ABSTRACT
Colistin, which targets lipopolysaccharide (LPS), is used as a last-resort drug against severe infections caused by drug-resistant Acinetobacter baumannii. However, A. baumannii possesses two colistin-resistance mechanisms. LPS modification caused by mutations in pmrAB genes is often observed in clinical isolates of multidrug-resistant Gram-negative pathogens. In addition to LPS modification, A. baumannii has a unique colistin resistance mechanism, a complete loss of LPS due to mutations in the lpxACD genes, which are involved in LPS biosynthesis. This study aimed to elucidate the detailed mechanism of the emergence of colistin-resistant A. baumannii using strains with the same genetic background. Various colistin-resistant strains were generated experimentally using colistin alone and in combination with other antimicrobials, such as meropenem and ciprofloxacin, and the mutation spectrum was analyzed. In vitro selection of A. baumannii in the presence of colistin led to the emergence of strains harboring mutations in lpxACD genes, resulting in LPS-deficient colistin-resistant strains. However, combination of colistin with other antimicrobials led to the selection of pmrAB mutant strains, resulting in strains with modified LPS (LPS-modified strains). Further, the LPS-deficient strains showed decreased fitness and increased susceptibility to many antibiotics and disinfectants. As LPS-deficient strains have a higher biological cost than LPS-modified strains, our findings suggested that pmrAB mutants are more likely to be isolated in clinical settings. We provide novel insights into the mechanisms of resistance to colistin and provide substantial solutions along with precautions for facilitating current research and treatment of colistin-resistant A. baumannii infections.
IMPORTANCE Acinetobacter baumannii has developed resistance to various antimicrobial drugs, and its drug-resistant strains cause nosocomial infections. Controlling these infections has become a global clinical challenge. Carbapenem antibiotics are the frontline treatment drugs for infectious diseases caused by A. baumannii. For patients with infections caused by carbapenem-resistant A. baumannii, colistin-based therapy is often the only treatment option. However, A. baumannii readily acquires resistance to colistin. Many patients infected with colistin-resistant A. baumannii undergo colistin treatment before isolation of the colistin-resistant strain, and it is hypothesized that colistin resistance predominantly emerges under selective pressure during colistin therapy. Although the concomitant use of colistin and carbapenems has been reported to have a synergistic effect in vitro against carbapenem-resistant A. baumannii strains, our observations strongly suggest the need for attention to the emergence of strains with a modified lipopolysaccharide during treatment.
KEYWORDS: Acinetobacter baumannii, LPS, colistin, lpxACD, meropenem, pmrAB
INTRODUCTION
Acinetobacter baumannii is a Gram-negative bacterium widely distributed in nature. Although A. baumannii is detected on the skin of healthy individuals and is generally harmless, it can cause opportunistic infections in immunocompromised hosts. The ability of this pathogen to survive in harsh environments, such as under desiccated conditions, enables its survival in hospital facilities and on medical devices, causing a spectrum of infectious diseases of the respiratory tract, bloodstream, urinary tract, surgical sites, and wounds (1, 2). Although health care professionals are paying attention to A. baumannii infections, this pathogen is responsible for a vast array of nosocomial infections, and the difficulty of controlling these infections remains a problem of global importance. One reason for this difficulty is its ability to readily acquire antimicrobial resistance. Acinetobacter baumannii exhibits multiple mechanisms of antimicrobial resistance, including production of several intrinsic and acquired β-lactamase enzymes, efflux pumps that prevent accumulation of antimicrobials, and acquired resistance through plasmids carrying resistance genes. Consequently, pandrug-resistant A. baumannii is a growing and alarming global health concern (2–8). Although carbapenem antibiotics are used as frontline treatment for A. baumannii, carbapenem-resistance is widely observed in several international clones, and the WHO classifies A. baumannii as a bacterium that requires new antibiotic development.
Currently, colistin-based therapy is the most efficient treatment option for patients with infections caused by carbapenem-resistant A. baumannii (9–13). Colistin is classified as a cationic amphiphilic polypeptide antibacterial drug that interacts with the lipid A component of the outer membrane lipopolysaccharide (LPS) and disrupts bacterial membrane integrity, ultimately causing cell death. Colistin is used as a last resort drug to combat infections caused by severe multidrug-resistant (MDR) A. baumannii and other MDR Gram-negative bacteria (14–17). The increased use of colistin in human pharmacotherapy has led to an increase in colistin-resistant bacterial strains, which has caused major concern worldwide due to the lack of antimicrobials available for treatment of these resistant strains (18, 19). In particular, the high frequency of acquisition of colistin resistance by A. baumannii is concerning. Epidemiological surveillance shows that colistin-resistant strains of A. baumannii are not phylogenetically restricted and most colistin-resistant clinical isolates have acquired mutations in chromosomal genes related to colistin resistance (20–24).
The general mechanism of resistance to colistin is due to modification of LPS with phosphoethanolamine, which reduces the electrostatic interaction between colistin and LPS (14, 17). Acinetobacter baumannii has an endogenous phosphoethanolamine transferase (PmrC) that is regulated by the two-component regulatory system PmrAB, and it is the main enzyme responsible for LPS modification and colistin resistance (25, 26). Notably, A. baumannii has another colistin resistance mechanism, which is the complete loss of LPS due to mutations in lpxACD genes, which are responsible for LPS biosynthesis. LPS is thought to be essential for the survival of Gram-negative bacteria; therefore, inhibition of its synthesis is a target for novel antimicrobial agents (27–31). However, a few species, such as Neisseria meningitidis, Moraxella catarrhalis, A. baumannii, and Acinetobacter nosocomialis can survive with complete loss of LPS (32–34). The emergence of lpxACD mutants of A. baumannii under significant outer membrane stress caused by polymyxin antibiotics in both laboratory and clinical settings has been reported (29, 35, 36). However, most clinically isolated colistin-resistant strains exhibit LPS modification (referred to here as LPS-modified strains) due to the pmrAB mutations (37, 38). Additionally, some A. baumannii strains have been reported to exhibit a colistin-dependent transient phenotype for survival under colistin pressure (39, 40). The detailed mechanism through which A. baumannii acquires colistin resistance remains unknown. In particular, fundamental data representing the spectrum of mutation types have not been obtained.
RESULTS
Mutation spectrum of colistin-resistant strains obtained in the laboratory and clinical settings.
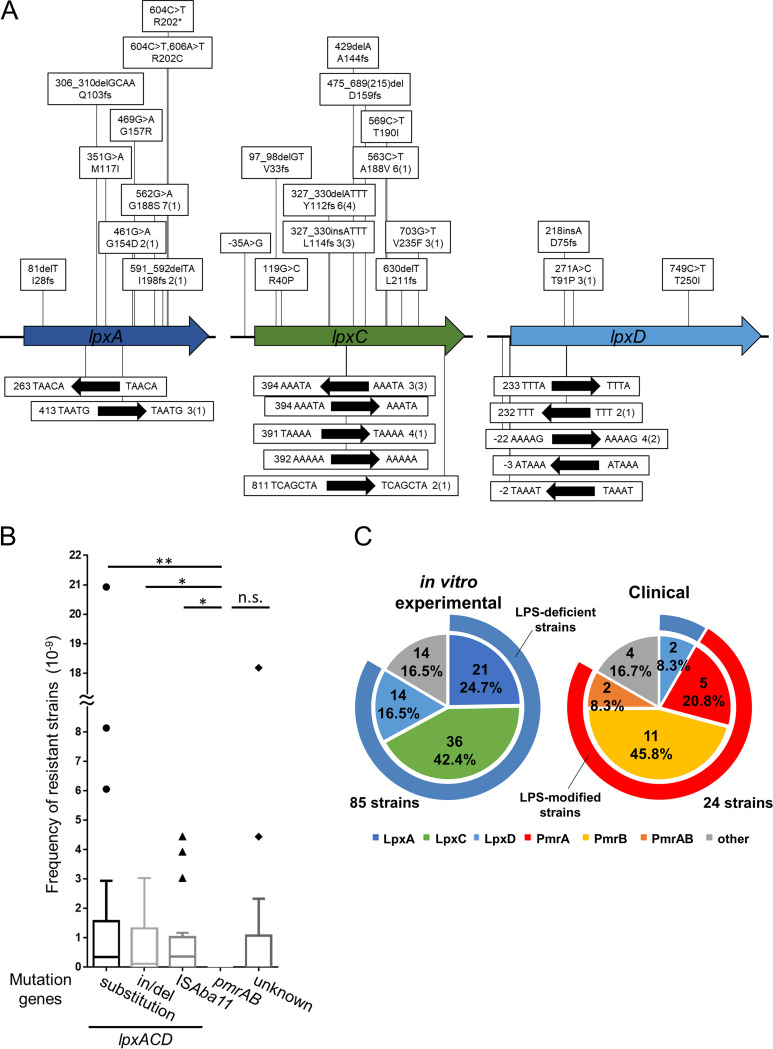
To analyze how A. baumannii acquires resistance to colistin, we used A. baumannii type strain ATCC 19606 as a model strain. The bacterium adopts two different resistance mechanisms: loss-of-function mutations in genes involved in LPS biosynthesis (lpxACD), resulting in LPS-deficient strains, and gain-of-function mutations in pmrAB, which regulate the expression of the LPS modification enzyme (PmrC), resulting in LPS-modified strains (1, 17, 28). To assess frequently occurring mutations in A. baumannii, we independently cultured 16 single colonies; aliquots were spread on plates supplemented with 10 μg/mL (5× MIC) of colistin, and the frequency of mutation types was analyzed. Eighty-five of the isolated colistin-resistant mutants of A. baumannii ATCC 19606 were subjected to PCR amplification of the lpxA, lpxC, lpxD, pmrA, and pmrB genes. The PCR products were sequenced to determine mutations that lead to colistin resistance (Fig. 1A). To compare the occurrence of each mutation, the frequency of each mutation was calculated by dividing the number of colonies with each mutation by the CFU obtained on colistin-containing plates. The frequencies of colistin-resistant strains harboring substitutions, short indels, and insertion of ISAba11 in lpx genes were similar, but strains harboring mutations in pmrAB were not detected (Fig. 1B). Mutations in lpxA, lpxC, and lpxD genes were detected in 21, 36, and 14 colistin-resistant strains, respectively, and the locations of these mutations were spread broadly throughout the genes (Fig. 1A; also, see Table S1 in the supplemental material). In 34% (24 strains) of the colistin-resistant strains, ISAba11 was inserted into the lpx genes, disrupting each gene. Mutations (KL068) or ISAba11 insertions (KL003, -045, -070, and -083) upstream of the lpx genes were also identified, and we confirmed that these mutations downregulated mRNA expression of downstream lpx genes (Fig. S1). In summary, we found nonsynonymous mutations in the lpx genes of 71 colistin-resistant A. baumannii strains, but none harbored mutations in pmrAB (Fig. 1A; Tables S1 and 2).
