Abstract
(1) Background: Cartilage degeneration with the natural aging process and the role of physical activity on cartilage wellness is still not clear. The objective of the present review was to understand how different physical activity interventions affect the cartilage and to propose a Standard Operating Procedure for an exercise program to maintain knee joint health; (2) Methods: Articles were collected on three different electronic databases and screened against the eligibility criteria. Results were collected in tables and the main outcomes were discussed narratively; (3) Results: A total of 24 studies have been included after the screening process and aerobic, strength, flexibility, postural balance, and mobility interventions were detected. Different protocols and types of interventions were adopted by the authors; (4) Conclusions: Physical activity interventions have mainly positive outcomes on cartilage structure, but the protocols adopted are different and various. A Standard Operating Procedure has been proposed for a physical intervention focalized on cartilage wellness that could be adopted as an intervention in the clinical setting. Furthermore, the creation of a standardized protocol wants to help scientific research to move in the same direction.
Keywords: exercise, physical fitness, osteoarthritis, prevention, health
1. Introduction
The natural process of aging has an impact on articular cartilage with chondrocyte loss and a decline in metabolic response, alterations to the matrix and synovial tissue composition, and impairing the ability to maintain and repair these tissues [1]. Chondrocyte senescence contributes to cartilage degeneration, characterized by oxidative stress and the production of cytokines causing the so-called stress-induced senescent state [2]. A sedentary lifestyle with the consequent absence of loading for the cartilage accelerates the progression of cartilage degeneration [3]. On the opposite, physical activity (PA) practiced by young adults is positively associated with cartilage volume, and this seems protective against the development of osteoarthritis (OA) [4]. Fortunately, cartilage deforms during physiological activities and usually recovers after loading [5], making physical exercise ideal to increase blood flow to the connective tissues of the joints and prevent cartilage deterioration.
Even if the literature suggests positive outcomes of exercise in young adults [4], the effects of PA in adults and older adults are contradictory. Some studies suggest that high PA levels do not damage the cartilage of the knee [6] also if practiced for years [7], and it is suggested that it could reduce cartilage loss [8], slowing the progression of cartilage degeneration [3]. Differently, other studies suggest that PA has a deleterious effect on cartilage [9] causing degeneration [10] or worsening the composition [11], and accelerating the disruption of joint stability [3]. Higher PA levels may be at greater risk for cartilage, meniscus and ligament abnormalities [12].
These contradictory ideas on the effects of PA on cartilage should be due to the different dosages of the interventions, indeed, an underload or an overload are associated with cartilage damage [13]. Consequently, it is important the development of a PA intervention that includes aerobic and muscle resistance exercises to protect joints, promote health, and reduce disability in older age [14]. The importance of understanding the effects of a structured PA program increases in people with mild to moderate OA; indeed, the intervention should have to reduce pain and disability levels [15] not presenting deleterious effects.
The so-called Standard Operating Procedures are being widely adopted in other fields to proceed correctly and limit possible errors [16]. Their use could help standardize the interventions concerning PA in OA individuals and foster the clarification of the PA preventive and protective role towards cartilage health. The objective of the present review is to understand how different PA typologies interventions affect cartilage; and then propose a Standard Operating Procedure for an exercise program to prevent joint cartilage degeneration and maintain knee joint health, especially during aging.
2. Materials and Methods
This systematic review and meta-analysis follows the principles outlined by the PRISMA guidelines [17], and the checklist can be found in the Supplementary Materials. The protocol is not registered in a specific database, but it was written before the systematic review performance.
2.1. Eligibility Criteria
Population, Intervention, Comparison, Outcomes, and Study (PICO-S) criteria are followed. The population of interest is composed of people aged 18 years and older, both males and females, and no restrictions were adopted on body composition. Participants were excluded if they were professional or elite athletes; or if they presented physical (i.e., muscular dystrophy, hypotonia, injury to tendons or ligaments) or movement disorders (i.e., akinesia, bradykinesia, or dystonia). For the intervention, manuscripts are included if PA training is proposed without any other complementary intervention, to limit the confounding factors. Consequently, studies were excluded if presented with other complementary factors that could influence cartilage. The comparators considered are the cartilage evaluation pre- and post-intervention. Outcomes considered are related to magnetic resonance imaging (MRI) acquisition and blood and urinary samples. Only English written, original, peer-reviewed articles that adopted cross-sectional, correlational, randomized, and nonrandomized controlled, as well as quasi-randomized studies are included (study design). Other studies’ design typologies were excluded.
2.2. Data Collection
The systematic search includes studies published until the 20 January 2022 (the day of the search) performed through the electronic databases PubMed, Web of Science, and Scopus. The following keywords were adopted:
Keywords 1: articular cartilage; fibrocartilage; gristle.
Keywords 2: exercise; physical activity; fitness; movement; sport.
The keywords were matched adopting the Boolean operators OR/AND, and the following string has been created:
(“Articular Cartilage” OR fibrocartilage OR gristle) AND (exercise OR “physical activity” OR fitness OR movement OR sport).
2.3. Study Record
EndNote software (EndNote version X8; Thompson Reuters, New York, NY, USA) was adopted to identify duplicates. The selection process was performed by two independent investigators, who screened the titles first, then the abstracts, and the full-length articles. In case of disagreement in categorizing a manuscript, the principal investigator considered the studies independently and provided the tie-breaking decision. Investigators were not blinded to the study title, authors, or associated institutions during the selection process.
2.4. Data Extraction and Analysis
A Microsoft Excel® (Microsoft Corp., Redmond, WA, USA) spreadsheet was compiled with the following information: first author; year of publication; type of study; sample size; participants’ age (range or mean ± SD); gender; intervention characteristics; and outcomes. The main outcomes are discussed narratively.
