Abstract
Pathogenic Escherichia coli strains are known to cause edema disease (ED) and postweaning diarrhea (PWD) in piglets. Although the exact mechanisms of pathogenicity that lead to ED-PWD remain to be elucidated, E. coli-borne Shiga-like toxin and adhesion-mediating virulence factors such as F18 adhesin or F4 fimbriae are believed to play a central role in ED-PWD. In light of these observations we investigated whether another E. coli adhesin, the plasmid-encoded AIDA (adhesin involved in diffuse adherence) might also be present in ED-PWD-causing E. coli isolates. For rapid screening for the AIDA system in large numbers of isolates, a multiplex PCR method along with a duplex Western blot procedure was developed. When screening 104 strains obtained from pigs with or without ED-PWD, we observed a high prevalence of the AIDA operon in porcine E. coli isolates, with over 25% of all strains being AIDA positive, and we could demonstrate a significant association of the intact AIDA gene (orfB) with ED-PWD, while defects in orfB were associated with the absence of disease. Although our data hint toward a contribution of AIDA to ED-PWD, further studies will be necessary since the presence of the AIDA genes was also associated with the presence of the Shiga-like toxin and F18 adhesin genes, two reported virulence factors for ED-PWD.
Postweaning diarrhea (PWD) and edema disease (ED) are serious infectious diseases for piglets and are responsible for major economic losses worldwide. ED and PWD are caused by certain groups of Escherichia coli strains including enterotoxigenic E. coli (ETEC) and Shiga toxin-producing E. coli (STEC) 18, 23. E. coli strains isolated from piglets with PWD typically belong to serogroups O8, O141, and O149, are hemolytic, and produce thermostable and/or thermolabile enterotoxins 8, 9, 27. E. coli strains that cause ED are often members of the STEC group. Typically, these strains exhibit serogroups O138, O139, and O141, are hemolytic as well, and produce the Shiga-like toxin Stx2e 4, often in conjunction with enterotoxins.
Although detailed information about the mechanisms of pathogenicity which lead to PWD and ED is missing, Shiga-like toxins such as Stx2e are considered the preeminent virulence factors for these diseases 19. The toxin damages the vascular endothelium of the small intestine, subcutis, and brain and ultimately leads to subcutaneous edema and neurological disorders 10, 16, 20. Besides the toxins, bacterial surface molecules that mediate microbial adhesion to the intestinal surface are likely to play an important role. The closest link to PWD and ED was found for the F18 family of adhesins, which mediate colonization of ETEC and STEC strains that cause PWD and ED in weaned pigs 12, 24, 29. Imberechts and collegues 11 observed in about 80% of strains investigated a correlation for the distribution of fedA, the gene encoding the major subunit of F18, with stx2e, the gene encoding Stx2e. Further studies 21 confirmed that correlation and showed that F18 is also associated with serotypes O139 and O141.
Members of the pathotypically heterogeneous group of diffuse adhering E. coli (DAEC) were shown to be diarrheagenic pathogens for humans 13. Their molecular equipment for attachment to host cell surfaces appears to be different from that of locally adhering E. coli strains. So far, four DAEC-specific adhesins have been described: the fimbrial adhesin F1845 5 and three afimbrial adhesins: a 57-kDa adhesion-mediating protein 30, the CF16K molecule 14, and the AIDA-I system 1.
The genes encoding AIDA (adhesin involved in diffuse adherence) are located on two open reading frames, orfA and orfB. The latter codes for the AIDA autotransporter system 1, 25, which is synthesized as a pre-pro-protein that is C-terminally processed to generate the mature adhesin AIDA-I and the outer membrane integrated AIDAC, which functions as the translocator for AIDA-I 1, 3, 25. orfA codes for a 45-kDa cytoplasmic protein which is required to modify AIDA-I such that it adheres to target cells 2. Although the AIDA system has first been characterized in a clinical E. coli isolate responsible for infant diarrhea, AIDA genes have been detected only in about 2 to 4% of human isolates 13, 14. Thus, an animal reservoir for AIDA may exist.
As adhesins appear to play a role in PWD and ED of piglets, we wanted to know whether AIDA might be important in ED-PWD and whether porcine E. coli strains could be a reservoir of AIDA-positive E. coli strains. We could demonstrate that the AIDA system indeed occurs at a much higher frequency in porcine E. coli isolates than in human and other mammalian E. coli isolates. Moreover, the AIDA system was shown to be associated with two other described virulence factors for ED-PWD, namely, F18 and Stx2e. This might indicate a potential involvement of AIDA in the pathogenesis of ED and PWD.
(This study represents part of the Ph.D. thesis of U. Niewerth.)
MATERIALS AND METHODS
Bacterial strains.
E. coli strains of porcine origin were sampled at different farms in Hessia, Germany. The health profiles of the pigs from which the E. coli strains were isolated are based on the diagnoses of local veterinarians and do not distinguish between ED and PWD. Follow-up data about the pathogenicity, virulence, or lethality of invididual strains were not available. We therefore discriminated between sick animals (representative for manifestation of ED and/or PWD at the time of sampling) or apparently healthy animals (no signs of either ED or PWD at the time of sampling) and assigned the 104 strains tested to two groups accordingly: group I contained 44 strains isolated from animals that were diagnosed with ED or PWD. Group II contained 60 strains isolated from animals that were not diagnosed with ED or PWD and that were apparently healthy at the time of sampling. E. coli isolates from other animal species (cattle, horses, sheep, cats, rabbits) were from the strain repository of the Institut Pasteur (Paris, France). Human clinical isolate E. coli 2787 (O126:H27) and E. coli K-12 strain C600 were used as positive and negative controls, respectively.
