Abstract
Secondary lymphedema of the extremities affects millions of people in the world as a common side effect of oncological treatments with heavy impact on every day life of patients and on the health care system. One of the surgical techniques for lymphedema treatment is the creation of a local connection between lymphatic vessels and veins, facilitating drainage of lymphatic fluid into the circulatory system. Successful results, however, rely on using a functional vessel for the anastomosis, and vessel function, in turn, depends on its structure. The structure of lymphatic collecting vessels changes with the progression of lymphedema. They appear initially dilated by excess interstitial fluid entered at capillary level. The number of lymphatic smooth muscle cells in their media then increases in the attempt to overcome the impaired drainage. When lymphatic muscle cells hyperplasia occurs at the expenses of the lumen, vessel patency decreases hampering lymph flow. Finally, collagen fiber accumulation leads to complete occlusion of the lumen rendering the vessel unfit to conduct lymph. Different types of vessels may coexist in the same patient but usually the distal part of the limb contains less affected vessels that are more likely to perform efficient lymphatic–venular anastomosis. Here we review the structure of the lymphatic collecting vessels in health and in lymphedema, focusing on the histopathological changes of the lymphatic vessel wall based on the observations on segments of the vessels used for lymphatic–venular anastomoses.
Keywords: lymphedema, lymphatic vessels, lymphatic–venular anastomosis, LVA
Introduction
Lymphatic vessels, due to the difficulty of identifying them in common hematoxylin–eosin stained sections, have long been neglected. New interest in this field started three decades ago with the discovery of two specific growth factors, vascular endothelial growth factors C and D and of their receptor, namely VEGFR3, and of specific markers in particular Prox1, LYVE1, and D2-40 that allowed expansion of knowledge in lymphatic vessels.1,2
Since then, the lymphatic system in health and disease has become a hot topic in vascular research. The main pathology affecting the lymphatic system is lymphedema, a chronic, progressive, and invalidating condition that affects millions of people in the world, most commonly as the consequence of oncological treatments involving lymph node removal and/or radiotherapy. The interruption of physiological lymph flow causes the accumulation of a protein-rich interstitial fluid that in turn triggers inflammation, fat deposition, and fibrosis.3 If collateral lymphatics do not compensate for the injury, these pathological changes worsen fluid accumulation, which further impairs lymphatic function in a positive feedback loop.
Studies on the inflammatory nature of lymphedema in mouse models have implicated CD4+ T cells in the inflammatory responses leading to lymphedema.4,5 Similar changes were seen in human specimens collected from patients with breast cancer-related lymphedema, where the number of tissue-infiltrating CD4+ T cells was positively correlated with disease severity.6
The traditional therapeutic approach consists in manual drainage and constant use of compressive garments. In those patients who no longer benefit from decongestive therapy, the surgical approach may be of use in reducing the edema and avoiding its progression.7 Among the various surgical treatments now available, lymphatic–venular anastomoses (LVA) represents a natural way of draining lymph into the circulatory system.8 During this mini-invasive surgery, it is easy to obtain a short segment of the lymphatic collecting vessel chosen for the anastomosis to analyze its structure providing precious insights into the pathophysiological modifications that occur in lymphedema.
Organization of the Lymphatic Vessel Tree
The lymphatic vasculature starts with blind-ended initial lymphatics in the connective tissue surrounding blood vessels.9,10 Initial lymphatics have a thin wall composed only of endothelial cells laying on a discontinuous basement membrane and connected to the extracellular matrix, by anchoring filaments made of fibrillin.11,12 Stress within the interstitium creates radial tension on the anchoring filaments, locally increasing the luminal volume of the lymphatic capillary.13 This creates a slight and temporary pressure difference, driving interstitial fluid into the lymphatic capillary. Initial lymphatic endothelial cells have a typical oak-leaf shape with overlapping flaps sealed on their borders by “buttons.”14
Fluid entry seems to occur in the junction-free region at the tip of the flap that may open and close to allow fluid entrance without disrupting junctional integrity. Initial lymphatic vessels merge into precollectors, sparsely wrapped by lymphatic muscle cells15,16 and precollectors drain into collecting vessels that have a continuous muscular coat and are divided by unidirectional valves into pumping units called “lymphangions.”9,10,17 In addition to their intrinsic contractility, lymph collectors respond to mechanical stimuli in the surrounding environment: arterial pulsations, skeletal muscle contraction, and respiratory movements form an “external pump” that contributes to lymph progression.3
After the filter of regional lymph nodes, where microorganisms and antigens enter in contact with immunocompetent cells for immune surveillance, lymph reaches the major lymphatic trunks that will eventually drain into the large veins at the base of the neck. Interstitial fluid, therefore, ultimately returns to blood. The presence of lymph nodes along lymphatic routes provides the lymphatic vasculature with a critical role in adaptive immune responses and removal of substances that might cause harmful reactions. Lymph carries immune cells, such as dendritic cells, that have engulfed and processed antigens for immune presentation in the lymph node, along with lymphocytes.18
In addition to its roles in the maintenance of interstitial fluid homeostasis and immune surveillance, the lymphatic system plays a critical role in regulating transport of dietary lipids to the blood circulation.10 Recent study in the past two decades has identified important new roles of lymphatics, associating defects in lymphatic vasculature with obesity, cardiovascular diseases, neurological disorders, and Crohn's disease.19
Lymphedema
Lymphedema consists in an abnormal accumulation of interstitial fluid and macromolecules due to malformations of the lymphatic system (primary lymphedema) or to external causes disrupting a previously normal lymphatic system (secondary lymphedema). Primary lymphedema is a rare condition mainly affecting the lower limbs. The genetic mutations causing primary lymphedema are beyond the scope of this review.20 Secondary lymphedema may be the consequence of trauma, infections, and, in tropical countries, filariasis.
