ABSTRACT
Microbiological surveillance of airway secretions is central to clinical care in cystic fibrosis (CF). However, the efficacy of microbiological culture, the diagnostic gold standard for pathogen detection, has been increasingly questioned. Here we compared culture with targeted quantitative PCR (QPCR) for longitudinal detection of 2 key pathogens, Pseudomonas aeruginosa and Staphylococcus aureus. Prospectively collected respiratory samples taken from 20 pediatric and 20 adult CF patients over a period of 3-years were analyzed. Patients were eligible if considered free of chronic Pseudomonas infection within 12-months prior to start of study. QPCR revealed high levels of infection with both pathogens not apparent from culture alone. Pseudomonas and Staphylococcus were detected by culture on at least one sampling occasion in 12 and 29 of the patients, respectively. Conversely, both pathogens were detected in all 40 patients by QPCR. Classification of infection status also significantly altered in both pediatric and adult patients, where the number of patients deemed chronically infected with Pseudomonas and Staphylococcus increased from 1 to 28 and 9 to 34, respectively. Overall, Pseudomonas and Staphylococcus infection status classification changed respectively for 36 and 27 of all patients. In no cases did molecular identification lead to a patient being in a less clinically serious infection category. Pathogen detection and infection status classification significantly increased when assessed by QPCR in comparison to culture. This could have implications for clinical care of CF patients, including accuracy of infection diagnosis, relevant and timely antibiotic selection, antimicrobial resistance development, establishment of chronic infection, and cross-infection control.
IMPORTANCE Chronic lung infection is the leading cause of morbidity and early mortality for people with cystic fibrosis (pwCF). Microbiological surveillance to detect lung pathogens is recommended as best practise in CF patient care. Here we studied pathogen detection in 40 pwCF over several years. We found that microbiological culture, the diagnostic gold standard, was significantly disparate to targeted culture-independent approaches for detection and determination of chronic infection status of two important pathogens in CF. Pathogen detection was significantly lower by culture and consequently infection status was also misclassified in most cases. In particular, the extent of chronic infection by both P. aeruginosa and S. aureus not realized with culture was striking. Our findings have implications for the development of infection and clinical care of pwCF. Future longitudinal studies with greater patient numbers will be needed to establish the full extent of the clinical implications indicated from this study.
KEYWORDS: lung infection, infection status, chronic infection, QPCR, Pseudomonas aeruginosa, Staphylococcus aureus
INTRODUCTION
Cystic fibrosis (CF) is characterized by chronic airway infection and consequent inflammation (1, 2). This starts early in life, most commonly with Staphylococcus aureus and Haemophilus influenzae infection. As time progresses, CF microbiology evolves and becomes increasingly dominated by a small number of Gram-negative pathogens rarely encountered in immunocompetent hosts. Chief among these is Pseudomonas aeruginosa, which increases in prevalence in adolescence and is cultured from sputum in over half of all adult CF patients (3, 4). Other important pathogens isolated in CF include Achromobacter spp., Stenotrophomonas maltophilia, and members of the Burkholderia cepacia complex.
Clinical diagnosis of microbial infection relies on microbiological culture as the gold standard for pathogen detection (5). Regular microbiological surveillance throughout the life of a CF patient is considered best practice (6). This is used to guide targeted treatment, provide an indication of the effectiveness of treatment against pre-existing infection, and enable discovery of recently acquired infections to allow timely eradication (6, 7). Following diagnosis of acute pulmonary exacerbation, further regular surveillance for the duration of treatment is recommended to direct antibiotic therapy (6). Furthermore, CF patients are typically categorized by infection type and attend segregated outpatient clinics to prevent cross-infection between patient cohorts colonized with, for example, P. aeruginosa and B. cepacia complex members (6, 8).
Over the last decade, culture-independent molecular microbiology techniques have become increasingly investigated. These methods are more sensitive than culture and are able to identify infections which are harder to detect using traditional microbiological culture alone (9). As such, molecular-based approaches like quantitative PCR (QPCR) have been increasingly proposed as alternatives to culture (7, 10). QPCR is well suited to this application, offering targeted organism identification and quantification with inherent high-target specificity and sensitivity, and can be performed on small amounts of material (as found in cough swabs) (7). Despite many cross-sectional studies reporting on improved sensitivity using QPCR in CF, little progress has been made in establishing such molecular techniques in clinical care, and there are few longitudinal studies which have explicitly followed individual patients over time. Furthermore, none of those studies considered the impact that more sensitive detection methods would have on infection status or clinical outcomes (see Supplementary Materials and Table S1).
In this study, respiratory samples from pediatric and adult CF patients were prospectively collected over a period of up to 3 years. These were all patients believed to be free of chronic Pseudomonas infection by culture prior to start of study. The aim of the study was to longitudinally compare the diagnostic gold standard of microbiological culture with targeted QPCR for detection of two prominent pathogens of concern, P. aeruginosa and S. aureus; both of which are deemed to be readily detected by culture (5). We also assessed the impact of the resulting disparity in detection methods on clinical classification and infection status (e.g., chronic or intermittent infection). Potential clinical implications for CF and for pathogen detection and surveillance more broadly are considered and discussed.
