Abstract
Cognitive decline is linked to decreased cerebral blood flow, particularly in women after menopause. Impaired cerebrovascular function precedes the onset of dementia, possibly because of reduced functional dilation in parenchymal arterioles. These vessels are bottlenecks of the cerebral microcirculation, and dysfunction can limit functional hyperemia in the brain. Large-conductance Ca2+-activated K+ channels (BKCa) are the final effectors of several pathways responsible for functional hyperemia, and their expression is modulated by estrogen. However, it remains unknown whether BKCa function is altered in cerebral parenchymal arterioles after menopause. Using a chemically induced model of menopause, the 4-vinylcyclohexene diepoxide (VCD) model, which depletes follicles while maintaining intact ovaries, we hypothesized that menopause would be associated with reduced functional vasodilatory responses in cerebral parenchymal arterioles of wild-type mice via reduced BKCa function. Using pressure myography of isolated parenchymal arterioles, we observed that menopause (Meno) induced a significant increase in spontaneous myogenic tone. Endothelial function, assessed as nitric oxide production and dilation after cholinergic stimulation or endothelium-dependent hyperpolarization pathways, was unaffected by Meno. BKCa function was significantly impaired in Meno compared with control, without changes in voltage-gated K+ channel activity. Cerebral functional hyperemia, measured by laser-speckle contrast imaging during whisker stimulation, was significantly blunted in Meno mice, without detectable changes in basal perfusion. However, behavioral testing identified no change in cognition. These findings suggest that menopause induces cerebral microvascular and neurovascular deficits.
NEW & NOTEWORTHY Cerebral parenchymal arterioles from menopause mice showed increased myogenic tone. We identified an impairment in smooth muscle cell BKCa channel activity, without a reduction in endothelium-dependent dilation or nitric oxide production. Microvascular dysfunction was associated with a reduction in neurovascular responses after somatosensory stimulation. Despite the neurovascular impairment, cognitive abilities were maintained in menopausal mice.
Keywords: BK channels, cerebral functional hyperemia, cerebral parenchymal arterioles, menopause, myogenic tone
INTRODUCTION
Menopause is accompanied by an increased risk of chronic diseases, and with the accelerated growth in the aging population, it is estimated that the number of menopausal women worldwide will be ∼1.1 billion by the year 2025 (1). Menopause is linked to cerebrovascular impairments (2), possibly a result of impaired arteriolar function (3). These impairments can significantly limit the real-time regulation of localized hemodynamic responses in the brain resulting from increased neuronal activity, the process of functional hyperemia (4). In the long term, nutrient delivery to active neuronal populations is reduced, resulting in neuronal loss and cognitive decline (5). Epidemiological evidence and population-based studies suggest that cognitive decline, including that observed in patients with Alzheimer’s disease, is more prevalent in postmenopausal women than age-matched men (6), further suggesting a role for menopause in progression of cognitive impairment.
Functional hyperemia relies on the real-time vasodilatory capacity of parenchymal arterioles, which are the bottlenecks of the cerebral microcirculation (7, 8). Parenchymal arterioles have few branches and anastomoses, and their perfusion territory encompasses an entire column of neurons and glial cells (8). Cerebral microvascular dysfunction can occur via maladaptive alterations in different vascular properties, including excessive myogenic tone (9, 10), loss of endothelium-dependent dilation (11–13), or alterations in smooth muscle cell (SMC) mechanisms that modulate excitability and contraction (14). Myogenic tone is an intrinsic property of SMCs that is initiated by a pressure-induced stretch of the membrane sensed by mechanosensors, leading to constriction (15–17). Arteriolar myogenic tone is regulated by diffusible or electrical signals from endothelial cells, as well as opening of K+-permeable channels in the vascular SMC plasmalemma. In SMCs, voltage-gated K+ channels (KV) (18) and large-conductance Ca2+-activated K+ channels (BKCa) (19–21) are vital in contractile regulation, as they lead to SMC relaxation and vasodilation. Under physiological conditions, these molecular systems are in equilibrium to regulate tissue perfusion; however, under supraphysiological or pathological conditions, failure of one of these systems can impair functional vasodilatory responses.
Menopause is linked to vascular dysfunction in both the peripheral and cerebral circulation, observed as endothelial impairment (22, 23) and hypercontractility (3, 24) because of improper SMC-dependent regulation. Endothelium-dependent arteriolar dilation occurs downstream of different signals, of which nitric oxide (NO) generation and endothelial cell hyperpolarization (termed endothelium-dependent hyperpolarization, EDH) play a central role. NO production by endothelial cells occurs after activation of nitric oxide synthase enzymes downstream from vasodilatory mediators, including muscarinic cholinergic receptors. In addition, endothelial cell hyperpolarization is a consequence of opening of small- and intermediate-conductance Ca2+-activated K+ channels (KCa2.3/KCa3.1) and inwardly rectifying K+ channels (KIR2). A previous study suggested that NO-dependent dilation is impaired in rodents after ovariectomy (25). Furthermore, KCa2.3 expression is upregulated by estrogen (26), supporting the possibility that they may be impaired after menopause. Both NO-dependent dilation and endothelium-dependent hyperpolarization pathways are central to functional hyperemia responses (27–30); thus, possible impairments can lead to impaired cerebral hemodynamics in menopause.
Furthermore, K+ channels in vascular SMCs control membrane potential, which is a major determinant of Ca2+ influx and myogenic constriction. BKCa, in particular, is composed of α-, β-, and γ-subunits, where the α-subunit forms the K+-selective pore and β/γ-subunits are regulatory (31, 32). Previous studies have shown that the β1-subunit is regulated by estrogen (33, 34) and has a protective effect in females against hypertension and stroke (35). Alongside BKCa in the SMCs, KV are also known to play an important role in the regulation of myogenic tone (14). KV are activated by membrane depolarization downstream from opening of voltage-gated Ca2+ channels, thus exerting a balancing influence on membrane potential and limiting contractility (6, 36). Despite previous studies showing acute effects of estrogen on neuronal KV (37, 38), little is known of how menopause affects their function in cerebral vascular SMCs.
Using the 4-vinylcyclohexene diepoxide (VCD) model of menopause, which depletes follicles (estrogen-producing cells) while maintaining the rest of the ovarian tissue intact (39), we tested the hypothesis that menopause will impair cerebral parenchymal arterioles via reduction in BKCa function, consequently blunting functional hyperemia and resulting in cognitive impairment in mice. We observed that parenchymal arterioles of menopausal mice exhibited an exacerbated myogenic tone linked to a reduction in SMC BKCa activity despite intact endothelial function. This microvascular impairment was associated with blunted functional hyperemia responses during somatosensory stimulation, although cognition seems to be preserved in the model. Together, our data highlight a new mechanism of menopause-induced cerebral microvascular dysfunction that affects neurovascular regulation and may precede cognitive decline.
METHODS
Animals
All animal procedures in this study were approved by the Institutional Animal Care and Use Committee of the University of Arizona College of Medicine (IACUC Protocol No. 18–473) and are in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (8th ed.). All animal experiments are reported in compliance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments). Female adult C57BL/6 background mice were purchased from Jackson Laboratories at 10–12 wk of age. All mice were kept in plastic cages with at least four mice per cage to maintain social interactions, cages were housed in rooms with controlled environmental conditions and a 12:12-h light-dark cycle, and mice had free access to water and commercial rodent chow.
The VCD Model of Menopause
We used the VCD model of chemically induced ovotoxicity to induce ovarian failure. C57BL/6 mice received daily intraperitoneal injections of 4-vinylcyclohexene diepoxide at a concentration of 160 mg/kg or equivolume sesame oil (vehicle) for 20 consecutive days (39, 40) starting at 12 wk of age. At this concentration and dosage regimen, the toxic effects of VCD are restricted to gonads, and no toxicity is observed in the liver, kidneys, adrenal glands, and spleen (41). Vaginal cytology was monitored daily to determine the onset of menopause (ovarian failure), defined as 10 consecutive days of diestrus (39), which took 62 days on average. After confirmation of menopause, mice were allowed to age for 3 mo before experimenting to capture any structural changes that may have occurred because of the VCD treatment. Throughout the article, vehicle-treated mice are referred to as control and VCD-treated mice as Meno. In a cohort of mice, we collected the ovaries and measured circulating levels of estradiol to validate that VCD induced menopause. Serum levels of estradiol were assessed by ELISA using a commercially available kit (estradiol ELISA Kit, catalog no. 501890, Cayman Chemicals, Ann Arbor, MI), following the manufacturer’s instructions.
Measurement of Blood Pressure
Blood pressures were measured noninvasively via tail-cuff plethysmography (Hatteras Instruments, MC4000) before the onset of VCD dosing and once the mice became menopausal on ∼day 62. All blood pressure readings were made at 8:00 am, after completion of the dark cycle. Mice were acclimated to the tail-cuff measurement system for three consecutive days before measurements were taken (42).
Isolation of Cerebral Parenchymal Arterioles
On the day of experiments, mice were anesthetized with isoflurane (3%) and euthanized by decapitation and exsanguination. Brains were carefully excised and placed in ice-cold tissue collection physiological saline solution (tissue collection PSS, in mM: 140 NaCl, 5 KCl, 2 MgCl2, 10 dextrose, and 10 HEPES, pH 7.4), supplemented with 0.1% bovine serum albumin (Fisher Scientific, Hampton, NH, catalog no. BP1600) inside a water-jacketed dissection dish connected to a cooled circulating water bath. Isolation of parenchymal arterioles followed previously published protocols (9, 43, 44) with minor modifications. Briefly, a rectangle of brain around the middle cerebral artery, ∼3 cm in depth, was excised, and the superficial cortex was carefully separated from deeper cortical areas at the level of the corpus callosum. Parenchymal arterioles below the corpus callosum were carefully separated from the surrounding tissue and used for pressure myography experiments.
Pressure Myography
Parenchymal arterioles were transferred to a custom-made pressure myograph chamber filled with tissue collection PSS with a microplunger and a 100-µL glass pipette (VWR, Visalia, CA). One end of the vessel was cannulated onto a glass cannula (∼30-µm inner diameter at the tip); the other end was tied to the cannula in a blind-sac experimental setup. The cannula was filled with artificial cerebrospinal fluid (aCSF, in mM: 124 NaCl, 3 KCl, 2 MgCl2, 1.085 NaH2PO4·2H2O, 26 NaHCO3, 2.0 CaCl2, and 4 dextrose). For endothelium-denudation experiments, one end of the artery was cannulated and an air bubble was slowly passed through the intraluminal side to damage the endothelial cell layer (45); then, the cannulas and arterioles were filled with aCSF and the other end was tied off. The pressure myography chamber was then transferred to an inverted microscope and connected to a gravity-dependent column of water for pressurization. Preparations were superfused with warm (37°C), oxygenated (21% O2-5% CO2-74% N2) aCSF exchanged at a rate of 2–3 mL/min for ∼30 min at a pressure of 15 mmHg to equilibrate. Intraluminal pressure was then increased to 40 mmHg until generation of spontaneous myogenic tone. Outer and lumen diameters were recorded in real time at 15 Hz with IonWizard v.7.3 software (IonOptix, Westwood, MA). Spontaneous myogenic tone was calculated as follows: myogenic tone (%) = [1 − (LDT/LDP)] × 100 (46), where LDT is the lumen diameter at stable spontaneous myogenic tone and LDP is the passive lumen diameter. Preparations that did not reach at least 15% myogenic tone were considered nonviable and excluded from further analysis.
After generation of spontaneous myogenic tone, preparations were exposed to different experimental protocols to assess endothelial cell-dependent or SMC mechanisms balancing myogenic constriction and vasodilatory pathways present in the cerebral parenchymal circulation. Arterioles were only exposed to one experimental protocol, to avoid possible artifacts caused by sequential exposure to different pharmacological agents.
