
Keywords: core temperature, hot bath, thermoregulation, thyroxine, triiodothyronine
Abstract
This research compared thermal and perceptual adaptations, endurance capacity, and overreaching markers in men after 3, 6, and 12 days of post-exercise hot water immersion (HWI) or exercise heat acclimation (EHA) with a temperate exercise control (CON), and examined thyroid hormones as a mechanism for the reduction in resting and exercising core temperature (Tre) after HWI. HWI involved a treadmill run at 65% V̇o2peak at 19°C followed by a 40°C bath. EHA and CON involved a work-matched treadmill run at 65% V̇o2peak at 33°C or 19°C, respectively. Compared with CON, resting mean body temperature (Tb), resting and end-exercise Tre, Tre at sweating onset, thermal sensation, and perceived exertion were lower and whole-body sweat rate (WBSR) was higher after 12 days of HWI (all P ≤ 0.049, resting Tb: CON −0.11 ± 0.15°C, HWI −0.41 ± 0.15°C). Moreover, resting Tb and Tre at sweating onset were lower after HWI than EHA (P ≤ 0.015, resting Tb: EHA −0.14 ± 0.14°C). No differences were identified between EHA and CON (P ≥ 0.157) except WBSR that was greater after EHA (P = 0.013). No differences were observed between interventions for endurance capacity or overreaching markers (mood, sleep, Stroop, P ≥ 0.190). Thermal adaptations observed after HWI were not related to changes in thyroid hormone concentrations (P ≥ 0.086). In conclusion, 12 days of post-exercise hot water immersion conferred more complete heat acclimation than exercise heat acclimation without increasing overreaching risk, and changes in thyroid hormones are not related to thermal adaptations after post-exercise hot water immersion.
INTRODUCTION
It is well established that exercise in hot and hot-humid environments is detrimental to endurance capacity (1, 2) and may expose individuals to the risk of exertional heat illness (3). To reduce these deleterious effects of heat stress, athletes, military personnel, and occupational workers should prepare by completing a period of heat acclimation (4, 5).
Previous research in both recreationally active (6) and endurance-trained individuals (7) demonstrates that taking a hot bath for up to 40 min immediately after submaximal exercise in temperate conditions on six consecutive days reduces resting core body temperature. This reduction in resting core body temperature leads to a subsequent reduction in core body temperature during exercise-heat stress, a hallmark heat acclimation adaptation. post-exercise hot water immersion (HWI) also presents a more practical heat acclimation strategy than conventional exercise heat acclimation (EHA), as it eliminates the requirement for access to an environmental chamber and can be more easily incorporated into normal training and an athlete’s taper (8). Moreover, McIntyre et al. (9) recently demonstrated that despite a similar endogenous thermal stimulus for adaptation, 6 days of HWI elicited larger thermal adaptations than EHA. Although the 6-day HWI intervention presents an effective, practical, and time-efficient short-term (< 7 days) heat acclimation strategy, previous literature suggests that medium- (7–14 days) (10, 11) and long-term (>14-day) (10) interventions provide a more complete state of heat acclimation. It is yet to be determined whether extending the 6-day HWI intervention provides additional thermal benefits. In addition, the true benefit of medium-term conventional exercise-based heat acclimation strategies beyond exercising in temperate conditions is unknown due to the lack of work-matched interventions within the literature and hence further research is warranted. In contrast to the beneficial adaptations of medium-term heat acclimation, the physical demands of prolonged interventions can disrupt training and may trigger overreaching, which has detrimental effects on exercise performance and mood (4, 12). The effects of medium-term heat acclimation on overreaching are currently unknown and, given the applied implications, warrant investigation.
The reduction in thermal strain after HWI heat acclimation can be largely attributed to a reduction in resting core temperature (6, 7, 9, 13, 14). The underlying mechanism for this reduction in resting core temperature is currently unknown but may involve a reduction in metabolic heat production via reduced circulating thyroid hormone concentrations (15), a decrease in the thermoregulatory balance point (16, 17), or hypothalamic neural network remodeling (18, 19). The release of thyroid-stimulating hormone by the anterior pituitary gland stimulates the release of two protein-iodine-bound hormones: triiodothyronine (T3) and thyroxine (T4). When unbound, free thyroid hormones are metabolically active and stimulate glucose uptake, gluconeogenesis, lipolysis, and thermogenesis (20). Reductions in thyroid hormones have been demonstrated after 3 weeks of heat exposure in rats (21, 22) and previous research also shows rats with lower circulating thyroid hormones have a lower core temperature at rest and during heat stress (23, 24). However, no study to date in humans has investigated the effect of heat acclimation on thyroid hormone concentrations or thyroid hormone influences on heat acclimation thermal adaptations. Specifically, it is unknown whether reductions in thyroid hormones are responsible for the pronounced reduction in resting core temperature observed after HWI heat acclimation (6, 7, 9).
This research is presented in two parts. Part 1 compared heat acclimation thermal and endurance capacity adaptations, overreaching markers, and changes in plasma thyroid hormone concentrations after 3, 6, and 12 days of HWI and EHA with a work-matched temperate exercise control (CON) in 21 active males. Given larger thermal adaptations were observed after short-term HWI than short-term EHA (9), we hypothesized that extending the HWI intervention to 12 days would augment thermal adaptations and that these would confer more complete heat acclimation than EHA. In addition, we expected that compared with CON the high physical demands of daily exercise and heat stress during HWI and EHA would lead to increased markers of overreaching (i.e., low mood and physical/cognitive performance decrements). Part 2 examined, in a larger cohort of 48 active males, the effect of 6 days of HWI in comparison with CON on plasma thyroid hormone concentrations, and additionally examined the relationship of thyroid hormone changes with hallmark heat acclimation adaptations. We hypothesized that 6 days of HWI would elicit reductions in plasma thyroid hormone concentrations and that these reductions would be associated with heat acclimation thermal adaptations, in particular a reduction in resting and exercising core temperature.
METHODS
Experimental Approaches
In part 1, a mixed-methods (between and within) repeated-measures design was used to assess the effect of 12 days of HWI, EHA, and CON on thermal and perceptual adaptations, overreaching markers, and plasma thyroid hormone concentrations in 21 recreationally active males. This is a subset of participants of a larger cohort that completed six intervention days (9). Participants in part 1 completed experimental trials before (PRE) and after 3 (POST3), 6 (POST6), and 12 days (POST12) of their assigned intervention (Fig. 1). To enable work-matching with EHA, CON participants completed the same external work ≥1 day after EHA participants. In part 2, data from four previously published heat acclimation studies from our laboratory (6, 7, 9, 14) were amalgamated in a between-groups design to assess the effect of 6 days of HWI and CON on thermal adaptations, thyroid hormone concentrations, and the relationship between plasma thyroid hormones and thermal adaptations. Thyroid hormones were not previously investigated in these studies. Amalgamating the four studies enabled the relationship between thyroid hormones and thermal adaptations to be examined more robustly in a larger sample. Testing was halted during summer months (June–August) to reduce the potential effect of seasonal heat acclimatization. All studies received ethical approval (829/MoDREC/17, PO5-17/18, S/PhD10-15/16, PhD19-13/14), were conducted in accordance with the Declaration of Helsinki (2013) but were not registered in a database.
Figure 1.

Schematic of the study design (part 1). CON; temperate exercise control; EHA, exercise heat acclimation; HWI, post-exercise hot water immersion.
Participant Recruitment and Randomization
Part 1 participant flow and attrition before protocol completion and biochemical and statistical analyses are summarized in Fig. 2. Participants were matched for V̇o2peak in groups of three and randomly assigned to either HWI, EHA, or CON (randomizer.org). Participants were excluded from the final analysis if they failed to complete the 12-day study protocol. The participant characteristics of the 21 male participants included in the final analysis are summarized in Table 1. A sample size of 21 (7 participants per group) was estimated (G*Power 3.1.9) (25) as adequate to detect a significant difference in the change in end-exercise rectal core temperature (Tre) between heat acclimation and temperate exercise control interventions using a mixed-model analysis of covariance (ANCOVA), standard α (0.05), and power (0.80), and a Cohen’s F effect size of 0.88. This effect size was calculated from the average reduction in end-exercise Tre change after HWI (−0.36°C) (6) and exercise heat acclimation [−0.44°C (26) and −0.49°C (27)] compared with exercise in temperate conditions (0.00°C) (6) and a pooled SD of 0.21°C (control group) (6). Part 2 participants were 48 active males (age, 22 ± 3 yr; height, 178 ± 6 cm; body mass, 72 ± 7 kg; V̇o2peak, 58 ± 8 mL·kg−1·min−1). Data of 14 participants (HWI, n = 7; CON, n = 7) were included in both parts 1 and 2. All participants in parts 1 and 2 provided written informed consent and were healthy, nonsmokers, free from any known cardiovascular or metabolic diseases, were not taking any medication and had not been regularly (more than once a week) exposed to the heat (including sauna and hot bath use) in the 6 weeks before commencing testing.
Figure 2.

Flow diagram indicating the numbers of participants assessed for eligibility, commenced testing, and withdrew, were excluded, or completed the study protocol (part 1). CON, temperate exercise control; EHA, exercise heat acclimation; HWI, post-exercise hot water immersion.
Table 1.
Part 1 participant characteristics of post-exercise hot water immersion, exercise heat acclimation, and temperate exercise control interventions
| HWI | EHA | CON | |
|---|---|---|---|
| Age, yr | 22 ± 3 | 21 ± 2 | 22 ± 2 |
| Height, cm | 176 ± 4 | 183 ± 5 | 177 ± 6 |
| Body mass, kg | 70 ± 6 | 75 ± 6 | 70 ± 7 |
| V̇o2peak, mL·kg−1·min−1 | 53 ± 7 | 54 ± 3 | 53 ± 4 |
| Running economy, kcal·kg−1·min−1 | 3.3 ± 0.1 | 3.6 ± 0.4 | 3.5 ± 0.3 |
Data are represented as means ± SD; n = 7 participants in each group. Analyzed by one-way ANOVA. CON, temperate exercise control; EHA, exercise heat acclimation; HWI, hot water immersion.