RESULTS
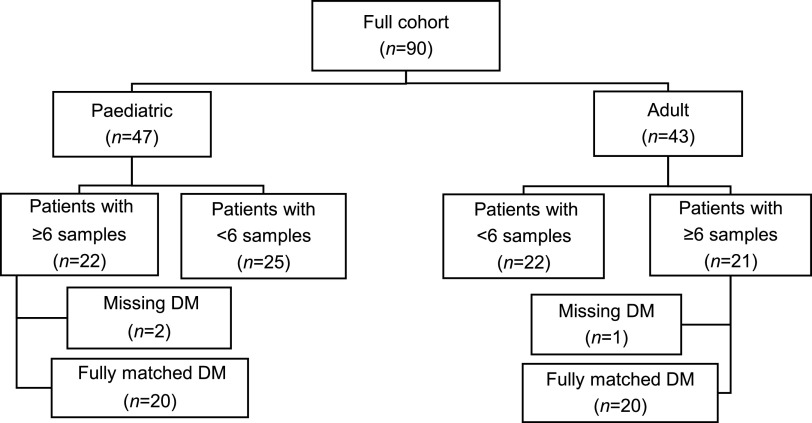
Ninety pediatric and adult patients were recruited for the study. However, subsequent longitudinal analysis was restricted to patients who contributed ≥6 samples with contemporaneous diagnostic microbiology data (Fig. 1). The 20 pediatric and 20 adult patients that met those criteria contributed 327 respiratory samples over the 3-year study period, with a mean ± standard deviation (SD) of 8.2 (± 2.8) samples per patient (minimum 6 samples, maximum 20) (Table S2). Overall, 233 (71%) of samples were sputum, the remainder (n = 94) being cough swabs taken by clinical staff, with rates of cough swab higher in children compared to adults (42% vs 19%). Clinical characteristics of all patients are summarized in Table 1, with characteristics of individual patients presented in Table S3. Overall, this was a population with mild CF lung disease, with a mean FEV1 of 80.6% predicted.
FIG 1.
Flow diagram detailing patient selection process. Only patients who contributed ≥6 samples with contemporaneous diagnostic microbiology data (DM) were included in the final analyses. Using a modification of the Leeds criteria, patients were deemed to be chronically or intermittently colonized with a given pathogen if >50% or ≤50% of samples, respectively, over the 3-year study period were positive by diagnostic microbiology or targeted QPCR. A minimum of ≥6 samples was chosen as less samples would have increased the likelihood of misclassifying infection status.
TABLE 1.
Summary of clinical characteristics for all patients
| Characteristics | Paediatric | Adult | All patients |
|---|---|---|---|
| No. of patients | 20 | 20 | 40 |
| Mean no. of samples per patient (±SD) | 7.0 (1.0) | 9.4 (3.5) | 8.2 (2.8) |
| No. of sample types (sputum/swab) | 81/58 | 152/36 | 233/94 |
| Sex (female/male) | 10/10 | 8/12 | 18/22 |
| Mean age (±SD)a | 10.5 (2.7) | 25.1 (4.6) | 18.9 (8.1) |
| Mean %FEV1 (±SD) | 91.0 (15.3) | 73.0 (19.5) | 80.6 (19.9) |
| Pancreatic insufficiency (sufficient/insufficient) | 3/17 | 8/12 | 11/29 |
| CF related diabetes (Y/N) | 0/20 | 1/19 | 1/39 |
| CFTR genotypeb | |||
| Phe508del homozygousc | 9 | 7 | 16 |
| Phe508del heterozygous | 7 | 8 | 15 |
| Non-Phe508del | 4 | 5 | 9 |
SD denotes standard deviation of the mean. Based on age at time of first sample for each patient.
CFTR genotype - cystic fibrosis transmembrane conductance regulator genotype.
Homozygous Phe508del, 2 copies of the Phe508del gene mutation. Heterozygous Phe508del, single copy of this mutation plus another mutation.
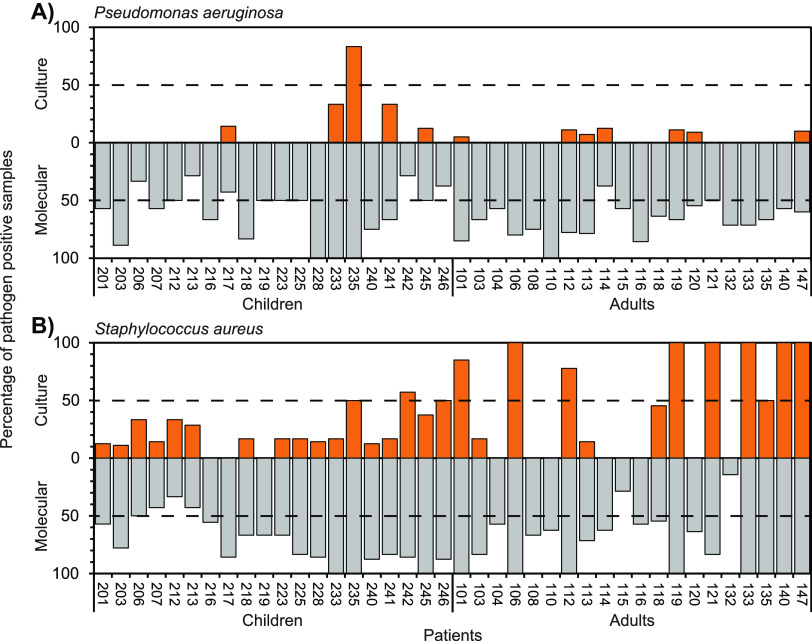
Pathogen detection by culture and QPCR approaches was compared across the patients included in the study (Fig. 2). In all instances, culture significantly underestimated pathogen detection. For P. aeruginosa, the mean percentage of pathogen detection (± SD) across all pediatric patients by culture was 8.8 ± 20.4% compared to 60.9 ± 23.4% by QPCR (Kruskal-Wallis test, H = 24.96, P < 0.0001). For P. aeruginosa in adults, the means were 3.3 ± 4.8% and 68.5 ± 15.7% by culture and QPCR, respectively (H = 30.34, P < 0.0001). For S. aureus in pediatric patients the mean detection by culture and QPCR were 21.6 ± 16.8% and 72.2 ± 20.8%, respectively (H = 24.98, P < 0.0001). Within adult patients, S. aureus mean detection was 44.5 ± 45.3 and 75.1 ± 25.9 by culture and QPCR, respectively (H = 4.51, P = 0.034).