SMC-Dependent Control of Parenchymal Arteriole Myogenic Tone
Endothelium-denuded preparations were exposed to 30 nM of the BKCa-channel inhibitor iberiotoxin (IbTox; AnaSpec, Fremont, CA), or 1 mM and 5 mM of 4-aminopyridine (4-AP), a blocker of KV channels (14), to assess the contribution of SMC K+ channels to the regulation of parenchymal arteriolar myogenic tone. Constriction data are shown as vasoconstriction (%), calculated as [(LDC − LDB)/LDP] × 100, where LDC is the lumen diameter at peak constriction, LDB is lumen diameter at baseline (immediately before addition of drug), and LDP is the passive lumen diameter. With this equation, all data regarding constriction have a negative value.
Assessment of NO Generation in Pressurized Parenchymal Arterioles
Parenchymal arterioles from control and Meno mice were pressurized onto glass cannulas to assess NO generation after exposure to the muscarinic cholinergic receptor agonist carbachol (10 µM; Tocris Bioscience, Bristol, UK, catalog no. 2810). Arterioles were loaded with the NO indicator DAF-FM (4-amino-5-methylamino-2′,7′-difluorofluorescein, 10 μM; ThermoFisher Scientific, Eugene, OR, catalog no. D23844) supplemented with equivolume 20% pluronic acid (ThermoFisher Scientific) in tissue collection PSS without BSA for 30 min at 37°C. After DAF-FM loading, preparations were moved to a Leica DM6 microscope with a ×40 water immersion objective (numerical aperture 0.8), pressurized to 40 mmHg, and superfused with aCSF supplemented with 10 mM HEPES, pH 7.4, at 37°C for 15 min to allow complete deesterification of the intracellular DAF-FM and equilibration. Z-stack (16-bit) confocal images through the entire thickness of the arteriole were acquired with a X-Light V2 spinning disk (CrestOptics, Rome, Italy) coupled to a high-sensitivity Evolve Delta EMCDD camera (Teledyne Photometrics, Tucson, AZ), controlled by μManager software (47). DAF fluorescence (excitation 495 nm and emission 515 nm) was captured with a 470-nm excitation laser and a 510/50-nm band-pass filter. Acquisition parameters (laser intensity, camera gain, exposure time) were the same for all images. Baseline images were taken to capture resting fluorescence signal from the arteriolar wall, the laser was then shut down, and the preparation was incubated with carbachol for 10 min. At the end of the incubation, another Z-stack image was acquired. Z-stacks were then compressed into maximum-intensity projections with FIJI software (ImageJ, version 1.53q), and a manual region of interest (ROI) was drawn on the limits of the arteriole. Mean fluorescence intensity of the ROI was recorded at baseline and after carbachol incubation. Data are expressed as percent changes in fluorescence from baseline.
Evaluation of NO-Dependent Dilation and Endothelial K+ Channel Function
NO-dependent dilation was assessed by incubation of parenchymal arterioles with the muscarinic cholinergic receptor agonist carbachol (10 µM) ± the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 µM). Similarly, dilation to the Ca2+ ionophore A23187 (1 µM; Tocris Bioscience, Bristol, UK, catalog no. 1234) ± l-NAME was evaluated. Endothelium-dependent hyperpolarization pathways were studied by activation of endothelial KCa2.3/KCa3.1 channels in endothelium-intact arterioles by a cumulative concentration-response curve to the pharmacological activator NS309 (0.1 nM to 1 µM; Tocris Bioscience, catalog no. 3895). KIR2 activity was tested by exposing arterioles to aCSF containing 15 mM KCl [extracellular K+ concentration ([K+]E) and 112 mM NaCl to balance cationic charges]. At the end of the experiment, aCSF + test agent was replaced by fresh aCSF, and arterioles were allowed to return to basal myogenic tone and then exposed to 60 mM KCl aCSF (balanced NaCl) to ensure viability of the preparation until the end of the experiment. Dilation data are shown as vasodilation (%), calculated as [(LDD − LDB)/LDP] × 100, where LDD is the lumen diameter at peak dilation, LDB is lumen diameter at baseline (immediately before addition of drug), and LDP is the passive lumen diameter. With this equation, all data regarding vasodilation have a positive value.
Microvascular Structural and Biomechanical Properties
Parenchymal arterioles were bathed in Ca2+-free aCSF supplemented with 2 mM EGTA (a Ca2+ chelator) and 10 µM diltiazem (a L-type voltage-gated Ca2+ channel blocker; Tocris) to record passive lumen diameter, as well as structural and biomechanical properties (8). Intraluminal pressure was increased from 5 to 160 mmHg in 20-mmHg increments, and arterioles were allowed to equilibrate for 3 min at each pressure before outer and lumen diameters were recorded. Wall thickness was calculated as (outer diameter − lumen diameter). Distensibility and wall-to-lumen ratio were calculated as previously described (48, 49). Microvascular stiffness was assessed by the β-coefficient exponential model, which is the slope of exponential fit from the stress/strain curves, calculated as (y = aeβx), where y is circumferential stress, x is circumferential strain, a is intercept, and β is the slope, which is an index of vascular stiffness (50).
Laser-Speckle Contrast Imaging of Basal Cerebral Perfusion
Each mouse was placed in an induction chamber with 2–3% isoflurane mixed with breathing air to induce anesthesia. Ventilation was maintained constant via an automated ventilator (RoVent Small Animal Ventilator; Kent Scientific Corporation) inserted into the trachea after tracheotomy. After intubation the mouse was moved to a self-heated stereotaxic frame (Stoelting Co., Wood Dale, IL), the head was immobilized in ear bars, a veterinary ophthalmic ointment was placed over the eyes to prevent dryness during the procedure, and a rectal temperature probe was inserted to provide body temperature feedback. The hair covering the scalp was shaved with electrical clippers, and a midline incision was performed to expose the underlying skull. The periosteum was gently removed with cotton-tipped applicators, and a small stainless steel tissue retractor was used to keep the cranium exposed throughout the procedure. The laser-speckle contrast imaging system (PSI-Z; Perimed AB, Järfälla, Sweden) was placed ∼11 cm above the skull, and basal cerebral perfusion was recorded for 2 min at a rate of 20 images/s. All perfusion data were acquired and analyzed with the manufacturer’s software (PIMsoft v. 1.6; Perimed). Data showing average perfusion for whole dorsal surface of the brain are expressed as perfusion units (PU).
Hemodynamic Responses of the Whisker Barrel Cortex after Somatosensory Stimulation
After basal cerebral perfusion measurements, a thinned-skull cranial window was prepared over the left somatosensory cortex on top of the whisker barrel cortex (51). Isoflurane was then lowered to 1.5%, a dosage shown to maintain stable, near-physiological cardiovascular function while providing acceptable depth of anesthesia (52). Furthermore, this anesthesia regimen still allows for a large hemodynamic response after stimulation (53). Whisker stimulation was performed by manually stroking the contralateral (right) vibrissae with a brush at 5 Hz for 20 s, followed by a 3-min recovery period. The stimulation was repeated three times, and at the end we stimulated the ipsilateral vibrissae to ensure that no movement artifacts were occurring in the recordings. Perfusion was monitored constantly in real time with the PSI-Z system at the same imaging frequency described above (20 Hz), and all data were acquired with PIMsoft software. Baseline functional hyperemia measurements consisted of the average perfusion in the 10 s before the onset of stimulus; perfusion during whisker stimulation was averaged over the duration of the 20-s stimulation. Data are presented as percent increase in perfusion from baseline.
Novel Object Recognition Test
Episodic memory was assessed by the novel object recognition test to analyze discrimination index, which represents recognition memory sensitivity (54). The novel object recognition test was administered over 2 days, with a total of five trials. During each trial, the mice explored their environment freely for 5 min before returning to their home cage. The five trials were as follows:
Trial 1: Mice were acclimated to an empty rectangular box (12 cm × 16 cm).
Trials 2 and 3: Mice were exposed to the three familiar objects placed in three corners of the rectangular box.
Trial 4: Mice were exposed to the same three familiar objects.
Trial 5: Mice were exposed to two familiar objects and one novel object.
Trials 1–3 were performed on day 1, and trials 4 and 5 took place on day 2 and were the trials recorded for analysis. Between animals, the maze was cleaned thoroughly with Versa-Clean (Fisher Scientific). Performance videos were recorded via an aerial view with a digital video camera, and data were acquired with ANY-maze software (version 5.4, Stoelting Co.). Discrimination index was calculated as follows: timenovel/(timefamiliar + timenovel) (55, 56). Data shown are the discrimination index and total exploration time.
Nesting Test
Five days after completion of the novel object recognition test, mice underwent the nesting test to measure their executive function or ability to perform activities related to daily living (57). Approximately 24 h before the start of nesting, mice were individually housed in cages with woodchip bedding, but no other environmental enrichment items were added. The individual housing was done to reduce any anxiety and stress that may occur because of social separation. The following day, 1 h before the dark cycle, two cotton nestlets were placed in each cage to allow the mouse to build nests. Nests were assessed the following morning on a rating scale of 1–5: 1, cotton nestlets are 90% intact; 2, nestlets are 50–90% intact; 3, nestlets mostly shredded but no identifiable nest; 4, >90% of nestlets are torn with an identifiable nest, albeit flat; and 5, a near-perfect nest with >90% of the nestlets torn and nest walls are higher than the height of the mouse for at least 50% of its circumference (57). Nest scoring was performed by three investigators blinded to experimental groups to remove bias, and a half-point scale was used to “split the difference” when scores were variable between the three investigators.
Chemicals
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Data Analysis
Data are expressed as means ± SE. All data analyses were performed with GraphPad Prism 9. Differences between means of two experimental groups were analyzed with two-tailed Student’s t tests or Mann–Whitney test if the data did not follow a normal distribution. Constrictions to 4-AP were analyzed by an ordinary two-way ANOVA, whereas vasodilation to carbachol ± l-NAME and A23187 ± l-NAME were analyzed by a mixed-model two-way ANOVA followed by a Bonferroni post hoc correction for multiple comparisons. Concentration-response curves to NS309 were analyzed by a nonlinear regression model to assess the curve fit, followed by a two-way ANOVA to identify possible differences between groups. All data were tested for the possible presence of outliers by two methods: 1) the ROUT method, which is based on a false discovery rate with a Q = 1 and 2) any data point that was two standard deviations away from the mean. No outliers were detected in our data set with either method.
RESULTS
Physiological Parameters of Control and Meno C57BL/6 Mice
There were no significant differences in body weight, systolic blood pressure, and organ weight-to-body weight ratio (Table 1) between control and Meno mice at the time points measured. Ovary weights were significantly reduced in Meno mice, and circulating levels of estradiol were undetectable in Meno mice (Table 1).
Table 1.
Physiological parameters assessed
| Control | Meno | |
|---|---|---|
| Age, moa | 8–12 | 8–12 |
| Body weight, ga | 27.1 ± 0.8 | 28.5 ± 1.3 |
| Systolic blood pressure, mmHga | 105 ± 4.7 | 106.8 ± 6.6 |
| Heart wt-to-body wt ratio, g/ga | 0.006 ± 0.001 | 0.005 ± 0.001 |
| Kidney wt-to-body wt ratio, g/ga | 0.012 ± 0.001 | 0.014 ± 0.001 |
| Ovaries wet weight, mgb | 5.23 ± 0.47 | 2.91 ± 0.34* |
| Ovaries-to-body weight ratio, mg/gb | 0.222 ± 0.020 | 0.123 ± 0.014* |
| Circulating estradiol, pg/mLb | 5.641 ± 1.614 | Undetected |
Values are means ± SE; an = 16 mice/group; bn = 7 and 5 mice/group, control vs. menopause (Meno). *P < 0.05, 2-tailed Student’s t test.
