Abstract
Background: Breast cancer affects 2.3 million women and kills 685,000 globally, making it the most prevalent cancer. The telemedicine modality has been used to treat the symptoms associated with breast cancer recovery. Objectives: To analyze the effectiveness of telemedicine to help women recover from the treatment-associated effects and promote overall recovery from breast cancer. Methods: Four databases were queried for published literature from the last 10 years. The systematic literature review was conducted in accordance with the Kruse Protocol and reported in accordance with PRISMA 2020. Results: Five interventions were identified in the literature, with the most dominant being eHealth and mHealth. The other interventions were telephone, video teleconference, and a combination of eHealth and mHealth. There were positive effects of these telemedicine interventions in 88% of the studies analyzed. Telemedicine is shown to positively affect physical and mental health, sleep outcomes, quality of life, and body image. The largest barriers to the adoption of telemedicine interventions are training, cost, workflow, time of providers, and low reimbursement. Conclusion: Telemedicine offers promise to both providers and breast cancer survivors to improve the physical and mental health detriments of both cancer and its associated treatments. It also helps women develop healthy habits to reduce the risk of reoccurrence.
Keywords: mHealth, telemedicine, breast cancer
1. Introduction
1.1. Rationale
Breast cancer is a disease, originating in the breast, in which breast cells grow out of control [1]. The incidence of breast cancer is extensive. In 2020, for example, over 2.3 million women were diagnosed with this condition, and this resulted in 685,000 deaths globally. The 5-year prevalence was estimated at 7.8 million women, which establishes it as the world’s most prevalent cancer [2]. Breast cancer treatment is effective when caught early. Treatment often includes surgical removal, radiation therapy, and medication, but all of these treatments come at a physical and emotional cost to the survivor. Providers have sought new and innovative means to help women through the treatment process and the aftermath of the emotional devastation it brings. Telemedicine offers some interventions.
Telemedicine is defined as healing at a distance through the use of information and communications technologies (ICT) [3]. Telemedicine takes on many forms, but in general, it provides clinical support and overcomes geographical boundaries to improve health outcomes through ICT. Although many distinguish between telehealth and telemedicine, the World Health Organization does not distinguish between them, therefore, telehealth and telemedicine will be used interchangeably in this study. One form of telemedicine is mHealth and eHealth, or mobile-based health and computer-based health, respectively. These take the form of mobile apps, text messages through short message service (SMS), telephonic calls, websites, and computer programs. Many eHealth interventions can now be accessed on mobile devices, therefore the lines between the modalities have become blurred.
Several forms of telehealth have been used for the last several years in the area of oncology, and specifically breast cancer. mHealth apps have shown effectiveness in improving mood, symptom interference, self-efficacy, self-esteem, and emotional functioning [4]. mHealth apps provide education and improve health literacy [5,6]. They improve medication adherence and help women with coping strategies [7,8]. Overall, mHealth apps have shown positive effects on the perception of physical benefits, psychological factors such as motivation, social factors such as group practice, and organizational factors including preplanning physical activity [9]. The paucity of evidence for clinical efficacy begs additional research. This is the justification for this study.
In 2021, a systematic review was published examining mHealth interventions’ ability to improve the quality of life for cancer patients. They identified 25 articles over a period of 10 years. They found the most common issues addressed by mHealth were physical activity, mindfulness, and stress management. Overall, mHealth had a positive effect on patients [10].
In 2022, a scoping review was published that examined mHealth’s ability to increase screening rates among Hispanic communities. Ten articles were selected out of an original result of 597 from a search that spanned ten years. The reviewers reported mHealth was effective at providing education and increasing health literacy [6].
1.2. Objectives
The purpose of this review is to analyze the effectiveness of telehealth interventions to manage breast cancer care and recovery.
2. Methods
2.1. Eligibility Criteria
To be included in the group of articles for analysis, studies had to be published in the English language in the last 10 years in peer-reviewed, academic journals, and used human adult females as subjects. To avoid confounding results, other reviews were excluded. Systematic reviews summarize the findings of previous results (from a set number of years). Including a systematic review from 2022 in the analysis, for instance, would include results from articles also analyzed separately. This would double count instances of findings, which would confound the results.
2.2. Information Sources
Four databases were queried: The U.S. Library of Medicine’s PubMed (MEDLINE), the Cumulative Index of Nursing and Allied Health Literature (CINAHL), Web of Science, and Embase’s Science Direct. These databases were searched on 2 August 2022. We also performed a journal-specific search of Healthcare.
2.3. Search Strategy
We used the U.S. Library of Medicine’s Medical Subject Headings (MeSH) to create a Boolean search string to combine key terms into an exhaustive search: (mHealth OR telemedicine OR “mobile apps”) AND (“breast cancer” AND “treatment”). The same search string was used in all databases, and as much as possible, we used the same filters in each database. MEDLINE was excluded from all databases except PubMed since PubMed includes the MEDLINE database. This action helped eliminate duplicates.
2.4. Selection Process
Search results were filtered and abstracts were screened in accordance with the Kruse Protocol [11] and reported in accordance with PRISMA 2020 [12]. The Kruse Protocol was written to demonstrate the veracity of using the systematic literature review in higher education, but it outlines a proven methodology that has been published over 50 times in high-quality journals [11]. The PRISMA 2020 standard provides a systematic methodology to ensure standardized fields are reported for all systematic reviews and meta-analyses. Abstracts were screened by at least two reviewers.
2.5. Data Collection Process
An Excel spreadsheet, standardized in the Kruse Protocol, was utilized as a data extraction tool, collecting additional data at each step of the process. Three consensus meetings were held to identify articles for analysis, perform a narrative or thematic analysis, and perform additional analysis on the results to identify trends [11,13]. Abstracts were screened and studies were analyzed by at least two reviewers throughout the process.
2.6. Data Items
We collected the following fields of data: research database source, year of publication, authors, title of study, journal, study participants, experimental intervention, results compared to the control, medical outcomes, study design, study sample size, observations of bias, effect size (Cohen’s d), sensitivity, specificity, and F1 (when reported), country of origin, statistics used, patient satisfaction, effectiveness, barriers to adoption, strength of evidence, and quality of evidence.
2.7. Study Risk of Bias Assessment
Each reviewer noted observations of bias (e.g., selection bias), and we assessed the quality of each study using the Johns Hopkins Nursing Evidence Based Practice tool (JHNEBP) [14]. These observations were recorded because they affect how to interpret the results, and because bias can limit external validity [15].
2.8. Effect Measures
Summary measures were not standardized because we accepted mixed methods and qualitative studies. Measures of effect were summarized in tables for those studies in which it was reported.
2.9. Synthesis Methods
Once data extraction was completed, a thematic analysis was performed to make sense of the data. [13] Themes were tabulated and summarized. Results across studies were analyzed for additional inferences and to identify heterogeneity.
2.10. Reporting Bias Assessment
We identified the strength and quality of evidence in accordance with the JHNEBP to provide us with an assessment of the applicability of the cumulative evidence and the limit of external validity.
2.11. Additional Analyses and Certainty Assessment
We performed a narrative/thematic analysis of the observations to convert them into themes, or common threads between articles. This helped us make sense of the data. We calculated the frequency of occurrence and reported them in affinity matrices. The frequency provided the probability of occurrence in the group of articles analyzed, and it provided confidence in the data analyzed.
2.12. Statistical Analysis
Measures of effect were collected during the data extraction process. Where possible, each effect was translated into an effect size equivalent to Cohen’s d [16]. These measures were converted into a weighted average effect size by using the sample size for the weight.
3. Results
3.1. Study Selection
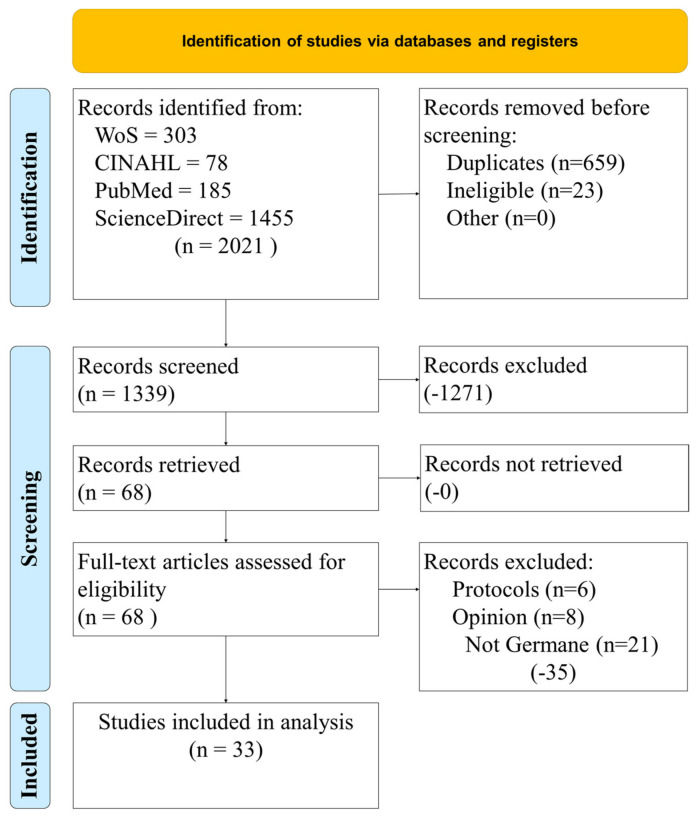
Figure 1 illustrates the study selection process with four databases. A kappa statistic was calculated to estimate the level of agreement between reviewers, (k = 0.92, almost perfect agreement) [17,18]. Results from four research databases presented 2021 results. Duplicates and those outside the date range were removed from screening. Using database filters, 1399 records were screened for full text, human subjects, English language, peer-reviewed, and academic journals. Anything except peer-reviewed, published work was excluded along with other systematic literature reviews and meta-analyses. The remaining 68 records were assessed for eligibility. Protocols, editorials, and studies that would not address the objective statement were removed. The remaining group for analysis was 33.
Figure 1.
Study selection process.
3.2. Study Characteristics
PRISMA 2020 and the Kruse Protocol were followed throughout this review. Part of that process is to create a table that lists the characteristics of each study analyzed: participants, intervention, results, medical outcomes, and study design (see Table 1: PICOS). The 33 studies are broken down into the following years: 2012(0), 2013(0), 2014(1) [19], 2015(2) [20,21], 2016(1) [22], 2017(4) [23,24,25,26], 2018(4) [27,28,29,30], 2019(1) [31], 2020(7) [32,33,34,35,36,37,38], 2021(8) [39,40,41,42,43,44,45,46], 2022(5) [47,48,49,50,51]. All studies involved adults as participants. About 76% of the studies were RCT or true experiments, 3 were quasi-experimental, and the rest were a combination of non-experimental, pre-post, qualitative, or mixed methods. About half (16/33, 48%) of the interventions were web-based (eHealth), 13/33 (39%) were mHealth, 3/33 (9%) were telephone-based, and one was a combination of mHealth and eHealth. About 40% of the studies were conducted in the United States, 12% were from Spain, 9% were from the Netherlands, and the rest were from Taiwan, Turkey, Sweden, Norway, India, Iran, and Australia. Almost all studies reported strong positive satisfaction from users, with only one exception [29].