FIG 2.
Pathogen detection by conventional culture and molecular-based approaches in pediatric and adult cystic fibrosis patients. Given for each patient, is the percentage of respiratory samples over the 3-year study duration that were culture (orange) or QPCR (gray) positive for (A) Pseudomonas aeruginosa and (B) Staphylococcus aureus. In each instance, the dashed line denotes the threshold for chronic (>50%) or intermittent (≤50%) colonization. Numbers on the x axis represent individual patient study numbers.
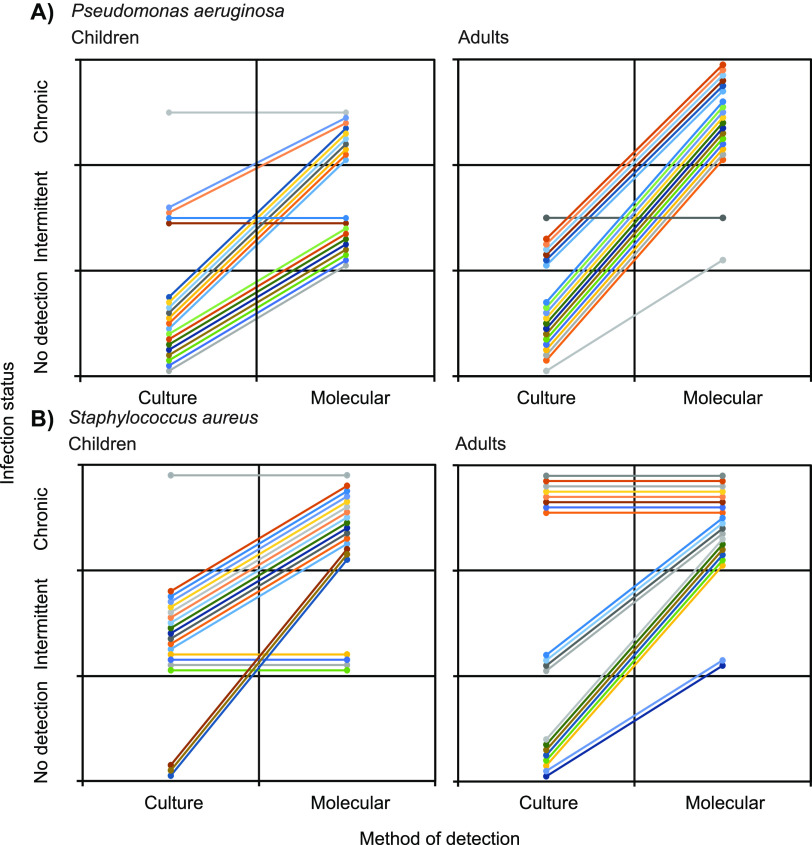
More specifically, P. aeruginosa and S. aureus were detected by culture on at least one occasion in 12 (30%) and 29 (73%) of the patients, respectively. Conversely, both pathogens were detected in all 40 patients on at least one occasion by QPCR (Fig. 2). For P. aeruginosa, culture determined only one patient to be classified as chronically infected and a further 11 classified as intermittently infected (Fig. 2 and 3). That increased to 28 classified as chronically infected and 12 classified as intermittently infected with P. aeruginosa when using QPCR to identify presence of infection. For S. aureus, 9 patients were deemed to be chronically infected by culture compared to 34 patients classified as chronically infected by QPCR. Change in bacterial infection status classification was seen in both the adult and pediatric patients. Overall, P. aeruginosa and S. aureus infection status classification changed for 36 and 27 out of all patients, respectively. In no cases did molecular identification lead to a patient being allocated to a negative or intermittent infection category when culture placed them with an intermittent or chronically infected classification (Fig. 3).
FIG 3.
Changes in pathogen infection status classification from when defined by diagnostic culture to then by molecular-based detection in pediatric and adult patients. Given are changes in infection status classification for (A) Pseudomonas aeruginosa and (B) Staphylococcus aureus in each of the children and adult CF patients. In each instance, colored lines represent individual patients.
