Abstract
In this study, we used PCR to measure the levels of the peroxisome proliferator activated receptor genes PPARα1, PPARα2, PPARβ, and PPARγ in the intestine, liver, gill, heart, kidney, brain, muscle, spleen, skin, and stomach of turbot (Scophthalmus maximus) cultured under different temperature conditions (14, 20, 23, 25, and 28 °C). We used split-split-plot (SSP) analysis of variance, additive main effects and multiplicative interaction (AMMI) analysis, and genotype main effects and genotype × environment interaction (GGE) biplot analysis to evaluate the genotype × tissue interaction effects on gene expression. The results of the SSP analysis of variance showed that temperature and tissue × gene have highly significant (p < 0.01) effect on the expression of S. maximus PPAR genes. The AMMI analysis results revealed that the expression of PPAR genes at the appropriate temperature (14 °C) mainly depended on genotype × tissue interaction and tissue effects. Under stress temperatures, genotype effects, tissue effects, and genotype × tissue interaction, all had significant effects on the expression of PPAR genes. The contribution of the genotype effect slowly increased with increasing temperature; it increased faster at 20 °C and then slowly declined at 25 °C. The contribution of the tissue effect slowly increased from 14 to 20 °C, where it sharply decreased, and then it stabilized after a slight fluctuation. The contribution of the genotype × tissue interaction effect showed a fluctuating upward trend throughout the experiment, and it had a significant impact on PPAR gene expression. The key temperature at which the three effects changed was 20 °C, indicating that it is the limit temperature for active lipid metabolism under high-temperature stress. The GGE biplot analysis results showed that under suitable water temperature, the expression difference of PPAR genes in the liver was the largest; at 20 and 23 °C, the expression difference in the gill was the largest; and at 25 and 28 °C, the expression difference in the brain was the largest. Overall, our results suggest that the mechanism responsible for PPAR gene expression under the three high temperatures (23, 25, and 28 °C) was relatively consistent, but it differed from that at 20 °C.
Keywords: genotype, tissue, interactions, PPAR genes, turbot Scophthalmus maximus
1. Introduction
The growth and reproduction of fish are closely related to environmental conditions, and temperature is one of the most important environmental factors in the aquaculture environment [1]. This not only affects growth, development, and survival of aquatic animals but also significantly affects their immune system [2,3]. High temperature induces a stress response in fish and causes a series of changes, including changes in genes, mRNA, proteins, and metabolites [4,5,6,7,8], which directly affect the biological functions of cells, tissues, and organs and cause fish growth and metabolism disorders, a decline in disease resistance, and changes in meat quality [1].
Turbot (Scophthalmus maximus) is an economically valuable fish species native to the coast of Europe. Since it was introduced into China in 1992, it has become one of the leading varieties of industrially cultured fish along the northern coast of China [9]. Turbot has strict requirements for environmental conditions, especially temperature. Its optimum growth temperature is 14–17 °C, and its maximum lethal temperature is 28–30 °C [1]. Because of its requirement for relatively low temperature, cool underground seawater must be used for breeding during the high-temperature summer months in northern China. However, the limited underground seawater resources cannot meet the needs of breeding, and this increases breeding costs and limits the breeding scope of this important mariculture fish species. Therefore, understanding the heat-resistance mechanism of turbot and cultivating heat-tolerant varieties are very important research goals [1].
Studies have shown that under stress conditions, the stress response of cells is closely related to changes in energy metabolism (glucose and lipid metabolism) [1,10]. High temperature significantly changes the metabolism of lipids and carbohydrates, and the metabolism of lipids plays an important role in resisting high-temperature stress [10]. As one of the three macronutrients required by fish, fat plays an important role in maintaining fish survival and health. Disordered lipid metabolism directly affects the growth, development, and a variety of physiological activities of fish, including stress resistance [11]. Therefore, the study of lipid metabolism has important biological significance. Numerous studies have shown that the lipid metabolic response pathway of fish is precisely regulated [1]. Peroxisome proliferator activated receptors (PPARs) are a class of nuclear hormone superfamily receptors. They are one of the main nuclear receptors controlling lipid metabolism, and they play an important role in regulating the transcription of genes related to lipid metabolism [1,12,13,14,15,16].
PPARs were first found in the frog Xenopus laevis [17] and then were cloned in the mouse Mus musculus [18]. Three subtypes of PPARs (PPARα, PPARβ (also known as PPARδ), and PPARγ) have been identified in mammals, birds, amphibians, and fish [1,12,13,14,15,16]. PPARα1 and PPARα2 have been found in fugu (Takifugu rubripes), zebrafish (Danio rerio), Japanese rice fish (Oryzias latipes), turbot, and grass carp (Ctenopharyngodon idella) [1,13,14,15,16,17,18,19]. When activated by corresponding ligands in vivo, these receptors form heterodimers with the retinoid X receptor (RXR) and bind to DNA sequences to regulate gene transcription related to glucose and lipid metabolism [1]. Researchers have conducted extensive research on the structure, function, expression mode, and regulation of lipid metabolism of PPAR genes in rodents, humans, poultry, and livestock. Genes of PPARs are mostly related to lipid transport and metabolism and play an important role in fatty acid oxidation and fat metabolism [18]. PPARα and PPARβ activate enzymes related to lipolysis and metabolism, regulate the expression of enzymes encoded by genes, and participate in mitochondria β-oxidation. PPARγ controls fat accumulation and regulates fat and bone formation. Research of the three subtypes of PPARs in fish started late. At present, sequence cloning and identification of PPARs have been completed in zebrafish [20], Dicentrarchus labrax [21], and T. rubripes [22]. Because the research of the three subtypes of PPARs in fish started late, studies in this field remain at the level of sequence cloning and tissue-specific or development-specific expression pattern analysis.
To explore the heat-resistance mechanism of turbot, Zhao et al. (2020) [1] used fluorescence quantitative PCR (qPCR) to detect the expression of the three subtypes of PPAR genes in different tissues of turbot and the expression of PPARs in the kidney of turbot under high-temperature stress. They identified PPARα, PPARβ, and PPARγ in turbot and reported that they may participate in the regulation of lipid metabolism in a tissue-specific way. This study was also the first to report the expression changes of PPAR subtypes under temperature stress. However, the same gene often shows different expression levels in different tissues in the same organism. This has been reported in some livestock [23,24,25,26], plants [27], fish [1,28,29,30], and humans [31]. There are also many reports about the tissue-specific expression of PPAR genes in various organisms [1,12,13,14,15,32,33,34,35,36,37,38]. The differential expression of the same gene in different tissues can be attributed to a genotype effect, a tissue effect, and/or genotype × tissue interactions. However, the genetic mechanism of tissue-specific expression has not been reported so far.
In this study, we used split-split-plot (SSP) analysis of variance, additive main effects and multiplicative interaction (AMMI) analysis [39], and genotype main effects and genotype × environment interaction (GGE) biplot analysis [40] to evaluate genotype × tissue interaction effects on PPARα1, PPARα2, PPARβ, and PPARγ genes in turbot cultured under different temperature conditions. The purpose of this study was to identify the genetic mechanism responsible for the differential expression of PPARs in different tissues in turbot to apply the formulation of high-temperature-tolerance breeding program.
2. Results
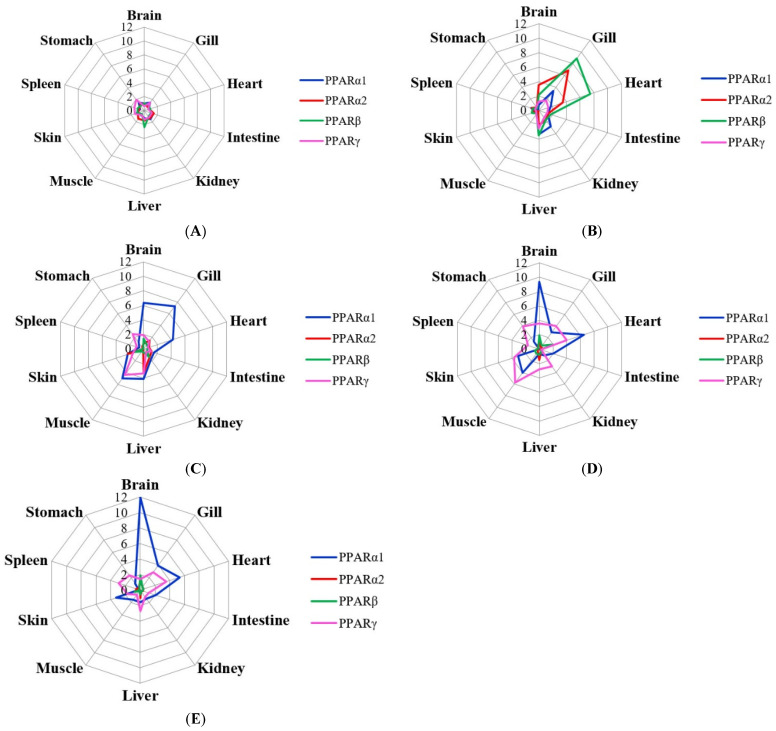
Figure 1 shows the expression of PPARα1, PPARα2, PPARβ, and PPARγ in different tissues from fish cultured at different temperatures. On the whole, the expression of PPARα1 and PPARγ at 23, 25, and 28 °C were higher than that at the appropriate temperature (14 °C); the expression of PPARα2 and PPARβ peaked at 20 °C.