Oligonucleotide primers.
Primers for detection of fedA or stx2e genes were taken from the literature 10, 15. AIDA primer sequences are based on the DNA sequence of orfA (I. Benz, unpublished results) and orfB (EMBL/GenBank/DDBJ nucleotide accession no. X65022) of the AIDA operon and were optimized for multiplex PCR (M-PCR) with the program Primer (HUSAR program package; German Cancer Research Center, Heidelberg, Germany). All primers are summarized in Table 1. Primers were synthesized by Eurogentec (Seraing, Belgium).
TABLE 1.
Primer sequences used for amplification of fragments derived from AIDA, F18, and Stx2e
| Target region | Primer | Oligonucleotide sequence (5′→3′) | Product size (bp) | Reference |
|---|---|---|---|---|
| orfAa | UN19 | CTGGGTGACATTATTGCTTGG | 370 | This study |
| UN20 | TTTGCTTGTGCGGTAGACTG | |||
| orfBb | UN21 | TGCAAACATTAAGGGCTCG | 450 | This study |
| UN22 | CCGGAAACATTGACCATACC | |||
| orfBc | UN23 | CAGTTTATCAATCAGCTCGGG | 543 | This study |
| UN24 | CCACCGTTCCGTTATCCTC | |||
| fedAd | fedA1 | GTGAAAAGACTAGTGTTTATTTC | 230 | 10 |
| fedA2 | CTTGTAAGTAACCGCGTAAGC | |||
| stx2ee | Vte-a | CCTTAACTAAAAGGAATATA | 510 | 15 |
| Vte-b | CTGGTGGTGTATGATTAATA |
Giving rise to a fragment from the coding region for ORFA.
Giving rise to a fragment from the coding region for AIDA-I (nucleotides 1014 to 1556).
Giving rise to a fragment from the coding region for AIDAC (nucleotides 3131 to 3580).
Giving rise to a fragment from the coding region for fedA, the major subunit of F18.
Giving rise to a fragment from the coding region for Stx2e.
PCR.
PCR reagents were obtained from Roche Diagnostics (Mannheim, Germany). PCR was performed in a 25-μl volume containing 5 μl of cell lysates, 0.2 mM each deoxynucleoside triphosphate, 2.5 mM MgCl2, 10 pmol of each primer, and 0.75 U of Taq polymerase. For M-PCR three sets of primers were used in parallel. PCRs were started with a double-strand melting step of 2 min at 94°C, amplification for 30 cycles, and an extended polymerization step of 10 min at 72°C. Parameters for each amplification cycle were as follows: 1 min at 94°C, 1 min at 63.5°C, and 1 min and 30 s at 72°C (orfA and orfB M-PCRs); 1 min at 94°C, 1 min at 56°C, and 1 min at 72 °C (fedA PCR); and 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C (stx2e PCR). Ten microliters from each PCR run was analyzed on a 2.0% agarose gel, and fragments were visualized by ethidium bromide staining. DNAs of E. coli strains 2787 and C600 served as controls.
DNA sequencing.
The following PCR-amplified fragments (both strands) were sequenced by SEQLAB (Göttingen, Germany): fragments a (central region of orfA) of strains 2787, S103G, S116G, S125G, S130G, S134G, S142G, S147G, S158G, S162G, S190G, S192G, S199G, and S209G; fragments c (5′ region of orfB ≈ AIDA-I) of strains 2787, S103G, S116G, S142G, S162G, and S192G; and fragments b (3′ region of orfB ≈ AIDAC) of strains 2787, S103G, S116G, S142G, S162G, and S192G. Sequences were analyzed with the ClustalW program of the HUSAR program package.
Southern blotting.
Fragments obtained by PCR were separated on a 2.0% agarose gel, transferred to positively charged nylon membranes (Roche Diagnostics), and immobilized by UV cross-linking. DNA probes for AIDA were generated by M-PCR as described above with DNA from E. coli 2787 as the template. The fragments were labeled with digoxigenin (PCR DIG Probe Synthesis kit; Roche Diagnostics) and purified with a PCR purification kit (Roche Diagnostics). Hybridization and visualization of the bands were carried out as described elsewhere 7.
Preparation of whole-cell lysates.
Bacterial cultures were grown to the exponential phase in Standard I medium (Merck, Darmstadt, Germany), aliquoted into portions of approximately 2.0 × 108 bacteria, harvested by centrifugation at 12,000 × g for 5 min at room temperature (RT), washed in 1 ml of phosphate-buffered saline–2 mM NaH2PO4–8 mM Na2HPO4–140 mM NaCl per aliquot, centrifuged again, resuspended in 200 μl of sodium dodecyl sulfate (SDS)-sample buffer 17, and lysed at 100°C for 10 min. Cell debris was removed by centrifugation before the supernatant was transferred to a fresh tube and stored at −20°C.
Detection of AIDA-I and AIDAC by Western blotting.