There was only a single instance of a greater percentage of pathogen positive samples detected by culture than by QPCR; specifically, S. aureus in patient 121 (Fig. 2B). That patient was however still deemed to be chronically colonized with S. aureus irrespective of method of detection. Out of the 18 samples culture positive for P. aeruginosa samples, only 5 of those did not have corresponding detection by QPCR. For S. aureus, from the 121 culture positive samples, 8 did not have matching molecular detection.
Pathogen abundance was assessed to ascertain whether it contributed to the underestimation of pathogen detection by culture; whereby low bacterial cell abundances could contribute to false negatives by culture. However, abundance derived by QPCR for both pathogens was relatively high across all samples (Fig. S1). The mean abundance (± SD) for P. aeruginosa in pediatric and adult patients was 1.91 × 109 ± 1.11 × 101 CFU (CFU) mL−1 equivalents and 4.75 × 108 ± 1.34 × 101 CFU mL−1 equivalents, respectively. For S. aureus, mean abundances were 7.37 × 108 ± 2.54 × 101 and 1.85 × 108 ± 1.14 × 101 CFU mL−1 equivalents for pediatric and adult patients, respectively. Similarly, the influence of respiratory sample type was assessed, with an expectation that cough swabs would have lower pathogen abundances when compared to sputum as a source sample. With the expectation of P. aeruginosa in pediatric samples, there was no significant differences in pathogen abundances when derived from sputum or cough swab samples (Fig. S2).
DISCUSSION
In this study, we compared the detection of key pathogens on repeated samples from the same cohort of CF children and adults over several years. Since the patients were considered free of chronic Pseudomonas infection by culture, we have focused on the identification of this pathogen along with S. aureus in sputum and cough swabs collected at the same time as diagnostic microbiology. Both pathogens are broadly accepted as readily detectable by culture-based diagnostic microbiology (5, 11). Strikingly, detection of both P. aeruginosa and S. aureus was significantly lower by culture, which in, many instances, did not detect either pathogen despite these being found repeatedly by targeted QPCR (Fig. 2). Consequently, the infection status of patients was also altered in the majority of instances (Fig. 3), with potential implications for understanding of infection in CF and clinical management of emergent infections.
Current infection surveillance approaches in CF are guided by classical aerobic culture-based diagnostic microbiology (5, 6), and these data are collated across CF centers and used to inform national annual CF patient registry reports (3, 4). Based on these culture-based microbiology results, patients are also cohorted into groups and segregated to avoid cross-infection (6, 8). In this study, patients were specifically drawn from Pseudomonas-free clinics, containing patients with no new growth of P. aeruginosa for at least 12 months and considered free of chronic infection by this pathogen. Although it was accepted that some would subsequently become infected by this pathogen over the 3-year study period, culture indicated all patients were free of chronic infection throughout the study, with only one exception (Fig. 2). Conversely, QPCR indicated that 28 (70%) of all patients were chronically infected with P. aeruginosa, and the remainder intermittently infected. Based on culture-based reporting alone, none of the pediatric and adult patients would have been redirected into P. aeruginosa based outpatient clinics.
In the case of Pseudomonas in particular, this has implications for the treatments that patients are offered. Eradication treatments, targeted oral or intravenous therapies for exacerbations, and long-term inhaled anti-pseudomonal antibiotics are not prescribed unless P. aeruginosa is isolated on culture. Our data would indicate that this may significantly underestimate the true prevalence of chronic infection. Suboptimal antimicrobial therapy could further contribute to continued infection, and an increase likelihood of developing antimicrobial resistance or transitioning to chronic infection with time (12). Importantly, underestimation or non-detection may miss the critical period in early P. aeruginosa infection typically used as an ideal time frame for targeted eradication therapy to prevent establishment of chronic infection (13).
Over the last 10 years or so, molecular microbiology techniques, have challenged our fundamental understanding of CF airway pathogen epidemiology (14); with previous literature focused on QPCR in CF summarized in the supplemental materials and Table S1. In support of our findings, a CF lung microbiome study of 297 patients attending 13 CF centers in Europe and the USA, representing a broad cross-section of respiratory disease, found P. aeruginosa in all patients and S. aureus in the majority of those patients (93%) (15). However, there have been surprising few studies though that have addressed this issue in longitudinal sampling. Most notably, in a study of 80 Danish CF patients, whole genome sequencing approaches and positive culture history of P. aeruginosa infections demonstrated that the same clone type could persist from cultures taken before and after a judged Pseudomonas-free eradication period in many of those patients (16). This suggested that the original Pseudomonas strain had not been cleared, and was missed by routine culture-based surveillance, as we have shown. Similar to the current study, Héry-Arnaud et al. (17) looked at patients apparently free of Pseudomonas and compared QPCR with culture. They showed that QPCR would often become positive before culture and showed high rates of molecular identification of Pseudomonas in those in whom it was never cultured (17). Results were presented in aggregate however, and the impact on classification of microbiological status of individual patients was not reported.
