Abstract
Background:
Breast cancer survivors who report chronic pain in the affected ipsilateral upper limb or body are nearly twice as likely to develop lymphedema. Little is known about lymphatic pain, defined as co-occurring pain and swelling in the affected ipsilateral upper limb or body. The study aimed to examine the predictors and effects of lymphatic pain on breast cancer survivors' activities of daily living (ADLs).
Materials and Methods:
A sample of 568 patients was recruited in a metropolitan cancer center in the United States. Demographic and clinical data were collected. Body mass index (BMI) and limb volume were measured using infra-red perometer. Lymphatic pain and ADLs were measured by the Lymphedema and Breast Cancer Symptom Experience Index. Parametric and nonparametric tests and generalized linear models were used to analyze data.
Results:
Lymphatic pain affected 33% of survivors. Significant predictors of lymphatic pain included younger age, higher BMI, financial hardship, and a diagnosis of lymphedema. Patients with a diagnosis of lymphedema had 9.68 odds (confidence interval [CI]: 5.78–16.63; p < 0.001) and those with financial hardship had 4.64 odds (CI: 1.99–11.32; p = 0.001) of experiencing lymphatic pain. Patients with lymphatic pain had more impairments in ADLs (p < 0.001) compared to patients with only pain, only swelling, and no symptoms. Significantly more patients with lymphatic pain had a limb volume difference of >5% and >10% compared to patients with only pain and no symptom.
Conclusion:
This study is the first to report that in a large sample of patients, 33.1% experienced lymphatic pain and that lymphatic pain was associated with significant impairments in ADLs. Findings suggest that lymphatic pain may be due to abnormal accumulation of lymph fluid. Research is needed to ascertain the physiological mechanisms that underlie lymphatic pain and determine whether strategies to prevent and treat lymphedema can decrease lymphatic pain.
Keywords: lymphatic pain, pain, limb volume, lymph fluid, lymph fluid accumulation, activities of daily living, lymphedema, breast cancer
Introduction
Chronic pain and swelling in the affected ipsilateral upper limb or body following breast cancer treatment remain the most common and debilitating complications that impact breast cancer survivors' quality of life (QOL).1–3 Among the 3.8 million breast cancer survivors in the United States,4 one-third of them report chronic pain in the affected ipsilateral upper limb or body, making it the second most common chronic pain syndrome following breast cancer treatment.5 Breast cancer survivors who report pain on the affected ipsilateral upper limb or body are nearly twice as likely to develop lymphedema.6 The abnormal accumulation of lymph fluid may be the major cause of lymphatic pain that includes a variety of pain sensations (i.e., pain, aching, and soreness) and swelling.7–9 To make a distinction between pain in the affected ipsilateral upper limb without co-occurring swelling, we conceptualized “lymphatic pain” as the co-occurrence of pain AND swelling in the affected ipsilateral upper limb or body following breast cancer treatment.
Most studies of chronic cancer pain among breast cancer survivors focus on occurrence and severity of general body pain and impact of pain on patients.10,11 While these studies provide information on the burden of chronic pain in breast cancer survivors, they do not differentiate among specific pain causes by specific pain conditions (e.g., lymphatic pain due to abnormal lymph fluid accumulation, postmastectomy pain, chemotherapy-induced peripheral neuropathy, and arthralgias from hormonal treatments). Therefore, opportunities to link specific pain conditions with effective treatments are limited.10,11 While risk factors for abnormal lymph fluid accumulation or lymphedema are known (e.g., higher body mass index [BMI], receipt of radiation, and lymph node removal),12–14 it is unknown whether these risk factors are associated with lymphatic pain. In addition, while self-reported swelling of the upper limb is a known marker of early stage of lymphedema and negatively influences breast cancer survivors' QOL,6,15–18 no study has investigated the impact of lymphatic pain on breast cancer survivors' abilities to perform activities of daily living (ADLs). Finally, the relationship between lymphatic pain and limb volume changes as a potential marker for lymphedema has not been evaluated. Therefore, the purposes of this study in a sample of breast cancer survivors (N = 568) were to (1) determine the predictors of lymphatic pain; (2) examine the relationship between lymphatic pain and limb volume changes; and (3) evaluate the impact of lymphatic pain on ADLs.
Materials and Methods
Study design
This study was part of the larger, cross-sectional, observational study. The primary aim of the larger study was to use machine learning methods to detect and diagnose breast cancer-related lymphedema.
Ethical considerations
This study (IRB #16-01665) was approved by the Institutional Review Board of a metropolitan cancer center in New York of the United States. All the participants signed the written informed consent. This study does not contain any animal subjects.
Participants
Between December 2016 and March 2020, we recruited 568 breast cancer survivors at a metropolitan cancer center in New York City. Participants were women older than 21 years, who had completed acute treatment (i.e., surgery, radiation, and chemotherapy) greater than 3 months before enrollment and had no sign of metastatic disease, recurrence, or other bulk diseases.
