Abstract
For the diagnosis of Chagas' disease, the trans-sialidase inhibition assay was able to resolve the results for samples with borderline results, to detect as positive 60% of samples that were negative by conventional serology, and to discriminate idiopathic from chagasic megaviscera or cardiopathy. No cross-reaction with sera from patients with other diseases was observed.
Trypanosoma cruzi is the agent of Chagas' disease, an endemic illness in Latin America. The trans-sialidase activity present in T. cruzi 10 has not been detected in Trypanosoma rangeli, Leishmania spp., or Plasmodium spp., parasites that share geographical distributions with T. cruzi, or in mammals.
We have previously communicated that sera from chronically infected patients display high levels of neutralizing activity against trans-sialidase, while sera from patients in the acute phase have lower and variable titers, depending on the time of the evolution of infection 6, 9. These antibodies are transferred via the placenta, but in the absence of congenital infection, titers decrease with the time after birth. In longitudinal follow-up of patients treated during the acute phase and cured after chemotherapy treatment (under the accepted criteria of parasitological cure 12), a persistence of neutralizing antibodies even 14 years after treatment was found 7. When serum samples from Amerindians who were at high risk of infection and who were negative by conventional serology (CS) and parasitologic assays (hemoculture and xenodiagnosis) were analyzed, they showed neutralizing values similar to those obtained for serum from patients with chronic Chagas' disease 8. These findings might indicate that neutralizing antibodies detected by the trans-sialidase inhibition assay (TIA) could identify infections that are undetectable by CS. TIA uses a recombinant trans-sialidase 2, 3 that is preincubated with the serum to be tested, and the remnant ability to transfer the sialyl residues from sialyllactose to [d-glucose-1-14C]lactose is evaluated 5. Pooled normal human sera were used as negative controls, and the value of inhibition obtained was taken as 0% with the cutoff value set at 50% inhibition 6, 7.
The CS means of diagnosis of T. cruzi infections, indirect immunofluorescence (IIF) assay and enzyme-linked immunosorbent assay (ELISA), use raw preparations of the parasite, although different defined antigens have been used 4. PCR assay has shown similar or even less sensitivity than serology 1, 11. Since TIA seems to be a highly sensitive diagnostic technique, it was used to analyze a panel of serum samples with different degrees of diagnostic difficulty. Every serum sample in this panel was previously analyzed at least by IIF assay and ELISA.
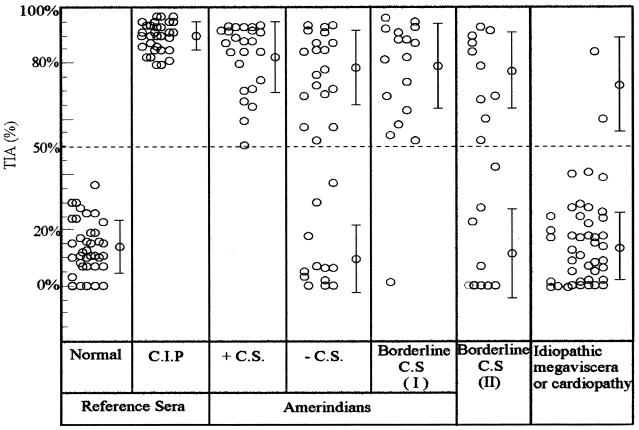
The panel included the following serum samples. (i) Sera were obtained from Amerindians from Paraguay to increase the number of samples for which results were reported previously 8. Amerindians are at high risk of infection and reside in shacks with elevated number of infected vector bugs. However, a high percentage of this population is parasitologically and serologically negative by conventional assays. Among 73 samples that were analyzed, 24 were simultaneously positive for T. cruzi by CS and TIA. Among the 32 serum samples negative by CS, 19 (60%) were reactive by TIA. When samples with borderline results by CS (group I; n = 17) were analyzed, 16 (94%) were found to be positive by TIA (Fig. 1).
FIG. 1.
Sensitivity of TIA. TIA values are for sera from Amerindians from Paraguay that were positive, negative, and borderline by CS (group I) and samples from patients from regions of endemicity in Paraguay with borderline results by CS (group II) and patients with idiopathic cardiopathy or megaviscera. TIA values for sera from healthy individuals and chronically infected patients (CIP) obtained from blood banks in Argentina (negative and positive control groups, respectively) are shown as a reference. Pooled normal human serum from a region of nonendemicity was used to assign 0% inhibition. Cutoff values were set at 50% inhibition. The mean ± standard deviation is indicated for each group.