Although methodological strengths and weaknesses of molecular and culture detection methods have been reviewed by others (7, 10), it is important to consider potential factors that might contribute to the observed disparity between the sets of outcomes. For instance, low pathogen abundance within a sample or low abundance resulting from different respiratory sample types (e.g., cough swab versus sputum) could be factors leading to misdetection by culture (7, 10). However, given the relatively high abundances observed for both pathogens by QPCR across all patients this is unlikely to have been a major factor here (Fig. S1 and S2). It is also possible that artifacts of cell death were amplified by QPCR, creating false positives. Again, this seems unlikely to be a major or constant factor given the observed abundances across all patients over the 3-year study period. Viable but not culturable (VBNC) status may explain to some degree why these readily culturable pathogens were undetected in many cases (17–19). VBNC is believed to be a protective state in response to some form of environmental stressor, including changes from aerobic or anaerobic conditions, prevalence of energy and nutrient sources, or as a defense mechanism against antibiotics. Pertinent to this study, the ability of antibiotics to induce a VBNC state have been proposed from studies of S. aureus and P. aeruginosa, which may also support pathogen survival to extended and repeated antibiotic interventions (18, 19). The clinical impact of bacteria in such states on either current or future health remains to be established. In our study, there were a few instances where there was detection by culture but not by QPCR. However, it is possible for culture to misidentify pathogens and generate false positives. For example, other Staphylococcus species can be mistaken for S. aureus in routine testing (11, 20).
Microbiological surveillance to detect pathogens is the foundation of CF clinical care and treatment. Our longitudinal data substantially builds on previous observations to demonstrate that the diagnostic gold standard of microbiological detection by culture may significantly underestimate prevalence (Table S1). Crucially, we have further shown how classification of a patient’s infection status would change using findings from QPCR in comparison with culture even for what are believed to be readily culturable pathogens from pediatric and adult patients. Despite following patients over several years, the current study was limited to 40 patients. To further advance this work, future studies will need to have larger patient numbers representative of the wider CF population, encompassing, for example, differing disease severities, disease states, and exacerbation and infection histories. That said, it remains that our findings have potential implications for understanding the evolution of infection and the clinical care of patients. This issue will become even more important in CF patients treated with highly effective cystic fibrosis transmembrane (CFTR) modulators. These treatments often lead to dramatic reductions in sputum volumes, increasing our reliance on culture of other specimens than sputum, such as cough swabs, with known reduced sensitivity in detection of pathogens. Thus, molecular detection may have an increasing potential role for detection of potential CF pathogens, but there is a need to establish its relevance in comparison with current culture-based techniques (21). These findings also have implications beyond CF, since microbiological detection and surveillance are critical to guide therapeutics in other airways diseases. As targeted molecular identification becomes quicker and cheaper, it is likely to become increasingly deployed in clinical scenarios. Subsequently, treatment approaches developed in response to conventional clinical microbiology need reevaluation to deal with these more sensitive techniques. Again, future longitudinal studies with larger patient numbers will be needed to establish the extent of the clinical implications arising from this study. Moreover, such studies will be required to thoroughly assess targeted QPCR approaches before they could be approved for routine and widespread diagnostic use in clinical settings.
MATERIALS AND METHODS
Study and patient sampling.
Patients were recruited as part of a longitudinal observational study of adults and children with CF cared for at two different CF centers (22). Adults were recruited from the Manchester Adult CF Centre (center patient population n = 466 patients), and children from the Royal Manchester Children’s Hospital (n = 342 patients). Patients were required to be at least 5 years old, with a FEV1 of >50% predicted at study entry. Patients were drawn from Pseudomonas-free clinics and were eligible if considered free of chronic infection with P. aeruginosa and with no new growth of this pathogen for at least 12 months using diagnostic culture approaches and as defined by the Leeds criteria (23). Patients or parents/guardians provided written informed consent and children provided assent. This study was reviewed and approved by the NHS Research Ethic Committee North West, Lancaster (Ref 14/NW/1195).
Patients were assessed at their usual clinic appointments by their regular clinical team and included routine and emergency visits. Spirometry was performed by the usual clinical team. Normal ranges for spirometry were those from the Global Lung Initiative (24). At study entry, 85% of adults (17/20) were on long-term azithromycin treatment, while one patient (patient 110) was receiving long-term colistin and the two remaining patients (133 and 135) were not prescribed any long-term antibiotics. Seventy percent of pediatric patients (14/20) were also on long-term azithromycin, the remaining 6 (201, 206, 212, 216, 228 and 241) were not prescribed any long-term antibiotics (Table S2 and S3). Sputum or cough swab samples were taken at each clinic visit for diagnostic and molecular microbiology. All sputum samples were spontaneously expectorated. Molecular microbiology samples were transported to the lab within 3 h and stored at −80°C prior to DNA extraction and PCR (25, 26). Sputum samples were mixed and weighed prior to splitting and storage. To assess longitudinal microbiology, only patients with a minimum of six respiratory samples along with accompanying full diagnostic microbiology data were included (Fig. 1 and Table 1).
Diagnostic microbiology.
Diagnostic culture-based microbiology data was provided by the microbiology service within Manchester University NHS Foundation Trust who perform microbiology testing for the participating centers, in line with international guidance and standards (5, 6).
DNA Extraction and QPCR.
Nucleic acid extraction was performed on sputum and cough swab samples as previously described, with a modification for the latter sample type (27). As an alternative to the wash stage for sputum, cough swabs were saturated in sterile phosphate buffer solution for 5 min, then squeezed with sterile tweezers to extract as much material as possible. The resulting solution was then introduced at the bead beating stage and the protocol continued as normal as for the sputum samples thereafter.
It was not within the scope of the current study to design and assess new primers for targeted QPCR against P. aeruginosa and S. aureus, as many effective primer sets have been developed and evaluated over at least the last 2 decades; for example, see Table S1 for P. aeruginosa based primers. Instead, the approach here was to use primer sets already established as highly effective.