FIG 1.
Mutation sites of colistin-resistant A. baumannii strains in laboratory and clinical settings. (A) Mutation site analysis of in vitro experimental laboratory-isolated strains. A. baumannii ATCC 19606 strains were isolated by direct plating on LB agar plates containing 5× MIC of colistin (10 μg/mL) to select resistant strains. Sanger sequencing was performed on the genomic regions containing the lpxACD genes. In the boxes above the genes, base changes are shown on top, while amino acid changes are shown below. The numerical values are the numbers of isolated strains, and numbers in parentheses are the numbers of independent events. The black arrows indicate ISAba11, and repetitive sequences are shown. (B) Frequency analysis of occurrence for each mutation. Data are shown in a box plot; 18 independent assays were compared by one-way ANOVA. n.s., not significant; **, P < 0.01; *, P < 0.05. Of the 110 colistin-resistant strains, 28 could not be sequenced because of overlapping colonies. (C) Pie chart showing mutation rates. Data for isolated in vitro experimental strains are on the left, and the results of NCBI BioSample analysis are on the right.
The MICs of colistin for most strains with amino acid substitutions in LpxACD were similar to those determined for strains with frameshift mutations. Only strain KL052, which harbored a mutation leading to a T91P substitution in LpxD, exhibited a low MIC of colistin (Table S3). The observed mutations in lpxACD genes comprised nonsynonymous substitutions, short indels, and transposition of the insertion sequence ISAba11 and possibly resulted in loss of function of enzymes involved in lipid A synthesis. In the remaining 14 colistin-resistant strains, no mutations were detected in either lpxACD or pmrAB, and the resistance mechanism remains unknown. In total, 151 colistin-resistant strains of A. baumannii ATCC 19606 were identified, including the strains that were not sequenced (66 strains). Among these, only one strain showed growth in the presence of colistin (5× MIC) and meropenem (1/5× MIC), and subsequent sequence analysis revealed mutations in pmrB (KL148; pmrB 697C→T, P233S). These results indicate that the colistin-resistant mutations in A. baumannii are more likely to occur in lpxACD genes than in pmrAB genes in vitro.
Next, we performed in silico analysis using the NCBI BioSample database to understand how clinical isolates of A. baumannii acquire resistance to colistin. Among the 24 clinically isolated colistin-resistant strains analyzed, only two harbored an amino acid substitution in LpxD, and 18 harbored an amino acid substitution in PmrAB (Table S4). Thus, in contrast to our in vitro experimental observations that indicated a higher frequency of LPS-deficient strains, most colistin-resistant clinical isolates were PmrAB mutants and therefore had LPS modifications (Fig. 1C).
Change in colistin-resistant mutation type due to combination with meropenem.
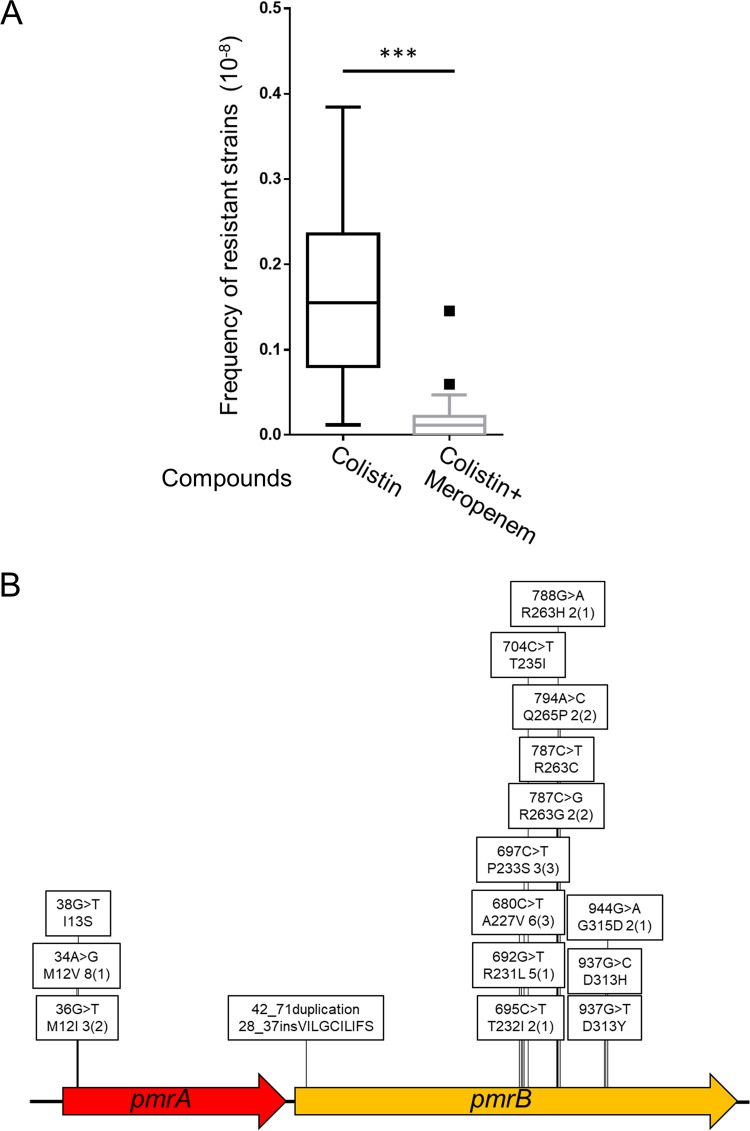
The colistin resistance mutations differed between in vitro experimental and clinically isolated strains of A. baumannii. In clinical practice, colistin is often used with other antibiotics, such as carbapenem, after failure of first-line drugs (22, 23). In cases where the use of colistin is necessary, these first-line drugs may still act at sub-MICs, as a withdrawal period cannot be established. Therefore, we investigated colistin-resistant mutants obtained in combination with meropenem, which is commonly used to treat A. baumannii infections. The frequency of colistin resistance was determined when A. baumannii was treated with a combination of colistin (5× MIC) and meropenem (sub-MIC [1/5× MIC]). Although no growth disparity was observed following treatment with 1/5× MIC of meropenem alone, combinational treatment with meropenem and colistin decreased the frequency of colistin-resistant colonies to approximately 1/8 of that determined with colistin alone (Fig. 2A). We isolated 41 colistin-meropenem-resistant A. baumannii strains, and their genetic mutations were analyzed. As shown in Fig. 2B, nonsynonymous point mutations or 30-bp duplications resulting in 10 amino acid insertions in pmrAB genes were identified in all 41 colistin-meropenem-resistant strains (Table S1). Mutations in pmrAB genes were restricted to several sites, and most of these sites were identical to those observed in clinical isolates (Fig. 2B and Table S5) (41, 42). Further, the lpxACD genes were analyzed in 21 of the 41 colistin-meropenem-resistant strains, but no mutations were observed (Table S5). Thus, although the dose of meropenem was quite low compared to that used in clinical settings, the addition of meropenem resulted in the preferential selection of pmrAB mutants possibly harboring modified LPS.
FIG 2.
Frequency of emergence of colistin-resistant A. baumannii (type strain, ATCC 19606) strains and changes in mutation tendency in combination with meropenem. (A) The frequency of colistin resistance was analyzed by plating 0.2 mL of ATCC 19606 culture (OD600 of 1.0) on LB agar containing 5× MIC (10 μg/mL) of colistin for 24 h at 37°C. The frequency of resistance to colistin, when combined with 1/5× MIC (0.1 μg/mL) of meropenem, was determined by plating 0.1 mL of ATCC 19606 culture (OD600 of 10) on LB agar containing 10 μg/mL of colistin and 0.1 μg/mL of meropenem. Data are shown in a box plot; 20 independent assays were compared by the Mann-Whitney U test. ***, P < 0.001. (B) Mutation site analysis of colistin-meropenem-resistant ATCC 19606 strains isolated by direct plating on LB agar plates containing 5× MIC (10 μg/mL) of colistin and 1/5× MIC (0.1 μg/mL) of meropenem. Sanger sequencing was performed on the genomic regions containing the pmrAB genes. In the boxes, base changes are shown on top, while amino acid changes are shown below. The numerical values are the numbers of isolated strains, and numbers in parentheses are the numbers of independent events.
Analysis of the mutation types of colistin resistance in combination with ciprofloxacin and in drug-resistant strains.
