ABSTRACT
Indole signaling is viewed as a potential target for antivirulence therapy against antibiotic-resistant pathogens because of its link with the production of virulence factors. This study examined the antimicrobial and antivirulence properties of 44 indoles toward Vibrio campbellii. Based on the results, 17 halogenated indole analogues were selected, as they significantly improved the survival of brine shrimp larvae challenged with V. campbellii. Specifically, 6-bromoindole, 7-bromoindole, 4-fluoroindole, 5-iodoindole, and 7-iodoindole showed a high protective effect, improving the survival of brine shrimp to over 80% even at a low concentration of 10 μM. To explore the impact of selected indole analogues on bacterial virulence phenotypes, swimming motility, biofilm formation, protease activity, and hemolytic activity of V. campbellii were determined. The results showed that all of the 17 selected indole analogues decreased swimming motility at both 10 μM and 100 μM. Most of the indole analogues decreased biofilm formation at a concentration of 100 μM. In contrast, only a slightly decreased protease activity and no effect on hemolytic activity were observed at both concentrations. To our knowledge, this is the first study of the structure-activity relation of halogenated indole analogues with respect to virulence inhibition of a pathogenic bacterium in an in vivo host model system, and the results demonstrate the potential of these compounds in applications aiming at the protection of shrimp from vibriosis, a major disease in aquaculture.
IMPORTANCE Bacterial diseases are a major problem in the aquaculture industry. In order to counter this problem, farmers have been using antibiotics, and this has led to the evolution and spread of antibiotic resistance. In order for the aquaculture industry to further grow in a sustainable way, novel and sustainable methods to control diseases are needed. We previously reported that indole signaling is a valid target for the development of novel therapies to control disease caused by Vibrio campbellii and related bacteria, which are among the major bacterial pathogens in aquaculture. In the present study, we identified indole analogues that are more potent in protecting brine shrimp (a model organism for shrimp) from V. campbellii. To our knowledge, this is the first study of the structure-activity relation of halogenated indole analogues with respect to virulence inhibition of a pathogenic bacterium in an in vivo host model system.
KEYWORDS: antivirulence therapy, indole signaling, quorum sensing, shrimp, virulence
INTRODUCTION
Shrimp and prawns have historically been one of the most heavily traded fish products, and shrimp farming is one of the major parts of the aquaculture sector (1–3). However, bacterial diseases, such as vibriosis caused by various vibrios, are limiting the further sustainable expansion of aquaculture, especially in the early life stages of the animals (4–6). Vibrios belonging to the Harveyi clade are causing severe losses in shrimp farming, with up to 100% mortality in postlarvae and juveniles (7–9). Vibrio harveyi and Vibrio campbellii are closely related species belonging to the Harveyi clade. Both of them are important pathogens causing disease in wild and cultured aquatic organisms, including shrimp, fish, and molluscs, and this is leading to huge losses in the aquaculture industry worldwide (9, 10). In this situation, antibiotics often are the only effective agents that farmers have to protect their animals from bacterial infections. However, the wide and frequent use of antibiotics has resulted in the emergence of multidrug-resistant strains and has led to an international health crisis of both aquatic animals and humans (11, 12). Therefore, there is an urgent need for a truly novel strategy instead of antibiotics to control bacterial diseases in aquaculture (13). Antivirulence therapy is one of the newly developed therapeutic strategies; it aims at disarming pathogens by inhibiting their virulence rather than killing them. This strategy has been deemed to be a promising alternative strategy, as bacteria will probably have a lower tendency to develop resistance against it (14, 15).
Indole signaling is viewed as a potential target for antivirulence therapy against antibiotic-resistant pathogens because of its ability to inhibit other quorum-sensing (QS) systems and the production of virulence factors. Indole and indole analogues are widespread in the natural environment, as a variety of bacteria and some plants produce large quantities of indole, which can be structurally modified by some non-indole-producing prokaryotes and eukaryotes (16–18). Indole is an interspecies and intercellular signal; it attends diverse aspects of bacterial physiology, such as spore formation (19), plasmid stability (20), drug resistance (21, 22), biofilm formation (16, 23), and virulence (24, 25). Furthermore, indole and its aromatic heterocyclic structure are popularly used as synthetic starting points in the pharmaceutical industry (26), and some indole-based drugs are currently in clinical trials (27, 28). Recently, several indole analogues have been reported to reduce bacterial virulence. For example, 4-iodoindole, 7-iodoindole, 4-chloroindole, and 7-chloroindole were found to effectively inhibit biofilm formation, bacterial motility, fimbrial activity, hydrophobicity, protease activity, and indole production in Vibrio parahaemolyticus (29). Furthermore, 7-fluoroindole (30), 7-hydroxyindole (31), and 3-indolylacetonitrile (32) reduced the production of virulence factors in Pseudomonas aeruginosa. Finally, 5-fluoroindole, 6-fluoroindole, 5-methylindole, and 7-methylindole were proven to inhibit quorum sensing-controlled violacein pigment production in Chromobacterium violaceum CV026 and suppressed prodigiosin production, biofilm formation, swimming motility, and swarming motility in Serratia marcescens (33). In addition, halogenated indoles have been reported to have nematicidal and insecticidal potential (34, 35). Although the antivirulence activity of indole in V. campbellii has been reported in our previous work (23, 36), the concentration at which the best virulence-inhibitory activity occurred was also toxic to invertebrates.
Therefore, the objectives of this study were to identify more potent indole analogues against V. campbellii, and to understand their structure-activity relationship. Initially, 44 indole analogues (Fig. 1) were investigated in terms of their abilities to protect brine shrimp larvae from infection by V. campbellii. Based on this test, 17 indoles were selected for further experiments aiming at testing their dose-response relations with respect to protecting brine shrimp larvae from vibriosis at lower concentrations and at verifying their impact on swimming motility, biofilm formation, protease activity, and hemolytic activity, which are major virulence factors of V. campbellii.
FIG 1.
Structure of the indole analogues used in this study.