Preliminary Measurements and Familiarization
Participants completed a fitness assessment within a week before their first experimental trial (PRE; Fig. 1). V̇o2peak was assessed using a continuous maximal incremental exercise test performed on a motorized treadmill (HP Cosmos Mercury 4.0, Nussdorf-Traunstein, Germany) in a temperate laboratory (19°C, 45% relative humidity) to volitional exhaustion. V̇o2peak was determined as the highest oxygen uptake attained over a 30-s period. The average values of breath-by-breath V̇o2 and V̇co2 during the final minute of each submaximal stage were used to calculate running economy, expressed as kilocalories per kilogram per minute (28). A running speed that elicited 65% V̇o2peak in temperate conditions was subsequently determined by the interpolation of the running speed-V̇o2 relationship and confirmed via Douglas bag method. All participants ran at a speed below their anaerobic threshold as determined by the onset of blood lactate accumulation (29). Participants were then familiarized with the treadmill running speed, Stroop test, venepuncture, and Profile of Mood States (POMS) questionnaire.
Experimental Trials
Twenty-four hours before the first experimental trial, participants were instructed to refrain from exercise, alcohol, diuretics, and caffeine and to complete a diet diary. Twenty-four hours before all subsequent experimental trials, participants were instructed to replicate this food and fluid intake. To ensure a similar circadian pattern, participants were instructed to sleep between 2200 and 0700 before experimental trials with their sleep duration and efficiency assessed by an Actigraph (Actigraph GT3X Version 4.4.0, Actigraph, Pensacola, FL). Sleep duration and efficiency were also assessed as overreaching markers (30).
On the day of the experimental trials, participants arrived at the laboratory at 0730 and were provided with a standardized breakfast (2,091 kJ, 71 g carbohydrate, 18 g fat, 17 g protein) and a bolus of water (7 mL·kg−1 of nude body mass). At 0800, dressed in a t-shirt, shorts, socks, and trainers, participants rested for 20 min in temperate conditions (19°C, 45% relative humidity). After the seated rest, participants completed the abbreviated POMS questionnaire (31) to determine total mood disturbance and energy index (vigor-fatigue) as markers of overreaching (30). A venous blood sample was then taken without stasis for the determination of plasma volume and plasma concentrations of free T3, free T4, total T3, and total T4. A urine sample was then analyzed to confirm urine specific gravity was <1.03 (32) and a flexible, sterile, single-use rectal thermistor (Henleys Medical Supplies Ltd., Herts, UK) was self-inserted 10 cm beyond the anal sphincter to measure Tre. A pre-exercise nude body mass was recorded using a digital platform scale (model 703; Seca, Hamburg, Germany) and skin thermistors were attached on the right side of the body for the determination of mean skin temperature (Tsk), as previously described (33). Mean body temperature (Tb) was estimated using the following formula (34):
After instrumentation, participants rested for a further 30 min in temperate conditions (19°C, 45% relative humidity) to establish baseline measures. Body surface area (AD) by the Du Bois equation (35), and V̇o2 and respiratory exchange ratio (RER) from a 60-s expired gas collection by Douglas bag method between 29 and 30 min of seated rest were used to estimate resting metabolic heat production (H) as follows (36):
At 0945, dressed in shorts, socks, and trainers, participants entered the environmental chamber (33°C, 40% relative humidity, 0.2 m·s−1 wind velocity) to complete a 40-min treadmill run at 65% V̇o2peak. Tre, skin temperatures, and heart rate were monitored continuously. Local forearm sweat rate was measured by dew point hygrometry (DS2000; Alpha Moisture Systems, UK). Anhydrous compressed nitrogen at a flow rate of 1 L·min−1 was passed through a 5-cm2 capsule, affixed to the ventral surface of the lower arm (halfway between the antecubital fossa and carpus). Local forearm sweat rate was calculated as the difference in water content between effluent and influent air, divided by the skin surface area under the capsule (expressed in milligrams per square centimeter per minute). Tre at sweating onset was determined by plotting the relationship between local forearm sweat rate and Tre (recorded at 20-s intervals) before using segmented linear regression to identify the breakpoint in the two line segments (37). Rating of perceived exertion (RPE) (38), thermal sensation (TS) (39), V̇o2, and RER (40) were recorded every 10 min. On completion of the exercise, participants rested for 20 min in temperate conditions (19°C, 45% relative humidity), during which they completed a modified Stroop test (41) to assess cognitive function as a marker of overreaching (30), and provided a nude body mass to estimate whole-body sweat rate.
Participants then re-entered the environmental chamber and completed a time to exhaustion (TTE) on a motorized treadmill at 65% V̇o2peak. Participants were instructed to “run for as long as possible.” TTE was terminated when participants stopped running owing to volitional exhaustion, thermal discomfort, or when Tre exceeded 39.5°C. No fluids were consumed, no feedback was provided, and Tre and heart rate were monitored continuously. After the cessation of exercise, capillary blood lactate concentration was assessed (Lactate Pro 2, Arkray, Australia) as a marker of overreaching (42, 43). Participants were provided with a bolus of water and were free to leave the laboratory when Tre ≤ 38.5°C.
Daily Intervention
All participants in part 1 and part 2 completed 12 and 6 days of their assigned intervention, respectively. During the intervention, participants were instructed to consume their normal diet and fluid intake, including caffeine and alcohol (≤3 units/day). Participants arrived at the laboratory each day between 0600 and 1300. Before exercise, a nude body mass was taken, and a rectal thermistor and heart rate monitor were fitted. After instrumentation, participants completed a 15-min seated rest in temperate conditions (19°C, 45% relative humidity) to establish baseline measures, before commencing their assigned intervention protocol. A bolus of water (5 mL·kg−1 of nude body mass) was consumed during the first 20 min of exercise.
Participants assigned to HWI completed a 40-min treadmill run dressed in shorts, socks, and trainers at a speed equivalent to their 65% V̇o2peak (9.1 ± 1.6 km·h−1) in temperate conditions (19°C, 45% relative humidity, 0.2 m·s−1 wind velocity). After exercise (2–3 min transition), dressed in shorts, participants began a semirecumbent ≤40-min HWI (40°C) to the neck, as previously described (6). Participants assigned to EHA completed a ≤60-min treadmill run at a speed equivalent to their 65% V̇o2peak (9.1 ± 1.1 km·h−1) in an environmental chamber (33°C, 40% relative humidity, 0.2 m·s−1 wind velocity). Participants assigned to CON completed a daily submaximal treadmill run equivalent to 65% V̇o2peak and work matched to EHA (8.8 ± 0.9 km·h−1) in temperate conditions (19°C, 45% relative humidity, 0.2 m·s−1 wind velocity). Owing to the nature of these interventions, it was not possible to blind the participants. In part 1, to maintain the endogenous thermal stimulus for adaptation after the first six intervention sessions (days 1–3 and days 6–8, Fig. 1), maximum immersion (HWI) and exercise duration (EHA and CON) increased by 25%, as of the seventh intervention session (days 11–16, intervention sessions 7–12), to ≤50 min and ≤75 min, respectively. All intervention sessions were terminated if the maximal immersion/exercise duration was reached, at the participant’s volition, or if Tre exceeded 39.5°C. Upon removal from the hot water/environmental chamber, participants rested in a seated position for 5 min in a temperate laboratory, were provided with a bolus of water, and were free to leave the laboratory when Tre ≤ 38.5°C.
Blood Sample Collection and Analysis
Venous blood samples were collected from an antecubital vein without stasis into two 6-mL EDTA vacutainers (BD, Oxford, UK). Aliquots of whole blood were used for the immediate determination of hemoglobin in duplicate (Hemocue, Sheffield, UK) and hematocrit in triplicate using a microcentrifuge and microhematocrit reader (Hawksley & Sons Limited, Lancing, UK). The change in plasma volume was estimated by correcting the initial plasma volume at PRE for the percentage change in plasma volume (%ΔPV) at POST3, POST6, and POST12, as previously described (44). The remaining whole blood was then centrifuged, and the plasma frozen at −80°C for later analysis.
Plasma concentrations of free and total triiodothyronine (T3) and thyroxine (T4) were measured in duplicate by ELISA (free T3: Cat. No. RE55231, detection limit: 0.1 pmol·L−1; free T4: Cat. No. RE55241, detection limit: 0.6 pmol·L−1; total T3: Cat. No. RE55251, detection limit: 0.2 nmol·L−1; total T4: Cat. No. RE55261, detection limit: 0.1 nmol·L−1; IBL International, Hamburg, Germany). The intra-assay coefficient of variation for duplicates were free T3, 5.1%; free T4, 2.6%; total T3, 5.6%; total T4, 5.9%. Thyroid hormone concentrations were adjusted for plasma volume changes using the following formula (45):
Statistical Analysis
Data were analyzed using SPSS version 27 (IBM Corporation) or GraphPad Prism Version 9.1 (GraphPad Software, Inc. La Jolla, CA). All data were checked for normality and sphericity; plasma free T4 data were reciprocal transformed to address statistical assumptions of sphericity. Data are presented as untransformed mean and SD unless otherwise stated, and statistical significance was accepted at P < 0.05. In part 1, the mean daily endogenous thermal stimulus and external work during HWI, EHA, and CON were compared using a two-way mixed model ANOVA. A two-way mixed-model ANCOVA, with baseline (PRE) as the covariate, was used to detect differences in heat acclimation adaptations, endurance capacity, overreaching markers, and plasma thyroid hormone concentrations after 3, 6, and 12 days of HWI, EHA, or CON. Bonferroni-adjusted pairwise comparisons were used where appropriate to determine where differences occurred. The size of the between-intervention differences was calculated using Cohen’s d effect size with values greater than 0.2, 0.5, and 0.8 representing small, medium, and large effects, respectively (46). In part 2, the mean daily endogenous thermal stimulus and external work during HWI and CON were compared using t tests, and a one-way ANCOVA was used to detect differences in heat acclimation adaptations and plasma thyroid hormone concentrations after 6 days of HWI or CON. Bonferroni-adjusted pairwise comparisons were used where appropriate to determine where differences occurred. Pearson’s correlations determined the strength of the relationship between the endogenous thermal stimulus, changes in resting Tre and plasma thyroid hormone concentrations after 12 days of heat acclimation by HWI and EHA. Pearson correlation coefficients of 0.00–0.19 were regarded as very weak, 0.20–0.39 as weak, 0.40–0.59 as moderate, and 0.60–0.79 as strong relationships (47).