Its most common cause in industrialized countries is, however, surgical ablation of lymph nodes in oncological therapy, particularly when followed by radiotherapy. Obesity increases the risk of developing secondary lymphedema.21 Experimentally induced obesity impairs lymphatic function by increasing perilymphatic inflammation and altering lymphatic endothelial cells gene expression.
Once lymphedema is established, the accumulation of protein-rich interstitial fluid causes chronic inflammation, adipose tissue hypertrophy, and eventually fibrosis.3,22 Protein-rich interstitial fluid constitutes a perfect growth media for bacterial proliferation, predisposing the subject to infections that, in turn, aggravate the edema and trigger fibrosis. It is well known that under an inflammatory environment, lymphatic vessels undergo pronounced enlargement and display increased leakiness. Drainage performance is influenced by various signals, including VEGF-C/VEGFR-3 signaling as well as inflammatory mediators such as tumor necrosis factor-α, interleukin-1β, nitric oxide, histamine, and prostaglandins.22
Mast cells, which are in proximity of lymphatic vessels, synthesize, store, and release various inflammatory and vasoactive mediators essential for the regulation of lymphatic vascular functions. In response to inflammatory signals, activation of mast cells can trigger adaptive immune responses by dendritic cell-directed T cell activation and, through the secretion of various mediators, influence lymphatic permeability, contractility, as well as perilymphatic inflammatory cell accumulation.23
The edema is initially soft and pitting but, with time, collagen fiber deposition causes induration and pitting is no longer present.8,24 The affected limb enlargement causes disfigurement with heavy consequences on every day's life in terms of psychological impact, functional impairment, and invalidity. Patients need constant decongestive lymphatic therapy including manual drainage, compressive garments, exercise, and meticulous skin care. Surgical therapeutic approaches include excisional procedures aimed at removal of excessive tissue and fat and physiological procedures (lymph node transfer and LVA) that promote lymph drainage.25
Although excisional procedures are beneficial, they may result in the destruction of remaining lymphatics and poor cosmetic results. Vascularized lymph node transfer consists in transferring lymph nodes from an unaffected donor site to the affected limb. The transferred lymph nodes may act as a sponge or a pump to absorb the lymphatic fluid or stimulate the formation of new lymphatic channels by secreting growth factors or they may simply bridge the gaps between functional lymphatic vessels.25,26
As early as in 1977, O'Brien et al.27 opened the way to LVA by creating anastomoses between lymphatic vessels and superficial large veins. These pioneer results were promising but compression of the vein by interstitial fluid sometimes caused increased hydrostatic pressure in the vein and blood retrograde flow into the lymphatic vessel with thrombosis and obstruction of the anastomosis. Since there may be a lower pressure in smaller venules than in larger veins, using small venules as recipient vessels decreases the risk of occlusion of the anastomosis due to venous backflow.24
LVA are performed with a super-microsurgical minimally invasive technique under local anesthesia between lymphatic vessels with a diameter <0.8 mm and adjacent venules of similar dimensions.8,28,29 Since destruction of smooth muscle cells and occlusion of lymphatic vessels start at the proximal level of the limbs and progress to the distal end, the preferential site for LVA is the distal part of the extremities.30 The timing of the degeneration of smooth muscle cells in the wall of lymphatic vessels, however, may not correspond to the duration of edema and it is worth trying to perform anastomoses also in long duration edemas. Clinical improvement is often obtained even with a single anastomosis.
To perform LVA, it is necessary to obtain a detailed map of the lymphatic vessels and of the adjacent veins. For many years, lymphoscintigraphy has been the gold standard imaging technique in lymphedema.31 Radiation exposure, long examination time needed, and the possibility of life-threatening complications, such as pulmonary embolism, have limited its clinical applicability: indocyanine green fluorescence (ICG) lymphography is now preferred32 due to its safety, low cost, high resolution, and real-time imaging.33,34 ICG is intraoperatively injected subcutaneously in the interdigital spaces. It rapidly binds to plasma proteins and generates an infrared near fluorescence that allows accurate visualization of lymphatic vessels with the photo dynamic eye. The only disadvantage of ICG is that penetration of the near infrared rays is limited to 2 cm from the skin surface.33 This renders the technique unsuitable for follow-up control of the performed anastomosis in time unless the lymphatic collecting vessel used for the anastomosis was very superficial. Moreover, if the anastomosis was successful, as soon as the tracer reaches the vein, it is rapidly washed out, in other words the lymph course may be visualized only as far as the anastomosis. The ICG staging includes the linear pattern, more frequently found in early stages of lymphedema and dermal backflow typical of the more advanced stages.35 Biopsies of lymphatic vessels selected for LVA show no alterations of the wall in linear pattern, whereas endothelial disruption, loss of smooth muscle cells, and accumulation of collagen fibers are frequent in the more advanced patterns.