RESULTS AND DISCUSSION
Impact of the indole analogues on the growth of V. campbellii.
First, we determined the impact of the indole analogues on the growth of V. campbellii, as we aimed for analogues that affect the virulence but not the viability of the pathogen. Results showed that most of the indole analogues at a concentration of 200 μM did not affect bacterial growth, except for the isatins 40, 41, 42, 43, and 44 (see Fig. S1 in the supplemental material). Therefore, these compounds were excluded for further experiments.
Impact of the indole analogues on the virulence of V. campbellii toward gnotobiotic brine shrimp (Artemia franciscana) larvae.
Based on the results of bacterial growth, indole analogues 1 to 39, which did not affect bacterial growth, were selected to explore the impact on the virulence of V. campbellii toward brine shrimp larvae. We added the indoles directly into the brine shrimp rearing water at a concentration of 20 μM together with V. campbellii. After 2 days of culture, an increased survival of brine shrimp larvae indicated the protective effect of several indole analogues. Results showed that 10 out of 13 brominated indoles, 4 out of 7 fluorinated indoles, 3 out of 3 iodinated indoles, 5 out of 6 chlorinated indoles, 1 out of 3 multiple halogenated indoles, 2 out of 3 aminoindoles, and 3 out of 5 nitroindoles improved the survival of challenged brine shrimp larvae (Table 1). Among these, 17 indole analogues increased the survival rate of challenged brine shrimp larvae to over 60%, including 7 brominated indoles, 2 fluorinated indoles, 3 iodinated indoles, 4 chlorinated indoles, and 1 multiple halogenated indole. Importantly, the protection offered by the compounds was obtained at a concentration (20 μM) that was well below the concentration that could inhibit the growth of V. campbellii, as there was no impact on growth up to 200 μM (Fig. S1). Hence, the MIC of the compounds is above 200 μM, whereas the virulence inhibitory concentration is 20 μM or lower.
TABLE 1.
Percent survival of brine shrimp (Artemia franciscana) larvae after 2 days of challenge with V. campbellii and 20 μM indole analoguesa
| Experiment and treatment | Survival (%) | P value summaryb | Live cell density (×105 CFU/mL) |
|---|---|---|---|
| Expt 1 | |||
| Unchallenged shrimp larvae | 87 ± 6 | 0 | |
| V. campbellii | 2 ± 2 | 238 ± 61 | |
| V. campbellii + 4-bromoindole | 79 ± 12 | *** | 203 ± 69 |
| V. campbellii + 5-bromoindole | 73 ± 7 | *** | 230 ± 85 |
| V. campbellii + 6-bromoindole | 63 ± 17 | *** | 163 ± 46 |
| V. campbellii + 7-bromoindole | 82 ± 12 | *** | 197 ± 45 |
| V. campbellii + 5-bromoindole-3-carboxaldehyde | 50 ± 0 | *** | 207 ± 56 |
| V. campbellii + 5-bromoindole-2-carboxylic acid | 2 ± 2 | 213 ± 63 | |
| V. campbellii + 7-acetyl-5-bromoindole | 10 ± 7 | 158 ± 55 | |
| V. campbellii + 7-amino-5-bromoindole | 69 ± 7 | *** | 222 ± 102 |
| V. campbellii + 5-bromo-1-methylindole | 20 ± 9 | * | 212 ± 31 |
| V. campbellii + 6-bromoindole-3-carboxaldehyde | 88 ± 10 | *** | 178 ± 12 |
| V. campbellii + 6-bromoindole-2-carboxylic acid | 8 ± 7 | 178 ± 42 | |
| V. campbellii + 7-bromoindole-2-carboxylic acid | 19 ± 4 | * | 188 ± 60 |
| V. campbellii + 7-bromoindole-3-carboxaldehyde | 86 ± 14 | *** | 165 ± 49 |
| Expt 2 | |||
| Unchallenged shrimp larvae | 100 ± 0 | 0 | |
| V. campbellii | 6 ± 5 | 228 ± 38 | |
| V. campbellii + 4-fluoroindole | 96 ± 2 | *** | 180 ± 41 |
| V. campbellii + 5-fluoroindole | 31 ± 2 | *** | 185 ± 62 |
| V. campbellii + 6-fluoroindole | 53 ± 12 | *** | 162 ± 60 |
| V. campbellii + 7-fluoroindole | 82 ± 7 | *** | 160 ± 64 |
| V. campbellii + 5-fluoroindole-2-carboxylic acid | 4 ± 2 | 172 ± 29 | |
| V. campbellii + 6-fluoroindole-3-acetic acid | 7 ± 3 | 187 ± 58 | |
| V. campbellii + 5-iodoindole | 73 ± 12 | *** | 182 ± 10 |
| V. campbellii + 7-iodoindole | 68 ± 11 | *** | 187 ± 50 |
| V. campbellii + 7-iodoindole-3-carboxaldehyde | 81 ± 4 | *** | 197 ± 13 |
| V. campbellii + 4-chloroindole | 90 ± 0 | *** | 203 ± 20 |
| V. campbellii + 5-chloroindole | 82 ± 8 | *** | 180 ± 68 |
| V. campbellii + 6-chloroindole | 68 ± 15 | *** | 170 ± 65 |
| Expt 3 | |||
| Unchallenged shrimp larvae | 96 ± 2 | 0 | |
| V. campbellii | 0 ± 0 | 243 ± 50 | |
| V. campbellii + 7-chloroindole | 82 ± 5 | *** | 235 ± 46 |
| V. campbellii + 6-chloroindole-3-carboxaldehyde | 41 ± 28 | *** | 215 ± 36 |
| V. campbellii + 5-chloroindole-2-carboxylic acid | 6 ± 5 | 190 ± 13 | |
| V. campbellii + 6-chloro-5-fluoroindole | 83 ± 7 | *** | 172 ± 35 |
| V. campbellii + 7-bromo-5-fluoroindole | 17 ± 3 | 235 ± 46 | |
| V. campbellii + 7-iodo-5-chloroindole | 4 ± 2 | 222 ± 28 | |
| V. campbellii + 4-aminoindole | 32 ± 8 | ** | 262 ± 21 |
| V. campbellii + 5-aminoindole | 18 ± 5 | 148 ± 15 | |
| V. campbellii + 6-aminoindole | 21 + 17 | * | 320 ± 84 |
| V. campbellii + 5-nitroindole | 12 ± 10 | 178 ± 62t | |
| V. campbellii + 7-nitroindole | 47 ± 30 | *** | 248 ± 31 |
| V. campbellii + 4-nitroindole-3-carboxaldehyde | 11 ± 4 | 242 ± 40 | |
| V. campbellii + 2-methyl-5-nitroindole | 42 ± 15 | *** | 205 ± 35 |
| V. campbellii + 2,3-dimethyl-5-nitroindole | 50 ± 10 | *** | 213 ± 28 |
Average ± standard deviation of three shrimp cultures. Density of V. campbellii with or without 20 μM indole analogues in the brine shrimp larvae-rearing water after 2 days of challenge.