RESULTS
Part 1 Daily Intervention Thermal Stimulus and External Work
Throughout the 12-day intervention, the daily endogenous thermal stimulus for adaptation was similar between HWI and EHA (Table 2; all P ≥ 0.407), but lower in CON (P < 0.001); there were no main effects of time or interaction effects (P ≥ 0.252). The daily endogenous thermal stimulus was maintained throughout the 12 days by an increase in mean daily immersion on HWI (days 1–3, 33 ± 4 min; days 6–8, 35 ± 5 min; days 11–16, 39 ± 5 min, P < 0.001) and an increase in exercise duration on EHA (days 1–3, 51 ± 9 min; days 6–8, 55 ± 8 min; days 11–16, 61 ± 11 min, P < 0.001). The similar daily thermal stimulus during HWI and EHA was achieved with a lower mean daily external work in HWI than EHA (Table 2; P = 0.006), and mean daily external work also tended to be lower in HWI than CON (P = 0.053).
Table 2.
Daily endogenous thermal stimulus and external work during temperate exercise control, exercise heat acclimation, and post-exercise hot water immersion interventions
|
Days 1–3
|
Days 6–8
|
Days 11–16
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | EHA | HWI | CON | EHA | HWI | CON | EHA | HWI | |
| Duration Tre ≥ 38.5°C, min | 7 ± 12 | 35 ± 14†† | 36 ± 5†† | 8 ± 12 | 38 ± 11†† | 38 ± 6†† | 7 ± 10 | 38 ± 13†† | 39 ± 8†† |
| AUC, °C·min−1 | 1 ± 3 | 17 ± 10†† | 17 ± 5†† | 2 ± 4 | 16 ± 8†† | 18 ± 4†† | 1 ± 1 | 12 ± 6†† | 20 ± 6†† |
| End intervention Tre, °C | 38.24 ± 0.34 | 39.17 ± 0.28†† | 39.24 ± 0.16†† | 38.22 ± 0.46 | 39.11 ± 0.22†† | 39.27 ± 0.14†† | 38.23 ± 0.22 | 38.99 ± 0.25†† | 39.31 ± 0.18†† |
| External work, km | 7.4 ± 1.1 | 7.7 ± 1.6 | 6.1 ± 1.1‡ | 7.6 ± 1.7 | 8.1 ± 1.7 | 6.1 ± 1.1‡ | 8.7 ± 1.7 | 9.0 ± 1.5 | 6.1 ± 1.1‡ |
Data are represented as means ± SD of days 1–3, days 6–8, and days 11–16. n = 7 participants in each group (part 1). Analyzed by two-way mixed model ANOVA;
††group difference to CON, P < 0.01; ‡group difference to EHA, P < 0.05. AUC, area under the curve for Tre >38.5°C; CON, temperate exercise control; EHA, exercise heat acclimation; HWI, postexercise hot water immersion, Tre, rectal core temperature.
Part 1 Experimental Trials
Before the experimental trial, standardization ensured sleep duration (6 ± 1 h, P ≥ 0.184) and hydration status, as assessed by urine specific gravity (1.020 ± 0.007, P ≥ 0.268), were similar between the interventions, as evidenced by no main effects of group or time, and no interaction effects.
Thermal responses at rest in temperate conditions.
Thermal responses at rest in temperate conditions were different between interventions after 12 days. Resting Tb was lower after HWI than EHA (Fig. 3A, P = 0.009, d = 1.86) and CON (P = 0.005, d = 2.04). Resting Tb was not different between EHA and CON over the 12 days (P = 1.000, d = 0.20). The average reduction in resting Tb over the 12 days was −0.41 ± 0.15°C for HWI, −0.14 ± 0.14°C for EHA, and −0.11 ± 0.15°C for CON. Resting Tre was lower after HWI (Fig. 3B, −0.41 ± 0.15°C) than CON (−0.12 ± 0.15°C, P = 0.007, d = 1.93) but not EHA (−0.20 ± 0.15°C, P = 0.061, d = 1.37). Resting Tre was not different between EHA and CON over the 12 days (P = 0.936, d = 0.56). Conversely, there were no differences between interventions for resting Tsk (Fig. 3C), resting Tre − Tsk gradient, resting H (Fig. 3D), or plasma volume (all P ≥ 0.096; Table 3).
Figure 3.

Influence of 3 (POST3), 6 (POST6), and 12 days (POST12) of a temperate exercise control (CON, n = 7 participants), exercise heat acclimation (EHA, n = 7 participants), or post-exercise hot water immersion (HWI, n = 7 participants) on resting mean body temperature (Tb, A), rectal core temperature (Tre, B), mean skin temperature (Tsk, C), and metabolic heat production (H, D) in temperate conditions (19°C, 45% relative humidity). Bars represent baseline-adjusted means; circles represent individual participant responses. Analyzed by two-way mixed model ANCOVA, with baseline (PRE) as the covariate and Bonferroni-adjusted pairwise comparisons; †HWI lower than CON, P < 0.05; ††HWI lower than CON, P < 0.01; ‡HWI lower than EHA, P < 0.05.
Table 3.
Change from baseline in heat acclimation adaptations at rest (19 °C, 45% RH) and during 40-min submaximal exercise in the heat (33 °C, 40% relative humidity) after 3, 6 and 12 days of temperate exercise control, exercise heat acclimation, and post-exercise hot water immersion interventions
| CON |
EHA |
HWI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| POST3 | POST6 | POST12 | POST3 | POST6 | POST12 | POST3 | POST6 | POST12 | |
| Rest | |||||||||
| Resting Tb, °C | −0.12 ± 0.20 | −0.11 ± 0.22 | −0.09 ± 0.20 | −0.19 ± 0.20 | −0.18 ± 0.21 | −0.03 ± 0.20 | −0.26 ± 0.20††,‡‡ | −0.48 ± 0.22††,‡‡ | −0.48 ± 0.20††,‡‡ |
| Resting Tre, °C | −0.10 ± 0.19 | −0.13 ± 0.18 | −0.13 ± 0.19 | −0.19 ± 0.19 | −0.24 ± 0.18 | −0.17 ± 0.19 | −0.35 ± 0.19†† | −0.41 ± 0.18†† | −0.46 ± 0.19†† |
| Resting Tsk, °C | −0.17 ± 0.63 | −0.09 ± 0.36 | −0.05 ± 0.42 | −0.19 ± 0.62 | −0.07 ± 0.36 | −0.23 ± 0.41 | −0.10 ± 0.63 | −0.60 ± 0.36 | −0.50 ± 0.42 |
| Resting H, W·m−2 | 7 ± 20 | −9 ± 15 | 4 ± 17 | 2 ± 20 | 4 ± 15 | 9 ± 17 | 3 ± 21 | 0 ± 15 | 3 ± 17 |
| Plasma volume, % | 3 ± 7 | 3 ± 5 | 2 ± 7 | 3 ± 7 | 6 ± 5 | 5 ± 7 | 1 ± 7 | 4 ± 5 | 3 ± 7 |
| Submaximal exercise | |||||||||
| End-exercise Tb, °C | −0.27 ± 0.24 | −0.36 ± 0.24 | −0.52 ± 0.25 | −0.33 ± 0.25 | −0.44 ± 0.25 | −0.62 ± 0.26 | −0.39 ± 0.25 | −0.58 ± 0.25 | −0.83 ± 0.26 |
| End-exercise Tre, °C | −0.21 ± 0.23 | −0.36 ± 0.21 | −0.41 ± 0.20 | −0.32 ± 0.24 | −0.33 ± 0.21 | −0.44 ± 0.21 | −0.32 ± 0.23† | −0.56 ± 0.21† | −0.64 ± 0.20† |
| ΔTre during exercise, °C | −0.10 ± 0.26 | −0.22 ± 0.29 | −0.28 ± 0.29 | −0.16 ± 0.28 | −0.09 ± 0.30 | −0.29 ± 0.30 | −0.06 ± 0.27 | −0.15 ± 0.30 | −0.18 ± 0.30 |
| Tre at sweating onset, °C | −0.15 ± 0.16 | −0.15 ± 0.19 | −0.18 ± 0.15 | −0.19 ± 0.17 | −0.29 ± 0.19 | −0.19 ± 0.15 | −0.30 ± 0.17††,‡ | −0.47 ± 0.19††,‡ | −0.50 ± 0.15††,‡ |
| Whole-body sweat rate, L·h−1 | −0.05 ± 0.09 | −0.05 ± 0.06 | 0.07 ± 0.11 | 0.06 ± 0.09† | 0.04 ± 0.06† | 0.09 ± 0.10† | 0.08 ± 0.09†† | 0.08 ± 0.06†† | 0.10 ± 0.10†† |
| End-exercise Tsk, °C | −0.38 ± 0.49 | −0.38 ± 0.46 | −0.73 ± 0.54 | −0.39 ± 0.50 | −0.66 ± 0.47 | −0.95 ± 0.55 | −0.50 ± 0.52 | −0.60 ± 0.48 | −1.15 ± 0.57 |
| End-exercise HR, beats·min−1 | −8 ± 5 | −12 ± 7 | −14 ± 8 | −12 ± 5 | −15 ± 7 | −20 ± 8 | −11 ± 5 | −17 ± 7 | −20 ± 8 |
| Mean V̇o2, L·min−1 | −0.10 ± 0.13 | −0.10 ± 0.15 | −0.16 ± 0.13 | −0.01 ± 0.13 | 0.00 ± 0.15 | −0.06 ± 0.13 | −0.04 ± 0.13 | −0.04 ± 0.14 | −0.06 ± 0.12 |
| Mean RER | −0.01 ± 0.04 | −0.02 ± 0.03 | −0.03 ± 0.04 | −0.02 ± 0.04 | −0.02 ± 0.03 | −0.01 ± 0.04 | −0.02 ± 0.04 | −0.02 ± 0.03 | −0.02 ± 0.04 |
| End-exercise RPE, 6–20 scale | 0 ± 2 | 0 ± 1 | 0 ± 2 | −1 ± 2 | −1 ± 1 | −1 ± 2 | −2 ± 2† | −2 ± 1† | −2 ± 2† |
| End-exercise TS, 1–13 scale | 0 ± 1 | 0 ± 1 | 0 ± 1 | 0 ± 1 | −1 ± 1 | −1 ± 1 | −1 ± 1† | −1 ± 1† | −1 ± 1† |
Data are baseline-adjusted mean change ± SD after 3 (POST3), 6 (POST6), and 12 days (POST12). n = 7 participants in each group (part 1). CON, temperate exercise control; EHA, exercise heat acclimation; HWI, post-exercise hot water immersion; H, metabolic heat production; HR, heart rate; RER, respiratory exchange ratio; RPE, rating of perceived exertion; TS, thermal sensation; Tb, mean body temperature; Tre, rectal core temperature; Tsk, mean skin temperature. Analyzed by two-way mixed model ANCOVA, with baseline (PRE) as the covariate and Bonferroni-adjusted pairwise comparisons;
†group difference to CON, P < 0.05; ††group difference to CON, P < 0.01; ‡group difference to EHA, P < 0.05.