Parenchymal Arteriolar Myogenic Tone Is Increased in Menopausal Mice
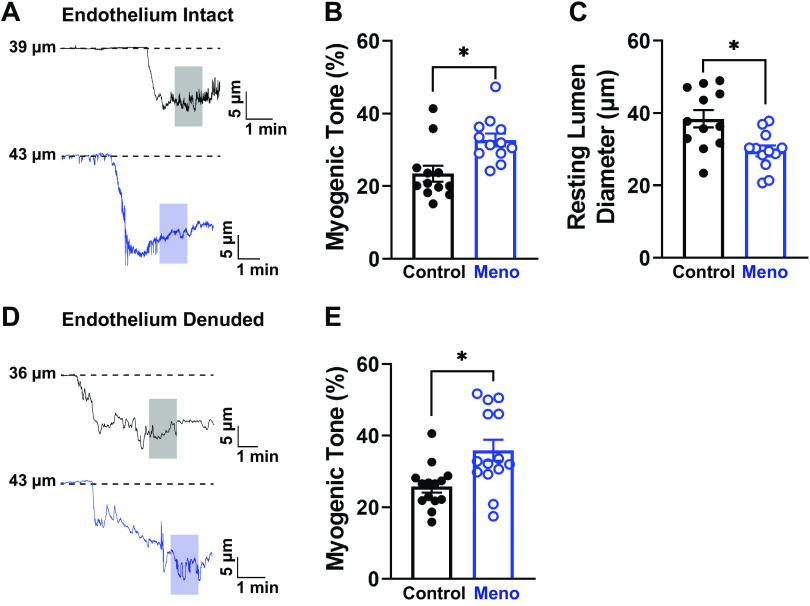
Parenchymal arterioles were cannulated for pressure myography experiments to assess myogenic tone in the cerebral microcirculation. When the pressure was raised to 40 mmHg, arterioles from control and Meno mice showed an expected generation of spontaneous myogenic tone (Fig. 1A). However, arterioles from Meno mice demonstrated a significant increase in spontaneous myogenic tone (myogenic tone, 23.5 ± 2.2 vs. 32.8 ± 1.8%, control vs. Meno, P < 0.05; Fig. 1B) and a ∼20% reduction in resting lumen diameter (i.e., the diameter after stable myogenic tone) compared with arterioles isolated from control mice (lumen diameter, 38.45 ± 2.36 vs. 29.54 ± 1.52 µm, control vs. Meno, P < 0.05; Fig. 1C). On the basis of these findings, we performed experiments to identify the possible mechanisms underlying this augmented myogenic tone.
Figure 1.
Menopause increases myogenic tone and decreases resting lumen diameter in C57BL/6 mice. A: representative traces of the lumen diameter of parenchymal arterioles from control (top, black) and menopause (Meno) (bottom, blue) mice showing increased myogenic tone, observed as a larger contraction to the physiological intraluminal pressure of 40 mmHg. Shaded areas indicate where measurements were taken. B: summary data showing that Meno induced a statistically significant increase in spontaneous myogenic tone of isolated, pressurized parenchymal arterioles. C: consequently, the resting lumen diameter of parenchymal arterioles isolated from Meno mice was significantly reduced compared with those isolated from control mice. Data are means ± SE; N = 12 arterioles from 8 different mice. *P < 0.05, 2-tailed Student’s t test. D and E: removal of the endothelium did not affect the increased myogenic tone observed in Meno mice, as shown by the representative traces (D) and the summary bar graphs (E). Data are means ± SE; N = 14 arterioles from 7 different mice. *P < 0.05, 2-tailed Student’s t test.
Endothelium Denudation Does Not Change Myogenic Tone
To assess whether changes in myogenic tone were endothelium dependent, we denuded parenchymal arterioles from control and Meno mice. We demonstrated that removal of the endothelium had no effect on the myogenic tone of either group and spontaneous myogenic tone remained higher in parenchymal arterioles from Meno mice than control mice (myogenic tone, 25.8 ± 1.6 vs. 35.9 ± 3.0%, control vs. Meno, P < 0.05; Fig. 1, D and E).
BKCa Function Is Impaired in Menopausal Cerebral Parenchymal Arterioles
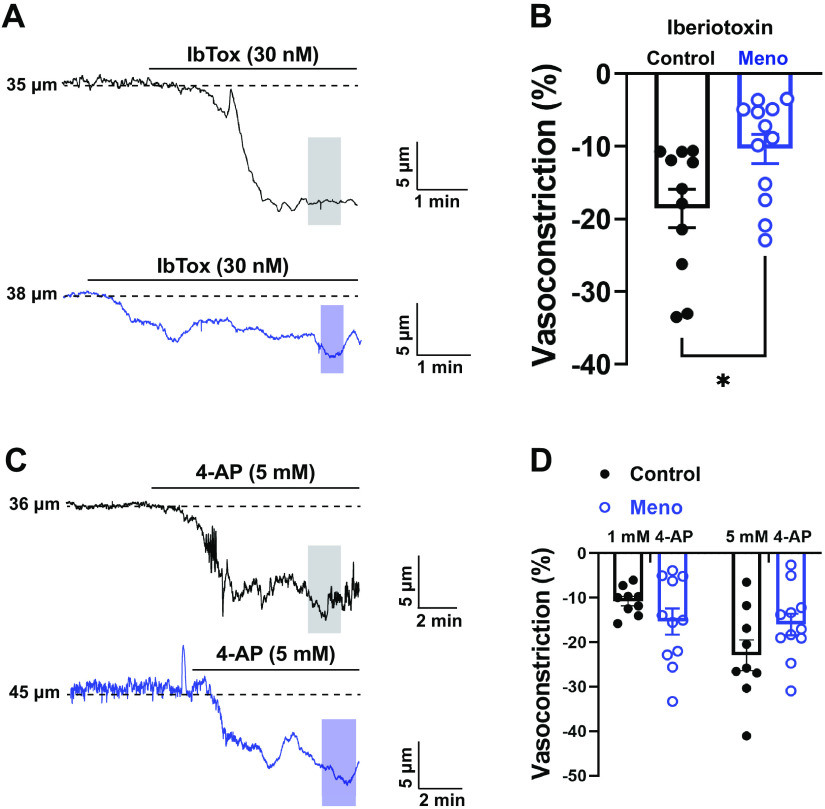
BKCa channels are important regulators of myogenic tone in the cerebral circulation (58, 59); therefore, we hypothesized that the observed increase in myogenic tone in menopausal mice was caused by impaired BKCa function. To assess this, cerebral parenchymal arterioles were treated with IbTox (30 nM), a selective BKCa inhibitor, and BKCa channel function was measured. Arterioles from control and Meno mice constricted after exposure to IbTox, but the vasoconstriction response was blunted in Meno (vasoconstriction, −18.56 ± −2.66 vs. −10.37 ± −2.01%, control vs. Meno, P < 0.05; Fig. 2, A and B). These data suggest that BKCa channel function is impaired in parenchymal arterioles of Meno mice.
Figure 2.
Large-conductance Ca2+-activated K+ channel (BKCa) function is impaired in menopausal cerebral parenchymal arterioles, whereas voltage-gated K+ channels (KV) remained stable. A: representative traces of the lumen diameter of endothelium-denuded parenchymal arterioles from control (top, black) and menopause (Meno) (bottom, blue) mice demonstrating impaired response to the BKCa inhibitor iberiotoxin (IbTox, 30 nM), observed as a smaller constriction after incubation with IbTox in the superfusing bath. Shaded areas indicate where measurements were taken. B: summary data showing lower vasoconstriction after administration of IbTox in Meno mice, suggesting that menopause impairs BKCa function. Data are means ± SE; N = 11 to 12 arterioles from 7 to 8 mice. *P < 0.05, 2-tailed Student’s t test. C: representative traces of the lumen diameter of endothelium-denuded pressurized arterioles before and after incubation with the KV blocker 4-aminopyridine (4-AP). D: summary graph showing that arteriolar constriction after 1 mM and 5 mM 4-AP is not significantly different between control and Meno. Data are means ± SE; N = 9–11 arterioles from 5 different mice; 1-way ANOVA with a Bonferroni post hoc correction for multiple comparisons.
Function of KV Channels Remained Stable in Cerebral Parenchymal Arterioles after Menopause
After confirming that the increase in myogenic tone was likely due to SMC dysfunction, we explored the function of KV channels by exposing the arterioles to two different concentrations of the KV blocker 4-AP (1 mM and 5 mM; Fig. 2, C and D). Parenchymal arterioles from control mice showed a concentration-dependent contraction to 4-AP (Fig. 2D) that was not present in arterioles from Meno mice, which were maximally constricted at 1 mM 4-AP. However, no significant differences were observed in KV channel function between control and Meno mice (vasoconstriction at 1 mM 4-AP: −11.48 ± −3.62 vs. −13.98 ± −4.03%, control vs. Meno, P > 0.05; vasoconstriction at 5 mM 4-AP: −21.91 ± −6.92 vs. −14.98 ± −4.33%, control vs. Meno, P > 0.05; Fig. 2, C and D).
Menopause Does Not Affect NO Production and NO-Dependent Dilation in Cerebral Parenchymal Arterioles
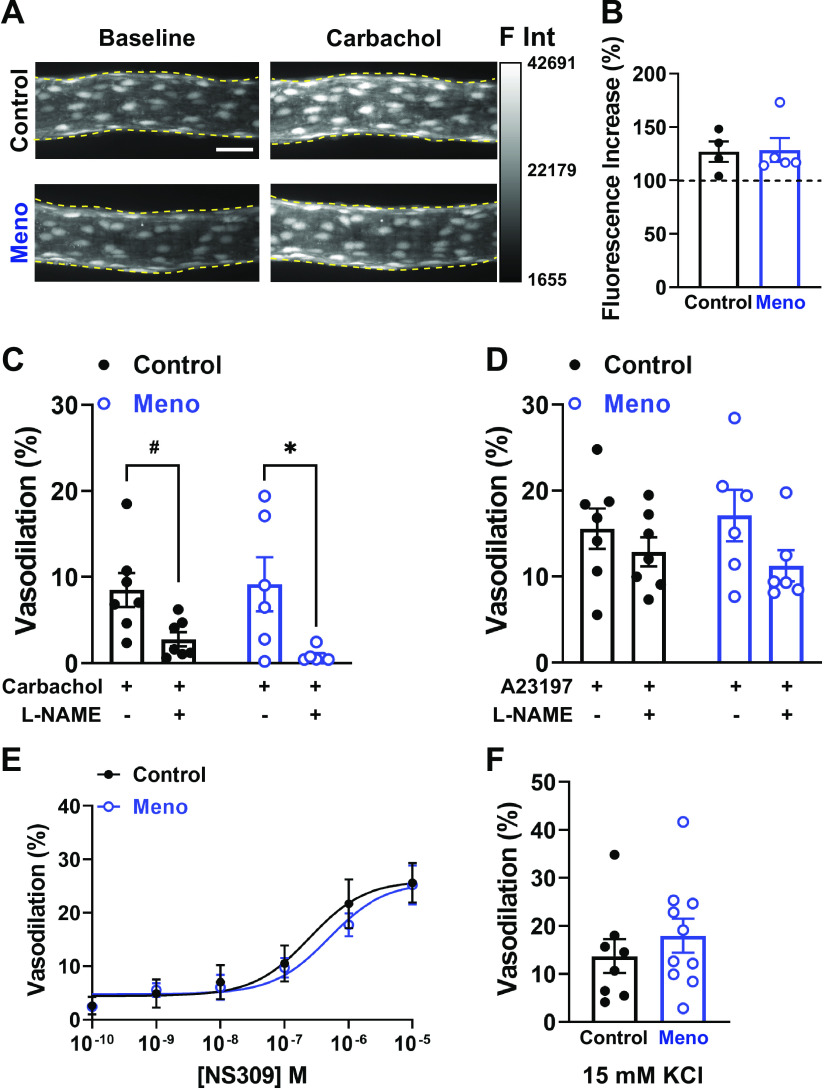
NO-dependent dilation is an important mechanism that underlies functional hyperemia in the brain (29, 30), and NO signaling has been previously shown to be altered in ovariectomized rats (25). Thus, we assessed whether NO generation and NO-dependent dilation were affected in parenchymal arterioles of chemically induced menopausal mice. Using spinning-disk confocal microscopy, we observed that the ability of pressurized parenchymal arterioles to generate NO upon cholinergic stimulation with carbachol (10 µM) was similar between groups [fluorescence intensity (% from baseline), 127.0 ± 9.5 vs. 128.4 ± 11.3, control vs. Meno, P > 0.05; Fig. 3, A and B].