2.5. Quality and Risk of Bias Assessment
Selection bias was analyzed by dividing the participants of the studies in groups based on age and health status. The studies were divided based on their design, and the randomized controlled trials were evaluated by two investigators through the PEDro scale, a third investigator was involved only in case of disagreement. This scale is composed of 11 items [18], and a score between 0 to 10 (high quality: excellent (9–10 points), good (6–8 points), fair (4–5 points), and poor (less than 4 points) quality. This scale presents good reliability [18] and validity [19].
3. Results
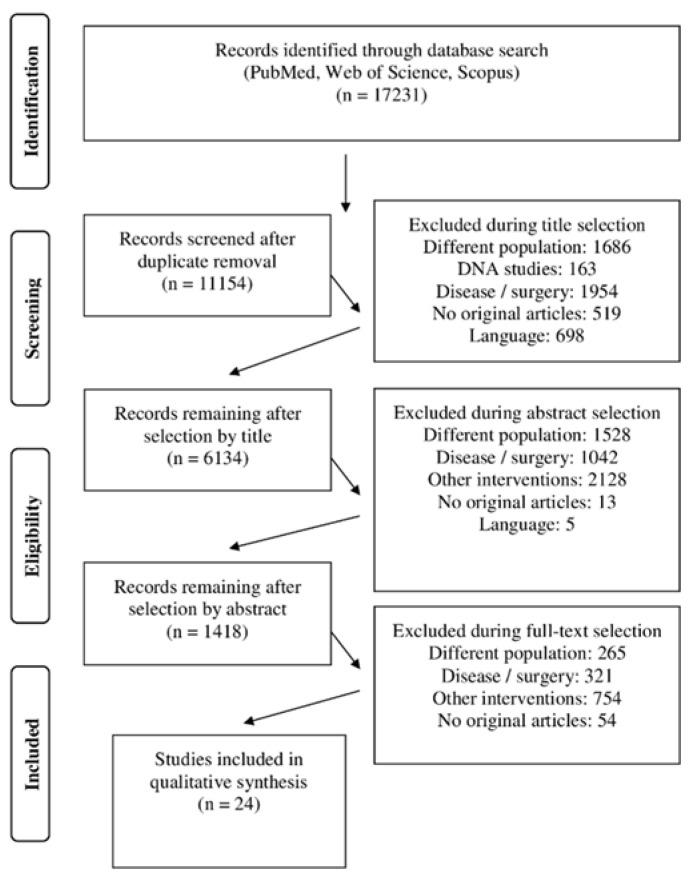
From a total number of 17,231 collected after the electronic search, 11,154 is the number of studies left after the duplicate’s removal. After the screening process, the final number of included studies is 24 studies. The flow chart is presented in Figure 1.
Figure 1.
Flow chart of the study collection process.
A total of 1222 (age range 20–70) participants, 748 of them with OA, have been included. The mean age was 47 (18), 542 were females, 380 were males; for 300, the gender was not specified. The main study characteristics are summarized in Table 1.
Table 1.
Overview of the studies characteristics.
| 1st Author | Study Design | Pop | Number (m) | Age (sd) | Method | Biomarkers | Conclusions |
|---|---|---|---|---|---|---|---|
| Azukizawa 2019 [20] | IS | OA | 42 (0) | 59 (6) | blood and urine sample ELISA | sPiiCP; uCtX-ii; uC2C; sCOMP | Well-rounded exercise improves PA and has beneficial effects on type 2 collagen metabolism |
| Bautch 2000 [21] | RCT | OA | 21 (7) | 70 (2) | synovial fluid (knee joints) ELISA | chondroitin sulphate epitopes 3B3, 7D4, GAG | No deleterious effects on osteoarthritic joints and ameliorated joint pain |
| Boocock 2009 [22] | IS | H | 20 (10) | 33 (9) | MRI | Cartilage volume | Running resulted in deformation of femoral, medial, and lateral tibial articular cartilage volume. |
| Celik 2013 [23] | RCT | H | 44 (44) | 22 (2) | blood samples ELISA | sCOMP | Regular, weight-bearing, high-impact physical exercise consolidates cartilage tissue |
| Centeno 2018 [24] | RCT | OA | 48 | 55 (9) | none | none | PA is an effective alternative therapy for KOA |
| Dinçer 2016 [25] | RCT | OA | 30 (6) | 51 (5) | MRI | Cartilage volume | No significant effect of closed kinetic chain exercise on the cartilage volume or morphology. |
| Esculier 2019 [26] | Pilot study | OA | 20 (0) | 52 (8) | MRI | T2_s | No changes after 30 min of running. People with KOA need more time to recover |
| Gatti, 2017 [27] | IS | H | 15 (15) | 26 (4) | MRI | T2_s, cartilage volume | Run shortened tibiofemoral cartilage T2, not bike |
| Hartley 2019 [28] | RCT | H | 42 (0) | 55–70 | MRI | T2_s | A high-impact exercise intervention has no negative effects on KOA. |
| Helmark 2010 [29] | RCT | OA | 29 (19) | 66 (6) | blood and urine samples ELISA | COMP, Aggrecan, CTX-II, IL6, IL8, IL10; TNF-α. | Positive effect of PA on a chondroprotective anti-inflammatory cytokine response in KOA |
| Horga 2020 [30] | PS | H | 44 (17) | 45 | MRI | Lesions | The knees achieved sustained improvement, for at least 6 months post-marathon |
| Ikuta 2020 [31] | RCT | OA | 26 (3) | 68 (9) | MRI | T2_m | PA could be a treatment to improve the course of KOA |
| Kangeswari 2021 [32] | RCT | OA | 200 | 45–65 | none | none | Isometric exercise program reduce pain, stiffness and improve physical function in KOA |
| Kessler 2020 [33] | PS | H | 19 (10) | 30 (6) | MRI | T2_s | Joint-loading with a stepping activity resulted in T1ρ and T2 changes above background measurement error |
| Kingsley 2012 [34] | IS | H | 8 (8) | 21 (1) | MRI | T2_s, Cartilage volume | Changes were observed throughout the thoracic and lumbar vertebral regions |
| Knoop, 2014 [35] | RCT | OA | 95 (31) | 61 (7) | MRI | Cartilage lesions | Effectiveness of PA is independent of OA severity |
| Liangyu 2014 | RCT | H | 120 | 42 | MRI | Cartilage volume | Decrease the total knee cartilage volume |
| Mikesky 2006 [36] | RCT | H | 221 (93) | 69 | radiographic evaluation | OA severity | Strength training retained more strength and exhibited less frequent progressive joint space narrowing |
| Multanen 2014 [37] | RCT | OA | 80 (80) | MRI | T2_s | PA improve balance, force, and endurance. No effect on cartilage | |