Ten microliters of whole-cell lysates was separated by SDS-polyacrylamide gel electrophoresis (10% gel) and transferred to a nitrocellulose membrane in 11.5 mM Tris-Cl (pH 8.0) by tank electroblotting 26. The membrane was blocked for 60 min in Tris-buffered saline (TBS; 10 mM Tris-Cl [pH 7.4], 150 mM NaCl) containing 5% (wt/vol) nonfat dry milk, (TBS-BLOTTO) before the blot was reacted for 60 min at RT with 1:30,000 and 1:50,000 (wt/vol) dilutions of AIDA-I- and AIDAC-specific rabbit antisera raised against AIDA-I of E. coli 2787 and a His6-tagged fusion protein of AIDAC of E. coli 2787, respectively, in TBS-BLOTTO containing 0.1% (vol/vol) Tween 20 (TBST-BLOTTO). After 60 min, the membrane was washed three times at RT for 10 min each time in TBS with Tween 20, followed by incubation with alkaline phosphatase-conjugated goat anti-rabbit antibody (1:7,500; Jackson Immunoresearch Laboratories, West Grove, Pa.) in TBST-BLOTTO for 60 min at RT. After another three washes in TBS the blot was developed at RT in 60 mM Nitro Blue Tetrazolium–115 mM 5-bromo-4-chloro-3-indolylphosphate in 100 mM Tris-HCl–1 mM MgCl2 (pH 9.5).
Data analysis and statistics.
Two-by-two tables of the data were analyzed by Fisher's exact test; larger tables were analyzed by chi-square analysis. In cases in which orphan serotypes, virulence factor combinations, or expression patterns yielded expected values <1 or more than 20% of the expected values were <5, chi-square tests were considered invalid 6. When possible and meaningful, the respective groups were reorganized and/or combined to obtain sufficiently large sample sizes for unbiased statistical anlysis. Results were considered significant only if P was <0.05. All statistical analyses were carried out with the Statview 4.5 program (Abacus Concepts, Berkeley, Calif.).
RESULTS
Rapid analysis of AIDA genes and gene products in E. coli isolates by M-PCR and duplex Western blotting.
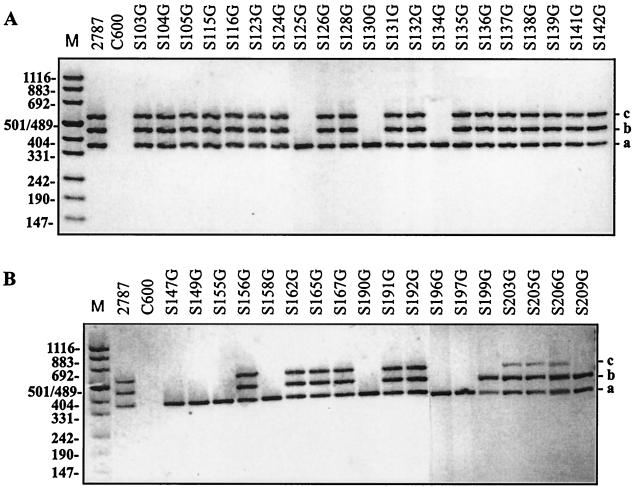
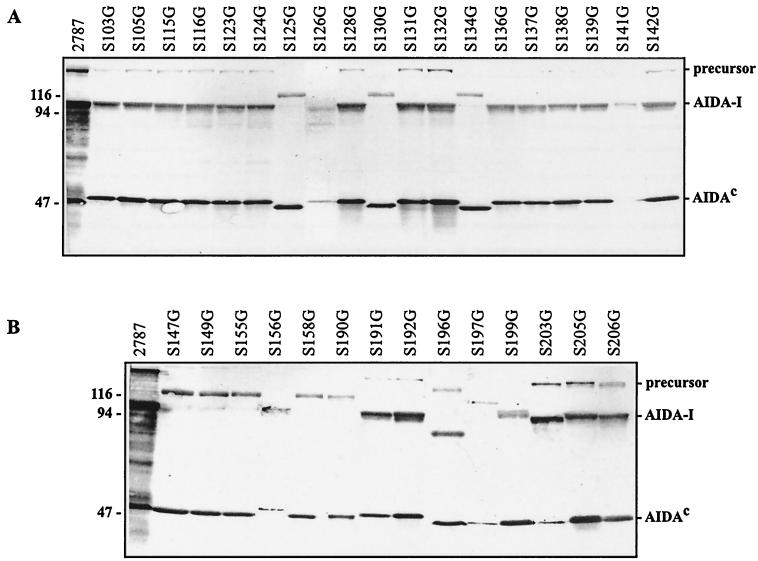
To minimize false-negative results for the detection of AIDA genes, we devised an M-PCR detection system in which two primer pairs detect fragments of orfB, while a third primer pair amplifies a portion of orfA. The primer pairs displayed an excellent specificity for the target genes for a positive control (E. coli 2787) and no signal for a negative control (E. coli C600) and could be used simultaneously without any interference (Fig. 1). The primers and the expected amplification products are listed in Table 1. To check for the expression of AIDA we used duplex Western blotting by simultaneously applying two specific antisera; one directed against AIDA-I and the second directed against AIDAC (Fig. 2). The results for 104 E. coli field isolates subjected to this analytical regimen are given in Table 2.
FIG. 1.
Analysis by M-PCR of strains positive for the AIDA system. (A) Samples from PCR of strains obtained from pigs diagnosed with ED or PWD. (B) Samples from PCR of strains isolated from pigs which at the time of sampling showed no ED or PWD. a, fragment amplified with primers UN19 and UN20 derived from orfA; b, fragment amplified with primers UN23 and UN24 derived from orfB in the region coding for the AIDAC part of the AIDA system; c, fragment amplified with primers UN21 and UN22 derived from orfB in the region coding for the AIDA-I part of the AIDA system.
FIG. 2.