Thermal and perceptual responses to exercise in the heat.
Thermal and perceptual responses to submaximal exercise in the heat were different between the interventions after 12 days. End-exercise Tre after exercise-heat-stress was lower after HWI (Fig. 4B, −0.50 ± 0.19°C) than CON (−0.33 ± 0.13°C; P = 0.049, d = 1.13) but not EHA (−0.37 ± 0.13°C; P = 0.196, d = 0.88); no difference was observed between EHA and CON (P = 1.000, d = 0.30). Tre at sweating onset was lower after HWI (Fig. 4C, −0.43 ± 0.12°C) than EHA (−0.22 ± 0.12°C; P = 0.015, d = 1.75) and CON (−0.16 ± 0.12°C; P = 0.002, d = 2.27). Conversely, EHA did not reduce Tre at sweating onset compared with CON (P = 1.000, d = 0.52). Whole-body sweat rate was greater after HWI (Fig. 4D, +0.08 L·h−1; P = 0.003, d = 2.13) and EHA (+0.06 L·h−1; P = 0.013, d = 1.78) than CON (−0.06 L·h−1), but no difference was detected between HWI and EHA (P = 1.000, d = 0.35). In accordance with thermal adaptations, perceptual responses to exercise-heat-stress were lower after HWI (RPE Fig. 4E, −2 ± 1; TS Fig. 4F, −1 ± 1) than CON (RPE, 0 ± 1, P = 0.036, d = 1.57; TS, 0 ± 1, P = 0.047, d = 1.55) but not EHA (RPE, −1 ± 1, P = 0.951, d = 0.54; TS, −1 ± 1, P = 1.000, d = 0.55); no differences were observed between EHA and CON (P ≥ 0.157, d = 1.07). There were no differences between interventions for the change in Tre during the 40-min treadmill run in the heat, end-exercise Tb (Fig. 4A), end-exercise Tsk, end-exercise Tre − Tsk gradient, end-exercise heart rate, exercising V̇o2, or exercising RER (Table 3; all P ≥ 0.059). The rate of thermal and perceptual adaptations was not different among HWI, EHA, or CON from POST3 to POST12, as indicated by no interaction effects (all P ≥ 0.087). There were also no main effects of time (all P ≥ 0.148).
Figure 4.

Influence of 3 (POST3), 6 (POST6), and 12 days (POST12) of a temperate exercise control (CON, n = 7 participants), exercise heat acclimation (EHA, n = 7 participants), or post-exercise hot water immersion (HWI, n = 7 participants) on end-exercise mean body temperature (Tb, A), end-exercise rectal core temperature (Tre, B), Tre at sweating onset (C), whole-body sweat rate (D), end-exercise rating of perceived exertion (RPE, E), and end-exercise thermal sensation (TS, F) in the heat (33°C, 40% relative humidity). Bars represent baseline-adjusted means; circles represent individual participant responses. Analyzed by two-way mixed model ANCOVA, with baseline (PRE) as the covariate and Bonferroni-adjusted pairwise comparisons; †group difference to CON, P < 0.05; ††group difference to CON, P < 0.01; ‡HWI lower than EHA, P < 0.05.
Overreaching markers and endurance capacity.
There was no evidence to suggest that 12 days of HWI or EHA induced overreaching to a greater extent than CON, with no interaction effects, main effects of group or time detected for total mood disturbance, energy index, Stroop reaction time, Stroop accuracy, sleep duration, or sleep efficiency (Table 4; all P ≥ 0.190). Five participants were removed from the TTE endurance capacity test analysis owing to reaching the Tre ethical cut-off (HWI, n = 2); going to the toilet (EHA, n = 1); exercise-induced bronchoconstriction (CON, n = 1); and an obvious lack of effort without markers of overreaching at rest (CON, n = 1). Analysis of the remaining 16 participants (HWI, n = 5; EHA, n = 6; CON, n = 5) who completed the TTE revealed no statistical differences between interventions or across time (Table 4; P ≥ 0.219). In addition, no differences were detected between interventions for end-TTE Tre, end-TTE heart rate, or end-TTE blood lactate concentration (Table 4; all P ≥ 0.198).
Table 4.
Change from baseline in markers of overreaching and endurance capacity in the heat (33°C, 40% relative humidity) after 3, 6 and 12 days of temperate exercise control, exercise heat acclimation, and post-exercise hot water immersion interventions
| CON |
EHA |
HWI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| POST3 | POST6 | POST12 | POST3 | POST6 | POST12 | POST3 | POST6 | POST12 | |
| Markers of overreaching | |||||||||
| Total mood disturbance | 5 ± 10 | 2 ± 12 | 2 ± 10 | 5 ± 10 | 7 ± 12 | 2 ± 10 | 4 ± 10 | 4 ± 12 | 2 ± 10 |
| Energy index | −3 ± 4 | −2 ± 6 | −3 ± 5 | −3 ± 4 | −5 ± 6 | −3 ± 5 | −2 ± 4 | −4 ± 6 | −3 ± 5 |
| Stroop reaction time, ms | −29 ± 58 | −25 ± 40 | −11 ± 64 | −13 ± 58 | −32 ± 40 | −15 ± 63 | −16 ± 62 | −18 ± 43 | −28 ± 68 |
| Stroop accuracy, % | 0 ± 2 | −1 ± 3 | 1 ± 4 | −1 ± 3 | −1 ± 3 | −2 ± 4 | 2 ± 3 | 1 ± 3 | 0 ± 4 |
| Sleep duration, h | 6 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 |
| Sleep efficiency, % | 0 ± 9 | −2 ± 7 | −1 ± 8 | −6 ± 9 | −5 ± 7 | 1 ± 8 | −2 ± 9 | 2 ± 7 | −2 ± 8 |
| Endurance capacity | |||||||||
| TTE, s | −27 ± 676 | 75 ± 808 | 212 ± 991 | 101 ± 627 | 539 ± 749 | 323 ± 919 | 321 ± 743 | 686 ± 888 | 1,030 ± 1,089 |
| End-TTE Tre, °C | −0.14 ± 0.30 | −0.24 ± 0.34 | −0.32 ± 0.47 | −0.20 ± 0.31 | −0.06 ± 0.34 | −0.29 ± 0.47 | −0.20 ± 0.31 | −0.03 ± 0.34 | −0.25 ± 0.47 |
| End-TTE HR beats·min−1 | −8 ± 8 | −10 ± 7 | −16 ± 10 | −10 ± 8 | −12 ± 7 | −20 ± 10 | −8 ± 8 | −10 ± 7 | −14 ± 10 |
| End-TTE blood lactate, mmol·L−1 | 0.2 ± 1.4 | −0.1 ± 0.7 | 0.2 ± 0.6 | 0.5 ± 1.3 | −0.2 ± 0.7 | −0.9 ± 0.6 | −0.2 ± 1.3 | −0.1 ± 0.7 | 0.2 ± 0.6 |
Data are baseline-adjusted mean change ± SD after 3 (POST3), 6 (POST6), and 12 days (POST12). n = 21, except n = 16 participants for TTE (part 1). CON, temperate exercise control; EHA, exercise heat acclimation; HR, heart rate; HWI, post-exercise hot water immersion; Tre, rectal core temperature; TTE, time to exhaustion. Analyzed by two-way mixed model ANCOVA, with baseline (PRE) as the covariate.
Thyroid hormones.
Twelve days of HWI elicited a reduction in thyroid hormones, evidenced by an interaction effect for plasma concentrations of free T3 (P = 0.006). Follow-up analyses showed that free T3 was lower after 12 days of HWI (−23%) than EHA (+4%, P = 0.008) and CON (+1%, P = 0.015; Fig. 5A). No differences were detected for free T3 between EHA and CON (P = 1.000). Conversely, there were no interaction effects or main effects of group or time detected for plasma concentrations of free T4 (P ≥ 0.148, Fig. 5B), total T3 (P ≥ 0.057, Fig. 5C), or total T4 (P ≥ 0.156, Fig. 5D).