Variables and measures
Lymphatic pain
Lymphatic pain was defined as the co-occurrence of pain and swelling in the affected ipsilateral upper limb following breast cancer treatment. We operationalized lymphatic pain as the report of co-occurring pain/aching/soreness and arm/hand swelling in the affected ipsilateral upper limb or body.
Breast Cancer and Lymphedema Symptom Experience Index (BCLE-SEI) Part I of this reliable and valid self-report instrument was used to assess lymphatic pain (i.e., pain, aching, soreness, and arm/hand swelling) and additional symptoms related to lymph fluid accumulation or lymphedema.6,19–21 Patients were asked to report whether they experienced pain, aching, soreness, and arm/hand swelling in the affected ipsilateral upper limb during the past 3 months. We used a response frame of the past 3 months to ensure the chronic nature of lymphatic pain, that is, the symptoms had persisted for ≥3 months.11 Each item was rated on a 5-point Likert scale (i.e., 0 = no presence of a given symptom to 4 = greatest severity of a given symptom). For this study, four symptom phenotype groups were created: no symptom (i.e., absence of pain, aching, soreness, and arm/hand swelling), only swelling (i.e., only arm/hand swelling without pain, aching, or soreness), only pain (i.e., only pain, aching, or soreness without arm/hand swelling), and lymphatic pain (i.e., presence of pain, aching, or soreness, and arm/hand swelling).
Activities of daily living
Breast Cancer and Lymphedema Symptom Experience Index (BCLE-SEI) Part II of this reliable and valid self-reported instrument was used to evaluate symptom distress (i.e., the negative impact and suffering evoked by an individual's experience of symptoms related to lymph fluid accumulation or lymphedema).19,20,22 For this study, the subscale of ADLs was used.19,20,22 This subscale consists of thirteen items that assess self-reported difficulty in performing 13 ADLs, including cooking, using a knife, writing, cleaning the house, vacuuming, laundry, bathing, caring for kids, lifting, yard work, dressing, driving, and making the bed. Each item was rated on a 5-point Likert scale (i.e., 0 = no difficulty, 1 = a little, 2 = somewhat, 3 = quite a bit, and 4 = a lot). Patients were asked to indicate if a particular activity did not apply to them (e.g., if a patient did not have children, the item about caring for kids did not apply). Cronbach's alpha for the ADLs subscale in this sample was 0.94. In this study, we calculated the number of activities impaired divided by the number of activities performed. This value represented proportion of ADLs that were impaired for each patient.
Limb volume
Limb volumes were measured using an infra-red perometer (Perometry 350S). Interlimb volume difference was calculated using the following formula: percentage increase in limb volume = (affected frustum limb volume – contralateral frustum limb volume) × 100/contralateral frustum limb volume.23 While the widely accepted diagnostic criterion for breast cancer-related lymphedema is an interlimb volume difference of >10%, it is known that a 5% difference in interlimb volume causes symptoms8,9 and impairments in ADLs.22 Therefore, we categorized interlimb volume differences as >5% and >10% in the affected ipsilateral limb compared to the nonaffected contralateral limb.
Anthropometric measurements of height and BMI
Height was measured to the nearest 0.1 cm with a portable stadiometer without shoes.6,8,9,21 A bioimpedance device (InBody 520, Biospace Co., Ltd.) was used to measure weight and the device automatically calculated BMI.6,8,9,21
Lymphedema diagnosis
Two criteria were used to define lymphedema diagnosis: (1) patients self-reported of being diagnosed with and treated for lymphedema and (2) medical record review to confirm that patients had an existing medical diagnosis of and treatment for lymphedema following breast cancer treatment.
Demographic and clinical data
Demographic data included the following: age, education, marital status, employment status, and ethnicity. Financial status was assessed by asking participants to choose one of the three responses regarding their household income (i.e., comfortable finances = have more than enough to make ends meet; adequate finances = have enough to make ends meet; and financial hardship = do not have enough to make ends meet). Medical records were reviewed to obtain information on breast cancer diagnosis, stage of the disease, cancer location, types of surgeries, lymph node procedure, and type of adjuvant therapy (radiation, chemotherapy, and hormonal therapy).
Data analysis
Data were analyzed using SPSS (version 27) and R version 3.6.2. Differences in demographic and clinical characteristics among the four symptom phenotype groups (i.e., lymphatic pain, only pain, only swelling, and no symptom) were evaluated using parametric and nonparametric tests.
