Abstract
A novel blocking enzyme-linked immunosorbent assay (BL-ELISA) was developed for detection of antibodies to human group C rotavirus (CHRV). The specificity of the BL-ELISA was confirmed by using animal sera hyperimmunized to group A and group C rotaviruses and paired sera from five patients with acute CHRV gastroenteritis. Furthermore, there was concordance between the BL-ELISA and a neutralization assay for CHRV in 226 (95%) of 238 samples. By using the BL-ELISA, we determined the seroprevalence of CHRV in 704 serum samples obtained from nine different age groups of inhabitants of Okayama Prefecture, Japan, in 1992, 1994, and 1996. As a result, 211 sera (30%) were found to be positive for CHRV antibodies. The seroprevalence gradually increased with age and reached 52.7% in the oldest individuals. A further analysis of the youngest age group suggested that CHRVs predominantly prevail in persons older than 3 years of age in Japan. When comparing the three sampling years, a larger percentage of antibody-positive sera was detected in 1994 than in either 1992 or 1996 in individuals between 6 and 15 years of age, reflecting the occurrence of a CHRV outbreak among children during the winter of 1992 to 1993 that was previously documented. These results indicate that CHRV infections may occur more frequently in spite of the relatively low detection rate of the virus.
Rotaviruses are members of the Reoviridae family and are recognized as important causative agents of acute infantile gastroenteritis. The genomes of rotaviruses consist of 11 segments of double-stranded RNA enclosed in a double-shelled particle. Rotaviruses are classified into at least seven groups (groups A, B, C, D, E, F, and G) on the basis of their double-stranded RNA electropherotypes and a common group antigen on the inner-capsid protein (protein VP6) 1, 25. Three of these groups (A, B, and C) are known to infect humans 1, 25. Human group A rotaviruses (AHRVs) are the major agents of severe diarrheal diseases in infants and young children. Epidemics caused by group B rotaviruses have occurred mainly among both adults and children in China 30. Human group C rotaviruses (CHRVs) have been detected in patients with either sporadic cases of diarrhea 12, 21, 23 or outbreaks of gastroenteritis 5, 9, 17.
Group C rotaviruses were first recognized as a causative agent of gastroenteritis in animals 2, 26. Thereafter, rotaviruses with electropherotypes and antigenic properties similar to the porcine virus were identified in humans 4. Since then, it has been demonstrated that CHRVs were associated with several outbreaks of acute gastroenteritis in Asia 9, 17, 19, Europe 3, 5, and South America 8. CHRVs have recently been detected in sporadic cases of diarrhea in the United States 12 and South Africa 27. Thus, CHRVs are globally distributed and may be an emerging pathogen.
The seroprevalence of group C rotaviruses in human sera was first determined by an immunofluorescence test with a porcine virus (strain Cowden) as antigen in 1986 4. In 1990, Oseto conducted the first serological survey of CHRV using an immune adherence hemagglutination assay with a human virus as antigen 21. Thereafter, seroepidemiological studies of group C rotaviruses have been undertaken by using reagents derived from porcine viruses 18, 24, 29 and human viruses 11, 28. However, serological information about CHRV is still limited because of the lack of a constant supply of the virus antigen. Efficient growth of CHRV in vitro had been very difficult to achieve until Oseto et al. 20 demonstrated the growth of CHRV in CaCo-2 cells.
We used this CHRV culture system to establish a neutralizing assay for CHRV by using a reverse passive hemagglutination test for endpoint determination 6. This neutralizing assay is very specific and useful for precise analysis of the serological status. However, the assay is not suitable for a large-scale seroepidemiological study because it is complicated and requires many steps. For simple detection of antibody to CHRV, we developed a blocking enzyme-linked immunosorbent assay (BL-ELISA) as part of the present study. The methodology was based on an antigen-detection ELISA using monoclonal antibodies previously developed by our group 7. In the BL-ELISA, diluted sera were first mixed with purified virion antigen and then the mixture was subjected to the antigen-detection ELISA. The presence of antibodies was demonstrated by blockage of antigen binding. The specificity of the method was evaluated with hyperimmunized animal sera and paired sera obtained from patients with CHRV gastroenteritis (i.e., sera obtained from the same patients during the acute and convalescent phases). Furthermore, we used the BL-ELISA to conduct a seroepidemiological survey of CHRV in Okayama Prefecture, Japan, in 1992, 1994, and 1996.