All QPCR was performed on a Bio-Rad CFX connect machine (Bio-Rad, Deeside, UK), Each plate had a blank consisting of the master mix, probes, primers and water, and pure strains of each pathogen were run on each plate in a 10-fold dilution as both a positive control and a detection standard.
P. aeruginosa – oprL.
While there are several targets for P. aeruginosa amplification, studies vary in their usage. Since development in the late 1990s, the oprL gene target has consistently shown good sensitivity and specificity across multiple studies (28). Multiple studies have utilized the oprL gene to identify P. aeruginosa in CF sputum and swabs (See Table S1). While some studies have further characterized the P. aeruginosa positive samples using confirmative QPCR for the gyrB/ecfX gene targets (See Table S1), the sensitivity and specificity of oprL alone (100% and 70–75% respectively) (28, 29) was sufficient for this study, particularly in light of the further expense needed for confirmation with other gene targets. For this study, the average r2 (efficiency) value was 0.97 across 5 runs (min: 0.96, max: 0.99). The r2 in this study sat between 0.99 and 0.96 for all five runs.
P. aeruginosa detection targeting the oprL was performed using the following forward primer (0.2 μL from 100 μM stock) CGAGTACAACATGGCTCTGG, reverse primer (0.2 μL from 100 μM stock) ACCGGACGCTCTTTACCATA, and probe (0.2 μL from 100 μM stock) FAM–CCTGCAGCACC-AGGTAGCGC-TAMRA (30, 31). A further 2 μL of DNA, 20 μL of TaqMan Gene expression Mastermix (Applied Biosystems) and 17.4 μL of molecular grade water was added to a final volume of 40 μL. Samples underwent an initial denaturation for 10 min at 950C, followed by 40 cycles consisiting of 10 s denaturation at 950C, 30 s of annealing at 580C, and a 1 min elongation at 720C.
S. aureus – nuc.
The nuc gene used for S. aureus detection was developed 1992 (32). The gene is highly specific for S. aureus and is not found in other key staphylococcal strains (33). The nuc gene is a well-established S. aureus QPCR primer, with a sensitivity and specificity of around 98% and 100% respectively (34–37). For this study, the average r2 (efficiency) value for this target was 0.97 over 4 runs (min: 0.94, max: 1).
S. aureus detection targeting the nuc was performed using the following forward primer (0.06 μL from a 100 μM stock) CGCTACTAGTTGCTTAGTGTTAACTTTAGTTG, reverse primer (0.06 μL from a 100 μL stock) TGCACTATATACTGTTGGATCTTCAGAA, and probe (0.02 μL from 100 μM stock) FAM-TGCATCACAAACAGATAACGGCGTAAATAGAAG-TAMRA (34). A further 2 μL of DNA, 10 μL of TaqMan Gene expression Mastermix and 7.86 μL of molecular grade water was added to a final volume of 20 μL. Samples underwent an initial denaturation for 10 min at 950C, followed by 40 cycles consisiting of 30 s denaturation at 950C, 30 s of annealing at 600C, and a 1 min elongation at 72°C.
Data analysis.
For longitudinal analysis, this was restricted to patients who contributed ≥6 samples with contemporaneous diagnostic microbiology data (Fig. 1). Using a modification of the Leeds criteria (23), patients were deemed to be chronically or intermittently colonized with a given pathogen if >50% or ≤50% of samples, respectively, over the 3-year study period were positive by diagnostic microbiology or targeted QPCR. A minimum of 6 samples were chosen as fewer samples would have increased the likelihood of misclassifying infection status. Summary statistics, including means and standard deviations (SD), were calculated using XLSTAT v2018.1 (Addinsoft). Kruskal-Wallis analyses in conjunction with post hoc Dunn tests were performed in XLSTAT. Significance was set at P < 0.05.
Data availability.
Culture and QPCR data from the study has been deposited at figshare.com under https://doi.org/10.6084/m9.figshare.14483394.v2.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients who took part in this and the clinical teams who supported this work. This work was supported by the CF Trust (VIA 045), the NIHR (CS012-13), and the North West Lung Centre. This work was also supported by the NIHR Manchester Clinical Research Facility.
The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication. All authors declare support from the CF Trust. A.H. reports personal fees for advisory services (Mylan Pharmaceuticals) and educational and presentation activities (Vertex Pharmaceuticals).
C.v.d.G., A.H., and H.G. conceived the study. H.G., L.H., and D.R. performed sample processing and analysis. H.G., D.R., and C.v.d.G. performed data and statistical analysis. A.H., A.M., and A.J. were responsible for sample collection, clinical care records and documentation. H.G., A.H., and C.v.d.G. verified the underlying data. H.G., A.H., and C.v.d.G. were responsible for the creation of the original draft of the manuscript. All authors contributed to the development of the final manuscript. C.v.d.G. and A.H. are guarantors of this work. All authors read and approved the final manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Christopher van der Gast, Email: C.vanderGast@mmu.ac.uk.