Figure 3.
Endothelial nitric oxide (NO) and K+-dependent dilation are maintained in menopausal parenchymal arterioles. A: representative maximal intensity projections of pressurized parenchymal arterioles loaded with the NO indicator 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) under baseline conditions (left) and after 10-min incubation with the muscarinic cholinergic agonist carbachol (10 µM, right). Note that the increase in fluorescence after carbachol is similar between control (top) and menopause (Meno) (bottom) mice. Bar, 30 µm. F Int, fluorescence intensity (arbitrary units) within the yellow dashed lines. B: summary data showing that the increase in fluorescence from baseline was similar between control and Meno mice. Data are means ± SE; N = 4 to 5 arterioles from 3 to 4 different mice. C: dilation of parenchymal arterioles to carbachol (10 µM) was similar between control and Meno mice and was significantly blocked by the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME) (100 µM) in Meno, whereas there was a trend toward a significant decrease in control. Data are means ± SE; N = 7 to 6 arterioles from 5 different mice. #P = 0.058, *P < 0.05, mixed-model 2-way ANOVA. D: similarly, no differences in dilation to A23187 were observed between control and Meno mice. Data are means ± SE; N = 7 to 6 arterioles from 5 different mice. E: dilation to the KCa2.3/KCa3.1 activator NS309 is similar in parenchymal arterioles from control vs. Meno mice. Data are means ± SE; N = 8 to 8 arterioles from 8 mice/group, analyzed by 2-way ANOVA with a Bonferroni post hoc correction for multiple comparisons. F: similarly, dilation to 15 mM KCl, dependent on inwardly rectifying K+ channels (KIR2), was unchanged by menopause. Data are means ± SE; N = 8 to 9 arterioles from 8 to 9 mice, analyzed by Student’s t test.
We next assessed the ability of parenchymal arterioles from control and Meno mice to dilate after exposure to carbachol (10 µM) or the Ca2+ ionophore A23187 (1 µM), as well as the NO component of both responses by inhibiting NO synthases with l-NAME (100 µM). We observed that parenchymal arterioles from control mice dilated to carbachol in a NO-dependent manner, as most of the dilation was prevented by l-NAME (vasodilation, 8.51 ± 1.98 vs. 2.79 ± 1.06%, control carbachol vs. control carbachol + l-NAME, P < 0.05; Fig. 3C). Similarly, carbachol-induced vasodilation in parenchymal arterioles from Meno mice was blocked by l-NAME (vasodilation, 10.21 ± 3.86 vs. 0.61 ± 0.24%, Meno carbachol vs. Meno carbachol + l-NAME, P < 0.05; Fig. 3C). However, no differences were observed in carbachol-induced dilation between control and Meno mice (Fig. 3C).
We then assessed vasodilation in response to increases in intracellular Ca2+ induced by A23187 (1 μM). Similar to carbachol, A23187 induced dilation of cerebral parenchymal arterioles from control mice; however, the dilation was not blocked by l-NAME (vasodilation, 15.59 ± 5.89 vs. 12.89 ± 4.87%, control A23187 vs. control A23187 + l-NAME, P > 0.05; Fig. 3D). Arterioles isolated from Meno displayed the same patterns of response (vasodilation, 17.11 ± 3.01 vs. 11.28 ± 1.82%, Meno A23187 vs. Meno A23187 + l-NAME, P > 0.05; Fig. 3D). Vasodilation to A23187 was not statistically different between control and Meno mice. Therefore, we conclude that menopause did not affect NO-dependent dilation in cerebral parenchymal arterioles.
Dilation to Activation of Endothelial K+ Channels Is Unaltered by Menopause
To evaluate whether endothelial KCa2.3/KCa3.1 channel functions were affected in mice, we incubated pressurized parenchymal arterioles to cumulative concentrations of NS309 (0.01 nM to 1 µM), a pharmacological activator of KCa2.3/KCa3.1. NS309 induced a concentration-dependent vasodilation of cerebral parenchymal arterioles that was similar between control and Meno mice (Fig. 3E), suggesting that the function of endothelial KCa2.3/KCa3.1 remains intact in parenchymal arterioles after menopause.
We next examined the dilatory function of KIR2 channels in parenchymal arterioles by exposing them to 15 mM [K+]E. Like NS309, 15 mM [K+]E induced a similar vasodilatory response in arterioles from control and Meno mice (vasodilation, 13.7 ± 3.5 vs. 18.0 ± 3.6%, control vs. Meno, P > 0.05; Fig. 3F). Thus, we conclude that menopause did not disrupt KIR2 function within cerebral parenchymal arterioles.
Structural and Biomechanical Properties of Cerebral Parenchymal Arterioles Remain Unchanged after Menopause
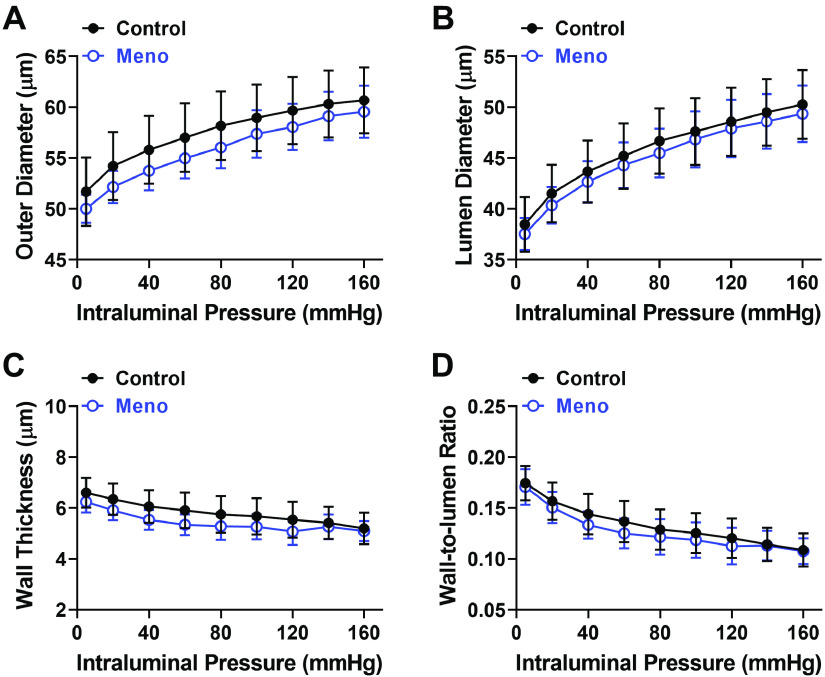
To determine whether menopause induced a structural remodeling in cerebral parenchymal arterioles, passive outer and lumen diameter, wall thickness, and wall-to-lumen ratio were measured. There was no significant difference observed between control and Meno mice in passive outer diameter, lumen diameter, wall thickness, or wall-to-lumen ratio (Fig. 4, A–D, respectively). Similarly, biomechanical properties (distensibility, vascular stiffness, and compliance) were not different between control and Meno mice (Fig. 5, A–C).
Figure 4.
Menopause does not change structural properties of cerebral parenchymal arterioles. Summary graphs showing that outer diameter (A), lumen diameter (B), wall thickness (C), and wall-to-lumen ratio (D) in parenchymal arterioles were unchanged by menopause. Data are means ± SE; N = 8 to 8 arterioles from 8 mice/group, analyzed by 2-way ANOVA with a Bonferroni post hoc correction for multiple comparisons. Passive structural measurements were obtained in pressurized arterioles incubated in Ca2+-free conditions with addition of vasodilators to the superfusing bath.
Figure 5.
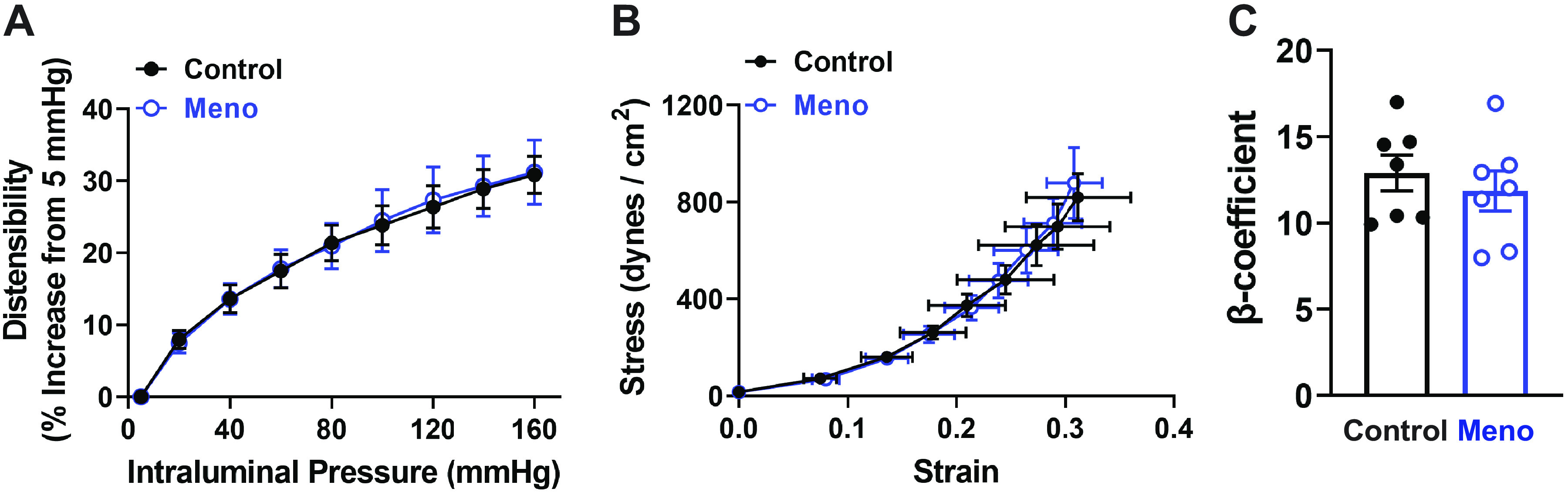
Biomechanical properties of cerebral parenchymal arterioles remain unchanged after menopause. A: summary graph showing that distensibility, measured as % increase in diameter from a low-pressure baseline (5 mmHg), is unchanged by menopause. B: microvascular compliance, assessed by stress-strain relationships of parenchymal arterioles, is unaffected by menopause. Data are means ± SE; N = 8 arterioles from 8 mice/group, analyzed by 2-way ANOVA with a Bonferroni post hoc correction for multiple comparisons. C: summary bar graph demonstrating that the β-coefficient, calculated as the slope of the individual stress-strain curves, is not altered by menopause. Data are means ± SE; N = 7 arterioles from 7 mice/group, analyzed by 2-tailed Student’s t test. Passive biomechanical measurements were obtained in pressurized arterioles incubated in Ca2+-free conditions with addition of vasodilators to the superfusing bath.