(ii) Another set of serum samples (n = 19) from regions of Paraguay endemic for Chagas' disease with borderline results by CS was included (group II). Among the samples in this group, 53% (n = 10) were positive by TIA (Fig. 1).
Serum samples with borderline results (groups I and II) were those with low antibody titers, as detected by IIF assay and ELISA.
(iii) Sera were obtained from patients with idiopathic megaesophagus or megacolon (n = 38) or cardiopathy (n = 4) from regions of Brazil and Paraguay where Chagas' disease is endemic. Two positive samples were detected by TIA: one from a patient (from Paraguay) with idiopathic megacolon and another obtained from a patient (from Brazil) with idiopathic cardiopathy with a complete bundle branch block as determined by electrocardiography (Fig. 1).
The results described for the first three groups of serum samples (i to iii) show the high sensitivity of TIA (Fig. 1).
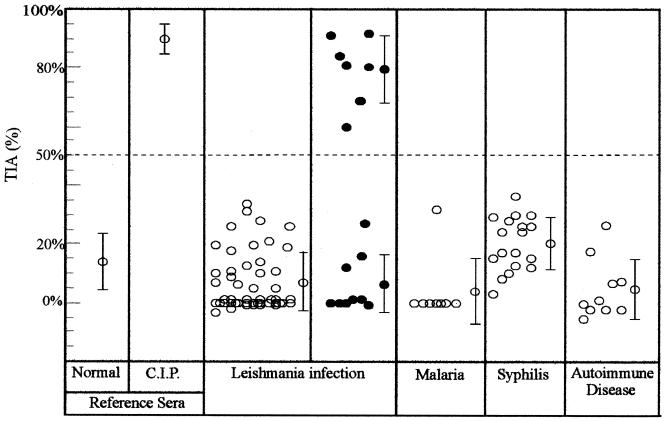
To further analyze cross-reactivity with overlapping endemic infections or interference with other disorders, we also studied the following serum samples. (iv) Sera were obtained from patients from Paraguay with cutaneous leishmaniosis who were serologically and parasitologically positive. Among the first group (Fig. 2) of serum samples serologically negative by CS for T. cruzi (n = 49), every serum sample was TIA negative. Among the second group of serum samples (n = 16) (Fig. 2) from patients with suspected mixed infection (diagnosed by CS to be positive for T. cruzi), only seven samples were reactive by TIA. The remaining nine samples that were unreactive by TIA coincidentally displayed a low level but positive reactivity for T. cruzi by CS. In addition, (v) sera from patients with malaria (n = 8) from Brazil, (vi) sera from patients with syphilis (n = 18) from Paraguay, and (vii) sera from patients with autoimmune disease (systemic lupus erythematosus, (n = 6); rheumatoid arthritis, n = 1; pigmentary scleroderma, n = 1; myositis, n = 1; mixed connective tissue disease, (n = 1) from regions of Argentina not endemic for Chagas' disease were also tested.
FIG. 2.
Specificity of TIA. TIA values are for sera from patients with cutaneous leishmaniasis, malaria, syphilis, and autoimmune diseases that were negative by CS assays (open circles). Solid circles represents TIA values for sera from patients with cutaneous leishmaniasis and suspected simultaneous T. cruzi infection as determined by CS. Reference sera were from healthy individuals and chronically infected patients (CIP), as described in the legend to Fig. 1. The mean ± standard deviation is indicated for each group.
Serum samples from patients suffering from malaria, syphilis, or autoimmune disease were negative for T. cruzi antigens by CS as well as by TIA (Fig. 2). Among the group of patients with cutaneous leishmaniasis, those who were negative by T. cruzi-specific CS were also unreactive by TIA. However, among those who were positive by T. cruzi-specific CS, seven were found to be TIA positive and nine were found to be TIA negative. Therefore, the low level of reactivity to T. cruzi could be due to cross-reactions with epitopes from Leishmania sp. antigens. These results demonstrate the specificity of TIA (Fig. 2).
Taken together with the results presented in our previous reports, the results presented here strongly support the view that TIA is a highly specific and sensitive technique for the serological diagnosis of T. cruzi infection. It lacks cross-reactivity with other infections like leishmaniasis, malaria, or syphilis, as well as with autoimmune disorders. Its specificity is especially relevant to the solution of the diagnosis problem for those parasitic diseases that have epidemiological distributions similar to those of T. cruzi and that carry cross-reactive antigens, like Leishmania spp. It is usually hard to discriminate by CS T. cruzi and Leishmania spp. co-infections. TIA allowed discrimination of those patients infected with both parasites from those whose positivity for T. cruzi by CS was due to cross-reactive epitopes from Leishmania spp. TIA was also able to resolve samples with borderline results, to detect as positive 60% of CS-negative samples, and to discriminate between idiopathic and infectious megaviscera or cardiopathy.