MATERIALS AND METHODS
Serum specimens.
A total of 704 serum samples were obtained from nine different age groups of inhabitants of Okayama Prefecture, Japan, in 1992 (n = 201), 1994 (n = 261), and 1996 (n = 242). These sera were collected in outpatient sections of general hospitals in Okayama Prefecture. Five paired sera collected from patients with acute gastroenteritis caused by CHRV were kindly provided by S. Hasegawa, Toyama Institute of Health, Toyama, Japan.
Antisera.
Hyperimmune goat antiserum to a bovine group A rotavirus strain (NCDV) was purchased from Biogenesis, Ltd. (Poole, United Kingdom) and Chemicon International Inc. (Temecula, Calif.). Hyperimmune rabbit antiserum to the Wa strain of AHRV was obtained from DAKO Japan Ltd., Kyoto, Japan, and Denka Seiken Co. Ltd., Tokyo, Japan. Guinea pig and mouse hyperimmune antisera to three CHRV strains (OK118, OK450, and K9304) which were isolated in Okayama Prefecture from 1988 to 1993 13, 14 were prepared by inoculating animals with each purified virus as previously described 6. Monoclonal antibody (MAb) 13A3, which recognizes the inner capsid of CHRV 15, was used as a detector antibody for the BL-ELISA. The MAb was purified from ascites fluid with an Econo-Pac protein A column (Bio-Rad Laboratories, Richmond, Calif.) and conjugated with N-biotinyl-ω-aminocaproic acid-N-hydroxysuccinimide ester (Enzotin; Enzo Biochemicals Inc., New York, N.Y.).
BL-ELISA.
The BL-ELISA to detect antibody against CHRV was developed by modifying our ELISA method 7. The virus antigen was prepared from a single CHRV strain (K9304) isolated in Okayama Prefecture 13. Briefly, the K9304 strain was propagated in CaCo-2 cells and the culture supernatant was overlaid on 30% (wt/vol) sucrose solution. After centrifugation at 150,000 × g for 1 h at 4°C, the precipitate was resuspended in phosphate-buffered saline (PBS; pH 7.2) and the concentration of the virus antigen was subsequently determined by using our ELISA 7. The virus antigen was diluted with PBS until the absorbance of the ELISA reached between 1.0 and 1.5, and then it was stored at −80°C.
For the BL-ELISA, the wells of 96-well flat-bottom microplates (MaxiSorp; Nunc, Inc., Roskilde, Denmark) were coated with 100 μl of the immunoglobulin fraction of the guinea pig hyperimmune antiserum to the K9304 strain (10 μg/ml in PBS). The plates were incubated at 4°C overnight, washed three times with PBS containing 0.05% Tween 20 (PBS-T), and blocked with borate-buffered saline (pH 8.0) containing 0.1% nonfat dry milk (BBS-M) for 1 h at 37°C. Serum samples were diluted with BBS-M (initial dilution 1:50). Fifty microliters of the diluted serum was mixed with an equal volume of the virus antigen and then incubated at 37°C for 1 h. After washing the microplates with PBS-T, the antigen-serum mixtures were transferred to the wells and incubated for 1 h at 37°C. After three washes, 100 μl of the biotin-conjugated MAb 13A3 (0.2 μg/ml in BBS-M) was added to each well, and the plates were kept for 1 h at 37°C. The plates were washed four times with PBS-T, and then 100 μl of horseradish peroxidase-conjugated streptavidin (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) diluted 1:1,000 in BBS-M was added to the wells and incubated for 30 min at room temperature. After a final five washes with PBS-T, 100 μl of 0.04% 3,3′, 5,5′-tetramethylbenzidine in phosphate-citrate buffer (pH 5.0) containing 0.02% H2O2 was added, and the plates were allowed to stand for 10 min at room temperature. The reaction was stopped with 100 μl of 1 M phosphoric acid per well, and the absorbance at 450 nm was read with an Immunoreader NJ-2000 (Nunc, Inc.).
