Abstract
We present a case of Bartonella quintana infective endocarditis requiring valvular surgery in an Indigenous patient from northern Alberta that was identified months after initial presentation to hospital with undifferentiated laboratory abnormalities. Syndromes caused by B. quintana are often challenging to diagnose due to their non-specific presentation and the difficulty in detecting this organism using traditional culture methods. Additionally, risk factors for B. quintana include marginal housing and alcohol use disorder, which often impede access to health care. Indigenous patients in northern Canada often face worse health outcomes compared with other regions owing to poor economic conditions, substandard housing, and limited access to health care resources. Given that risk factors for B. quintana are prevalent throughout northern Canada and that this infection is difficult to diagnose, we surmise that the prevalence of B. quintana infection is underestimated in northern Canada.
Keywords: bacterial, Bartonella quintana, endocarditis, vulnerable populations
Mots-clés: bactérien, Bartonella quintana, endocardite, populations vulnérables
Abstract
Les auteurs présentent un cas d’endocardite infectieuse à Bartonella quintana exigeant une chirurgie valvulaire chez un patient autochtone du nord de l’Alberta, dépisté des mois après la première consultation à l’hôpital, alors que les anomalies de laboratoires étaient indifférenciées. Les syndromes causés par le Bartonella quintana sont souvent difficiles à diagnostiquer à cause de leur présentation non spécifique et de la difficulté à déceler cet organisme au moyen des méthodes de culture classiques. De plus, les facteurs de risque de Bartonella quintana incluent des logements inférieurs aux normes et des troubles de l’usage de l’alcool, qui nuisent souvent à l’accès aux soins. Les patients autochtones du nord du Canada présentent souvent des résultats cliniques pires que ceux d’autres régions à cause des mauvaises conditions économiques, des logements inférieurs aux normes et de l’accès limité aux ressources de santé. Puisque les facteurs de risque de Bartonella quintana prévalent dans tout le nord du Canada et que cette infection est difficile à diagnostiquer, les auteurs postulent que la prévalence d’infection à Bartonella quintana est sous-estimée dans cette région.
Case Summary
An otherwise healthy 36-year-old Indigenous male with alcohol use disorder presented to his community hospital with a 2-day history of asymptomatic hematuria and no associated constitutional symptoms. He resided in a small dwelling with his wife, four children, and two extended family members, and regularly hunted and skinned large mammals, including bison. Their home was located on a remote northern Alberta reserve, near the border with the Northwest Territories.
Initial physical examination findings were not documented, and basic laboratory investigations were significant for bicytopenia (hemoglobin of 115 g/L with MCV of 93 fL and platelet count of 128 × 109/L), renal dysfunction (creatinine of 119 µmol/L, baseline of 72 µmol/L), and a urinalysis demonstrating hematuria and proteinuria. Urine culture was negative, and blood cultures were not performed. Nevertheless, he was treated with a 5-day course of intravenous ceftriaxone therapy, and his gross hematuria resolved. Further investigation of his hematuria with cystoscopy and computed tomography revealed a normal urinary tract but incidentally noted mild splenomegaly.
Four months later, he sought medical attention for subacute dyspnea and progressive constitutional symptoms. Repeat laboratory evaluation revealed worsening bicytopenia (hemoglobin of 69 g/L, a platelet count of 122 × 109/L), progressive renal failure (creatinine of 241 µmol/L), and microscopic hematuria with proteinuria. Initial EKG showed non-specific T wave inversion in the anterior leads but was otherwise unremarkable. No PR interval prolongation was evident. Chest radiography demonstrated cardiomegaly and pulmonary edema, and he was transferred to a tertiary care hospital for further workup.
Physical examination revealed a febrile, overweight, unkempt male with poor dentition. Cardiac examination suggested volume overload with a diffuse grade III/VI pansystolic murmur. There were no stigmata of infective endocarditis, although clubbing in both hands and a non-blanching, erythematous, petechial rash on both legs resembling leukocytoclastic vasculitis were noted.