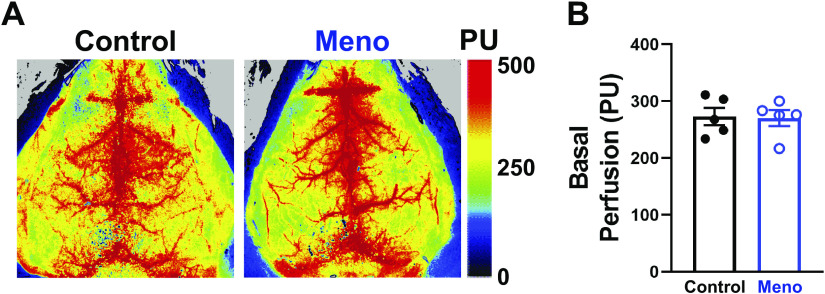
Basal Cerebral Perfusion Is Unaltered by Menopause
Using laser-speckle contrast imaging, we assessed whether the increase in arteriolar myogenic tone translated into a reduction in basal cerebral perfusion in Meno mice. Mechanical ventilation parameters used are shown in Table 2. No significant difference was observed in basal cerebral perfusion within the entire dorsal surface of the cortex between control and Meno mice, as shown in representative perfusion maps (Fig. 6A) and summary graph (perfusion, 273.0 ± 15.1 vs. 270.4 ± 14.1 PU, control vs. Meno, P > 0.05; Fig. 6B). These data suggest that menopause does not alter basal cerebral perfusion.
Table 2.
Ventilation parameters used in cerebral perfusion experiments
| Parameter | Values | ||||
|---|---|---|---|---|---|
| Body weight, g | 26 | 27 | 28 | 29 | 30 |
| Respiratory rate, breaths/min | 138 | 136 | 135 | 134 | 133 |
| Pressure, cmH2O | 15 | 15 | 15 | 15 | 15 |
| Tidal volume, mL | 0.172 | 0.179 | 0.186 | 0.193 | 0.200 |
| Minute volume, mL/min | 23 | 24 | 25 | 25 | 26 |
Figure 6.
Basal cerebral perfusion is unaltered by menopause. A: representative heat maps from laser-speckle contrast imaging showing no change in basal cerebral perfusion between control (left) and menopause (Meno; right) mice. PU, perfusion units. B: summary bar graph showing that basal cerebral perfusion is not significantly altered by menopause in mice. Data are means ± SE; N = 5 mice/group, analyzed by 2-tailed Student’s t test.
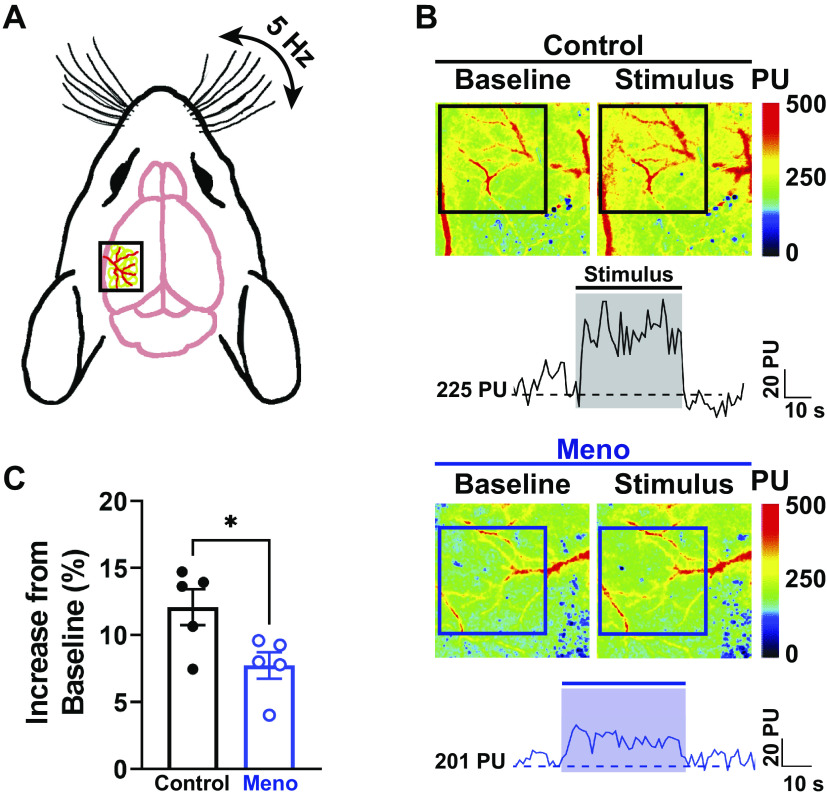
Cerebral Functional Hyperemia Is Impaired by Menopause
Mice were subjected to mechanical whisker stimulations (Fig. 7A) to determine whether menopause alters localized hemodynamic responses in the brain, a hallmark of functional hyperemia and an index of neurovascular health. Baseline perfusion of the whisker barrel cortex following thinning of the skull and lower isoflurane percentage was similar between groups (perfusion, 269 ± 20 vs. 273 ± 30 PU, control vs. Meno). As shown in Fig. 7B, contralateral mechanical stimulation of the whiskers induced a rapid increase in perfusion in control mice, followed by a rapid return to baseline (Fig. 7B, top and trace). This rapid increase in perfusion was also observed in Meno mice, albeit significantly blunted (Fig. 7B, bottom and trace), suggesting that localized hemodynamic responses are blunted in menopausal mice [increase in perfusion from baseline (%), 12.1 ± 1.4 vs. 7.8 ± 1.0%, control vs. Meno, P < 0.05; Fig. 7C). These data suggest that functional hyperemia following somatosensory stimulation is impaired by menopause.
Figure 7.
Menopause impairs neurovascular coupling. A: diagram of a mouse head showing laser-speckle contrast imaging recording on top of the whisker-barrel cortex (black box) during contralateral whisker stimulation. B: representative heat maps and traces at baseline (left) and during mechanical stimulation of the whiskers (right). Squares show the area where measurements were performed (whisker-barrel cortex). Note the increase in perfusion (warmer colors) in the microcirculation of control females (top), which was blunted in menopause (Meno) (bottom). This can also be observed in the representative traces shown below the images. The shaded areas on the traces show the region where the perfusion measurements were taken. PU, perfusion units. C: summary data showing a significantly blunted hemodynamic response upon whisker stimulation in Meno. Data are means ± SE; N = 5 mice/group. *P < 0.05, 2-tailed Student’s t test.
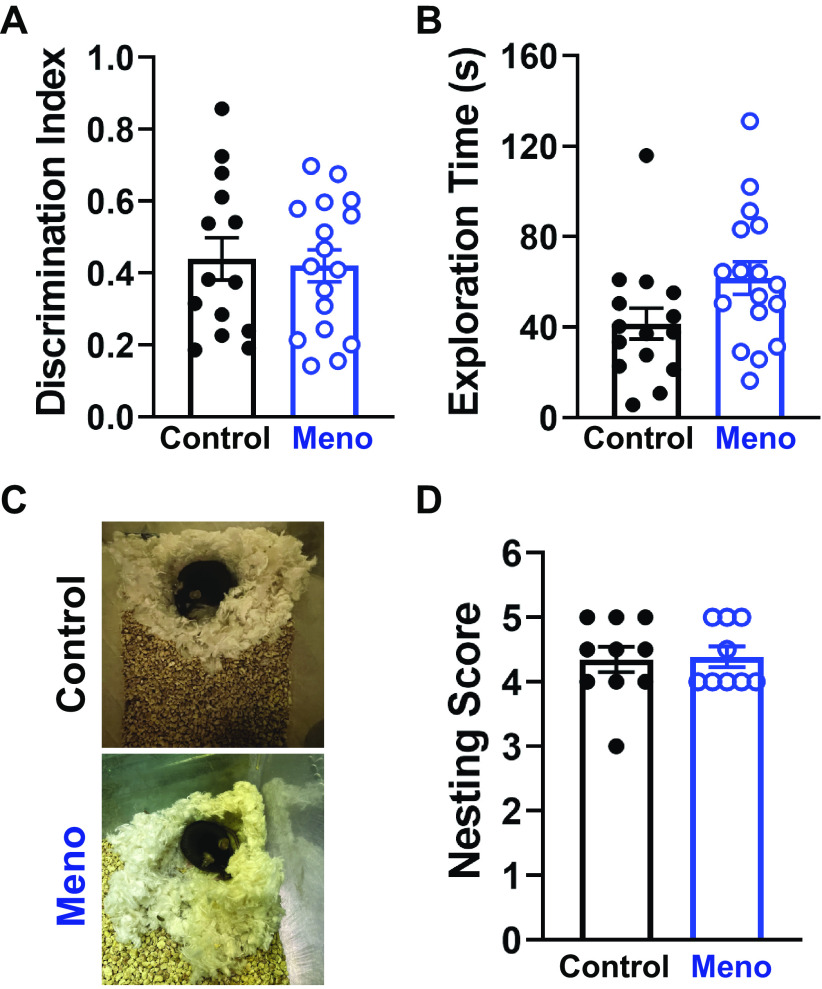
Cognitive Abilities Are Preserved after Menopause in Mice
Because of the observed reduction in cerebral functional hyperemia, we then hypothesized that menopause would impair cognition as measured by novel object recognition and nesting tests in mice. The discrimination index, a measure of the ability of mice to retain memory, was not different between control and Meno (discrimination index, 0.44 ± 0.06 vs. 0.42 ± 0.05, control versus Meno, P > 0.05; Fig. 8A), although there was a trend toward an increase in mobility in Meno mice (total exploration time, 41.6 ± 6.8 vs. 61.71 ± 7.2 s, control vs. Meno, P = 0.0538; Fig. 8B). Nesting scores showed no significant difference in postmenopausal females in their nest-building capabilities (nest score, 4.35 ± 0.2 vs. 4.40 ± 0.16, control vs. Meno, P > 0.05; Fig. 8, C and D). These findings show that cognitive performance is unchanged by menopause in mice.
Figure 8.
Cognitive abilities are preserved in postmenopause females. A: summary data from the novel object recognition test showing no change in discrimination index, suggesting that recognition memory remains intact after menopause in C57BL/6 mice. Data are means ± SE. P > 0.05, 2-tailed Student’s test. B: there was a trend toward an increase in total exploration time in menopause (Meno) mice. Data are means ± SE; N = 14–17 mice/group. P > 0.054, 2-tailed Student’s test. C: representative images demonstrating that nests built by control and Meno mice are similar in shape and completely built. D: summary graph showing no difference in nesting scores between control and Meno. Data are means ± SE; N = 10 to 9 mice/group. P > 0.05, 2-tailed Mann–Whitney test.