A BL-ELISA for detection of antibody to AHRV was performed by the same procedure as the BL-ELISA for CHRV, with reagents to AHRV. In the BL-ELISA for AHRV, the coating antibody was a 10-μg/ml concentration of hyperimmune rabbit antiserum to the Wa strain, the antigen was partially purified Wa strain propagated in MA-104 cells, and the detector antibody was a 1:50 dilution of horseradish peroxidase-conjugated hyperimmune rabbit antiserum to AHRV (DAKO Japan Ltd.).
A ≥50% reduction of absorbance produced by the serum samples compared with that of the buffer control was considered positive for the antibody to group C or group A rotaviruses. Antibody titers to group C and group A rotaviruses were expressed as the reciprocal of the highest serum dilution that was positive in the respective BL-ELISAs.
Neutralization assay for CHRV.
A neutralization reverse passive hemagglutination test (N-RPHA test) for CHRV was carried out as described elsewhere 6. Briefly, heat-inactivated (56°C, 30 min) serum samples were diluted in twofold steps with Eagle's minimum essential medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). The diluted serum (25 μl) was mixed with an equal volume of a virus antigen (strain K9304) that would yield an RPHA test titer of 8 at 3 days after infection. The mixtures were incubated for 1 h at 37°C and then were inoculated onto CaCo-2 cells prepared in 96-well microplates. The RPHA titer of each well was determined at 3 days after inoculation. The reciprocal of the maximum dilution of the serum that exhibited a fourfold (75%) or greater reduction of the RPHA titer was defined as the neutralization titer.
RESULTS
Development of the BL-ELISA to detect antibodies against CHRV.
The specificity of the BL-ELISA for CHRV was first evaluated with seven hyperimmune sera prepared against group A and group C rotavirus strains (Table 1). All hyperimmune sera against group C rotaviruses tested positive in the BL-ELISA for CHRV (each BL-ELISA titer was 1,280), whereas hyperimmune sera against group A rotaviruses were all negative (BL-ELISA titers were <50). Inverse results were obtained in the BL-ELISA for AHRV. The specificity of the BL-ELISA for CHRV was also examined with five paired sera collected from patients with acute gastroenteritis caused by CHRV. These sera were also tested by the BL-ELISA for AHRV. As shown in Table 2, seroconversion for CHRV was clearly demonstrated in all sets of the paired sera. In contrast, none of the sera revealed a fourfold or greater rise in titer for antibody to AHRV. Thus, the BL-ELISA for CHRV successfully detected antibodies to CHRV and no cross-reactivity between group A and group C rotaviruses was detected in our BL-ELISA system.
TABLE 1.
Antibody titers in animals hyperimmunized with group A or group C rotavirus strains
| Strain used for immunizationa | Immunized animal | BL-ELISA titer of antibody to
|
|
|---|---|---|---|
| AHRV | CHRV | ||
| Wa (human, A) | Rabbit | 1,280 | <50 |
| Wa (human, A) | Rabbit | 320 | <50 |
| NCDV (bovine, A) | Goat | 1,280 | <50 |
| NCDV (bovine, A) | Goat | 2,560 | <50 |
| OK118 (human, C) | Mouse | <50 | 1,280 |
| OK450 (human, C) | Mouse | <50 | 1,280 |
| K9304 (human, C) | Guinea pig | <50 | 1,280 |
Host species and group specificity are indicated in parentheses.
TABLE 2.
Antibody titers to group A or group C rotaviruses in paired (acute- and convalescent-phase) sera from patients with CHRV gastroenteritis
| Patient no. | Sex | Age (yr) | Sampling time (no. of days after onset) | BL-ELISA titers of antibody to
|
|
|---|---|---|---|---|---|
| AHRV | CHRV | ||||
| 1 | Female | 11 | 3 | <50 | 80 |
| 22 | <50 | 2,560 | |||
| 2 | Male | 33 | 3 | 80 | <50 |
| 21 | 160 | 640 | |||
| 3 | Female | 26 | 4 | 160 | <50 |
| 22 | 320 | 640 | |||
| 4 | Female | 9 | 4 | 80 | <50 |
| 22 | 160 | 640 | |||
| 5 | Male | 6 | 3 | 80 | <50 |
| 21 | 160 | 640 | |||
Comparison of seroprevalence of CHRV determined by BL-ELISA and N-RPHA tests.