*, Significantly different from treatment with unchallenged shrimp larvae (P ≤ 0.05); **, significantly different from treatment with unchallenged shrimp larvae (P ≤ 0.01); ***, significantly different from treatment with unchallenged shrimp larvae (P ≤ 0.001).
Although some halogenated indole analogues have been shown to affect quorum sensing and virulence factor production in various kinds of pathogens (Table 2), none of them have been tested in vivo in animals challenged with a pathogen of interest before. In the present study, we found that within the brominated, fluorinated, and chlorinated indoles, in general, the 4- and 7-substituted compounds showed the highest activity, increasing the survival of challenged brine shrimp larvae to over 80%. The only exception was 5-chloroindole, which also increased the survival of challenged brine shrimp larvae to over 80%. None of the iodinated indoles was able to increase the survival of challenged brine shrimp larvae to this level. Further, 2 out of the 3 multiple halogenated indoles did not improve the survival of challenged brine shrimp larvae, with the exception of 5-fluoro,6-chloroindole, which also increased the survival of challenged brine shrimp larvae to over 80%. Most of the halogenated indoles with another second substitution were not able to increase the survival of challenged brine shrimp larvae to over 80%, with the only three exceptions being those with a 3-carboxaldehyde substitution (i.e., analogues 10, 13, and 22). Finally, aminoindoles and nitroindoles did not protect brine shrimp from V. campbellii at the concentration that was tested.
TABLE 2.
Published data of halogenated indole analogues and their impact on pathogens
| Name | Structure | Phenotypic changes affected by indoles | Target pathogen | Reference |
|---|---|---|---|---|
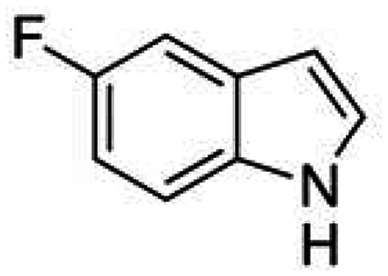
| 4-Fluoroindole |

|
Inhibited biofilm formation | Candida albicans | 47 |
| 5-Fluoroindole |
|
Inhibited QS of Chromobacterium violaceum CV026; inhibited prodigiosin production, biofilm formation, swimming motility, and swarming motility | Serratia marcescens | 33 |
| 6-Fluoroindole |

|
Inhibited QS of Chromobacterium violaceum CV026; inhibited prodigiosin production, biofilm formation, swimming motility, swarming motility, protease activity, and lipase activity | Serratia marcescens | 33 |
| 7-Fluoroindole |

|
Inhibited biofilm formation, blood hemolysis of Pseudomonas aeruginosa; inhibited QS of Chromobacterium violaceum CV026; inhibited prodigiosin production, biofilm formation, swimming motility, and swarming motility of Serratia marcescens | Pseudomonas aeruginosa, Serratia marcescens | 30, 33 |
| 4-Iodoindole |

|
Inhibited biofilm formation, bacterial motility, fimbrial activity, hydrophobicity, protease activity, and indole production | Vibrio parahaemolyticus | 29 |
| 5-Iodoindole |

|
Inhibited biofilm formation of Candida albicans; inhibited prodigiosin production, biofilm formation, swimming motility, and swarming motility of Serratia marcescens; inhibited swimming motility, biofilm formation of Acinetobacter baumannii | Candida albicans, Serratia marcescens, Acinetobacter baumannii | 47, 33, 50 |
| 6-Iodoindole |

|
Inhibited biofilm formation, swimming motility, protease activity | Agrobacterium tumefaciens | 48 |
| 7-Iodoindole |

|
Inhibited biofilm formation, bacterial motility, fimbrial activity, hydrophobicity, protease activity, and indole production | Vibrio parahaemolyticus | 29 |
| 4-Chloroindole |

|
Inhibited biofilm formation, bacterial motility, fimbrial activity, hydrophobicity, protease activity, and indole production of V. parahaemolyticus; inhibited biofilm formation, swimming motility, protease activity of Agrobacterium tumefaciens; inhibited swarming and swimming motility and downregulated the expressions of virulence genes of Escherichia coli | Vibrio parahaemolyticus, Agrobacterium tumefaciens, Escherichia coli | 29, 48, 49 |
| 5-Chloroindole |

|
Inhibited swarming and swimming motility and downregulated the expressions of virulence genes of Escherichia coli | Escherichia coli | 49 |
| 7-Chloroindole |

|
Inhibited biofilm formation, bacterial motility, fimbrial activity, hydrophobicity, protease activity, and indole production | Vibrio parahaemolyticus | 29 |