Given his examination, transthoracic echocardiography was performed, revealing severe aortic regurgitation with speckling and thickening of the aortic valve. Further characterization by transesophageal echocardiography revealed multivalvular vegetations on the aortic and tricuspid valve, aortic valve leaflet perforation, and an echolucent crescent on the anterior root of the valve consistent with an aortic root abscess. Three sets of blood cultures drawn prior to antimicrobial therapy did not yield a microbiological diagnosis. Autoimmune investigations revealed a positive antineutrophil cytoplasmic antibody with a concordant anti-proteinase 3 titre, a positive anti-nuclear antibody with a speckled pattern, an elevated rheumatoid factor, a weakly positive anti-double stranded DNA titre, and hypocomplementemia. Enlarging splenomegaly (18.9 × 18.4 cm) was also noted radiographically.
Blood for serological assays against Coxiella, Brucella, and Bartonella species were requested, and ceftriaxone and vancomycin were started empirically for endocarditis. Due to progressive congestive heart failure, a mechanical aortic valve replacement and a tricuspid valve reconstruction were performed 14 days into his admission. Intraoperatively, the aortic valve was noted to be anatomically bicuspid, with its leaflets destroyed (Figure 1a); furthermore, an aortic root abscess was appreciated. Anatomical pathology examination was consistent with bicuspid aortic valve with active endocarditis, microabscess formation, focal tissue necrosis, and calcific degeneration. Bacterial culture and 16S rRNA PCR were performed on the aortic valve. Culture had no growth; however, 16S rRNA PCR identified the presence of Bartonella quintana. Detailed histopathologic examination confirmed the presence of gram-negative bacilli on Gram stain of the aortic valve specimen, which stained positive on Warthin–Starry silver stain and Giemsa stain (Figure 1b–e). These organisms were also appreciated on electron microscopy, which confirmed high valvular organism burden (Figure 1f). Bartonella serology results were subsequently released 18 calendar days after collection and supported the diagnosis of B. quintana infective endocarditis with titres of 1:4096 for B. henselae IgG and 1:2048 for B. quintana IgG.
Figure 1:
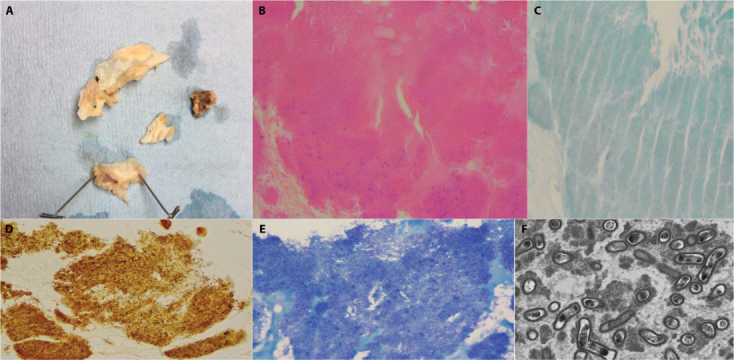
(a) Gross valve specimen; (b) H&E stain of the valve; (c) Gram stain of the valve, with faint pink weakly staining Gram-negative bacilli; (d) Warthin–Starry stain of the valve, with silver staining revealing a high organism burden within the specimen; (e) Giemsa stain confirming the burden of B. quintana within the valve; (f) electron microscopy showing B. quintana at 8000x magnification
H&E = Hematoxylin and eosin
Given his concomitant renal injury, a kidney biopsy was pursued following his valve replacement, and a cryoglobulin level was sent. Renal biopsy revealed a C3 dominant diffuse proliferative glomerulonephritis, consistent with post- infectious glomerulonephritis. Cryoglobulins were positive, with electrophoresis of the cryoprecipitate showing a pattern of non-specific staining with a heterogenous background and no identifiable monoclonal component, consistent with a type III cryoglobulinemia. This secondary type III cryoglobulinemia potentially may have explained his skin findings and some of his systemic disease.
Once a microbiological diagnosis was achieved post-operatively, the patient was immediately started on a 2-week course of rifampin and a 6-week course of doxycycline; vancomycin and ceftriaxone were discontinued. His renal dysfunction and constitutional symptoms resolved following his antibiotic course. Subsequently, after hospital discharge, the patient was lost to clinical follow-up.
Discussion
B. quintana is a pathogen transmitted by the body louse Pediculus humanus corporis and is responsible for a variety of clinical syndromes. Initially isolated in 1920 as the etiologic agent of “trench fever” (1), B. quintana has emerged to be implicated in multiple syndromes including chronic asymptomatic bacteremia, bacillary angiomatosis, lymphadenopathy, and infective endocarditis (2). The syndromes noted above often present with innocuous or non-specific constitutional symptoms (2,3).