Table 1.
PICOS.
| Authors | Participants | Experimental Intervention | Results (Compared to Control Group) | Medical Outcomes Reported | Study Design |
|---|---|---|---|---|---|
| Borosund et al. [19] | Adults ≥ 18, avg age 51.4 | Internet-based patient-provider communication service | Intervention group reported significantly lower symptom distress, anxiety, and depression | nurse-administered IPPC alone can significantly reduce depression, decreased symptom distress, decreased anxiety | RCT |
| Freeman et al. [20] | Adults ≥ 18, avg age 55.4 | Telemedicine (TD) [vs live vs. wait list] | TD (and Live) reported less fatigue, cognitive dysfunction, and sleep disturbance with WL | improvements in multiple QOL domains for breast cancer survivors compared with WL.Less fatigue, less cognitive dysfunction, fewer sleep disturbances | RCT |
| Wheelock et al. [21] | Adults ≥ 18, average age 52.85, 73% Caucasian | SIS.NET (online questionnaire with remove NP overview and follow-up) | patients reported more new or changed symptoms compared with standard care patients | This intervention facilitated symptom reporting and may provide a means of convenient symptom assessment | RCT |
| Galiano-Castillo et al. [22] | Adults ≥ 18 | Internet-based, tailored exercise program | telerehabilitation group improved significantly global health status, physical, role, cognitive functioning and arm symptoms, as well as pain severity, and pain interference, compared with the control group. | Improved physical health, cognitive functioning, pain severity, and pain interference | RCT |
| Admiraal et al. [23] | Adults ≥ 18, average age 53.2 | web-based psychoeducation for breast cancer (ENCOURAGE) | No statistically significant differences between control and intervention for optimism or control over future | For clinical distressed patients, use of the intervention increased optimism and control over future | RCT |
| Fazzino et al. [24] | Adults ≥ 18 | telephone (weekly) | No control group. Distance-based weight loss program can be successful | Moderate-to-vigorous physical activity significantly increased from baseline to 6 months. | Non-experimental (no randomization, no control) |
| Han et al. [25] | Adults ≥ 18, average age 52.2, 88% Caucasian | eHealth system (Comprehensive Health Enhancement Support System, CHESS) | No control group. cancer patients’ access to more complex tools generates more use with their time spreading out over the diverse services. | Communication functions drive long-term engagement with the system. | Pre-post |
| Uhm et al. [26] | Adults ≥ 18 | mHealth | Improved exercise, but not statistically different than control | Improved exercise, but not statistically different than control | Quasi-experimental |
| Kim et al. [27] | Adults ≥ 18 | mHealth (mobile game) | Improved drug adherence, lower side effects of chemotherapy (nausea, fatigue, numbness of hand or foot, and hair loss). Improved quality of life. No significant difference in depression or anxiety | Improved drug adherence, lower side effects of chemotherapy (nausea, fatigue, numbness of hand or foot, and hair loss). Improved quality of life. Improved medication adherence. No significant difference in depression or anxiety | RCT |
| McCarthy et al. [28] | Adults ≥ 18 | nurse-led telemedicine delivered, cognitive behavioral therapy | participants reported improvements in sleep outcomes, including SE and SL. QOL and daily functioning improved, but anxiety and depression did not. | participants reported improvements in sleep outcomes, including SE and SL. QOL and daily functioning improved, but anxiety and depression did not. | Quasi-experimental |
| Visser et al. [29] | Adults ≥ 18 | tablet online support group | No statistically significant differences between control and intervention for distress and empowerment. Greater peer support identified in control. | No improvement with intervention. Satisfaction very low. | RCT |
| Zachariae et al. [30] | Adults ≥ 18, average age 52.3 | Internet-delivered cognitive-behavioral therapy (iCBT) | Statistically significant improvements observed for all sleep-related outcomes (fatigue, sleep disturbances, total sleep time). | Reduced insomnia, increased sleep quality, increases sleep efficiency, increased total sleep time, improved time in bed, reduced fatigue | RCT |
| Ariza-Garcia et al. [31] | Adults ≥ 18 | web-based exercise system (e_CuidateChemo) | Functional capacity improved significantly, abdominal strength, lower body strength, back strength | Intervention increased exercise capacity by 10.8% (33.4% reached a normal exercise capacity compared with 12.3% in control). Functional capacity, abdominal strength, lower body strength, back strength improved significantly. | RCT |
| Crafoord et al. [32] | Adults ≥ 18 | mHealth app for symptom self-management | Daily symptom reporting created feelings of having continuous contact with health care professionals, being acknowledged, and safe. | Engagement was very high for intervention. The app promoted patient participation in their care. | Mixed Methods |
| Ferrante et al. [33] | Adults ≥ 60, African American only | mHealth/eHealth tools | No statistically significant differences between weight lost in both groups. Waist circumference improved more, quality of life more, and use of strategies for healthy eating and decreasing calories. | Effective at weight loss, but not statistically significant | RCT |
| Fjell et al. [34] | Adults ≥ 18, average age 48 | mHealth app (Interaktor) during neoadjuvant chemo | statistically significant less symptom prevalence in nausea, vomiting, feeling sad, appetite loss and constipation. Overall symptom distress and physical symptom distress were rated statistically significant lower in the intervention group. Further, emotional functioning was rated statistically significant higher in the intervention group. | statistically significant less symptom prevalence in nausea, vomiting, feeling sad, appetite loss and constipation. Overall symptom distress and physical symptom distress were rated statistically significant lower in the intervention group. Further, emotional functioning was rated statistically significant higher in the intervention group. | RCT |
| Hou et al. [35] | Adults ≥ 50 | mHealth app for self-management support (BCSMS) | Mean quality of life scores and global health higher | Mean quality of life scores and global health higher | RCT |
| Lally et al. [36] | Adults ≥ 18 | we-based, psychoeducational distress self-management program (CaringGuidance) | post hoc analysis showed significant group differences in slopes occurring between study months 2 and 3 on distress and depressive symptoms | post hoc analysis showed significant group differences in slopes occurring between study months 2 and 3 on distress and depressive symptoms | True experiment |
| Lozano-Lozano et al. [37] | Adults ≥ 18 | mHealth (BENECA) + rehab | Both groups showed improved outcomes, but global QoL was significantly better with intervention. Improvement in upper-limb functionality also higher | Both groups showed improved outcomes, but global QoL was significantly better with intervention. Improvement in upper-limb functionality also higher | RCT |
| van der Hout et al. [38] | Adults ≥ 56 | eHealth (Oncokompas) symptom self-management app | Oncokompas did not improve the amount of knowledge, skills, and confidence for self-management in cancer survivors. | No difference between groups | RCT |
| Çınar et al. [39] | Adults ≥ 18 | mHealth app for education, symptom tracking, and management | QoL of the treatment group after intervention increased and distress level was lower | QoL of the treatment group after intervention increased and distress level was lower | True experiment |
| Fang et al. [40] | Adults ≥ 20 | decision-support app (Pink Journey) | body image distress declined significantly for the intervention group but increased for the control group. no significant difference in decision conflict, decision regret, anxiety, or depression. | Decrease in body image, regret, anxiety, & distress | RCT |
| Krzyzanowska et al. [41] | Adults ≥ 40 | telephone based management of toxicities | No differences in self-efficacy, anxiety, or depression | No differences in self-efficacy, anxiety, or depression | RCT |
| Kumar et al. [42] | Adult, aged 27 | Teleconsultation | No control group. Concerns and questions answered through intervention | Breast conservation surgery | Qualitative |
| Lai et al. [43] | Adults ≥ 18, avg age 56.8, 53% Caucasian | Telemedicine (VTC) Occupational Therapy | No control group. Patients regained baseline function within a mean of 42.4 days after surgery and after an average of three sessions | all regained baseline functional status and full range of motion | Non-experimental (no randomization, no control) |
| Öztürk et al. [44] | Adults ≥ 18 | mHealth symptom monitoring app | Effective at decreasing nausea-vomiting, raising sexual function and sexual enjoyment | Symptom monitoring with mHealth highly effective in controlling physical symptoms | True experiment |
| Reeves et al. [45] | Adults ≥ 45 | mHealth weight-loss | Improved weight reduction (over control) fat mass, metabolic syndrome risk score, waist circumference, fasting plasma glucose, and quality of life | Improved weight reduction (over control) fat mass, metabolic syndrome risk score, waist circumference, fasting plasma glucose, and quality of life | RCT |
| Wagner et al. [46] | Adults ≥ 18 | eHealth (Fear of recurrence, FoR) Telecoaching | Significantly reduced fear of recurrence. Telecoaching improved adherence and retention. | Reduced fear of recurrence. Telecoaching improved adherence and retention. | RCT |
| Bandani-Susan et al. [47] | Adults ≥ 18, average age 46.34 | mHealth education | Mean score of cancer fatigue decreased and body image increased significantly | Decreased fatigue, increased body image | RCT |
| Fu et al. [48] | Adults ≥ 18 | mHealth pain-management | Participants in the intervention were more likely to experience complete reduction in pain and soreness, lower median severity scores and general body pain, less arm/hand swelling, heaviness, redness, and limited movement in shoulder | Less pain, less soreness, less swelling, less heaviness, less redness, less limited movement in shoulder | RCT |
| Gao et al. [49] | Adults ≥ 18, average age 56.17 | mHealth Tai Chi and health education | A significant time effect for mental health, physical health, but not for stress. | Tai Chi participants had a significantly better mental health at follow up. | RCT |
| Medina et al. [50] | Adults ≥ 18, average age 52.35 | eHealth ecosystem (ICOnnecta) | Strong social support led to better psychosocial course | ICOnnecta supports the development of a digital relation with healthcare services | Quasi-experimental |
| Oswald et al. [51] | Adults ≥ 18 | eHealth cognitive-behavioral therapy (iCBT) | Improvements in insomnia, sleep efficiency, and sleep disturbance | Improvements in insomnia, sleep efficiency, and sleep disturbance | RCT |
BCMSM: Breast cancer self-management support; CHESS: Comprehensive Health Enhancement Support System; FoR: Fear of reoccurrence; QoL: Quality of Life; iCBT: Internet Cognitive Behavior Therapy; IPPC: Internet-based provider communications service; SIS:NET: System for Individualized Survivorship Care; SE: Sleep efficiency; SL: Sleep latency; TD: Telemedicine delivery; VTC: Video tele-conference; WL: Wait list.
3.3. Risk of Bias in and across Studies
Reviewers used the JHNEBP quality assessment tool to identify the strength and quality of evidence. Due to the strong methodologies chosen for review, the JHNEBP tool identified 76% of the articles as Strength I, which means the methodologies were experimental or RCTs (studies had control groups and used randomization). Only 2 studies were identified as Strength II, reserved for quasi-experimental studies. The rest were Strength III, which were a combination of non-experimental, qualitative, observational, pre-post, or mixed methods. Additionally, the JHNEBP tool identified the quality of evidence based on sample size and consistency of evidence. Our group of articles chosen for analysis was 90% (30/33) Quality Q, and only 9% (3/33) were quality B.