Figure 5.
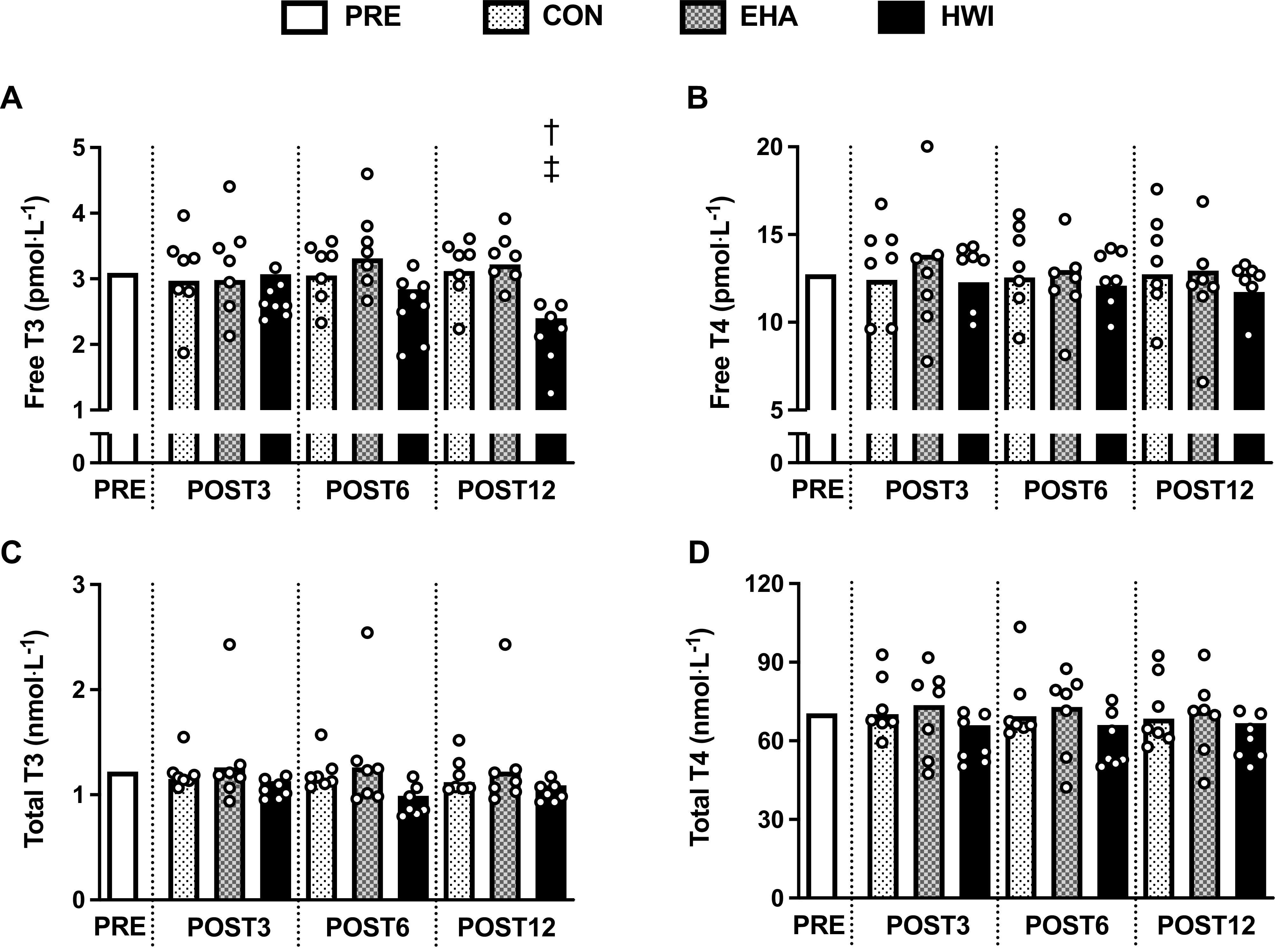
Influence of 3 (POST3), 6 (POST6), and 12 days (POST12) of a temperate exercise control (CON, n = 7 participants), exercise heat acclimation (EHA, n = 7 participants), or post-exercise hot water immersion (HWI, n = 7 participants) on plasma concentrations of free triiodothyronine (T3, A), free thyroxine (T4, B), total T3 (C), and total T4 (D). Bars represent baseline-adjusted means; circles represent individual participant responses. Analyzed by two-way mixed model ANCOVA, with baseline (PRE) as the covariate and Bonferroni-adjusted pairwise comparisons; †HWI lower than CON, P < 0.05; ‡HWI lower than EHA, P < 0.05.
Part 2 Daily Intervention Thermal Stimulus and External Work
All 48 participants completed 6 days of their assigned intervention. The HWI intervention caused a greater daily endogenous thermal stimulus than CON as indicated by greater daily duration Tre > 38.5°C (HWI, 41 ± 13 min; CON, 7 ± 8; P < 0.001), area under the curve (AUC) for Tre > 38.5°C (HWI, 23 ± 10 °C·min−1; CON, 1 ± 2 °C·min−1; P < 0.001), and end-intervention Tre (HWI, 39.3 ± 0.2°C; CON, 38.3 ± 0.4°C; P < 0.001). Daily external work was similar between HWI and CON (HWI, 7.0 ± 1.1 km; CON 7.3 ± 1.3 km; P = 0.065).
Part 2 Experimental Trials
Thermal responses at rest in temperate conditions.
Resting Tb was lower after 6 days of HWI than CON (HWI, −0.31 ± 0.32°C; CON, −0.04 ± 0.32°C; P = 0.009). In accordance with resting Tb, resting Tre was also lower after 6 days of HWI than CON (HWI, −0.33 ± 0.20°C; CON, −0.09 ± 0.21°C; P = 0.001, Fig. 6A). Conversely, no differences were detected for resting Tsk (P = 0.083), resting Tre − Tsk gradient (P = 0.509), resting H (P = 0.711, Fig. 6B), or plasma volume (P = 0.387).
Figure 6.

Influence of 6 days of a temperate exercise control (CON, n = 16 participants) or post-exercise hot water immersion (HWI, n = 32 participants) on resting rectal core temperature (Tre, A), resting metabolic heat production (H, B), and resting plasma concentrations of free triiodothyronine (T3, C), free thyroxine (T4, D), total T3 (E), and total T4 (F). Bars represent baseline-adjusted means; circles represent individual participant responses. Analyzed by one-way ANCOVA, with baseline (PRE) as the covariate; ††HWI lower than CON, P < 0.01.
Thermal and perceptual responses to exercise in the heat.
Compared with CON, 6 days of HWI also resulted in a lower end-exercise Tb (HWI, −0.54 ± 0.24°C; CON, −0.18 ± 0.24°C; P < 0.001), end-exercise Tre (HWI, −0.42 ± 0.24°C; CON, −0.13 ± 0.24°C; P < 0.001), Tre at sweating onset (HWI, −0.31 ± 0.20°C; CON, −0.08 ± 0.19°C; P = 0.01), end-exercise Tsk (HWI, −0.74 ± 0.54°C; CON, −0.30 ± 0.54°C; P < 0.001), end-exercise RPE (HWI, −1 ± 1; CON, 0 ± 1; P = 0.010), and end-exercise TS (HWI, −1 ± 1; CON, 0 ± 1; P = 0.003). No differences were detected for whole-body sweat rate (P = 0.228).
Thyroid hormones.
Despite 6 days of HWI causing pronounced heat acclimation adaptations, including reductions in Tb and Tre at rest and during exercise in the heat, no differences between HWI and CON were detected in resting plasma thyroid hormone concentrations; free T3 (HWI, 0 ± 12%; CON, −1 ± 12%; P = 0.802; Fig. 6C), free T4 (HWI, −8 ± 10%; CON, −3 ± 10%; P = 0.108; Fig. 6D), total T3 (HWI, −3 ± 10%; CON, −2 ± 17%; P = 0.873; Fig. 6E), or total T4 (HWI, −4 ± 8%; CON, −1 ± 8%; P = 0.180; Fig. 6F). Moreover, after 6 days of HWI, only weak nonsignificant relationships were observed between the reduction in resting Tb, resting Tre (Fig. 7), resting Tsk, resting Tre − Tsk gradient, end-exercise Tb, end-exercise Tre, Tre at sweating onset, end-exercise Tsk or end-exercise TS and changes in free T3 (r ≤ 0.21, P ≥ 0.269), free T4 (r ≤ 0.20, P ≥ 0.274), total T3 (r ≤ 0.31, P ≥ 0.086), and total T4 (r ≤ 0.24, P ≥ 0.193).
Figure 7.