Magnetic resonance lymphangiography with the contrast agent gadolinium has recently emerged as a simple and minimally invasive method for assessing lymphatic disorders.36 It has high resolution and allows to evaluate the preservation of the lymphatic pump, a prerequisite to perform functional LVA.
Histology of Normal Collecting Vessels
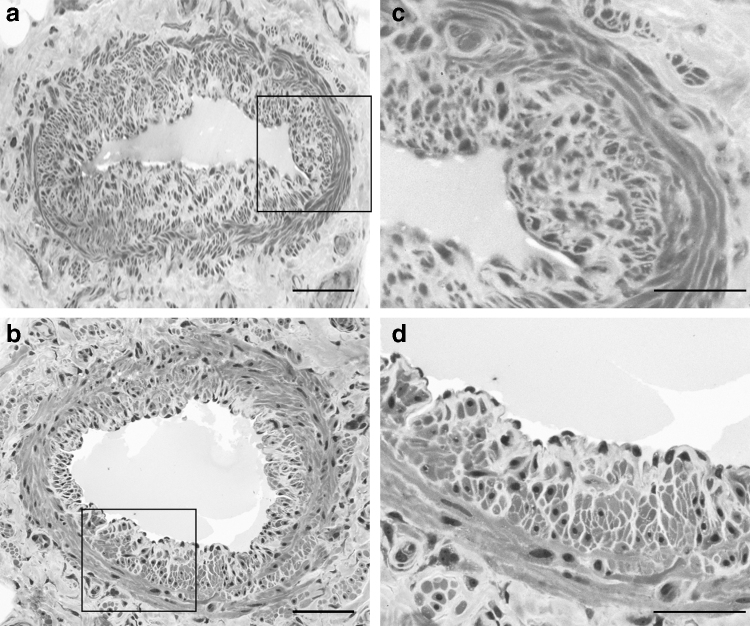
The wall of collecting vessels has three layers: intima, media, and adventitia similarly to that of blood vessels but with no distinct boundaries. The intima is made of a single layer of endothelial cells, and, unlike in the arteries, there is no internal elastic lamina. The media contains extracellular matrix with collagen and elastin fibers with varying proportions of smooth muscle cells.37 Lymphatic smooth muscle cells form two layers38: a thick inner layer with predominantly longitudinal orientation and a thin outer layer with predominant circular orientation (Fig. 1).
FIG. 1.
Semithin sections stained with toluidine blue of two superficial lymphatic collecting vessels of the human thigh (a, b); scale bar 100 μm. (c, d) Enlargements of the same vessels showing the two different layers of the media: the inner (i) longitudinal and the outer (o) circular layer. Scale bar 50 μm.
The functional importance of the lymphatic muscle layer is that it establishes both vessel tone and rapid phasic contractions that allow lymph progression.39 These rapid large-amplitude spontaneous contractions of collecting lymphatic vessels are driven by an intrinsic electrical pace-making activity.40 They play a crucial role in moving lymph uphill against gravity in the extremities. Contractions of the lymphangion muscle coat induces an increase in the local lymph pressure, closure of the upstream valve to prevent backflow, and opening of the downstream valve to allow ejection of lymph in the following lymphangion.17
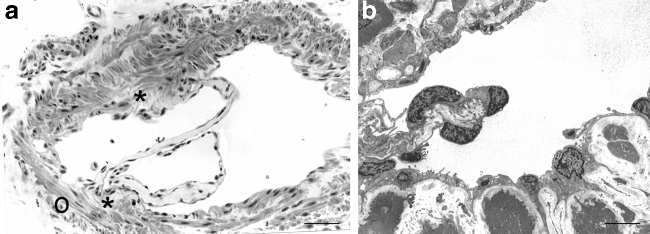
Valves are made of two leaflets with a thick buttress or base and a thinner free edge (Fig. 2). Each leaflet has a connective tissue core rich in elastic and collagen fibers covered in endothelium as previously described in precollectors.15 Valve regions are mostly free of lymphatic muscle cell coverage.41 Interestingly during development, the regions at which valves will form are also devoid of lymphatic muscle cells.42 Initiation of valve formation during development is marked by the appearance of a cluster of cells, often near vessel branch points, where shear stress is higher, that exhibit high levels of the transcription factors PROX1, FOXC2, and of the upstream regulator GATA2.43,44
FIG. 2.
Semithin section (a; scale bar 100 μm) and transmission electron microscopy (b) of a valve (scale bar 5 μm). The valve leaflets are made of a connective tissue core covered on both sides by endothelial cells. At the valve buttress (*), the inner muscular coat is disrupted whereas the outer (o) circular layer is still present.