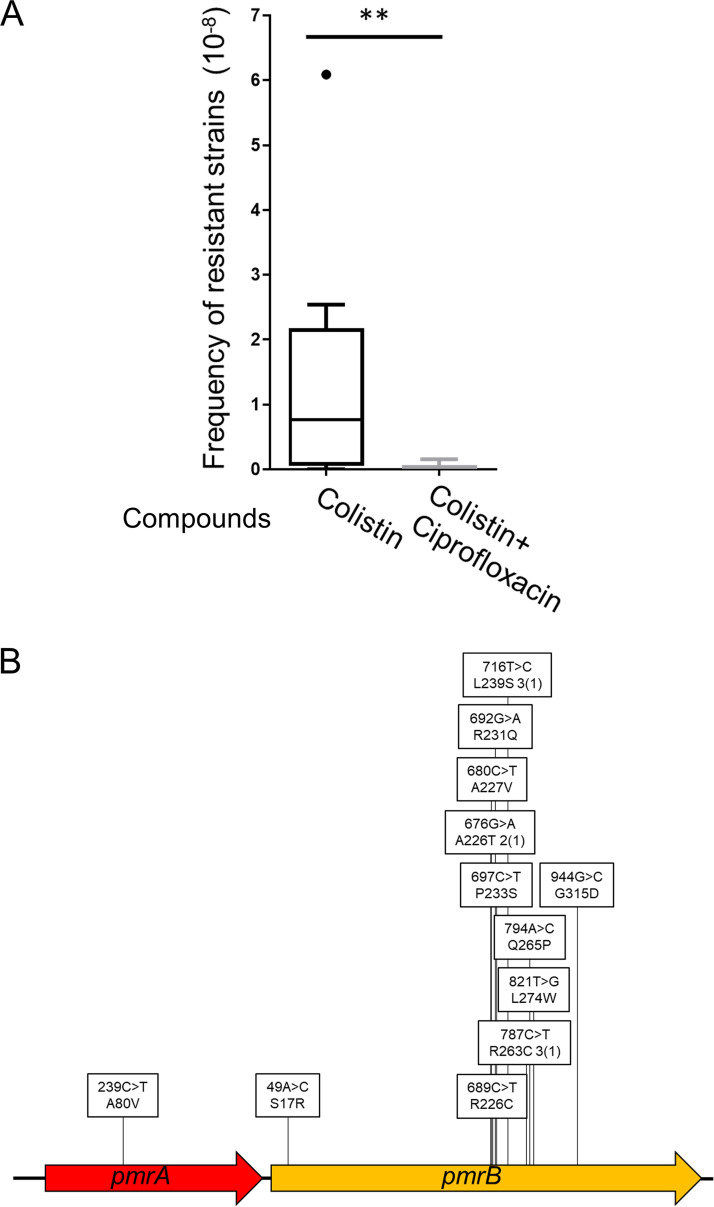
We next investigated the frequency of colistin resistance and the mutation spectrum using a combination of colistin (5× MIC) and ciprofloxacin (1/5× MIC). Ciprofloxacin is used against resistant bacterial strains, and its mechanism of action differs from that of meropenem by targeting DNA (43). Combination with ciprofloxacin reduced the frequency of colistin resistance (Fig. 3A), and all 17 colistin-ciprofloxacin-resistant strains harbored mutations in the pmrAB genes (Fig. 3B and Table S6).
FIG 3.
Frequency of emergence of colistin-resistant A. baumannii ATCC 19606 strains and altered mutation tendencies in combination with ciprofloxacin. (A) The frequency of colistin resistance was analyzed by plating 0.2 mL culture (OD600 of 1.0) on LB agar containing 5× MIC (10 μg/mL) of colistin and incubating for 24 h at 37°C. The frequency of colistin resistance in combination with ciprofloxacin was determined by plating 0.1 mL culture (OD600 of 10) on LB agar containing 10 μg/mL of colistin and 0.4 μg/mL (1/5× MIC) of ciprofloxacin. Data are shown as box plots (n = 10 independent assays). Comparisons were made using the Mann-Whitney U test. **, P < 0.01. (B) Mutation site analysis of colistin-ciprofloxacin-resistant ATCC 19606 strains isolated by direct plating on LB agar containing 10 μg/mL colistin and 0.4 μg/mL of ciprofloxacin. Sanger sequencing was performed on genomic regions containing the pmrAB genes. In the boxes, base changes are shown on top, while amino acid changes are shown below. The numerical values are the numbers of isolated strains, and numbers in parentheses are the numbers of independent events.
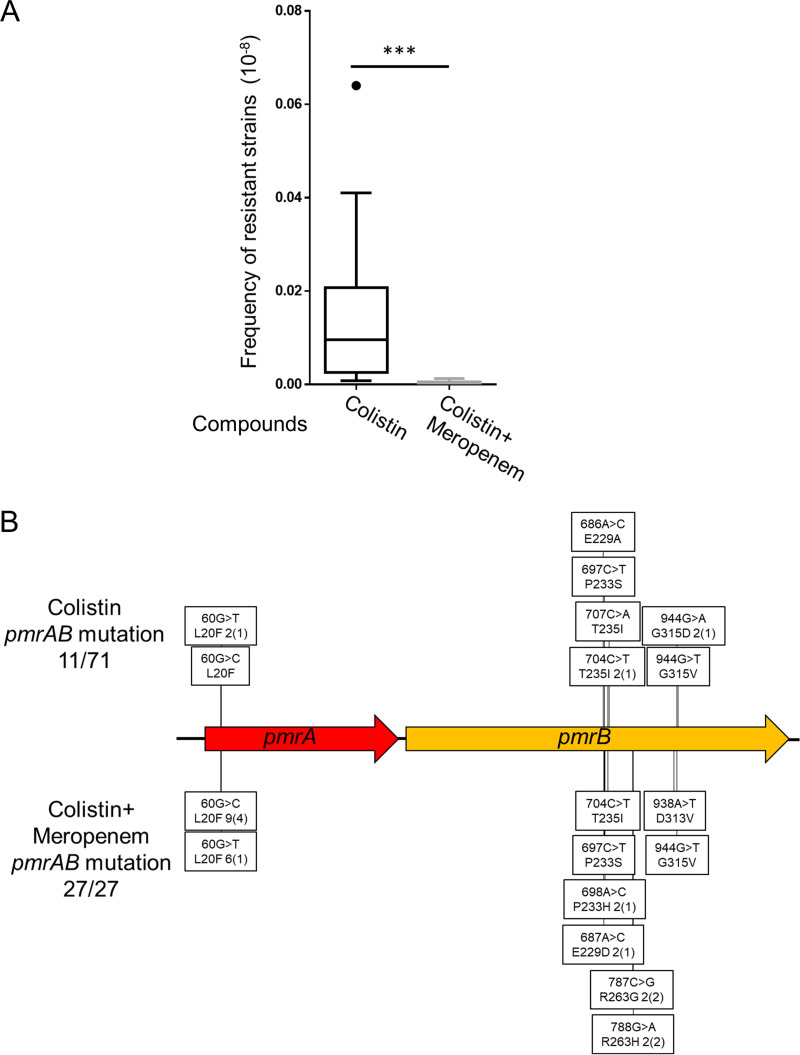
Furthermore, to test whether these observations are accurate in a clinical multidrug-resistant strain of A. baumannii, we analyzed the frequency of colistin-resistant mutants in the strain ATCC BAA-1605. It was originally resistant to ceftazidime, gentamicin, ticarcillin, piperacillin, aztreonam, cefepime, ciprofloxacin, imipenem, and meropenem; the MIC of meropenem was 10 μg/mL. Additionally, this strain was sensitive to amikacin and tobramycin (44, 45). Among the 71 colistin-resistant strains obtained in the absence of meropenem, 11 (approximately 15%) strains showed growth in the presence of both colistin (5× MIC) and meropenem (1/5× MIC). Analysis of the related genes showed that these strains harbored mutations in pmrAB (Fig. 4B and Table S7). Sequence analysis of a proportion of the remaining 60 strains that did not grow on the plate containing colistin and meropenem revealed that these strains harbored mutations in lpxACD genes but not in pmrAB genes (data not shown). Interestingly, although the ATCC BAA-1605 strain lacks ISAba11 in its genome, PCR analysis targeting lpx genes in colistin-resistant strains resulted in the amplification of fragments larger than the expected size in 33 strains (lpxA [2 strains], lpxC [28 strains], and lpxD [3 strains]) among the 71 strains (46%) (Table S8). Sequence analysis of some of these fragments demonstrated that the change in size was due to the insertion of ISAba1 (data not shown). The frequency of colistin-resistant mutants of ATCC BAA-1605 in the absence of meropenem was approximately 10-fold lower than that of ATCC 19606. The combination with 1/5× MIC of meropenem reduced the frequency of colistin resistance in ATCC BAA-1605 (Fig. 4A). As observed for ATCC 19606, all 27 ATCC BAA-1605 colistin-resistant strains identified in combination with meropenem were pmrAB mutants (Fig. 4B and Table S7). Thus, preferential selection of pmrAB mutants by the combined use of colistin with sub-MIC of antimicrobials occurred in laboratory and clinical drug-resistant strains.
FIG 4.
Frequency of emergence of colistin-resistant A. baumannii (ATCC BAA-1605) strains and change in mutation tendency in combination with meropenem. (A) The frequency of colistin resistance was analyzed by plating 0.1 mL culture (OD600 1.0) on LB agar containing 5× MIC (10 μg/mL) of colistin for 24 h at 37°C. The frequency of colistin resistance in combination with 1/5× MIC (2 μg/mL) of meropenem was determined by plating 0.1 mL culture (OD600 of 25) on LB agar containing 10 μg/mL of colistin and 2 μg/mL of meropenem. Data are shown as a box plot; 20 independent assays were compared by the Mann-Whitney U test. ***, P < 0.001. (B) Mutation site analysis of colistin- and colistin-meropenem-resistant ATCC BAA-1605 strains. Sanger sequencing of the genomic regions containing the pmrAB genes was performed. Mutations in colistin-resistant strains and colistin-meropenem-resistant strains are shown above and below the genes, respectively. In the boxes, base changes are shown on top, while amino acid changes are shown below. The numerical values are the numbers of isolated strains, and numbers in parentheses are the numbers of independent events.
Susceptibility to other antibiotics and phenotype in colistin-resistant mutants.