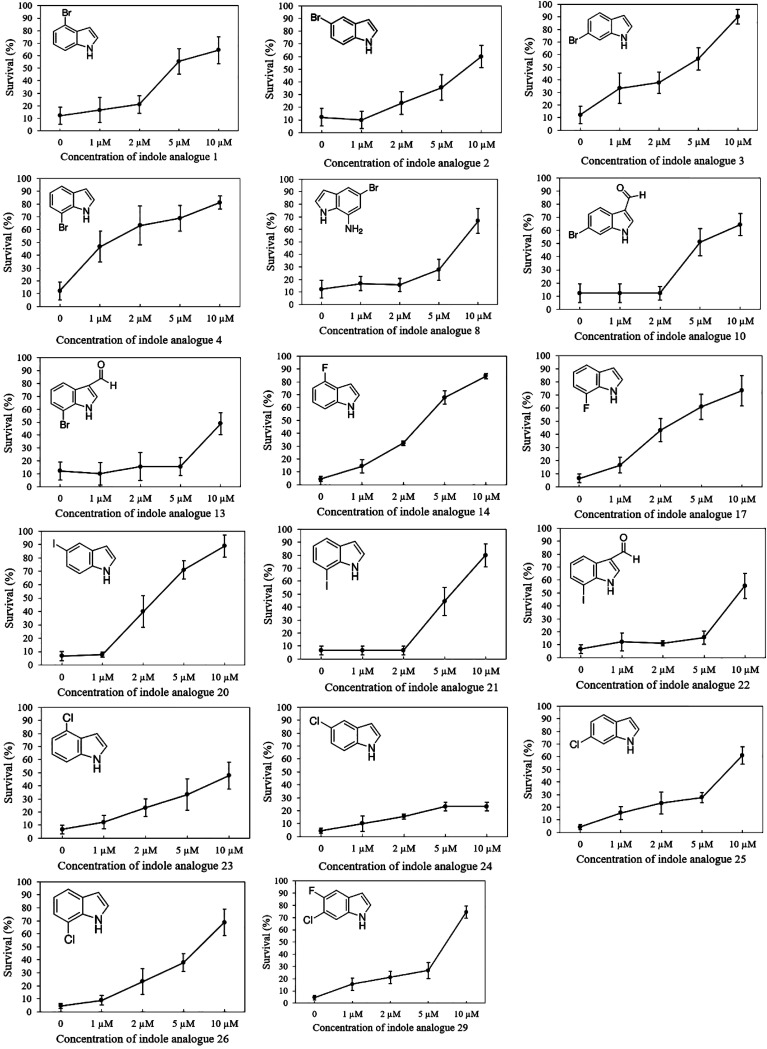
Seventeen indole analogues (i.e., indole analogues 1, 2, 3, 4, 8, 10, 13, 14, 17, 20, 21, 22, 23, 24, 25, 26, and 29, which increased the survival of challenged brine shrimp larvae to over 60% at 20 μM) were selected for more detailed experiments. The selected indole analogues were added into the brine shrimp rearing water at concentrations of 1, 2, 5, and 10 μM, respectively. After 2 days of culture, the survival of the brine shrimp larvae was found to be proportional to the concentration of the indole analogues (Fig. 2). Most of the selected indoles were able to increase the survival of challenged brine shrimp larvae to over 60% when added at 10 μM, except for analogues 13, 22, 23, and 24. The most active compounds increasing the survival of challenged brine shrimp to over 60% were 7-bromoindole (at 2 μM or more), 4-fluoroindole (5 μM), 7-fluoroindole (5 μM), and 5-iodoindole (5 μM). Five of the indoles were able to increase the survival of challenged brine shrimp larvae to over 80% (all at 10 μM) as follows: 6-bromoindole, 7-bromoindole, 4-fluoroindole, 5-iodoindole, and 7-iodoindole. The results of this second batch of brine shrimp challenge tests indicated that, in general, all of the compounds with a halogen (bromo, fluoro, iodo, or chloro) at position 7 are highly active in protecting brine shrimp larvae against V. campbellii. Several 7-substituted halogenated indole analogues, such as 7-fluoroindole, 7-iodoindole, and 7-chloroindole, have been reported before to affect bacterial virulence factors in vitro at higher concentrations than the active concentration found in this study. More specifically, 7-fluoroindole inhibited biofilm formation at a concentration of 500 μM and blood hemolysis at a concentration of 100 μM in Pseudomonas aeruginosa (30); 7-iodoindole and 7-chloroindole inhibited biofilm formation at a concentration of 10 μg/mL (about 40 to 60 μM), bacterial motility at a concentration of 50 μg/mL (about 200 to 300 μM), and protease activity at a concentration of 10 μg/mL in V. parahaemolyticus (29).
FIG 2.
Percent survival of brine shrimp (Artemia franciscana) larvae after 2 days of challenge with Vibrio campbellii BB120 and different concentrations of selected indole analogues (average ± standard deviation of three shrimp cultures). The indole analogues were added to the brine shrimp rearing water at the start of the experiment. Error bars represent the standard deviation of three shrimp cultures. The survival of unchallenged larvae that were otherwise treated in the same way as challenged larvae was 98% ± 3%.
Impact of selected indole analogues on swimming motility, biofilm formation, protease activity, and hemolytic activity of V. campbellii.
Since the 17 selected indole analogues showed protective effect on brine shrimp larvae, further experiments were set up to explore how these indole analogues improved the survival by affecting the production of virulence factors of the pathogen, including swimming motility, biofilm formation, protease activity, and hemolytic activity. The results showed that all of the selected indole analogues decreased swimming motility of V. campbellii at both 10 μM and 100 μM (Fig. 3A). Brominated indoles (analogues 1, 2, 3, 4, 8, 10, and 13) and chlorinated indoles (analogues 23, 24, 25, and 26) showed stronger suppression of swimming motility than fluorinated, iodinated, and multiple halogenated indoles. Bacterial motility is viewed as a specific therapeutic target to cure or prevent disease, as it helps the cells colonize the host or abiotic surfaces (37, 38). Several halogenated indole analogues have also been reported before to decrease bacterial motility, including fluorinated indoles (5-fluoroindole, 6-fluoroindole, and 7-fluoroindole in Serratia marcescens), iodinated indoles (4-iodoindole and 7-iodoindole in V. parahaemolyticus and 5-iodoindole and 6-iodoindole in Acinetobacter baumannii), and chlorinated indoles (4-chloroindole and 5-chloroindole in Escherichia coli and 7-chloroindole in V. parahaemolyticus) (Table 2), which confirms that halogenated indole analogues have a significant effect on bacterial motility in different species of bacteria.