Figure 1.
Expression values of PPAR genes in ten tissues at five temperatures ((A), 14 °C; (B), 20 °C; (C), 23 °C; (D), 25 °C; (E), 28 °C).
2.1. SSP Analysis of Variance
The results of the SSP analysis of variance are listed in Table 1. Table 1 shows that the p values of factors temperature, tissue, gene, temperature × tissue, temperature × gene, tissue × gene, and temperature × tissue × gene were 0.0004, 0, 1 × 10−7, 0, 1 × 10−7, 1 × 10−7, and 1 × 10−7, respectively, indicating that the expression of the four PPAR genes was significantly (p < 0.05) affected by the seven effects (Table 1).
Table 1.
SSP analysis of variance for S. maximus high temperature experiment with four PPAR genes, five temperature gradients, and ten tissues.
| Source of Variation | Sum of Square | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Blocks (replicates) | 1.2927 | 2 | 0.6464 | ||
| Temperature | 58.603 | 4 | 14.651 | 18.398 ** | 0.0004 |
| Main-plot error | 6.3707 | 8 | 0.7963 | ||
| Tissue | 270.57 | 9 | 30.063 | 128.02 ** | 0 |
| Temperature × tissue | 328.25 | 36 | 9.1181 | 38.83 ** | 0 |
| Split-plot error | 21.134 | 90 | 0.2348 | ||
| Gene | 180.69 | 3 | 60.229 | 249.7 ** | 1 × 10−7 |
| Temperature × gene | 321.96 | 12 | 26.83 | 111.23 ** | 1 × 10−7 |
| Tissue × gene | 222.42 | 27 | 8.2378 | 34.153 ** | 1 × 10−7 |
| Temperature × tissue × gene | 485.05 | 108 | 4.4912 | 18.62 ** | 1 × 10−7 |
| Split-split-plot error | 72.361 | 300 | 0.2412 |
Notes: Asterisks denote that correlations were significant at ** p < 0.01.
2.2. AMMI Analysis
The AMMI analysis was carried out based on expressions of PPAR genes in different tissues at different temperatures (Supplementary Material Table S1). The results of the AMMI analysis showed that the expression of the four genes was significantly affected by genotype effects, tissue effects, and genotype × tissue interactions at different temperatures (Table 2).
Table 2.
AMMI analysis results for PPARα1, PPARα2, PPARβ, and PPARγ in different tissues at different temperatures.
| Temperatures | Source of Variation | df | SS | MS | F | Prob. | % of Total SS |
|---|---|---|---|---|---|---|---|
| Total | 119 | 21.5253 | 0.1809 | ||||
| Treatment | 39 | 15.2082 | 0.39 | 4.9384 ** | 0 | ||
| Gene | 3 | 0.0548 | 0.0183 | 0.2311 | 0.87444 | 0.2545 | |
| Tissue | 9 | 5.9496 | 0.6611 | 8.3718 ** | 0 | 27.6400 | |
| 14 °C | Interaction | 27 | 9.2039 | 0.3409 | 4.317 ** | 0 | 42.7585 |
| IPCA1 | 11 | 4.39599 | 0.39964 | 5.06101 ** | 6 × 10−6 | 47.7622 | |
| IPCA2 | 9 | 3.2454 | 0.3606 | 4.56665 ** | 7.1 × 10−5 | 35.2611 | |
| Residual | 7 | 1.5625 | 0.22321 | ||||
| Error | 80 | 6.31709 | 0.07896 | ||||
| Total | 119 | 480.995 | 4.042 | ||||
| Treatment | 39 | 447.293 | 11.4691 | 27.2247 ** | 0 | ||
| Gene | 3 | 47.154 | 15.718 | 37.3106 ** | 0 | 9.8034 | |
| Tissue | 9 | 256.9 | 28.5444 | 67.7573 ** | 0 | 53.4100 | |
| 20 °C | Interaction | 27 | 143.239 | 5.3052 | 12.5931 ** | 0 | 29.7797 |
| IPCA1 | 11 | 120.236 | 10.9305 | 25.9463 ** | 0 | 83.9403 | |
| IPCA2 | 9 | 17.053 | 1.89477 | 4.49772 ** | 8.5 × 10−5 | 11.9052 | |
| Residual | 7 | 5.9508 | 0.85011 | ||||
| Error | 80 | 33.702 | 0.42127 | ||||
| Total | 119 | 370.434 | 3.1129 | ||||
| Treatment | 39 | 347.314 | 8.9055 | 30.8149 ** | 0 | ||
| Gene | 3 | 127.261 | 42.4204 | 146.784 ** | 0 | 34.3546 | |
| Tissue | 9 | 74.5141 | 8.2793 | 28.6483 ** | 0 | 20.1153 | |
| 23 °C | Interaction | 27 | 145.539 | 5.3903 | 18.6517 ** | 0 | 39.2886 |
| IPCA1 | 11 | 95.9195 | 8.71996 | 30.1729 ** | 0 | 65.9065 | |
| IPCA2 | 9 | 37.9649 | 4.21832 | 14.5963 ** | 0 | 26.0857 | |
| Residual | 7 | 11.6543 | 1.6649 | ||||
| Error | 80 | 23.12 | 0.289 | ||||
| Total | 119 | 524.157 | 4.4047 | ||||
| Treatment | 39 | 498.347 | 12.7781 | 39.6067 ** | 0 | ||
| Gene | 3 | 195.432 | 65.1441 | 201.919 ** | 0 | 37.2850 | |
| Tissue | 9 | 133.402 | 14.8224 | 45.9432 ** | 0 | 25.4507 | |
| 25 °C | Interaction | 27 | 169.512 | 6.2782 | 19.4598 ** | 0 | 32.3400 |
| IPCA1 | 11 | 132.551 | 12.0501 | 37.3501 ** | 0 | 78.1954 | |
| IPCA2 | 9 | 35.5725 | 3.9525 | 12.2511 ** | 0 | 20.9852 | |
| Residual | 7 | 1.38891 | 0.19842 | ||||
| Error | 80 | 25.8101 | 0.32263 | ||||
| Total | 119 | 512.987 | 4.3108 | ||||
| Treatment | 39 | 500.778 | 12.8405 | 84.1362 ** | 0 | ||
| Gene | 3 | 132.745 | 44.2483 | 289.934 ** | 0 | 25.8768 | |
| Tissue | 9 | 128.053 | 14.2281 | 93.229 ** | 0 | 24.9622 | |
| 28 °C | Interaction | 27 | 239.979 | 8.8881 | 58.2389 ** | 0 | 46.7808 |
| IPCA1 | 11 | 224.367 | 20.397 | 133.65 ** | 0 | 93.4943 | |
| IPCA2 | 9 | 15.3029 | 1.70032 | 11.1412 ** | 0 | 6.3767 | |
| Residual | 7 | 0.30941 | 0.0442 | ||||
| Error | 80 | 12.2092 | 0.15261 |
Notes: 1 df: degree of freedom; SS: sum of squares; MS: mean squares; 2 **: significant at 1% probability level.
At 14 °C, the results of the AMMI analysis of variance indicated that 0.2545, 27.6400, and 42.7585% of the total sum of squares (SS) were attributable to the genotype, tissue, and genotype × tissue interactions, respectively. IPCA1 and IPCA2 were obtained, which contributed 47.7622 and 35.2611% of the genotype × tissue interaction, respectively.
At 20 °C, the results of the AMMI analysis of variance indicated that 9.8034, 53.4100, and 29.7797% of the total SS were attributable to the genotype, tissue, and genotype × tissue interactions, respectively. IPCA1 and IPCA2 contributed 83.9403 and 11.9052% of the genotype × tissue interaction, respectively.
At 23 °C, the results of the AMMI analysis of variance indicated that 34.3546, 20.1153, and 39.2886% of the total SS were attributable to the genotype, tissue, and genotype × tissue interactions, respectively. IPCA1 and IPCA2 contributed 65.9065 and 26.0857% of the genotype × tissue interaction, respectively.
At 25 °C, the results of the AMMI analysis of variance indicated that 37.28508, 25.4507, and 32.3400% of the total SS were attributable to the genotype, tissue, and genotype × tissue interactions, respectively. IPCA1 and IPCA2 contributed 78.1954 and 20.9852% of the genotype × tissue interaction, respectively.
At 28 °C, the results of the AMMI analysis of variance indicated that 25.8768, 24.9622, and 46.7808% of the total SS were attributable to the genotype, tissue, and genotype × tissue interactions, respectively. IPCA1 and IPCA2 contributed 93.4943 and 6.3767% of the genotype × tissue interaction, respectively.
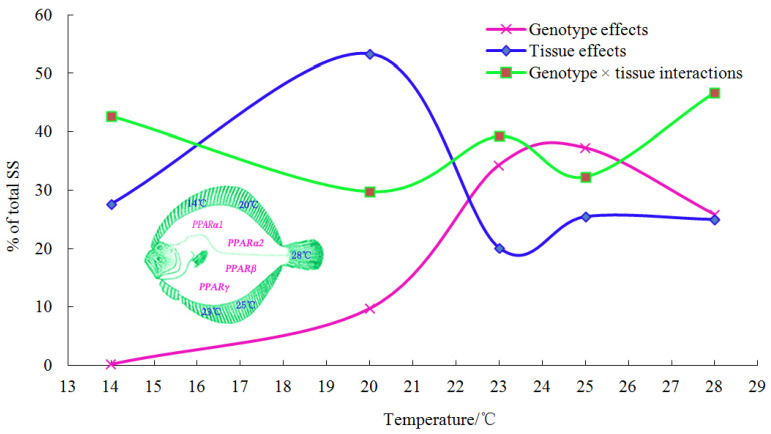
The contribution trends of the three effects are shown in Figure 2. The contribution of the genotype effect slowly increased with increasing temperature; it increased faster at 20 °C and then slowly declined at 25 °C. The contribution of the tissue effect slowly increased from 14 °C to 20 °C, where it sharply decreased, and then it stabilized after a slight fluctuation. The contribution of the genotype × tissue interaction effect showed a fluctuating upward trend throughout the experiment, and it had a significant impact on PPAR gene expression.