The LPS-deficient mutant of A. baumannii is more susceptible to some antibiotics, such as β-lactams, than the wild-type strain (35, 36, 40). Representative strains of each mutation were selected to examine colistin susceptibility as a function of resistance mutations. To exclude the possibility of contamination with genetically different strains during the isolation of representative strains from a large population, single colonies were isolated on plates supplemented with 10 μg/mL (5 × MIC) colistin. For example, KL001 was selected as a representative lpxA mutant and subjected to single-colony isolation, and the resultant monoclonal strain was named KL001S. The analysis of the whole-genome sequence obtained by next-generation sequencing confirmed that the lpxACD or pmrAB mutation was the sole mutation, suggesting that they are responsible for colistin resistance. Three strains (KL001S, KL037S, and KL055S) had mutations in the lpxACD genes responsible for LPS biosynthesis. The MIC of colistin for these lpx mutant strains was 128 μg/mL. In pmrAB mutant strains (CM022S [pmrA] and CM012S [pmrB]), the MIC of colistin was 1,024 μg/mL (Table 1). Next, we examined susceptibility to other antibiotics viz meropenem, amikacin, ciprofloxacin, and tigecycline. As expected, the MICs of these antibiotics in pmrAB mutant strains were similar to those determined in the wild-type strain. In contrast, the MICs of meropenem, amikacin, and ciprofloxacin decreased substantially in lpxACD mutant strains; however, the MIC of tigecycline was similar to that in the wild type or pmrAB mutants (Table 2).
TABLE 1.
Gene mutation analysis, MIC of colistin, and LAL assay results for representative colistin-resistant A. baumannii strains
| Mutated gene | Strain | Mutation | Colistin MIC (μg/mL) | Endotoxin concn (EU/mL)a |
|---|---|---|---|---|
| None (wild type) | ATCC 19606 | 2 | 9.2 × 103 ± 6.0 × 103 | |
| lpxA | KL001S | 591_592delTA: I198fs(I198*) | 128 | <0.1 |
| lpxC | KL037S | 475_689(215)del: D159fs(D159C)D161* | 128 | <0.1 |
| lpxD | KL055S | 218insA: D75fs(D75R)N76* | 128 | <0.1 |
| pmrA | CM022S | 36G>T: M12I | 1024 | 17 × 103 ± 8.7 × 103 |
| pmrB | CM012S | 697C>T: P233S | 1024 | 13 × 103 ± 9.8 × 103 |
Determined by the LAL assay at an OD of 0.1. EU, endotoxin units. Values are means ± SD.
TABLE 2.
MICs of antimicrobials and disinfectants against representative colistin-resistant A. baumannii strains
| Agent (MIC units) | MIC for: |
|||||
|---|---|---|---|---|---|---|
| Wild-type ATCC 19606 |
lpxACD mutants |
pmrAB mutants |
||||
| KL001S | KL037S | KL055S | CM022S | CM012S | ||
| Colistin (μg/mL) | 2 | 128 | 128 | 128 | 1,024 | 1,024 |
| Meropenem (μg/mL) | 0.5 | <0.016 | <0.016 | <0.016 | 0.5 | 0.25 |
| Amikacin (μg/mL) | 16 | 1 | 0.5 | 0.5 | 16 | 16 |
| Ciprofloxacin (μg/mL) | 2 | 0.125 | 0.125 | 0.0625 | 1 | 1 |
| Tigecycline (μg/mL) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ethanol (%) | 3.125 | 3.125 | 3.125 | 3.125 | 3.125 | 3.125 |
| H2O2 (%) | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| SDS (%) | 0.5 | 0.004 | 0.004 | 0.004 | 0.25 | 0.25 |
| Benzalkonium (%) | 0.0006 | 0.00016 | 0.00016 | 0.000078 | 0.0006 | 0.0006 |
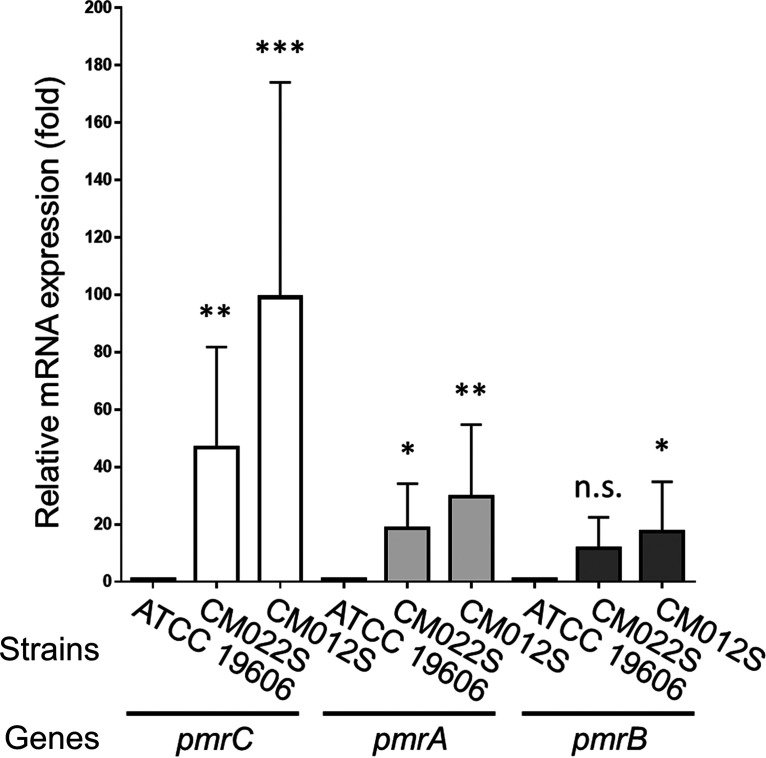
The quantity of LPS was analyzed using representative strains of each mutation. Mutations in the lpxACD genes (KL001S, KL037S, and KL055S) resulted in endotoxin levels below the detection limit with a possible LPS-deficient phenotype. In contrast, endotoxin levels in the two pmrAB mutant strains (CM022S and CM012S) were not significantly different from that in the wild-type strain (Table 1). Mutations in pmrAB have been reported to increase the expression of the upstream pmrC gene, encoding lipid A phosphoethanolamine transferase, and pmrAB itself (26, 42, 46). Therefore, the expression level of pmrCAB mRNA in the representative pmrAB mutant strains was measured by quantitative reverse transcription-PCR (qRT-PCR). Levels of pmrCAB mRNA were enhanced in the pmrAB mutants compared to that in the wild-type strain (Fig. 5).
FIG 5.
The expression of pmrCAB mRNA in representative A. baumannii strains of pmrAB mutants (CM022S [pmrA], CM012S [pmrB], and wild-type strain ATCC 19606) was analyzed by qRT-PCR. The strains were grown in LB broth at 37°C with shaking until they reached an OD600 of 0.75. Total RNA was extracted and subjected to qRT-PCR by the intercalator method (TB Green). Data are means and SD; n = 3 per group, compared by one-way ANOVA. n.s., not significant; ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Previous studies have reported that the LPS-deficient A. baumannii strains have a higher biological cost than the LPS-modified strains (36, 47, 48). We confirmed the biological cost of representative strains by analyzing their proliferative and biofilm-forming activities. As expected, lpxACD mutant strains (KL001S, KL037S, and KL055S) exhibited considerably decreased proliferative and biofilm-forming activities than did the wild-type strain. However, growth defects and suppressed biofilm-forming activity were not observed in the pmrAB mutant strains (CM022S and CM012S) (Fig. S2A and B).
In addition to antimicrobials, we analyzed the MICs of the disinfectants ethanol, H2O2, sodium dodecyl sulfate (SDS), and benzalkonium in the representative strains (Table 2). The MICs of disinfectants in the pmrAB mutant strains were almost the same as those in the wild-type strain. In contrast, the MICs of SDS, benzalkonium, and anionic and cationic detergents for the lpxACD mutant strains were decreased. The MICs of ethanol and H2O2 did not change in any strains. These results suggest that pmrAB mutant strains are likely to be more pathogenic than lpxACD mutant strains. It is also noteworthy that the MICs of some antimicrobials and disinfectants, such as tigecycline, ethanol, and H2O2, did not decrease in the lpxACD mutant strains despite complete loss of LPS.
DISCUSSION
In this study, we focused on the mechanism by which A. baumannii acquire resistance to colistin under the selective pressure of colistin alone and in combination with other antimicrobials. Many patients with infections caused by colistin-resistant A. baumannii undergo colistin treatment before isolation of the colistin-resistant strain, and it is hypothesized that colistin resistance predominantly emerges under selective pressure during colistin therapy in individual patients (22, 49). There are reports that most clinically isolated colistin-resistant strains are LPS-modified strains (20, 23, 38, 50), which was also the case in our in silico analysis. Consistent with the findings of the present study, a recent systemic review (37) concluded that the in vivo emergence of colistin resistance in A. baumannii is mediated by mutations in pmrAB rather than by LPS loss. Although LPS-deficient strains were predominantly obtained when colistin was used alone for selection, we observed preferential selection of pmrAB mutants, probably harboring a modified LPS, when meropenem was added to the selection medium in vitro. These results are consistent with a previous report that when colistin and other antimicrobial agents were used to treat carbapenem-resistant A. baumannii in clinical settings, resistance developed during treatment, resulting in an LPS-modified colistin-resistant strain (22).