FIG 3.
Impact of 10 and 100 μM selected indole analogues on swimming motility, biofilm formation, protease, and hemolytic activity of V. campbellii BB120. Data are presented as the mean ± standard deviation (SD) of six replicates for swimming motility and three independent experiments for biofilm formation, protease, and hemolytic activity. Control refers to untreated V. campbellii. The activities in this treatment were set at 100%, and other treatments were normalized accordingly (by multiplying with the same factor). For each analogue (each time comparing control, 10 μM, and 100 μM), different letters indicate significant differences in activity, whereas treatments receiving the same letter are not significantly different from each other (one-way analysis of variance [ANOVA] with Tukey’s post hoc test; P < 0.01).
In the biofilm experiment, biofilm levels were decreased in the presence of indole analogues 1, 2, 8, 10, 13, 20, 21, 22, 23, and 26 at a concentration of 100 μM, while they were not affected at a concentration of 10 μM (Fig. 3B). Biofilm formation is important for the transmission of infections and the persistence of bacterial pathogens in hostile environments (39), and biofilm-forming Vibrio species are a major threat to the aquaculture industry (40). When in the biofilm state, bacteria are usually more resistant to antibiotics and disinfectants than planktonic bacteria (41). Therefore, it is desirable to select antimicrobial agents with the ability to decrease biofilm formation. It has been reported before that indole inhibited biofilm formation in V. campbellii, V. harveyi, and V. parahaemolyticus at a relatively high concentration of 200 μM (23), and 6-fluoroindole can inhibit bacterial biofilm formation in S. marcescens at a concentration of 250 μM (33). Interestingly, 4-fluoroindole and 7-fluoroindole were shown to decrease biofilm formation at lower concentrations of 20 μM and 100 μM in Candida albicans and S. marcescens (30, 42), whereas neither of them affected biofilm formation of V. campbellii in this study. Finally, 5-iodoindole, 7-iodoindole, 4-chloroindole, 5-chloroindole, and 7-chloroindole inhibited biofilm formation in C. albicans, V. parahaemolyticus, and E. coli at a concentration of 5 to 20 μg/mL (about 20 to 100 μM) (29, 42–44), which is consistent with the results obtained in this study.
In the protease activity and hemolytic activity experiments, the effect of selected indole analogues was smaller than in the swimming motility assay and the biofilm formation assay. Most selected indole analogues showed a small inhibitory effect on protease activity of V. campbellii at both 10 and 100 μM (except for analogues 3 and 8) (Fig. 3C). Most of the selected indole analogues did not affect the hemolytic activity of V. campbellii (Fig. 3D). As important virulence factors of pathogens, the impact of indole analogues on protease activity and hemolytic activity has also been explored before. It has been reported that 6-fluoroindole decreased protease activity of Serratia marcescens at a concentration of 500 μM (33). Further, 4-iodoindole, 7-iodoindole, 4-chloroindole, and 7-chloroindole inhibited protease activity of V. parahaemolyticus at a concentration of 10 to 100 μg/mL (about 40 to 600 μM) (29). Finally, 7-fluoroindole was shown to inhibit blood hemolysis without inhibiting the growth of Pseudomonas aeruginosa at a concentration of 100 μM (30). These results indicate that the impact of indoles on protease and hemolytic activities might be species dependent.
Conclusions.
In conclusion, the present study demonstrated the abilities of halogenated indole analogues to protect brine shrimp larvae from pathogenic V. campbellii at a low concentration (≤20 μM) without affecting bacterial growth (no impact on growth curves up to 200 μM). This is consistent with the concept of antivirulence therapy, which does not kill the pathogens but rather blocks their virulence (15). We selected 17 halogenated indole analogues that improved the survival of brine shrimp larvae to over 60% at 20 μM and found that the most active compounds were 7-bromoindole (increasing the survival of challenged brine shrimp to over 60% at 2 μM or more), 4-fluoroindole (5 μM), 7-fluoroindole (5 μM), and 5-iodoindole (5 μM). Five of the indoles were able to increase the survival of challenged brine shrimp larvae to over 80% (all at 10 μM) as follows: 6-bromoindole, 7-bromoindole, 4-fluoroindole, 5-iodoindole, and 7-iodoindole. Further, indole analogues with another second substituent were found to work less well, and aminoindoles and nitroindoles did not affect the virulence of V. campbellii. Further in vitro work showed that all of the 17 selected halogenated indoles decreased swimming motility at both 10 μM and 100 μM, and most of them decreased biofilm formation at a concentration of 100 μM, whereas only a slightly decreased protease activity and no effect on hemolytic activity were observed. The fact that the compounds protected brine shrimp larvae at lower concentrations than those needed to inhibit the tested virulence factors suggests that the indole analogues also block a yet unknown virulence factor that is important for the virulence of V. campbellii to brine shrimp. However, further research will be needed to identify this virulence factor and to further establish the mode of action of the indole analogues.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and chemicals.
Vibrio campbellii BB120 was used in this study. The bacteria were cultured in Luria-Bertani medium containing 35 g/L of sea salt (LB35) at 28°C under constant agitation (100 min−1). Cell densities were measured spectrophotometrically at 600 nm. Indole analogues were purchased from Sigma-Aldrich (Belgium) and are listed in Fig. 1. Each indole analogue was dissolved in dimethyl sulfoxide (DMSO) at 1, 2, 5, 10, 20, 100, and 200 mM, respectively. In all experiments, all treatments received the same volume of DMSO (0.1% vol/vol).
Determination of the impact of the analogues on the growth curve of V. campbellii.