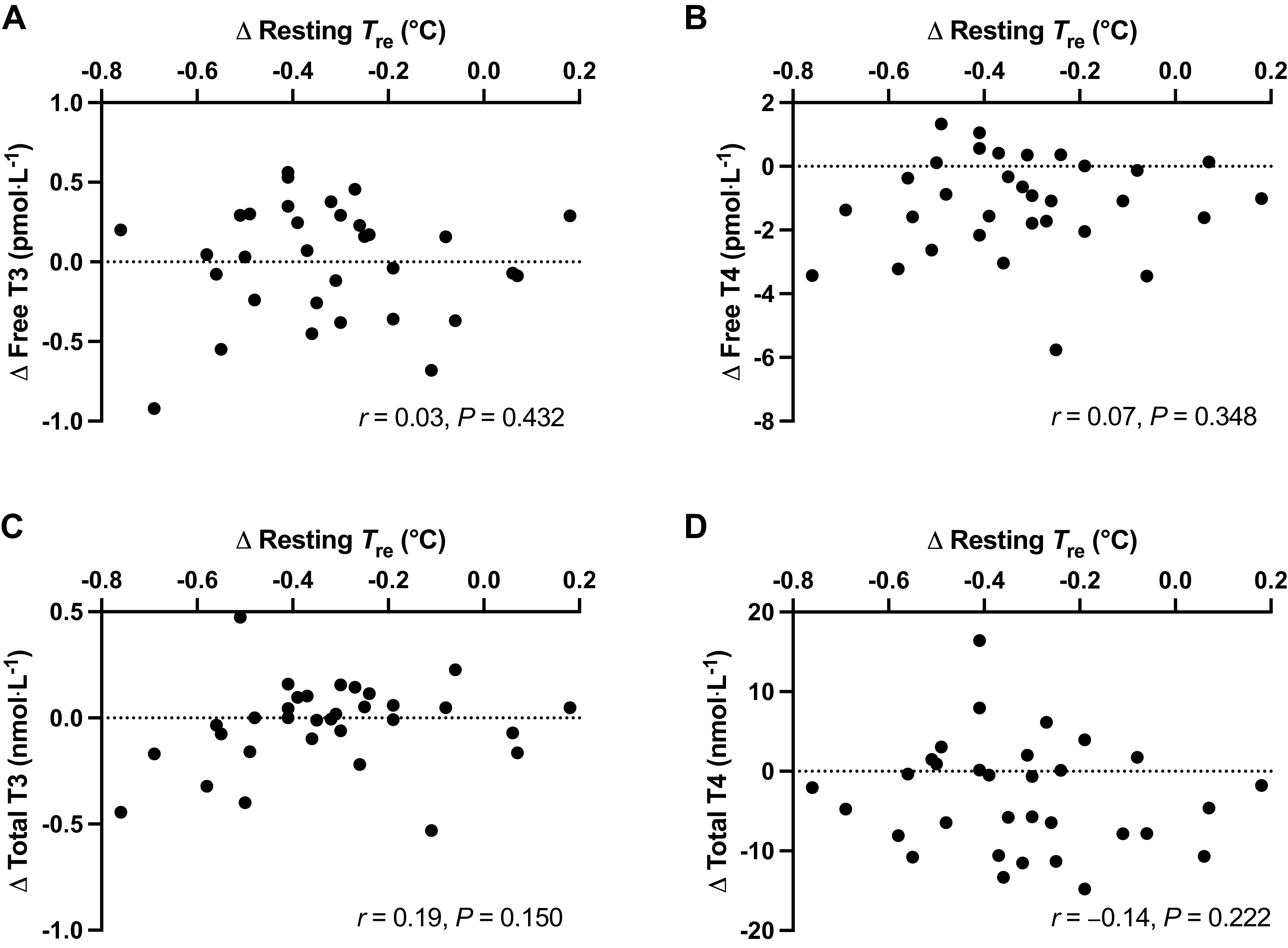
Relationships between the changes in resting core temperature (Tre) and plasma concentrations of thyroid hormones free triiodothyronine (T3, A), free thyroxine (T4, B), total T3 (C), and total T4 (D) after 6 days of post-exercise hot water immersion (n = 32 participants). Analyzed by Pearson's correlation.
DISCUSSION
This research is the first to compare hallmark heat acclimation adaptations, endurance capacity, and overreaching markers after 12 days of HWI and EHA with work-matched CON. This study is also the first in humans to examine the potential role of plasma thyroid hormone changes as a mechanism for thermal adaptations after heat acclimation, specifically HWI heat acclimation. The three primary findings of this research conducted in recreationally active men are the following: 1) In line with our hypothesis, HWI elicited a larger and a greater number of thermal adaptations, and reductions in perceived strain during exercise-heat-stress compared with CON and EHA over the 12-day interventions. Conventional EHA provided only modest further heat acclimation benefits to work-matched CON, 2) Contrary to our hypothesis, and previous literature examining short-term heat acclimation (12), there was no evidence to suggest that HWI or EHA induced overreaching risk more than with exercise in temperate conditions, and 3) Also contrary to our hypothesis, changes in plasma thyroid hormone concentrations were not significantly associated with changes in thermal adaptations over the 12 days of HWI, indicating that a reduction in thyroid hormones is unlikely the cause of the pronounced reduction in resting and end-exercise core temperature observed consistently after HWI heat acclimation. Instead, we provide evidence that the reduction in core temperature elicited by postexercise HWI intervention represents the establishment of a new lower thermal balance point (17).
Previous research has demonstrated that short-term (<7 days) HWI provides beneficial heat acclimation adaptations in comparison with CON and conventional EHA in recreationally active males (6, 9, 13, 14). The current study furthers this work by showing that 12 days of HWI heat acclimation led to more pronounced resting and exercising thermal adaptations than EHA and CON (Figs. 3 and 4). Resting Tb and Tre at sweating onset were lower over the 12-day HWI intervention than the 12-day EHA intervention. Compared with CON, HWI led to a greater number of thermal adaptations than EHA, i.e., HWI reduced resting Tb, resting Tre, end-exercise Tre, Tre at sweating onset, end-exercise RPE, end-exercise TS, and increased whole-body sweat rate, whereas EHA increased whole-body sweat rate only. The data also suggests that improvements in endurance capacity in the heat may be more readily observed after HWI than EHA, which has practical implications for applied practitioners and coaches. However, due to dropout, future studies are required to confirm (or reject) this preliminary finding. In combination, these findings indicate that HWI leads to larger and more complete heat acclimation than conventional EHA, even when the endogenous thermal stimulus for adaptation is similar. Heat acclimation adaptations developed throughout the 12 days, with the largest proportion of the adaptations occurring within the first 3 days, for example, ∼58% of the 12-day reduction in end-exercise Tre was observed on day 3 (Fig. 4B). Nevertheless, we observed no further statistically significant thermal benefits or improvements in endurance capacity by extending the 6-day heat acclimation interventions to 12 days. These findings align with the majority of previous studies that show no further thermal adaptations in males after medium- compared with short-term interventions (10, 26, 48, 49), even when a progressive heat acclimation method was used (27). Far less studied is the influence of additional heat acclimation days on exercise performance. In contrast with our findings, previous research showed additional improvements in exercise performance in the heat when extending exercise heat acclimatization from 6 to 14 days (50). The disparity with our findings may be explained by the small sample size for the TTE outcome in the current study and/or by differences in intervention methods and/or participants’ training status (recreationally active vs. competitive) (51).
The change in Tre during the 40-min submaximal treadmill run in the heat was similar on all interventions; hence, the lower end-exercise Tre (i.e., lower thermal strain) after HWI can be attributed to larger reductions in resting Tre than observed after CON. The induction of large reductions in resting Tre after HWI is likely due to exposure to a large dual thermal stimulus (i.e., maintained elevation in both core and skin temperature), as it is purported to induce a more complete state of heat acclimation (52). We anticipated the larger thermal adaptations from HWI would be associated with larger reductions in thyroid hormone concentrations in accordance with previous literature, which demonstrate a lower core temperature in hypothyroid compared with control rats (23, 24). However, despite large reductions in resting and end-exercise Tre after 3, 6, and 12 days of HWI, a concomitant reduction in plasma thyroid hormone concentrations (free T3) was only observed after 12 days (Fig. 5A). The temporal disconnect and the absence of significant relationships between changes in thyroid hormones and thermal adaptations indicate that circulating thyroid hormone changes are unlikely the cause of short- and medium-term heat acclimation adaptations. Indeed, the change in free T3 observed after 12 days appears a consequence of HWI heat acclimation. We can further refute the notion that HWI heat acclimation reduces core temperature via alterations in thyroid hormones and metabolism as we did not observe differences between interventions or a reduction in resting H after HWI (Fig. 3D and Fig. 6B). The lower resting core temperature after HWI is also unlikely explained by increased heat loss mechanisms as skin temperature, an index of skin blood flow, was not higher after HWI. In fact, a trend (P < 0.1) was observed for lower skin temperature after HWI in both parts 1 and 2 (Fig. 3C). The large reduction in resting core temperature observed after HWI heat acclimation may alternatively be explained by the establishment of a new lower thermal balance point (17). In this study, the new lower thermal balance point is indicated by a lower resting whole body temperature with no change in resting core to skin temperature gradient (Fig. 3A).
A combined stimulus of exercise and heat stress is generally considered the “gold standard” method for inducing heat acclimation adaptations (53). As expected, we found that conventional EHA caused thermal adaptations in comparison with baseline (end-exercise: Tre −0.37 ± 0.13°C, Table 3). However, there is a dearth of medium-term heat acclimation studies with an appropriate control intervention; hence, the true effect of conventional exercise heat acclimation is poorly understood. In the current study, the inclusion of a work-matched temperate exercise intervention allowed the independent effectiveness of the exercise and heat stress stimuli to be determined. We found that, aside from an increase in whole-body sweat rate, which was greater after EHA, no additional heat acclimation adaptations existed between EHA and CON. Our findings align with studies that demonstrate aerobic training in temperate conditions initiates adaptations commonly associated with heat acclimation in recreationally active individuals (54–57). These studies suggest it is principally the endogenous heat production incurred during exercise rather than the external environmental temperature that is important for initiating heat acclimation adaptations. When considered together with these investigations, the benefits of conventional exercise-based heat acclimation beyond work-matched exercise in temperate conditions are modest.
Previous research has shown that intensified training during exercise heat acclimation can trigger markers of overreaching including increased perceived fatigue and decreased performance (12). In contrast, we observed no evidence of overreaching after EHA or HWI; a discrepancy that might be explained by the lower exercise intensity and the inclusion of three rest days in our study compared with previous research. More participants did however withdraw with lower limb discomfort (i.e., knee/ankle pain, etc.) in EHA (25%) and CON (25%) than in HWI (7%; Fig. 2); a finding that might be explained by the ∼35% greater external work during EHA and CON interventions than HWI. This finding provides insight into the practical feasibility of these interventions but is difficult to compare with previous research as heat acclimation studies do not often report participant flow and attrition. Based on our findings, athletes and coaches may be more inclined to choose HWI in the knowledge it carries less injury risk than EHA. Although this is a reasonable hypothesis, future studies with adequate sample sizes are required to specifically evaluate the injury and illness risks of heat acclimation.