Figure 2.
Genotype effect, tissue effect, and genotype × tissue interaction changes with temperature.
2.3. GGE Biplot Analysis
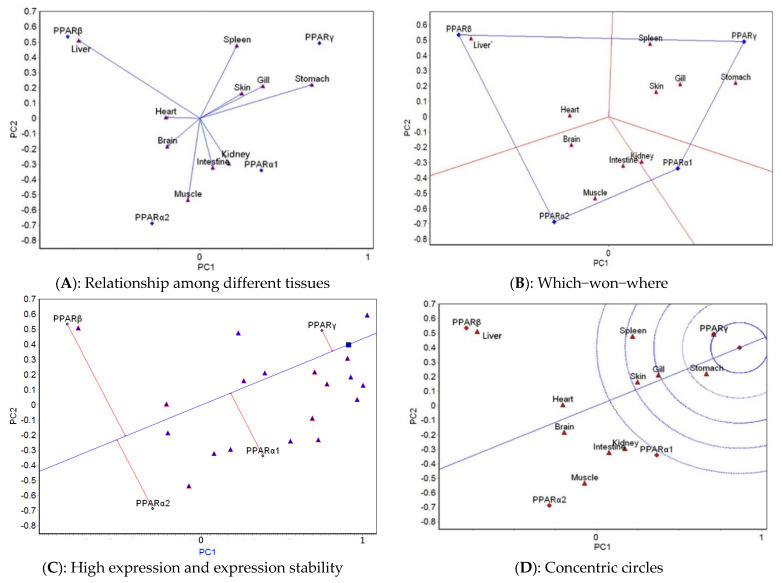
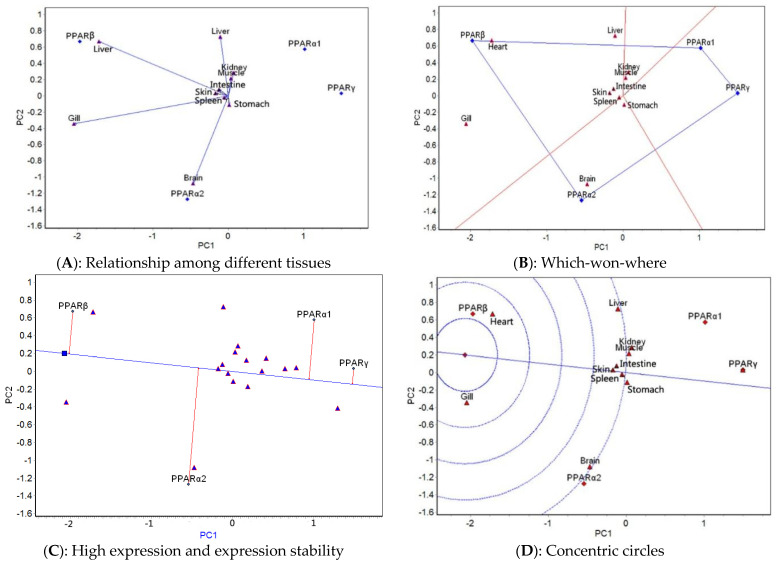
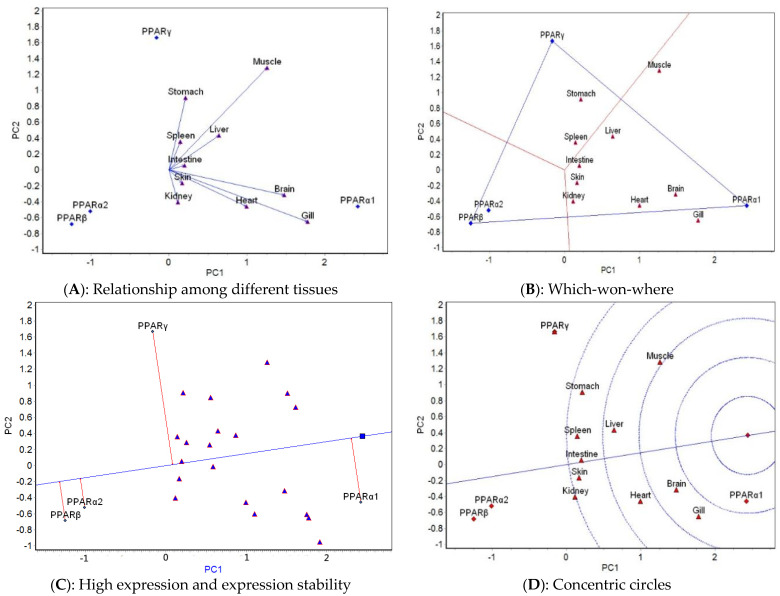
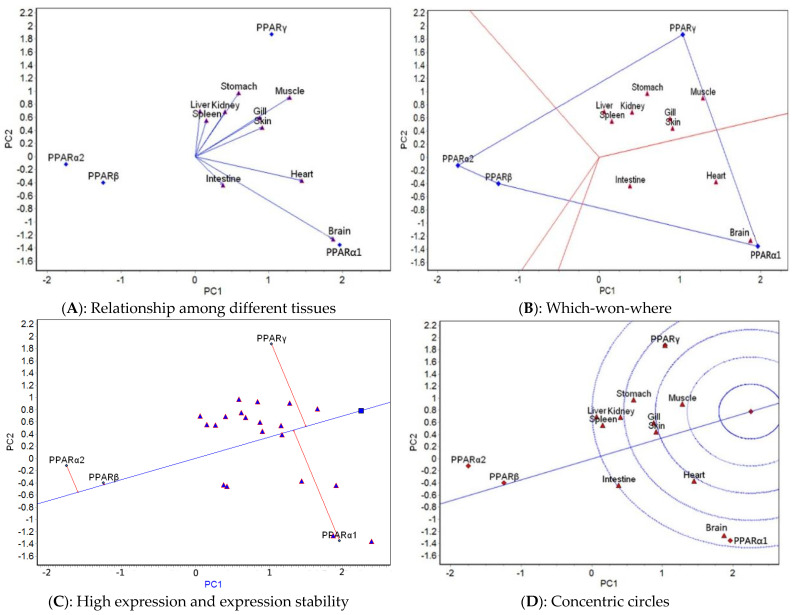
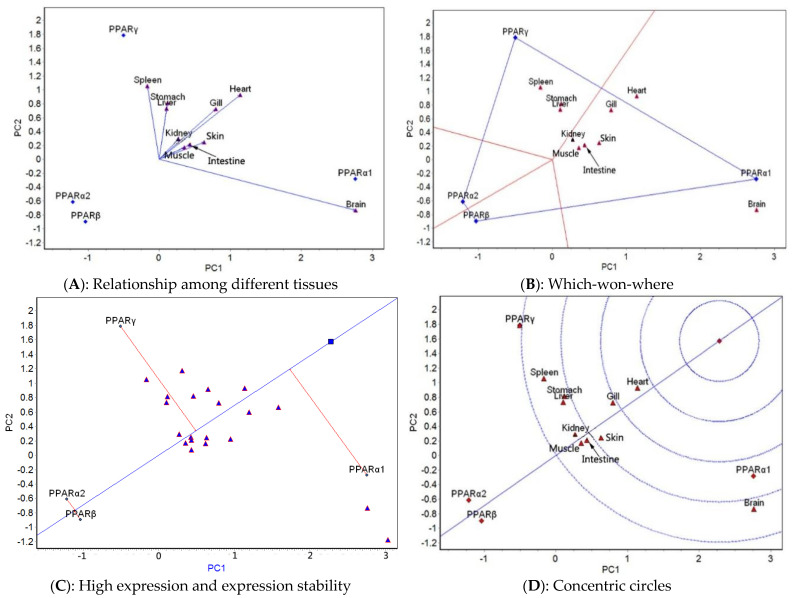
The GGE biplot analysis was carried out based on the mean expression of four genes in 10 tissues at different temperatures. The relationship among different tissues, which-won-where, high expression and expression stability, and concentric circle GGE biplots for each of the five temperatures tested (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7) were drawn, respectively, based on the results of the GGE biplot analysis shown in Table 3.
Figure 3.
GGE biplots of PPAR genes in different tissues at 14 °C.
Figure 4.
GGE biplots of PPAR genes in different tissues at 20 °C.
Figure 5.
GGE biplots of PPAR genes in different tissues at 23 °C.
Figure 6.
GGE biplots of PPAR genes in different tissues at 25 °C.
Figure 7.
GGE biplots of PPAR genes in different tissues at 28 °C.
Table 3.