A generalized linear model with a logit link was used to identify predictors of lymphatic pain. Characteristics included in the regression analysis were based on known risk factors for lymphedema and chronic cancer pain, as well as characteristics that were different among the four symptom phenotype groups.24,25 These potential predictors included known clinical risk factors for lymphedema and chronic pain (i.e., types of cancer surgeries, radiation, axillary lymph nodes dissection, time since breast cancer diagnosis, and lymphedema diagnosis) and demographic factors (i.e., age, BMI, and financial status).24,25
To examine the relationships among the different symptom phenotype groups and degrees of impairments in ADLs, we used a general linear model that compared the mean proportion of ADLs impaired among the four symptom phenotype groups. Pairwise comparisons using Benjamini-Hochberg adjusted p-values were conducted to compare mean scores of ADL impairments among the four symptom phenotype groups.26 Finally, odds ratios (ORs) were calculated to examine the odds of impairment in each ADL between the lymphatic pain and the only pain groups. For these tests, the reported p-values were also adjusted using the Benjamini-Hochberg approach.
Results
Characteristics of participants
Of the 568 women who completed the study, their mean age was 56.8 years (standard deviation [SD] = 11.3; range of 26–85 years); 73.2% had a bachelors' or professional degree; 58.6% were married; and 64.8% were employed. Among the participants, 47.2% had lumpectomy and 52.8% mastectomy; 70.7% received adjuvant chemotherapy; and 70.1% had radiation (Table 1). Significant differences were found between symptom phenotype groups in financial hardship (Pearson's x2 = 28.8, p < 0.001), types of surgeries (Pearson's x2 = 18.8, p < 0.001), lymph node procedures (Pearson's x2 = 20.4, p < 0.001), receipt of radiation (Pearson's x2 = 10, p = 0.018), and presence of a lymphedema diagnosis (Pearson's x2 = 149.9, p < 0.001). Posthoc chi-square residual tests with Bonferroni corrected p-values demonstrated that participants in the lymphatic pain group were more likely to have financial hardship (p < 0.001). Participants in the only pain group were more likely to have a lumpectomy than mastectomy (p = 0.027), while participants in the only swelling group were more likely to have mastectomy than lumpectomy (p = 0.035). Participants in the no symptom group were less likely to have the receipt of radiation (p = 0.041).
Table 1.
Differences in Demographic and Clinical Characteristics Among the Four Symptom Groups
| Variables | All sample (N = 568) | No symptom (n = 142) | Only swelling (n = 34) | Only pain (n = 204) | Lymphatic pain (n = 188) | p |
|---|---|---|---|---|---|---|
| Age, mean ± SD (range) | 58.2 ± 11.3 (26–85) | 59.2 ± 11.3 (32–82) | 63.8 ± 10.3 (36–82) | 58.0 ± 12.0 (28–81) | 56.4 ± 10.4 (26–85) | 0.003* |
| BMI, mean ± SD (range) | 26.8 ± 5.9 (16.0–58.6) | 26.5 ± 5.4 (18.4–46.6) | 26.4 ± 5.3 (20.1–38.8) | 26.0 ± 6.0 (16.0–58.6) | 30.0 ± 6.1 (17.7–48.4) | 0.010* |
| Years since cancer treatment, mean ± SD (range) | 5.3 ± 5.2 (0.1–31.8) | 4.9 ± 5.0 (0.2–27.4) | 8.2 ± 7.5 (0.8–31.8) | 4.8 ± 4.9 (0.1–24.2) | 5.6 ± 5.2 (0.3–28.3) | 0.003* |
| Education, n (%) | ||||||
| Associate Degree or less | 152 (26.8) | 26 (18.3) | 9 (26.5) | 57 (27.9) | 60 (31.9) | 0.184 |
| Bachelors' Degree | 216 (38.0) | 59 (41.5) | 12 (35.3) | 72 (35.3) | 71 (37.8) | |
| Graduate/professional degree | 200 (35.2) | 57 (40.1) | 13 (38.2) | 73 (35.8) | 57 (30.3) | |
| Marital status, n (%) | ||||||
| Married or Partnered | 333 (58.6) | 85 (59.9) | 17 (50.0) | 125 (61.3) | 106 (56.4) | 0.891 |
| Divorced | 82 (14.4) | 23 (16.2) | 5 (14.7) | 24 (11.8) | 30 (16.0) | |
| Widowed | 37 (6.5) | 9 (6.3) | 3 (8.8) | 14 (6.9) | 11 (5.9) | |
| Single | 116 (20.4) | 25 (17.6) | 9 (26.5) | 41 (20.1) | 41 (21.8) | |