DISCUSSION
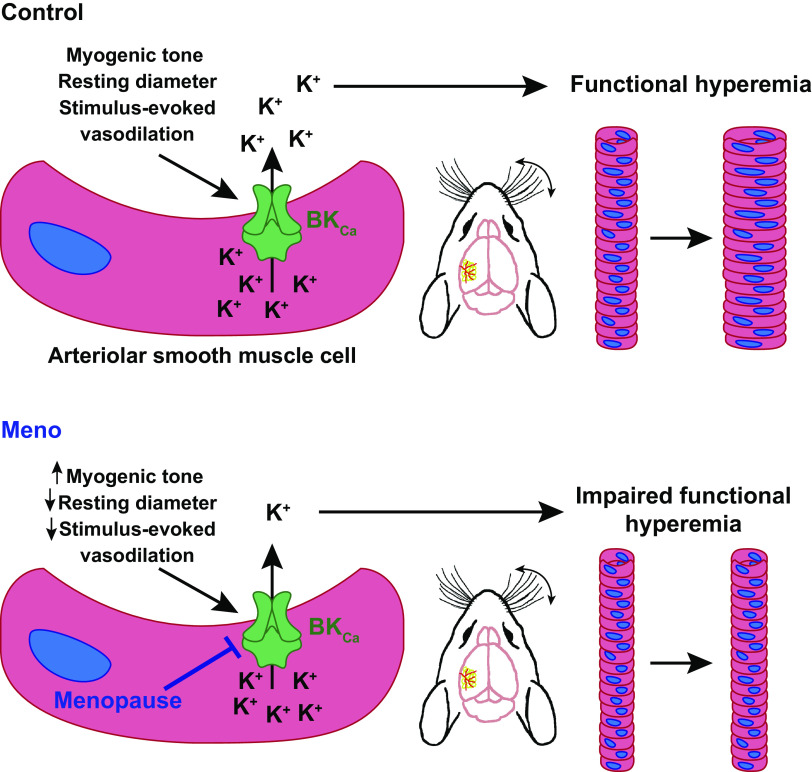
The present study investigated cerebral microvascular alterations in postmenopausal females with a model of chemically induced and progressive ovarian failure, the VCD model of menopause. Our findings show that menopause exaggerates myogenic tone in cerebral parenchymal arterioles, with a concomitant decrease in resting lumen diameter despite an absence of vascular remodeling or apparent endothelial dysfunction, suggesting an impairment in SMC-dependent mechanisms regulating basal myogenic tone. In support of this possibility, our data show that constriction resulting from SMC BKCa channel blockade is smaller in parenchymal arterioles from Meno mice. The impairments in myogenic tone regulation/BKCa activity were associated with a reduction in stimulus-induced hemodynamic responses (i.e., functional hyperemia) in Meno mice but did not affect basal cerebral perfusion. Interestingly, the microvascular and hemodynamic impairments did not lead to cognitive decline. Together, our data show that a physiologically relevant model of menopause, in which there is a gradual decline in circulating levels of estrogen while androgen production remains unaltered (39), induces cerebral microvascular and neurovascular impairments in mice, likely via affecting SMC pathways important for fine-tuning myogenic tone and stimulus-evoked vasodilation (Fig. 9).
Figure 9.
Diagram summarizing findings of this study. Top: in regularly cycling females (control), somatosensory stimulation leads to the release of a stimulus-evoked vasodilation onto parenchymal arteriole smooth muscle cells (SMCs), which leads to activation of large-conductance Ca2+-activated K+ channels (BKCa), K+ efflux, arteriolar dilation, and functional hyperemia. Bottom: after menopause (Meno), BKCa function in arteriolar SMC is impaired, associated with an increase in spontaneous myogenic tone and lower resting lumen diameter. In addition, functional hyperemia is blunted, leading to a smaller increase in perfusion after whisker stimulation.
Maintenance of proper brain function, necessary to sustain cognitive health, is dependent on the ability of the cerebral microcirculation to supply necessary nutrients in real time to active neurons. Despite their high metabolic rate, neurons have limited energy storage and are highly sensitive to conditions of reduced nutrient availability, and neuronal death can occur as early as 3–5 min after nutrient depletion (60). In the brain, functional hyperemia depends on two main factors: 1) the ability of neurons/glia to communicate to parenchymal arterioles the need for increased nutrient delivery and 2) a functionally intact microvasculature to properly respond to this demand, particularly in arterioles, the bottlenecks of the cerebral circulation. Problems arise when there is a breakdown in these factors, which can lead to neuronal death and, over time, cognitive decline.
The link between menopause and cognitive decline is becoming increasingly clear. Epidemiological studies show that the incidence of cognitive impairment is higher in menopausal women than in age-matched men (6), which becomes even more evident in the presence of genetic risk factors for development of dementia (61). Despite the evidence, underlying mechanisms responsible for this increase in susceptibility remain incompletely understood. Estrogens are important to maintain brain oxidative metabolism of glucose, as they regulate glucose uptake by both capillary endothelial cells and neurons (62), and a drop in estrogens induces a ketogenic metabolic profile (63, 64), which can ultimately lead to neurodegeneration by inducing oxidative stress and chronic neuroinflammation (65). In addition, estrogens, or lack thereof, also have substantial effects on the cerebral circulation. Previous studies in rats show that ovariectomy induces an increase in myogenic tone in middle cerebral arteries (24) and parenchymal arterioles (3). Our study agrees with these previous findings, as we also observed a significant increase in basal myogenic tone of parenchymal arterioles of Meno mice, which resulted in a reduction in resting lumen diameter (i.e., the arteriolar diameter after stable myogenic tone). We predicted that these factors combined would result in basal hypoperfusion of the cortex; however, we observed that perfusion was similar between control and Meno mice. A possible explanation for the lack of basal hypoperfusion is that surface arteries and arterioles, located in the dorsal pial surface (the region recorded by laser-speckle contrast imaging), may not show changes in myogenic tone as observed in the parenchymal arterioles used for our pressure myography experiments. Future studies are needed to use arterial spin labeling magnetic resonance imaging to assess blood flow in deeper brain regions of Meno mice.
Regulation of myogenic tone is a complex mechanism that involves the endothelium and SMC, as well as communication between them. In general, endothelial cells exert an inhibitory influence on SMC contractility and myogenic tone via paracrine (release of soluble factors such as NO) and/or electrotonic communication after endothelial cell hyperpolarization (66). The role of NO in regulating cerebral microvascular function is well characterized (for reviews, see Refs. 67–69), and, under physiological conditions, NO release by endothelial cells reduces SMC contractility. Additionally, NO signaling is modulated by estrogen (70, 71), and NO bioavailability and NO-dependent dilation are reduced in menopause (25, 72). Thus, it was logical to investigate whether changes in NO contributed to the increase in myogenic tone and neurovascular impairment observed in Meno mice. However, our data suggest that this pathway seems preserved after menopause, as NO generation and arteriolar dilation after exposure to a muscarinic cholinergic agonist were similar between groups. We then investigated a possible impairment in endothelium-dependent hyperpolarization mechanisms by focusing on dilation after activation of KCa2.3/KCa3.1 (73) and KIR2 (74). In particular, KCa2.3 was previously shown to be impaired in ovariectomized mice (22); thus, we hypothesized that parenchymal arterioles from Meno mice would show a similar impairment. Like NO signaling, we observed that KCa2.3/KCa3.1 and KIR2 functions were not affected by menopause. Finally, we observed that endothelium removal also had no effect on the difference between control and Meno myogenic tone. Together, these data suggest that menopause did not affect endothelial function in parenchymal arterioles, which contrasts with studies in the periphery that show loss of endothelium-dependent vasodilation in ovariectomized rodents (75–77). These findings propelled us to focus on SMC mechanisms regulating vascular reactivity.
There are two major molecular pathways in SMC that counterbalance myogenic tone by influencing membrane voltage: opening of KV and/or BKCa. The current hypothesis on the regulation of SMC contractility and myogenic tone states that mechanosensors present in the plasma membrane of SMCs are activated by stretch as a consequence of increases in intraluminal pressure. These mechanosensors will then generate signals that lead to opening of voltage-gated Ca2+ channels, resulting in membrane depolarization and SMC contraction (15, 17). The resulting depolarization increases the open probability of KV, which conduct an outward current of K+, thus balancing charges in the SMCs to maintain membrane potential and contractility in equilibrium (78). Changes in KV function are known to lead to alterations in vascular contractility, such as observed in a mouse model of cerebral small vessel disease (14). However, to the best of our knowledge, no studies have evaluated SMC KV activity after menopause. We observed that in control mice KV blockade induced a robust constriction of parenchymal arterioles in a concentration-dependent manner. Interestingly, the constriction in Meno mice was also present and statistically similar to control mice, but it was not concentration dependent: increasing 4-AP from 1 mM to 5 mM did not induce further contraction. The underlying reason for the observed response remains unidentified, but it is possible that menopause is reducing expression of KV in SMCs or affecting its regulation by protein kinase A, a known target of estradiol in cardiac cells (79). These possibilities need to be explored in future studies.
The interplay between SMC BKCa activity and menopause is better characterized, and it is clear that sex hormones are important regulators of these channels. BKCa channels are activated by localized increases in intracellular Ca2+ (Ca2+ sparks), as well as membrane depolarization (80), and conduct large outward K+ currents to hyperpolarize SMCs, leading to vasorelaxation (81). The importance of BKCa in regulating cerebral vascular myogenic tone has been shown in different studies, where loss of BKCa activity in SMCs leads to a hypercontractile phenotype (58, 59) or overactivation causes a breakdown in cerebral arterial autoregulation (82). BKCa channels consist of an α1-subunit, which forms the pore, and two regulatory subunits, β and γ. Expression of the β1-subunit is regulated by estrogen (83), and estrogen can bind directly to the β1-subunit, which leads to an increase in the opening probability of BKCa channels and, consequently, higher membrane hyperpolarization (33). On the other hand, estrogen depletion leads to a reduction in expression of the β1-subunit in rat cerebral arteries, which was associated with enhanced contractility (24). This agrees with our findings, as we observed that the BKCa channel blockade induced a smaller contraction of parenchymal arterioles from Meno than control females, although we did not explore whether a reduction in expression of the β1-subunit occurred in our model. Interestingly, the reduced BKCa channel function in our mice may also be explained by effects of androgens over the α1-subunit, as the VCD model depletes only estrogen-producing cells and androgen production is maintained (84). Androgens were previously shown to block BKCa currents in adenohypophyseal cells by acting on the α1-subunit (85), and it is possible that the same effect may be occurring in SMCs from Meno mice. Independent of the molecular mechanism, our study highlights the importance of estrogen in regulating BKCa function, and consequently myogenic constriction, in the cerebral microcirculation.
BKCa is also an important effector of functional hyperemia responses in the brain. Cerebral functional hyperemia is a multicellular response that involves the integration of neuronal signals, activation of astrocytes, and, ultimately, parenchymal arteriole dilation. The current dogma proposes that extensions of astrocytes monitor neuronal synapses and relay increases in synaptic activity to the vasculature, thus leading to functional hyperemia by releasing vasodilators onto parenchymal arterioles (86). Some of these vasoactive molecules elicit dilation by activating SMC BKCa, including 11,12-epoxyeicosatrienoic acids (87, 88). Interestingly, a previous study showed that ovariectomy alone did not affect functional hyperemia responses in mice unless an additional challenge was added to the females, such as hypertension (89). Although seemingly in disagreement with our findings, the explanation for the different results may reside in the models used (VCD vs. ovariectomy) and the presence of circulating androgens. Clinical studies suggest that androgens are deleterious to the female cardiovascular system, leading to endothelial dysfunction (90) and hypertension (91). It is possible that in VCD-induced menopause, the loss of protective estrogen signaling with maintenance of androgens causes an endocrine imbalance that exacerbates cerebral microvascular dysfunction, resulting in neurovascular impairment. However, Meno mice did not present cognitive deficits, and the reasons underlying the preservation of cognitive function remain unclear.
A final point is the effect of menopause on the blood-brain barrier and its possible consequences for neurovascular function. A study using ovariectomized mice showed that the blood-brain barrier is leaky in menopausal females, likely because of the increase in circulating gonadotropins rather than loss of sex hormones (92). In addition, another study with humanized ApoE4 mice fed a high-fat diet showed that VCD-induced menopause is associated with cognitive dysfunction (93). Increased blood-brain barrier permeability would allow for neurotoxic substances to move from the plasma into the brain parenchyma, thus leading to neurodegeneration and, over time, cognitive decline. However, to our knowledge, little is known of the interplay between blood-brain barrier integrity and neurovascular coupling/functional hyperemia. Such studies would be of great interest in the future.