| Multanen 2017 [38] | RCT | OA | 78 (0) | 58 (4) | MRI | T2_s | High-impact training increase femoral neck strength not affecting knee cartilage on KOA |
| Pruksakorn 2013 [39] | IS | H | 82 (32) | 20 | blood sample; ELISA | COMP, WF6, HA | Articular cartilage is susceptible to the increasing load |
| Subburaj 2012 [40] | IS | H | 20 (10) | 29 | MRI | T2_s, Cartilage volume | Acute effect of run on knee cartilage and meniscus composition |
| Vassao 2021 [41] | RCT | OA | 23 | 64 (4) | Blood sample ELISA |
IL6, IL8, IL10, IL1β, TNF-α | Physical exercise increases the functional capacity |
| Yanagisawa 2021 [42] | IS | H | 15 (11) | 23 (3) | MRI | Apparent Diffusion Coefficient | High-load deadlift exercise stress the lumbar intervertebral discs |
Note: chondroitin sulfate-WF6: WF6; Enzyme-linked immunosorbent assays: ELISA; healthy: H; hyaluronic acid: HA; knee osteoarthritis: KOA; Interleukin: IL; intervention study: IS; magnetic resonance imaging: MRI; osteoarthritis: OA; physical activity: PA; prospective study: PS; population: Pop; randomized controlled trial: RCT; serum cartilage oligomeric matrix protein: sCOMP; serum cartilage type ii procollagen carboxy propeptide: sPiiCP; sulphated glycosaminoglycan: GAG; Transverse relaxation time: T2; T2 maps (non-contrast compositional MRI): T2_m; T2 MRI sequence; T2_s; Tumor Necrosis Factor: TNF-α; urine C-terminal telopeptide of collagen type ii: uCtX-ii; urine cleavage of type ii collagen by collagenases: uC.
3.1. Characteristics of the Included Studies
The intervention ranges from 7 days to 120 weeks, with four studies that propose 12 weeks. The frequency ranges from one to five times per week (the most adopted is three times a week). The session duration ranges from 30 to 90 min. Eight interventions study the acute effects of PA on cartilage. Eight studies propose a supervised intervention, at least in the first period during which participants learned the exercise routine. Six studies move the intervention to participants’ homes making the intervention possible outside the gym. Some of the studies evaluate the effects of amatorial sports practice such as swimming (n = 1), running (n = 7) and cycling (n = 3).
Related to the association between exercise training, pain, and functional outcomes, only 10 studies provided data pre- and post-intervention. Azukizawa et al. [20], Centeno et al. [24], Mikesky et al. [36], and Multanen et al. [37] generally detected no significant changing after physical exercise related to pain and functional outcomes. Dinçer et al. [25], Ikuta et al. [31], Kangeswari et al. [32], Knoop et al. [35], and Vassao et al. [41] detected better results after a physical exercise treatment both for the pain scale and functional outcomes.
3.2. Example of Interventions
Well-rounded exercise programs include stretching, balance exercises, aerobic activity, functional strengthening, and resistance exercises [20,24]. The body alignment of the core, pelvis and low extremities, and a balance/neuromuscular training could help the interventions and enhance the results [24].
Specific muscle strengthening interventions [25,28,31,35,36,41] and isometric exercises are proposed [32]. One of the intervention proposals is individually progressed and consisted of 5 min warm-up and hopping (3–5 sets, 15 s of rest between sets) exercises of approximately 3–4 min [28]. Resistance training interesting leg press, leg curl, seated chest press, and seated back row, upper body exercises [36], or bodyweight squat exercises [25] are also proposed. Similarly, an intervention proposes muscle strengthening exercises for the gluteal area, hamstrings, quadriceps, and triceps surae muscles [31]. Another intervention consists of 5 min on treadmill, six strength exercises (3 sets; 8 repetitions; 60% of 1-RM; rest interval of 2–3 min: hip abductors and adductors chair, seated leg raise, glute bridge, knee flexors and extensors chair), and stretching of major muscle groups [41]. The interventions could be supported also by knee joint stabilization exercises [35].
Related to cardiovascular interventions, aerobic exercise includes high-impact loading (jumping exercises) and rapid change of direction with music [37,38]. Other aerobic activities consisted of amateur swimming, running, and cycling (60–70% of their heart rate) [23]. More information related to the intervention’s proposal are provided in Table 2.
Table 2.