To examine the usefulness of the BL-ELISA for CHRV in a seroepidemiological study, a total of 238 selected human sera were tested for CHRV antibodies by the BL-ELISA and for neutralization antibodies by the N-RPHA test. The serum samples were screened at a dilution of 1:50 in the BL-ELISA and at a dilution of 1:64 in the N-RPHA test. The overall seroprevalence of CHRV was 23.9% by the BL-ELISA and 27.3% by the N-RPHA test (Table 3). There was concordance between the two assays in 226 (95%) of 238 samples.
TABLE 3.
Comparison of seroprevalence of CHRV determined by BL-ELISA and N-RPHA
| N-RPHA result | No. of samples with BL-ELISA result
|
||
|---|---|---|---|
| Positivea | Negative | Total | |
| Positiveb | 54 | 9 | 63 |
| Negative | 3 | 172 | 175 |
| Total | 57 | 181 | 238 |
Samples that showed positive reactions at a dilution of 1:50.
Samples that showed neutralization activity at a dilution of 1:64.
Seroprevalence of CHRV in Japan.
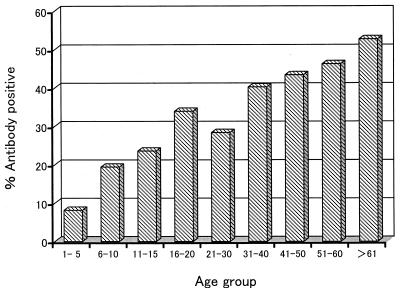
The prevalence of CHRV antibodies in a total of 704 human sera collected in Okayama Prefecture, Japan, was determined by the BL-ELISA for CHRV at a serum dilution of 1:50 (Table 4). The seroprevalence data are also displayed graphically in Fig. 1. In total, 211 sera (30%) were found to be positive for CHRV antibodies. The lowest seroprevalence (8.2%) was noted in the 1- to 5-year-old group, and then the seroprevalence gradually increased with age and reached 52.7% in the oldest individuals (more than 60 years of age). A further analysis of the youngest age group (Table 4) revealed that the seroprevalence in individuals aged 4 and 5 years (13% [7/54]) was apparently higher than that in individuals between 1 and 3 years of age (4.4% [3/68]), suggesting that CHRVs predominantly prevail in persons older than 3 years of age in Japan. Comparing the three sampling years, it should be noted that about two times larger percentages of antibody-positive sera were detected in 1994 than in either 1992 or 1996 in the 6- to 10- and 11- to 15-year-old groups (Table 4). Little difference in the seroprevalence was found between males (27.1%) and females (32.8%). The antibody-positive individuals were not localized in a certain geographical area (data not shown).
TABLE 4.
Prevalence of antibodies to CHRV in sera from inhabitants of Okayama Prefecture in 1992, 1994, and 1996a
| Age group (yrs) | 1992
|
1994
|
1996
|
Total
|
||||
|---|---|---|---|---|---|---|---|---|
| No. of samples | No. positive (%) | No. of samples | No. positive (%) | No. of samples | No. positive (%) | No. of samples | No. positive (%) | |
| 1–5 | 14 | 0 (0) | 60 | 6 (10) | 48 | 4 (8.3) | 122 | 10 (8.2) |
| 6–10 | 16 | 2 (12.5) | 45 | 13 (28.9) | 42 | 5 (11.9) | 103 | 20 (19.4) |
| 11–15 | 21 | 4 (19) | 30 | 10 (33.3) | 21 | 3 (14.3) | 72 | 17 (23.6) |
| 16–20 | 12 | 4 (33.3) | 30 | 8 (26.7) | 14 | 7 (50) | 56 | 19 (33.9) |
| 21–30 | 25 | 7 (28) | 29 | 7 (24.1) | 27 | 9 (33.3) | 81 | 23 (28.4) |
| 31–40 | 25 | 10 (40) | 12 | 6 (50) | 35 | 13 (37.1) | 72 | 29 (40.3) |
| 41–50 | 30 | 16 (53.3) | 21 | 7 (33.3) | 25 | 10 (40) | 76 | 33 (43.4) |
| 51–60 | 29 | 17 (58.6) | 20 | 6 (30) | 18 | 8 (44.4) | 67 | 31 (46.3) |
| >60 | 29 | 18 (62.1) | 14 | 8 (57.1) | 12 | 3 (25) | 55 | 29 (52.7) |
| Total | 201 | 78 (38.8) | 261 | 71 (27.2) | 242 | 62 (25.6) | 704 | 211 (30) |
Prevalence of antibodies to CHRV was determined by the BL-ELISA for CHRV at a serum dilution of 1:50.