Limitations
A few limitations to our study should be mentioned. First, we did not perform molecular measurements to assess BKCa channel expression in the cerebral parenchymal arterioles. These experiments are important, as they would allow for a better understanding on whether menopause induced by VCD affects expression of the different subunits of BKCa channels or whether the deficits observed are in the functional regulation of the channel. Second, we are not yet set up to perform electrophysiology recordings of SMC BKCa channel behavior in the cerebral microvasculature and how its activity is modulated by estrogens or androgens. Furthermore, in vivo assessment of cerebral blood flow with magnetic resonance imaging would allow us to elucidate whether the observed increase in myogenic tone of parenchymal arterioles isolated from deeper cortical regions results in deficits in basal perfusion.
Another limitation in our study is that we did not investigate how menopause may affect neurons or astrocytes in regard to modulation of neurovascular coupling. Estrogen was shown to stimulate expression of neuronal nitric oxide synthase in neutrophils (94), and this enzyme is an important mediator of functional hyperemia responses (95). However, whether menopause affects expression of neuronal nitric oxide synthase in mice, and its association to disrupted functional hyperemia, remains unknown. Similarly, to our knowledge, little is known regarding how menopause affects astrocyte physiology. A previous study showed that ovariectomy did not affect the number of astrocytes in the rat dentate gyrus, but it did reduce their surface density (96); however, it is unclear whether there are alterations in the astrocyte end feet. Finally, BKCa in astrocyte end feet mediate neuronal-stimulated parenchymal arteriolar dilation (97); thus, it is possible that, in our model, menopause also impaired this pathway and may contribute to the neurovascular dysfunction observed.
In summary, we show that an increase in myogenic tone is associated with cerebral microvascular SMC BKCa channel dysfunction in menopausal mice. This reduction led to in vivo consequences, observed as impaired functional hyperemia. Despite such impairment, we found no differences in basal cerebral perfusion or cognitive abilities after menopause. Future studies are needed to focus on investigating the molecular mechanisms, signaling pathway disruptions, and changes in ion channel behavior underlying cerebral microvascular dysfunction after menopause.
GRANTS
The present work was funded by National Institutes of Health Grants R00 HL140106 and R01 AG073230 (to P.W.P.), R01 HL131834 (to H.L.B.), R01 NS099292 (to T.M.L.-M.), and P30 DA051355 (to T.M.L.-M., Behavioral Core Director) and Alzheimer’s Association Grant AARGD-21–850835 (to P.W.P.).
DISCLAIMERS
The data and interpretation presented in this article reflect the authors’ opinions and not those of the funding agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.B., J.F.S., H.L.B., and P.W.P. conceived and designed research; J.A.B., J.F.S., E.M.L., A.S., T.M.L.-M., and P.W.P. performed experiments; J.A.B., J.F.S., E.M.L., A.S., T.M.L.-M., and P.W.P. analyzed data; J.A.B., J.F.S., A.S., T.M.L.-M., H.L.B., and P.W.P. interpreted results of experiments; J.A.B., J.F.S., and P.W.P. prepared figures; J.A.B. and P.W.P. drafted manuscript; J.A.B., J.F.S., E.M.L., A.S., T.M.L.-M., H.L.B., and P.W.P. edited and revised manuscript; J.A.B., J.F.S., E.M.L., A.S., T.M.L.-M., H.L.B., and P.W.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Joshua Uhlorn for technical help with the VCD model of menopause and Kelly Karlage for providing training on the novel object recognition test.
REFERENCES
- 1. Stute P, Ceausu I, Depypere H, Lambrinoudaki I, Mueck A, Pérez-López FR, van der Schouw YT, Senturk LM, Simoncini T, Stevenson JC, Rees M. A model of care for healthy menopause and ageing: EMAS position statement. Maturitas 92: 1–6, 2016. doi: 10.1016/j.maturitas.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 2. Ruediger SL, Koep JL, Keating SE, Pizzey FK, Coombes JS, Bailey TG. Effect of menopause on cerebral artery blood flow velocity and cerebrovascular reactivity: Systematic review and meta-analysis. Maturitas 148: 24–32, 2021. doi: 10.1016/j.maturitas.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 3. Cipolla MJ, Godfrey JA, Wiegman MJ. The effect of ovariectomy and estrogen on penetrating brain arterioles and blood-brain barrier permeability. Microcirculation 16: 685–693, 2009. doi: 10.3109/10739680903164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5: 347–360, 2004. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 5. Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 100: 328–335, 2006. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 16: 391–460, 2020. doi: 10.1002/alz.12068. [DOI] [PubMed] [Google Scholar]
- 7. Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci USA 104: 365–370, 2007. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nature Neurosci 16: 55–63, 2013. doi: 10.1038/nn.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pires PW, Jackson WF, Dorrance AM. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am J Physiol Heart Circ Physiol 309: H127–H136, 2015. doi: 10.1152/ajpheart.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palomares SM, Cipolla MJ. Myogenic tone as a therapeutic target for ischemic stroke. Curr Vasc Pharmacol 12: 788–800, 2014. doi: 10.2174/15701611113116660155. [DOI] [PubMed] [Google Scholar]
- 11. Diaz-Otero JM, Yen TC, Ahmad A, Laimon-Thomson E, Abolibdeh B, Kelly K, Lewis MT, Wiseman RW, Jackson WF, Dorrance AM. Transient receptor potential vanilloid 4 channels are important regulators of parenchymal arteriole dilation and cognitive function. Microcirculation 26: e12535, 2019. doi: 10.1111/micc.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diaz-Otero JM, Yen TC, Fisher C, Bota D, Jackson WF, Dorrance AM. Mineralocorticoid receptor antagonism improves parenchymal arteriole dilation via a TRPV4-dependent mechanism and prevents cognitive dysfunction in hypertension. Am J Physiol Heart Circ Physiol 315: H1304–H1315, 2018. doi: 10.1152/ajpheart.00207.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Silva TM, Modrick ML, Dabertrand F, Faraci FM. Changes in cerebral arteries and parenchymal arterioles with aging: role of rho kinase 2 and impact of genetic background. Hypertension 71: 921–927, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dabertrand F, Krøigaard C, Bonev AD, Cognat E, Dalsgaard T, Domenga-Denier V, Hill-Eubanks DC, Brayden JE, Joutel A, Nelson MT. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc Natl Acad Sci USA 112: E796–E805, 2015. doi: 10.1073/pnas.1420765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCgamma1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal 7: ra49, 2014. doi: 10.1126/scisignal.2004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brayden JE, Li Y, Tavares MJ. Purinergic receptors regulate myogenic tone in cerebral parenchymal arterioles. J Cereb Blood Flow Metab 33: 293–299, 2013. doi: 10.1038/jcbfm.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiology (Bethesda) 29: 343–360, 2014. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tykocki NR, Boerman EM, Jackson WF. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr Physiol 7: 485–581, 2017. doi: 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett 584: 2033–2042, 2010. doi: 10.1016/j.febslet.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res 110: 285–294, 2012. doi: 10.1161/CIRCRESAHA.111.258145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dabertrand F, Nelson MT, Brayden JE. Ryanodine receptors, calcium signaling, and regulation of vascular tone in the cerebral parenchymal microcirculation. Microcirculation 20: 307–316, 2013. doi: 10.1111/micc.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yap FC, Taylor MS, Lin MT. Ovariectomy-induced reductions in endothelial SK3 channel activity and endothelium-dependent vasorelaxation in murine mesenteric arteries. PLoS One 9: e104686, 2014. doi: 10.1371/journal.pone.0104686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 24. Reed JT, Pareek T, Sriramula S, Pabbidi MR. Aging influences cerebrovascular myogenic reactivity and BK channel function in a sex-specific manner. Cardiovasc Res 116: 1372–1385, 2020. doi: 10.1093/cvr/cvz314. [DOI] [PubMed] [Google Scholar]
- 25. Pelligrino DA, Ye S, Tan F, Santizo RA, Feinstein DL, Wang Q. Nitric-oxide-dependent pial arteriolar dilation in the female rat: effects of chronic estrogen depletion and repletion. Biochem Biophys Res Commun 269: 165–171, 2000. doi: 10.1006/bbrc.2000.2206. [DOI] [PubMed] [Google Scholar]
- 26. Tang YR, Yang WW, Wang Y, Gong YY, Jiang LQ, Lin L. Estrogen regulates the expression of small-conductance Ca-activated K+ channels in colonic smooth muscle cells. Digestion 91: 187–196, 2015. doi: 10.1159/000371544. [DOI] [PubMed] [Google Scholar]
- 27. Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci 20: 717–726, 2017. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mughal A, Sackheim AM, Sancho M, Longden TA, Russell S, Lockette W, Nelson MT, Freeman K. Impaired capillary-to-arteriolar electrical signaling after traumatic brain injury. J Cereb Blood Flow Metab 41: 1313–1327, 2021. doi: 10.1177/0271678X20962594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayata C, Ma J, Meng W, Huang P, Moskowitz MA. L-NA-sensitive rCBF augmentation during vibrissal stimulation in type III nitric oxide synthase mutant mice. J Cereb Blood Flow Metab 16: 539–541, 1996. doi: 10.1097/00004647-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 30. Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol 309: H1837–H1845, 2015. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krishnamoorthy-Natarajan G, Koide M. BK channels in the vascular system. Int Rev Neurobiol 128: 401–438, 2016. doi: 10.1016/bs.irn.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 32. Dopico AM, Bukiya AN, Jaggar JH. Calcium- and voltage-gated BK channels in vascular smooth muscle. Pflugers Arch 470: 1271–1289, 2018. doi: 10.1007/s00424-018-2151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Wet H, Allen M, Holmes C, Stobbart M, Lippiat JD, Callaghan R. Modulation of the BK channel by estrogens: examination at single channel level. Mol Membr Biol 23: 420–429, 2006. doi: 10.1080/09687860600802803. [DOI] [PubMed] [Google Scholar]
- 34. Nagar D, Liu XT, Rosenfeld CR. Estrogen regulates β1-subunit expression in Ca2+-activated K+ channels in arteries from reproductive tissues. Am J Physiol Heart Circ Physiol 289: H1417–H1427, 2005. doi: 10.1152/ajpheart.01174.2004. [DOI] [PubMed] [Google Scholar]
- 35. Fernández-Fernández JM, Tomás M, Vázquez E, Orio P, Latorre R, Sentí M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest 113: 1032–1039, 2004. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nieves-Cintrón M, Syed AU, Nystoriak MA, Navedo MF. Regulation of voltage-gated potassium channels in vascular smooth muscle during hypertension and metabolic disorders. Microcirculation 25: e12423, 2018. doi: 10.1111/micc.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Du J, Wang Q, Hu F, Wang J, Ding H, Gao R, Xiao H, Wang L. Effects of estradiol on voltage-gated potassium channels in mouse dorsal root ganglion neurons. J Membr Biol 247: 541–548, 2014. doi: 10.1007/s00232-014-9670-z. [DOI] [PubMed] [Google Scholar]