Characteristics of the interventions adopted in the included studies.
| 1st Author, Year | Length (Weeks) | Frequency (Days a Week) | Duration (Minutes) | Tutoring | Intervention |
|---|---|---|---|---|---|
| Azukizawa 2019 [20] | 12 | 1 | 90 | supervised them HB | stretching, balance, walk, and isometric exercises |
| Bautch 2000 [21] | 1 | 3 | 60 | NI | strengthening; low-intensity walking |
| Boocock 2009 [22] | AE | NI | NI | NI | run |
| Celik 2013 [23] | 12 | 3 | 40 | NI | swimming, running, cycling. 60–70% of heart rate |
| Centeno 2018 [24] | NI | NI | NI | HB | strengthening, resistance, functional, balance/neuro-muscular, aerobic, ROM |
| Dinçer 2016 [25] | 2 | 5 | 30 | NI | strengthening |
| Esculier 2019 [26] | AE | NI | NI | NI | run |
| Gatti, 2017 [27] | AE | NI | NI | NI | bike and run |
| Hartley 2020 [28] | 24 | NI | 50 hops | NI | high impact exercise progressing |
| Helmark 2010 [29] | AE | NI | NI | NI | resistance training |
| Horga 2020 [30] | 28 | NI | NI | NI | run |
| Ikuta, 2020 [31] | NI | NI | NI | supervised them HB | ROM and muscle strengthening; leg stretching |
| Kangeswari 2021 [32] | 12 | 3 | 40 | supervised then HB | isometric exercises |
| Kessler 2020 [33] | AE | NI | NI | NI | step |
| Kingsley, 2012 [34] | AE | NI | NI | NI | walk |
| Knoop, 2014 [35] | 12 | 2 | 60 | supervised and HB | knee joint stabilization muscle strengthening |
| Mikesky, 2006 [36] | 120 | 3 | NI | supervised then HB | resistance training |
| Multanen 2014 [37] | 48 | 3 | 55 | supervised | aerobic and step-aerobic jumping exercise |
| Multanen 2017 [38] | 48 | 3 | 55 | supervised | high-impact aerobic and step aerobic |
| Pruksakorn 2013 [39] | AE | NI | NI | NI | walk |
| Subburaj 2012 [40] | AE | NI | NI | NI | run |
| Vassao, 2021 [41] | 8 | 2 | NI | supervised | strength exercises and stretching |
| Yanagisawa 2021 [42] | AE | NI | NI | NI | stretching and submaximal deadlift repetitions |
Acute effect: AE; Home base: HB; no info: NI; repetition maximum: RM.
3.3. Quality and Risk of Bias Assessments
The PEDro scale for the risk of bias and quality of the studies assessment for the randomized controlled trails of the included studies presents a good quality with a mean range score of 6.8. It ranged from 7 to 10. Results are summarized in Table 3.
Table 3.
PEDro scale for the risk of bias and quality of the studies assessment for the randomized controlled trails manuscript included.
| Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 10/10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bautch 2000 [21] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5/10 |
| Celik 2013 [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9/10 |
| Centeno 2018 [24] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 5/10 |
| Dinçer 2016 [25] | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 7/10 |
| Hartley 2019 [28] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 5/10 |
| Helmark 2010 [29] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5/10 |
| Ikuta 2020 [31] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5/10 |
| Kangeswari 2021 [32] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8/10 |
| Knoop 2014 [35] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7/10 |
| Mikesky 2006 [36] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 8/10 |
| Multanen 2014 [37] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8/10 |
| Multanen 2017 [38] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6/10 |
| Vassao 2021 [41] | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6/10 |
| Total | 6.8/10 |
Note: 1. Eligibility criteria (unscored); 2. Subject random allocation; 3. Allocation concealed; 4. The groups were similar at baseline; 5. Subject blinded; 6. Therapists blinded; 7. Assessors blinded; 8. more than 85% retention rate; 9. Intention to treat; 10. Between-group analysis; 11. Point measures and measures of variability of at least one key outcome.
4. Discussion
Generally, PA has positive outcomes on cartilage structure regardless of the duration, frequency, intensity, and type of the intervention, suggesting positive effects due to an active lifestyle also during elderly. In this review, we highlighted the different effects of PA on the health of cartilage; however, attention in the field is required because all the considered interventions adopted different and not structured protocols. A guideline to structure the intervention is required; therefore, we propose the Standard Operating Procedure to correctly address the intervention for cartilage health. It is important to consider our standard operating procedure proposal is always based on the cartilage health status of the people trained.
The Standard Operating Procedure includes aerobic, resistance and stretching exercises. Aerobic capacity and resistance training should be proposed on different days [43]. Flexibility is part of the intervention, postural balance and coordination should be added to obtain the best results [20], also in people with severe OA [44]. If the individual is overweight, it is important to include weight loss in the intervention [45]. Furthermore, if it is present knee malalignment, the intervention should include also exercises correcting the angle of the knee during gait to reduce the degeneration, especially of the posteromedial knee [31]. Ideally, the minimum length of the intervention should be of 12 weeks with a frequency of 3 times a week for 60 min each session. In the first week of the intervention, people should be adequately prepared by trainers to correctly perform the exercise routine without supervision. After this learning period, people should perform the exercises independently at home and throughout their life. The Standard Operating Procedure is presented in Table 4.
Table 4.