| Employment, n (%) | ||||||
| Employed | 364 (64.1) | 95 (66.9) | 15 (44.1) | 131 (64.2) | 123 (65.4) | 0.080 |
| Not employed | 204 (35.9) | 47 (33.1) | 19 (55.9) | 73 (35.8) | 65 (34.6) | |
| Ethinicity, n (%) | ||||||
| Non-Hispanic White | 404 (71.1) | 110 (77.5) | 24 (70.6) | 148 (72.5) | 122 (65.2) | 0.119 |
| Non-Hispanic Black/African American | 49 (8.6) | 11 (7.7) | 3 (8.8) | 11 (5.4) | 24 (12.8) | |
| Hispanic/Latino | 49 (8.6) | 8 (5.6) | 1 (2.9) | 21 (10.3) | 19 (10.2) | |
| Asian | 44 (7.7) | 12 (8.5) | 4 (11.8) | 15 (7.4) | 13 (7.0) | |
| Mixed or Other Ethnicity | 21 (3.7) | 1 (0.7) | 2 (5.9) | 9 (4.4) | 9 (4.8) | |
| Financial status, n (%) | ||||||
| Comfortable finances: Have more than enough to make ends meet | 348 (61.3) | 96 (67.6) | 25 (73.5) | 127 (62.3) | 100 (53.2) | <0.001* |
| Adequate finances: Have enough to make ends meet | 177 (31.2) | 42 (29.6) | 8 (23.5) | 68 (33.3) | 59 (31.4) | |
| Financial hardship: Do not have enough to make ends meet | 37 (6.5) | 2 (1.4) | 1 (2.9) | 8 (3.9) | 26 (13.8) | |
| No response | 6 (1.1) | 2 (1.4) | 0 (0.0) | 1 (0.5) | 3 (1.6) | |
| Surgery, n (%) | ||||||
| Lumpectomy | 268 (47.2) | 73 (51.4) | 8 (23.5) | 113 (55.4) | 74 (39.4) | <0.001* |
| Mastectomy | 300 (52.8) | 69 (48.6) | 26 (76.5) | 91 (44.6) | 114 (60.6) | |
| Lymph nodes procedure, n (%) | ||||||
| Sentinel lymph nodes biopsy | 232 (40.8) | 76 (53.5) | 6 (17.6) | 102 (50) | 48 (25.5) | <0.001* |
| Axillary lymph nodes dissection | 69 (12.1) | 9 (6.3) | 7 (20.6) | 22 (10.8) | 31 (16.5) | |
| Sentinel plus axillary lymph nodes dissection | 266 (46.8) | 57 (40.1) | 21 (61.8) | 79 (38.7) | 109 (58.0) | |
| Radiation, n (%) | ||||||
| Yes | 408 (71.8) | 89 (62.7) | 26 (76.5) | 146 (71.6) | 147 (78.2) | 0.010* |
| No | 160 (28.2) | 53 (37.3) | 8 (23.5) | 58 (28.4) | 41 (21.8) | |
Statistically significant test-statistic at p < 0.05. The test statistic is an ANOVA F statistic for continuous measures and Pearson's x2 for categorical measures.
ANOVA, analysis of variance; BMI, body mass index; SD, standard deviation.
Predictors of lymphatic pain
Among the 568 participants, 33.1% experienced lymphatic pain, while 35.9% women had only pain, 5.9% had only swelling, and 25% had no symptom (i.e., neither pain nor swelling). As shown in Table 2, significant predictors of lymphatic pain included the following: a lymphedema diagnosis (OR = 9.68, p < 0.001, 95% CI = [5.78–16.63]), financial hardship (OR = 4.64, p = 0.001, 95% CI = [1.99–11.32]), higher BMI (OR = 1.07, p < 0.001, 95% CI = [1.03–1.11]), and younger age (OR = 0.97, p = 0.011, 95% CI = [0.96–0.99]).
Table 2.
Predictors of Lymphatic Pain
| Predictors | Estimate | SE | z | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| (Intercept)a | −1.92 | 0.34 | −5.71 | 0.15 | 0.08–0.28 | <0.001 |
| Years since breast cancer diagnosis | −0.03 | 0.02 | −1.23 | 0.97 | 0.93–1.02 | 0.219 |
| BMI (kg/m2) | 0.06 | 0.02 | 3.61 | 1.07 | 1.03–1.11 | <0.001 |
| Age (years) | −0.03 | 0.01 | −2.56 | 0.97 | 0.96–0.99 | 0.011 |
| Comfortable finance: have more than enough to make ends meet | −0.10 | 0.23 | −0.44 | 0.90 | 0.58–1.43 | 0.662 |
| Financial hardship: do not have enough to make ends meet | 1.53 | 0.44 | 3.48 | 4.64 | 1.99–11.32 | 0.001 |
| Lymphedema diagnosis | 2.27 | 0.27 | 8.45 | 9.68 | 5.78–16.63 | <0.001 |
| Mastectomy | 0.26 | 0.25 | 1.05 | 1.30 | 0.80–2.10 | 0.292 |
| Radiation therapy | 0.53 | 0.27 | 1.94 | 1.69 | 1.00–2.90 | 0.052 |
| Axillary lymph nodes dissection | 0.19 | 0.31 | 0.61 | 1.21 | 0.65–2.23 | 0.544 |
| Observations | 568 | |||||
| R2 Tjur | 0.263 |
Intercept reflects the odds ratio for the reference group, including people who have enough to make ends meet (moderate income level), people who had lumpectomy (vs. mastectomy), those who had no radiation and no axillary lymph node dissection, and who were of mean age, BMI, and number of years since breast cancer diagnosis.