- 38. Druzin M, Malinina E, Grimsholm O, Johansson S. Mechanism of estradiol-induced block of voltage-gated K+ currents in rat medial preoptic neurons. PLoS One 6: e20213, 2011. doi: 10.1371/journal.pone.0020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda) 31: 250–257, 2016. doi: 10.1152/physiol.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pollow DP Jr, Uhlorn JA, Sylvester MA, Romero-Aleshire MJ, Uhrlaub JL, Lindsey ML, Nikolich-Zugich J, Brooks HL. Menopause and FOXP3+ Treg cell depletion eliminate female protection against T cell-mediated angiotensin II hypertension. Am J Physiol Heart Circ Physiol 317: H415–H423, 2019. doi: 10.1152/ajpheart.00792.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk JL, Bouxsein ML, Hoyer PB. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res 23: 1296–1303, 2008. doi: 10.1359/jbmr.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sylvester MA, Pollow DP Jr, Moffett C, Nunez W, Uhrlaub JL, Nikolich-Zugich J, Brooks HL. Splenocyte transfer from hypertensive donors eliminates premenopausal female protection from ANG II-induced hypertension. Am J Physiol Renal Physiol 322: F245–F257, 2022. doi: 10.1152/ajprenal.00369.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pires PW, Dabertrand F, Earley S. Isolation and cannulation of cerebral parenchymal arterioles. J Vis Exp 111: 53835, 2016. doi: 10.3791/53835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosehart AC, Johnson AC, Dabertrand F. Ex vivo pressurized hippocampal capillary-parenchymal arteriole preparation for functional study. J Vis Exp 154: 60676, 2019. doi: 10.3791/60676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ralevic V, Kristek F, Hudlická O, Burnstock G. A new protocol for removal of the endothelium from the perfused rat hind-limb preparation. Circ Res 64: 1190–1196, 1989. doi: 10.1161/01.res.64.6.1190. [DOI] [PubMed] [Google Scholar]
- 46. Pires PW, Ko EA, Pritchard HA, Rudokas M, Yamasaki E, Earley S. The angiotensin II receptor type 1b is the primary sensor of intraluminal pressure in cerebral artery smooth muscle cells. J Physiol 595: 4735–4753, 2017. doi: 10.1113/JP274310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N. Advanced methods of microscope control using muManager software. J Biol Methods 1: e10, 2014. doi: 10.14440/jbm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chan SL, Chapman AC, Sweet JG, Gokina NI, Cipolla MJ. Effect of PPARgamma inhibition during pregnancy on posterior cerebral artery function and structure. Front Physiol 1: 130, 2010. doi: 10.3389/fphys.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension 21: 816–826, 1993. doi: 10.1161/01.HYP.21.6.816. [DOI] [PubMed] [Google Scholar]
- 50. Baumbach GL, Walmsley JG, Hart MN. Composition and mechanics of cerebral arterioles in hypertensive rats. Am J Pathol 133: 464–471, 1988. [PMC free article] [PubMed] [Google Scholar]
- 51. Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc 5: 201–208, 2010. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 52: e21–e31, 2011. [PMC free article] [PubMed] [Google Scholar]
- 53. Franceschini MA, Radhakrishnan H, Thakur K, Wu W, Ruvinskaya S, Carp S, Boas DA. The effect of different anesthetics on neurovascular coupling. Neuroimage 51: 1367–1377, 2010. doi: 10.1016/j.neuroimage.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protoc 8: 2531–2537, 2013. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 55. Hay M, Polt R, Heien ML, Vanderah TW, Largent-Milnes TM, Rodgers K, Falk T, Bartlett MJ, Doyle KP, Konhilas JP. A Novel angiotensin-(1-7) glycosylated mas receptor agonist for treating vascular cognitive impairment and inflammation-related memory dysfunction. J Pharmacol Exp Ther 369: 9–25, 2019. doi: 10.1124/jpet.118.254854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pandit V, Khan M, Zakaria ER, Largent-Milnes TM, Hamidi M, O’Keeffe T, Vanderah TW, Joseph B. Continuous remote ischemic conditioning attenuates cognitive and motor deficits from moderate traumatic brain injury. J Trauma Acute Care Surg 85: 48–53, 2018. doi: 10.1097/TA.0000000000001835. [DOI] [PubMed] [Google Scholar]
- 57. Deacon RM. Assessing nest building in mice. Nat Protoc 1: 1117–1119, 2006. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 58. Pritchard HAT, Gonzales AL, Pires PW, Drumm BT, Ko EA, Sanders KM, Hennig GW, Earley S. Microtubule structures underlying the sarcoplasmic reticulum support peripheral coupling sites to regulate smooth muscle contractility. Sci Signal 10: eaan2694, 2017. doi: 10.1126/scisignal.aan2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pritchard HA, Griffin CS, Yamasaki E, Thakore P, Lane C, Greenstein AS, Earley S. Nanoscale coupling of junctophilin-2 and ryanodine receptors regulates vascular smooth muscle cell contractility. Proc Natl Acad Sci USA 116: 21874–21881, 2019. doi: 10.1073/pnas.1911304116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 67: 181–198, 2010. [Erratum in Neuron 68: 161, 2010]. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pertesi S, Coughlan G, Puthusseryppady V, Morris E, Hornberger M. Menopause, cognition and dementia—a review. Post Reprod Health 25: 200–206, 2019. doi: 10.1177/2053369119883485. [DOI] [PubMed] [Google Scholar]
- 62. Shi J, Simpkins JW. 17beta-Estradiol modulation of glucose transporter 1 expression in blood-brain barrier. Am J Physiol Endocrinol Metab 272: E1016–E1022, 1997. doi: 10.1152/ajpendo.1997.272.6.E1016. [DOI] [PubMed] [Google Scholar]
- 63. Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv Drug Deliv Rev 60: 1504–1511, 2008. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ding F, Yao J, Rettberg JR, Chen S, Brinton RD. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PLoS One 8: e79977, 2013. doi: 10.1371/journal.pone.0079977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Picca A, Calvani R, Coelho-Junior HJ, Landi F, Bernabei R, Marzetti E. Mitochondrial dysfunction, oxidative stress, and neuroinflammation: intertwined roads to neurodegeneration. Antioxidants (Basel) 9: 647, 2020. doi: 10.3390/antiox9080647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci 16: 23–30, 1995. [Erratum in Trends Pharmacol Sci 16: 177, 1995]. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- 67. Atochin DN, Huang PL. Role of endothelial nitric oxide in cerebrovascular regulation. Curr Pharm Biotechnol 12: 1334–1342, 2011. doi: 10.2174/138920111798280974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee TJ. Nitric oxide and the cerebral vascular function. J Biomed Sci 7: 16–26, 2000. doi: 10.1007/BF02255914. [DOI] [PubMed] [Google Scholar]
- 69. Faraci FM, Brian JE Jr.. Nitric oxide and the cerebral circulation. Stroke 25: 692–703, 1994. doi: 10.1161/01.str.25.3.692. [DOI] [PubMed] [Google Scholar]
- 70. McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke 33: 1685–1691, 2002. doi: 10.1161/01.str.0000016325.54374.93. [DOI] [PubMed] [Google Scholar]
- 71. Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension 28: 330–334, 1996. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- 72. Klawitter J, Hildreth KL, Christians U, Kohrt WM, Moreau KL. A relative L-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol Rep 5: e13409, 2017. doi: 10.14814/phy2.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol 553: 183–189, 2003. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Longden TA, Dabertrand F, Gonzales AL, Koide M, Nelson M. Potassium sensing by capillary kir channels regulates cerebral blood flow (Abstract). J Gen Physiol 146: 10A, 2015. [Google Scholar]
- 75. Camporez JP, Akamine EH, Davel AP, Franci CR, Rossoni LV, Carvalho CR. Dehydroepiandrosterone protects against oxidative stress-induced endothelial dysfunction in ovariectomized rats. J Physiol 589: 2585–2596, 2011. doi: 10.1113/jphysiol.2011.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cola MS, Gava AL, Meyrelles SS, Vasquez EC. Endothelial dysfunction of resistance vessels in female apolipoprotein E-deficient mice. Lipids Health Dis 9: 51, 2010. doi: 10.1186/1476-511X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wassmann S, Bäumer AT, Strehlow K, van Eickels M, Grohé C, Ahlbory K, Rösen R, Böhm M, Nickenig G. Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circulation 103: 435–441, 2001. doi: 10.1161/01.cir.103.3.435. [DOI] [PubMed] [Google Scholar]
- 78. Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol Heart Circ Physiol 269: H348–H355, 1995. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- 79. Maslov PZ, Kim JK, Argulian E, Ahmadi A, Narula N, Singh M, Bax J, Narula J. Is cardiac diastolic dysfunction a part of post-menopausal syndrome? JACC Heart Fail 7: 192–203, 2019. doi: 10.1016/j.jchf.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 80. Gonzalez-Perez V, Lingle CJ. Regulation of BK channels by beta and gamma subunits. Annu Rev Physiol 81: 113–137, 2019. doi: 10.1146/annurev-physiol-022516-034038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pérez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol 113: 229–238, 1999. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pritchard HA, Pires PW, Yamasaki E, Thakore P, Earley S. Nanoscale remodeling of ryanodine receptor cluster size underlies cerebral microvascular dysfunction in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 115: E9745–E9752, 2018. doi: 10.1073/pnas.1804593115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li XT, Qiu XY. 17beta-estradiol upregulated expression of alpha and beta subunits of larger-conductance calcium-activated K+ channels (BK) via estrogen receptor beta. J Mol Neurosci 56: 799–807, 2015. doi: 10.1007/s12031-015-0502-0. [DOI] [PubMed] [Google Scholar]
- 84. Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod 71: 130–138, 2004. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 85. Suárez L, Bilal U, Bordallo J, Cantabrana B, Sánchez M. Androgens block outward potassium currents and decrease spontaneous action potentials in GH3 cells. Naunyn Schmiedebergs Arch Pharmacol 388: 67–78, 2015. doi: 10.1007/s00210-014-1057-2. [DOI] [PubMed] [Google Scholar]
- 86. Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke 29: 229–234, 1998. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 87. Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol 263: H519–H525, 1992. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 88. Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol 294: H2855–H2863, 2008. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am J Physiol Heart Circ Physiol 294: H156–H163, 2008. doi: 10.1152/ajpheart.01137.2007. [DOI] [PubMed] [Google Scholar]
- 90. Calderon-Margalit R, Schwartz SM, Wellons MF, Lewis CE, Daviglus ML, Schreiner PJ, Williams OD, Sternfeld B, Carr JJ, O’Leary DH, Sidney S, Friedlander Y, Siscovick DS. Prospective association of serum androgens and sex hormone-binding globulin with subclinical cardiovascular disease in young adult women: the “Coronary Artery Risk Development in Young Adults” women’s study. J Clin Endocrinol Metab 95: 4424–4431, 2010. doi: 10.1210/jc.2009-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gulanski BI, Flannery CA, Peter PR, Leone CA, Stachenfeld NS. Compromised endothelial function in transgender men taking testosterone. Clin Endocrinol (Oxf) 92: 138–144, 2020. doi: 10.1111/cen.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wilson AC, Clemente L, Liu T, Bowen RL, Meethal SV, Atwood CS. Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochim Biophys Acta 1782: 401–407, 2008. doi: 10.1016/j.bbadis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 93. Pontifex MG, Martinsen A, Saleh RN, Harden G, Fox C, Muller M, Vauzour D, Minihane AM. DHA-Enriched Fish Oil Ameliorates Deficits in Cognition Associated with Menopause and the APOE4 Genotype in Rodents. Nutrients 14: 1698, 2022. doi: 10.3390/nu14091698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. García-Durán M, de Frutos T, Díaz-Recasens J, García-Gálvez G, Jiménez A, Montón M, Farre J, Sánchez de Miguel L, González-Fernández F, Arriero MD, Rico L, García R, Casado S, López-Farré A. Estrogen stimulates neuronal nitric oxide synthase protein expression in human neutrophils. Circ Res 85: 1020–1026, 1999. doi: 10.1161/01.res.85.11.1020. [DOI] [PubMed] [Google Scholar]
- 95. Lourenço CF, Santos RM, Barbosa RM, Cadenas E, Radi R, Laranjinha J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic Biol Med 73: 421–429, 2014. doi: 10.1016/j.freeradbiomed.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 96. Luquin S, Naftolin F, Garcia-Segura LM. Natural fluctuation and gonadal hormone regulation of astrocyte immunoreactivity in dentate gyrus. J Neurobiol 24: 913–924, 1993. doi: 10.1002/neu.480240705. [DOI] [PubMed] [Google Scholar]
- 97. Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]