These factors are also required for valve maintenance. The shear stress-activated ion channel PIEZO1 is a key regulator in valve formation: the process of protrusion in the valve leaflets, collective cell migration, actin polymerization, and remodeling of cell junctions was impaired in a mouse model lacking PIEZO1 in endothelial cells with the result of a dramatic reduction in the number of lymphatic valves.45 Mutations in PIEZO1 were identified in patients suffering from lymphedema associated with pleural effusions and ascites.46 Petrova et al.43 showed that Foxc-2 inactivation leads to agenesis of valves in lymphatic vessels in mice and to lymph reflux.
The same gene defect and defective valves, accompanied by abnormal recruitment of mural cells also to the valve regions, underlie the clinical manifestations of lymphedema distichiasis in humans. Several other molecules play an important role in valve formation, among them the connexin family proteins that, by forming gap junctions, allow collective behavior of cells.47 Connexin 47 mutations increased risk for secondary lymphedema after breast cancer treatment and support the hypothesis that genetic susceptibility is an important risk factor for developing secondary lymphedema.48
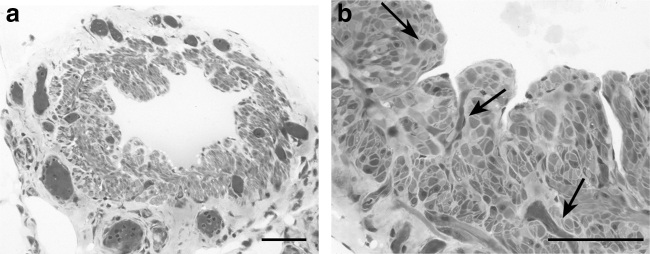
The fat-rich adventitia contains several blood microvessels (Fig. 3) that in the intervalvular portions, where the muscle coat is thicker, penetrate among the lymphatic muscle cells.37,38 Vasa vasorum sometimes extend even to the subendothelial space as shown in bovine mesenteric lymphatics49 and in superficial collecting lymphatics of human thigh.50
FIG. 3.
Semithin sections stained with toluidine blue. (a) Network of blood microvessels surrounding this lymphatic collecting vessel of the human thigh. Scale bar 100 μm. (b) Vasa vasorum (arrows) penetrate deep into the media of this vessel reaching as far as the subendothelial space. Scale bar 50 μm.
Although the relevance of arterial vasa vasorum in atherosclerosis has been recently reviewed51 and their density correlated with the formation of atheromatous plaques, it is yet unknown whether the rich supply in vasa vasorum of the lymphatic collecting vessel wall may influence their alterations in lymphedema. This interesting topic requires further studies.
Histology of Lymphatic Collecting Vessels in Lymphedema
The first study on the histological changes of lymphatic collecting vessels in lymphedema is that of Mihara et al.52 These authors studied segments of collecting vessels taken at time of LVA from patients affected by secondary lymphedema of the lower limb and classified lymphatic collecting vessels in four histological types of increasing gravity: normal, ectasis, contraction, and sclerosis. The first histological change, corresponding to the ectasis type, interestingly appears before the onset of lymphedema. Removal of draining lymph nodes in fact causes an increase in endolymphatic pressure accompanied by dilatation of the vessel lumen and thinning of the wall.
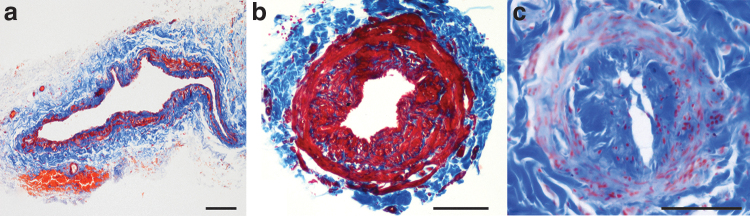
Endothelial cells flatten, smooth muscle cells change from the contractile to the synthetic state, and collagen fibers become thin and elongated. With the continuing increase in pressure inside them, the vessels change to the contraction type and some smooth muscle cells proliferate and migrate directly beneath the endothelium secreting collagen fibers. Contraction type vessels have a thickened wall and a narrowed lumen. The next step is the sclerosis type: cell junctions between endothelial cells become loose, lymph leaks outside, smooth muscle cells decrease in number, and collagen fibers increase. The lumen becomes markedly narrowed and eventually completely occluded (Fig. 4).
FIG. 4.
Masson's trichrome staining, red: lymphatic muscle cells, blue: collagen fibers. (a) Ectasis type vessel: the lumen is dilated and the wall is thin; (b) contraction type vessel: the lumen is restricted, and the wall is mainly made of lymphatic muscle cells stained red by Masson's trichrome; (c) sclerosis type vessel: the lumen is almost completely occluded, the inner longitudinal layer of the media is stained blue by Masson's trichrome, whereas the outer circular layer is stained in red. Scale bar 100 μm.
Ogata et al.53 analyzed smooth muscle cells in lymphatic vessels of lymphedematous legs evaluating the expression of α-smooth muscle actin (α-SMA) and of the smooth muscle myosin heavy chain (MyHC) isoforms SM1 and SM2. Compared with normal lymphatic vessels that have a thin wall with 1–3 layers of smooth muscle cells, the walls of lymphatic vessels from lymphedematous legs are thick, with 3–10 rows of smooth muscle cells in the media.