The underlying mechanism for the preferential selection of pmrAB mutants was further deciphered. As the spectrum of mutants obtained through selection is based on both frequency of occurrence and fitness of each mutant, it is necessary to analyze both factors. To further investigate this, we evaluated the bactericidal activity of various antimicrobial agents toward LPS-deficient (KL001S) and LPS-modified (CM012S) strains. We observed that the LPS-deficient strains could not proliferate in the presence of colistin and sub-MIC of meropenem, while no such defects were observed in the LPS-modified strains under the same conditions (Fig. S3). These results suggest that the main mechanism of preferential selection of pmrAB mutants may be that LPS-deficient strains are sensitive to colistin and sub-MIC of meropenem. However, the frequency of pmrAB mutants might be higher when colistin and meropenem are simultaneously added to the selection medium than when only colistin is added. The pmrAB mutants grew well even when meropenem was added to the medium, and the population plated onto the selection plates contained a similar number of pmrAB mutants, suggesting that pmrAB mutations occurred under selection pressure. We further sought to determine whether the addition of meropenem exerted mutagenic effects on A. baumannii. Therefore, the frequency of resistance to rifampicin, a commonly used drug during mutation rate analysis, was measured (51). Contrary to expectations, concomitant use of 1/5× MIC of meropenem and 5× MIC of rifampicin (10 μg/mL) did not alter the frequency of rifampicin-resistant colonies (Fig. S4). On the other hand, colistin resistance by LPS modification was dependent on the activity of phosphoethanolamine transferase, which was determined by both expression level and specific activity. This assumption prompted us to consider another possibility: that the addition of low-dose meropenem or ciprofloxacin enhanced the activity of phosphoethanolamine transferase, resulting in preferential selection of pmrAB mutants. The combination with antimicrobials might give rise to a complex selection mechanism, which is one limitation of the present study. Future research should focus on determining the appropriate antimicrobial agents to be used in combination with colistin to change the mutation spectrum and elucidate the detailed mechanism.
In this study, all colistin-resistant strains harbored mutations in pmrAB genes after concomitant use of meropenem. PmrAB is a two-component regulatory system that responds to presently unidentified signal(s), although it can also be activated by specific amino acid changes (42). Activation of the PmrAB system enhances the production of LPS-modifying enzymes. Most clinical isolates have mutations in PmrB, a membrane-bound histidine kinase. Among the frequently detected amino acid substitutions, P233S, R263C, and A226V were also identified in this study; however, P170L and P360Q were not (41, 42, 50). Most of the mutation sites detected in this study were located at specific sites in either the response regulatory domain of PmrA or the histidine kinase domain of PmrB. However, it has not been experimentally tested whether mutations in most clinical isolates are truly responsible for colistin resistance in A. baumannii. One study reported that a colistin-susceptible A. baumannii strain harbored the A226V substitution in PmrB, suggesting that PmrAB mutations alone do not confer colistin resistance (52). These findings suggest the importance of considering not only the single nucleotide polymorphisms in genes involved in colistin resistance but also their genetic background, as also pointed out by Gerson et al. (53). By confirming isogenic pmrAB mutants, we succeeded in producing mutation libraries in the background of two A. baumannii strains, ATCC 19606 and ATCC BAA-1605, and present useful data for understanding the colistin resistance mechanism and the PmrAB two-component system.
Insertion sequences (IS) are involved in colistin resistance in A. baumannii. In addition to gene disruption by IS insertion, transposition of ISAba1 containing an outward promoter upstream of eptA, a pmrC homolog, enhances the expression of phosphoethanolamine transferase, enabling LPS modification (53, 54). Transpositions of IS were observed in most colistin-resistant strains generated in this study; ISAba11 was observed in more than 30% of the colistin-resistant isolates of ATCC 19606 (Fig. 1A; Tables S1 and 2). Even with the ATCC BAA-1605 strain lacking ISAba11, other IS were present in more than 40% of the colistin-resistant strains (Table S8). In agreement with our results, previous reports have shown that ISAba11 mediates LPS deficiency through insertional inactivation of lpxACD genes. Moreover, a high number of insertions in lpxC compared to those in lpxA and lpxD, a semiconserved AT-rich consensus sequence upstream of the ISAba11 insertion site, has been reported, suggesting that ISAba11 insertion sites may be sequence dependent (55, 56). In addition to these reports, we analyzed the ratio and frequency of ISAba11 insertions, substitutions, and short indels (Fig. 1B). Furthermore, Olmeda-Lopez et al. reported that antimicrobials affect IS insertion; the sub-MIC of tetracycline significantly increased ISAba11 insertion, and rifampicin completely inhibited the emergence of colistin resistance due to ISAba11 inactivation of lpxC gene (55). IS may be involved, because the mutational spectrum of colistin resistance was altered in this study with sub-MIC antimicrobials. The detailed relationship between IS and colistin resistance mechanisms should be explored in future studies.
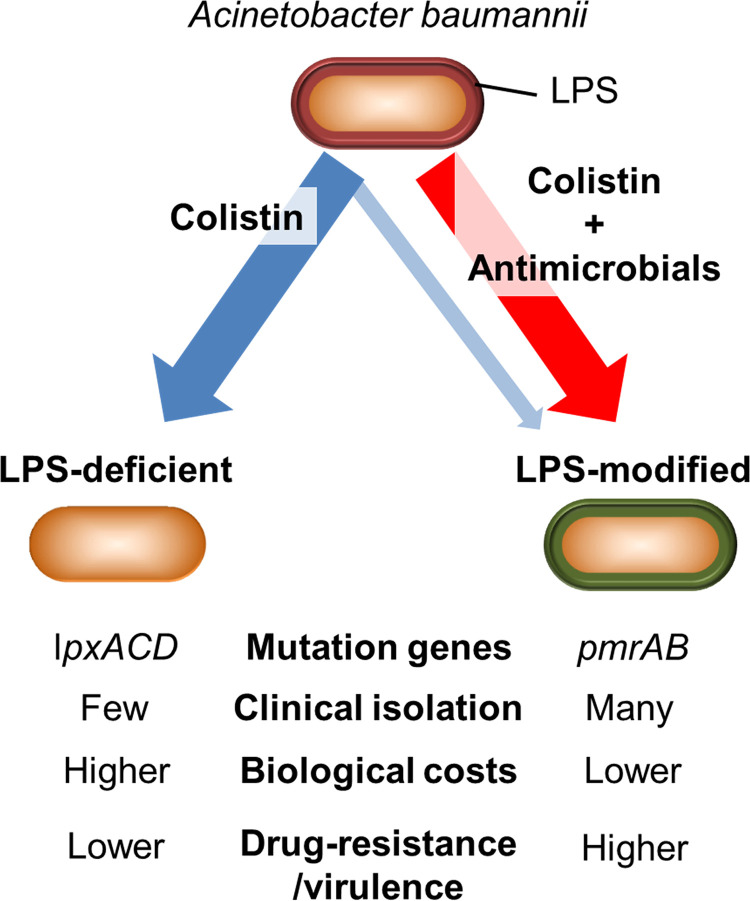
The schematic representation of the present study is shown in Fig. 6. LPS-deficient A. baumannii is more sensitive to host antimicrobials, such as lysozyme and lactoferrin (35, 57). We demonstrated that the LPS-modified strains had higher virulence than LPS-deficient strains in terms of antimicrobial and disinfectant resistance, proliferation, and biofilm-forming ability (Table 2 and Fig. S2), suggesting that colistin-resistant LPS-modified A. baumannii strains may be more difficult to treat than LPS-deficient strains in clinical settings. The LPS-modified strains selected using a combination of low-dose antimicrobials harbored mutations in pmrAB genes. These observations support the fact that most clinically isolated colistin-resistant strains are LPS-modified strains. Although the concomitant use of colistin and carbapenems has been reported to have a synergistic effect in vitro against carbapenem-resistant A. baumannii strains, and clinical trials have also been conducted to evaluate the same (58–63), our insights strongly suggest the importance of monitoring the emergence of LPS-modified strains during treatment. Additionally, the results of clinical trials suggest that combination therapy with colistin and meropenem is not superior to colistin monotherapy for carbapenem-resistant A. baumannii strains (64). Notably, a post hoc analysis of the AIDA study (the multicenter open-label randomized controlled trial study to compare colistin alone and combinational treatment of colistin and meropenem against severe infections caused by carbapenem-resistant Gram-negative infections) reported higher mortality for the cases where infection was treated with colistin-meropenem combination than with colistin alone in carbapenem-resistant A. baumannii infections (65). Hence, further investigation and reassessment of relevant methodology is needed to identify clinically useful synergistic combinations against A. baumannii (9, 58). The findings of this study suggest that substantial solutions and precautions should be taken for facilitating current research and clinical treatment of colistin-resistant A. baumannii infections.
FIG 6.
Proposed schematic diagram of the present study. A. baumannii was treated with colistin alone, which mainly selected mutations in lpxACD genes, resulting in LPS-deficient colistin-resistant strains. On the other hand, strains with modified LPS due to mutations in pmrAB were generated on treatment with combination of antimicrobials. LPS-modified colistin-resistant strains exhibit lower biological costs and higher drug resistance/virulence than LPS-deficient strains.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and isolation of colistin-resistant strains.
The type strain A. baumannii ATCC 19606 and ATCC BAA-1605 were purchased from ATCC (Manassas, VA, USA). Bacteria were cultured for 18 h in Luria-Bertani (LB) broth (BD Biosciences, San Diego, CA, USA) at 37°C with constant shaking (135 rpm). Colistin-resistant strains of A. baumannii were isolated by directly plating the parent strain onto LB agar (BD Biosciences) containing 10 μg/mL (5× MIC) of colistin sulfate (Fujifilm Wako Pure Chemical Industries, Osaka, Japan) for 24 h at 37°C (29). LPS-deficient strains were cultured for 24 h in LB broth at 37°C with constant shaking (180 rpm).
PCR-Sanger sequencing.
Genomic regions containing lpxACD and pmrAB genes were amplified by colony PCR using following specific primers (primer sequences are listed in Table 3): for lpxAD, lpxA_F and lpxD_R; for lpxC, lpxC_F1 and lpxC_R1 or lpxC_F2 and lpxC_R2; and for pmrAB, pmrA_F1 and pmrB_R. The PCR products were purified using the FastGene gel/PCR extraction kit (Nippon Genetics, Tokyo, Japan). Direct sequencing of each gene was performed by Eurofins Genomics (Tokyo, Japan) using the following primers: for lpxA from ATCC 19606, lpxA_F and lpxA_R1; for lpxC, lpxC_F1 and lpxC_R1 or lpxC_F2 and lpxC_R2; for lpxD, lpxD_F and lpxD_R; for pmrAB, pmrA_F1, pmrA_F2, and pmrB_R; and for lpxA from ATCC BAA-1605, lpxA_F and lpxA_R2.