Standard Operating Procedure proposal for an intervention for the cartilage wellness.
| Training/Intervention | Aerobic intervention/walking-running |
| Strength/isometric exercise | |
| Flexibility/all major muscles | |
| Person-specific necessity: postural balance; knee alignment; loss of weight | |
| Frequency | 3 times a week |
| Duration | 60 min |
| Intensity | Gradually increased |
| Supervision | Only in the first period. It is suggested that the intervention become a home-based exercise |
Walking is the simplest aerobic activity without adverse effects on articular cartilage metabolism [21]; indeed, there is no association between daily walking and structural changes over two years in people at risk of or with mild knee OA [46]. If walking is not sufficient, also running is a suitable intervention, the only precaution is that people with OA require longer recovery times [26]. Another precaution to consider when running is to increase the step rate frequency that decreases cartilage contact area, impact peak and cartilage contact pressure [47]. Running produces better results than swimming or cycling by inducing an impact on the cartilage that consolidates articular-cartilage tissue [23] making it ideal as an aerobic activity. Exercise in water presents good short-term effects but it appears deleterious in the long term [45,48]. Literature suggests that high-impact exercises do not negatively affect knee cartilage biochemical composition or knee pain [37]; so it is important to include this stimulus in the PA intervention.
Similar to aerobic intervention, resistance exercises have to be included in the ideal Standard Operating Procedure [46] also in people with advanced OA [35]. Isometric exercise reduces pain, stiffness, and physical dysfunction [32] with a beneficial effect on the cartilage [20]. Exercises to strengthen the quadriceps and hamstring muscles have no adverse effect on knee cartilage volume and thickness, reduce the pain and increase functionality [25]. Clinicians have to be aware that when alterations in the normal knee physiology are present, aerobic activities should be limited (no more than 10,000 steps/day), increasing weight-bearing activities to maintain PA level [49]. In order to measure the daily activities, individuals with OA could use smartwatches or fitness trackers to collect the PA during the day including both walking and exercising [50].
All grades of OA severity can benefit from PA, although effects might be reduced in patients with advanced OA [35]. It is important to propose a structured intervention especially for those people with OA because exercise reduces pain and disability [24,51]. On the opposite, sports prone to joint injury or vigorous high-impact should be avoided because they present an increased risk for OA [52]. Moderate to strenuous exercise and frequent knee-bending activities may accelerate cartilage degeneration and abnormalities [53].
Data on the mouse model suggest that moderate PA improves tribology and lubricative properties of articular cartilage, promoting lubricin synthesis and its elevation in synovial fluid, thus preventing cartilage degradation [54,55,56,57]. Consequently, PA, whatever the training, has benefits and can be considered a complementary and optional primary treatment for OA [58]. Regular moderate impact exercise does not increase the risk of OA, and there is some evidence that it does not increase symptoms in patients with mild OA [59]. Physical activity may not have a detrimental effect on the knee joint and thus be beneficial to joint health [60], suggesting this intervention as a preventive approach for cartilage degeneration.
The first limitation of the present study was related to the heterogeneity in the information provided about the health of the cartilage of the participants included. Furthermore, studies that included diet or other interventions proposed in a complementary way were not considered probably excluding some studies with interesting PA interventions. This decision was taken to limit the confounding factors. Another important limitation of the study it has been the impossibility to perform a meta-analysis due to the differences in the population (healthy people vs. people vs. OA with different gender and age), intervention (acute vs. long term effects), comparison (blood samples, MRI, other variables), and outcomes (knee or hip articulation, vertebrae). Future studies should investigate the effects of PA on people with different gravity in cartilage damage and determine the proper cut-off for the practice of the most appropriate PA typology. Second, studies should have to investigate accurately the interaction of different sports and their effects in the presence of OA. For instance, sports such as enduro motorcycling, where the individual is exposed to prolonged vibrations, may predispose the individual to early OA degeneration [61].
5. Conclusions
Structured PA interventions have mainly positive outcomes on cartilage structure. Because the interventions were different and various a Standard Operating Procedure has been proposed for a physical intervention focalized on cartilage wellness. Clinicians and researchers have to be aware when prescribing PA programs to select the most appropriate intervention based on the patient’s needs.
This study wants to provide the community with guidelines to prevent or limit cartilage degeneration and guarantee a healthy and active older age. Furthermore, a proper and accurate method to monitor the interventions is required to supervise the PA intervention and its outcomes. Finally, non-invasive techniques could help the researcher to detect early cartilage degenerations and plan structured PA programs personalized to the patients’ needs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare10101821/s1. PRISMA checklist.