In analogy with what happens in atherosclerosis, smooth muscle cells are also present in the subendothelial region particularly in the more obstructed vessels. In the more advanced stages of disease, SM2 levels are markedly reduced, suggesting smooth muscle cell phenotypic modulation from the mature contractile state to the synthetic state. The cause of this shift might be the disturbances in lymphatic flow that occur early in lymphedema.
Flow disturbances may also cause endothelial cell dysfunction. Endothelial cells are connected by continuous zipper-like junctions made of junctional proteins, including VE-cadherin.14 Interestingly it has been reported that lymphatic-specific deletion of VE-cadherin in a knockout mouse model causes chylous ascites,54 suggesting that VE-cadherin may regulate lymphatic permeability. Recently, ex vivo experiments showed that disruption of VE-cadherin in collecting lymphatic vessels significantly increased lymphatic permeability, indicating that VE-cadherin is required for ensuring barrier function in the lymphatic vasculature, and that its disruption would likely lead to the extravasation of most fluids and solutes into the interstitium, causing edema.55
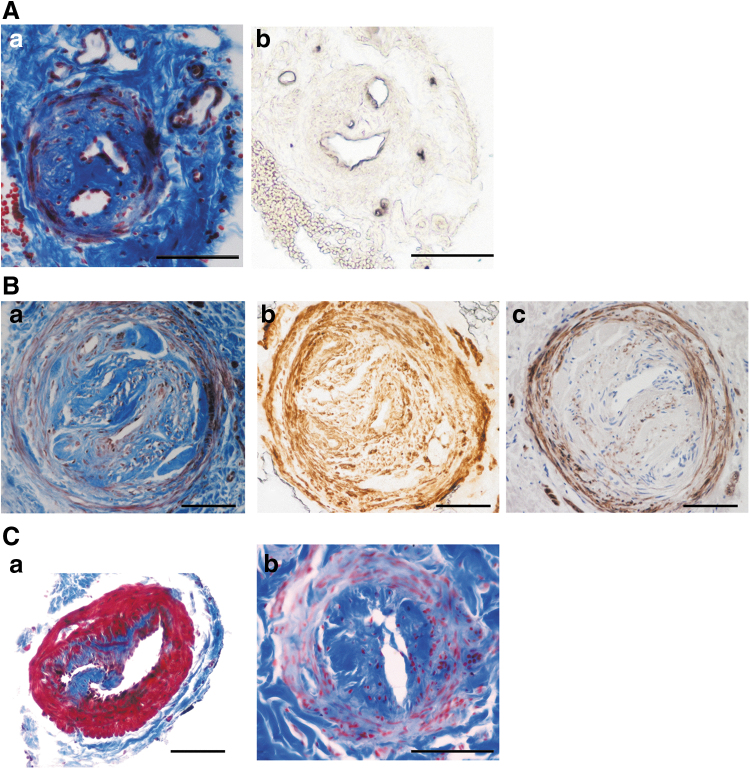
Mihara et al.52 reported that the expression of the endothelial marker podoplanin progressively decreases from ectasis to contraction to sclerosis type vessels. At variance with these authors, we, however, found that the expression of this marker was variable and unrelated with the vessel type or the stage of lymphedema.56 In some collecting vessels, podoplanin was uniformly present, whereas in others its expression was markedly reduced regardless of any clinical correlate (clinical stage, duration of disease, and morphological type of vessel) and of lumen patency. We even found vessels with a sclerosis morphology and a podoplanin-positive endothelium (Fig. 5A).
FIG. 5.
(A) Serial sections of the same sclerosis type vessel of the superior limb. (a) Masson's trichrome, the lumen is almost completely occluded and split in two. (b) Immunohistochemical staining with the endothelial marker podoplanin shows positive endothelial cells lining both lumens. Scale bar 100 μm. (B) A sclerosis type vessel of the inferior limb with an almost completely occluded lumen. (a) Masson's trichrome: the inner longitudinal layer of the media is stained blue, the outer circular layer is stained red. (b) Immunostaining for α-SMA: both the inner longitudinal and the outer circular layer of the media are positive for this smooth muscle cell marker. (c) Immunostaining for the differentiated smooth muscle cell marker MyH11: the inner longitudinal layer is negative, the outer circular layer is positive. Scale bar 100 μm. (C) Masson's trichrome stain of a valve in a contraction (a) and a sclerosis (b) type vessel.
Besides changes in smooth muscle cells and in endothelial cells, long-term lymphatic overload can also induce pathological changes in the surrounding adipose tissue, with hypertrophic adipocytes surrounded by thick collagen fibers and interstitial fluid. Adipose stromal cells that in normal conditions contribute to tissue homeostasis and repair, decrease in lymphedema; inflammatory macrophages increase, and anti-inflammatory macrophages decrease.57 Taken together, these findings indicate that lymphatic overload in the lymphedematous limb leads in time to chronic tissue inflammation, progressive fibrosis, impaired homeostasis and repair capacity, and immunological dysfunction.
