Abstract
Enzyme-linked immunosorbent assay (ELISA) and micro-ELISA were evaluated for their ability to detect anti-Fasciola hepatica antibodies in humans by using excretory-secretory antigen. The sensitivity of each method was 100%, but the specificity was 100% for ELISA and 97% for micro-ELISA. The micro-ELISA could be used as a screening assay and ELISA could be used as a confirmatory method for the serodiagnosis of human fascioliasis.
Fascioliasis is a chronic helminthic disease predominant in domestic ruminants 13, 22, 26. It is now emerging as an important chronic disease of humans 1, 2, 5, 15, 17.
Diagnosis of Fasciola hepatica infection has traditionally relied on detecting the presence of eggs in fecal samples, but this method is unreliable and complicated 7, 18. At present, the routine diagnosis of human fascioliasis is based on the detection of antifluke antibodies in serum. Methods such as immunoelectrophoresis 6 and counterimmunoelectrophoresis 14, although they are very specific, have limited sensitivity. The diagnosis was improved by the development of enzyme-linked immunosorbent assay (ELISA), using crude extracts 17, excretory-secretory products 10, 23, and purified or recombinant molecules such as cathepsin L-1 21, 25 and by the detection of circulating antigens and coproantigens by sandwich ELISA 8.
We report the use of the ELISA technique and its modification as micro-ELISA for the serodiagnosis of human fascioliasis employing excretory-secretory products from adult F. hepatica.
Flukes were obtained from naturally infected bovine livers and treated following the technique described by Rivera Marrero et al. 23. Briefly, the flukes were washed three times with 0.01 M phosphate-buffered saline (PBS) (pH 7.2) and incubated at 37°C for 3 h in PBS containing 0.8 mM phenylmethylsulfonyl fluoride, 400 U of aprotinin per ml, and 0.1 mM dithiothreitol (one worm/5 ml). The suspension containing the excretory-secretory antigen of F. hepatica (FhESA) was centrifuged at 4°C (13,000 × g) for 2 h. Protein concentration was measured by the Bradford method 4.
One hundred negative sera were obtained from individuals living in areas where the worm is not known to exist and who did not recall any possible contact. Human positive sera (n = 22) were obtained from people diagnosed with F. hepatica infections identified by coprological analysis, surgical observation, or retrograde cholangiopancreatography. Two hundred nineteen sera from patients with other parasitic and nonparasitic infections were included: 20 with Toxoplasma gondii, 6 with Trypanosoma cruzi, 4 with Leishmania spp., 2 with Plasmodium vivax, 9 with cysticercosis, 10 with hydatid disease, 21 with trichinosis, 11 with toxocariasis, 2 with Schistosoma mansoni, 6 with Ascaris lumbricoides, 2 with Ancylostoma duodenale, 3 with Enterobius vermicularis, 3 with Strongyloides stercoralis, 1 with Trichuris trichiura, 4 with Taenia spp., 2 with Entamoeba histolytica, 5 with Giardia lamblia, 11 with syphilis, 9 with tuberculosis, 6 with anti-hepatitis A virus immunoglobulin M (IgM), 4 with IgG antibodies against hepatitis A virus, 50 with hepatitis B (positive for surface antigen), and 28 with hepatitis C.
The ELISA for the detection of antibodies to FhESA was performed essentially according to the protocol of Espino Hernandez et al. 11. FhESA was employed at a concentration of 40 μg/ml. Human sera were used at a 1:800 dilution, and peroxidase-conjugated anti-human IgG was used at 1:3,000. The substrate was 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (Sigma, St. Louis, Mo.). Plates were read on a Dynatech MR4000 plate reader at an absorbance of 410 nm.
The micro-ELISA was carried out by modification of the procedure previously described by Rosenzvit et al. 24. Glass slides covered by polystyrene (Cel-Line Associates, Inc., Newfield, N.J.) were used for this assay. Ten microliters of FhESA (20 ng/μl) was placed in each well. Slides were allowed to air dry, and then they were blocked with 10 μl of 0.01 M PBS (pH 7.2) containing 1.5% nonfat milk (PBS-M) per well and incubated for 40 min. Slides were washed twice (5 min each) with PBS and air dried. They were stored at 4°C until required, up to 1 year. Sera were diluted 1:600 in PBS-M, and 10-μl samples were placed in the wells. Then they were incubated for 20 min, rinsed in PBS, washed twice in PBS for 5 min each, and air dried. Immunoglobulin anti-human IgG peroxidase conjugate was diluted in PBS-M and 10 μl was spotted in each well. Incubation and washing steps were repeated and a final rinse in distilled water was added. Slides were air dried, and 5 μl of a substrate solution of ortho-phenylenediamine was placed in each well. Absorbance at 495 nm was read on a Metrolab 970 microplate reader. Absorbance measurement was carried out only for positive and negative control sera. For samples corresponding to other parasitic and nonparasitic infections, the reaction was visually evaluated.