Author Contributions
G.M. had the idea for the article and supervised the work. L.P., F.R. and B.T. wrote the protocol, structured and performed the data collection and the literature search, and performed the data analysis. L.P. and F.R. drafted the manuscript. M.Z. and C.G. critically revised the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the University Research Project Grant (PIACERI Found—NATURE-OA—2020–2022), Department of Biomedical and Biotechnological Sciences (BIOMETEC), University of Catania, Italy.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luria A., Chu C.R. Articular Cartilage Changes in Maturing Athletes: New Targets for Joint Rejuvenation. Sports Health. 2014;6:18–30. doi: 10.1177/1941738113514369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musumeci G., Szychlinska M.A., Mobasheri A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: Molecular markers of senescent chondrocytes. Histol. Histopathol. 2015;30:1–12. doi: 10.14670/hh-30.1. [DOI] [PubMed] [Google Scholar]
- 3.Lin W., Alizai H., Joseph G.B., Srikhum W., Nevitt M.C., Lynch J.A., McCulloch C.E., Link T.M. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2013;21:1558–1566. doi: 10.1016/j.joca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antony B., Venn A., Cicuttini F., March L., Blizzard L., Dwyer T., Cross M., Jones G., Ding C. Association of physical activity and physical performance with tibial cartilage volume and bone area in young adults. Arthritis Res. Ther. 2015;17:298. doi: 10.1186/s13075-015-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckstein F., Hudelmaier M., Putz R. The effects of exercise on human articular cartilage. J. Anat. 2006;208:491–512. doi: 10.1111/j.1469-7580.2006.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bricca A., Wirth W., Juhl C.B., Kemnitz J., Hunter D.J., Kwoh C.K., Eckstein F., Culvenor A.G. Moderate Physical Activity and Prevention of Cartilage Loss in People With Knee Osteoarthritis: Data From the Osteoarthritis Initiative. Arthritis Care Res. 2019;71:218–226. doi: 10.1002/acr.23791. [DOI] [PubMed] [Google Scholar]
- 7.Kwee R.M., Wirth W., Hafezi-Nejad N., Zikria B.A., Guermazi A., Demehri S. Role of physical activity in cartilage damage progression of subjects with baseline full-thickness cartilage defects in medial tibiofemoral compartment: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2016;24:1898–1904. doi: 10.1016/j.joca.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Teichtahl A.J., Wluka A.E., Forbes A., Wang Y., English D.R., Giles G.G., Cicuttini F.M. Longitudinal effect of vigorous physical activity on patella cartilage morphology in people without clinical knee disease. Arthritis Rheum. 2009;61:1095–1102. doi: 10.1002/art.24840. [DOI] [PubMed] [Google Scholar]
- 9.Stahl R., Luke A., Li X., Carballido-Gamio J., Ma B.C., Majumdar S., Link T.M. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients—A 3.0-Tesla MRI study. Eur. Radiol. 2009;19:132–143. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 10.Hovis K.K., Stehling C., Souza R.B., Haughom B.D., Baum T., Nevitt M., McCulloch C., Lynch J.A., Link T.M. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum. 2011;63:2248–2256. doi: 10.1002/art.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar D., Souza R.B., Singh J., Calixto N.E., Nardo L., Link T.M., Li X., Majumdar S. Physical activity and spatial differences in medial knee T1rho and T2 relaxation times in knee osteoarthritis. J. Orthop. Sports Phys. Ther. 2014;44:964–972. doi: 10.2519/jospt.2014.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehling C., Lane N.E., Nevitt M.C., Lynch J., McCulloch C.E., Link T.M. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: Analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthr. Cartil. 2010;18:776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voinier D., Neogi T., Stefanik J.J., Guermazi A., Roemer F.W., Thoma L.M., Master H., Nevitt M.C., Lewis C.E., Torner J., et al. Using Cumulative Load to Explain How Body Mass Index and Daily Walking Relate to Worsening Knee Cartilage Damage Over Two Years: The MOST Study. Arthritis Rheumatol. 2020;72:957–965. doi: 10.1002/art.41181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minor M.A. Exercise in the management of osteoarthritis of the knee and hip. Arthritis Care Res. Off. J. Arthritis Health Prof. Assoc. 1994;7:198–204. doi: 10.1002/art.1790070407. [DOI] [PubMed] [Google Scholar]
- 15.van Baar M.E., Assendelft W.J., Dekker J., Oostendorp R.A., Bijlsma J.W. Effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: A systematic review of randomized clinical trials. Arthritis Rheum. 1999;42:1361–1369. doi: 10.1002/1529-0131(199907)42:7<1361::AID-ANR9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Petrigna L., Pajaujiene S., Delextrat A., Gómez-López M., Paoli A., Palma A., Bianco A. The importance of standard operating procedures in physical fitness assessment: A brief review. Sport Sci. Health. 2021;18:21–26. doi: 10.1007/s11332-021-00849-1. [DOI] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 19.de Morton N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009;55:129–133. doi: 10.1016/S0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 20.Azukizawa M., Ito H., Hamamoto Y., Fujii T., Morita Y., Okahata A., Tomizawa T., Furu M., Nishitani K., Kuriyama S., et al. The Effects of Well-Rounded Exercise Program on Systemic Biomarkers Related to Cartilage Metabolism. Cartilage. 2019;10:451–458. doi: 10.1177/1947603518767998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bautch J.C., Clayton M.K., Chu Q., Johnson K.A. Synovial fluid chondroitin sulphate epitopes 3B3 and 7D4, and glycosaminoglycan in human knee osteoarthritis after exercise. Ann. Rheum. Dis. 2000;59:887–891. doi: 10.1136/ard.59.