A marked wall thickening in most of the collecting lymphatic vessels analyzed was reported both in primary and in secondary lymphedema.56
The wall thickening was due to smooth muscle cells hyperplasia in the contraction type and to collagen deposition in the sclerosis type. In whatever way it is generated, wall thickening may decrease the vessel responsivity to the mechanical stimuli of the surrounding environment, such as the “external pump.”3 Moreover, wall thickening influences vessel patency (the ratio between the lumen and the whole vessel area), which directly affects the amount of lymph that can be transported. Patency is reduced not only in vessels of the sclerosis but also in those of the contraction type, suggesting that both types may hamper lymph flow and thus induce lymphedema worsening.56
Possibly, valve incompetence may also play a role in lymphedema. No direct evidence of valve morphological defects has been reported to date in human patients with either congenital or secondary lymphedema.58 In ectasis type vessels, luminal dilatation might lead to valve incompetence.59 In contraction and sclerosis type vessels, the wall thickening present also at the valve buttress might hamper the lymphangion pumping (Fig. 5C).
Similarly, lumen reduction also occurs in resistance arteries in essential hypertension. It is not, however, associated with wall thickening.60 This process, called eutrophic inward remodeling, is due to repositioning of vascular smooth muscle cells around a smaller luminal diameter. If insufficient to achieve compensation, wall hypertrophy occurs. The purpose of inward remodeling in small arteries would be to protect downstream arterioles and capillaries from elevated blood pressure. In the lymphatic system, capillaries are upstream of collecting vessels, and this may account for the differences in their adaptation to increased intraluminal pressure.
The cellular population of the media has been frequently reported positive for the smooth muscle cell marker α-SMA. Since α-SMA may also be expressed by other cell types including myofibroblasts, this suggests that myofibroblasts may be responsible for the collagen deposition that leads to the sclerosis type. Indeed, fully differentiated smooth muscle cells positive for both MyHC and α-SMA were always visible in the outer circular layer, whereas the inner circular layer (Fig. 5B) contained cells that were sometimes positive for both markers and sometimes positive for α-SMA but negative for MyHC.56,61
The α-SMA+-MyHC− cells might be modulated smooth muscle cells that have switched from a contractile to a synthetic state or myofibroblasts that may originate not only from smooth muscle cells but also from endothelial cells through endothelial–mesenchymal transition triggered by inflammation. In agreement, Asano et al.61 reported an increase of the inflammatory cytokine tumor necrosis factor-α in the wall of collecting vessels of primary lymphedema.
Chronic inflammation is presumed to initiate lymphedema clinical progression. Macromolecules not efficiently drained by lymphatic vessels in fact accumulate in the interstitium, inducing an inflammatory reaction that triggers fibroblast recruitment.1 Activated fibroblasts synthesize collagen fibers that further entrap the lymphatic vessels in a self-perpetuating cycle. This vicious cycle will cause lymphedema progression until interrupted.
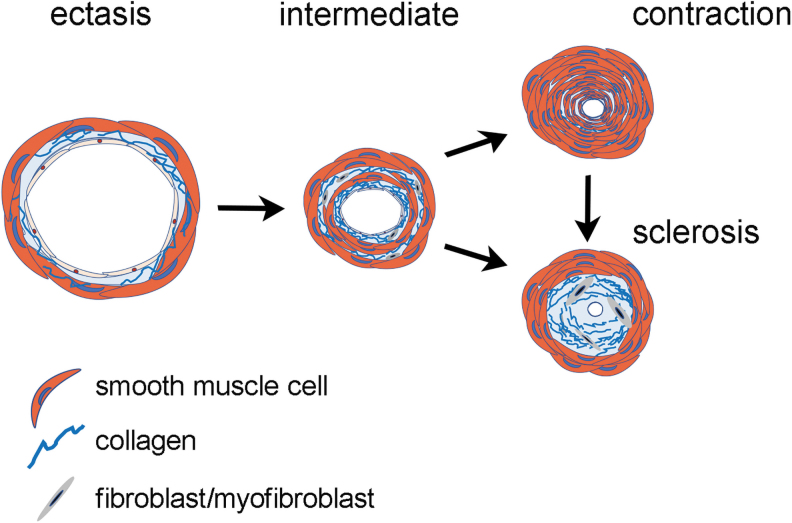
The schematic drawing in Figure 6 recapitulates what happens in lymphedema: at early stages the lumen is patent and the vessel wall is thin (ectasis type). With disease progression, the patency is reduced, and the vessel wall becomes thick due either to smooth muscle cells hyperplasia (contraction type) or to collagen deposition (sclerosis type). Smooth muscle cells of a contraction type vessel may also switch to the synthetic state and deposit collagen fibers, the vessel thus becomes a sclerosis type vessel. Myofibroblasts, evidenced by α-SMA immunostaining in the subintimal layer, may also account for this collagen deposition. Notably the outer circular layer of smooth muscle cells is preserved even in sclerosis type vessels.
FIG. 6.
Schematic drawing of the histopathological changes of the lymphatic wall in lymphedema.