Athletes and coaches should consider HWI rather than EHA before traveling to hot climates as it leads to a more complete state of heat acclimation, can be incorporated into the post-exercise washing routine, and eliminates the requirement for an increased training load or access to an environmental chamber. These benefits reduce the disruption to normal training compared with conventional exercise-based strategies, which is especially important during tapering in the lead-up to sporting events. Whereas adverse events after HWI, including syncope, have not been observed by us (6, 7, 13, 14), or reported by others (58, 59), practitioners should follow protocol guidelines carefully. In particular, hot water immersions should be terminated at the participant’s volition or if Tre exceeds 39.5°C rather than attempting to complete 40 min. In our study, this led to a gradual daily increase in hot water immersion duration up to a maximum of 40 min for the first six (days 1–3, 33 ± 4 min; days 6–8, 35 ± 5 min), and then 50 min for the seventh to twelfth immersions (days 11–16, 39 ± 5 min). The current and previous studies demonstrate the effectiveness of HWI to prepare young, healthy, active males (6, 7, 9, 13, 14) and elderly males and females for heat stress (59). Further research is required to confirm that HWI is effective to cause beneficial thermal, perceptual, and performance adaptations in pediatric, female, and older athletic populations. We hypothesize that HWI will be an effective strategy in these populations as Mee et al. (60) demonstrated that combining both active and passive heat acclimation strategies can accelerate thermal adaptations in females. The large dual thermal stimulus from 6 days of HWI should be sufficient to initiate heat acclimation adaptations in these populations as they typically have smaller body masses than adult males and consequently gain heat more quickly (61). Because of the smaller body masses these future investigations might require shorter maximum HWI durations to cause the beneficial thermal adaptations.
Perspectives and Significance
Our findings show that medium-term post-exercise HWI confers more complete heat acclimation than conventional exercise heat acclimation, without increasing the risk of overreaching. Compared with conventional exercise heat acclimation, post-exercise HWI caused a greater reduction in resting whole body temperature (core and periphery), which highlights the importance of a large dual (endogenous and exogenous) thermal stimulus for optimizing adaptation to the heat. The consistently reported large reduction in resting core temperature after HWI is most likely explained by the establishment of a new lower thermal balance point and not initiated by thyroid hormone alterations, changes in heat production, or heat loss mechanisms. In addition to lowering resting whole-body temperature, post-exercise HWI also caused more pronounced beneficial exercising thermal and perceptual adaptations than conventional exercise heat acclimation. Future research should assess whether the reduction in thermal strain after post-exercise HWI translates to “real-world” performance improvements and reduces the incidence of exertional heat illness.
GRANTS
The work was funded by the Chief Scientific Advisers research program, Ministry of Defence UK (to N. P. Walsh and J. A. Mee), through the Defence Science and Technology Laboratory (DSTLX-1000111028).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.D.M., M.J.Z., J.A.M., N.P.W., and S.J.O. conceived and designed research; R.D.M., M.J.Z., J.A.M., and N.P.W. performed experiments; R.D.M. and S.J.O. analyzed data; R.D.M., M.J.Z., J.A.M., N.P.W., and S.J.O. interpreted results of experiments; R.D.M. and S.J.O. prepared figures; R.D.M., M.J.Z., J.A.M., N.P.W., and S.J.O. drafted manuscript; R.D.M., M.J.Z., J.A.M., N.P.W., and S.J.O. edited and revised manuscript; R.D.M., M.J.Z., J.A.M., N.P.W., and S.J.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jason Edwards and Kevin Williams for their valuable technical assistance with data collection. We are also indebted to the participants for their time and cooperation.
REFERENCES
- 1. Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 29: 1240–1249, 1997. doi: 10.1097/00005768-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 2. Maughan RJ, Otani H, Watson P. Influence of relative humidity on prolonged exercise capacity in a warm environment. Eur J Appl Physiol 112: 2313–2321, 2012. doi: 10.1007/s00421-011-2206-7. [DOI] [PubMed] [Google Scholar]
- 3. Casa DJ, DeMartini JK, Bergeron MF, Csillan D, Eichner ER, Lopez RM, Ferrara MS, Miller KC, O'Connor F, Sawka MN, Yeargin SW. National athletic trainers' association position statement: exertional heat illnesses. J Athl Train 50: 986–1000, 2015. [Erratum in J Athl Train 52: 401, 2017]. doi: 10.4085/1062-6050-52.4.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saunders PU, Garvican-Lewis LA, Chapman RF, Périard JD. Special environments: altitude and heat. Int J Sport Nutr Exerc Metab 29: 210–219, 2019. doi: 10.1123/ijsnem.2018-0256. [DOI] [PubMed] [Google Scholar]
- 5. Ashworth ET, Cotter JD, Kilding AE. Methods for improving thermal tolerance in military personnel prior to deployment. Mil Med Res 7: 58, 2020. doi: 10.1186/s40779-020-00287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zurawlew MJ, Walsh NP, Fortes MB, Potter C. Post-exercise hot water immersion induces heat acclimation and improves endurance exercise performance in the heat. Scand J Med Sci Sports 26: 745–754, 2016. doi: 10.1111/sms.12638. [DOI] [PubMed] [Google Scholar]
- 7. Zurawlew MJ, Mee JA, Np W. Post-exercise hot water immersion elicits heat acclimation adaptations in endurance trained and recreationally active individuals. Front Physiol 9: 1824, 2018. doi: 10.3389/fphys.2018.01824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casadio JR, Kilding AE, Siegel R, Cotter JD, Laursen PB. Periodizing heat acclimation in elite Laser sailors preparing for a world championship event in hot conditions. Temperature (Austin) 3: 437–443, 2016. doi: 10.1080/23328940.2016.1184367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McIntyre RD, Zurawlew MJ, Oliver SJ, Cox AT, Mee JA, Walsh NP. A comparison of heat acclimation by post-exercise hot water immersion and exercise in the heat. J Sci Med Sport 24: 729–734, 2021. doi: 10.1016/j.jsams.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 10. Moss JN, Bayne FM, Castelli F, Naughton MR, Reeve TC, Trangmar SJ, Mackenzie RWA, Tyler CJ. Short-term isothermic heat acclimation elicits beneficial adaptations but medium-term elicits a more complete adaptation. Eur J Appl Physiol 120: 243–254, 2020. doi: 10.1007/s00421-019-04269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheung SS, McLellan TM. Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J Appl Physiol (1985) 84: 1731–1739, 1998. doi: 10.1152/jappl.1998.84.5.1731. [DOI] [PubMed] [Google Scholar]
- 12. Schmit C, Duffield R, Hausswirth C, Brisswalter J, Le Meur Y. Optimizing heat acclimation for endurance athletes: high- vs low-intensity training. Int J Sports Physiol Perform 13: 816–823, 2018. doi: 10.1123/ijspp.2017-0007. [DOI] [PubMed] [Google Scholar]
- 13. Zurawlew MJ, Mee JA, Walsh NP. Post-exercise hot water immersion elicits heat acclimation adaptations that are retained for at least two weeks. Front Physiol 10: 1080, 2019. doi: 10.3389/fphys.2019.01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zurawlew MJ, Mee JA, Walsh NP. Heat acclimation by post-exercise hot water immersion in the morning reduces thermal strain during morning and afternoon exercise-heat-stress. Int J Sports Physiol Perform 13: 1281–1286, 2018. doi: 10.1123/ijspp.2017-0620. [DOI] [PubMed] [Google Scholar]
- 15. Buguet A, Gati R, Soubiran G, Straboni JP, Hanniquet AM, Livecchi-Gonnot G, Bittel J. Seasonal changes in circadian rhythms of body temperatures in humans living in a dry tropical climate. Eur J Appl Physiol Occup Physiol 58: 334–339, 1988. doi: 10.1007/BF00417272. [DOI] [PubMed] [Google Scholar]
- 16. Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292: R37–R46, 2007. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 17. Romanovsky AA. The thermoregulation system and how it works. Handb Clin Neurol 156: 3–43, 2018. doi: 10.1016/B978-0-444-63912-7.00001-1. [DOI] [PubMed] [Google Scholar]
- 18. Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, Knight ZA. Warm-sensitive neurons that control body temperature. Cell 167: 47–59.e15, 2016. doi: 10.1016/j.cell.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao ZD, Yang WZ, Gao C, Fu X, Zhang W, Zhou Q, Chen W, Ni X, Lin JK, Yang J, Xu XH, Shen WL. A hypothalamic circuit that controls body temperature. Proc Natl Acad Sci USA 114: 2042–2047, 2017. doi: 10.1073/pnas.1616255114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwen KA, Oelkrug R, Brabant G. Effects of thyroid hormones on thermogenesis and energy partitioning. J Mol Endocrinol 60: R157–R170, 2018. doi: 10.1530/JME-17-0319. [DOI] [PubMed] [Google Scholar]
- 21. Horowitz M, Peyser YM, Muhlrad A. Alterations in cardiac myosin isoenzymes distribution as an adaptation to chronic environmental heat stress in the rat. J Mol Cell Cardiol 18: 511–515, 1986. doi: 10.1016/S0022-2828(86)80916-6. [DOI] [PubMed] [Google Scholar]