FIG. 1.
Seroprevalence of CHRV in 704 serum samples obtained from inhabitants of Okayama Prefecture, Japan, in 1992, 1994, and 1996.
DISCUSSION
Seroepidemiological studies of group C rotaviruses have previously been carried out by indirect immunofluorescence tests 4, 18, 24 or ELISA-based tests 11, 28, 29. The ELISA-based tests were more appropriate for testing many serum samples. Tsunemitsu et al. 29 first reported a blocking ELISA for the detection of antibodies to group C rotavirus. However, their method would not be suitable for the detection of CHRV antibodies in human sera because the reagents of that ELISA originate from a porcine virus that is genetically distinct from human viruses 16. Furthermore, we were unable to achieve the same sensitivity using their protocol, in which the viral antigens, serum samples, and detecting antibodies were sequentially added. To overcome these drawbacks, we developed a new BL-ELISA system with reagents derived from CHRV, and we also adopted an alternative protocol, in which viral antigens were premixed with serum samples and then the mixtures were added to the wells precoated with the capture antibody. James et al. 11 described an antibody-detecting ELISA that used the recombinant VP6 of CHRV as antigen. However, their ELISA cannot detect antibodies that recognize the outer-capsid proteins (VP4 and VP7), in which major neutralization epitopes of CHRV should exist. The present BL-ELISA should detect all the antibodies that are thought to recognize the neutralizing epitopes. In fact, a good correlation was found between the BL-ELISA and the N-RPHA test. Our BL-ELISA is therefore useful for a screening test prior to the neutralization test.
To date, there have been only two other reports on the seroprevalence of group C rotaviruses in Japan. Oseto 21 reported seroprevalence rates of 13.5 to 29.2% in residents of Ehime Prefecture, and Tsunemitsu et al. 29 reported rates of 2.6% in children (less than 16 years of age) and 13.1% in adults (20 to 84 years of age) in Hokkaido Prefecture. The seroprevalence rates of CHRV determined in this study (25.6 to 38.8%) were similar to those in Ehime Prefecture and were higher than those in Hokkaido Prefecture. These differences in seroprevalence may be due to differences in the past occurrence of CHRV epidemics: CHRVs have been detected in Okayama and Ehime Prefectures 13, 21, 22 but not in Hokkaido Prefecture. To confirm the relationship between seroprevalence and antigen detection, we are now planning to determine the seroprevalence of CHRV in several other prefectures.
Both seroepidemiological data and detection profiles from previous studies suggest that the target age groups of CHRV are distinct from those of AHRV. AHRVs typically infect children younger than 3 years of age, whereas CHRVs have been detected mainly in older children and adults 1, 13, 16, 22. Serological data in this study are consistent with this finding. The lowest seroprevalence was noted in the youngest age group, and then the seroprevalence gradually increased with age. A further analysis of the youngest age group suggested that CHRVs predominantly prevail in persons older than 3 years of age. Nilsson et al. 18 have also reported that CHRV infections are relatively common in older Swedish children and adults but appear to be less common in children younger than 5 years of age. Although similar seroprevalence patterns were reported in both the United Kingdom 11 and South Africa 28, a detailed analysis of the youngest age group (between 1 and 5 years of age) was not performed in these reports. In any case, additional seroepidemiological studies that cover all age groups are required to more precisely define the major target age groups of CHRV.
Our previous epidemiological survey of CHRV by using an antigen-detecting RPHA 13 demonstrated that a large-scale outbreak of CHRV occurred during the winter of 1992 to 1993 in Japan and that the viruses were predominantly detected in children between 3 and 8 years of age. In this study, larger percentages of antibody-positive sera were detected in 1994 than in either 1992 or 1996 in sera from individuals between 6 and 15 years of age. Taken together, these results suggest that the higher seroprevalence among the children in 1994 is a reflection of the occurrence of the CHRV outbreak during the winter of 1992 to 1993.
