PGL-I was considered to be the titer of PGL-I in this study in order to compare the PGL-I level between serum samples.
In each experiment, PGL-I was included at concentrations of 2.5, 1.0, 0.5, and 0.25 ng per spot as a positive control, and hexane was included as a negative control. The detection limit was set at 0.5 to 1.0 ng of PGL-I, and any experiment giving a higher detection limit was considered invalid and repeated.
Detection of antibodies to PGL-I.
An ELISA described by Voller et al. 15 was used with minor modification as reported previously 3, 6; however, instead of the native glycolipid, the semisynthetic neoglycoprotein O-(3,6-di-O-methyl-β-d-glucopyranosyl)-(1→4)-(2,3-di-O-methyl-α-1-rhamnopyranosyl)-(1→9)-oxynonanoyl–bovine serum albumin (natural disaccharide-octyl-BSA [ND-O-BSA]) 2 was used. Briefly, 50 μl of diluted ND-O-BSA (20 ng of sugar/ml) in carbonate buffer (pH 9.6) was added to the wells of U-bottom microtiter plates (Dynatech Laboratories, Inc., Alexandria, Va.), and incubated overnight at 37°C in a moist chamber. The wells were then washed with phosphate-buffered saline (PBS) solution (pH 7.4) containing 0.05% Tween 20 (PBST) and blocked by the addition of 100 μl of PBST–0.05% BSA at 37°C for 1 h. After the wells were emptied, 50 μl of serum diluted 1:300 in PBST–5% normal goat serum (Gibco Laboratories, Grand Island, N.Y.) was added to the wells, which were then incubated at 37°C for 90 min. After the wells were washed, 50 μl of affinity-purified peroxidase-conjugated goat anti-human IgM (Behring Diagnostics, San Diego, Calif.) diluted 1:5,000 in PBST–5% normal goat serum was added and incubation was continued at 37°C for 1 h. After another wash, 50 μl of substrate solution, H2O2–o-phenylenediamine, was added to the wells, which were then incubated at room temperature for about 15 min. The reaction was stopped with 50 μl of 2.5 N H2SO4, and the absorbance was read at 490 nm.
Each test was performed in duplicate, and the mean absorbance of BSA-only wells was subtracted from that of wells with ND-O-BSA before analysis.
RESULTS
Detection of PGL-I in untreated patients.
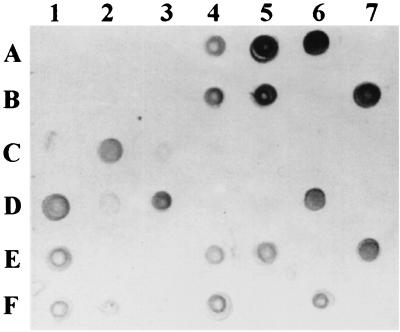
Crude lipids were extracted from serum samples obtained from leprosy patients before starting chemotherapy, and the lipid fractions containing the PGL-I antigen were purified using the Florisil column. The purified lipids were then applied to a polysulfone membrane and subjected to dot ELISA. The results were read visually, and some of the dot ELISA results are shown in Fig. 1. In general, the size and color intensity of dots varied markedly from 1+ (Fig. 1 dot 5E) to 2+ (dot 4B) to 3+ (dot 6A) between serum samples. This thus indicated that the circulating PGL-I level in blood is markedly different between patients.
FIG. 1.
Dot ELISA for the detection of the PGL-I-containing lipid fraction from serum samples of leprosy patients. A 5-μl portion of serum lipid dissolved in hexane was applied to each square, and the rest of the ELISA steps were then carried out. Any visible dot was considered PGL-I antigen positive. Note the difference in the sizes and intensities of dots because of different quantities of PGL-I in serum.