3.4. Results of Individual Studies
Following the Kruse Protocol, reviewers independently extracted data and recorded observations about each study on a standardized Excel spreadsheet. As part of a thematic analysis, observations that occurred more than once were identified as themes [13]. These themes are tabulated in Table 2. Multiple observations of a similar nature are listed multiple times for studies, but an observation-to-theme match can be found in Appendix A and Appendix B. In 29/33 (88%) studies analyzed, an improvement in at least one area was noted. Additional observations collected in the data extraction step (sample size, bias, effect size, country of origin, statistics used, patient satisfaction, and the strength and quality of evidence from the JHNEBP tool) can be found in Appendix C. Effect sizes were only reported for 22 of the 33 studies (67%). The weighted average effect size was 0.21 (small).
Table 2.
Summary of analysis, sorted chronologically.
| Authors | Intervention Themes | Results Themes | Medical Outcome Themes | Effectiveness Themes | Barrier Themes |
|---|---|---|---|---|---|
| Borosund et al. [19] | Web-based (eHealth) | Improved in at least one area | Improved mental health | Improved mental health | Must train users |
| Improved mental health | Improved mental health | Improved mental health | |||
| Improved mental health | |||||
| Freeman et al. [20] | Web-based (eHealth) | Improved sleep outcomes | Improved sleep outcomes | Improved sleep outcomes | Must train users |
| Wheelock et al. [21] | Web-based (eHealth) | Improved in at least one area | Provided education/answered questions | Provided education/answered questions | Time of providers/workflow |
| Low reimbursement of treatment | Improved social support/answered questions | ||||
| Galiano-Castillo et al. [22]. | Web-based (eHealth) | Improved in at least one area | Improved physical health | Improved physical health | Cost of intervention |
| Improved global health/baseline function | Improved sleep outcomes | Improved sleep outcomes | |||
| Improved sleep outcomes | Less pain | Less pain | |||
| Less numbness/pain/swelling | Improved quality of life | Improved quality of life | |||
| Improved quality of life | |||||
| Admiraal et al. [23] | Web-based (eHealth) | Improved in at least one area | Improved mental health | Improved mental health | Cost of intervention |
| Improved mental health | Improvements not statistically significant | Improvements not statistically significant | |||
| No statistically significant differences | |||||
| Fazzino et al. [24] | Telephone | Improved in at least one area | Improved physical health | Improved physical health | Cost of intervention |
| Improved body image | Improved body image | Improved body image | |||
| No statistically significant differences | Improvements not statistically significant | Improvements not statistically significant | |||
| Han et al. [25] | Web-based (eHealth) | Complexity of tool takes more time to process | Provided education/answered questions | Provided education/answered questions | Cost of intervention |
| Uhm et al. [26] | mHealth | Improved in at least one area | Improved physical health | Improved physical health | Cost of intervention |
| Improved exercise | Improvements not statistically significant | Improvements not statistically significant | |||
| No statistically significant differences | |||||
| Kim et al. [27] | mHealth | Improved in at least one area | Less nausea/vomiting | Improved medication adherence | Cost of intervention |
| Less nausea/vomiting | Less numbness | Less nausea/vomiting | Must train users | ||
| Less numbness/pain/swelling | Improved physical health | Improved sleep outcomes | |||
| Improved physical health | Improved quality of life | Less numbness | |||
| Improved quality of life | Improved medication adherence | Improved quality of life | |||
| McCarthy et al. [28] | Web-based (eHealth) | Improved in at least one area | Improved sleep outcomes | Improved sleep outcomes | Time of providers/workflow |
| Improved sleep outcomes | Improved quality of life | Improved quality of life | |||
| Improved quality of life | |||||
| Visser et al. [29] | mHealth | Improved in at least one area | Provided education/answered questions | Improvements not statistically significant | Intervention not effective |
| Improved social support/answered questions | Cost of intervention | ||||
| Zachariae et al. [30] | Web-based (eHealth) | Improved in at least one area | Improved sleep outcomes | Improved sleep outcomes | Must train users |
| Improved sleep outcomes | Improved sleep outcomes | Improved sleep outcomes | |||
| Improved sleep outcomes | Improved sleep outcomes | Improved sleep outcomes | |||
| Improved sleep outcomes | Improved mental health | Improved mental health | |||
| Ariza-Garcia et al. [31] | Web-based (eHealth) | Improved in at least one area | Improved physical health | Improved physical health | Must train users |
| Improved physical health | Improved physical health | Improved physical health | |||
| Improved physical health | Improved physical health | Improved physical health | |||
| Improved physical health | Improved physical health | Improved physical health | |||
| Crafoord et al. [32] | mHealth | Improved in at least one area | long-term engagement with intervention | long-term engagement with intervention | Must train users |
| Provided education/answered questions | Provided education/answered questions | Provided education/answered questions | |||
| Ferrante et al. [33] | mHealth + eHealth | Improved in at least one area | Improved body image | Improved physical health | Must train users |
| Improved body image | Improvements not statistically significant | Improved body image | |||
| Improved quality of life | Improved quality of life | ||||
| No statistically significant differences | |||||
| Fjell et al. [34] | mHealth | Improved in at least one area | Less nausea/vomiting | Less nausea/vomiting | Must train users |
| Less nausea/vomiting | |||||
| Less nausea/vomiting | Less nausea/vomiting | Less nausea/vomiting | |||
| Improved mental health | Improved mental health | Improved mental health | |||
| Improved mental health | Improved mental health | Improved mental health | |||
| Improved physical health | Improved physical health | Improved physical health | |||
| Hou et al. [35] | mHealth | Improved in at least one area | Improved quality of life | Improved quality of life | Must train users |
| Improved quality of life | Improved physical health | Improved physical health | |||
| Improved global health/baseline function | |||||
| Lally et al. [36] | Web-based (eHealth) | Improved in at least one area | Improved mental health | Improved mental health | Must train users |
| Improved mental health | Improved mental health | Improved mental health | |||
| Improved mental health | |||||
| Lozano-Lozano et al. [37] | mHealth | Improved in at least one area | Improved quality of life | Improved quality of life | Time of providers/workflow |
| Improved quality of life | Improved arm symptoms/upper limb functionality | Improved arm symptoms/upper limb functionality | |||
| Improved arm symptoms/upper limb functionality | |||||
| van der Hout et al. [38] | Web-based (eHealth) | No statistically significant differences | Improvements not statistically significant | Improvements not statistically significant | Intervention not effective |
| Cost of intervention | |||||
| Çınar et al. [39] | mHealth | Improved in at least one area | Improved quality of life | Improved quality of life | Must train users |
| Improved quality of life | Improved mental health | Improved mental health | |||
| Improved mental health | |||||
| Fang et al. [40] | Web-based (eHealth) | Improved in at least one area | Improved body image | Improved body image | Intervention not statistically effective |
| Improved body image | Improved mental health | Improved mental health | Must train users | ||
| Improved mental health | Improved mental health | Improved mental health | |||
| Improved mental health | Improved mental health | Improved mental health | |||
| Improved mental health | |||||
| Krzyzanowska et al. [41] | Telephone | No statistically significant differences | Improvements not statistically significant | Improvements not statistically significant | Intervention not statistically effective |
| Kumar et al. [42] | Telephone | Improved in at least one area | Provided education/answered questions | Provided education/answered questions | Cost of intervention |
| Provided education/answered questions | Time of providers/workflow | ||||
| Lai et al. [43] | Web-based (eHealth) | Improved in at least one area | Improved physical health | Provided education/answered questions | Cost of intervention |
| Improved global health/baseline function | Time of providers/workflow | ||||
| Öztürk et al. [44] | mHealth | Improved in at least one area | Less nausea/vomiting | Less nausea/vomiting | Cost of intervention |
| Less nausea/vomiting | Less nausea/vomiting | Less nausea/vomiting | Must train users | ||
| Less nausea/vomiting | Improved quality of life | Improved quality of life | |||
| Improved quality of life | Improved physical health | Improved physical health | |||
| Improved physical health | |||||
| Reeves et al. [45] | mHealth | Improved in at least one area | Improved body image | Improved body image | Cost of intervention |
| Improved body image | Improved body image | Improved body image | Must train users | ||
| Improved body image | Improved body image | Improved body image | |||
| Improved body image | Improved fasting plasma glucose | Improved fasting plasma glucose | |||
| Improved fasting plasma glucose | Improved quality of life | Improved quality of life | |||
| Improved quality of life | |||||
| Wagner et al. [46] | Web-based (eHealth) | Improved in at least one area | Improved mental health | Improved mental health | Cost of intervention |
| Improved mental health | long-term engagement with intervention | long-term engagement with intervention | Time of providers/workflow | ||
| Improved medication adherence | Must train users | ||||
| Bandani-Susan et al. [47] | mHealth | Improved in at least one area | Improved sleep outcomes | Improved sleep outcomes | Cost of intervention |
| Improved sleep outcomes | Improved body image | Improved body image | Must train users | ||
| Improved body image | |||||
| Fu et al. [48] | mHealth | Improved in at least one area | Less pain | Less pain | Cost of intervention |
| Less numbness/pain/swelling | Less pain | Less pain | Must train users | ||
| Less numbness/pain/swelling | Less pain | Less pain | |||
| Less numbness/pain/swelling | Less numbness | Less numbness | |||
| Less numbness/pain/swelling | Improved arm symptoms/upper limb functionality | Improved arm symptoms/upper limb functionality | |||
| Improved arm symptoms/upper limb functionality | |||||
| Gao et al. [49] | mHealth | Improved in at least one area | Improved mental health | Improved mental health | Cost of intervention |
| Improved mental health | Improved physical health | Improved physical health | Must train users | ||
| Improved physical health | |||||
| Medina et al. [50] | Web-based (eHealth) | Improved in at least one area | Improved mental health | Improved mental health | Cost of intervention |
| Improved social support/answered questions | Must train users | ||||
| Improved mental health | |||||
| Oswald et al. [51] | Web-based (eHealth) | Improved in at least one area | Improved sleep outcomes | Improved sleep outcomes | Cost of intervention |
| Improved sleep outcomes | Improved sleep outcomes | Improved sleep outcomes | Must train users | ||
| Improved sleep outcomes | Improved sleep outcomes | Improved sleep outcomes | |||
| Improved sleep outcomes |
3.5. Results of Syntheses, Additional Analysis and Certainty of Evidence
Thematic analysis was performed on all studies. Themes and additional observations were summarized into affinity matrices. Results are sorted by frequency. Frequency is reflected not to imply importance, but only to identify the probability a theme or observation was found in the group of studies analyzed.