Lymphatic pain, limb volume, and lymphedema diagnosis
As Table 3 demonstrates, more patients in lymphatic pain (41.0%) and only swelling (64.7%) groups had a limb volume difference of >5% compared to patients in the only pain (17.2%) and no symptom (11.3%) groups (p < 0.001). Patients in the lymphatic pain and only swelling groups were also more likely to have limb volume different >5% compared to the no symptom group (11.3%) (p = 0.002; p < 0.001). Similarly, more patients in lymphatic pain (24.5%) and only swelling (47.1%) groups had a limb volume difference of >10% compared to patients in the only pain (5.4%) and no symptom (3.5%) groups (p < 0.001 for all comparisons, except lymphatic pain to only swelling, p = 0.001). Furthermore, significantly more patients (24.5%) in the lymphatic pain group had a limb volume difference of >10% compared to patients (5.4%) in the only pain group (p < 0.001). Patients in the lymphatic pain group and only swelling group were more likely to have a diagnosis of lymphedema (p < 0.001). Compared to patients with only pain (4.9%), significantly more (46.8%) patients in the lymphatic pain group had a diagnosis of lymphedema (p < 0.001).
Table 3.
Pairwise Differences in Limb Volumes and Lymphedema Diagnosis Among the Four Symptom Groups
| Variables | All sample; N = 568, n (%) | No symptom (1); n = 142, n (%) | Only swelling (2); n = 34, n (%) | Only pain (3); n = 204, n (%) | Lymphatic pain (4); n = 188, n (%) | Fisher's exact p-value; with B-Ha adjustment |
|---|---|---|---|---|---|---|
| Lymphedema diagnosis | 1 < 2; p- < 0.001*; 1:3; p = 1.00; 1 < 4; p < 0.001*; 2 > 3; p < 0.001*; 2 > 4; p = 0.427; 3 < 4; p < 0.001* | |||||
| Yes | 124 (21.8) | 7 (4.9) | 19 (55.9) | 10 (4.9) | 88 (46.8) | |
| No | 444 (78.2) | 135 (95.1) | 15 (44.1) | 194 (95.1) | 100 (53.2) | |
| Interlimb volume >5% | 1 < 2; p < 0.001*; 1:3; p = 0.165; 1 < 4; p < 0.001*; 2 > 3; p < 0.001*; 2 > 4; p = 0.002*; 3 < 4; p < 0.001* | |||||
| Yes | 150 (26.4) | 16 (11.3) | 22 (64.7) | 35 (17.2) | 77 (41.0) | |
| No | 418 (73.6) | 126 (88.7) | 12 (35.3) | 169 (82.8) | 111 (59.0) | |
| Interlimb volume >10% | 1 < 2; p < 0.001*; 1:3; p = 0.604; 1 < 4; p < 0.001*; 2 > 3; p < 0.001*; 2 > 4; p = 0.001*; 3 < 4; p < 0.001* | |||||
| Yes | 78 (13.7) | 5 (3.5) | 16 (47.1) | 11 (5.4) | 46 (24.5) | |
| No | 490 (86.3) | 137 (96.5) | 18 (52.9) | 193 (94.6) | 142 (75.5) |
Benjamini-Hochberg adjustment to control for false discovery rate.
Denotes statistical significance at an alpha level of 0.05.