Risk factors for B. quintana infection include homelessness, alcohol use disorder, poor hygiene, and cold weather (4). These risk factors predispose to B. quintana infection as they are the ideal growth condition for its vector, the human body louse (5). Louse infestation and associated disease outbreaks correlate with poverty, low socio-economic status, and political instability; these factors lead to poor hygiene and close contact, which facilitate spread of the body louse (5). Although the endemicity of Pediculus humanus corporis in Canada is unknown due to its non-reportable status, remote areas of northern Canada with overcrowding, chronic housing shortages, substandard plumbing infrastructure, and low temperatures conceivably are at higher risk (6).
B. quintana is a fastidious organism with prolonged incubation time (4). Traditional cultures of blood or infected tissue have a yield of approximately 30% (7). Histological examination of affected tissues using stains such as the Warthin–Starry silver or Giemsa stain can assist in Bartonella identification, but these are non-specific for Bartonella (3). Serology is an effective means of confirming Bartonella infection, but cross-reactivity between antibodies against B. quintana and B. henselae can be problematic for definitive species identification (8), as seen in this case. Both organisms can cause culture-negative endocarditis (3). However, epidemiology differs between the two species; therefore, clinical differentiation may be attempted in the absence of tissue for molecular confirmation (9). In Canada, Bartonella serology is performed at the National Microbiology Laboratory (Public Health Agency of Canada, Winnipeg, Manitoba). Routine turnaround time for this test is 21 calendar days. Molecular diagnostics, such as 16S rRNA PCR of excised tissue, have emerged as a useful means for identifying B. quintana and other slow-growing or fastidious organisms, particularly in the context of culture-negative endocarditis (10). Where tissue is available, 16S rRNA PCR is specific to the species level and the turnaround time for the test is rapid. If the diagnosis is strongly suspected, two-way communication between the clinician and the clinical microbiologist is critical to optimize and expedite serological and molecular testing wherever feasible.
Treatment of B. quintana endocarditis typically includes systemic aminoglycosides for 2 weeks along with doxycycline for 6 weeks (11). Aminoglycosides are usually used initially as a second antimicrobial agent as they have been associated with improved outcomes in Bartonella endocarditis (12), thought to be related to their bactericidal activity (13). In this case, given our patient’s severe renal injury, we opted to avoid aminoglycoside therapy due to potential nephrotoxicity. Rifampin has been used as an alternative to aminoglycosides in Bartonella infections (11), which is why we opted to use rifampin in conjunction with doxycycline.
This is the fifth reported case of B. quintana endocarditis in Canada, and the second reported case in northern Canada (14,15). Both endocarditis cases in northern Canada had an advanced presentation requiring transfer to a tertiary care centre and valvular surgery, with the diagnosis secured through 16S rRNA PCR and histologic examination of the excised valve (14).
We surmise an underestimation of the prevalence of B. quintana infection in northern Canada for multiple reasons. First, population overcrowding, poor housing conditions, and challenges in accessing health care resources abound given the remote geography that exists. Second, syndromes associated with B. quintana are non-specific and challenging to confirm microbiologically. Third, treatment of individuals experiencing poverty, poor housing conditions, or alcohol use disorder may sometimes be complicated by financial constraints and lack of adherence (16).
Indigenous people in northern Canada are at greater risk of infectious and non-communicable diseases due to structural inequities that have led to Indigenous populations becoming marginalized within Canada (6,17). Tuberculosis, diabetes, and asthma are disproportionately prevalent in northern Canada, mirroring the known higher rates of poverty and lower socio-economic status (17). Given the risk factors for louse-borne disease in the region, the prevalence of B. quintana may be disproportionately higher as well.
We report the second reported case of B. quintana infection in northern Canada. In this case, the diagnosis was delayed but was ultimately made by 16S PCR on excised valvular tissue. Identification of B. quintana endocarditis in marginalized populations may be rising due to increasing usage of molecular diagnostics. Our case highlights the importance of working in partnership with the surgeon and the microbiologist to coordinate molecular testing on excised valve tissue in cases where B. quintana endocarditis is suspected, as well as the importance of histopathological review of excised valve tissue. In this case, molecular testing resulted in a specific and expedited diagnosis relative to serology, and facilitated earlier initiation of definitive treatment. Furthermore, given the destructive nature of unmitigated B. quintana disease as demonstrated in our case, it is prudent for clinicians working with vulnerable populations to consider this diagnosis in the appropriate clinical context.