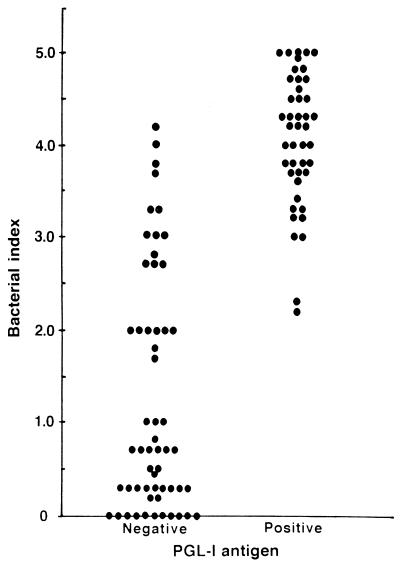
To determine the range of BI in leprosy patients whose serum samples were PGL-I positive, the leprosy patients were grouped based on PGL-I detection results, and the BI of leprosy patients were plotted in each group. As shown in Fig. 2, PGL-I was detectable in patients with BI as low as 2.2, although the majority of PGL-I-positive patients had BI over 3.0. On the other hand, a portion of leprosy patients with BI over 3.0 had no circulating PGL-I antigen in sera detectable by the method employed in this study. When the prevalence of PGL-I antigenemia was analyzed based on the average BI, the PGL-I antigen was detectable in sera from 27 (93.1%) of 29 patients with BI of 4.0 to 5.0, from 15 (68.2%) of 22 patients with BI of 3.0 to 3.9, and from 2 (16.7%) of 12 patients with BI of 2.0 to 2.9, respectively (Table 1). However, none of the 37 patients with BI of less than 2.0 had circulating PGL-I antigen in their sera. When the results were analyzed based on the clinical spectrum of leprosy, PGL-I was detectable in 25 (89.3%) of 28 LL patients, 18 (56.3%) of 32 BL patients, and 1 (3.7%) of 27 BT patients (data not shown). None of 13 TT and 1 indeterminate patients had PGL-I antigenemia.
FIG. 2.
Distribution of BI of patients with or without detectable PGL-I antigen in their serum specimens.
TABLE 1.
Detection of PGL-I in sera from untreated leprosy patients
| BI | No. of sera assayed | No. (%) of PGL-I-positive seraa |
|---|---|---|
| 0.0–1.9 | 37 | 0 (0) |
| 2.0–2.9 | 12 | 2 (16.7) |
| 3.0–3.9 | 22 | 15 (68.2) |
| 4.0–5.0 | 29 | 27 (93.1) |
Determined by dot ELISA.
Monitoring the PGL-I level after chemotherapy.
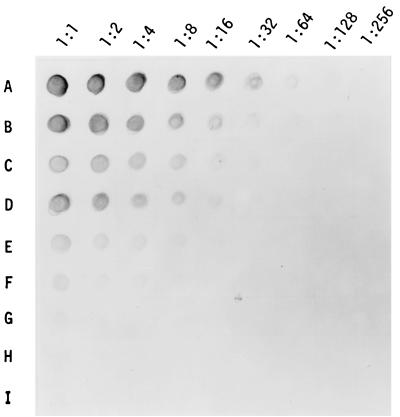
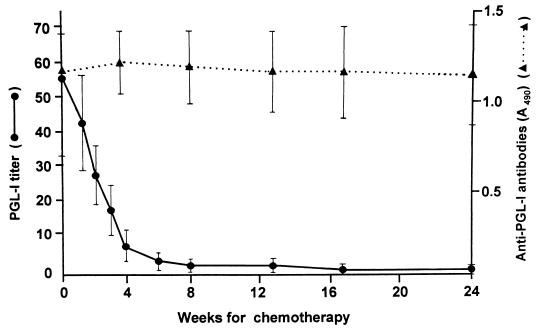
A semiquantitative assay was used to compare PGL-I antigen levels before and after starting chemotherapy. The purified lipid fraction containing PGL-I was serially diluted twofold in hexane, and each diluted lipid was assayed for the presence of PGL-I. Figure 3 shows some of the results of PGL-I titer determinations in sera serially obtained from a leprosy patient. The PGL-I titer in serum obtained before treatment was 1:128, and it decreased rapidly to 1:8 2 months after starting chemotherapy and was barely detectable at 6 months. Likewise, eight patients with BI of 4.5 or over were examined for their PGL-I antigen levels weekly during the first month of chemotherapy and after 2, 4, and 6 months. In addition, anti-PGL-I IgM antibodies were assayed in the serum samples and the results were compared with the PGL-I antigen level. As expected, the mean titer of PGL-I antigen decreased rapidly soon after starting chemotherapy: by about 50% within 2 weeks of chemotherapy, by about 90% after 1 month, and by more than 95% after 2 months (Fig. 4). Six of eight patients had no PGL-I detectable 6 months after starting MDT. In contrast, there was no significant decrease in mean anti-PGL-I IgM antibody levels during the first 6 months, particularly in the patient group. Thus, PGL-I antigen seems to be a good marker for monitoring the effectiveness of chemotherapy during the early phase of MDT.