To investigate the effect of the halogenated indole analogues on the growth of V. campbellii BB120, overnight grown cells were inoculated into fresh LB35 medium at an initial optical density at 600 nm (OD600) of 0.01. Indole analogues were added at 0, 20, 100, or 200 μM, respectively. Then, 200-μL aliquots of these suspensions were pipetted into the wells of a polystyrene 96-well plate and cultured at 28°C for 24 h. The OD600 of each sample was measured by a Tecan Infinite M200 Pro plate reader every hour. Growth curves were determined for three independent cultures.
Axenic hatching of brine shrimp larvae.
Five hundred milligrams of high-quality hatching cysts of Artemia franciscana (EGVR type; INVE Aquaculture, Baasrode, Belgium) were hydrated in 45 mL of filter-sterilized tap water for 1 h. Sterile cysts and larvae were obtained by decapsulation according to Marques et al. (45). In brief, 1.65 mL of NaOH (32%) and 25 mL of NaOCl (50%) were added to the hydrated cyst suspension to facilitate decapsulation. The process was stopped after 2 min by adding 35 mL of Na2S2O3 (10 g/L). Filtered (0.22 μm) aeration was provided during the reaction. The decapsulated cysts were washed with filtered and autoclaved artificial seawater (containing 35 g/L of instant ocean synthetic sea salt; Aquarium Systems, Sarrebourg, France). The cysts were resuspended in a bottle containing 1 L of filtered and autoclaved synthetic seawater and hatched for at least 28 h at 28°C with aeration and constant illumination (2,000 lx). The sterility of the cysts was verified by inoculating 1 mL of culture water into 9 mL of LB35 and incubating at 28°C for 24 h. After 28 h of hatching, batches of 30 larvae were counted and transferred into fresh, sterile 50-mL tubes containing 30 mL of filtered and autoclaved seawater. Finally, the tubes were put on a rotor (4 rotations per min) and kept at 28°C. All manipulations were performed in a laminar flow hood in order to maintain sterility of the cysts and larvae.
Brine shrimp challenge tests.
The impact of indole analogues on the virulence of V. campbellii BB120 was determined using a standardized challenge test with gnotobiotic brine shrimp larvae as described by Defoirdt et al. (46). Briefly, V. campbellii BB120 was added into the sterile 50-mL tubes containing 30 mL of filtered and autoclaved seawater and 30 brine shrimp larvae at 106 CFU/mL. The indole analogues (at the concentrations indicated in Results) were added into the brine shrimp rearing water at the start of the experiment. A suspension of autoclaved LVS3 bacteria (47) in filtered and autoclaved artificial seawater was added into all of the cultures as feed at the start of the challenge test at 107 cells/mL. Brine shrimp cultures to which only DMSO and autoclaved LVS3 bacteria were added were used as controls. For each tube, the survival of the larvae was counted 48 h after the addition of the pathogen, and this value was converted to a percentage. Each treatment was carried out in triplicate. In each test, the sterility of the control treatments was checked at the end of the challenge by inoculating 1 mL of rearing water of the control treatment (no pathogens added) to 9 mL of LB35 and incubating the mixture for 2 days at 28°C. The concentrations of V. campbellii BB120 in the brine shrimp-rearing water at the end of the experiment were determined by plate counting on LB35 agar.
Determination of swimming motility of V. campbellii.
The swimming motility assay was determined on LB35 soft agar plates containing 0.2% agar (48). Vibrio campbellii BB120 was grown overnight in LB35 medium and diluted to an OD600 of 1. The LB35 soft agar was cooled down to approximately 50°C after autoclaving. Then, the selected indole analogues were added at concentrations of 10 and 100 μM, respectively. The agar was poured into petri plates and left open at room temperature for 15 min. Five-microliter aliquots of the bacterial suspensions were added to the center of soft agar plates (6 replicate plates per treatment). The plates were incubated upright at 28°C, and the motility halo diameters were measured after 1 day.
Determination of biofilm levels of V. campbellii.
Biofilm formation was quantified by crystal violet staining as described previously (49). Briefly, an overnight culture of V. campbellii BB120 was diluted to an OD600 of 0.01, and indole analogues were added at 10 and 100 μM. Then, 200-μL aliquots of these suspensions were pipetted into the wells of a polystyrene 96-well plate and cultured without agitation at 28°C for 24 h. After that, unattached cells were washed away with phosphate-buffered saline (PBS) three times. Subsequently, the remaining attached bacteria were fixed with 200 μL methanol per well for 20 min, after which the methanol was removed, and the plates were air-dried. Then, biofilms were stained for 15 min with 200 μL per well of a 1% crystal violet solution. Plates were then rinsed with tap water to remove nonadherent cells by three times. After the plates were air-dried, bound crystal violet was dissolved in 200 μL of 95% ethanol per well for 30 min, and absorbance was measured at 570 nm with a Tecan Infinite M200 Pro plate reader. Sterile medium served as a negative control, and the reported values are blank-corrected.
Determination of protease and hemolytic activity of V. campbellii.
Protease and hemolytic activity assays were performed according to Natrah et al. (50) with some modifications. The protease assay plates were prepared by mixing double strength LB35 agar (30g/L) with a 4% skim milk powder suspension (Oxoid, Basingstoke, Hampshire, UK) sterilized separately at 121°C for 5 min. Hemolytic assay plates were prepared by supplementing LB35 agar with 5% defibrinated sheep blood (Oxoid). The selected indole analogues were added into agar before it was poured into petri plates at concentrations of 10 and 100 μM, respectively. All agar plates were opened at room temperature for 15 min to dry. Then the bacterial suspension was applied to the surface of an agar plate (at least 3 replicates per treatment). The plates were incubated upright at 28°C, and the clearing zone diameter and colony diameter were measured after 4 days of incubation.
ACKNOWLEDGMENTS
This work was supported by the China Scholarship Council, the Scientific Research Fund of Flanders (FWO project no. 1500219N) and the Special Research Fund of Ghent University (BOF-UGent).
Footnotes
[This article was published on 26 September 2022 with an error in Table 1. The table was corrected in the current version, posted on 28 September 2022.]
Supplemental material is available online only.
Contributor Information
Tom Defoirdt, Email: tom.defoirdt@ugent.be.