Analysis of whole-cell lysates of strains positive for the AIDA system by immunoblot assays. Whole-cell lysates were separated on SDS–10% polyacrylamide gels, and proteins were detected by Western blot analysis with specific antibodies raised against AIDA-I and AIDAC. (A) Samples from PCR of strains obtained from animals diagnosed with ED or PWD. (B) Samples from PCR of strain isolated from animals not diagnosed with ED or PWD. precursor, proteins resembling the uncleaved pre-pro-protein of the AIDA system; AIDA-I, proteins resembling the adhesin of the AIDA system; AIDAC, proteins resembling the autotransporter of the AIDA system.
TABLE 2.
Results obtained by PCR and immunoblot assaysa
| Isolate group and serotype | stx2e | fedA | orfA | AIDA | Strain |
|---|---|---|---|---|---|
| O138:K81 | + | + | + | − | S104G |
| O138:K81 | − | + | +a | + | S134G |
| O138:K81 | − | − | − | − | S140G |
| O138:K81 | − | − | − | − | S144G |
| O139:K12 | + | + | + | + | S105G |
| O139:K82 | − | − | − | − | S101G |
| O139:K82 | − | − | − | − | S108G |
| O139:K82 | + | + | + | + | S115G |
| O139:K82 | + | + | + | + | S116G |
| O139:K82 | + | + | + | + | S123G |
| O139:K82 | + | + | + | + | S124G |
| O139:K82 | + | + | +a | + | S125G |
| O139:K82 | + | + | + | + | S126G |
| O139:K82 | − | − | − | − | S127G |
| O139:K82 | + | + | + | + | S131G |
| O139:K82 | + | + | + | + | S132G |
| O139:K82 | + | + | + | + | S138G |
| O139:K82 | + | + | + | + | S139G |
| O141:K85 | + | + | + | + | S103G |
| O141:K85 | + | + | − | − | S106G |
| O141:K85 | + | + | + | + | S128G |
| O141:K85ab | − | − | − | − | S107G |
| O141:K85ab | − | − | − | − | S117G |
| O141:K85ab | − | − | − | − | S122G |
| O141:K85ab | − | − | − | − | S133G |
| O141:K85ab | + | + | + | − | S135G |
| O141:K85ab | + | + | + | + | S136G |
| O141:K85ab | + | + | + | + | S137G |
| O141:K85ab | + | + | + | + | S142G |
| O141:K85ac | − | − | − | − | S110G |
| O141:K85ac | − | − | − | − | S111G |
| O141:K85ac | − | − | − | − | S112G |
| O141:K85ac | − | − | − | − | S113G |
| O141:K85ac | − | − | − | − | S114G |
| O141:K85ac | − | − | − | − | S118G |
| O141:K85ac | − | + | − | − | S119G |
| O141:K85ac | + | + | + | + | S141G |
| O141:K85ac | − | − | − | − | S143G |
| O147:K89 | − | − | − | − | S120G |
| O149:K91 | − | − | − | − | S102G |
| O149:K91 | − | − | − | − | S109G |
| O149:K91 | − | − | − | − | S121G |
| O149:K91 | − | − | − | − | S129G |
| O149:K91 | + | + | +a | + | S130G |
| Isolates from pigs without symptoms of PWD | |||||
| O138:K81 | − | − | − | − | S146G |
| O138:K81 | − | + | +a | + | S147G |
| O138:K81 | − | + | +a | + | S149G |
| O138:K81 | − | − | − | − | S151G |
| O138:K81 | − | − | − | − | S154G |
| O138:K81 | − | + | +a | + | S155G |
| O138:K81 | − | + | +a | + | S158G |
| O138:K81 | − | − | − | − | S163G |
| O138:K81 | − | − | − | − | S160G |
| O138:K81 | − | − | +a | + | S190G |
| O138:K81 | − | − | − | − | S194G |
| O138:K81 | − | − | +a | + | S196G |
| O139:K82 | − | + | + | + | S192G |
| O139:K82 | − | − | − | − | S148G |
| O141:K85ab | − | − | − | − | S159G |
| O141:K85ab | − | − | − | − | S161G |
| O141:K85ab | + | + | + | − | S162G |
| O141:K85ab | + | + | + | − | S165G |
| O141:K85ab | + | + | + | − | S167G |
| O141:K85ab | − | − | − | − | S168G |
| O141:K85ab | + | + | + | + | S156G |
| O141:K85ab | − | + | + | + | S191G |
| O141:K85ac | − | − | − | − | S150G |
| O141:K85ac | − | − | − | − | S152G |
| O141:K85ac | − | − | − | − | S157G |
| O147:K89 | − | − | − | − | S153G |
| O149:K88ac | − | − | − | − | S166G |
| NTb | − | − | − | − | S170G |
| NT | − | − | − | − | S171G |
| NT | − | − | − | − | S172G |
| NT | − | − | − | − | S173G |
| NT | − | − | − | − | S174G |
| NT | − | − | − | − | S175G |
| NT | − | − | − | − | S176G |
| NT | − | − | − | − | S177G |
| NT | − | − | − | − | S178G |
| NT | − | − | − | − | S179G |
| NT | − | − | − | − | S180G |
| NT | − | − | − | − | S181G |
| NT | − | − | − | − | S182G |
| NT | − | − | − | − | S183G |
| NT | − | − | − | − | S184G |
| NT | − | − | − | − | S185G |
| NT | − | − | − | − | S186G |
| NT | − | − | − | − | S187G |
| NT | − | − | − | − | S188G |
| NT | − | − | − | − | S189G |
| NT | − | − | +a | + | S197G |
| NT | − | − | − | − | S198G |
| NT | − | − | +a,b | + | S199G |
| NT | − | − | − | − | S200G |
| NT | − | − | − | − | S201G |
| NT | − | − | − | − | S202G |
| NT | − | − | + | + | S203G |
| NT | − | − | − | − | S204G |
| NT | − | − | + | + | S205G |
| NT | − | − | + | + | S206G |
| NT | − | − | − | − | S207G |
| NT | − | − | − | − | S208G |
| NT | − | − | +a,b | − | S209G |
+, amplification of three fragments from sequences encoding ORFA, AIDA-I and AIDAC; +a,b, amplification of two fragments from sequences encoding ORFA and AIDAC; +a, amplification of one fragment from sequences encoding ORFA.