TABLE 3.
Primers
| Primer | Sequence |
|---|---|
| lpxA_F | 5′-TGGTAATGCAGAAGCGCGGTATCTACAA-3′ |
| lpxA_R1 | 5′-ATCCTCTAGAGTCGACCAATATTCAAAGTCTGAAGAAGCA-3′ |
| lpxA_R2 | 5′-CTGTGTCAGCAAATCAATACAAG-3′ |
| lpxC_F1 | 5′-TCAGCAACGTAAGTAATTTAGCGTACA-3′ |
| lpxC_R1 | 5′-GCCAAGCTTTACTACGTTTGGCAAGCAA-3′ |
| lpxC_F2 | 5′-GCAGAGCCAAGAAAGCGTAA-3′ |
| lpxC_R2 | 5′-AAATGTTACGTAGTGCCGCC-3′ |
| lpxD_F | 5′-AAGCTTGCATGCGTTAAGCAAGCTGCTGAGCAATTACGAA-3′ |
| lpxD_R | 5′-CCAATAAGAATGGGTAACGATGCGGCAA-3′ |
| pmrA_F1 | 5′-CCAACAAACTAAACAAAAGTTAA-3′ |
| pmrA_F2 | 5′-TTGAACAGCATATTGCGACGTT-3′ |
| pmrB_R | 5′-GCAAATGATGCGAGGAGCACAT-3′ |
| qPCR_pmrC_F | 5′-TTCTCGGGTATGCCACGTGTA-3′ |
| qPCR_pmrC_R | 5′-CCGCACGTTTTGCAATATCTAGT-3′ |
| qPCR_pmrA_F | 5′-TGATGAGTTGCTTGCCCGTAT-3′ |
| qPCR_pmrA_R | 5′-ATAGTTGATCTTGACTCGCAAGTTGA-3′ |
| qPCR_pmrB_F | 5′-CGTTTTTATCGCGTGCATCA-3′ |
| qPCR_pmrB_R | 5′-AAGCCTTTGAGTTGCACGATCT-3′ |
| qPCR_16S rRNA_F | 5′-CATGAAGTCGGAATCGCTAGTAATC-3′ |
| qPCR_16S rRNA_R | 5′-TGACGGGCGGTGTGTACA-3′ |
| qPCR_lpxC_F | 5′-TTGTGGAAGTGTCTGCTTCTGAAG-3′ |
| qPCR_lpxC_R | 5′-GCCACCTTGCATGAGCAAAT-3′ |
| qPCR_lpxD_F | 5′-CGGGAACCGGATTATTTGAAA-3′ |
| qPCR_lpxD_R | 5′-GGTCAATGGCACATCTGCTAATT-3′ |
Analysis of NCBI BioSample isolates.
We analyzed LpxACD and PmrAB mutations in colistin-resistant A. baumannii strains from clinical isolates available in the NCBI BioSample database with published whole genomes. Briefly, we compared the LpxACD and PmrAB amino acid sequences of 265 colistin-susceptible A. baumannii strains, defined the mutations found in more than 1% of the strains as single nucleotide polymorphisms, and created reference sequences. Mutation analysis of strains determined to be colistin resistant with a MIC of ≥4 μg/mL was performed using the above-mentioned sequences as queries. The results of this analysis are presented in Table S4.
Frequency analysis of the emergence of drug-resistant strains and isolating methods.
Bacterial culture density was adjusted with LB broth to an optical density at 600 nm (OD600) of 1.0 using overnight cultures. The culture OD600 was measured using a CO8000 Biowave (WPA; Biochrom, Cambridge, UK). The frequency of colistin resistance was analyzed by culturing 0.2 mL (for ATCC 19606) or 0.1 mL (for ATCC BAA-1605) of bacterial suspension (OD600 of 1.0) for 24 h on LB agar plates containing 10 μg/mL (5× MIC) of colistin at 37°C. The frequency of colistin resistance with a combination of colistin and 1/5× MIC of meropenem (Fujifilm Wako Pure Chemical Industries) was measured using 0.1 mL of bacterial culture (OD600 of 10 for ATCC 19606 or 25 for ATCC BAA-1605). Bacterial suspensions were plated on LB agar containing 10 μg/mL of colistin and 0.1 μg/mL (for ATCC 19606) or 2 μg/mL (for ATCC BAA-1605) of meropenem. To calculate the number of CFU, 0.1 mL of OD600 1.0 culture diluted to 10−7 (for ATCC 19606) or 10−8 (for ATCC BAA-1605) was plated on nonantibiotic LB agar. Also, overlapping colonies were counted when the frequency was calculated. The frequency of colistin resistance in the presence of colistin and 1/5× MIC (0.4 μg/mL) of ciprofloxacin (Fujifilm Wako Pure Chemical Industries) was measured by plating 0.1 mL of OD600 10 culture for ATCC 19606, as described above.
The frequency of resistance to rifampicin alone or in combination with 1/5× MIC of meropenem was measured using 0.1 mL of overnight culture (OD600 of approximately 30); the culture was plated on LB agar plates containing 10 μg/mL (5× MIC) of rifampicin alone or in combination with 0.1 μg/mL meropenem.
Whole-genome sequencing.
Genomic DNA was isolated from overnight cultures using a DNeasy blood and tissue kit (Qiagen, Hilden, Germany). Sequencing libraries were prepared using a NEBNext Ultra II FS DNA library preparation kit for Illumina (New England BioLabs, Ipswich, MA, USA) according to the manufacturer’s instructions. The libraries were subjected to 2 × 150-bp sequencing in an Illumina HiSeq X sequencer (Illumina, San Diego, CA, USA). To obtain the complete reference genome sequence of the parental strain, we conducted a long-read sequencing analysis using Oxford Nanopore F sequencing technology (Oxford Nanopore, Oxford, UK). After the generation of the barcoded library using a ligation sequencing kit (Oxford Nanopore) with native barcoding expansion (Oxford Nanopore), the library was subjected to sequence analysis using the MinION Mk1C (Oxford Nanopore) and sequencing kit (Oxford Nanopore). The circular genome sequence was assembled using Unicycler software (66). Open reading frames (ORFs), and RNA regions were annotated by Prokka v1.14.6 (67) using GCF_000737145.1_ASM73714v1 as the primary database. Some ORFs of interest were manually annotated.
Determination of antibiotic and disinfectant MICs.
MICs of antibiotics and disinfectants were determined by microdilution in LB broth for antibiotics or Mueller-Hinton broth (BD Biosciences) for disinfectants, as described previously (35). Antibiotic agents, such as colistin, meropenem, amikacin, and ciprofloxacin, and disinfectants, such as ethanol, H2O2, SDS, and benzalkonium, were purchased from Fujifilm Wako Pure Chemical Industries. Tigecycline was purchased from the Tokyo Chemical Industry (Tokyo, Japan). The bacterial culture was adjusted to an OD600 of 0.001 from the overnight cultures. MICs were monitored using a 2-fold dilution series for each antibiotic or disinfectant.
LAL assay.
The Limulus amebocyte lysate (LAL) assay was performed using a Toxicolor LS-50M set (Seikagaku Corporation, Tokyo, Japan) according to the manufacturer’s protocol. The bacterial culture was adjusted to an OD600 of 0.1 from overnight cultures, and the cultures were diluted in sterile, pyrogen-free saline (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan).
Quantitative qRT-PCR.
Cultures of A. baumannii were grown in LB broth at 37°C with constant shaking until the OD600 reached 0.75. The cells were harvested from 1 mL culture by centrifugation, and total RNA was prepared using the acid guanidinium thiocyanate-phenol-chloroform extraction (AGPC) method using TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. DNA contamination was removed by treatment with DNase I (Nippon Gene, Tokyo, Japan), according to the manufacturer’s instructions. RNA concentration was quantified using NanoDrop One (Thermo Fisher Scientific). Total RNA (500 ng) was reverse transcribed using PrimeScript RT master mix (TaKaRa Bio Inc., Shiga, Japan) according to the manufacturer’s protocol. TB Green Premix Ex Taq II (Tli RNaseH Plus) was purchased from TaKaRa Bio, Inc., and the Applied Biosystems StepOne Plus real-time PCR system (Thermo Fisher Scientific) was used to perform RT-qPCR. Table 3 lists the primers used for PCR. Data were generated using cDNA prepared from three independent RNA isolations, and qRT-PCR was performed in triplicate to ensure accuracy. Fold changes in gene expression relative to the control strain (ATCC 19606) and the control gene (16S rRNA gene) were determined using the 2−ΔΔCT method (68).
Growth curve.
Bacterial culture density was adjusted to an OD600 of 0.001 using overnight cultures. Bacteria were grown under static conditions in LB broth for 24 h at 37°C and the absorbance at 595 nm (A595 nm) was measured every hour using Multiskan FC (Thermo Fisher Scientific).
Biofilm formation assay.
The biofilm-forming ability of each strain was analyzed using the crystal violet staining method. Bacterial culture density was adjusted to an OD600 of 0.01 using overnight cultures. Bacteria were grown under static conditions for 24 h in LB broth at 37°C in a 96-well U-bottom plate (163320, Thermo Fisher Scientific). Following incubation, the culture was carefully removed, the plates were washed three times with phosphate-buffered saline (PBS), and each well was stained for 15 min with 0.1% crystal violet at about 25°C. After gently removing the crystal violet solution, each well was washed three times with PBS to remove excess stain and dried for 2 h at 37°C. The remaining crystal violet was solubilized in 95% (vol/vol) ethanol by incubating for 5 min. The eluate was transferred to a new plate, and A570 was measured (Varioskan; Thermo Fisher Scientific). Fold changes in biofilm formation were calculated using the control strain (ATCC 19606) at A570 of 1.0.