GGE biplot analysis of PPAR genes in different tissues at different temperatures.
| Temperature | PPARs Gene/Tissue | PCA1 | PCA2 | PCA3 | Distance From Center Point (Di) |
|---|---|---|---|---|---|
| PPARα1 | 0.3631 | -0.3388 | 0.5799 | 0.7635 | |
| PPARα2 | −0.2853 | −0.6881 | −0.4031 | 0.8470 | |
| PPARβ | −0.7873 | 0.5357 | 0.1337 | 0.9616 | |
| PPARγ | 0.7094 | 0.4912 | −0.3105 | 0.9171 | |
| Brain | −0.1959 | −0.1842 | 0.2120 | 0.3424 | |
| Gill | 0.3742 | 0.2135 | 0.3533 | 0.5571 | |
| Heart | −0.2033 | 0.0081 | −0.0187 | 0.2043 | |
| 14 °C | Intestine | 0.0757 | −0.3207 | 0.0136 | 0.3298 |
| Kidney | 0.1718 | −0.2942 | 0.1449 | 0.3703 | |
| Liver | −0.7228 | 0.5105 | −0.049 | 0.8863 | |
| Muscle | −0.0716 | −0.5338 | −0.4938 | 0.7307 | |
| Skin | 0.2494 | 0.1629 | −0.0821 | 0.3090 | |
| Spleen | 0.2178 | 0.4764 | −0.3899 | 0.6530 | |
| Stomach | 0.6664 | 0.2204 | −0.1291 | 0.7137 | |
| PPARα1 | 1.0137 | 0.5744 | 0.8333 | 1.4325 | |
| PPARα2 | −0.5413 | −1.2729 | 0.2258 | 1.4016 | |
| PPARβ | −1.9714 | 0.6681 | −0.2492 | 2.0964 | |
| PPARγ | 1.4990 | 0.0304 | −0.8098 | 1.7041 | |
| Brain | −0.4701 | −1.0778 | −0.1075 | 1.1808 | |
| Gill | −2.0521 | −0.3423 | 0.4680 | 2.1324 | |
| Heart | −1.7196 | 0.6663 | −0.5255 | 1.9176 | |
| 20 °C | Intestine | −0.1222 | 0.0793 | −0.0252 | 0.1479 |
| Kidney | 0.0731 | 0.2832 | 0.8371 | 0.8867 | |
| Liver | −0.1064 | 0.7261 | 0.2317 | 0.7696 | |
| Muscle | 0.0370 | 0.2154 | −0.1444 | 0.2620 | |
| Skin | −0.1720 | 0.0282 | 0.0462 | 0.1803 | |
| Spleen | −0.0496 | −0.0232 | 0.3393 | 0.3437 | |
| Stomach | 0.0133 | −0.1143 | −0.2517 | 0.2767 | |
| PPARα1 | 2.4252 | −0.4588 | 0.0502 | 2.4687 | |
| PPARα2 | −1.0131 | −0.5212 | −1.1106 | 1.5910 | |
| PPARβ | −1.2498 | −0.6838 | 0.9882 | 1.7339 | |
| PPARγ | −0.1622 | 1.6639 | 0.0721 | 1.6734 | |
| Brain | 1.4789 | −0.3157 | 0.4705 | 1.5838 | |
| Gill | 1.7790 | −0.6510 | −0.1629 | 1.9014 | |
| Heart | 0.9966 | −0.4567 | 0.0363 | 1.0969 | |
| 23 °C | Intestine | 0.1985 | 0.0565 | −0.2898 | 0.3558 |
| Kidney | 0.1161 | −0.4054 | −0.0442 | 0.4240 | |
| Liver | 0.6440 | 0.4309 | −1.2183 | 1.4438 | |
| Muscle | 1.2598 | 1.2849 | 0.3835 | 1.8399 | |
| Skin | 0.1683 | −0.1650 | −0.4971 | 0.5502 | |
| Spleen | 0.1445 | 0.3581 | 0.0620 | 0.3911 | |
| Stomach | 0.2155 | 0.9091 | −0.0159 | 0.9345 | |
| PPARα1 | 1.9616 | −1.3496 | 0.1036 | 2.3833 | |
| PPARα2 | −1.7486 | −0.1206 | 0.5524 | 1.8377 | |
| PPARβ | −1.2483 | −0.4003 | −0.6314 | 1.4550 | |
| PPARγ | 1.0353 | 1.8706 | −0.0247 | 2.1382 | |
| Brain | 1.8721 | −1.2646 | 0.3754 | 2.2902 | |
| Gill | 0.8757 | 0.5911 | 0.0162 | 1.0567 | |
| Heart | 1.4477 | −0.3709 | −0.5774 | 1.6021 | |
| 25 °C | Intestine | 0.3812 | −0.4361 | 0.1814 | 0.6070 |
| Kidney | 0.4074 | 0.6864 | −0.0198 | 0.7985 | |
| Liver | 0.0646 | 0.6955 | 0.4082 | 0.8091 | |
| Muscle | 1.2839 | 0.9041 | 0.0284 | 1.5706 | |
| Skin | 0.9093 | 0.4450 | −0.0753 | 1.0152 | |
| Spleen | 0.1557 | 0.5520 | −0.1217 | 0.5864 | |
| Stomach | 0.5938 | 0.9715 | 0.1387 | 1.1470 | |
| PPARα1 | 2.7550 | −0.2784 | 0.0241 | 2.7691 | |
| PPARα2 | −1.2142 | −0.6114 | 0.3761 | 1.4105 | |
| PPARβ | −1.0348 | −0.8981 | −0.3546 | 1.4154 | |
| PPARγ | −0.5059 | 1.7880 | −0.045 | 1.8588 | |
| Brain | 2.7607 | −0.7314 | 0.0951 | 2.8575 | |
| Gill | 0.7933 | 0.7247 | −0.1139 | 1.0805 | |
| Heart | 1.1408 | 0.9294 | −0.0646 | 1.4729 | |
| 28 °C | Intestine | 0.4358 | 0.2129 | 0.0948 | 0.4942 |
| Kidney | 0.2706 | 0.2908 | −0.0118 | 0.3974 | |
| Liver | 0.1054 | 0.7348 | 0.4034 | 0.8449 | |
| Muscle | 0.3547 | 0.1679 | −0.1111 | 0.4079 | |
| Skin | 0.6312 | 0.2448 | −0.1734 | 0.6989 | |
| Spleen | −0.1638 | 1.0535 | 0.0795 | 1.0691 | |
| Stomach | 0.1145 | 0.8142 | −0.1518 | 0.8362 |
The results of the GGE biplot analysis of the relationship among different tissues (panel A, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7) mainly reveal the similarity of gene expression among tissues. The included angle of the two line segments indicates the correlation of the gene expression ranking in the tissue represented by the two line segments. When the included angle of the two segments is acute, the gene expression ranking in the two tissues is positively correlated: the smaller the angle, the higher the correlation and the closer the gene expression ranking. When the angle between the two segments is obtuse, the gene expression ranking is negatively correlated in the two tissues, and when the angle between the two segments is a right angle, the gene expression ranking in the two tissues is not related. The length of the line segment indicates the ability of the tissue to distinguish gene expression, with a longer line segment indicating stronger ability to distinguish gene expression. The which-won-where view of the GGE biplot (panel B, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7) divides the experimental regions according to genotype × tissue interaction and identifies genes with the highest expression level in each region. The gene located at the top corner of the polygon in each region is the gene with the highest expression in this region. The high expression and expression stability biplot (panel C, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7) shows the genes with high and stable expression. The direction of the transverse oblique line to the ideal value is the approximate average expression of the gene in all tissues: the closer to the ideal value, the higher the average gene expression. The straight line perpendicular to the transverse slash represents the tendency of the gene × tissue interaction, and a greater deviation of the vertical line from the transverse oblique line indicates greater instability of gene expression. The GGE biplot with concentric circles (panel D, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7) comprehensively evaluates high expression and expression stability based on the distance of various genes from the central point of the genes. A smaller distance indicates higher and more stable gene expression [41].
At 14 °C, the relationship among different tissues biplot (Figure 3A) showed that the angle between skin and gill was the smallest, which indicated that they had basically the same ranking of gene expression. The liver had the longest line segment length, indicating that this tissue had the strongest ability to distinguish the expression of the four genes. The which-won-where view (Figure 3B) divided the 10 tissues into four regions consisting of spleen, skin, gill, and stomach; brain, muscle, and intestine; heart and liver; and kidney. The expression of PPARγ was the highest in the spleen–skin–gill–stomach region, that of PPARα2 was the highest expression in the brain–muscle–intestine region, PPARβ had the highest expression in the heart–liver region, and PPARα1 had the highest expression in the kidney region. The high expression and expression stability biplot (Figure 3C) showed that the expression of PPARγ was the highest, followed by PPARα1, PPARβ, and PPARα2; PPARγ had the most stable expression, followed by PPARα1, PPARα2, and PPARβ. The concentric circles biplot (Figure 3D) showed that PPARγ had the best expression and stability, followed by PPARα1, PPARα2, and PPARβ.