Joanna B. Goldberg, Emory University School of Medicine
REFERENCES
- 1.Elborn JS. 2016. Cystic fibrosis. Lancet 388:2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 2.Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, Ranganathan S, Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) . 2011. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med 184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation. 2019. Cystic Fibrosis Foundation Patient Registry 2019 Annual Data Report. Cystic Fibrosis Foundation, Bethesda, Maryland, USA. https://www.cff.org/sites/default/files/2021-10/2019-Patient-Registry-Annual-Data-Report.pdf. [Google Scholar]
- 4.Cystic Fibrosis Trust. 2019. UK Cystic Fibrosis Registry Annual Data Report 2019. Cystic Fibrosis Trust, London, UK. https://www.cysticfibrosis.org.uk/sites/default/files/2020-12/2019%20Registry%20Annual%20Data%20report_Sep%202020.pdf. [Google Scholar]
- 5.Public Health England. 2019. Investigation of bronchoalveolar lavage, sputum and associated specimens. UK Standard for Microbiology Investigations, England. B 57 Issue 3.5. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/800451/B_57i3.5.pdf. [Google Scholar]
- 6.Smyth AR, Bell SC, Bojcin S, Bryon M, Duff A, Flume P, Kashirskaya N, Munck A, Ratjen F, Schwarzenberg SJ, Sermet-Gaudelus I, Southern KW, Taccetti G, Ullrich G, Wolfe S, European Cystic Fibrosis S . 2014. European cystic fibrosis society standards of care: best practice guidelines. J Cyst Fibros 13:S23–S42. doi: 10.1016/j.jcf.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Pattison SH, Rogers GB, Crockard M, Elborn JS, Tunney MM. 2013. Molecular detection of CF lung pathogens: current status and future potential. J Cyst Fibros 12:194–205. doi: 10.1016/j.jcf.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doe SJ, McSorley A, Isalska B, Kearns AM, Bright-Thomas R, Brennan AL, Webb AK, Jones AM. 2010. Patient segregation and aggressive antibiotic eradication therapy can control methicillin-resistant Staphylococcus aureus at large cystic fibrosis centres. J Cyst Fibros 9:104–109. doi: 10.1016/j.jcf.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Stewart EJ. 2012. Growing unculturable bacteria. J Bacteriol 194:4151–4160. doi: 10.1128/JB.00345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JL, Rolain J-M. 2014. Culture-based diagnostic microbiology in cystic fibrosis: can we simplify the complexity? J Cyst Fibros 13:1–9. doi: 10.1016/j.jcf.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Public Health England. 2020. Identification of Staphylococcus species, Micrococcus species and Rothia species. UK Standards for Microbiology Investigations, England. ID 07 Issue 4. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832968/ID_7_dj_.pdf. [Google Scholar]
- 12.Flume PA, Waters VJ, Bell SC, Van Devanter DR, Stuart Elborn J, Antimicrobial Resistance in Cystic Fibrosis International Working Group . 2018. Antimicrobial resistance in cystic fibrosis: does it matter? J Cyst Fibros 17:687–689. doi: 10.1016/j.jcf.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Høiby N, Frederiksen B, Pressler T. 2005. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros 4:49–54. doi: 10.1016/j.jcf.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. 2012. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA 109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuthbertson L, Walker AW, Oliver AE, Rogers GB, Rivett DW, Hampton TH, Ashare A, Elborn JS, De Soyza A, Carroll MP, Hoffman LR, Lanyon C, Moskowitz SM, O'Toole GA, Parkhill J, Planet PJ, Teneback CC, Tunney MM, Zuckerman JB, Bruce KD, van der Gast CJ. 2020. Lung function and microbiota diversity in cystic fibrosis. Microbiome 8:45. doi: 10.1186/s40168-020-00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartell JA, Sommer LM, Marvig RL, Skov M, Pressler T, Molin S, Johansen HK. 2021. Omics-based tracking of Pseudomonas aeruginosa persistence in “eradicated” cystic fibrosis patients. Eur Respir J 57:2000512. doi: 10.1183/13993003.00512-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Héry-Arnaud G, Nowak E, Caillon J, David V, Dirou A, Revert K, Munck MR, Frachon I, Haloun A, Horeau-Langlard D, Le Bihan J, Danner-Boucher I, Ramel S, Pelletier MP, Rosec S, Gouriou S, Poulhazan E, Payan C, Férec C, Rault G, Le Gal G, Le Berre R. 2017. Evaluation of quantitative PCR for early diagnosis of Pseudomonas aeruginosa infection in cystic fibrosis: a prospective cohort study. Clin Microbiol Infect 23:203–207. doi: 10.1016/j.cmi.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Mangiaterra G, Amiri M, Di Cesare A, Pasquaroli S, Manso E, Cirilli N, Citterio B, Vignaroli C, Biavasco F. 2018. Detection of viable but non-culturable Pseudomonas aeruginosa in cystic fibrosis by qPCR: a validation study. BMC Infect Dis 18:701. doi: 10.1186/s12879-018-3612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasquaroli S, Zandri G, Vignaroli C, Vuotto C, Donelli G, Biavasco F. 2013. Antibiotic pressure can induce the viable but non-culturable state in Staphylococcus aureus growing in biofilms. J Antimicrob Chemother 68:1812–1817. doi: 10.1093/jac/dkt086. [DOI] [PubMed] [Google Scholar]