FIG. 3.
Titer determination of PGL-I-containing lipids purified from serum samples that were obtained serially from a leprosy patient following chemotherapy. Rows: A, before starting chemotherapy; B, 1 week after starting chemotherapy; C, 2 weeks; D, 3 weeks; E, 1 month; F, 2 months; G, 4 months; H, 6 months; I, 9 months.
FIG. 4.
Comparison between the PGL-I antigen level and anti-PGL-I serum antibody levels following chemotherapy of lepromatous leprosy patients. Each value represents the mean and standard deviation for eight patients.
To determine the duration of PGL-I antigenemia in patients undergoing MDT, serum samples obtained at predetermined intervals from leprosy patients were examined for the presence of the PGL-I antigen. In general, within 2 to 6 months of starting MDT, the PGL-I antigen level declined rapidly to undetectable in serum samples from patients who had a low (1+; group I) or moderate (2+; group II) level of PGL-I at the time of diagnosis (Table 2). Only one (6.3%) of 16 patients with a PGL-I level of 2+ had the antigen detectable in this study after 6 months of therapy. Although patients with a high level (3+; group III) of PGL-I at the time of diagnosis showed a longer duration of PGL-I antigenemia, 14 (77.8%) of 18 patients were PGL-I antigen negative after 6 months of MDT and only 1 patient had circulating PGL-I antigen after 12 months of MDT. In contrast, there was no substantial decrease in BI in the majority of patients after 12 months of MDT. The mean BI declined from 4.3 ± 0.5 at the time of diagnosis to 3.4 ± 0.8 after 12 months of MDT in group III. This was in comparison to the other groups, in which the BI declined from 4.2 ± 0.7 to 2.9 ± 0.9 after 12 months for group II patients and from 3.4 ± 0.7 to 1.5 ± 0.5 for group I patients. The results clearly indicate that PGL-I antigen is a better indicator for monitoring the effects of chemotherapy on leprosy.
TABLE 2.
Detection of PGL-I in sera obtained serially from leprosy patients after starting MDT
| Patient groupa | No. of PGL-I-positive patients
|
||||||
|---|---|---|---|---|---|---|---|
| Before MDT | After MDT
|
||||||
| 1 mo | 2 mo | 4 mo | 6 mo | 9 mo | 12 mo | ||
| I | 5 | 2 | 1 | 0 | |||
| II | 16 | 15 | 8 | 4 | 1 | 0 | |
| III | 18 | 18 | 16 | 9 | 4 | 2 | 1 |
Groups are based on the initial PGL-I level. Groups I, 1+; group II, 2+; group III, 3+ (by visual grading of dot ELISA results as shown in Fig. 1).
DISCUSSION
This study showed that the majority of MB leprosy patients with BI over 3.0 had PGL-I antigenemia and that the PGL-I antigen disappeared from the circulation within 6 months of starting MDT. This thus indicated that the PGL-I antigen is a useful marker for monitoring the effect of MDT in leprosy patients with high bacterial loads. Since PGL-I is the M. leprae-specific antigen, the presence of the antigen in serum and other clinical samples may indicate an active M. leprae infection in the body. Previous studies showed that the PGL-I antigen was detectable in serum, urine, and biopsy samples from certain groups of leprosy patients, primarily MB patients. However, it has not been fully evaluated what proportion of MB patients would have PGL-I antigenemia and how long the antigen would be present in blood after starting MDT in leprosy patients.