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boocock M., McNair P., Cicuttini F., Stuart A., Sinclair T. The short-term effects of running on the deformation of knee articular cartilage and its relationship to biomechanical loads at the knee. Osteoarthr. Cartil. 2009;17:883–890. doi: 10.1016/j.joca.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Celik O., Salci Y., Ak E., Kalaci A., Korkusuz F. Serum cartilage oligomeric matrix protein accumulation decreases significantly after 12 weeks of running but not swimming and cycling training—A randomised controlled trial. Knee. 2013;20:19–25. doi: 10.1016/j.knee.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Centeno C., Sheinkop M., Dodson E., Stemper I., Williams C., Hyzy M., Ichim T., Freeman M. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: A randomized controlled trial with 2 year follow-up. J. Transl. Med. 2018;16:355. doi: 10.1186/s12967-018-1736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinçer Ü., Arıba S., Saygın H., İncedayı M., Rodop O. The effects of closed kinetic chain exercise on articular cartilage morphology: Myth or reality? A randomized controlled clinical trial. Turk. Fiz. Tip Ve Rehabil. Derg. 2016;62:28–36. doi: 10.5606/tftrd.2016.93899. [DOI] [Google Scholar]
- 26.Esculier J.F., Jarrett M., Krowchuk N.M., Rauscher A., Wiggermann V., Taunton J.E., Wilson D.R., Gatti A.A., Hunt M.A. Cartilage recovery in runners with and without knee osteoarthritis: A pilot study. Knee. 2019;26:1049–1057. doi: 10.1016/j.knee.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Gatti A.A., Noseworthy M.D., Stratford P.W., Brenneman E.C., Totterman S., Tamez-Peña J., Maly M.R. Acute changes in knee cartilage transverse relaxation time after running and bicycling. J. Biomech. 2017;53:171–177. doi: 10.1016/j.jbiomech.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Hartley C., Kerslake R., Folland J., Brooke-Wavell K. Effect of high impact exercise on femoral neck bone mineral density and T2 relaxation times of articular cartilage in postmenopausal women. J. Bone Miner. Res. 2018;33:171. doi: 10.1002/jbmr.3867. [DOI] [PubMed] [Google Scholar]
- 29.Helmark I.C., Mikkelsen U.R., Børglum J., Rothe A., Petersen M.C., Andersen O., Langberg H., Kjaer M. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: A randomized controlled trial. Arthritis Res. Ther. 2010;12:R126. doi: 10.1186/ar3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horga L.M., Henckel J., Fotiadou A., Hirschmann A.C., Di Laura A., Torlasco C., D’Silva A., Sharma S., Moon J.C., Hart A.J. Is the immediate effect of marathon running on novice runners’ knee joints sustained within 6 months after the run? A follow-up 3.0 T MRI study. Skelet. Radiol. 2020;49:1221–1229. doi: 10.1007/s00256-020-03391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikuta F., Takahashi K., Hashimoto S., Mochizuki Y., Yuzawa Y., Inanami H., Takai S. Effect of physical therapy on early knee osteoarthritis with medial meniscal posterior tear assessed by MRI T2 mapping and 3D-to-2D registration technique: A prospective intervention study. Mod. Rheumatol. 2020;30:738–747. doi: 10.1080/14397595.2019.1646193. [DOI] [PubMed] [Google Scholar]
- 32.Kangeswari P., Murali K., Arulappan J. Effectiveness of Isometric Exercise and Counseling on Level of Pain Among Patients With Knee Osteoarthritis. SAGE Open Nurs. 2021;7:2377960821993515. doi: 10.1177/2377960821993515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessler D.A., MacKay J.W., McDonald S., McDonnell S., Grainger A.J., Roberts A.R., Janiczek R.L., Graves M.J., Kaggie J.D., Gilbert F.J. Effectively Measuring Exercise-Related Variations in T1ρ and T2 Relaxation Times of Healthy Articular Cartilage. J. Magn. Reson. Imaging. 2020;52:1753–1764. doi: 10.1002/jmri.27278. [DOI] [PubMed] [Google Scholar]
- 34.Kingsley M.I., D’Silva L.A., Jennings C., Humphries B., Dalbo V.J., Scanlan A.T. Moderate-intensity running causes intervertebral disc compression in young adults. Med. Sci. Sports Exerc. 2012;44:2199–2204. doi: 10.1249/MSS.0b013e318260dbc1. [DOI] [PubMed] [Google Scholar]
- 35.Knoop J., Dekker J., Van Der Leeden M., Van Der Esch M., Klein J.P., Hunter D.J., Roorda L.D., Steultjens M.P.M., Lems W.F. Is the severity of knee osteoarthritis on magnetic resonance imaging associated with outcome of exercise therapy? Arthritis Care Res. 2014;66:63–68. doi: 10.1002/acr.22128. [DOI] [PubMed] [Google Scholar]
- 36.Mikesky A.E., Mazzuca S.A., Brandt K.D., Perkins S.M., Damush T., Lane K.A. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Rheum. 2006;55:690–699. doi: 10.1002/art.22245. [DOI] [PubMed] [Google Scholar]
- 37.Multanen J., Nieminen M.T., Häkkinen A., Kujala U.M., Jämsä T., Kautiainen H., Lammentausta E., Ahola R., Selänne H., Ojala R., et al. Effects of high-impact training on bone and articular cartilage: 12-month randomized controlled quantitative MRI study. J. Bone Miner. Res. 2014;29:192–201. doi: 10.1002/jbmr.2015. [DOI] [PubMed] [Google Scholar]
- 38.Multanen J., Rantalainen T., Kautiainen H., Ahola R., Jämsä T., Nieminen M.T., Lammentausta E., Häkkinen A., Kiviranta I., Heinonen A. Effect of progressive high-impact exercise on femoral neck structural strength in postmenopausal women with mild knee osteoarthritis: A 12-month RCT. Osteoporos. Int. 2017;28:1323–1333. doi: 10.1007/s00198-016-3875-1. [DOI] [PubMed] [Google Scholar]
- 39.Pruksakorn D., Tirankgura P., Luevitoonvechkij S., Chamnongkich S., Sugandhavesa N., Leerapun T., Pothacharoen P. Changes in the serum cartilage biomarker levels of healthy adults in response to an uphill walk. Singap. Med. J. 2013;54:702–708. doi: 10.11622/smedj.2013245. [DOI] [PubMed] [Google Scholar]