Impairments in ADLs
Differences in ADLs among the four symptom phenotype groups
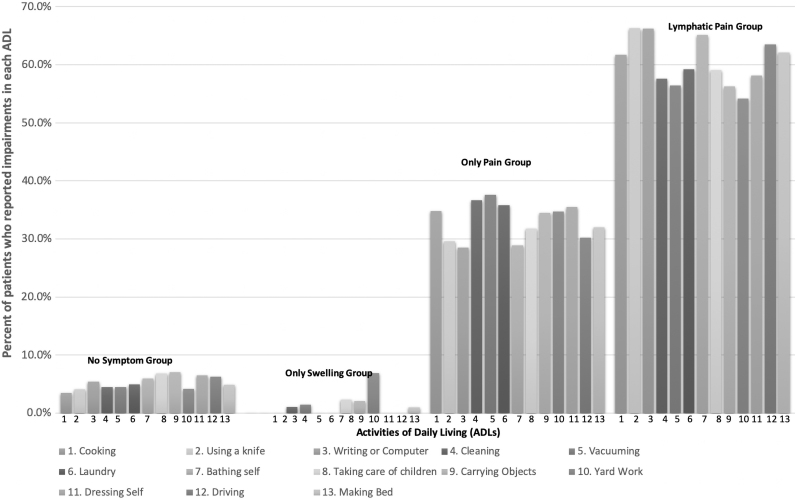
The mean degree of impairment in ADLs in each group is as follows: 0.06 (SD = 0.17) for no symptom group; 0.04 (SD = 0.09) for the only swelling group; 0.25 (SD = 0.30) for the only pain group; and 0.45 (SD = 0.36) for the lymphatic pain group. In other words, participants with lymphatic pain reported impairments in 45% of the ADLs, compared to 25% in the only pain group, 4% in the only swelling group, and 6% in the no symptom group. A general linear model that compared the proportion of impaired ADLs among the four symptom phenotype groups found significant differences among the groups, F(3,544) = 56.43, p < 0.001. Posthoc pairwise comparisons (with Benjamini-Hochberg adjusted p-values) demonstrated that patients who experienced only pain were impaired in more activities than those who experienced no symptom (p < 0.001) or only swelling (p < 0.001). No difference in proportion of ADLs impaired was found between patients in the no symptom and only swelling groups (p = 0.726). Patients who experienced lymphatic pain were significantly more impaired than patients in the other three groups (all p < 0.001). The linear model demonstrated that symptom phenotype alone explained about 23% of the between-person variance in the degree of impairment in ADLs (R2 = 0.233). Figure 1 shows the percent of each group that reported impairment in each ADL.
FIG. 1.
Percentage of patients who reported impairments in ADLs. ADLs, activities of daily living.
Odds of impairments in ADLs between the only pain and lymphatic pain groups
To better understand the magnitude of lymphatic pain on impairment in ADLs in comparison to only pain, ORs were calculated for each ADL that was evaluated. As shown in Table 4, the lymphatic pain group had significantly higher odds of being impaired in 12 of the 13 activities of ADLs (i.e., cooking, using a knife, writing, cleaning, vacuuming, laundry, bathing self, caring for children, carrying objects, dressing oneself, driving, and making a bed). Yardwork was the only activity that did not differ between the only pain and lymphatic pain groups.
Table 4.
Odds Ratios for Impairments in Each Activity of Daily Living in the Lymphatic Pain Group Compared to the Only Pain Group
| ADLs | OR | 95% CI | B-H adjusted p-valuea |
|---|---|---|---|
| Cooking | 2.42 | 1.52–3.85 | <0.001 |
| Using a knife | 3.12 | 1.91–5.21 | <0.001 |
| Writing, typing, or using a computer | 3.71 | 2.35–5.96 | <0.001 |
| Cleaning | 2.45 | 1.61–3.76 | <0.001 |
| Vacuuming | 2.03 | 1.28–3.23 | 0.003 |
| Laundry | 2.20 | 1.39–3.47 | <0.001 |
| Bathing self | 2.96 | 1.75–5.14 | <0.001 |
| Taking care of children | 3.18 | 1.52–6.90 | 0.003 |
| Carrying objects | 3.87 | 2.45–6.22 | <0.001 |
| Yard work | 2.53 | 1.35–4.84 | 0.004 |
| Dressing self | 2.07 | 1.27–3.42 | 0.004 |
| Driving | 3.29 | 1.80–6.20 | <0.001 |
| Making bed | 2.59 | 1.60–4.25 | <0.001 |
ns, Nonsignificant posthoc pairwise test based on adjusted p-values and an alpha level of 0.05.
Benjamini-Hochberg adjusted p-values to control for pairwise comparisons. ADLs, activities of daily living; CI, confidence interval.
Discussion
This study is the first to report that in a large sample of breast cancer survivors, 33.1% experienced lymphatic pain and that lymphatic pain was associated with significant impairments in ADLs. These results suggest that our conceptualization of lymphatic pain may be a clinically useful approach to identify breast cancer survivors who are experiencing significant impairments in ADLs. It is not surprising that a diagnosis of lymphedema was associated with a 9.68 increase in the odds of being in lymphatic pain group. More importantly, lymphatic pain was associated with >5% interlimb volume differences, which suggests early stage of lymphedema. Findings from this study demonstrate that lymphatic pain and lymphedema have similar predictors, including younger age27 and higher BMI.12–14 These findings suggest that one component of lymphatic pain may be related to increased fluid accumulation and/or associated inflammatory responses. The findings are essential to lay the foundation for examining the underlying mechanisms of lymphatic pain that may lead to a cure.