At 20°C, the relationship among different tissues (Figure 4A) showed that the angles between the spleen and gill and between the kidney and muscle were the smallest, which indicated that the ranking of gene expression was the same for each of these pairs. The gill has the longest line segment length, indicating that it had the strongest ability to distinguish the expression of the four genes. The which-won-where view (Figure 4B) divided the 10 tissues into three regions: spleen and muscle; liver, heart, gill, spleen, skin, and intestine; and brain and stomach. The highest expression of PPARα1 occurred in the spleen–muscle region, that of PPARβ was in the liver–heart–gill–spleen–skin–intestine region, and that of PPARα2 was detected in the brain–stomach region. The high expression and expression stability biplot (Figure 4C) showed that the expression of PPARβ was the highest, followed by PPARα2, PPARα1, and PPARγ; PPARγ had the most stable expression, followed by PPARβ, PPARα1, and PPARα2. The concentric circles biplot (Figure 4D) showed that PPARβ had the best expression and stability, followed by PPARα2, PPARα1, and PPARγ.
At 23 °C, the relationship among different tissues (Figure 5A) showed that the angles between the heart and gill and between the spleen and stomach were the smallest, which indicated that the ranking of gene expression was the same for each pair of tissues. The gill had the longest line segment length, indicating that it had the strongest ability to distinguish the expression of the four genes. The which-won-where view (Figure 5B) showed that the 10 tissues were divided into two regions: the spleen and stomach belonged to one area, and the other eight tissues belonged to another area. The expression of PPARγ was the highest in the spleen–stomach region, and PPARα1 expression was the highest in the other area. The high expression and expression stability biplot (Figure 5C) showed that the expression of PPARα1 was the highest, followed by PPARγ, PPARα2, and PPARβ; PPARα2 had the most stable expression, followed by PPARβ, PPARα1, and PPARγ. The GGE biplot with concentric circles (Figure 5D) showed that PPARα1 had the best expression and stability, followed by PPARγ, PPARα2, and PPARβ.
At 25 °C, the relationship among different tissues biplot (Figure 6A) showed that the angle between the muscle and gill was the smallest, which indicated that they had basically the same ranking of expression of the four genes. The brain had the longest line segment length, indicating that this tissue had the strongest ability to distinguish the expression of the four genes. The which-won-where view (Figure 6B) showed that the 10 tissues were divided into two regions: intestine, heart, and brain belonged to one area, and the other seven tissues belonged to the other area. PPARα1 had the highest expression in the intestine–heart–brain region, and PPARγ had the highest expression in the other region. The high expression and expression stability view (Figure 6C) showed that the expression of PPARγ was the highest, followed by PPARα1, PPARβ, and PPARα2; PPARβ had the most stable expression, followed by PPARα2, PPARγ, and PPARα1. The GGE biplot with concentric circles (Figure 6D) showed that PPARγ had the best expression and stability, followed by PPARα1, PPARβ, and PPARα2.
At 28 °C, the relationship among different tissues biplot (Figure 7A) showed that the angles among the intestine, muscle, and skin as well as those among the kidney, gill, and heart were the smallest, which indicated that the ranking of the expression of the four genes was the same for each set of tissues. The brain had the longest line segment length, indicating that it had the strongest ability to distinguish the expression of the four genes. The which-won-where view (Figure 7B) showed that the 10 tissues were divided into two regions, consisting of spleen, stomach, and liver in one region and the other seven tissues in the second region. The expression of PPARγ was the highest in the spleen–stomach–liver region, and that of PPARα1 was the highest in the other region. The high expression and expression stability biplot (Figure 7C) showed that the expression of PPARα1 was the highest, followed by PPARγ, PPARα2, and PPARβ; PPARβ had the most stable expression, followed by PPARα2. The concentric circles biplot (Figure 7D) showed that PPARα1 had the best expression and stability, followed by PPARγ.
The ranking of high expression, stable expression, and comprehensive evaluation of high and stable expression of PPAR genes at different temperatures are shown in Table 4. The top two genes in the comprehensive ranking of the high expression of PPAR genes at different temperatures were PPARγ and PPARα1; for stable expression, they were PPARα2 and PPARβ; and for high and stable expression, they were PPARγ and PPARα1. Thus, the comprehensive ranking of high and stable expression mainly depended on the high expression of PPAR genes.
Table 4.
Ranking of high expression, stable expression, and comprehensive evaluation of high and stable expression of PPAR genes at different temperatures.
| Temperatures | ||||||
|---|---|---|---|---|---|---|
| Evaluation Index | Ranking | 14 °C | 20 °C | 23 °C | 25 °C | 28 °C |
| High expression | 1 | PPARγ | PPARβ | PPARα1 | PPARγ | PPARα1 |
| 2 | PPARα1 | PPARα2 | PPARγ | PPARα1 | PPARγ | |
| 3 | PPARβ | PPARα1 | PPARα2 | PPARβ | PPARα2 | |
| 4 | PPARα2 | PPARγ | PPARβ | PPARα2 | PPARβ | |
| Stable expression | 1 | PPARγ | PPARγ | PPARα2 | PPARβ | PPARβ |
| 2 | PPARα1 | PPARβ | PPARβ | PPARα2 | PPARα2 | |
| 3 | PPARα2 | PPARα1 | PPARα1 | PPARγ | PPARα1 | |
| 4 | PPARβ | PPARα2 | PPARγ | PPARα1 | PPARγ | |
| Comprehensive evaluation of high and stable expression | 1 | PPARγ | PPARβ | PPARα1 | PPARγ | PPARα1 |
| 2 | PPARα1 | PPARα2 | PPARγ | PPARα1 | PPARγ | |
| 3 | PPARα2 | PPARα1 | PPARα2 | PPARβ | PPARα2 | |
| 4 | PPARβ | PPARγ | PPARβ | PPARα2 | PPARβ | |
3. Discussion
Many studies have shown that three PPAR family members have distinct patterns of tissue distribution [1,12,13,14,15,32,33,34,35,36,37,38]. In mammals, PPARα is usually expressed in metabolically active tissues, such as the liver, and it induces the expression of a series of genes related to lipid transport, oxidation, and thermal metabolism. PPARβ is widely expressed in the brain, adipose tissue, and skin of the body, but its expression level in the liver is low, and its function is not clear at present. PPARγ is present in white adipose tissue and is involved in lipid synthesis [42].
Tissue specificity in the expression of PPARs has also been reported in fish [15]. Ibabe et al. (2002) [43] reported that in zebrafish, PPARα was mainly expressed in the liver, kidney, intestine, and pancreas; PPARβ was expressed in the liver, proximal and distal tubules of the kidney, glomerulus, and pancreas; and PPARγ expression in the pancreas, intestine, and gonads was very weak. In the red sea bream Pagrus major, three kinds of PPARs were widely expressed in the adipose tissue, gills, heart, and hepatocytes of young fish and in the ovaries of adult fish [44]. Studies of brown trout (Salmo trutta. fario) showed that PPARα was mainly present in the white muscle, liver, and heart; PPARβ was expressed in the testis, liver, heart, trunk kidney, and white muscle; and PPARγ was expressed in the liver and trunk kidney [45]. In their study of sea bream, Leaver et al. (2005) [46] found that PPARα was mainly expressed in the heart and liver, PPARγ was mainly expressed in the intestine and adipose tissue, and PPARβ was expressed in all tissues. In grass carp, PPARα was dominant in the liver; PPARβ was enriched in the liver, heart, and muscle; and PPARγ was rich in the liver but less abundant in the muscle, visceral adipose tissue, and brain [47].
The literature shows that tissue-specific expression of PPARs is very common in mammals and fish, and it can be attributed to genotype effects, tissue effects, and genotype × tissue interactions. Therefore, it is of great significance to explore the heat-resistance mechanism of turbot by analyzing the genotype × tissue interactions of PPAR genes at different temperatures to elucidate the genetic mechanism responsible for the tissue-specific expression of these genes. In the current study, the tissue was regarded as the “environment”, and the interactions between PPAR genes and tissues in turbot were evaluated using SSP, AMMI, and GGE biplot analyses.
The results of the SSP analysis of variance showed that temperature and tissue × gene have highly significant (p < 0.01) effect on the expression of S. maximus PPAR genes, which indicates that it is of great significance to explore the heat-resistance mechanism of turbot by analyzing the genotype × tissue interactions of PPAR genes at different temperatures to elucidate the genetic mechanism responsible for the tissue-specific expression of these genes.
The results of the AMMI analysis revealed that the expression of PPAR genes at the appropriate temperature (14 °C) mainly depended on genotype × tissue interactions and tissue effects; however, under stress temperatures, genotype effects, tissue effects, and genotype × tissue interactions, all had significant effects on the expression of PPAR genes (Figure 2). Overall, the contribution of genotype effects slowly increased with increasing temperature; it increased faster at 20°C and slowly decreased at 25 °C. The contribution of tissue effects slowly increased and then sharply decreased at 20 °C, and then it reached a stable state after a slight fluctuation. The contribution of genotype × tissue interactions showed a fluctuating upward trend throughout the experiment, and it had a significant impact on PPAR gene expression. The trends of the contributions of the three effects to PPAR gene expression at the different temperatures clearly showed that 20 °C was the key point at which changes occurred, which may be related to the temperature range in which turbot can adapt. This species has strict requirements for temperature and other environmental indicators. Its suitable growth water temperature is 14–17 °C; its maximum growth temperature is 21–22 °C; it can tolerate temperatures of 25–26 °C but only for short duration; and 28 °C is its lethal temperature [48]. We found that at 20 °C, which is close to the maximum growth temperature of turbot, the mean value of PPAR gene expression was the highest (1.9118 U/mg), which indicates that 20 °C is the limit for active lipid metabolism under high-temperature stress. At 14, 20, 23, 25, and 28 °C, the average expression levels of the four PPAR genes in the 10 tissues were 1.1166, 1.9118, 1.7727, 1.9112, and 1.5856 U/mg, respectively. The expression levels under high-temperature stress were significantly higher than those under the suitable water temperature. This result was likely related to the regulation of lipid metabolism under high-temperature stress, which involved increasing the overall expression of PPAR genes.