3.5.1. Results of Studies Compared with Control Group
Table 3 summarizes the results of the studies compared with a control group. For non-experimental studies, the “no control group” leads the results. This is done to avoid confounding the results. facilitators observed. Thirteen themes and four individual observations were identified by the reviewers for a total of 111 occurrences in the literature. The theme most often observed was “improved mental health”, which occurred 16/111 (14%) occurrences [19,23,34,36,39,40,46,49,50]. This theme combined observations of anxiety, distress, fear of reoccurrence, depression, optimism, self-efficacy, and self-actualization. Sleep outcome was the next most frequently identified theme. It occurred 12/111 (11%) of the occurrences [20,22,28,30,47]. This theme included the following observations: sleep disturbance, insomnia, sleep efficiency, cognitive function, fatigue, and cancer fatigue. The next theme is an improved quality of life, which appeared in 9/111 (8%) of the occurrences [22,27,28,33,35,37,39,44,45]. Two themes appeared in 7/111 (6%) of the occurrences: improved body image [22,31,38,43,45] and improved physical health [27,31,34,44,49]. The body image theme was comprised of the following observations: waist circumference, fat mass, and weight. Two themes were identified in 6/111 (5%) of the occurrences: less numbness, pain, or swelling [22,27,48], and no statistical differences between the intervention and control groups [23,24,26,33,38,41]. Next was less nausea or vomiting [27,34,44]. This occurred in 5/111 (5%) of the observations. Although nausea and vomiting are highly correlated, they are not synonymous, so reviewers chose to report them separately, but they appeared together in two studies. Two themes appeared in 3/111 (3%) of the occurrences: improved global health/return to baseline functioning [22,35,43] and improved social support, and questions were answered by providers [21,29,50]. Two themes occurred in 2/111 (2%) of the occurrences: improved arm symptoms/upper limb functionality [37,48], and the app provided education and answered questions [32,42]. There were four observations that could not be fit into themes: improved exercise, improved medication adherence, improved fasting plasma glucose, and the complexity of the tool (app) takes more time for users to process [25,26,45,46].
Table 3.
Results of studies, compared to control group.
| Results Themes and Observations | Frequency |
|---|---|
| Improved in at least one area [19,21,22,23,24,26,27,28,29,30,31,32,33,34,35,36,37,39,40,42,43,44,45,46,47,48,49,50,51] | 29 |
| Improved mental health [19,23,34,36,39,40,46,49,50] | 16 |
| Improved sleep outcomes [20,22,28,30,47] | 12 |
| Improved quality of life [22,27,28,33,35,37,39,44,45] | 9 |
| Improved body image [24,33,40,45,47] | 7 |
| Improved physical health [27,31,34,44,49] | 7 |
| Less numbness/pain/swelling [22,27,48] | 6 |
| No statistically significant differences [23,24,26,33,38,41] | 6 |
| Less nausea/vomiting [27,34,44] | 5 |
| Improved global health/baseline function [22,35,43] | 3 |
| Improved social support/answered questions [21,29,50] | 3 |
| Improved arm symptoms/upper limb functionality [37,48] | 2 |
| Provided education/answered questions [32,42] | 2 |
| Improved exercise [26] | 1 |
| Improved medication adherence [46] | 1 |
| Improved fasting plasma glucose [45] | 1 |
| Complexity of tool takes more time to process [25] | 1 |
| 111 |
3.5.2. Medical Outcome and Effectiveness Commensurate with the Intervention
Table 4 summarizes the medical outcomes and effectiveness observed. Twelve themes and two individual observations were recorded commensurate with the adoption of the intervention for a total of 85 occurrences. Due to the high level of overlap with study results, reviewers chose to only report the differences. In 2/87 (2%) of the occurrences, the intervention was credited with long-term engagement with treatment programs [32,46].
Table 4.
Medical outcomes and effectiveness commensurate with the adoption of the intervention.
| Medical Outcomes and Effectiveness Themes and Observations | Frequency |
|---|---|
| Improved mental health [19,23,30,34,36,39,40,46,49,50] | 17 |
| Improved physical health [22,24,26,27,31,34,35,43,44,49] | 13 |
| Improved sleep outcomes [20,22,28,30,47,51] | 12 |
| Improved quality of life [22,27,28,35,37,39,44,45] | 8 |
| Improved body image [24,33,40,45,47] | 7 |
| Improvements not statistically significant [23,24,26,33,38,41] | 6 |
| Less nausea/vomiting [27,34,44] | 5 |
| Provided education/answered questions [21,25,29,32,42] | 5 |
| Less pain [22,48] | 4 |
| Less numbness [27,48] | 2 |
| Improved arm symptoms/upper limb functionality [37,48] | 2 |
| long-term engagement with intervention [32,46] | 2 |
| Improved medication adherence [27] | 1 |
| Improved fasting plasma glucose [45] | 1 |
| 85 |
3.5.3. Barriers to the Adoption of Telehealth for Breast Cancer
Table 5 tabulates the barriers identified in the literature. Five themes and one observation were recorded in 49 occurrences. The most frequently observed theme was the need to train users, which occurred in 20/49 (41%) of the occurrences [19,20,27,30,31,32,33,34,35,36,39,40,44,45,46,47,48,49,50,51]. The second barrier was the cost (set up, maintenance, and equipment), which appeared in 18/87 (37%) of the occurrences [22,23,24,25,26,27,29,38,42,43,44,45,46,47,48,49,50,51]. The intervention took time of the providers and presented unusual workflow appeared in 6/49 (12%) of the occurrences [21,28,37,42,43,46]. The intervention was not effective [29,38] or not statistically significant in 2/49 (4%) of the occurrences [40,41]. Finally, there is low reimbursement for the time spent on the intervention that appeared once [21].
Table 5.
Barriers to the adoption of Telehealth for the treatment of Breast Cancer.
| Barrier Themes and Observations | Frequency |
|---|---|
| Must train users [19,20,27,30,31,32,33,34,35,36,39,40,44,45,46,47,48,49,50,51] | 20 |
| Cost of intervention [22,23,24,25,26,27,29,38,42,43,44,45,46,47,48,49,50,51] | 18 |
| Time of providers/workflow [21,28,37,42,43,46] | 6 |
| Intervention not effective [29,38] | 2 |
| Intervention not statistically effective [40,41] | 2 |
| Low reimbursement of treatment [21] | 1 |
| 49 |
3.5.4. Interactions between Observations
The intervention of mHealth resulted in the most observations of “improvement in at least one area”, but not all outcomes were statistically significant [26,27,29,32,34,35,37,39,44,45,47,48,49]. The mHealth intervention studies used strong methodologies: 11 were either RCT or experimental, while one was quasi-experimental and one used mixed methods [26,27,29,32,34,35,37,39,44,45,47,48,49].
4. Discussion
This systematic literature review examined 33 studies from 11 countries published over the last 10 years to analyze the effectiveness of telemedicine to treat the symptoms commensurate with the treatment and recovery of breast cancer. Five interventions were identified, however, the dominant interventions were eHealth and mHealth. Methodologies were strong among the group for analysis, and the results of the studies showed positive effects in at least one area [19,21,22,23,24,26,27,28,29,30,31,32,33,34,35,36,37,39,40,42,43,44,45,46,47,48,49,50,51]. Telehealth interventions showed improvements in both mental health [19,23,30,34,36,39,40,46,49,50], physical health [22,24,26,27,31,34,35,43,44,49], sleep outcomes [20,22,28,30,47,51], quality of life [22,27,28,35,37,39,44,45] and body image [24,33,40,45,47]. Telehealth interventions decreased nausea, vomiting [27,34,44], numbness, pain [27,48], improved arm symptoms and upper limb functionality [27,48]. Only a few studies reported non-statistically significant findings [23,24,26,33,38,41].
The findings of this systematic literature review are congruent with that of Buneviciene et al. [10]. The intervention of mHealth and eHealth addressed the quality of life of patients in the areas of physical activity, mindfulness, and stress management. This review found multiple instances of improvements in mental health, physical health, sleep outcomes, and quality of life. Our findings are also consistent with Watanabe et al., in that eHealth and mHealth augmented medical education and health literacy [6].
eHealth and mHealth offer several possible interventions that show promise as a treatment modality of care, however the clinical efficacy of this modality shows mixed results. The difference in results could be due to a difference of methodology or a difference of measurement. While older patients do not often prefer eHealth and mHealth interventions, many other patients do prefer this modality. Even when the results of using the eHealth and mHealth modalities of care show equivalent, but not statistically greater efficacy, offering the modality may meet the preference of the patient. These issues should be addressed in future research considerations.
Future research should examine the reasons for the lack of significant results in some of the studies. Standardization of methodology and measurement should yield consistent results. The results reported in this review were inconsistent. This systematic review focused on breast cancer. Future reviews should examine other types of cancer, then a review of reviews should be conducted for all cancer. The results did not seem to follow any particular intervention. This means it could have been a bias in the sample. Many examples of both sample bias and selection bias were observed, which affect the external and internal validity, respectively.
The results of this review should give practitioners confidence that telehealth can provide viable interventions to help their patients assuage the effects of breast cancer recovery and chemotherapy. The results from the studies analyzed in this review demonstrate healthy habits, less nausea, lost weight, more strength, and an increase in personal confidence. Policy makers should explore other reimbursement mechanisms to ensure the extra time and money these interventions require is reimbursed.
Limitations
No study is without its limitations, and this literature review is no different. Only four databases were queried over 10 years for published works. A broader scope of databases, years, and sources of literature, such as grey literature, may have identified additional interventions and results. However, the reviewers chose these databases due to their wide availability, 10 years because telemedicine is a rapidly growing field, and published literature to ensure a peer review. Within the studies analyzed were multiple examples of selection and sample bias, which affect the internal and external validity, respectively.
5. Conclusions
Telehealth offers promise to help breast cancer survivors cope with the side effects of treatment, the mental anguish that shakes confidence, and the physical ailments that accompany chemotherapy. Several exercise applications show promise educating and helping survivors establish healthy habits to lower the risk of reoccurrence. The most significant barrier is training followed by cost, but these are not significant barriers to overcome.