Acknowledgements:
The authors would like to acknowledge the patient for providing his consent for publication of this report, and the electron microscopy technicians for providing high resolution figures for the purposes of this publication.
Informed consent:
Informed consent was obtained from the patient.
Funding:
No funding was received for this work.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
References
- 1.Anstead GM. The centenary of the discovery of trench fever, an emerging infectious disease of World War 1. Lancet Infect Dis. 2016;16(8):e164–72. 10.1016/S1473-3099(16)30003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelakis E, Raoult D. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents. 2014;44(1):16–25. 10.1016/j.ijantimicag.2014.04.006. Medline: [DOI] [PubMed] [Google Scholar]
- 3.Okaro U, Addisu A, Casanas B, Anderson B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev. 2017;30(3):709–46. 10.1128/CMR.00013-17. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12(2):217–23. 10.3201/eid1202.050874. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raoult D, Roux V. The body louse as a vector of reemerging human diseases. Clin Infect Dis. 1999;29(4):888–911. 10.1086/520454. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Anaya J. The situation of Indigenous peoples in Canada. Report of the Special Rapporteur on the rights on Indigenous peoples, James Anaya. Geneva: United Nations Human Rights Office of the High Commissioner; 2014. Jul 4. [Google Scholar]
- 7.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162–73. 10.1097/01.md.0000165658.82869.17. Medline: [DOI] [PubMed] [Google Scholar]
- 8.McGill SL, Regnery RL, Karem KL. Characterization of human immunoglobulin (Ig) isotype and IgG subclass response to Bartonella henselae infection. Infect Immun. 1998;66(12):5915–20. 10.1128/IAI.66.12.5915-5920.1998. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel S, Richert ME, White R, Lambing T, Saleeb P. A case of Bartonella quintana culture-negative endocarditis. Am J Case Rep. 2019;20:602–6. 10.12659/AJCR.915215. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marín M, Muñoz P, Sánchez M, et al. Molecular diagnosis of infective endocarditis by real-time broad-range polymerase chain reaction (PCR) and sequencing directly from heart valve tissue. Medicine (Baltimore). 2007;86(4):195–202. 10.1097/MD.0b013e31811f44ec. Medline: [DOI] [PubMed] [Google Scholar]
- 11.Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48(6):1921–33. 10.1128/AAC.48.6.1921-1933.2004. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raoult D, Fournier P, Vandenesch F, et al. Outcome and treatment of Bartonella endocarditis. Arch Intern Med. 2003;163(2):226–30. 10.1001/archinte.163.2.226. Medline: [DOI] [PubMed] [Google Scholar]
- 13.Rolain J, Maurin M, Raoult D. Bactericidal effect of antibiotics on Bartonella and Brucella spp.: clinical implications. J Antimicrob Chemother. 2000;46(5):811–4. 10.1093/jac/46.5.811. Medline: [DOI] [PubMed] [Google Scholar]
- 14.Keynan Y, MacKenzie L, Lagacé-Wiens P. Quintessential culture-negative endocarditis. Can J Cardiol. 2016;32(3):395. e9,– 10. 10.1016/j.cjca.2015.05.027. Medline: [DOI] [PubMed] [Google Scholar]
- 15.Lam JC, Fonseca K, Pabbaraju K, Meatherall BL. Case report: Bartonella quintana endocarditis outside of the Europe–African gradient: comprehensive review of cases within North America. Am J Trop Med Hyg. 2019;100(5):1125–9. 10.4269/ajtmh.18-0929. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raoult D, Foucault C, Brouqui P. Infections in the homeless. Lancet Infect Dis. 2001;1(2):77–84. 10.1016/S1473-3099(01)00062-7. [DOI] [PubMed] [Google Scholar]
- 17.Public Health Agency of Canada. Key health inequalities in Canada [Internet]. Ottawa: Public Health Agency of Canada; c2018. [updated 2018 Nov 14]. Available from: https://www.canada.ca/en/public-health/services/publications/science-research-data/key-health-inequalities-canada-national-portrait-executive-summary.html. [Google Scholar]