The results of the GGE biplot analysis indicated that, on the whole, the ranking of PPAR gene expression in different tissues under the appropriate water temperature (14 °C) was more different than that under high-temperature stress, which likely was due to changes of the regulation mechanism of lipid metabolism under high-temperature stress. At 23, 25, and 28 °C, the difference in the PPAR gene expression ranking was relatively small. At 14 °C, the expression difference of PPAR genes was the greatest in the liver; at 20 and 23 °C, it was the greatest in the gill; and at 25 and 28 °C, it was the greatest in the brain. These results showed that the tissue specificity of PPAR gene expression differed at different water temperatures and that under the appropriate water temperature, the liver played a very important role in maintaining normal lipid metabolism. To deal with respiratory function problems at 20 and 23 °C, PPAR genes were significantly expressed in the gill. Temperatures of 25 and 28 °C affected the brain, and, therefore, PPAR gene expression was high in this tissue.
At different temperatures, the tissue regions with high expression of PPAR genes differed, which further indicated that the expression of PPAR genes in different tissues at different temperatures has different tissue specificity. The top-ranked genes in terms of high expression at different temperatures were PPARγ and PPARα1; those for stable expression were PPARα2 and PPARβ; and those for high and stable expression were PPARγ and PPARα1 (Table 4). These were also the patterns for the four high temperatures. The comprehensive ranking of high and stable expression and that of high expression were almost the same, but they differed from that of stable expression. This result indicated that the comprehensive ranking of high and stable expression mainly depended on the high expression of the PPAR gene. Overall, the mechanism responsible for PPAR gene expression under the three highest temperatures appears to be relatively consistent, but it differs from that at 20 °C. The average value of PPAR gene expression was the highest at 20 °C (1.9118 U/mg), which may mean that 20 °C is the temperature limit at which active lipid metabolism can occur under high-temperature stress.
4. Materials and Methods
4.1. Experimental Materials
The turbot used in the experiment were obtained from Tianyuan Aquatic Limited Corporation (Yantai, China). These fish were artificially bred in a healthy way, with a body mass of 28.19 ± 1.38 g and a body length of 10.1 ± 0.7 cm. Sixty healthy fish with fresh body surface; good vitality; and absence of redness, swelling, or other trauma were selected and put into three experimental barrels with a volume of 2 m3, with 20 fish in each barrel. The temperature experiment was carried out after acclimation period for 1 week. During the period of temporary care and during the experiment, the fish were not fed, and the water was changed once a day (the rate of daily water exchange is 50%) and was continuously aerated.
The water-heating process followed that of Ndong et al. (2007) [49] with slight modifications. That is, after 12 h at 14 ± 0.5°C, the test fish were heated to the experimental temperature via a 1 °C increase every 12 h. The hot air pipe was used to heat the seawater in the reservoir tank to a suitable temperature to achieve water temperature regulation. Seawater was pumped into the experimental tank by a water pump. The water flow was 0.05 m3/h. We used five experimental temperatures (14, 20, 23, 25, and 28 °C), of which 14 °C was the normal temperature control group [1]. After 12 h at 14 ± 0.5 °C, we removed three fish from each barrel. After being anesthetized with 200 mg/L of MS222 (tricaine methane sulfonate) (Maya Reagent Limited Corporation, Jiaxing, China) [50], we quickly removed the intestine, liver, gill, heart, kidney, brain, muscle, spleen, skin, and stomach and put them on ice and rinsed with 0.9% sodium chloride solution. The remaining turbot were heated to the experimental temperature via a 1 °C increase every 12 h, and the tissues were removed after the fish had been at each experimental temperature for 12 h. Samples were immersed in 10 times the volume of RNA preservation solution (Tiangen Biotech Co., Ltd., Beijing, China) and stored at –80 °C after storage at 4 °C for 24 h. The whole process of the experiment followed the hydrostatic method, and the temperature was controlled by automatic thermostatic heaters to ensure the synchronous temperature increase of each experimental barrel.
4.2. Experimental Methods
4.2.1. Total RNA Extraction, Quantification, Integrity Detection, and cDNA Synthesis
The total RNA was extracted from animal tissues (intestine, liver, gill, heart, kidney, brain, muscle, spleen, skin, and stomach) using total RNA Extraction Kits (RNAprep pure Tissue Kit, Tiangen Biotech Co., Ltd., Beijing, China), and the quality and concentration of RNA were detected by 1% agarose gel electrophoresis and Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA). The RNAs that met the quality criteria (28S:18S = 2:1) were used to synthesize cDNA according to the steps of the reverse transcription kit (TranScript One-Step gDNA Removal and cDNA Synthesis SuperMix, TransGen Biotech Co., Ltd., Beijing, China), and the samples were stored at −20 °C for analysis.
4.2.2. Fluorescence qPCR
We used the housekeeping gene β-actin as the internal reference gene [51] to evaluate the quality of the synthesized cDNA template. We used Primer premier 5.0 software to design fluorescent qPCR primers based on the PPARα1, PPARα2, PPARβ, and PPARγ gene sequences of the turbot genome (Assembly GCA_003186165.1). The synthesized primers were subjected to PCR, and the products were sequenced to detect the specificity of primers. Primer synthesis and related sequencing were completed by Sangon Biotech Co., Ltd. (Shanghai, China).
The tissue distribution of PPAR RNAs in turbot was detected by fluorescence qPCR. The specific operation method was as follows: The reverse-transcribed cDNAs of the 10 tissues were used as the templates, and the specific primers P1/P2 (PPARα1), P3/P4 (PPARα2), P5/P6 (PPARβ), and P7/P8 (PPARγ) of each gene (Table 5) were used on an Applied Biosystems StepOnePlus PCR amplification instrument (Applied Biosystems, Foster City, CA, USA). According to the instructions of the TORO Green qPCR Master Mix (TOROIVD, Shanghai, China), the amplification reaction was carried out using the SYBR Green I chimeric fluorescence method. The PCR system in a 20 μL volume consisted of 10.0 μL of TORO Green qPCR Master Mix, 0.8 μL (10 μMol/L) of each upstream and downstream primer, 2.0 μL of the cDNA template, and 6.8 μL of RNase-free water. The reaction procedure was as follows: pre-denaturation at 95 °C for 60 s followed by 40 cycles of denaturation at 95 °C for 10 s and annealing at 60 °C for 30 s. After the fluorescence qPCR procedure, the relative expression of mRNA was calculated by the △△CT (2−ΔΔCt) method [52] based on the Ct values of the PPARα1, PPARα2, PPARβ, PPARγ, and β-actin genes.
Table 5.
Primer sequences used in the experiment.
| Primer Name | Gene Name | Sequence (5′ to 3′) | Annealing Temperature |
Amplification Efficiency |
|---|---|---|---|---|
| P1(S) | PPARα1 | CTACTCAAGCCTGGACCTCAACGA | 6 °C | 93.095% |
| P2(AS) | PPARα1 | TCACTGAAGGGACGCCGCA | ||
| P3(S) | PPARα2 | CCCTGATAACACCTTCCTCTTTCCC | 60 °C | 93.92% |
| P4(AS) | PPARα2 | TGTCTCGGTCGTCTTGATGTCCTG | ||
| P5(S) | PPARβ | ACGGCAAAGGCTTCGTTACC | 60 °C | 96.944% |
| P6(AS) | PPARβ | CTAATGGCAGCAACAAACAGG | ||
| P7(S) | PPARγ | ATCTGAAATACTTCCCCCTCACCAC | 60 °C | 106.12% |
| P8(AS) | PPARγ | GCTGATGCTCGTCATTCCCAA | ||
| P9(S) | β-actin | CATGTACGTTGCCATCCAAG | 60 °C | 97.36% |
| P10(AS) | β-actin | ACCAGAGGCATACAGGGACA |
Notes: S represents the upstream primer and AS represents the downstream primer.
4.3. Data Analysis
4.3.1. SSP Analysis of Variance
Referring to Piepho et al. (2018) [53], we used an SSP design for this experiment with temperature as the main-plot factor. We assigned the five experimental temperatures (14, 20, 23, 25, and 28 °C) to the five main plots in each of the three complete replicate blocks. We used tissue as the subplot (or split-plot) factor, with the ten tissues (intestine, liver, gill, heart, kidney, brain, muscle, spleen, skin, and stomach) assigned to ten subplots within each main plot, and PPAR genes as the sub-subplot (or SSP) factor, with the four PPAR genes (PPARα1, PPARα2, PPARβ, and PPARγ) assigned to individual sub-subplots within each subplot. The SSP analysis model is written according to Equation (1):
| (1) |
where is the expression of the i-th temperature treatment for the h-th tissue and j-th PPAR gene in the k-th complete block; , , and are the general intercept, effect of the k-th block, and ihj-th treatment effect, respectively; is the main-plot error associated with the k-th block and i-th temperature gradient, assumed to be random with zero mean and variance ; is the subplot error associated with the k-th block, i-th temperature, and h-th tissue (assumed to be random with zero mean and variance ); and is a residual sub-subplot error with zero mean and variance 2.