It should be noted that, compared to the only pain group, 41% of patients in the lymphatic pain group had interlimb volume differences of >5%. In addition, about 60% of the patients with lymphatic pain had impairments in ADLs. Our findings suggest that a >5% increase in interlimb volume needs to be considered a diagnostic criterion for an early lymphedema diagnosis because of its debilitating effects on ADLs and QOL.8,9,22,28 In addition, our findings support the hypothesis that symptoms of lymphedema (e.g., lymphatic pain, swelling, and heaviness) often precede changes in limb volume and a lymphedema diagnosis.29 Taken together, the results of our study suggest that self-reported lymphatic pain is an important marker of early lymphedema.
Patients with financial hardship were 4.64 times more likely to report lymphatic pain. This finding is noteworthy because, while the occurrence of lymphedema and chronic pain is influenced by a variety of social determinants of health,30,31 our study provides new evidence for an association with lymphatic pain. Women with lymphatic pain may face additional demands and have few resources to assist with ADLs, which may exacerbate both lymphatic pain and functional impairments. In addition, as noted in previous studies,32,33 50% of breast cancer survivors reported that lymphedema had a negative impact on their work or job, which may contribute to additional financial hardships.32,33
While chronic cancer pain and lymphedema are known to lead to decreases in physical function,5,24,25 findings of our study detailed that patients with lymphatic pain reported impairments in 45% of daily activities that were evaluated. In addition, patients with lymphatic pain had a significant increase in the odds of having difficulty performing 12 of the 13 ADLs that were evaluated compared to patients with only pain. This finding extends recent work on the effects of increased limb volumes on performance of ADLs.22
Limitations and strength of the study
Limitations of the study included its cross-sectional design that prevented an evaluation of changes in lymphatic pain over time. As ADLs may be influenced by other chronic illnesses, future research should include comorbilities as a covariate. A strength of the study includes the use of a valid and reliable instrument to evaluate symptoms associated with lymph fluid accumulation, which allowed for the creation of the symptom phenotype groups. Because previous research found that traditional measures of ADLs (i.e., toileting, ambulation, continence, and feeding) were less relevant to breast cancer survivors,2,23–25,27,33 the breast cancer-specific measures of ADLs is another strength.2,23
Conclusion
A substantial portion of breast cancer surivors (33.1%) reported lymphatic pain. Given that lymphatic pain was associated with an objective increase in limb volume, it is possible that lymphatic pain may have different underlying mechanisms from the only pain phenotype. Future research is needed to test the hypothesis that lymphatic pain has unique underlying mechanisms. Importantly, lymphatic pain results in greater impairments in ADLs. Studies are needed to evaluate the efficacy of current prevention and treatment strategies for lymphedema to decrease lymphatic pain and to improve functional impairments.
Acknowledgments
We thank Ms. Alejandra Yancey for helping manage the study and data collection. We thank all the patients who participated in the study. We thank nurses, physicians, and staff at NYU Perlmutter Cancer Center for their support for the study.
Authors' Contributions
Conception and design: M.R.F., Y.W., and K.F.J. Administrative support: M.R.F., Y.W., D.A., and A.A.G. Provision of study material or patients: M.R.F., D.A., and A.A.G. Collection and assembly of data: M.R.F., E.K., and S.Y. Data analysis and interpretation: K.F.J., M.R.F., and M.L.M. Article drafting: K.F.J., M.R.F., and M.L.M. Article revision and editing: all authors. Final approval of article: all authors.
Disclaimer
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by the National Institute of Health/National Science Foundation/National Cancer Institute (1R01CA214085-01) with M.R.F. and Y.W. as the multiple principal investigators. This study was also supported by a research grant from Judges and Lawyers Breast Cancer Alert with M.R.F. as the principal investigator. Katie Fitzgerald Jones is 2021–2023 Jonas Scholar and supported by the National Institute of Nursing Research Ruth L. Kirschstein National Research Service Award (F31NR019929) as a predoctoral fellow with Dr. Mei R Fu and Dr. Lisa Wood as her sponsors.
References
- 1. Burckhardt CS, Jones KD. Effects of chronic widespread pain on the health status and quality of life of women after breast cancer surgery. Health Qual Life Outcomes 2005; 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fu MR, Rosedale M. Breast cancer survivors' experiences of lymphedema-related symptoms. J Pain Symp Manage 2009; 38:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu MR, Ridner SH, Hu SH, Stewart BR, Cormier JN, Armer JM. Psychosocial impact of lymphedema: A systematic review of literature from 2004 to 2011. Psychooncology 2013; 22:1466–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breast Cancer Facts & Figures 2019–2020. Atlanta: American Cancer Society https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf Accessed June 18, 2020.