Overall, after an initial dilatation, the progression of lymphedema causes lymphatic muscle cells proliferation as an adaptive mechanism to force lymph flow interruption. When hyperplasia of lymphatic muscle cells restricts the lumen however, the resistance to lymph flow increases. The lymphatic muscle cells' switch to the synthetic state and/or the transformation of endothelial cells into myofibroblasts leads to increased collagen deposition. The lumen is thus further restricted and lymph flow hampered. Interrupting this vicious cycle appears imperative. The realization of LVA in the distal part of the affected limb is effective in draining part of the lymph into small caliber veins and may greatly improve the patient's quality of life.
A dilated ectasis type vessel or a contraction type with a not yet restricted lumen, when available, would probably carry more lymph than an almost obstructed vessel. It would be interesting to evaluate whether regression from one type to the other, for instance from the contraction to the ectasis or normal type, may occur after releasing the trigger of external pressure exerted by edema on the vessel wall. This evaluation would probably be possible only with ICG lymphography or magnetic resonance lymphangiography preoperatively and at different postoperative time points.
Disease Specifity in Lymphedema
The here reported data mainly concern cancer-related secondary lymphedema, in which the different types of collecting lymphatic vessels (i.e., ectasis, contraction, and sclerosis) have been associated with different clinical stages, suggesting that collecting lymphatic vessels gradually transformed from one type into the next.52,56 Interestingly, in our casuistry, we noted that in primary lymphedema, unlike secondary lymphedema, most of the vessels are of the sclerosis type, and the contraction type vessel is very rare.56 A possible explanation for this difference could be that patients with prolonged disease and early onset (primary lymphedema) might establish compensatory physiological mechanisms.
Lymphatic vessel dilatation is an early event in filariasis-related lymphedema62 and, at variance with cancer-related lymphedema, is maintained throughout the disease course. It initiates when the adult worms are still alive and the offspring larvae are released causing antigenic stimulation. The death of worms, their toxins, and phagocytic uptake of degenerate larvae increase inflammation and trigger innate immunity with the release of inflammatory cytokines and lymphangiogenic molecules. Secondary infections exacerbate the situation. Insufficient lymph transport leads to fluid extravasation and lymphedema.
Further lymphatic remodeling includes collateral formation, fibrosis, and extensive infiltration of immune cells. In the wall of these dilated lymphatic vessels, areas of fibrosis alternate with areas of muscle hyperplasia, leading to valve damage and lymph backflow. Interestingly, gene variants in the genes that are important in valve formation and maintenance have been found in patients affected by lymphatic filariasis,63,64 suggesting that these genes, together with immune responses, may contribute to lymphedema susceptibility.
Growing evidence also indicates a connection between lymphatic dysfunction and obesity.65 It is well known that obesity is a risk factor for secondary lymphedema, but more recently it has also been shown that extreme obesity can cause lymphedema in the absence of other risk factors.66 As the amount of adipose tissue increases, lymphatic vessels may become dysfunctional, possibly because of compression and/or inflammation.
Obesity-induced pathological changes in the lymphatic system result, at least in part, from the accumulation of inflammatory cells around lymphatic vessels, leading to impaired lymphatic collecting vessel pumping capacity, leaky initial and collecting lymphatics, alterations in lymphatic endothelial cell gene expression, and degradation of junctional proteins.67 Obese mice were shown to have impaired lymphatic transport and decreased dendritic cell migration to lymph nodes,68 and in a mouse model of diet-induced lymphedema, obese mice were shown to have impaired lymphatic function associated with increased inflammation, fibrosis, and adipose tissue deposition.69
All types of lymphedema are strictly associated with inflammation. Common pathological features are vessel dilatation (at least initially) and valve incompetence, but further research is needed on the histological changes of lymphatic collecting vessels, particularly in obesity-associated lymphedema.
Authors' Contributions
E.W., V.B., E.B., and P.G. contributed to the study design and data interpretation. E.W. and V.B. contributed to writing/revision of the article. P.G. and G.G. performed surgery and provided the samples for histological analysis. V.B., E.W., and M.A. performed immunohistochemistry, semithin section staining, transmission electron microscopy, and data analysis.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by PSR Piano di Sostegno alla Ricerca 2267-2020-BV-PAR_001 and 2267-2021-BV-PAR_001 to V.B. and E.W., University of Siena.