The seroprevalence of CHRV in Japan presented here (30%) is similar to the seroprevalences reported in Sweden (38%) 18, western New York State (30%) 24, and South Africa (34.4%) 28. However, it is slightly lower than the level found in Southampton, United Kingdom (43.4%) 11. These seroepidemiological data suggest that CHRV infections occur more frequently in spite of the relatively low detection rates (less than 7%) reported in antigen detection studies 3, 10, 12, 13. Further epidemiological studies, including those in other countries, are needed to define the distribution of CHRV in the world and to establish its importance in human diarrheal diseases. The present BL-ELISA and our RPHA 15 or ELISA 7 should be useful for this purpose.
ACKNOWLEDGMENT
We are grateful to S. Hasegawa, Toyama Institute of Health, for providing the paired serum samples collected from patients with acute gastroenteritis caused by CHRV.
REFERENCES
- 1.Bass D M, Greenberg H B. Group A rotaviruses. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 967–982. [Google Scholar]
- 2.Bohl E H, Saif L J, Theil K W, Agnes A G, Cross R F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982;15:312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonsdorf C H V, Svensson L. Human serogroup C rotavirus in Finland. Scand J Infect Dis. 1988;20:475–478. doi: 10.3109/00365548809032493. [DOI] [PubMed] [Google Scholar]
- 4.Bridger J C, Pedley S, McCrae M A. Group C rotaviruses in humans. J Clin Microbiol. 1986;23:760–763. doi: 10.1128/jcm.23.4.760-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caul E O, Ashley C R, Darville J M, Bridger J C. Group C rotavirus associated with fatal enteritis in a family outbreak. J Med Virol. 1990;30:201–205. doi: 10.1002/jmv.1890300311. [DOI] [PubMed] [Google Scholar]
- 6.Fujii R, Kuzuya M, Hamano M, Ogura H, Yamada M, Mori T. Neutralization assay for human group C rotaviruses using a reverse passive hemagglutination test for endpoint determination. J Clin Microbiol. 2000;38:50–54. doi: 10.1128/jcm.38.1.50-54.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii R, Kuzuya M, Hamano M, Yamada M, Yamazaki S. Detection of human group C rotaviruses by an enzyme-linked immunosorbent assay using monoclonal antibodies. J Clin Microbiol. 1992;30:1307–1311. doi: 10.1128/jcm.30.5.1307-1311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabbay Y B, D'Arc J, Mascarenas P, Linhares A C, Freitas R B. Atypical rotavirus among diarrhoeic children living in Belem, Brazil. Mem Inst Oswaldo Cruz. 1989;84:5–7. doi: 10.1590/s0074-02761989000100002. [DOI] [PubMed] [Google Scholar]
- 9.Hamano M, Kuzuya M, Fujii R, Ogura H, Mori T, Nakayama T, Yuen E, Katayama K, Mitsunobu Y, Inoue K. Outbreak of acute gastroenteritis caused by human group C rotavirus in a primary school. Jpn J Infect Dis. 1999;52:170–171. [PubMed] [Google Scholar]
- 10.James V L A, Lambden P R, Caul E O, Clarke I N. Enzyme-linked immunosorbent assay based on recombinant human group C rotavirus inner capsid protein (VP6) to detect human group C rotaviruses in fecal samples. J Clin Microbiol. 1998;36:3178–3181. doi: 10.1128/jcm.36.11.3178-3181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James V L A, Lambden P R, Caul E O, Cooke S J, Clarke I N. Seroepidemiology of human group C rotavirus in the UK. J Med Virol. 1997;52:86–91. doi: 10.1002/(sici)1096-9071(199705)52:1<86::aid-jmv14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Jiang B M, Dennehy P H, Spangenberger S, Gentsch J R, Glass R I. First detection of group C rotavirus in fecal specimens of children with diarrhea in the United States. J Infect Dis. 1995;172:45–50. doi: 10.1093/infdis/172.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Kuzuya M, Fujii R, Hamano M, Yamada M, Shinozaki K, Sasagawa A, Hasegawa S, Kawamoto H, Matsumoto K, Kawamoto A, Itagaki A, Funatsumaru S, Urasawa S. Survey of human group C rotaviruses in Japan during the winter of 1992 to 1993. J Clin Microbiol. 1998;36:6–10. doi: 10.1128/jcm.36.1.6-10.