NT, not tested.
High prevalence of AIDA in E. coli strains of porcine origin.
Since AIDA has rarely been identified in human E. coli isolates 13, 14, E. coli strains from various animal species were analyzed by M-PCR for the presence of AIDA genes. The analysis revealed a significantly higher prevalence of AIDA (orfB) in porcine isolates (27 of 104 [26.0%] isolates) than in strains of human origin (3 of 352 [0.9%] orfB-positive strains) 23 (by Fisher's exact test, P < 0.0001), while strains from other species (cattle, rabbits, horses, sheep) tested negative (data not shown). By considering isolates from sick individuals only, the prevalence for pigs is even more pronounced, with 18 of 44 (40.9%) porcine strains carrying orfB, in whereas 3 of 262 (1.1%) diarrheagenic strains of human origin were orfB positive 13 (by Fisher's exact test, P < 0.0001). Thus, porcine E. coli strains appear to be a major reservoir for AIDA genes.
Genetic variability of AIDA operon in porcine E. coli strains.
M-PCR also provided insight into the variability of the two AIDA genes orfA and orfB. By PCR all 39 AIDA-positive porcine E. coli isolates were positive for orfA, but only 27 (69.2%) also carried an intact orfB. Among the 12 strains affected by mutations, 10 lacked the PCR products both for AIDA-I and for AIDAC, while 2 were still positive for the AIDAC amplification product. Surprisingly, the mutations did not abrogate expression of orfB in 11 of the 12 strains affected, as shown by duplex Western blot analysis. However, the gene products displayed considerable polymorphisms in their apparent molecular masses, with AIDA-I being shifted in most strains toward a higher molecular mass and AIDAC being shifted toward a lower molecular mass (Fig. 2). To gain further information about the mutation rates of orfA and orfB, we sequenced the PCR-amplified orfA fragments of 13 randomly selected strains and the PCR-amplified orfB fragments of 5 strains and compared them to the corresponding sequences of the positive control, E. coli 2787. No significant differences in the genetic variabilities of both orf genes could be detected. The between-strain similarity of orfA was 91.9% ± 5.8% (mean ± standard error of the mean), while the two orfB fragments exhibited similarities of 93.9% ± 4.9% for the AIDA-I fragment and 93.6% ± 3.1% for the AIDAC fragment. Thus, the average genetic variabilities of both orf genes of the AIDA operon between different porcine E. coli isolates appear to be ≤8%.
Extrachromosomal location of the AIDA operon in porcine E. coli strains.
All strains found to harbor AIDA genes by M-PCR contained at least one but often several plasmids of about 100 kb. Additionally, smaller plasmids could be detected in some strains. Since AIDA is known to be plasmid borne in the prototype strain E. coli 2787 1, we subjected all AIDA-positive porcine E. coli isolates to Southern blot analysis against orfA and orfB. The results show that AIDA is encoded on large plasmids of different sizes (between 100 and 150 kb) in strains of porcine origin.
Association of AIDA with PWD and/or ED.
The high prevalence of AIDA in sick hogs indicated an association of AIDA with ED-PWD, which was confirmed by correlation analysis. There is a significant correlation between the presence of orfB, as detected by PCR (by Fisher's exact test, P = 0.0036) or the expression of intact AIDA-I and AIDAC, as analyzed by Western blotting (by Fisher's exact test, P = 0.0015) and the onset of ED-PWD. Interestingly, in orfA-positive strains, PCR-detectable variations in orfB are associated with the absence of disease (by Fisher's exact test, P = 0.035). The expression of polymorphic AIDA-I and AIDAC proteins showed a similar tendency. Moreover, the mere presence of orfA was not associated with ED-PWD (by Fisher's exact test, P > 0.10).
Although the results presented above hint toward the involvement of AIDA in the pathogenesis of ED-PWD, the presence of the AIDA operon alone is not sufficient to cause disease since 9 of 60 E. coli strains (15.0%) from animals not suffering from ED-PWD turned out to carry the intact AIDA operon, while the AIDA operon was completely absent from 23 of 44 strains (52.3%) from sick animals. This indicates that AIDA may act as an adjuvant for other virulence or pathogenicity factors in ED-PWD.
Linkage of AIDA with Stx2e and F18 adhesin in porcine E. coli isolates.