- 40.Subburaj K., Kumar D., Souza R.B., Alizai H., Li X., Link T.M., Majumdar S. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am. J. Sports Med. 2012;40:2134–2141. doi: 10.1177/0363546512449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vassão P.G., de Souza A.C.F., da Silveira Campos R.M., Garcia L.A., Tucci H.T., Renno A.C.M. Effects of photobiomodulation and a physical exercise program on the expression of inflammatory and cartilage degradation biomarkers and functional capacity in women with knee osteoarthritis: A randomized blinded study. Adv. Rheumatol. 2021;61 doi: 10.1186/s42358-021-00220-5. [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa O., Oshikawa T., Matsunaga N., Adachi G., Kaneoka K. Acute Physiological Response of Lumbar Intervertebral Discs to High-load Deadlift Exercise. Magn. Reson. Med. Sci. MRMS: Off. J. Jpn. Soc. Magn. Reson. Med. 2021;20:290–294. doi: 10.2463/mrms.mp.2020-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juhl C., Christensen R., Roos E.M., Zhang W., Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: A systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622–636. doi: 10.1002/art.38290. [DOI] [PubMed] [Google Scholar]
- 44.Rogind H., Bibow-Nielsen B., Jensen B., Moller H.C., Frimodt-Moller H., Bliddal H. The effects of a physical training program on patients with osteoarthritis of the knees. Arch. Phys. Med. Rehabil. 1998;79:1421–1427. doi: 10.1016/S0003-9993(98)90238-6. [DOI] [PubMed] [Google Scholar]
- 45.McAlindon T.E., Bannuru R.R., Sullivan M.C., Arden N.K., Berenbaum F., Bierma-Zeinstra S.M., Hawker G.A., Henrotin Y., Hunter D.J., Kawaguchi H., et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Oiestad B.E., Quinn E., White D., Roemer F., Guermazi A., Nevitt M., Segal N.A., Lewis C.E., Felson D.T. No association between daily walking and knee structural changes in people at risk of or with mild knee osteoarthritis. prospective data from the multicenter osteoarthritis study. J. Rheumatol. 2015;42:1685–1693. doi: 10.3899/jrheum.150071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenhart R.L., Smith C.R., Vignos M.F., Kaiser J., Heiderscheit B.C., Thelen D.G. Influence of step rate and quadriceps load distribution on patellofemoral cartilage contact pressures during running. J. Biomech. 2015;48:2871–2878. doi: 10.1016/j.jbiomech.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartels E.M., Juhl C.B., Christensen R., Hagen K.B., Danneskiold-Samsoe B., Dagfinrud H., Lund H. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst. Rev. 2016;3:CD005523. doi: 10.1002/14651858.CD005523.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doré D.A., Winzenberg T.M., Ding C., Otahal P., Pelletier J.P., Martel-Pelletier J., Cicuttini F.M., Jones G. The association between objectively measured physical activity and knee structural change using MRI. Ann. Rheum. Dis. 2013;72:1170–1175. doi: 10.1136/annrheumdis-2012-201691. [DOI] [PubMed] [Google Scholar]
- 50.Ravalli S., Roggio F., Lauretta G., Di Rosa M., D’Amico A.G., D’Agata V., Maugeri G., Musumeci G. Exploiting real-world data to monitor physical activity in patients with osteoarthritis: The opportunity of digital epidemiology. Heliyon. 2022;8:e08991. doi: 10.1016/j.heliyon.2022.e08991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roddy E., Zhang W., Doherty M. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann. Rheum. Dis. 2005;64:544–548. doi: 10.1136/ard.2004.028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter D.J., Eckstein F. Exercise and osteoarthritis. J. Anat. 2009;214:197–207. doi: 10.1111/j.1469-7580.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gahunia H.K., Pritzker K.P. Effect of exercise on articular cartilage. Orthop. Clin. N. Am. 2012;43:187–199. doi: 10.1016/j.ocl.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Musumeci G., Castrogiovanni P., Trovato F.M., Imbesi R., Giunta S., Szychlinska M.A., Loreto C., Castorina S., Mobasheri A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports. 2015;25:e222–e230. doi: 10.1111/sms.12290. [DOI] [PubMed] [Google Scholar]
- 55.Castrogiovanni P., Di Rosa M., Ravalli S., Castorina A., Guglielmino C., Imbesi R., Vecchio M., Drago F., Szychlinska M.A., Musumeci G. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int. J. Mol. Sci. 2019;20:511. doi: 10.3390/ijms20030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravalli S., Federico C., Lauretta G., Saccone S., Pricoco E., Roggio F., Di Rosa M., Maugeri G., Musumeci G. Morphological Evidence of Telocytes in Skeletal Muscle Interstitium of Exercised and Sedentary Rodents. Biomedicines. 2021;9:807. doi: 10.3390/biomedicines9070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Rosa M., Castrogiovanni P., Musumeci G. The Synovium Theory: Can Exercise Prevent Knee Osteoarthritis? The Role of “Mechanokines”, A Possible Biological Key. J. Funct. Morphol. Kinesiol. 2019;4:11. doi: 10.3390/jfmk4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castrogiovanni P., Musumeci G. Which is the best physical treatment for osteoarthritis? J. Funct. Morphol. Kinesiol. 2016;1:54–68. doi: 10.3390/jfmk1010054. [DOI] [Google Scholar]
- 59.Shrier I. Muscle dysfunction versus wear and tear as a cause of exercise related osteoarthritis: An epidemiological update. Brit. J. Sport Med. 2004;38:526–535. doi: 10.1136/bjsm.2003.011262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urquhart D.M., Tobing J.F., Hanna F.S., Berry P., Wluka A.E., Ding C., Cicuttini F.M. What is the effect of physical activity on the knee joint? A systematic review. Med. Sci. Sports Exerc. 2011;43:432–442. doi: 10.1249/MSS.0b013e3181ef5bf8. [DOI] [PubMed] [Google Scholar]
- 61.Roggio F., Trovato B., Ledda C., Rapisarda V., Musumeci G. Kinesiological Treatment of Early Spine Osteoarthritis in a Motorcyclist. Int. J. Environ. Res. Public Health. 2022;19:961. doi: 10.3390/ijerph19020961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.