The data collected were compared by regression, correlation, and analysis of variance. P values lower than 0.05 were considered significant.
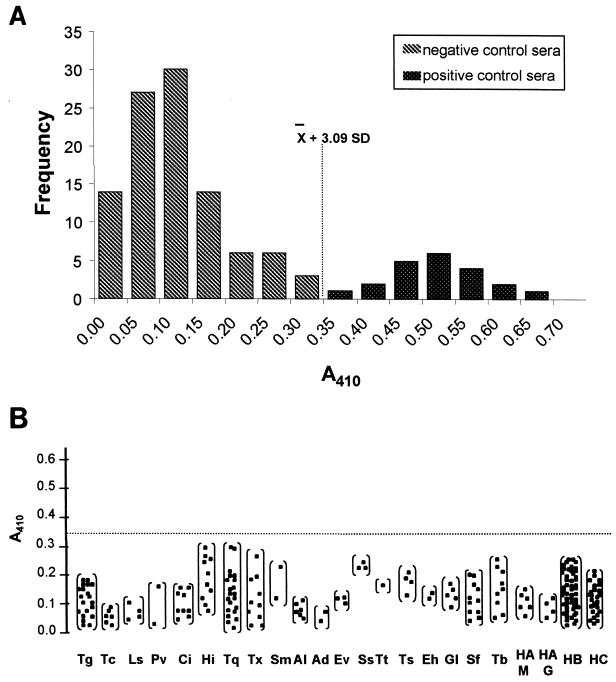
ELISA results from patients with and without evidence of fascioliasis were examined by plotting the frequency of absorbance measurement as a histogram (Fig. 1A). The mean A410 of the negative control group was 0.12, with a standard deviation (SD) of 0.08 and a range between 0.01 and 0.32. Sera from patients with fascioliasis showed a range of A410 values between 0.38 and 0.79, with a mean value of 0.52 (SD, 0.08). The cutoff point was 0.35 (Fig. 1). Sera from patients with other diseases (Fig. 1B) showed a range of A410 values between 0.02 and 0.29, with a mean value of 0.10 (SD, 0.07). The mean was not significantly different from that obtained for the negative control group (P > 0.05). All samples showed absorbance values lower than the calculated cutoff point, avoiding false-positive determinations. The sensitivity and the specificity for this assay were 100%.
FIG. 1.
ELISA absorbances of serum samples using FhESA. (A) Analysis of sera obtained from 100 negative control patients and 22 individuals parasitologically positive for F. hepatica infection. The y axis shows the frequency of absorbance measurements. The vertical dashed line represents the cutoff point, which was calculated as 3.09 SDs from the mean of the seronegative group. (B) Specificity of ELISA using sera from groups of patients with proven infections with Toxoplasma gondii (Tg), Trypanosoma cruzi (Tc), Leishmania spp. (Ls), Plasmodium vivax (Pv), cysticercosis (Ci), hydatid disease (Hi), trichinosis (Tq), toxocariasis (Tx), Schistosoma mansoni (Sm), Ascaris lumbricoides (Al), Ancylostoma duodenale (Ad), Enterobius vermicularis (Ev), Strongyloides stercoralis (Ss), Trichuris trichiura (Tt), Taenia spp. (Ts), Entamoeba histolytica (Eh), Giardia lamblia (Gl), syphilis (Sp), tuberculosis (Tb), anti-hepatitis A virus IgM (HAM), IgG antibodies against hepatitis A virus (HAG), hepatitis B (positive for surface antigen) (HB), and hepatitis C (HC). The dashed horizontal line represents the cutoff point. The individuals are indicated by closed squares.
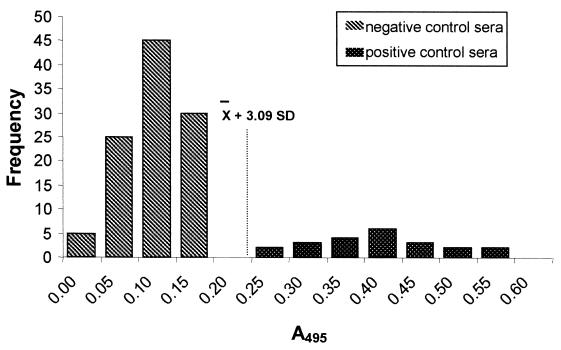
For the micro-ELISA we performed two steps of analysis: in the first, we considered only the positive and negative control groups and determined absorbance values, and in the second, we included the group of patients with other infections and evaluated the results by visual observation. In the first step, analysis was carried out in the same fashion as for the ELISA test (Fig. 2). Negative control sera showed a range of A492 values between 0.09 and 0.19, with a mean value of 0.13 (SD, 0.04). The mean A492 of sera with fascioliasis was 0.41 (SD, 0.09), with a range between 0.28 and 0.59. The calculated cutoff point was 0.25 (Fig. 2), and no false-negative results were observed. When positive sera for other infections were visually analyzed to determine cross-reactivities, 12 of 50 (25%) of the samples positive for hepatitis B and 5 of 28 (18%) of the samples positive for hepatitis C were seropositive by this test. These results give a sensitivity of 100% and a specificity of 97% for the micro-ELISA.