- 20.Notarnicola SM, Zamarchi GR, Onderdonk AB. 1985. Misidentification of mucoid variants of Staphylococcus aureus by standard laboratory techniques. J Clin Microbiol 22:459–461. doi: 10.1128/jcm.22.3.459-461.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, Radey M, Accurso FJ, Wolter DJ, Cooke G, Adam RJ, Carter S, Grogan B, Launspach JL, Donnelly SC, Gallagher CG, Bruce JE, Stoltz DA, Welsh MJ, Hoffman LR, McKone EF, Singh PK. 2017. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horsley AR, Belcher J, Bayfield K, Bianco B, Cunningham S, Fullwood C, Jones A, Shawcross A, Smith JA, Maitra A, Gilchrist FJ. 2022. Longitudinal assessment of lung clearance index to monitor disease progression in children and adults with cystic fibrosis. Thorax 77:357–363. doi: 10.1136/thoraxjnl-2021-216928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. 2003. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros 2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 24.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J, Initiative E, ERS Global Lung Function Initiative . 2012. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuthbertson L, Rogers GB, Walker AW, Oliver A, Hafiz T, Hoffman LR, Carroll MP, Parkhill J, Bruce KD, van der Gast CJ. 2014. Time between collection and storage significantly influences bacterial sequence composition in sputum samples from cystic fibrosis respiratory infections. J Clin Microbiol 52:3011–3016. doi: 10.1128/JCM.00764-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuthbertson L, Rogers GB, Walker AW, Oliver A, Hoffman LR, Carroll MP, Parkhill J, Bruce KD, van der Gast CJ. 2015. Implications of multiple freeze-thawing on respiratory samples for culture-independent analyses. J Cyst Fibros 14:464–467. doi: 10.1016/j.jcf.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers GB, Hart CA, Mason JR, Hughes M, Walshaw MJ, Bruce KD. 2003. Bacterial diversity in cases of lung infection in cystic fibrosis patients: 16S ribosomal DNA (rDNA) length heterogeneity PCR and 16S rDNA terminal restriction fragment length polymorphism profiling. J Clin Microbiol 41:3548–3558. doi: 10.1128/JCM.41.8.3548-3558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vos D, Lim A, Jr, Pirnay JP, Struelens M, Vandenvelde C, Duinslaeger L, Vanderkelen A, Cornelis P. 1997. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J Clin Microbiol 35:1295–1299. doi: 10.1128/jcm.35.6.1295-1299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutin S, Weitnauer M, Hassel S, Graeber SY, Stahl M, Dittrich AS, Mall MA, Dalpke AH. 2018. One time quantitative PCR detection of Pseudomonas aeruginosa to discriminate intermittent from chronic infection in cystic fibrosis. J Cyst Fibros 17:348–355. doi: 10.1016/j.jcf.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Feizabadi MM, Majnooni A, Nomanpour B, Fatolahzadeh B, Raji N, Delfani S, Habibi M, Asadi S, Parvin M. 2010. Direct detection of Pseudomonas aeruginosa from patients with healthcare associated pneumonia by real time PCR. Infect Genet Evol 10:1247–1251. doi: 10.1016/j.meegid.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Rogers GB, Marsh P, Stressmann AF, Allen CE, Daniels TVW, Carroll MP, Bruce KD. 2010. The exclusion of dead bacterial cells is essential for accurate molecular analysis of clinical samples. Clin Microbiol Infect 16:1656–1658. doi: 10.1111/j.1469-0691.2010.03189.x. [DOI] [PubMed] [Google Scholar]
- 32.Brakstad OG, Aasbakk K, Maeland JA. 1992. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol 30:1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graber HU, Casey MG, Naskova J, Steiner A, Schaeren W. 2007. Development of a highly sensitive and specific assay to detect Staphylococcus aureus in bovine mastitic milk. J Dairy Sci 90:4661–4669. doi: 10.3168/jds.2006-902. [DOI] [PubMed] [Google Scholar]
- 34.Alarcón B, Vicedo B, Aznar R. 2006. PCR-based procedures for detection and quantification of Staphylococcus aureus and their application in food. J Appl Microbiol 100:352–364. doi: 10.1111/j.1365-2672.2005.02768.x. [DOI] [PubMed] [Google Scholar]
- 35.Hein I, Lehner A, Rieck P, Klein K, Brandl E, Wagner M. 2001. Comparison of different approaches to quantify Staphylococcus aureus cells by real-time quantitative PCR and application of this technique for examination of cheese. Appl Environ Microbiol 67:3122–3126. doi: 10.1128/AEM.67.7.3122-3126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palomares C, Torres MJ, Torres A, Aznar J, Palomares JC. 2003. Rapid detection and identification of Staphylococcus aureus from blood culture specimens using real-time fluorescence PCR. Diagn Microbiol Infect Dis 45:183–189. doi: 10.1016/s0732-8893(02)00542-4. [DOI] [PubMed] [Google Scholar]
- 37.Pinto B, Chenoll E, Aznar R. 2005. Identification and typing of food-borne Staphylococcus aureus by PCR-based techniques. Syst Appl Microbiol 28:340–352. doi: 10.1016/j.syapm.2005.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3 and Fig. S1 and S2. Download spectrum.00419-22-s0001.pdf, PDF file, 1.4 MB (1.4MB, pdf)
Data Availability Statement
Culture and QPCR data from the study has been deposited at figshare.com under https://doi.org/10.6084/m9.figshare.14483394.v2.