Brian Conlon, University of North Carolina at Chapel Hill.
REFERENCES
- 1.Kumar G, Engle CR. 2016. Technological advances that led to growth of shrimp, salmon, and tilapia farming. Rev Fish Sci Aquac 24:136–152. doi: 10.1080/23308249.2015.1112357. [DOI] [Google Scholar]
- 2.Sivaraman I, Krishnan M, Radhakrishnan K. 2019. Better management practices for sustainable small-scale shrimp farming. J Clean Prod 214:559–572. doi: 10.1016/j.jclepro.2018.12.172. [DOI] [Google Scholar]
- 3.FAO. 2020. The state of world fisheries and aquaculture 2020. Sustainability in action. FAO, Rome, Italy. [Google Scholar]
- 4.Vadstein O, Bergh Ø, Gatesoupe F-J, Galindo-Villegas J, Mulero V, Picchietti S, Scapigliati G, Makridis P, Olsen Y, Dierckens K, Defoirdt T, Boon N, De Schryver P, Bossier P. 2013. Microbiology and immunology of fish larvae. Rev Aquacult 5:S1–S25. doi: 10.1111/j.1753-5131.2012.01082.x. [DOI] [Google Scholar]
- 5.Novriadi R. 2016. Vibriosis in aquaculture. Omni-Akuatika 12:24. doi: 10.20884/1.oa.2016.12.1.24. [DOI] [Google Scholar]
- 6.Defoirdt T. 2016. Implications of ecological niche differentiation in marine bacteria for microbial management in aquaculture to prevent bacterial disease. PLoS Pathog 12:e1005843. doi: 10.1371/journal.ppat.1005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karunasagar I, Pai R, Malathi GR, Karunasagar I. 1994. Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture 128:203–209. doi: 10.1016/0044-8486(94)90309-3. [DOI] [Google Scholar]
- 8.Prayitno SB, Latchford JW. 1995. Experimental infections of crustaceans with luminous bacteria related to Photobacterium and Vibrio. Effect of salinity and pH on infectiosity. Aquaculture 132:105–112. doi: 10.1016/0044-8486(94)00374-W. [DOI] [Google Scholar]
- 9.Austin B, Zhang XH. 2006. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol 43:119–124. doi: 10.1111/j.1472-765X.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 10.Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. 2007. Alternatives to antibiotics to control bacterial infections: luminescent vibriosis in aquaculture as an example. Trends Biotechnol 25:472–479. doi: 10.1016/j.tibtech.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Rasul MG, Majumdar BC. 2017. Abuse of antibiotics in aquaculture and it’s effects on human, aquatic animal and environment. Saudi J Life Sci 2:81–88. [Google Scholar]
- 12.Scott HM, Acuff G, Bergeron G, Bourassa MW, Gill J, Graham DW, Kahn LH, Morley PS, Salois MJ, Simjee S, Singer RS, Smith TC, Storrs C, Wittum TE. 2019. Critically important antibiotics: criteria and approaches for measuring and reducing their use in food animal agriculture. Ann N Y Acad Sci 1441:8–16. doi: 10.1111/nyas.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defoirdt T, Sorgeloos P, Bossier P. 2011. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14:251–258. doi: 10.1016/j.mib.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Dickey SW, Cheung GY, Otto M. 2017. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov 16:457–471. doi: 10.1038/nrd.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Defoirdt T. 2018. Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol 26:313–328. doi: 10.1016/j.tim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Jayaraman A, Wood TK. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 7:42. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Lee J. 2010. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Wood TK, Lee J. 2015. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol 23:707–718. doi: 10.1016/j.tim.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y-G, Lee J-H, Cho MH, Lee J. 2011. Indole and 3-indolylacetonitrile inhibit spore maturation in Paenibacillus alvei. BMC Microbiol 11:119. doi: 10.1186/1471-2180-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chant EL, Summers DK. 2007. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol Microbiol 63:35–43. doi: 10.1111/j.1365-2958.2006.05481.x. [DOI] [PubMed] [Google Scholar]
- 21.Vega NM, Allison KR, Khalil AS, Collins JJ. 2012. Signaling-mediated bacterial persister formation. Nat Chem Biol 8:431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Shao Y, Zhao X, Li C, Guo M, Lv Z, Zhang W. 2020. Indole contributes to tetracycline resistance via the outer membrane protein OmpN in Vibrio splendidus. World J Microbiol Biotechnol 36:36. doi: 10.1007/s11274-020-02813-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SS, Yang Q, Defoirdt T. 2022. Indole decreases the virulence of pathogenic vibrios belonging to the Harveyi clade. J Appl Microbiol 132:167–176. doi: 10.1111/jam.15227. [DOI] [PubMed] [Google Scholar]
- 24.Hirakawa H, Kodama T, Takumi-Kobayashi A, Honda T, Yamaguchi A. 2009. Secreted indole serves as a signal for expression of type III secretion system translocators in enterohaemorrhagic Escherichia coli O157:H7. Microbiology (Reading) 155:541–550. doi: 10.1099/mic.0.020420-0. [DOI] [PubMed] [Google Scholar]
- 25.Bommarius B, Anyanful A, Izrayelit Y, Bhatt S, Cartwright E, Wang W, Swimm AI, Benian GM, Schroeder FC, Kalman D. 2013. A family of indoles regulate virulence and Shiga toxin production in pathogenic E. coli. PLoS One 8:e54456. doi: 10.1371/journal.pone.0054456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosales PF, Bordin GS, Gower AE, Moura S. 2020. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia 143:104558. doi: 10.1016/j.fitote.2020.104558. [DOI] [PubMed] [Google Scholar]