Appendix A. Observation-to-Theme Conversion
| Authors | Experimental Intervention | Intervention Themes |
Results (Compared to
Control Group) |
Results Themes | Medical Outcomes Reported | Medical Outcome Themes | Study Design |
| Borosund et al. | Internet-based patient-provider communication service | Web-based (eHealth) | Intervention group reported significantly lower symptom distress, anxiety, and depression | Improved in at least one area | nurse-administered IPPC alone can significantly reduce depression, decreased symptom distress, decreased anxiety | Improved mental health | RCT |
| Improved mental health | Improved mental health | ||||||
| Improved mental health | Improved mental health | ||||||
| Improved mental health | Improved mental health | ||||||
| Improved mental health | |||||||
| Freeman et al. | Telemedicine (TD) [vs live vs wait list] | Web-based (eHealth) | TD (and Live) reported less fatigue, cognitive dysfunction, and sleep disturbance with WL | Improved sleep outcomes | improvements in multiple QOL domains for breast cancer survivors compared with WL. Less fatigue, less cognitive dysfunction, fewer sleep disturbances |
Improved sleep outcomes | RCT |
| Improved sleep outcomes | Improved sleep outcomes | ||||||
| Improved sleep outcomes | Improved sleep outcomes | ||||||
| Wheelock et al. | SIS.NET (online questionnairre with remove NP overview and follow-up) | Web-based (eHealth) | patients reported more new or changed symptoms compared with standard care patients | Improved in at least one area | This intervention facilitated symptom reporting and may provide a means of convenient symptom assessment | Provided education/answered questions | RCT |
| Improved social support/answered questions | |||||||
| Galiano-Castillo et al. | Internet-based, tailored exercise program | Web-based (eHealth) | telerehabilitation group improved significantly global health status, physical, role, cognitive functioning and arm symptoms, as well as pain severity, and pain interference, compared with the control group. | Improved in at least one area | Improved physical health, cognitive functioning, pain severity, and pain interference | Improved physical health | RCT |
| Improved global health/baseline function | Improved sleep outcomes | ||||||
| Improved sleep outcomes | Less pain | ||||||
| Less numbness/pain/swelling | Improved quality of life | ||||||
| Improved quality of life | |||||||
| Admiraal et al. | web-based psychoeducation for breast cancer (ENCOURAGE) | Web-based (eHealth) | No statistically significant differences between control and intervention for optimism or control over future | Improved in at least one area | For clinical distressed patients, use of the intervention increased optimism and control over future | Improved mental health | RCT |
| Improved mental health | Improvements not statistically significant | ||||||
| No statistically significant differences | |||||||
| Fazzino et al. | telephone (weekly) | Telephone | No control group. Distance-based weight loss program can be successful | Improved in at least one area | Moderate-to-vigorous physical activity significantly increased from baseline to 6 months. | Improved physical health | Non-experimental (no randomization, no control) |
| Improved body image | Improved body image | ||||||
| No statistically significant differences | Improvements not statistically significant | ||||||
| Han et al. | eHealth system (Comprehensive Health Enhancement Support System, CHESS) | Web-based (eHealth) | No control group. cancer patients’ access to more complex tools generates more use with their time spreading out over the diverse services. | Complexity of tool takes more time to process | Communication functions drive long-term engagement with the system. | Provided education/answered questions | Pre-post |
| Uhm et al. | mHealth | mHealth | Improved exercise, but not statistically different than control | Improved in at least one area | Improved exercise, but not statistically different than control | Improved physical health | Quasi-experimental |
| Improved exercise | Improvements not statistically significant | ||||||
| No statistically significant differences | |||||||
| Kim et al. | mHealth (mobile game) | mHealth | Improved drug adherence, lower side effects of chemotherapy (nausea, fatigue, numbness of hand or foot, and hair loss). Improved quality of life. No significant difference in depression or anxiety | Improved in at least one area | Improved drug adherence, lower side effects of chemotherapy (nausea, fatigue, numbness of hand or foot, and hair loss). Improved quality of life. Improved medication adherence. No significant difference in depression or anxiety | Less nausea/vomiting | RCT |
| Less nausea/vomiting | Less numbness | ||||||
| Less numbness/pain/swelling | Improved physical health | ||||||
| Improved physical health | Improved quality of life | ||||||
| Improved quality of life | Improved medication adherence | ||||||
| McCarthy et al. | nurse-led telemedicine delivered, cognitive behavioral therapy | Web-based (eHealth) | participants reported improvements in sleep outcomes, including SE and SL. QOL and daily functioning improved, but anxiety and depression did not. | Improved in at least one area | participants reported improvements in sleep outcomes, including SE and SL. QOL and daily functioning improved, but anxiety and depression did not. | Improved sleep outcomes | Quasi-experimental |
| Improved sleep outcomes | Improved quality of life | ||||||
| Improved quality of life | |||||||
| Visser et al. | tablet online support group | mHealth | No statistically significant differences between control and intervention for distress and empowerment. Greater peer support identified in control. | Improved in at least one area | No improvement with intervention. Satisfaction very low. | Provided education/answered questions | RCT |
| Improved social support/answered questions | |||||||
| Zachariae et al. | Internet-delivered cognitive-behavioral therapy (iCBT) | Web-based (eHealth) | Statistically significant improvements observed for all sleep-related outcomes (fatigue, sleep disturbances, total sleep time). | Improved in at least one area | Reduced insomnia, increased sleep quality, increases sleep efficiency, increased total sleep time, improved time in bed, reduced fatigue | Improved sleep outcomes | RCT |
| Improved sleep outcomes | Improved sleep outcomes | ||||||
| Improved sleep outcomes | Improved sleep outcomes | ||||||
| Improved sleep outcomes | Improved mental health | ||||||
| Ariza-Garcia et al. | web-based exercise system (e_CuidateChemo) | Web-based (eHealth) | Functional capacity improved significantly, abdominal strength, lower body strength, back strength | Improved in at least one area | Intervention increased exercise capacity by 10.8% (33.4% reached a normal exercise capacity compared with 12.3% in control). Functional capacity, abdominal strength, lower body strength, back strength improved significantly. | Improved physical health | RCT |
| Improved physical health | Improved physical health | ||||||
| Improved physical health | Improved physical health | ||||||
| Improved physical health | Improved physical health | ||||||
| Crafoord et al. | mHealth app for symptom self-management | mHealth | Daily symptom reporting created feelings of having continuous contact with health care professionals, being acknowledged, and safe. | Improved in at least one area | Engagement was very high for intervention. The app promoted patient participation in their care. | long-term engagement with intervention | Mixed Methods |
| Provided education/answered questions | Provided education/answered questions | ||||||
| Ferrante et al. | mHealth/eHealth tools | mHealth + eHealth | No statistically significant differences between weight lost in both groups. Waist circumference improved more, quality of life more, and use of strategies for healthy eating and decreasing calories. | Improved in at least one area | Effective at weight loss, but not statistically significant | Improved body image | RCT |
| Improved body image | Improvements not statistically significant | ||||||
| Improved quality of life | |||||||
| No statistically significant differences | |||||||
| Fjell et al. | mHealth app (Interaktor) during neoadjuvant chemo | mHealth | statistically significant less symptom prevalence in nausea, vomiting, feeling sad, appetite loss and constipation. Overall symptom distress and physical symptom distress were rated statistically significant lower in the intervention group. Further, emotional functioning was rated statistically significant higher in the intervention group. | Improved in at least one area | statistically significant less symptom prevalence in nausea, vomiting, feeling sad, appetite loss and constipation. Overall symptom distress and physical symptom distress were rated statistically significant lower in the intervention group. Further, emotional functioning was rated statistically significant higher in the intervention group. | Less nausea/vomiting | RCT |
| Less nausea/vomiting | |||||||
| Less nausea/vomiting | Less nausea/vomiting | ||||||
| Improved mental health | Improved mental health | ||||||
| Improved mental health | Improved mental health | ||||||
| Improved physical health | Improved physical health | ||||||
| Hou et al. | mHealth app for self-management support (BCSMS) | mHealth | Mean quality of life scores and global health higher | Improved in at least one area | Mean quality of life scores and global health higher | Improved quality of life | RCT |
| Improved quality of life | Improved physical health | ||||||
| Improved global health/baseline function | |||||||
| Lally et al. | we-based, psychoeducational distress self-management program (CaringGuidance) | Web-based (eHealth) | post hoc analysis showed significant group differences in slopes occurring between study months 2 and 3 on distress and depressive symptoms | Improved in at least one area | post hoc analysis showed significant group differences in slopes occurring between study months 2 and 3 on distress and depressive symptoms | Improved mental health | True experiment |
| Improved mental health | Improved mental health | ||||||
| Improved mental health | |||||||
| Lozano-Lozano et al. | mHealth (BENECA) + rehab | mHealth | Both groups showed improved outcomes, but global QoL was significantly better with intervention. Improvement in upper-limb functionality also higher | Improved in at least one area | Both groups showed improved outcomes, but global QoL was significantly better with intervention. Improvement in upper-limb functionality also higher | Improved quality of life | RCT |
| Improved quality of life | Improved arm symptoms/upper limb functionality | ||||||
| Improved arm symptoms/upper limb functionality | |||||||
| van der Hout et al. | eHealth (Oncokompas) symptom self-management app | Web-based (eHealth) | Oncokompas did not improve the amount of knowledge, skills, and confidence for self-management in cancer survivors. | No statistically significant differences | No difference between groups | Improvements not statistically significant | RCT |
| Çınar et al. | mHealth app for education, symptom tracking, and management | mHealth | QoL of the treatment group after intervention increased and distress level was lower | Improved in at least one area | QoL of the treatment group after intervention increased and distress level was lower | Improved quality of life | True experiment |
| Improved quality of life | Improved mental health | ||||||
| Improved mental health | |||||||
| Fang et al. | decision-support app (Pink Journey) | Web-based (eHealth) | body image distress declined significantly for the intervention group but increased for the control group. no significant difference in decision conflict, decision regret, anxiety, or depression. | Improved in at least one area | Decrease in body image, regret, anxiety, & distress | Improved body image | RCT |
| Improved body image | Improved mental health | ||||||
| Improved mental health | Improved mental health | ||||||
| Improved mental health | Improved mental health | ||||||
| Improved mental health | |||||||