We propose that use of TIA would be of special interest in blood banks to confirm the results for negative donors and in public health laboratories to confirm or exclude infections in patients.
Acknowledgments
This study was supported by grants from the Universidad de Buenos Aires, the Consejo Nacional de Investigaciones Cientificas y Técnicas (CONICET) of Argentina, and the World Bank/UNDP/WHO Special Program for Research and Training in Tropical Disease (TDR). O.C., S.M.G.C., and M.S.L., are researchers from CONICET.
We are indebted to Jorge Manny from the Instituto de Investigaciones Médicas, Buenos Aires, Argentina, for supplying the samples from patients with autoimmune diseases and Carlos C. Frasch for critical reading of the manuscript.
REFERENCES
- 1.Avila H A, Pereira J B, Thieman O, De Paiva E, Degrave W, Morel C M, Simpson L. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J Clin Microbiol. 1993;31:2421–2426. doi: 10.1128/jcm.31.9.2421-2426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buschiazzo A, Frasch A C C, Campetella O. Medium scale production and purification to homogeneity of a recombinant trans-sialidase from Trypanosoma cruzi. Cell Mol Biol. 1996;42:703–710. [PubMed] [Google Scholar]
- 3.Campetella O, Uttaro A, Parodi A J, Frasch A C C. A recombinant Trypanosoma cruzi trans-sialidase lacking the amino acid repeats retains the enzymatic activity. Mol Biochem Parasitol. 1994;64:337–340. doi: 10.1016/0166-6851(94)00036-0. [DOI] [PubMed] [Google Scholar]
- 4.Frasch A C C, Cazzulo J J, Aslund L, Petterson U. Comparison of genes encoding Trypanosoma cruzi antigens. Parasitol Today. 1991;7:148–151. doi: 10.1016/0169-4758(91)90284-u. [DOI] [PubMed] [Google Scholar]
- 5.Leguizamón M S, Campetella O, González Cappa S M, Frasch A C C. Mice infected with Trypanosoma cruzi produce antibodies against the enzymatic domain of trans-sialidase that inhibit its activity. Infect Immun. 1994;62:3441–3446. doi: 10.1128/iai.62.8.3441-3446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leguizamón M S, Campetella O, Russomando G, Almirón M, Guillén L, González Cappa S M, Frasch A C C. Antibodies inhibiting Trypanosoma cruzi trans-sialidase activity in sera from human infections. J Infect Dis. 1994;170:1570–1574. doi: 10.1093/infdis/170.6.1570. [DOI] [PubMed] [Google Scholar]
- 7.Leguizamón M S, Russomando G, Luquetti A, Rassi A, Almirón M, González Cappa S M, Frasch A C C, Campetella O. Long-lasting antibodies detected by a trans-sialidase inhibition assay of sera from parasite-free, serologically cured chagasic patients. J Infect Dis. 1997;175:1272–1275. doi: 10.1086/593697. [DOI] [PubMed] [Google Scholar]
- 8.Leguizamón M S, Russomando G, Rojas de Arias A, Samudio M, Cabral M, González Cappa S M, Frasch A C C, Campetella O. Use of trans-sialidase inhibition assay in a population serologically negative for Trypanosoma cruzi but at high risk of infection. Clin Diagn Lab Immunol. 1998;5:254–255. doi: 10.1128/cdli.5.2.254-255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira-Chioccola V L, Schenkman S, Kloetzel J. Sera from chronic patients and rodents infected with Trypanosoma cruzi inhibit trans-sialidase by recognizing its amino-terminal and catalytic domain. Infect Immun. 1994;62:2973–2978. doi: 10.1128/iai.62.7.2973-2978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenkman S, Eichinger D, Pereira M E A, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 11.Wincker P, Bosseno M F, Britto C, Yaksic N, Cardoso M A, Morel C M, Breniere S F. High correlation between Chagas' disease serology and PCR-based detection of Trypanosoma cruzi kinetoplast DNA in Bolivian children living in an endemic area. FEMS Microbiol Lett. 1994;124:419–423. doi: 10.1111/j.1574-6968.1994.tb07318.x. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Report of a WHO expert committee. WHO Technical Report Series No. 811. Geneva, Switzerland: World Health Organization; 1991. Control of Chagas Disease; p. 43. [PubMed] [Google Scholar]