FIG. 2.
Histogram showing the analysis of positive and negative sera by micro-ELISA with FhESA. The dashed line represents the calculated cutoff point.
Regression and correlation analysis of the data for positive and negative control individuals showed that there was a highly linear relationship between ELISA and micro-ELISA (r2 = 0.971, P < 0.05).
In previous studies, ELISA was shown to be a useful tool for diagnosing human fascioliasis 3, 7, 9, 12, 15, 16, 19, 20, 27, and it was demonstrated that antibody levels to FhESA remain uniformly high in all infected patients during prepatent and patent phases 8. In this study we evaluated two methods, ELISA (with only a few modifications made to the previously reported assays) and micro-ELISA, for the immunodiagnosis of human fascioliasis.
Both methods identified all patients that actually shed eggs or contained worms in the bile duct. No false-negative sera were encountered. ELISA was more specific than micro-ELISA, as the latter method scored 3% of false-positive samples corresponding to hepatitis B or C but not hepatitis A. This difference in cross-reactivity between the two methods is difficult to explain. There are no reports that evaluate immunoenzymatic assays for F. hepatica using sera positive for hepatitis.
We should consider the application of these methods under specific conditions. It is reasonable to assume that the micro-ELISA could be applied as a screening test when a large number of samples are involved, because of its low consumption of reagents (especially antigens and second-antibody conjugates). The conventional ELISA could be employed as a confirmatory test following micro-ELISA analysis.
Acknowledgments
We are grateful to Carlos Carmona and Ana Acuña, Instituto de Higiene, Montevideo, Uruguay, for kindly providing antigen for comparison purposes as well as serum samples. We acknowledge Jorge González, from the Departamento de Virología, I.N.E.I., ANLIS, for providing hepatitis serum samples. We appreciate the collaboration of Alvaro Islas, Dante Loayza, and Eduardo Silva.
REFERENCES
- 1.Apt W, Aguilera X, Vega F, Zulantay I, Retamal C, Apt P, Sandoval J. Human fascioliasis in rural areas of Central Chile. Rev Med Chile. 1992;120:621–626. [PubMed] [Google Scholar]
- 2.Arjona R, Riancho J, Aguado J, Salesa R, Gonzalez-Macias J. Fascioliasis in developed countries: a review of classic and aberrant forms of the disease. Medicine. 1995;74:13–23. doi: 10.1097/00005792-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bjorland J, Byran R T, Strauss W, Hillyer G V, McAuley J B. An outbreak of acute fascioliasis among Aymara Indians in the Bolivian Altiplano. Clin Infect Dis. 1995;21:1228–1233. doi: 10.1093/clinids/21.5.1228. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Bryan R T, Michelson M K. Parasitic infections of the liver and biliary tree. In: Surawicz C, Owen R L, editors. Gastrointestinal and hepatic infections. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 405–454. [Google Scholar]
- 6.Capron A, Biguet J, Tran Vansky P, Rose G. Possibilities nouvelles dans le diagnostic immunologique de la distomatose humaine a ‘Fasciola hepatica’. Mise en evidence d'anticorps seriques par immunoelectrophorèse. Presse Med. 1964;72:3103–3107. [PubMed] [Google Scholar]
- 7.Chen M G, Mott K E. Progress in morbidity due to Fasciola hepatica infection. Trop Dis Bull. 1990;87:1–37. [Google Scholar]
- 8.Espino A M, Díaz A, Pérez A, Finlay C M. Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. J Clin Microbiol. 1998;36:2723–2726. doi: 10.1128/jcm.36.9.2723-2726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espino A M, Finlay C M. Sandwich enzyme-linked immunosorbent assay for detection of excretory secretory antigens in humans with fascioliasis. J Clin Microbiol. 1994;32:190–193. doi: 10.1128/jcm.32.1.190-193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espino A M, Dumenigo B E, Fernandez R, Finlay C M. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory-secretory products. Am J Trop Med Hyg. 1990;37:605–608. doi: 10.4269/ajtmh.1987.37.605. [DOI] [PubMed] [Google Scholar]