To address a potential association of AIDA with other agents in ED-PWD, we included Stx2e and the F18 adhesin, two virulence factors described for ED-PWD, in our PCR analysis. The PCR data revealed strong correlations for both Stx2e and the F18 adhesin with ED-PWD (by Fisher's exact test, P < 0.0001 and P = 0.0002, respectively). Moreover, the presence of stx2e and fedA also correlated with the presence of orfA (by Fisher's exact test, P < 0.0001) for both correlations), intact orfB (by Fisher's exact test, P < 0.0001 for both correlations), and the expression of intact AIDA-I and AIDAC (by Fisher's exact test, P < 0.0001 for both correlations). Finally, a strong correlation between the presence of stx2e and fedA was observed (by Fisher's exact test, P < 0.0001). Taken together, these mutual correlations strongly indicate that AIDA is either functionally or physically, or in both ways, linked to Stx2e and the F18 adhesin in ED-PWD-causing E. coli strains.
Association of AIDA with certain E. coli serotypes.
For all ED-PWD-causing E. coli strains and 27 control strains, a preferential association of AIDA, Stx2e, or the F18 adhesin with a particular serotype was investigated. Serotypes O149:K91, O139:K12, O147:K89, and O149:K88ac were not abundant enough to allow valid chi-square calculations. For serotypes O138:K81, O139:K82, and O141:K85, including serotype O141:K85 subtypes K85ab and K85ac, statistical analyses demonstrated that orfB or stx2e does not occur with the same frequency (by the chi-square test, P = 0.0023 for both correlations). orfB and stx2e as well as intact AIDA (P was not significant) were most frequently associated with serotype O139:K82 and were underrepresented in serotype O138:K81 strains. The presence of fedA or orfA was not preferentially associated with either serotype. Thus, the structural gene orfB for the AIDA adhesin-autotransporter system and the gene for Stx2e appear to be associated with serotype O139:K82 in porcine E. coli. isolates.
Diagnostics.
The correlations between ED-PWD and the expression of intact AIDA-I and AIDAC or the presence of intact orfB, fedA, and stx2e pointed toward a diagnostic or predictive potential of these genes and their expression products. As measures for the predictive power of individual gene fragments or combinations of fragments, we determined the detectability of disease (sensitivity), the detectability of health (specificity), the reliabilities of positive and negative test results, and the overall assay validity (Youden index, i.e., [sensitivity + specificity] − 1) 31 for each analytical setup (Table 3). The detectability of the absence of ED-PWD was consistently above 83% when the expression of intact AIDA-I and AIDAC and the presence of intact orfB, fedA, or stx2e, or combinations thereof, were investigated. The reliability of a negative test result, however, was only ≤72%. This was due to a considerable rate of false-negative results (sick animals testing negative). Consequently, an overall low rate of detectability of disease (≤52%) was obtained, irrespective of the gene investigated, how PCRs were combined for multiplex runs, or whether Western blot analysis was included. On the other hand, the reliability of a positive test result reached up to 94% when the results for Western blots for intact AIDA and PCR assays for stx2e were combined. Mainly because of the low assay sensitivity, Youden indices did not exceed 0.41. When individual assays and combinations thereof were ranked on the basis of the Youden index, a single PCR for stx2e turned out to be the most powerful diagnostic tool for ED-PWD, although the reliability of positivity benefits from assays for the expression of intact AIDA-I and AIDAC, in addition to a stx2e-based PCR assay for ED-PWD. Taken together, our results indicate that the probes tested in this study are of limited value in completely ruling out as well as safely predicting the ED-PWD-causing potential of unknown E. coli isolates.
TABLE 3.
Determination of sensitivity, sensibility, and Youden index of several analytical setups
| Marker | Sensitivity | Specificity | Youden index |
|---|---|---|---|
| stx2e | 0.48 | 0.93 | 0.41 |
| fedA | 0.52 | 0.83 | 0.36 |
| orfA | 0.48 | 0.70 | 0.18 |
| orfB | 0.41 | 0.85 | 0.26 |
| stx2e + fedA | 0.48 | 0.93 | 0.41 |
| stx2e + orfA | 0.45 | 0.93 | 0.39 |
| stx2e + orfB | 0.41 | 0.93 | 0.34 |
| fedA + orfA | 0.48 | 0.83 | 0.31 |
| fedA + orfB | 0.41 | 0.90 | 0.31 |
| orfA + orfB | 0.41 | 0.85 | 0.26 |
| stx2e + fed | 0.45 | 0.93 | 0.39 |
| stx2e + fed | 0.41 | 0.93 | 0.34 |
| stx2e + orf | 0.41 | 0.93 | 0.34 |
| fedA + orf | 0.41 | 0.93 | 0.34 |
| stx2e + fedA + orfA + orfB | 0.41 | 0.93 | 0.34 |
DISCUSSION
The adhesin AIDA was originally identified in a clinical E. coli isolate derived from patient with infantile diarrhea 1, 2, 3. Subsequent studies, however, detected the AIDA genes in a small percentage of diarrheagenic human E. coli isolates only 13, 14, which indicates that a gene reservoir for AIDA distinct from human E. coli strains is likely to exist. As bacterial adhesins are believed to play an important role in E. coli-borne PWD and ED of piglets, we pondered whether AIDA might be present in porcine E. coli isolates and perhaps be involved in ED-PWD. We therefore investigated the distribution of the genes of the AIDA system and their expression products in E. coli isolates derived from pigs with and without symptoms of PWD and ED and in strains isolated from other mammalian species.
Since colony hybridization for genotyping usually requires highly defined reaction conditions in order to prevent erroneous results, Southern blots often are required to increase assay reliability. While this procedure may be workable for a small quantity of bacterial strains, it is clearly not the method of choice for routine analysis of large numbers of bacterial field isolates. The PCR analysis has proved to be a rapid, reliable, and cost-effective alternative to these assays. However, unexpected mutations are a considerable problem in PCR analysis of viral or bacterial genes. Therefore, to minimize false-negative results for detection of AIDA, we devised an M-PCR detection system in which two primer pairs detect the genes coding for the adhesive domain AIDA-I and the translocator domain AIDAC, respectively, while a third primer pair amplifies the gene of a protein which postranslationally modifies AIDA-I such that it is able to bind to mammalian cells.