- 22. Mirit E, Gross C, Hasin Y, Palmon A, Horowitz M. Changes in cardiac mechanics with heat acclimation: adrenergic signaling and SR-Ca regulatory proteins. Am J Physiol Regul Integr Comp Physiol 279: R77–R85, 2000. doi: 10.1152/ajpregu.2000.279.1.R77. [DOI] [PubMed] [Google Scholar]
- 23. Maloyan A, Horowitz M. β-Adrenergic signaling and thyroid hormones affect HSP72 expression during heat acclimation. J Appl Physiol (1985) 93: 107–115, 2002. doi: 10.1152/japplphysiol.01122.2001. [DOI] [PubMed] [Google Scholar]
- 24. Yang Y, Gordon CJ. Regulated hypothermia in the hypothyroid rat induced by administration of propylthiouracil. Am J Physiol 272: R1390–R1395, 1997. doi: 10.1152/ajpregu.1997.272.5.R1390. [DOI] [PubMed] [Google Scholar]
- 25. Faul F, Erdfelder E, Lang AG, Buchner AG. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 26. Mee JA, Gibson OR, Doust J, Maxwell NS. A comparison of males and females' temporal patterning to short- and long-term heat acclimation. Scand J Med Sci Sports 25, Suppl 1: 250–258, 2015. doi: 10.1111/sms.12417. [DOI] [PubMed] [Google Scholar]
- 27. Gibson OR, Mee JA, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J Therm Biol 49–50: 55–65, 2015. doi: 10.1016/j.jtherbio.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 28. Shaw AJ, Ingham SA, Folland JP. The valid measurement of running economy in runners. Med Sci Sports Exerc 46: 1968–1973, 2014. doi: 10.1249/MSS.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 29. Spurway N, Jones AM. Lactate testing. In: Sport and Exercise Physiology Testing Guidelines: The British Association of Sport and Exercise Science Guide, edited by Winter EM, Jones AM, Davison RCR, Bromley PD, Mercer TH.. Abingdon, Oxon: Routledge, 2017, p. 112–119. [Google Scholar]
- 30. Meeusen R, Duclos M, Foster C, Fry A, Gleeson M, Nieman D, Raglin J, Rietjens G, Steinacker J, Urhausen A. Prevention, diagnosis and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science(ECSS) and the American College of Sports Medicine (ACSM). Eur J Sport Sci 13: 1–24, 2013. doi: 10.1080/17461391.2012.730061. [DOI] [PubMed] [Google Scholar]
- 31. Grove JR, Prapavessis H. Preliminary evidence for the reliability and validity of an abbreviated Profile of Mood States. Int J Sport Psychol 23: 93–109, 1992. [Google Scholar]
- 32. Armstrong LE. Hydration assessment techniques. Nutr Rev 63: 40–54, 2005. doi: 10.1111/j.1753-4887.2005.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 33. Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol 19: 531–533, 1964. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- 34. Burton AC. Human calorimetry. II. The average temperature of the tissues of the body: three figures. J Nutr 9: 261–280, 1935. doi: 10.1093/jn/9.3.261. [DOI] [Google Scholar]
- 35. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic) XVII: 863–871, 1916. [PubMed] [Google Scholar]
- 36. Gagge AP, Gonzalez RR. Mechanisms of heat exchange: biophysics and physiology. In: Handbook of Physiology Section 4, Environmental Physiology, edited by Fregly MJ, Blatteis CM.. New York: Oxford University Press, 1996, p. 45–85. [Google Scholar]
- 37. Cheuvront SN, Chinevere TD, Ely BR, Kenefick RW, Goodman DA, McClung JP, Sawka MN. Serum S-100beta response to exercise-heat strain before and after acclimation. Med Sci Sports Exerc 40: 1477–1482, 2008. doi: 10.1249/MSS.0b013e31816d65a5. [DOI] [PubMed] [Google Scholar]
- 38. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2: 92–98, 1970. [PubMed] [Google Scholar]
- 39. Hollies NRS, Goldman GRF. Psychological scaling in comfort assessment. In: Clothing Comfort: Interaction of Thermal, Ventilation, Construction, and Assessment Factors, edited by Hollies NRS, Goldman GRF.. Ann Arbor, MI: Ann Arbor Science Publishers, 1977, p. 107–120. [Google Scholar]
- 40. James DVB, Sandals LE, Wood DM, Jones AM. Pulmonary gas exchange. In: Sport and Exercise Physiology Testing Guidelines: The British Association of Sport and Exercise Science Guide, edited by Jones AM, Winter EM, Davidson RCR, Bromley PD, Mercer T.. Abingdon, Oxon: Routledge, 2017, p. 101–111. [Google Scholar]
- 41. Dupuy O, Lussier M, Fraser S, Bherer L, Audiffren M, Bosquet L. Effect of overreaching on cognitive performance and related cardiac autonomic control. Scand J Med Sci Sports 24: 234–242, 2014. doi: 10.1111/j.1600-0838.2012.01465.x. [DOI] [PubMed] [Google Scholar]
- 42. Le Meur Y, Hausswirth C, Natta F, Couturier A, Bignet F, Vidal PP. A multidisciplinary approach to overreaching detection in endurance trained athletes. J Appl Physiol (1985) 114: 411–420, 2013. doi: 10.1152/japplphysiol.01254.2012. [DOI] [PubMed] [Google Scholar]
- 43. Schaal K, Meur YLE, Louis J, Filliard JR, Hellard P, Casazza G, Hausswirth C. Whole-body cryostimulation limits overreaching in elite synchronized swimmers. Med Sci Sports Exerc 47: 1416–1425, 2015. doi: 10.1249/MSS.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 44. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 45. Sherk VD, Chrisman C, Smith J, Young KC, Singh H, Bemben MG, Bemben DA. Acute bone marker responses to whole-body vibration and resistance exercise in young women. J Clin Densitom 16: 104–109, 2013. doi: 10.1016/j.jocd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 47. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg 126: 1763–1768, 2018. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 48. Karlsen A, Nybo L, Nørgaard SJ, Jensen MV, Bonne T, Racinais S. Time course of natural heat acclimatization in well-trained cyclists during a 2-week training camp in the heat. Scand J Med Sci Sports 25, Suppl 1: 240–249, 2015. doi: 10.1111/sms.12449. [DOI] [PubMed] [Google Scholar]
- 49. Daanen HA, Jonkman AG, Layden JD, Linnane DM, Weller AS. Optimising the acquisition and retention of heat acclimation. Int J Sports Med 32: 822–828, 2011. doi: 10.1055/s-0031-1279767. [DOI] [PubMed] [Google Scholar]
- 50. Racinais S, Périard JD, Karlsen A, Nybo L. Effect of heat and heat acclimatization on cycling time trial performance and pacing. Med Sci Sports Exerc 47: 601–606, 2015. doi: 10.1249/MSS.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pandolf KB, Burse RL, Goldman RF. Role of physical fitness in heat acclimatisation, decay and reinduction. Ergonomics 20: 399–408, 1977. doi: 10.1080/00140137708931642. [DOI] [PubMed] [Google Scholar]
- 52. Regan JM, Macfarlane DJ, Taylor NAS. An evaluation of the role of skin temperature during heat adaptation. Acta Physiol Scand 158: 365–375, 1996. doi: 10.1046/j.1365-201X.1996.561311000.x. [DOI] [PubMed] [Google Scholar]
- 53. Racinais S, Alonso JM, Coutts AJ, Flouris AD, Girard O, González-Alonso J, Hausswirth C, Jay O, Lee JK, Mitchell N, Nassis GP, Nybo L, Pluim BM, Roelands B, Sawka MN, Wingo JE, Périard JD. Consensus recommendations on training and competing in the heat. Br J Sports Med 49: 1164–1173, 2015. doi: 10.1136/bjsports-2015-094915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shvartz E, Shapiro Y, Magazanik A, Meroz A, Birnfeld H, Mechtinger A, Shibolet S. Heat acclimation, physical fitness, and responses to exercise in temperate and hot environments. J Appl Physiol Respir Environ Exerc Physiol 43: 678–683, 1977. doi: 10.1152/jappl.1977.43.4.678. [DOI] [PubMed] [Google Scholar]
- 55. Kampmann B, Bröde P, Schütte M, Griefahn B. Lowering of resting core temperature during acclimation is influenced by exercise stimulus. Eur J Appl Physiol 104: 321–327, 2008. doi: 10.1007/s00421-007-0658-6. [DOI] [PubMed] [Google Scholar]
- 56. Ravanelli N, Gagnon D, Imbeault P, Jay O. A retrospective analysis to determine if exercise training-induced thermoregulatory adaptations are mediated by increased fitness or heat acclimation. Exp Physiol 106: 282–289, 2021. doi: 10.1113/EP088385. [DOI] [PubMed] [Google Scholar]
- 57. Avellini BA, Shapiro Y, Fortney SM, Wenger CB, Pandolf KB. Effects on heat tolerance of physical training in water and on land. J Appl Physiol Respir Environ Exerc Physiol 53: 1291–1298, 1982. doi: 10.1152/jappl.1982.53.5.1291. [DOI] [PubMed] [Google Scholar]
- 58. Gerrett N, Alkemade P, Daanen H. Heat reacclimation using exercise or hot water immersion. Med Sci Sports Exerc 53: 1517–1528, 2021. doi: 10.1249/MSS.0000000000002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Waldock KAM, Gibson OR, Relf RL, Eichhorn G, Hayes M, Watt PW, Maxwell NS. Exercise heat acclimation and post-exercise hot water immersion improve resting and exercise responses to heat stress in the elderly. J Sci Med Sport 24: 774–780, 2021. doi: 10.1016/j.jsams.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 60. Mee JA, Peters S, Doust JH, Maxwell NS. Sauna exposure immediately prior to short-term heat acclimation accelerates phenotypic adaptation in females. J Sci Med Sport 21: 190–195, 2018. doi: 10.1016/j.jsams.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 61. Foster J, Hodder SG, Lloyd AB, Havenith G. Individual responses to heat stress: implications for hyperthermia and physical work capacity. Front Physiol 11: 541483, 2020. doi: 10.3389/fphys.2020.541483. [DOI] [PMC free article] [PubMed] [Google Scholar]