One of the objectives of this study was therefore to determine the proportion of patients with PGL-I antigenemia in comparison to the average BI of leprosy patients. As expected, PGL-I was detectable in most serum samples from untreated leprosy patients with an average BI over 4.0. Its prevalence declined markedly among patients with a BI of 3.0 to 4.0, and it was hardly detectable in sera from patients with a BI less than 3.0. These results supported reports by Roche et al. 12 and Chanteau et al. 1, in which the majority of leprosy patients with a BI over 4.0 or LL patients had PGL-I antigenemia before starting MDT. The presence of PGL-I in sera thus clearly reflects the higher bacterial loads. It was also of interest that several patients whose BI was over 3.0 had no detectable PGL-I in sera, despite having a relatively high BI, up to 4.0 to 4.2. The patients with a BI over 4.0 had a relatively high level of anti-PGL-I antibodies despite the absence of the antigen in serum (data not shown), indicating production of PGL-I by M. leprae. Such an absence of PGL-I antigenemia in MB patients with BI over 4.0 was also reported by Roche et al. 12. One possible explanation would be previous medication with anti-leprosy drugs despite denial by the patients at the time the clinical history was obtained.
The other objective of this study was to closely monitor the PGL-I level immediately after the start of MDT in leprosy patients. When the PGL-I level in sera were measured semiquantitatively in serial serum samples, PGL-I declined rapidly. Even 1 week after the start of MDT, there was an indication of a decrease in PGL-I level, and the mean PGL-I titer was reduced by one-half after 2 weeks of MDT in this study. Previously, it was shown that the PGL-I titer apparently declined in serum samples obtained at least 1 or 2 months after starting MDT 1, 4, 12, 17. The results suggested that PGL-I was actively secreted from live M. leprae into surrounding tissues, and as soon as the bacilli were killed by chemotherapy, the PGL-I antigen was no longer produced and only the residual antigens in tissues and in blood disappeared from the circulation with a half-life of about 2 weeks.
The duration of PGL-I antigenemia was apparently well correlated with the initial level of PGL-I antigen in sera, which also reflected the bacterial loads in leprosy patients. PGL-I was barely detectable in sera from patients after 6 months of MDT. Only about 13% of patients who had PGL-I antigenemia before chemotherapy had PGL-I antigen in sera detectable by the method used in this study. Chanteau et al. 1 also showed that 3 of 10 patients had residual PGL-I in sera 6 months after the start of MDT and that PGL-I level decreased by 97% 1 year after MDT in MB leprosy patients. Therefore, the PGL-I level in serum or other clinical samples may be a useful marker for monitoring the effectiveness of MDT among MB patients, particularly during the early stage of chemotherapy.
Interestingly, Roche et al. 12 reported that PGL-I levels decreased very rapidly in patients infected with drug-sensitive M. leprae but decreased slowly in patients infected with drug-resistant bacilli or with the erythema nodosum leprosum (ENL) reaction. In our study, however, no patients relapsed from WHO MDT even 3 to 4 years after their release from treatment. Therefore, it was not possible to correlate the antigen clearance rate with the outcome of relapse of drug-resistant M. leprae infection. In addition, although there were 10 ENL patients among the study patients, we did not note any significant delay in PGL-I antigen clearance compared to those in patients with the same BI at the time of diagnosis. The slow clearance of PGL-I antigen in sera from ENL patients may reflect a high BI at the beginning of treatment.
As indicated in the literature, therefore, the PGL-I antigen detection assay can be added to the existing methods for laboratory assessment of leprosy patients after starting MDT. To date, the morphological index has been used for early assessment of MDT, but it has not been reliable, mainly due to variation in the results. BI has thus been most widely used for monitoring the effectiveness of MDT in leprosy patients. However, at least 1 year is required to see any measurable decrease in BI. Anti-PGL-I antibody levels also decreased following MDT, but the reduction rate was measured at 50% after 2 years. PGL-I antigen detection thus seems to have an advantage over morphological index, BI, and anti-PGL-I antibody assay for monitoring the effects of chemotherapy of leprosy.
ACKNOWLEDGMENTS
This study was supported in part by grants from the Korean Science and Engineering Foundation (951-0705-026-2), Seoul, Korea; the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR); and the Leonard Wood Memorial (American Leprosy Foundation), Rockville, Md. The native PGL-I antigen was provided through funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, contract N01-AI-05074.