Although not predictable in the first place, our choice of primers turned out to be an excellent one not only because they were highly specific and the amplified fragments could readily be distinguished from each other but also because they were able to detect point mutations or deletions in the AIDA genes which in turn were associated with the absence of ED-PWD. These mutations usually led to a loss of the respective PCR product and gave rise to size variations in the orfB gene products AIDA-I and AIDAC. This may indicate that our orfB primers bind to a region of the AIDA gene which codes for a protein sequence motif that is important for the posttranslational cleavage of the AIDA precursor. It will be interesting to see how the adhesive properties mediated by the AIDA system will be influenced by the sequence variations detected in the respective strains. By comparing the sequences of fragments from strains without PCR-detectable variations with the sequence of AIDA from E. coli strain 2787, a similarity of more than 90% for all three PCR products was found, indicating that the AIDA gene of our reference strain, strain 2787, may be considered representative for the AIDA genes of most E. coli isolates.
The epidemiological outcome of our PCR studies proved our initial hypothesis. It clearly showed that porcine E. coli strains are a major reservoir for AIDA genes, with AIDA being present in 26% of all isolates. Interestingly, E. coli isolates of bovine, equine, sheep, and rabbit origin did not carry the AIDA operon, which indicates that AIDA might have a certain host-related function in porcine E. coli. Our analysis could not rule out the existence of additional reservoirs for AIDA in other hosts, for instance, rodents or game. The presence of an AIDA gene pool in the gut flora of a domesticated animal indicates that the rare cases in which AIDA was found in human isolates are likely to be caused by strains of porcine origin, perhaps via contaminated food or porcine excretions.
The widespread occurence of AIDA in E. coli isolates from pigs suffering from ED-PWD along with its association with F18 and Stx2e, two reported virulence factors for ED-PWD, indicate that a physical and/or functional link may exist between AIDA, F18, Stx2e, and ED-PWD. In pathogenic E. coli, the genetic determinants for virulence factors are often carried on large plasmids, which, in the case of a physical linkage, would imply that all three gene loci can be found on a single plasmid. A plasmid location could indeed be demonstrated for AIDA in E. coli strain 2787 3, as well as in all porcine AIDA-positive isolates tested in this study. The fimbrial adhesin F18 has also been described as being plasmid encoded 28. The exact localization of the genes encoding Stx2e, however, has not been clearly demonstrated. The gene for Shiga-like toxin Stx2e, however, is located on the chromosome, namely, on a defective prophage 22. Thus, our data rather point toward a functional linkage between AIDA, F18, and Stx2e in ED-PWD.
Nevertheless, AIDA cannot be a sole reason for ED-PWD or a dominant virulence factor in this disease since a number of strains sampled from pigs without symptoms of ED-PWD carried an intact AIDA operon as well. This was the main reason for the poor diagnostic value of AIDA and its genes for ED-PWD compared to that of stx2e. Only a slight improvement in the reliability of positivity could be achieved when a Western blot for AIDA was included in the assays. On the other hand, stx2e is not a perfect tool either since the absence of stx2e does not render an E. coli strain harmless, as 52% of the strains isolated from sick piglets did not carry stx2e. Thus, ED and PWD appear to be multifactorial diseases to which several bacterial virulence factors may contribute. The AIDA system could possibly be one of these factors.
ACKNOWLEDGMENT
This study was funded in part by grant Schm770/7-1/2 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Benz I, Schmidt M A. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect Immun. 1989;57:1506–1511. doi: 10.1128/iai.57.5.1506-1511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz I, Schmidt M A. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27) Infect Immun. 1992;60:13–18. doi: 10.1128/iai.60.1.13-18.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 4.Bertschinger H U, Gyles C L. Oedema disease of pigs. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1994. pp. 193–220. [Google Scholar]
- 5.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to Hep-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochran W G. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 7.Engler-Blum G, Meier M, Frank J, Müller G A. Reduction of background problems in nonradioactive Northern and Southern blot analyses enables higher sensitivity than 32P-based hybridizations. Anal Biochem. 1993;210:235–244. doi: 10.1006/abio.1993.1189. [DOI] [PubMed] [Google Scholar]
- 8.Gyles C L. Escherichia coli. In: Gyles C L, Thoen C O, editors. Pathogenesis of bacterial infections in animals. Ames: Iowa State University Press; 1993. pp. 164–187. [Google Scholar]