| Krzyzanowska et al. | telephone based management of toxicities | Telephone | No differences in self-efficacy, anxiety, or depression | No statistically significant differences | No differences in self-efficacy, anxiety, or depression | Improvements not statistically significant | RCT |
| Kumar et al. | Teleconsultation | Telephone | No control group. Concerns and questions answered through intervention | Improved in at least one area | Breast conservation surgery | Provided education/answered questions | Qualitative |
| Provided education/answered questions | |||||||
| Lai et al. | Telemedicine (VTC) Occupational Therapy | Web-based (eHealth) | No control group. Patients regained baseline function within a mean of 42.4 days after surgery and after an average of three sessions | Improved in at least one area | all regained baseline functional status and full range of motion | Improved physical health | Non-experimental (no randomization, no control) |
| Improved global health/baseline function | |||||||
| Öztürk et al. | mHealth symptom monitoring app | mHealth | Effective at decreasing nausea-vomiting, raising sexual function and sexual enjoyment | Improved in at least one area | Symptom monitoring with mHealth highly effective in controlling physical symptoms | Less nausea/vomiting | True experiment |
| Less nausea/vomiting | Less nausea/vomiting | ||||||
| Less nausea/vomiting | Improved quality of life | ||||||
| Improved quality of life | Improved physical health | ||||||
| Improved physical health | |||||||
| Reeves et al. | mHealth weight-loss | mHealth | Improved weight reduction (over control) fat mass, metabolic syndrome risk score, waist circumference, fasting plasma glucose, and quality of life | Improved in at least one area | Improved weight reduction (over control) fat mass, metabolic syndrome risk score, waist circumference, fasting plasma glucose, and quality of life | Improved body image | RCT |
| Improved body image | Improved body image | ||||||
| Improved body image | Improved body image | ||||||
| Improved body image | Improved fasting plasma glucose | ||||||
| Improved fasting plasma glucose | Improved quality of life | ||||||
| Improved quality of life | |||||||
| Wagner et al. | eHealth (Fear of recurrence, FoF) telecoaching | Web-based (eHealth) | Significantly reduced fear of recurrence. Telecoaching improved adherence and retention. | Improved in at least one area | Reduced fear of recurrence. Telecoaching improved adherence and retention. | Improved mental health | RCT |
| Improved mental health | long-term engagement with intervention | ||||||
| Improved medication adherence | |||||||
| Bandani-Susan et al. | mHealth education | mHealth | Mean score of cancer fatigue decreased and body image increased significantly | Improved in at least one area | Decreased fatigue, increased body image | Improved sleep outcomes | RCT |
| Improved sleep outcomes | Improved body image | ||||||
| Improved body image | |||||||
| Fu et al. | mHealth pain-management | mHealth | Participants in the intervention were more likely to experience complete reduction in pain and soreness, lower median severity scores and general body pain, less arm/hand swelling, heaviness, redness, and limited movement in shoulder | Improved in at least one area | Less pain, less soreness, less swelling, less heaviness, less redness, less limited movement in shoulder | Less pain | RCT |
| Less numbness/pain/swelling | Less pain | ||||||
| Less numbness/pain/swelling | Less pain | ||||||
| Less numbness/pain/swelling | Less numbness | ||||||
| Less numbness/pain/swelling | Improved arm symptoms/upper limb functionality | ||||||
| Improved arm symptoms/upper limb functionality | |||||||
| Gao et al. | mHealth Tai Chi and health education | mHealth | A significant time effect for mental health, physical health, but not for stress. | Improved in at least one area | Tai Chi participants had a significantly better mental health at follow up. | Improved mental health | RCT |
| Improved mental health | Improved physical health | ||||||
| Improved physical health | |||||||
| Medina et al. | eHealth ecosystem (ICOnnecta) | Web-based (eHealth) | Strong social support led to better psychosocial course | Improved in at least one area | ICOnnecta supports the development of a digital relation with healthcare services | Improved mental health | Quasi-experimental |
| Improved social support/answered questions | |||||||
| Improved mental health | |||||||
| Oswald et al. | eHealth cognitive-behavioral therapy (iCBT) | Web-based (eHealth) | Improvements in insomnia, sleep efficiency, and sleep disturbance | Improved in at least one area | Improvements in insomnia, sleep efficiency, and sleep disturbance | Improved sleep outcomes | RCT |
| Improved sleep outcomes | Improved sleep outcomes | ||||||
| Improved sleep outcomes | Improved sleep outcomes | ||||||
| Improved sleep outcomes |
Appendix B. Observation-to-Theme Conversion
| Authors | Effectiveness | Effectiveness Themes | Barriers to Adoption | Barrier Themes |
| Borosund et al. | Decreased symptom distress, decreased depression, increased self-efficacy | Improved mental health | Must train users | Must train users |
| Improved mental health | ||||
| Improved mental health | ||||
| Improved mental health | ||||
| Freeman et al. | Less fatigue, less cognitive dysfunction, fewer sleep disturbances | Improved sleep outcomes | Must train users | Must train users |
| Improved sleep outcomes | ||||
| Improved sleep outcomes | ||||
| Wheelock et al. | This intervention facilitated symptom reporting and may provide a means of convenient symptom assessment, Intervention reduced feedback time to patient |
Provided education/answered questions | Adds workflow that may not be reimbursed | Time of providers/workflow |
| Low reimbursement of treatment | ||||
| Galiano-Castillo et al. | Improved quality of life, physical health, cognitive functioning, pain severity, and pain interference | Improved physical health | cost | Cost of intervention |
| Improved sleep outcomes | ||||
| Less pain | ||||
| Improved quality of life | ||||
| Admiraal et al. | Not statistically significant for primary and secondary outcome, however, clinically distressed patients increased optimism and control over future | Improved mental health | Setup costs | Cost of intervention |
| Improvements not statistically significant | ||||
| Fazzino et al. | Moderate-to-vigorous physical activity significantly increased from baseline to 6 months. | Improved physical health | Cost of equipment. Time of providers | Cost of intervention |
| Improved body image | ||||
| Improvements not statistically significant | ||||
| Han et al. | the effectiveness of the Information and Support services was attenuated in more complex versions of Full CHESS or Full CHESS + Mentor | Provided education/answered questions | Cost of system | Cost of intervention |
| Uhm et al. | Improved exercise, but not statistically different than control | Improved physical health | cost of system | Cost of intervention |
| Improvements not statistically significant | ||||
| Kim et al. | Improved drug adherence, lower side effects of chemotherapy (nausea, fatigue, numbness of hand or foot, and hair loss). Improved quality of life. No significant difference in depression or anxiety | Improved medication adherence | Cost of system, must train users | Cost of intervention |
| Less nausea/vomiting | Must train users | |||
| Improved sleep outcomes | ||||
| Less numbness | ||||
| Improved quality of life | ||||
| McCarthy et al. | participants reported improvements in sleep outcomes, including SE and SL. QOL and daily functioning improved, but anxiety and depression did not. | Improved sleep outcomes | Provider’s time | Time of providers/workflow |
| Improved quality of life | ||||
| Visser et al. | Not effective. | Improvements not statistically significant | Not effective. Cost of equipment | Intervention not effective |
| Cost of intervention | ||||
| Zachariae et al. | Reduced insomnia, increased sleep quality, increases sleep efficiency, increased total sleep time, improved time in bed, reduced fatigue | Improved sleep outcomes | Must train users | Must train users |
| Improved sleep outcomes | ||||
| Improved sleep outcomes | ||||
| Improved mental health | ||||
| Ariza-Garcia et al. | Functional capacity improved significantly, abdominal strength, lower body strength, back strength | Improved physical health | Must train users | Must train users |
| Improved physical health | ||||
| Improved physical health | ||||
| Improved physical health | ||||
| Crafoord et al. | Engagement related to feeling of being valued which affected satisfaction | long-term engagement with intervention | Must train users | Must train users |
| Provided education/answered questions | ||||
| Ferrante et al. | Improved weight loss, improved waist circumference, improved quality of life, improved healthy eating, decreased calories consumed | Improved physical health | Must train users | Must train users |
| Improved body image | ||||
| Improved quality of life | ||||
| Fjell et al. | statistically significant less symptom prevalence in nausea, vomiting, feeling sad, appetite loss and constipation. Overall symptom distress and physical symptom distress were rated statistically significant lower in the intervention group. Further, emotional functioning was rated statistically significant higher in the intervention group. | Less nausea/vomiting | Must train users | Must train users |
| Less nausea/vomiting | ||||
| Improved mental health | ||||
| Improved mental health | ||||
| Improved physical health | ||||
| Hou et al. | Mean quality of life scores and global health higher | Improved quality of life | Must train users | Must train users |
| Improved physical health | ||||
| Lally et al. | post hoc analysis showed significant group differences in slopes occurring between study months 2 and 3 on distress and depressive symptoms | Improved mental health | Must train users | Must train users |
| Improved mental health | ||||
| Lozano-Lozano et al. | Both groups showed improved outcomes, but global QoL was significantly better with intervention. Improvement in upper-limb functionality also higher | Improved quality of life | Uses more time of clinicians | Time of providers/workflow |
| Improved arm symptoms/upper limb functionality | ||||
| van der Hout et al. | none | Improvements not statistically significant | No difference between groups, cost | Intervention not effective |
| Cost of intervention | ||||
| Çınar et al. | QoL of the treatment group after intervention increased and distress level was lower | Improved quality of life | Must train users | Must train users |
| Improved mental health | ||||
| Fang et al. | Decrease in body image & distress | Improved body image | Decrease in body image, regret, anxiety, & distress | Intervention not statistically effective |
| Improved mental health | Must train users | |||
| Improved mental health | ||||
| Improved mental health | ||||
| Krzyzanowska et al. | none | Improvements not statistically significant | No differences in self-efficacy, anxiety, or depression | Intervention not statistically effective |
| Kumar et al. | Distance was overcome through teleconsultation | Provided education/answered questions | Cost of equipment. Time of providers | Cost of intervention |
| Time of providers/workflow | ||||
| Lai et al. | Distance was overcome through teleconsultation. Patients regained full functional status and full range of motion | Provided education/answered questions | Cost of equipment. Time of providers | Cost of intervention |
| Time of providers/workflow | ||||
| Öztürk et al. | Effective at decreasing nausea-vomiting, raising sexual function and sexual enjoyment | Less nausea/vomiting | cost, training | Cost of intervention |
| Less nausea/vomiting | Must train users | |||
| Improved quality of life | ||||
| Improved physical health | ||||
| Reeves et al. | Improved weight reduction (over control) fat mass, metabolic syndrome risk score, waist circumference, fasting plasma glucose, and quality of life | Improved body image | cost, training | Cost of intervention |
| Improved body image | Must train users | |||
| Improved body image | ||||