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuzuya M, Fujii R, Hamano M, Nakamura J, Yamada M, Nii S, Mori T. Molecular analysis of outer capsid glycoprotein (VP7) genes from two isolates of human group C rotavirus with different genome electropherotypes. J Clin Microbiol. 1996;34:3185–3189. doi: 10.1128/jcm.34.12.3185-3189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzuya M, Fujii R, Hamano M, Nagabayashi T, Tsunemitsu H, Yamada M, Nii S, Mori T. Rapid detection of human group C rotaviruses by reverse passive hemagglutination and latex agglutination tests using monoclonal antibodies. J Clin Microbiol. 1993;31:1308–1311. doi: 10.1128/jcm.31.5.1308-1311.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackow E R. Group B and C rotaviruses. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 983–1008. [Google Scholar]
- 17.Matsumoto K, Hatano M, Kobayashi K, Hasegawa A, Yamazaki S, Nakata S, Chiba S, Kimura Y. An outbreak of gastroenteritis associated with acute rotaviral infection in schoolchildren. J Infect Dis. 1989;160:611–615. doi: 10.1093/infdis/160.4.611. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson M, Sigstam G, Svensson L. Antibody prevalence and specificity to group C rotavirus in Swedish sera. J Med Virol. 2000;60:210–215. doi: 10.1002/(sici)1096-9071(200002)60:2<210::aid-jmv17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Oishi I, Yamazaki K, Minekawa Y. An occurrence of diarrheal cases associated with group C rotavirus in adults. Microbiol Immunol. 1993;37:505–509. doi: 10.1111/j.1348-0421.1993.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 20.Oseto M, Yamashita Y, Hattori M, Mori M, Inoue H, Ishimaru Y, Matsuno S. Serial propagation of human group C rotavirus in a continuous cell line (CaCo-2) J Clin Exp Med. 1994;168:177–178. . (In Japanese.) [Google Scholar]
- 21.Oseto M. Epidemiological study of group C rotavirus. J Jpn Assoc Infect Dis. 1990;64:1264–1273. doi: 10.11150/kansenshogakuzasshi1970.64.1264. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 22.Oseto M, Yamashita Y, Okuyama M, Kuwabara H, Inoue H. Detection of atypical rotaviruses by polyacrylamide gel electrophoresis. J Clin Exp Med. 1986;136:223–224. . (In Japanese.) [Google Scholar]
- 23.Penaranda M E, Cubitt W D, Sinarachatanant P, Taylor D N, Likanonsakul S, Saif L, Glass R I. Group C rotavirus infection in patients with diarrhea in Thailand, Nepal, and England. J Infect Dis. 1989;160:392–397. doi: 10.1093/infdis/160.3.392. [DOI] [PubMed] [Google Scholar]
- 24.Riepenhoff-Talty M, Morse K, Wang C H, Shapiro C, Roberts J, Welter M, Allen M, Evans M J, Flanagan T D. Epidemiology of group C rotavirus infection in western New York women of childbearing age. J Clin Microbiol. 1997;35:486–488. doi: 10.1128/jcm.35.2.486-488.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saif L J. Nongroup A rotaviruses. In: Saif L J, Theil K W, editors. Viral diarrhea of man and animals. Boca Raton, Fla: CRC Press; 1990. pp. 73–95. [Google Scholar]
- 26.Saif L J, Bohl E H, Theil K W, Cross R F, House J A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol. 1980;12:105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebata T, Steele A D. Human group C rotavirus identified in South Africa. S Afr Med J. 1999;89:1073–1074. [PubMed] [Google Scholar]
- 28.Steele A D, James V L A. Seroepidemiology of human group C rotavirus in South Africa. J Clin Microbiol. 1999;37:4142–4144. doi: 10.1128/jcm.37.12.4142-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsunemitsu H, Jiang B, Saif L J. Detection of group C rotavirus antigens and antibodies in animals and humans by enzyme-linked immunosorbent assays. J Clin Microbiol. 1992;30:2129–2134. doi: 10.1128/jcm.30.8.2129-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Cai R, Chen J, Li R, Jiang R. Etiologic studies of the 1983 and 1984 outbreaks of epidemic diarrhea in Guangxi. Intervirology. 1985;24:140–146. doi: 10.1159/000149633. [DOI] [PubMed] [Google Scholar]