- 11.Espino Hernandez A M, Seuret N, Morlote C, Dumenigo Ripoll B. Antígenos de Fasciola hepatica. Su utilidad en el diagnóstico de la fascioliasis humana. Rev Cuba Med Trop. 1991;43:151–155. [PubMed] [Google Scholar]
- 12.Esteban J G, Flores A, Aguirre C, Strauss W, Angles R, Mas Coma S. Presence of very high prevalence and intensity of infection with Fasciola hepatica among Aymara children from the northern Bolivian Altiplano. Acta Trop. 1997;66:1–14. doi: 10.1016/s0001-706x(97)00669-4. [DOI] [PubMed] [Google Scholar]
- 13.Froyd G. Liver flukes in Great Britain: a survey of affected livers. Vet Rec. 1975;97:492–495. [PubMed] [Google Scholar]
- 14.Hillyer G V, Capron A. Immunodiagnosis of human fascioliasis by counterimmunoelectrophoresis. J Parasitol. 1976;62:1011–1013. [PubMed] [Google Scholar]
- 15.Hillyer G V, Soler de Galanes M, Rodriguez-Perez J, Bjorland J, De Lagrava M S, Ramirez Guzman S, Bryan R T. Use of the Falcon assay™ screening test-enzyme linked immunosorbent assay (FAST-ELISA) and the enzyme-linked immunoelectrotransfer blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am J Trop Med Hyg. 1992;46:603–609. doi: 10.4269/ajtmh.1992.46.603. [DOI] [PubMed] [Google Scholar]
- 16.Knobloch J. Human fascioliasis in Cajamarca/Peru. II. Humoral antibody response and antigenaemia. Trop Med Parasitol. 1985;36:91–93. [PubMed] [Google Scholar]
- 17.Knobloch J, Delgado A E, Alvarez G A, Reymann U, Bialek R. Human fascioliasis in Cajamarca/Perú. I. Diagnostic methods and treatment with praziquantel. Trop Med Parasitol. 1985;36:88–90. [PubMed] [Google Scholar]
- 18.Levine D M, Hillyer G V, Flores S I. Comparison of counterelectrophoresis, the enzyme-linked immunosorbent assay and Kato faecal examination for the diagnosis of fascioliasis in infected mice and rabbits. Am J Trop Med Hyg. 1980;29:602–608. doi: 10.4269/ajtmh.1980.29.602. [DOI] [PubMed] [Google Scholar]
- 19.Massoud J. Fascioliasis outbreak in man and drug test (triclabendazole) in Caspian littoral, northern part of Iran, 1989. Bull Soc Fr Parasitol. 1990;8:438. [Google Scholar]
- 20.O'Neill S M, Parkinson M, Dowd A J, Strauss W, Angles R, Dalton J P. Short report: immunodiagnosis of human fascioliasis using recombinant Fasciola hepatica cathepsin L1 cysteine proteinase. Am J Trop Med Hyg. 1999;60:749–751. doi: 10.4269/ajtmh.1999.60.749. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill S M, Parkinson M, Strauss W, Angles R, Dalton J P. Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Am J Trop Med Hyg. 1998;58:417–423. doi: 10.4269/ajtmh.1998.58.417. [DOI] [PubMed] [Google Scholar]
- 22.Rimbault C. Une épidémie de distomatose dans une communauté rurale de Haute-Loire. Ph.D. thesis. Clermont-Ferrand, France: Faculté de Médecine, Université de Clermont-Ferrand I; 1991. [Google Scholar]
- 23.Rivera Marrero C A, Santiago N, Hillyer G V. Evaluation of immunodiagnostic antigens in the excretory-secretory products of Fasciola hepatica. J Parasitol. 1988;74:646–652. [PubMed] [Google Scholar]
- 24.Rosenzvit M, Angel S, Alvarez L, Mellare V, Blanco J, Pszenny V, Garberi J C. A simple and economic slide micro-immunoenzymatic (Micro-SIA) test for epidemiological studies of toxoplasmosis. Mem Inst Oswaldo Cruz. 1994;89:47–51. doi: 10.1590/s0074-02761994000100009. [DOI] [PubMed] [Google Scholar]
- 25.Santiago de Weil N, Hillyer G V, Pacheco E. Isolation of Fasciola hepatica genus-specific antigens. Int J Parasitol. 1984;14:197–206. doi: 10.1016/0020-7519(84)90049-3. [DOI] [PubMed] [Google Scholar]
- 26.Sexton J L, Miner A R, Campbell N J. Fasciola hepatica: immunoprecipitation analysis of biosynthetically labelled antigens using sera from infected sheep. Parasite Immunol. 1991;13:105–108. doi: 10.1111/j.1365-3024.1991.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 27.Silva Sampaio M L, Correia da Costa J M, Viana da Costa A M, Pires M A, Lopes S A, Castro A M, Monjour L. Antigenic components of excretory-secretory products of adult Fasciola hepatica recognized in human infections. Am J Trop Med Hyg. 1996;54:146–148. doi: 10.4269/ajtmh.1996.54.146. [DOI] [PubMed] [Google Scholar]