- 9.Harel J, Lapointe H, Fallara A, Lortie L A, Bigras-Poulin A M, Larivière S, Fairbrother J M. Detection of genes for fimbrial antigens and enterotoxins associated with Escherichia coli serogroups isolated from pigs with diarrhea. J Clin Microbiol. 1991;29:745–752. doi: 10.1128/jcm.29.4.745-752.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imberechts H, De Greve H, Schlicker C, Bouchet H, Pohl P, Charlier G, Bertschinger H U, Wild P, Vanderkeckhove J, Van Damme J, Van Montagu M, Lintermans P. Characterization of F107 fimbriae of Escherichia coli 107/86, which causes edema disease in pigs, and nucleotide sequence of the major fimbrial subunit gene, fedA. Infect Immun. 1992;60:1963–1971. doi: 10.1128/iai.60.5.1963-1971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imberechts H, de Greve H, Hernalsteens J P, Schlicker C, Bouchet H, Pohl P, Charlier G, Bertschinger H U, Wild P, Vandekerckhove J. The role of adhesive F107 fimbriae and of SLT-llv toxin in the pathogenesis of edema disease in pigs. Zentbl Bakteriol Parasitenkd Infektkrankh Hyg Abt 1 Orig. 1993;278:445–450. doi: 10.1016/s0934-8840(11)80861-3. [DOI] [PubMed] [Google Scholar]
- 12.Imberechts H, Bertschinger H U, Nagy B, Deprez P, Pohl P. Fimbrial colonisation factors F18ab and F18ac of Escherichia coli isolated from pigs with postweaning diarrhea and edema disease. Adv Exp Med Biol. 1997;97:175–183. doi: 10.1007/978-1-4899-1828-4_26. [DOI] [PubMed] [Google Scholar]
- 13.Jallat C, Livrelli V, Darfeuille Michaud A, Rich C, Joly B. Escherichia coli strains involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J Clin Microbiol. 1993;31:2031–2037. doi: 10.1128/jcm.31.8.2031-2037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jallat C, Darfeuille-Michaud A, Rich C, Joly B. Survey of clinical isolates of diarrhoeagenic Escherichia coli: diffusely adhering E. coli strains with multiple adhesive factors. Res Microbiol. 1994;145:621–632. doi: 10.1016/0923-2508(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 15.Johnson W M, Pollard D R, Lior H, Tyler S D, Rozee K R. Differentiation of genes coding for Escherichia coli verotoxin 2 and the verotoxin associated with porcine edema disease (VTe) by the polymerase chain reaction. J Clin Microbiol. 1990;28:2351–2353. doi: 10.1128/jcm.28.10.2351-2353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kausche F M, Dean E A, Arp L H, Samuel J E, Moon H W. An experimental model for subclinical edema disease (Escherichia coli enterotoxemia) manifest as vascular necrosis in pigs. Am J Vet Res. 1992;53:281–287. [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Mainil J. Shiga/Verocytotoxins and Shiga/Verotoxigenic Escherichia coli in animals. Vet Res. 1999;30:235–257. [PubMed] [Google Scholar]
- 19.Marques L R M, Peiris J S M, Cryz S J, O'Brien A D. Escherichia coli strains isolated from pigs with edema disease produce a variant of Shiga-like toxin-II. FEMS Microbiol Lett. 1987;44:33–38. [Google Scholar]
- 20.McLeod D L, Gyles F L, Wilcock B P. Reproduction of edema disease of swine with purified Shiga-like toxin II variant. Vet Pathol. 1991;28:66–73. doi: 10.1177/030098589102800109. [DOI] [PubMed] [Google Scholar]
- 21.Moeller Aarestrup F, Jorsal S E, Ahrens P, Jensen N E, Meyling A. Molecular characterization of Escherichia coli strains isolated from pigs with edema disease. J Clin Microbiol. 1997;35:20–24. doi: 10.1128/jcm.35.1.20-24.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muniesa M, Recktenwald J, Bielaszewska M, Karch H, Schmidt H. Characterization of a Shiga toxin 2e-converting bacteriophage from an Escherichia coli strain of human origin. Infect Immun. 2000;68:4850–4855. doi: 10.1128/iai.68.9.4850-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy B, Fekete P Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet Res. 1999;30:259–284. [PubMed] [Google Scholar]
- 24.Ojeniyi B, Ahrens P, Meyling A. Detection of fimbrial and toxin genes in Escherichia coli and their prevalence in piglets with diarrhoea. The application of colony hybridization assay, polymerase chain reaction and phenotypic assays. J Vet Med B. 1994;41:49–59. doi: 10.1111/j.1439-0450.1994.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 25.Suhr M, Benz I, Schmidt M A. Processing of the AIDA-I precursor: removal of AIDAC and evidence for the outer membrane anchoring as a β-barrel structure. Mol Microbiol. 1996;22:31–42. doi: 10.1111/j.1365-2958.1996.tb02653.x. [DOI] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson R A, Francis D H. Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am J Vet Res. 1986;47:213–217. [PubMed] [Google Scholar]
- 28.Wittig W, Prager R, Stamm M, Streckel W, Tschape H. Expression and plasmid transfer of genes coding for the fimbrial antigen F107 in porcine Escherichia coli strains. Zentbl Bakteriol Parasitenkd Infektkrankh Hyg Abt 1 Orig. 1994;281:130–139. doi: 10.1016/s0934-8840(11)80563-3. [DOI] [PubMed] [Google Scholar]
- 29.Wittig W, Klie H, Gallien P, Lehmann S, Timm M, Tschäpe H. Prevalence of the fimbrial antigens F18ab and F18ac and of enterotoxins and verotoxins among Escherichia coli isolated from weaned pigs. Zentbl Bakteriol Parasitenkd Infektkrankh Hyg Abt 1 Orig. 1995;283:95–104. doi: 10.1016/s0934-8840(11)80895-9. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Wakisaka N, Nakae T, Kamano T, Serichantalergs O, Echeverria P. Characterization of a novel hemagglutinin of diarrhea-associated Escherichia coli that has characteristics of diffusely adhering Escherichia coli and enteroaggregative E. coli. Infect Immun. 1996;64:3694–3702. doi: 10.1128/iai.64.9.3694-3702.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youden D. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]