4.3.2. AMMI Analysis
The main feature of the AMMI model integrates the analysis of variance and principal component (PC) analysis [39]. The AMMI model for the g-th genotype (PPARα1, PPARα2, PPARβ, and PPARγ) in the e-th tissue (intestine, liver, gill, heart, kidney, brain, muscle, spleen, skin, and stomach) is written according to Equation (2):
| (2) |
where is the expression of the four genotypes g in tissue e; , , and are the grand mean, average deviation of genotypes (the average value of each genotype minus the grand average value), and average deviation of the tissue (the average of each tissue minus the grand average), respectively; is the eigenvalue of the n-th interaction PC axis; is the genotype PC score of the n-th PC; is the tissue PC score of the n-th PC; N is the total number of PC axes; and is the residual.
4.3.3. GGE Biplot Analysis
GGE biplot analysis can reveal the complex interactions between different factors [41,54,55]. The gene expression data obtained from different tissue experiments were sorted into a two-way table, in which each value was the average value of the expression of corresponding genes in corresponding tissues (i.e., the phenotype value ()). We used singular value decomposition of the first two PCs to fit the GGE biplot model [56], which is written according to Equation (3):
| (3) |
where is the trait mean expression for genotype g in tissue e; , , and are the grand mean, main effect of tissue e, and mean expression across all genotypes in tissue e, respectively; and are the singular values for the first and second PCs (PC1 and PC2), respectively; and are the eigenvectors of genotype g for PC1 and PC2, respectively; and are the eigenvectors of tissue e for PC1 and PC2, respectively; and is the residual associated with genotype g in tissue e.
The SSP, AMMI, and GGE biplot analyses were performed using the DPS Data Processing System (Hangzhou, China) [57].
5. Conclusions
In conclusion, at different temperatures, PPAR genes in turbot participate in the regulation of lipid metabolism in different tissue-specific ways. Therefore, at different temperatures, the tissues selected to analyze the heat-resistance mechanism of turbot differ in the activities of lipid metabolism-related genes. The gill should be used to study the heat resistance of turbot at 20/23 °C, and the brain should be used at 25/28 °C. Considering that PPARγ and PPARα1 had the best expression and stability, they can be used as indirect selection indexes for temperature tolerance breeding.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232012205/s1.
Author Contributions
Conceptualization and funding acquisition, X.W. and A.M.; data curation, formal analysis, and investigation, X.W. and T.Z.; methodology and writing—original draft preparation, X.W.; project administration and supervision, A.M.; resources, A.M. and T.Z.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All experimental treatments for artificially cultivated fish were performed according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, China. The study protocol followed the recommendations of the Experimental Animal Ethics Committee, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, China (Decision no: YSFRI-2022003).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets analyzed during the current study are available from Supplementary Material.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding Statement
This work was supported by the National Key R&D Program of China (2018YFD0900102), China Agriculture Research System of MOF and MARA (grant number CARS-47-G01), the Agricultural Fine Breed Project of Shandong (2019LZGC013), the AoShan Talents Cultivation Program (2017ASTCPOS04), and the Central Public-interest Scientific Institution Basal Research Fund (grant number 2020TD25).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao T.T., Yang S.S., Ma A.J., Huang Z.H., Sun Z.B., Wang X.A., Xia D.D. Tissue expression of lipid metabolism related genes PPARs and their responses to heat stress in turbot (Scophthalmus maximus) J. Fish. China. 2020;44:515–522. [Google Scholar]
- 2.An M.I., Choi C.Y. Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: Effects on hemolymph and biochemical parameters. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010;155:34–42. doi: 10.1016/j.cbpb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Li W.Y., Xu Z.J., Yi X.J., Chen S., Ma X.B., Zhang X., Qiu H.J., Zhang X.L., Jiang X.M. Effects of temperature on growth, immune factor activity and related gene expression of juvenile Centropristis striata. Oceanol. Et Limnol. Sin. 2021;52:708–717. [Google Scholar]
- 4.Mushtaq M.Y., Marçal R., Champagne D.L., van der Kooy F., Verpoorte R., Choi Y.H. Effect of acute stresses on zebra fish (Danio rerio) metabolome measured by NMR-based metabolomics. Planta Med. 2014;80:1227–1233. doi: 10.1055/s-0034-1382878. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y.C., Kang C.K., Tang C.H., Lee T.H., Chen T.Y. Transcriptomic Analysis of Metabolic Pathways in Milkfish That Respond to Salinity and Temperature Changes. PLoS ONE. 2015;10:e0134959. doi: 10.1371/journal.pone.0134959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan N.I., Tunnah L., Currie S., MacCormack T.J. Metabolic adjustments to short-term diurnal temperature fluctuation in the rainbow trout (Oncorhynchus mykiss) Physiol. Biochem. Zool. 2016;89:498–510. doi: 10.1086/688680. [DOI] [PubMed] [Google Scholar]
- 7.Forgati M., Kandalski P.K., Herrerias T., Zaleski T., Machado C., Souza M.R.D.P., Donatti L. Effects of heat stress on the renal and branchial carbohydrate metabolism and antioxidant system of Antarctic fish. J. Comp. Physiol. B. 2017;187:1137–1154. doi: 10.1007/s00360-017-1088-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang K.H., Liu Y.R., Tian J.L., Huang K.Y., Shi T.R., Dai X.X., Zhang W.J. Transcriptional profiling and identification of heat-responsive genes in perennial ryegrass by RNA-sequencing. Front. Plant Sci. 2017;8:1032. doi: 10.3389/fpls.2017.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X.A., Ma A.J. Dissection of genotype × tissue interactions for immunological factors in turbot (Scophthalmus maximus) infected with Vibrio anguillarum. Fish Shellfish. Immunol. 2021;119:60–66. doi: 10.1016/j.fsi.2021.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez M.V.S., Johnson J.S., Abuajamieh M., Stoakes S.K., Seibert J.T., Cox L., Kahl S., Elsasser T.H., Ross J.W., Isom S.C., et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 2015;3:e12315. doi: 10.14814/phy2.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins D.A., Rocha F., Castanheira F., Rocha F., Castanheira F., Mendes A., Pousão-Ferreira P., Bandarra N., Coutinho J., Morais S., et al. Effects of dietary arachidonic acid on cortisolproduction and gene expression in stressresponse in Senegalese sole (Solea senegalensis) post-larvae. Fish Physiol. Biochem. 2013;39:1223–1238. doi: 10.1007/s10695-013-9778-6. [DOI] [PubMed] [Google Scholar]
- 12.Qian L., Qian Y.X., Tong L.J. Cloning of full-length cDNA and RT-PCR expression analysis of PPARβ in Larimichthys crocea. J. Biol. 2010;27:1–4. [Google Scholar]
- 13.Carlota G.Z. Tissue Distribution of Peroxisome Proliferator-Activated Receptors in Salmon Fish (Salmo salar L.) Sver. Lantbr. Inst. För Livsmed. 2013;373:1–22. [Google Scholar]
- 14.Fang L.L., Chen G., Wang Z.L., Tang B.G., Zhang J.D., Huang J.S., Zhou H. Molecular Cloning and Expression Distribution and Bioinformatics Analysis of Peroxisome Proliferator Activated Receptors-α in Trachinotus ovatus. J. Guangdong Ocean. Univ. 2015;35:1–9. [Google Scholar]
- 15.Gao J., Xu G.C., Du F.K., Gu R.B. Progress on tissue expression and function of PPARs gene in fish. J. Yangtze Univ. (Nat. Sci. Ed.) 2016;13:43–46. [Google Scholar]
- 16.Zhang Q.Z., Wang W.P. Progress of Cloning and expression of PPARs in fish. Sci. Technol. Inf. 2019;17:242–244. [Google Scholar]
- 17.Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y. The relationship between PPAR with animal lipocyte’s differentiation and lipometabolism. Guangdong J. Anim. Vet. Sci. 2007;32:9–11, 15. [Google Scholar]
- 19.Dong X., Xu H., Mai K., Xu W., Zhang Y.J., Ai Q.H. Cloning and characterization of SREBP-1 and PPAR-αin Japanese seabass Lateolabrax japonicus, and their gene expressions in response to different dietary fatty acid profiles. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014;180C:48–56. doi: 10.1016/j.cbpb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Ibabe A., Bilbao E., Cajaraville M.P. Expression of peroxisome proliferator-activated receptors in zebrafish (Danio rerio) depending on gender and developmental stage. Histochem. Cell Biol. 2005;123:75–87. doi: 10.1007/s00418-004-0737-2. [DOI] [PubMed] [Google Scholar]