Bactericidal activity of colistin alone and in combination with meropenem.
Bacterial culture density was adjusted with LB broth to an OD600 of 1.0 using overnight cultures. The culture was serially diluted (10−1 to 10−9), and 10 μL of each dilution was spotted on LB agar plates containing 10 μg/mL (5× MIC) of colistin alone or in combination with 0.1 μg/mL (1/5× MIC) of meropenem or no antibiotic. The CFU on non-antibiotic-containing LB agar was used to calculate the percent surviving bacteria. In this experiment, A. baumannii KL001S and CM012S were used as the LPS-deficient and LPS-modified strains, respectively, and ATCC 19606 was the wild-type strain.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Data are expressed as means and standard deviations (SD) and were compared using the Mann-Whitney U test and one-way and two-way analysis of variance (ANOVA). Differences with P values of <0.05 were considered statistically significant.
Data availability.
Original data sets are available in a publicly accessible repository. The whole-genome sequencing data of the representative A. baumannii isolates in this study were registered with the DDBJ (BioProject accession no. PRJDB12922). The GenBank file for the complete reference genome sequence of ATCC 19606 was registered with the DDBJ (nucleotide accession no. AP025740).
ACKNOWLEDGMENTS
We are grateful to Shinichi Kato, Masahiro Fujimuro, Yuichi Muraki, Yokotani Atsushi (Kyoto Pharmaceutical University), and Tsukasa Shiraishi (Sapporo Medical University) for their helpful discussions regarding this study. We are also grateful to Tohru Miyoshi-Akiyama (National Center for Global Health and Medicine) for their technical support on genome sequencing. We also thank Yui Furue, Mariko Hayata, Takuya Yada, Mayu Takagi, Sayaka Nagamine, Reika Yamauchi (Kyoto Pharmaceutical University), Kayo Shimada, Izumi Hojo, and Yu Sakurai (National Center for Global Health and Medicine) for their technical assistance. We thank Editage (www.editage.com) for English language editing.
This work was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Science Research Promotion Fund to G.K. (17K16230, 20K17475), the Kanehara Ichiro Memorial Foundation to G.K., the Kyoto Pharmaceutical University Fund for the Promotion of Scientific Research to G.K., and a grant from Agency for Medical Research and Development (AMED) to K.Y. (21fk0108611h0701). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Methodology: G.K. and N.T.; Formal analysis: G.K., N.Y., and N.T.; Investigation: G.K., T.N., D.Y., D.K., M.Y., C.H., N.K., M.S., H.M., T.S., T.O., Y.K., and N.T.; Writing - Original Draft: G.K. and N.T.; Supervision: K.Y.
We declare no competing financial interests.
Footnotes
Supplemental material is available online only.
Contributor Information
Go Kamoshida, Email: kamoshida@mb.kyoto-phu.ac.jp.
Norihiko Takemoto, Email: ntakemoto@ri.ncgm.go.jp.
Paolo Visca, Università Roma Tre.
REFERENCES
- 1.Harding CM, Hennon SW, Feldman MF. 2018. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol 16:91–102. doi: 10.1038/nrmicro.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayoub MC, Hammoudi HD. 2020. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics (Basel) 9:119. doi: 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karakonstantis S, Gikas A, Astrinaki E, Kritsotakis EI. 2020. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J Hosp Infect 106:447–453. doi: 10.1016/j.jhin.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Lee CR, Lee JH, Park M, Park KS, Bae IK, Kim YB, Cha CJ, Jeong BC, Lee SH. 2017. Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol 7:55. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antunes LC, Visca P, Towner KJ. 2014. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 71:292–301. doi: 10.1111/2049-632X.12125. [DOI] [PubMed] [Google Scholar]
- 7.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier PE, Richet H. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 9.Karakonstantis S, Ioannou P, Samonis G, Kofteridis DP. 2021. Systematic review of antimicrobial combination options for pandrug-resistant Acinetobacter baumannii. Antibiotics 10:1344. doi: 10.3390/antibiotics10111344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Li F, Awan F, Jiang H, Zeng Z, Lv W. 2021. Molecular epidemiology and clone transmission of carbapenem-resistant Acinetobacter baumannii in ICU rooms. Front Cell Infect Microbiol 11:633817. doi: 10.3389/fcimb.2021.633817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamidian M, Nigro SJ. 2019. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb Genom 5:e000306. doi: 10.1099/mgen.0.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isler B, Doi Y, Bonomo RA, Paterson DL. 2019. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 63:e01110-18. doi: 10.1128/AAC.01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins PG, Dammhayn C, Hackel M, Seifert H. 2010. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 14.Gogry FA, Siddiqui MT, Sultan I, Haq QMR. 2021. Current update on intrinsic and acquired colistin resistance mechanisms in bacteria. Front Med (Lausanne) 8:677720. doi: 10.3389/fmed.2021.677720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karakonstantis S, Kritsotakis EI, Gikas A. 2020. Pandrug-resistant Gram-negative bacteria: a systematic review of current epidemiology, prognosis and treatment options. J Antimicrob Chemother 75:271–282. doi: 10.1093/jac/dkz401. [DOI] [PubMed] [Google Scholar]
- 16.Karakonstantis S, Kritsotakis EI, Gikas A. 2020. Treatment options for K. pneumoniae, P aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection 48:835–851. doi: 10.1007/s15010-020-01520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bialvaei AZ, Samadi Kafil H. 2015. Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin 31:707–721. doi: 10.1185/03007995.2015.1018989. [DOI] [PubMed] [Google Scholar]
- 18.El-Sayed Ahmed MAE-G, Zhong L-L, Shen C, Yang Y, Doi Y, Tian G-B. 2020. Colistin and its role in the era of antibiotic resistance: an extended review (2000–2019). Emerg Microbes Infect 9:868–885. doi: 10.1080/22221751.2020.1754133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pormohammad A, Mehdinejadiani K, Gholizadeh P, Nasiri MJ, Mohtavinejad N, Dadashi M, Karimaei S, Safari H, Azimi T. 2020. Global prevalence of colistin resistance in clinical isolates of Acinetobacter baumannii: a systematic review and meta-analysis. Microb Pathog 139:103887. doi: 10.1016/j.micpath.2019.103887. [DOI] [PubMed] [Google Scholar]
- 20.Gerson S, Lucaßen K, Wille J, Nodari CS, Stefanik D, Nowak J, Wille T, Betts JW, Roca I, Vila J, Cisneros JM, Seifert H, Higgins PG. 2020. Diversity of amino acid substitutions in PmrCAB associated with colistin resistance in clinical isolates of Acinetobacter baumannii. Int J Antimicrob Agents 55:105862. doi: 10.1016/j.ijantimicag.2019.105862. [DOI] [PubMed] [Google Scholar]
- 21.Sheck EA, Edelstein MV, Sukhorukova MV, Ivanchik NV, Skleenova EY, Dekhnich AV, Azizov IS, Kozlov RS. 2017. Epidemiology and genetic diversity of colistin nonsusceptible nosocomial Acinetobacter baumannii strains from Russia for 2013–2014. Can J Infect Dis Med Microbiol 2017:1839190. doi: 10.1155/2017/1839190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qureshi ZA, Hittle LE, O'Hara JA, Rivera JI, Syed A, Shields RK, Pasculle AW, Ernst RK, Doi Y. 2015. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis 60:1295–1303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 24.Park YK, Jung SI, Park KH, Cheong HS, Peck KR, Song JH, Ko KS. 2009. Independent emergence of colistin-resistant Acinetobacter spp. isolates from Korea. Diagn Microbiol Infect Dis 64:43–51. doi: 10.1016/j.diagmicrobio.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 55:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson BW, Nieckarz M, Pinedo V, McLean AB, Cava F, Trent MS. 2021. Acinetobacter baumannii can survive with an outer membrane lacking lipooligosaccharide due to structural support from elongasome peptidoglycan synthesis. mBio 12:e03099-21. doi: 10.1128/mBio.03099-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers MJ, Trent MS. 2018. Expanding the paradigm for the outer membrane: Acinetobacter baumannii in the absence of endotoxin. Mol Microbiol 107:47–56. doi: 10.1111/mmi.13872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onishi HR, Pelak BA, Gerckens LS, Silver LL, Kahan FM, Chen MH, Patchett AA, Galloway SM, Hyland SA, Anderson MS, Raetz CR. 1996. Antibacterial agents that inhibit lipid A biosynthesis. Science 274:980–982. doi: 10.1126/science.274.5289.980. [DOI] [PubMed] [Google Scholar]
- 31.Goldman R, Kohlbrenner W, Lartey P, Pernet A. 1987. Antibacterial agents specifically inhibiting lipopolysaccharide synthesis. Nature 329:162–164. doi: 10.1038/329162a0. [DOI] [PubMed] [Google Scholar]
- 32.Vila-Farres X, Ferrer-Navarro M, Callarisa AE, Marti S, Espinal P, Gupta S, Rolain JM, Giralt E, Vila J. 2015. Loss of LPS is involved in the virulence and resistance to colistin of colistin-resistant Acinetobacter nosocomialis mutants selected in vitro. J Antimicrob Chemother 70:2981–2986. doi: 10.1093/jac/dkv244. [DOI] [PubMed] [Google Scholar]
- 33.Peng D, Hong W, Choudhury BP, Carlson RW, Gu XX. 2005. Moraxella catarrhalis bacterium without endotoxin, a potential vaccine candidate. Infect Immun 73:7569–7577. doi: 10.1128/IAI.73.11.7569-7577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steeghs L, den Hartog R, den Boer A, Zomer B, Roholl P, van der Ley P. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449–450. doi: 10.1038/33046. [DOI] [PubMed] [Google Scholar]