| Improved fasting plasma glucose | ||||
| Improved quality of life | ||||
| Wagner et al. | Reduced fear of recurrence. Telecoaching improved adherence and retention. | Improved mental health | Cost, time, training | Cost of intervention |
| long-term engagement with intervention | Time of providers/workflow | |||
| Bandani-Susan et al. | Decreased fagigue, increased body image | Improved sleep outcomes | cost, training | Cost of intervention |
| Improved body image | Must train users | |||
| Fu et al. | Less pain, less soreness, less swelling, less heaviness, less redness, less limited movement in shoulder | Less pain | cost, training | Cost of intervention |
| Less pain | Must train users | |||
| Less pain | ||||
| Less numbness | ||||
| Improved arm symptoms/upper limb functionality | ||||
| Gao et al. | Improved mental health at follow up. | Improved mental health | cost, training | Cost of intervention |
| Improved physical health | Must train users | |||
| Medina et al. | ICOnnecta supports the development of a digital relation with healthcare services | Improved mental health | cost, training | Cost of intervention |
| Must train users | ||||
| Oswald et al. | Improvements in insomnia, sleep efficiency, and sleep disturbance | Improved sleep outcomes | cost, training | Cost of intervention |
| Improved sleep outcomes | Must train users | |||
| Improved sleep outcomes |
Appendix C. Other Observations Incident to Review
| Authors |
Sample
Size (#s Only) |
Bias within Study (See Article)
Selection Bias, Sample Bias, etc. |
Effect Size
(Small, Medium, or Large with Cohen’s d Statistic) Sensitivity, Specificity, F1 |
Country of Origin (Where Was the Study Conducted?) | Statistics Used | Patient Satisfaction | Strength of Evidence | Quality of Evidence |
| Borosund et al. | 167 | One country only (selection bias) | Not reported | Norway | Linear mixed models | High levels of satisfaction | I | A |
| Selection bias | ||||||||
| Freeman et al. | 118 | One country only (selection bias)- two sites | Not reported | USA | Linear multilevel modeling, Bonferroni method | not reported | I | A |
| Selection bias | ||||||||
| Wheelock et al. | 102 | One region of one country (selection bias), 73% Caucasian (sample bias) | Not reported | USA | Descriptive statistics, Spearman rank test | not reported | I | A |
| Selection bias | ||||||||
| Sample bias | ||||||||
| Galiano-Castillo et al. | 81 | One country only (selection bias) | global health (d = 0.89, large), physical functioning (d = 0.90, large), role functioning (d = 0.78, medium), cognitive functioning (d = 0.75, medium), arm symptoms (d = −0.53, medium). | Spain | Descriptive statistics, Cronbach’s a, Chi-square, ANCOVA | 97.8% global satisfaction | I | A |
| Selection bias | ||||||||
| Admiraal et al. | 127 | One country only (selection bias) | (d = 0.65, medium) | Netherlands | Descriptive statistics, ANCOVA, logistic regression, chi-square | not reported | I | A |
| Selection bias | ||||||||
| Fazzino et al. | 142 | One region of one country (selection bias) | Not reported | USA | Linear mixed models | not reported | III | A |
| Selection bias | ||||||||
| Han et al. | 443 | One country only (selection bias), majority Caucasian (sample bias) | Not reported | USA | Descriptive statistics, Bonferroni adjustment | not reported | III | A |
| Selection bias | ||||||||
| Sample bias | ||||||||
| Uhm et al. | 356 | One region of one country (selection bias) | Not reported | Korea | Descriptive statistics, Chi-square, Fisher’s exact test, paired t-tests, ANCOVA | Strong satisfaction scores | II | A |
| Selection bias | ||||||||
| Kim et al. | 76 | One region of one country (selection bias) | Not reported | Korea | Descriptive statistics, independent t-tests, Mann–Whitney U-tests, Chi-square tests and Fisher’s exact test. | Strong satisfaction scores | I | A |
| Selection bias | ||||||||
| McCarthy et al. | 18 | One region of one country (selection bias) | Not reported | USA | Descriptive statistics, dependent t-tests | not reported | II | B |
| Selection bias | ||||||||
| Visser et al. | 109 | One country (selection bias) | Not reported | Netherlands | ANCOVA, ANOVA | satisfaction very low | I | A |
| Selection bias | ||||||||
| Zachariae et al. | 225 | One country (selection bias) | wake after sleep onset (d = 0.33, medium), large effect sizes identified for improvements in insomnia severity (d = 0.87), sleep quality, and sleep efficiency. Medium effects for total sleep time, less time in bed, and fewer EMAs; small effect sizes for shorter SOL, fewer NAs, reduction in fatigue, and less time spent awake after sleep onset | USA | Descriptive statistics, Chi-square, mixed linear models, generalized estimating equation models | High levels of satisfaction | I | A |
| Selection bias | ||||||||
| Ariza-Garcia et al. | 68 | One country (selection bias) | Large effect for all interactions | Spain | ANCOVA | not reported | I | A |
| Selection bias | ||||||||
| Crafoord et al. | 149 | One country (selection bias) | Not reported | Sweden | Descriptive statistics, independent t-tests, Fisher’s exact test, Chi-square test | Engagement and satisfaction was high | III | A |
| Selection bias | ||||||||
| Ferrante et al. | 35 | One country (selection bias), one race (sample bias) | Large effect for all interactions | USA | paired t-test, Fisher’s exact test | High levels of satisfaction | I | A |
| Selection bias | ||||||||
| Sample bias | ||||||||
| Fjell et al. | 150 | One country (selection bias) | Effect size small (d = 0.18) to medium (d = 0.34) | Sweden | ANCOVA, Chi-square, Fisher’s exact test | Satisfaction high | I | A |
| Selection bias | ||||||||
| Hou et al. | 112 | One country (selection bias) | Sensitivity calculated but not reported | Taiwan | Descriptive statistics, t-tests | Satisfaction high | I | A |
| Selection bias | ||||||||
| Lally et al. | 100 | One country (selection bias) | Not reported | USA | multilevel models, ANOVA, Fisher’s exact test | Satisfaction high | I | A |
| Selection bias | ||||||||
| Lozano-Lozano et al. | 80 | One country (selection bias) | large effect (d = 0.72) | Spain | Descriptive statistics, chi-square, ANCOVA | Satisfaction high | I | A |
| Selection bias | ||||||||
| van der Hout et al. | 138 | One country (selection bias) | effect size small (d < 0.2) | Netherlands | Descriptive statistics, t-tests | not reported | I | A |
| Selection bias | ||||||||
| Çınar et al. | 64 | One country (selection bias) | Not reported | Turkey | ANCOVA, Chi-square, Fisher’s exact test, ANOVA, t-test, and Mann–Whitney U test | Satisfaction was very high | I | A |
| Selection bias | ||||||||
| Fang et al. | 96 | One country (selection bias) | Not reported | Taiwan | Descriptive statistics, Chi-square, t-test | High levels of satisfaction | I | A |
| Selection bias | ||||||||
| Krzyzanowska et al. | 580 | Multiple locations of one country (selection bias) | Not reported | Canada | Descriptive statistics, Poisson model | not reported | I | A |
| Selection bias | ||||||||
| Kumar et al. | 1 | One country (selection bias) | Not reported | India | Natural language processing | High levels of satisfaction | III | B |
| Selection bias | ||||||||
| Lai et al. | 18 | One location (selection bias), majority Caucasian (sample bias) | Not reported | USA | Descriptive statistics, natural language processing | High levels of satisfaction | III | B |
| Selection bias | ||||||||
| Sample bias | ||||||||
| Öztürk et al. | 57 | One location (selection bias) | Not reported | Turkey | Descriptive statistics, Mann–Whitney U, Wilcoxon signed-rank test, Chi-square | High levels of satisfaction | I | A |
| Selection bias | ||||||||
| Reeves et al. | 159 | One location (selection bias) | D = −0.3 (medium) | Australia | Descriptive statistics, multivariable linear mixed models | High levels of satisfaction | I | A |
| Selection bias | ||||||||
| Wagner et al. | 196 | One location (selection bias) | medium effect sizes (ranged from d = −0.55–−0.69) | USA | Descriptive statistics, Chi-square, independent t-test | High levels of satisfaction | I | A |
| Selection bias | ||||||||
| Bandani-Susan et al. | 38 | One location (selection bias) | not reported | Iran | Descriptive statistics, Kolmogorov–Smirnov, Chi-square and Fisher’s exact, independent and paired t-test | not reported | I | A |
| Selection bias | ||||||||
| Fu et al. | 120 | One location (selection bias) | small effect size (ra = 0.05–0.29) | USA | Descriptive statistics, Wilcoxon R, odds ratio | High levels of satisfaction | I | A |
| Selection bias | ||||||||
| Gao et al. | 55 | One location (selection bias) | Not reported | USA | Descriptive statistics, | not reported | I | A |
| Selection bias | ||||||||
| Medina et al. | 189 | One location (selection bias) | Sensitivity 70%, specificity 73% | Spain | Descriptive statistics, multi-level linear models, Chi-square and student’s t-test | High levels of satisfaction | II | A |
| Selection bias | ||||||||
| Oswald et al. | 29 | One location (selection bias) | large group differences (d = 1.25–0.33) | USA | Descriptive statistics, Chi-square test, t-tests | High levels of satisfaction | I | A |
| Selection bias |
Author Contributions
Authors contributed equally to this manuscript. Conceptualization C.S.K.; methodology C.S.K.; formal analysis C.S.K., G.J.P., B.V., S.C., M.G., N.L.; resources C.S.K., G.J.P., B.V., S.C., M.G., N.L.; data curation C.S.K., G.J.P., B.V., S.C., M.G., N.L.; writing C.S.K.; original draft preparation C.S.K.; writing—review and editing C.S.K. and G.J.P.; supervision C.S.K.; project administration C.S.K.; public health aspects G.J.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This review is conducted in accordance with the Kruse Protocol for writing a systematic review. It is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). This review has been registered with PROSPERO: CRD42022347417.
Data Availability Statement
Data from this study can be obtained by asking the lead author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention Breast Cancer. [(accessed on 8 February 2022)]; Available online: https://www.cdc.gov/cancer/breast/basic_info/what-is-breast-cancer.htm.
- 2.World Health Organization Breast Cancer. [(accessed on 8 February 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
- 3.World Health Organization . Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 4.Akingbade O., Nguyen K.T., Chow K.M. Effect of mHealth interventions on psychological issues experienced by women diagnosed with breast cancer receiving chemotherapy: A systematic review and meta-analysis. medRxiv. 2022 doi: 10.1101/2022.02.17.22271140. [DOI] [PubMed] [Google Scholar]
- 5.Scholz S., Teetz L. Smart health via mHealth? Potentials of mobile health apps for improving prevention and adherence of breast cancer patients. Digit. Health. 2022;8:20552076221074127. doi: 10.1177/20552076221074127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe-Galloway S., Ratnapradipa K., Subramanian R., Ramos A., Famojuro O., Schmidt C., Farazi P. Mobile Health (mHealth) Interventions to Increase Cancer Screening Rates in Hispanic/Latinx Populations: A Scoping Review. Health Promot. Pract. 2022:15248399221103851. doi: 10.1177/15248399221103851. [DOI] [PubMed] [Google Scholar]
- 7.Akingbade O., Adediran V., Somoye I.E., Alade A.S., Chow K.M. Perception of mHealth Intervention for Psychoeducational Support Among Nigerian Women Receiving Chemotherapy for Breast Cancer: A Qualitative Study. Res. Sq. :2022. doi: 10.1007/s00520-022-07403-w. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inupakutika D., Rodriguez G., Akopian D., Lama P., Chalela P., Ramirez A.G. On the Performance of Cloud-Based mHealth Applications: A Methodology on Measuring Service Response Time and a Case Study. IEEE Access. 2022;10:53208–53224. doi: 10.1109/ACCESS.2022.3174855. [DOI] [Google Scholar]