- 5. Bao T, Seidman A, Li Q, et al. Living with chronic pain: Perceptions of breast cancer survivors. Breast Cancer Res Treat 2018; 169:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fu MR, Axelrod D, Cleland CM, et al. Symptom reporting in detecting breast cancer-related lymphedema. Breast Cancer (Dove Med Press) 2015; 7:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stanton AW, Modi S, Mellor RH, Levick, JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: Lymphatic pump failure and predisposing factors. Lymphatic Res Biol 2009; 7:29–45. [DOI] [PubMed] [Google Scholar]
- 8. Fu MR, Aouizerat BE, Yu G, et al. Model-based patterns of lymphedema symptomology: Phenotypic and biomarker characterization. Curr Breast Cancer Rep 2020; 13:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fu MR, Conley YP, Axelrod D, et al. Precision assessment of heterogeneity of lymphedema phenotype, genotypes and risk prediction. Breast 2016; 29:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang K, Yee C, Tam S, et al. Prevalence of pain in patients with breast cancer post-treatment: A systematic review. Breast 2018; 42:113–127. [DOI] [PubMed] [Google Scholar]
- 11. Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016; 34:3325–3345. [DOI] [PubMed] [Google Scholar]
- 12. Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GKD, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: A meta-analysis of treatment factors. Ann Surg Oncol 2009; 16:1959–1972. [DOI] [PubMed] [Google Scholar]
- 13. Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev 2007; 16:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mak SS, Yeo W, Lee YM et al. Predictors of lymphedema in patients with breast cancer undergoing axillary lymph node dissection in Hong Kong. Nurs Res 2008; 57:416–425. [DOI] [PubMed] [Google Scholar]
- 15. Sackey H, Johansson H, Sandelin K, et al. Self-perceived, but not objective lymphoedema is associated with decreased long-term health-related quality of life after breast cancer surgery. Eur J Surg Oncol 2015; 41:577–584. [DOI] [PubMed] [Google Scholar]
- 16. Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res 2003; 52:370–379. [DOI] [PubMed] [Google Scholar]
- 17. Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016; 34:3325–3345. [DOI] [PubMed] [Google Scholar]
- 18. Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain 2019; 20:146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu MR, Axelrod D, Guth AA, et al. mHealth self-care interventions: Managing symptoms following breast cancer treatment. mHealth 2016; 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi S, Lu Q, Fu MR, et al. Psychometric properties of the breast cancer and lymphedema symptom experience index: The Chinese version. Eur J Oncol Nurs 2016; 20:10–16. [DOI] [PubMed] [Google Scholar]
- 21. Fu MR, Chen C, Haber J, Guth A, Axelrod D. The effect of providing information about lymphedema on the cognitive and symptom outcomes of breast cancer survivors. Ann Surg Oncol 2010; 17:1847–1853. [DOI] [PubMed] [Google Scholar]
- 22. Park JH, Merriman J, Brody A, et al. Limb volume changes and activities of daily living: A prospective study. Lymphat Res Biol 2020; 19:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol 2005; 3:208–217. [DOI] [PubMed] [Google Scholar]
- 24. Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst 2006; 98:521–529. [DOI] [PubMed] [Google Scholar]
- 25. O'Toole JA, Ferguson CM, Swaroop MN, et al. The impact of breast cancer-related lymphedema on the ability to perform upper extremity activities of daily living. Breast Cancer Res Treat 2015; 150:381–388. [DOI] [PubMed] [Google Scholar]
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiplt testing. J R Stat Soc Series B 1995; 57:289–300. [Google Scholar]
- 27. Armer JM, Fu MR. Age differences in post-breast lymphedema signs and symptoms. Cancer Nurs 2005; 28:200–207. [DOI] [PubMed] [Google Scholar]
- 28. Cormier JN, Xing Y, Zaniletti I, Askew RL, Stewart BR, Armer JM. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology 2009; 42:161–175. [PMC free article] [PubMed] [Google Scholar]
- 29. Gençay Can A, Ekşioğlu E, Çakçı FA. Early detection and treatment of subclinical lymphedema in patients with breast cancer. Lymphat Res Biol 2019; 17:368–373. [DOI] [PubMed] [Google Scholar]
- 30. Jiang C, Wang H, Wang Q, Luo Y, Sidlow R, Han X. Prevalence of chronic pain and high-impact chronic pain in cancer survivors in the United States. JAMA Oncol 2019; 5:1224–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dean LT, Kumar A, Kim T, et al. Race or resource? BMI, race, and other social factors as risk factors for interlimb differences among overweight breast cancer survivors with lymphedema. J Obes 2016; 2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vignes S, Fau-Prudhomot P, Simon L, Sanchez-Bréchot M-L, Arrault M, Locher F. Impact of breast cancer–related lymphedema on working women. Support Care Cancer 2020; 28:79–85. [DOI] [PubMed] [Google Scholar]
- 33. Fu MR. Women at work with breast cancer-related lympheodema. J Lymphedema 2008; 3:30–36. [PMC free article] [PubMed] [Google Scholar]