- 35.Kamoshida G, Akaji T, Takemoto N, Suzuki Y, Sato Y, Kai D, Hibino T, Yamaguchi D, Kikuchi-Ueda T, Nishida S, Unno Y, Tansho-Nagakawa S, Ubagai T, Miyoshi-Akiyama T, Oda M, Ono Y. 2020. Lipopolysaccharide-deficient Acinetobacter baumannii due to colistin resistance is killed by neutrophil-produced lysozyme. Front Microbiol 11:573. doi: 10.3389/fmicb.2020.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carretero-Ledesma M, Garcia-Quintanilla M, Martin-Pena R, Pulido MR, Pachon J, McConnell MJ. 2018. Phenotypic changes associated with colistin resistance due to lipopolysaccharide loss in Acinetobacter baumannii. Virulence 9:930–942. doi: 10.1080/21505594.2018.1460187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karakonstantis S. 2021. A systematic review of implications, mechanisms, and stability of in vivo emergent resistance to colistin and tigecycline in Acinetobacter baumannii. J Chemother 33:1–11. doi: 10.1080/1120009X.2020.1794393. [DOI] [PubMed] [Google Scholar]
- 38.Mustapha MM, Li B, Pacey MP, Mettus RT, McElheny CL, Marshall CW, Ernst RK, Cooper VS, Doi Y. 2018. Phylogenomics of colistin-susceptible and resistant XDR Acinetobacter baumannii. J Antimicrob Chemother 73:2952–2959. doi: 10.1093/jac/dky290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JY, Lee H, Park M, Cha CJ, Shin D, Ko KS. 2019. Lytic transglycosylase contributes to the survival of lipooligosaccharide-deficient, colistin-dependent Acinetobacter baumannii. Clin Microbiol Infect 25:1156 e1–1156.e7. doi: 10.1016/j.cmi.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Lee JY, Chung ES, Ko KS. 2017. Transition of colistin dependence into colistin resistance in Acinetobacter baumannii. Sci Rep 7:14216. doi: 10.1038/s41598-017-14609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko KS, Choi Y, Lee J-Y. 2017. Old drug, new findings: colistin resistance and dependence of Acinetobacter baumannii. Precis Future Med 1:159–167. doi: 10.23838/pfm.2017.00184. [DOI] [Google Scholar]
- 42.Olaitan AO, Morand S, Rolain JM. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bou G, Otero FM, Santiso R, Tamayo M, Fernandez MC, Tomas M, Gosalvez J, Fernandez JL. 2012. Fast assessment of resistance to carbapenems and ciprofloxacin of clinical strains of Acinetobacter baumannii. J Clin Microbiol 50:3609–3613. doi: 10.1128/JCM.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ten KE, Md Zoqratt MZH, Ayub Q, Tan HS. 2021. Characterization of multidrug-resistant Acinetobacter baumannii strain ATCC BAA1605 using whole-genome sequencing. BMC Res Notes 14:83. doi: 10.1186/s13104-021-05493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chopra S, Torres-Ortiz M, Hokama L, Madrid P, Tanga M, Mortelmans K, Kodukula K, Galande AK. 2010. Repurposing FDA-approved drugs to combat drug-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:2598–2601. doi: 10.1093/jac/dkq353. [DOI] [PubMed] [Google Scholar]
- 46.Jeannot K, Bolard A, Plesiat P. 2017. Resistance to polymyxins in Gram-negative organisms. Int J Antimicrob Agents 49:526–535. doi: 10.1016/j.ijantimicag.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Farshadzadeh Z, Taheri B, Rahimi S, Shoja S, Pourhajibagher M, Haghighi MA, Bahador A. 2018. Growth rate and biofilm formation ability of clinical and laboratory-evolved colistin-resistant strains of Acinetobacter baumannii. Front Microbiol 9:153. doi: 10.3389/fmicb.2018.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mu X, Wang N, Li X, Shi K, Zhou Z, Yu Y, Hua X. 2016. The effect of colistin resistance-associated mutations on the fitness of Acinetobacter baumannii. Front Microbiol 7:1715. doi: 10.3389/fmicb.2016.01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lesho E, Yoon EJ, McGann P, Snesrud E, Kwak Y, Milillo M, Onmus-Leone F, Preston L, St Clair K, Nikolich M, Viscount H, Wortmann G, Zapor M, Grillot-Courvalin C, Courvalin P, Clifford R, Waterman PE. 2013. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis 208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- 50.Lima WG, Alves MC, Cruz WS, Paiva MC. 2018. Chromosomally encoded and plasmid-mediated polymyxins resistance in Acinetobacter baumannii: a huge public health threat. Eur J Clin Microbiol Infect Dis 37:1009–1019. doi: 10.1007/s10096-018-3223-9. [DOI] [PubMed] [Google Scholar]
- 51.Takemoto N, Numata I, Su'etsugu M, Miyoshi-Akiyama T. 2018. Bacterial EndoMS/NucS acts as a clamp-mediated mismatch endonuclease to prevent asymmetric accumulation of replication errors. Nucleic Acids Res 46:6152–6165. doi: 10.1093/nar/gky481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oikonomou O, Sarrou S, Papagiannitsis CC, Georgiadou S, Mantzarlis K, Zakynthinos E, Dalekos GN, Petinaki E. 2015. Rapid dissemination of colistin and carbapenem resistant Acinetobacter baumannii in Central Greece: mechanisms of resistance, molecular identification and epidemiological data. BMC Infect Dis 15:559. doi: 10.1186/s12879-015-1297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerson S, Betts JW, Lucaßen K, Nodari CS, Wille J, Josten M, Göttig S, Nowak J, Stefanik D, Roca I, Vila J, Cisneros JM, La Ragione RM, Seifert H, Higgins PG. 2019. Investigation of novel pmrB and eptA mutations in isogenic Acinetobacter baumannii isolates associated with colistin resistance and increased virulence in vivo. Antimicrob Agents Chemother 63:e01586-18. doi: 10.1128/AAC.01586-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trebosc V, Gartenmann S, Totzl M, Lucchini V, Schellhorn B, Pieren M, Lociuro S, Gitzinger M, Tigges M, Bumann D, Kemmer C. 2019. Dissecting colistin resistance mechanisms in extensively drug-resistant Acinetobacter baumannii clinical isolates. mBio 10:e01083-19. doi: 10.1128/mBio.01083-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olmeda-Lopez H, Corral-Lugo A, McConnell MJ. 2021. Effect of subinhibitory concentrations of antibiotics and disinfectants on ISAba-mediated inactivation of lipooligosaccharide biosynthesis genes in Acinetobacter baumannii. Antibiotics (Basel) 10:1259. doi: 10.3390/antibiotics10101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. 2011. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob Agents Chemother 55:3022–3024. doi: 10.1128/AAC.01732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Quintanilla M, Pulido MR, Moreno-Martinez P, Martin-Pena R, Lopez-Rojas R, Pachon J, McConnell MJ. 2014. Activity of host antimicrobials against multidrug-resistant Acinetobacter baumannii acquiring colistin resistance through loss of lipopolysaccharide. Antimicrob Agents Chemother 58:2972–2975. doi: 10.1128/AAC.02642-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karakonstantis S, Ioannou P, Kofteridis DD. 2022. In search for a synergistic combination against pandrug-resistant A. baumannii; methodological considerations. Infection 50:569–581. doi: 10.1007/s15010-021-01748-w. [DOI] [PubMed] [Google Scholar]
- 59.Abdul-Mutakabbir JC, Yim J, Nguyen L, Maassen PT, Stamper K, Shiekh Z, Kebriaei R, Shields RK, Castanheira M, Kaye KS, Rybak MJ. 2021. In vitro synergy of colistin in combination with meropenem or tigecycline against carbapenem-resistant Acinetobacter baumannii. Antibiotics 10:880. doi: 10.3390/antibiotics10070880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sertcelik A, Baran I, Akinci E, Mumcuoglu I, Bodur H. 2020. Synergistic activities of colistin combinations with meropenem, sulbactam, minocycline, disodium fosfomycin, or vancomycin against different clones of carbapenem-resistant Acinetobacter baumannii strains. Microb Drug Resist 26:429–433. doi: 10.1089/mdr.2019.0088. [DOI] [PubMed] [Google Scholar]
- 61.Bardbari AM, Arabestani MR, Karami M, Keramat F, Aghazadeh H, Alikhani MY, Bagheri KP. 2018. Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 37:443–454. doi: 10.1007/s10096-018-3189-7. [DOI] [PubMed] [Google Scholar]
- 62.Soudeiha MAH, Dahdouh EA, Azar E, Sarkis DK, Daoud Z. 2017. In vitro evaluation of the colistin-carbapenem combination in clinical isolates of A. baumannii using the checkerboard, Etest, and time-kill curve techniques. Front Cell Infect Microbiol 7:209. doi: 10.3389/fcimb.2017.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pankuch GA, Lin G, Seifert H, Appelbaum PC. 2008. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother 52:333–336. doi: 10.1128/AAC.00689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L. 2018. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 65.Dickstein Y, Lellouche J, Ben Dalak Amar M, Schwartz D, Nutman A, Daitch V, Yahav D, Leibovici L, Skiada A, Antoniadou A, Daikos GL, Andini R, Zampino R, Durante-Mangoni E, Mouton JW, Friberg LE, Dishon Benattar Y, Bitterman R, Neuberger A, Carmeli Y, Paul M, AIDA Study Group . 2019. Treatment outcomes of colistin- and carbapenem-resistant Acinetobacter baumannii infections: an exploratory subgroup analysis of a randomized clinical trial. Clin Infect Dis 69:769–776. doi: 10.1093/cid/ciy988. [DOI] [PubMed] [Google Scholar]
- 66.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 68.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.01928-22-s0001.pdf, PDF file, 0.6 MB (607.4KB, pdf)
Data Availability Statement
Original data sets are available in a publicly accessible repository. The whole-genome sequencing data of the representative A. baumannii isolates in this study were registered with the DDBJ (BioProject accession no. PRJDB12922). The GenBank file for the complete reference genome sequence of ATCC 19606 was registered with the DDBJ (nucleotide accession no. AP025740).