- 27.Rajasekharan SK, Lee JH, Ravichandran V, Lee J. 2017. Assessments of iodoindoles and abamectin as inducers of methuosis in pinewood nematode, Bursaphelenchus xylophilus. Sci Rep 7:6803. doi: 10.1038/s41598-017-07074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato N, Furutani S, Otaka J, Noguchi A, Kinugasa K, Kai K, Hayashi H, Ihara M, Takahashi S, Matsuda K, Osada H. 2018. Biosynthesis and structure–activity relationship studies of okaramines that target insect glutamate-gated chloride channels. ACS Chem Biol 13:561–566. doi: 10.1021/acschembio.7b00878. [DOI] [PubMed] [Google Scholar]
- 29.Sathiyamoorthi E, Faleye OS, Lee JH, Raj V, Lee J. 2021. Antibacterial and antibiofilm activities of chloroindoles against Vibrio parahaemolyticus. Front Microbiol 12:714371. doi: 10.3389/fmicb.2021.714371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Kim YG, Cho MH, Kim JA, Lee J. 2012. 7-Fluoroindole as an antivirulence compound against Pseudomonas aeruginosa. FEMS Microbiol Lett 329:36–44. doi: 10.1111/j.1574-6968.2012.02500.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Attila C, Cirillo SLG, Cirillo JD, Wood TK. 2009. Indole and 7- hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb Biotechnol 2:75–90. doi: 10.1111/j.1751-7915.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JH, Cho MH, Lee J. 2011. 3-Indolylacetonitrile decreases Escherichia coli o157:h7 biofilm formation and Pseudomonas aeruginosa virulence. Environ Microbiol 13:62–73. doi: 10.1111/j.1462-2920.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- 33.Sethupathy S, Sathiyamoorthi E, Kim YG, Lee JH, Lee J. 2020. Antibiofilm and antivirulence properties of indoles against Serratia marcescens. Front Microbiol 11:584812. doi: 10.3389/fmicb.2020.584812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajasekharan SK, Lee JH, Ravichandran V, Kim JC, Park JG, Lee J. 2019. Nematicidal and insecticidal activities of halogenated indoles. Sci Rep 9:1–4. doi: 10.1038/s41598-019-38561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajasekharan SK, Kim S, Kim JC, Lee J. 2020. Nematicidal activity of 5-iodoindole against root-knot nematodes. Pestic Biochem Physiol 163:76–83. doi: 10.1016/j.pestbp.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Yang Q, Pande GS, Wang Z, Lin B, Rubin RA, Vora GJ, Defoirdt T. 2017. Indole signalling and (micro) algal auxins decrease the virulence of Vibrio campbellii, a major pathogen of aquatic organisms. Environ Microbiol 19:1987–2004. doi: 10.1111/1462-2920.13714. [DOI] [PubMed] [Google Scholar]
- 37.Josenhans C, Suerbaum S. 2002. The role of motility as a virulence factor in bacteria. Int J Med Microbiol 291:605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 38.Verstraeten N, Braeken K, Debkumari B, Fauvart M, Fransaer J, Vermant J, Michiels J. 2008. Living on a surface: swarming and biofilm formation. Trends Microbiol 16:496–506. doi: 10.1016/j.tim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Lewis K. 2010. Persister cells. Annu Rev Microbiol 64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 40.Nithya C, Pandian SK. 2010. The in vitro antibiofilm activity of selected marine bacterial culture supernatants against Vibrio spp. Arch Microbiol 192:843–854. doi: 10.1007/s00203-010-0612-6. [DOI] [PubMed] [Google Scholar]
- 41.Worthington RJ, Richards JJ, Melander C. 2012. Small molecule control of bacterial biofilms. Org Biomol Chem 10:7457–7474. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manoharan RK, Lee JH, Lee J. 2018. Efficacy of 7-benzyloxyindole and other halogenated indoles to inhibit Candida albicans biofilm and hyphal formation. Microb Biotechnol 11:1060–1069. doi: 10.1111/1751-7915.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed B, Jailani A, Lee JH, Lee J. 2022. Effect of halogenated indoles on biofilm formation, virulence, and root surface colonization by Agrobacterium tumefaciens. Chemosphere 293:133603. doi: 10.1016/j.chemosphere.2022.133603. [DOI] [PubMed] [Google Scholar]
- 44.Boya BR, Lee JH, Lee J. 2022. Antibiofilm and antimicrobial activities of chloroindoles against uropathogenic Escherichia coli. Front Microbiol 13:872943. doi: 10.3389/fmicb.2022.872943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marques A, Dinh T, Ioakeimidis C, Huys G, Swings J, Verstraete W, Dhont J, Sorgeloos P, Bossier P. 2005. Effects of bacteria on Artemia franciscana cultured in different gnotobiotic environments. Appl Environ Microbiol 71:4307–4317. doi: 10.1128/AEM.71.8.4307-4317.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Defoirdt T, Bossier P, Sorgeloos P, Verstraete W. 2005. The impact of mutations in the quorum sensing systems of Aeromonas hydrophila, Vibrio anguillarum and Vibrio harveyi on their virulence toward gnotobiotically cultured Artemia franciscana. Environ Microbiol 7:1239–1247. doi: 10.1111/j.1462-2920.2005.00807.x. [DOI] [PubMed] [Google Scholar]
- 47.Verschuere L, Rombaut G, Huys G, Dhont J, Sorgeloos P, Verstraete W. 1999. Microbial control of the culture of Artemia juveniles through preemptive colonization by selected bacterial strains. Appl Environ Microbiol 65:2527–2533. doi: 10.1128/AEM.65.6.2527-2533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Q, Defoirdt T. 2015. Quorum sensing positively regulates flagellar motility in pathogenic Vibrio harveyi. Environ Microbiol 17:960–968. doi: 10.1111/1462-2920.12420. [DOI] [PubMed] [Google Scholar]
- 49.Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, Ruzicka F. 2007. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. APMIS 115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 50.Natrah FM, Ruwandeepika HD, Pawar S, Karunasagar I, Sorgeloos P, Bossier P, Defoirdt T. 2011. Regulation of virulence factors by quorum sensing in Vibrio harveyi. Vet Microbiol 154:124–129. doi: 10.1016/j.vetmic.2011.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.02689-22-s0001.pdf, PDF file, 2.0 MB (2MB, pdf)