- 9.Martin E., Di Meglio A., Charles C., Ferreira A., Gbenou A., Blond M., Fagnou B., Arvis J., Pistilli B., Saghatchian M. Use of mHealth to increase physical activity among breast cancer survivors with fatigue: Qualitative exploration. JMIR Cancer. 2021;7:e23927. doi: 10.2196/23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buneviciene I., Mekary R.A., Smith T.R., Onnela J.-P., Bunevicius A. Can mHealth interventions improve quality of life of cancer patients? A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021;157:103123. doi: 10.1016/j.critrevonc.2020.103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruse C.S. Writing a Systematic Review for Publication in a Health-Related Degree Program. JMIR Res. Protoc. 2019;8:e15490. doi: 10.2196/15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 14.Newhouse R., Dearholt S., Poe S., Pugh L., White K. The Johns Hopkins Nursing Evidence-Based Practice Rating Scale. The Johns Hopkins Hospital; Baltimore, MD, USA: 2005. [Google Scholar]
- 15.Pannucci C.J., Wilkins E.G. Identifying and avoiding bias in research. Plast. Reconstr. Surg. 2010;126:619. doi: 10.1097/PRS.0b013e3181de24bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salgado J.F. Transforming the area under the normal curve (AUC) into Cohen’sd, Pearson’s rpb, odds-ratio, and natural log odds-ratio: Two conversion tables. Eur. J. Psychol. Appl. Leg. Context. 2018;10:35–47. doi: 10.5093/ejpalc2018a5. [DOI] [Google Scholar]
- 17.Light R.J. Measures of response agreement for qualitative data: Some generalizations and alternatives. Psychol. Bull. 1971;76:365. doi: 10.1037/h0031643. [DOI] [Google Scholar]
- 18.McHugh M.L. Interrater reliability: The kappa statistic. Biochem. Med. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Børøsund E., Cvancarova M., Moore S.M., Ekstedt M., Ruland C.M. Comparing effects in regular practice of e-communication and Web-based self-management support among breast cancer patients: Preliminary results from a randomized controlled trial. J. Med. Internet Res. 2014;16:e3348. doi: 10.2196/jmir.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman L.W., White R., Ratcliff C.G., Sutton S., Stewart M., Palmer J.L., Link J., Cohen L. A randomized trial comparing live and telemedicine deliveries of an imagery-based behavioral intervention for breast cancer survivors: Reducing symptoms and barriers to care. Psycho-Oncol. 2015;24:910–918. doi: 10.1002/pon.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheelock A.E., Bock M.A., Martin E.L., Hwang J., Ernest M.L., Rugo H.S., Esserman L.J., Melisko M.E. SIS. NET: A randomized controlled trial evaluating a web-based system for symptom management after treatment of breast cancer. Cancer. 2015;121:893–899. doi: 10.1002/cncr.29088. [DOI] [PubMed] [Google Scholar]
- 22.Galiano-Castillo N., Cantarero-Villanueva I., Fernández-Lao C., Ariza-García A., Díaz-Rodríguez L., Del-Moral-Ávila R., Arroyo-Morales M. Telehealth system: A randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122:3166–3174. doi: 10.1002/cncr.30172. [DOI] [PubMed] [Google Scholar]
- 23.Admiraal J.M., van der Velden A.W., Geerling J.I., Burgerhof J.G., Bouma G., Walenkamp A.M., de Vries E.G., Schröder C.P., Reyners A.K. Web-based tailored psychoeducation for breast cancer patients at the onset of the survivorship phase: A multicenter randomized controlled trial. J. Pain Symptom Manag. 2017;54:466–475. doi: 10.1016/j.jpainsymman.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Fazzino T.L., Fabian C., Befort C.A. Change in Physical Activity during a Weight Management Intervention for Breast Cancer Survivors: Association with Weight Outcomes. Obesity. 2017;25:S109–S115. doi: 10.1002/oby.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han J.Y., Hawkins R., Baker T., Shah D.V., Pingree S., Gustafson D.H. How cancer patients use and benefit from an interactive cancer communication system. J. Health Commun. 2017;22:792–799. doi: 10.1080/10810730.2017.1360413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhm K.E., Yoo J.S., Chung S.H., Lee J.D., Lee I., Kim J.I., Lee S.K., Nam S.J., Park Y.H., Lee J.Y. Effects of exercise intervention in breast cancer patients: Is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res. Treat. 2017;161:443–452. doi: 10.1007/s10549-016-4065-8. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.J., Kim S.M., Shin H., Jang J.-S., Kim Y.I., Han D.H. A mobile game for patients with breast cancer for chemotherapy self-management and quality-of-life improvement: Randomized controlled trial. J. Med. Internet Res. 2018;20:e9559. doi: 10.2196/jmir.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy M.S., Matthews E.E., Battaglia C., Meek P.M. Feasibility of a Telemedicine-Delivered Cognitive Behavioral Therapy for Insomnia in Rural Breast Cancer Survivors. Oncol. Nurs. Forum. 2018;45:607–618. doi: 10.1188/18.ONF.607-618. [DOI] [PubMed] [Google Scholar]
- 29.Visser A., Prins J.B., Jansen L., Radema S.A., Schlooz M.S., van Dalen T., van Laarhoven H.W. Group medical consultations (GMCs) and tablet-based online support group sessions in the follow-up of breast cancer: A multicenter randomized controlled trial. Breast. 2018;40:181–188. doi: 10.1016/j.breast.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Zachariae R., Amidi A., Damholdt M.F., Clausen C.D., Dahlgaard J., Lord H., Thorndike F.P., Ritterband L.M. Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: A randomized controlled trial. J. Natl. Cancer Inst. 2018;110:880–887. doi: 10.1093/jnci/djx293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ariza-Garcia A., Lozano-Lozano M., Galiano-Castillo N., Postigo-Martin P., Arroyo-Morales M., Cantarero-Villanueva I. A web-based exercise system (e-CuidateChemo) to counter the side effects of chemotherapy in patients with breast cancer: Randomized controlled trial. J. Med. Internet Res. 2019;21:e14418. doi: 10.2196/14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crafoord M.-T., Fjell M., Sundberg K., Nilsson M., Langius-Eklöf A. Engagement in an interactive app for symptom self-management during treatment in patients with breast or prostate cancer: Mixed methods study. J. Med. Internet Res. 2020;22:e17058. doi: 10.2196/17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrante J.M., Devine K.A., Bator A., Rodgers A., Ohman-Strickland P.A., Bandera E.V., Hwang K.O. Feasibility and potential efficacy of commercial mHealth/eHealth tools for weight loss in African American breast cancer survivors: Pilot randomized controlled trial. Transl. Behav. Med. 2020;10:938–948. doi: 10.1093/tbm/iby124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fjell M., Langius-Eklöf A., Nilsson M., Wengström Y., Sundberg K. Reduced symptom burden with the support of an interactive app during neoadjuvant chemotherapy for breast cancer–A randomized controlled trial. Breast. 2020;51:85–93. doi: 10.1016/j.breast.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou I.-C., Lin H.-Y., Shen S.-H., Chang K.-J., Tai H.-C., Tsai A.-J., Dykes P.C. Quality of life of women after a first diagnosis of breast cancer using a self-management support mHealth app in Taiwan: Randomized controlled trial. JMIR mHealth uHealth. 2020;8:e17084. doi: 10.2196/17084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lally R.M., Kupzyk K.A., Bellavia G., Hydeman J., Gallo S., Helgeson V.S., Erwin D., Mills A.C., Brown J.K. CaringGuidance™ after breast cancer diagnosis eHealth psychoeducational intervention to reduce early post-diagnosis distress. Support. Care Cancer. 2020;28:2163–2174. doi: 10.1007/s00520-019-05028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lozano-Lozano M., Martín-Martín L., Galiano-Castillo N., Fernández-Lao C., Cantarero-Villanueva I., López-Barajas I.B., Arroyo-Morales M. Mobile health and supervised rehabilitation versus mobile health alone in breast cancer survivors: Randomized controlled trial. Ann. Phys. Rehabil. Med. 2020;63:316–324. doi: 10.1016/j.rehab.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Hout A., Van Uden-Kraan C.F., Holtmaat K., Jansen F., Lissenberg-Witte B.I., Nieuwenhuijzen G.A.P., Hardillo J.A., Baatenburg De Jong R.J., Tiren-Verbeet N.L., Sommeijer D.W., et al. Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: A randomised, controlled trial. Lancet Oncol. 2020;21:80–94. doi: 10.1016/S1470-2045(19)30675-8. [DOI] [PubMed] [Google Scholar]
- 39.Çınar D., Karadakovan A., Erdoğan A.P. Effect of mobile phone app-based training on the quality of life for women with breast cancer. Eur. J. Oncol. Nurs. 2021;52:101960. doi: 10.1016/j.ejon.2021.101960. [DOI] [PubMed] [Google Scholar]
- 40.Fang S.-Y., Lin P.-J., Kuo Y.-L. Long-Term Effectiveness of a Decision Support App (Pink Journey) for Women Considering Breast Reconstruction Surgery: Pilot Randomized Controlled Trial. JMIR mHealth uHealth. 2021;9:e31092. doi: 10.2196/31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krzyzanowska M.K., Julian J.A., Gu C.-S., Powis M., Li Q., Enright K., Howell D., Earle C.C., Gandhi S., Rask S., et al. Remote, proactive, telephone based management of toxicity in outpatients during adjuvant or neoadjuvant chemotherapy for early stage breast cancer: Pragmatic, cluster randomised trial. BMJ. 2021;375:e066588. doi: 10.1136/bmj-2021-066588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar J., Singh T., Singh A. Technology connects patients to tertiary care for non-COVID illnesses in pandemic times: A case study from India. Indian J. Community Health. 2021;33:202–204. doi: 10.47203/IJCH.2021.v33i01.029. [DOI] [Google Scholar]
- 43.Lai L.L., Player H., Hite S., Satyananda V., Stacey J., Sun V., Jones V., Hayter J. Feasibility of remote occupational therapy services via telemedicine in a breast cancer recovery program. Am. J. Occup. Ther. 2021;75:7502205030. doi: 10.5014/ajot.2021.042119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Öztürk E.S., Kutlutürkan S. The Effect of the Mobile Application-Based Symptom Monitoring Process on the Symptom Control and Quality of Life in Breast Cancer Patients. Semin. Oncol. Nurs. 2021;37:151161. doi: 10.1016/j.soncn.2021.151161. [DOI] [PubMed] [Google Scholar]
- 45.Reeves M.M., Terranova C.O., Winkler E.A.H., McCarthy N., Hickman I.J., Ware R.S., Lawler S.P., Eakin E.G., Demark-Wahnefried W. Effect of a Remotely Delivered Weight Loss Intervention in Early-Stage Breast Cancer: Randomized Controlled Trial. Nutrients. 2021;13:4091. doi: 10.3390/nu13114091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner L.I., Tooze J.A., Hall D.L., Levine B.J., Beaumont J., Duffecy J., Victorson D., Gradishar W., Leach J., Saphner T., et al. Targeted eHealth Intervention to Reduce Breast Cancer Survivors’ Fear of Recurrence: Results From the FoRtitude Randomized Trial. J. Natl. Cancer Inst. 2021;113:1495–1505. doi: 10.1093/jnci/djab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bandani-Susan B., Montazeri A., Haghighizadeh M.H., Araban M. The effect of mobile health educational intervention on body image and fatigue in breast cancer survivors: A randomized controlled trial. Ir. J. Med. Sci. 2022;191:1599–1605. doi: 10.1007/s11845-021-02738-5. [DOI] [PubMed] [Google Scholar]
- 48.Fu M.R., Axelrod D., Guth A.A., Scagliola J., Rampertaap K., El-Shammaa N., Qiu J.M., McTernan M.L., Frye L., Park C.S. A web-and mobile-based intervention for women treated for breast cancer to manage chronic pain and symptoms related to lymphedema: Results of a randomized clinical trial. JMIR Cancer. 2022;8:e29485. doi: 10.2196/29485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Z., Ryu S., Chen Y. Effects of Tai Chi App and Facebook health education programs on breast cancer survivors’ stress and quality of life in the Era of pandemic. Complement. Ther. Clin. Pract. 2022;48:101621. doi: 10.1016/j.ctcp.2022.101621. [DOI] [PubMed] [Google Scholar]
- 50.Medina J.C., Flix-Valle A., Rodríguez-Ortega A., Hernández-Ribas R., Lleras de Frutos M., Ochoa-Arnedo C. ICOnnecta’t: Development and Initial Results of a Stepped Psychosocial eHealth Ecosystem to Facilitate Risk Assessment and Prevention of Early Emotional Distress in Breast Cancer Survivors’ Journey. Cancers. 2022;14:974. doi: 10.3390/cancers14040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oswald L.B., Morales-Cruz J., Eisel S.L., Del Rio J., Hoogland A.I., Ortiz-Rosado V., Soto-Lopez G., Rodriguez-Rivera E., Savard J., Castro E. Pilot randomized controlled trial of eHealth cognitive-behavioral therapy for insomnia among Spanish-speaking breast cancer survivors. J. Behav. Med. 2022;45:503–508. doi: 10.1007/s10865-022-00313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study can be obtained by asking the lead author.