- 21.Boukouvala E., Antonopoulou E., Favre-Krey L., Diez A., Bautista J.M., Leaver M.J., Tocher D.R., Krey G. Molecular characterization of three peroxisome proliferator-activated receptors from the sea bass (Dicentrarchus labrax) Lipids. 2004;39:1085–1092. doi: 10.1007/s11745-004-1334-z. [DOI] [PubMed] [Google Scholar]
- 22.Kondo H., Misaki R., Gelman L. Ligand-dependent transcriptional activities of four torafugu pufferfish Takifugu rubripes peroxisome proliferator-activated receptors. Gen. Comp. Endocrinol. 2007;154:120–127. doi: 10.1016/j.ygcen.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Schwerin M., Voigt J., Wegner J., Kühn C., Ender K., Hagemeister H. Gene expression in different tissues of lactating cows of differing metabolic type: Comparison of mRNA patterns by the differential display method. J. Anim. Physiol. Anim. Nutr. 1999;81:113–123. doi: 10.1046/j.1439-0396.1999.813177.x. [DOI] [Google Scholar]
- 24.Chen Y., Yang S.L., Deng W.D., Mao H. Differential Gene Expression in Different Tissues of Black-Bone Sheep and Normal Sheep. Asian J. Anim. Vet. Adv. 2011;6:379–384. [Google Scholar]
- 25.Farzane A., Ali-Reza A., Zahra B., Abolfazl M. Novel Study of Model-Based Clustering Time Series Gene Expression in Different Tissues: Applications to Aging Process. Curr. Aging Sci. 2020;13:178–187. doi: 10.2174/1874609812666191015140449. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Mao S.M., Zhou D., Feng W.W., Li P., Chen D.F., Long Q.M., Li J.W., Xie Y., Wang S., et al. The study on mRNA expression of MC4R and SIMI genes in different tissues for congjiang pigs. Swine Prod. 2020;3:65–68. [Google Scholar]
- 27.Ling A., Li X., Hu X., Ma Z., Wu K., Zhang H., Hao M., Wei S. Dynamic changes in polyphenol compounds, antioxidant activity, and PAL gene expression in different tissues of buckwheat during germination. J. Sci. Food Agric. 2018;98:5723–5730. doi: 10.1002/jsfa.9119. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Ding L., Li Y., Hou N., Chang Y.M., Liang L.Q., Lei Q.Q. Influence on the expression quantity of five genes in the different tissues of common carps Cyprinus carpio under the low temperature. Acta Zool. Sin. 2008;54:460–466. [Google Scholar]
- 29.Sun J.H. Master’s Thesis. Shanghai Ocean University; Shanghai, China: 2017. Differential Gene Expression Analysis of Low Temperture Transcriptome and Genealogical Identification from Takifugu rubripes. [Google Scholar]
- 30.Yu W.B., Zhu K.H., Guo H.Y., Zhang N., Sun X.X., Wu N., Zhang D.C. Cloning and expression analysis of MHC II β gene in Trachinotus ovatus. South China Fish. Sci. 2017;13:69–79. [Google Scholar]
- 31.Wolfram J.P.K., Roger G., Dosch H.M. Quantitative analysis of gene expression in different tissues by template-calibrated RT-PCR and Laser-induced fluorescence. PCR Methods Appl. 1994;4:154–159. doi: 10.1101/gr.4.3.154. [DOI] [PubMed] [Google Scholar]
- 32.Braissant O., Foufelle F., Scotto C., Dauça M., Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 33.Braissant O., Wahli W. Differential Expression of Peroxisome Proliferator-Activated Receptor -α, -β, and -γ during Rat Embryonic Development. Endocrinology. 1998;139:2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- 34.Loviscach M., Rehman N., Carter L., Mudaliar S., Mohadeen P., Ciaraldi T.P., Veerkamp J.H., Henry R.R. Distribution of peroxisome proliferator-activated receptors (PPARs) in human skeletal muscle and adipose tissue: Relation to insulin action. Diabetologia. 2000;43:304–311. doi: 10.1007/s001250050048. [DOI] [PubMed] [Google Scholar]
- 35.Pascal E., Olivier B., Sharmila B.M., Liliane M., Walter W., Beatrice D. Rat PPARs: Quantitative Analysis in Adult Rat Tissues and Regulation in Fasting and Refeeding. Endocrinology. 2001;142:4195–4202. doi: 10.1210/endo.142.10.8458. [DOI] [PubMed] [Google Scholar]
- 36.Meng H., Lib H., Zhaob J.G., Gub Z.L. Differential expression of peroxisome proliferator-activated receptors alpha and gamma gene in various chicken tissues. Domest. Anim. Endocrinol. 2005;28:105–110. doi: 10.1016/j.domaniend.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y., Liu X.L., Xiao H.W., Zhang H.L. The differential expression of peroxisome proliferators-activated receptors in various duck tissues. Mol. Biol. Rep. 2010;37:1235–1240. doi: 10.1007/s11033-009-9494-6. [DOI] [PubMed] [Google Scholar]
- 38.Urbatzka B., Galante-Oliveira S., Rocha E., Castro L.F.C., Cunha I. Tissue expression of PPAR-alpha isoforms in Scophthalmus maximus and transcriptional response of genes in the heart after exposure to WY-14643. Fish Physiol. Biochem. 2013;39:1043–1055. doi: 10.1007/s10695-012-9761-7. [DOI] [PubMed] [Google Scholar]
- 39.Gollob H.F.A. Statistical model which combines features of factor analytic and analysis of variance techniques. Psychometrika. 1968;33:73–115. doi: 10.1007/BF02289676. [DOI] [PubMed] [Google Scholar]
- 40.Yan W.K., Kang M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists. CRC Press; Boca Raton, FL, USA: 2003. [Google Scholar]
- 41.Yan W.K., Holland J.B. A heritability-adjusted GGE Biplot for test environment evaluation. Euphytica. 2010;171:355–369. doi: 10.1007/s10681-009-0030-5. [DOI] [Google Scholar]
- 42.Trattner S. Doctoral Thesis. Swedish University of Agricultural Sciences Uppsala; Uppsala, Sweden: 2009. Quality of Lipids in Fish Fed Vegetable Oils. Diss. Uppsala: Sveriges Lantrbuksuniv. [Google Scholar]
- 43.Ibabe A., Grabenbauer M., Baumgart E., Fahimi D.H., Cajaraville M.P. Expression of peroxisome proliferator-activated receptors in zebrafish (Danio rerio) Histochem. Cell Biol. 2002;118:231–239. doi: 10.1007/s00418-002-0434-y. [DOI] [PubMed] [Google Scholar]
- 44.Oku H., Umino T. Molecular characterization of peroxisome proliferator-activated receptors (PPARs) and their gene expression in the differentiating adipocytes of red sea bream Pagrus major. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008;151:268–277. doi: 10.1016/j.cbpb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Batista-Pinto C., Rodrigues P., Rocha E., Lobo-da-Cunha A. Identification and organ expression of peroxisome proliferator activated receptors in brown trout (Salmo trutta f. fario) Biochim. Biophys. Acta. 2005;1731:88–94. doi: 10.1016/j.bbaexp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Leaver M.J., Boukouvala E., Antonopoulou E., Diez A., Favre-Krey L., Ezaz M.T., Bautista J.M., Tocher D.R., Krey G. Three peroxisome proliferator-activated receptor isotype from each of two species of marine fish. Endocrinology. 2005;146:3150–3162. doi: 10.1210/en.2004-1638. [DOI] [PubMed] [Google Scholar]
- 47.Pawar A., Xu J., Jerks E., Mangelsdorf D.J., Jump D.B. Fatty Acid Regulation of Liver X Receptors (LXR) and Peroxisome Proliferator-activated Receptor α (PPARα) in HEK293 Cells. J. Biol. Chem. 2002;277:39243–39250. doi: 10.1074/jbc.M206170200. [DOI] [PubMed] [Google Scholar]
- 48.Wang X.A., Ma A.J., Huang Z.H., Sun Z.B., Cui W.X., Qu J.B., Yu H. Estimation of genetic parameters for upper thermal tolerances and growth-related traits in turbot Scophthalmus maximus. J. Oceanol. Limnol. 2019;37:1736–1745. doi: 10.1007/s00343-019-7267-1. [DOI] [Google Scholar]
- 49.Ndong D., Chen Y.Y., Lin Y.H., Vaseeharan B., Chen J.C. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish. Immunol. 2007;22:686–694. doi: 10.1016/j.fsi.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Wang X.A., Liu Z.F., Ma A.J. Interpretation of the genotype by tissue Interactions of four genes (AFP1, CIRP, YB-1, and HMGB1) in Takifugu rubripes under different low-temperature conditions. Front. Mol. Biosci. 2022;9:897935. doi: 10.3389/fmolb.2022.897935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J.Q., Hou L., Li X.Y., Zhao X.T., Zhang R.F., Jiang L.J., Sun W.J., An J.L. The cloning and analysis of the β-actin gene in Turbot. Fish. Sci. 2007;26:214–217. [Google Scholar]
- 52.Schmittgen T.D., Zakrajsek B.A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods. 2000;46:69–81. doi: 10.1016/S0165-022X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 53.Piepho H.P., Edmondson R.N. A tutorial on the statistical analysis of factorial experiments with qualitative and quantitative treatment factor levels. J. Agron. Crop Sci. 2018;204:429–455. doi: 10.1111/jac.12267. [DOI] [Google Scholar]
- 54.Yan W.K. Methodology of Cultivar Evaluation Based on Yield Trial Data-with Special Reference to Winter Wheat in Ontario. University of Guelph; Guelph, ON, Canada: 1999. [Google Scholar]
- 55.Yan W.K. GGEbiplot—A windows application for graphical analysis of multienvironment trial data and other types of two-way data. Agron. J. 2001;93:1111–1118. doi: 10.2134/agronj2001.9351111x. [DOI] [Google Scholar]
- 56.Yan W. Singular value partitioning for biplot analysis of multi-environment trial data. Agron. J. 2002;94:990–996. [Google Scholar]
- 57.Tang Q. DPS Data Processing System. Science Press; Beijing, China: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analyzed during the current study are available from Supplementary Material.