We thank the technical staffs at the Leonard Wood Memorial Center in Cebu, Philippines, for their help in collection and processing of clinical samples.
REFERENCES
- 1.Chanteau S, Cartel J L, Celerier P, Plichart R, Desforges S, Roux J. PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy. Int J Lepr. 1989;57:735–743. [PubMed] [Google Scholar]
- 2.Chatterjee D, Douglas J T, Cho S N, Rea T H, Gelber R H, Aspinall G O, Brennan P J. Chemical synthesis and seroreactivity of O - (3,6 - di - O - methyl - β - d - glucopyranosyl) - (1→4) - O - (2,3 - di - O - methyl - α - 1 - rhamnopyranosyl)-(1→9)-oxynonanoyl-bovine serum albumin—the leprosy-specific, natural disaccharide-octyl-neoglycoprotein. Carbohydr Chem. 1986;156:39–56. doi: 10.1016/s0008-6215(00)90098-3. [DOI] [PubMed] [Google Scholar]
- 3.Cho S N, Cellona R V, Fajardo T T, Jr, Abalos R M, dela Cruz E C, Walsh G P, Kim J D, Brennan P J. Detection of phenolic glycolipid-I antigen and antibody in sera from new and relapsed lepromatous patients treated with various drug regimens. Int J Lepr. 1991;59:25–31. [PubMed] [Google Scholar]
- 4.Cho, S. N., S. W. Hunter, R. H. Gelber, T. H. Rea, and P. J. Brennan. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153:560–569. [DOI] [PubMed]
- 5.Cho S N, Shin J S, Choi I H, Kim S H, Kim D I, Kim J D. Detection of phenolic glycolipid I of Mycobacterium leprae and antibodies to the antigen in sera from leprosy patients and their contacts. Yonsei Med J. 1988;29:219–224. doi: 10.3349/ymj.1988.29.3.219. [DOI] [PubMed] [Google Scholar]
- 6.Cho S N, Yanagihara D L, Hunter S W, Gelber R H, Brennan P J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983;41:1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkes R, Niday E L, Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982;119:142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- 8.Hunter S W, Brennan P J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981;147:728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaldany P R J, Nurlign A. Development of a dot-ELISA for detection of leprosy antigenuria under field conditions. Lepr Rev. 1986;57(Suppl. 2):95–100. doi: 10.5935/0305-7518.19860059. [DOI] [PubMed] [Google Scholar]
- 10.Mahon A C, Nurlign A, Kebede B, Becx-Bleumink M, Lefford M J. Urinary phenolic glycolipid I in the diagnosis and management of leprosy. J Infect Dis. 1991;163:653–656. doi: 10.1093/infdis/163.3.653. [DOI] [PubMed] [Google Scholar]
- 11.Ridley D S, Jopling W H. Classification of leprosy according to immunity; a five-group system. Int J Lepr. 1966;34:255–273. [PubMed] [Google Scholar]
- 12.Roche P W, Britton W J, Failbus S S, Williams D, Pradhan H M, Theuyenet W J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int J Lepr. 1990;58:480–490. [PubMed] [Google Scholar]
- 13.Singh N B, Choudhary A, Bhatnagar S. Detection of M. leprae-specific antigens with dot-ELISA in urine and nasal samples from leprosy patients. Int J Lepr. 1991;59:398–404. [PubMed] [Google Scholar]
- 14.Vemuri N, Khandke L, Mahadevan P R, Hunter S W, Brennan P J. Isolation of phenolic glycolipid I from human lepromatous nodules. Intl J Lepr. 1985;53:487–489. [PubMed] [Google Scholar]
- 15.Voller A, Bidwell D E, Bartlett A. The enzyme-linked immunoassay (ELISA). Alexandria, Va: Dynatech Laboratories, Inc.; 1979. [Google Scholar]
- 16.World Health Organization. Action program for the elimination of leprosy. Status report. WHO/LEP/98.2. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 17.Young D B, Harnish J P, Knight J, Buchanan T M. Detection of phenolic glycolipid I in sera from patients with lepromatous leprosy. J Infect Dis. 1985;152:1078–1081. doi: 10.1093/infdis/152.5.1078. [DOI] [PubMed] [Google Scholar]