References
- 1. Norrmén C, Tammela T, Petrova TV, Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation 2011; 123:1335–1351. [DOI] [PubMed] [Google Scholar]
- 2. Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002; 21:1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manrique OJ, Bustos S, Ciudad P, Adabi K, Chen WF, Forte AJ, et al. Overview of lymphedema for physicians and other clinicians: A review of fundamental concepts. Mayo Clin Proc 2020; 20:S0025-6196(20)30033-1. [Epub ahead of print]; DOI: 10.1016/j.mayocp.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 4. Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012; 7:e49940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. García Nores GD, Ly CL, Cuzzone DA, Kataru RP, Hespe GE, Torrisi JS, Huang JJ, Gardenier JC, Savetsky IL, Nitti MD, Yu JZ, Rehal S, Mehrara BJ. CD4+T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat Commun 2018; 9:1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avraham T, Zampell JC, Yan A. Elhadad S, Weitman ES. Rockson SG, Bromberg J, Mehrara BJ. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 2013; 27:1114–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLaughlin SA, Stout NL, Schaverien MV. Avoiding the swell: Advances in lymphedema prevention, detection, and management. Am Soc Clin Oncol Educ Book 2020; 40:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Nagase T, Gonda K, Inoue K, Higashino T, Fukuda N, Gorai K, et al. Treatment of lymphedema with lymphaticovenular anastomoses. Int J Clin Oncol 2005; 10:304–310. [DOI] [PubMed] [Google Scholar]
- 9. Scavelli C, Weber E, Aglianò M, Cirulli T, Nico B, Vacca A, et al. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J Anat 2004; 204:433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic vessel network structure and physiology. Compr Physiol 2019; 9:207–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerli R, Ibba L, Fruschelli C. A fibrillar elastic apparatus around human lymph capillaries. Anat Embryol 1990; 181:281–286. [DOI] [PubMed] [Google Scholar]
- 12. Gerli R, Solito R, Weber E, Aglianó M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology 2000; 33:148–157. [PubMed] [Google Scholar]
- 13. Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev 2001; 50:3–20. [DOI] [PubMed] [Google Scholar]
- 14. Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007; 204:2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sacchi G, Weber E, Aglianò M, Raffaelli N, Comparini L. The structure of superficial lymphatics in the human thigh: Precollectors. Anat Rec 1997; 247:53–62. [DOI] [PubMed] [Google Scholar]
- 16. Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: Mechanics, mechanisms and malfunction. J Physiol 2016; 594:5749–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Breslin JW. Mechanical forces and lymphatic transport. Microvasc Res 2014; 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The lymphatic system: Integral roles in immunity. Annu Rev Immunol 2017; 35:31–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliver G, Kipnis J, Randolph GJ, Harvey NL. The lymphatic vasculature in the 21st Century: Novel functional roles in homeostasis and disease. Cell 2020; 182:270–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michelini S, Paolacci S, Manara E, Eretta C, MattassiR, Lee BB, et al. Genetic tests in lymphatic vascular malformations and lymphedema. J Med Genet 2018; 55:222–232. [DOI] [PubMed] [Google Scholar]
- 21. García Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, et al. Obesity but not high-fat diet impairs lymphatic function. Int J Obes 2016; 40:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwager S, Detmar M. Inflammation and lymphatic function. Front Immunol 2019; 10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pal S, Nath S, Meininger CJ, Gashev AA. Emerging roles of mast cells in the regulation of lymphatic immuno-physiology. Front Immunol 2020; 11:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winters H, Tielemans HP, Hameeteman M, Paulus VAA, Beurskens CH, Slater NJ, et al. The efficacy of lymphaticovenular anastomosis in breast cancer related lymphedema. Breast Cancer Res Treat 2017; 165:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ozturk CN, Ozturk C, Glasgow M, Platek M, Ashary Z, Kuhn J, et al. Free vascularized lymph node transfer for treatment of lymphedema: A systematic evidence based review. J Plast Reconstr Aesth Surg 2016; 69:1234–1247. [DOI] [PubMed] [Google Scholar]
- 26. Scaglioni MF, Arvanitakis M, Chen YC, Giovanoli P, Chia-Shen Yang J, Chang EI. Comprehensive review of vascularized lymph node transfers for lymphedema: Outcomes and complications. Microsurgery 2018; 38:222–229. [DOI] [PubMed] [Google Scholar]
- 27. O'Brien BM, Chait LA, Hurwitz PJ. Microlymphatic surgery. Orthop Clin N Am 1977; 8:405–424. [PubMed] [Google Scholar]
- 28. Gennaro P, Gabriele G, Mihara M, Kikuchi K, Salini C, Aboh I, Cascino F, Chisci G, Ungari C. Supramicrosurgical lymphatico-venular anastomosis (LVA) in treating lymphoedema: 36-months preliminary report. Eur Rev Med Pharmacol Sci 2016; 22:4642–4653. [PubMed] [Google Scholar]
- 29. Gennaro P, Gabriele G, Salini C, Chisci G, Cascino F, Xu JF, Ungari C. Our supramicrosurgical experience of lymphaticovenular anastomosis in lymphoedema patients to prevent cellulitis. Eur Rev Med Pharmacol Sci 2017; 21:674–679. [PubMed] [Google Scholar]
- 30. Koshima I, Kawada S, Moriguchi T, Kajiwara Y. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg 1996; 97:397–405. [DOI] [PubMed] [Google Scholar]
- 31. Akita S, Mitsukawa N, Kazama T, Kuriyama M, Kubota Y, Omori N, et al. Comparison of lymphoscintigraphy and indocyanine green lymphography for the diagnosis of extremity lymphoedema. J Plast Reconstr Aesth Surg 2013; 66:792–798. [DOI] [PubMed] [Google Scholar]
- 32. Gennaro P, Borghini A, Chisci G, Mazzei FG, Weber E, Tedone Clemente E, et al. Could MRI visualize the invisible? An Italian single center study comparing magnetic resonance lymphography (MRL), super microsurgery and histology in the identification of lymphatic vessels. Eur Rev Med Pharmacol Sci 2017; 21:687–694. [PubMed] [Google Scholar]
- 33. Unno N, Inuzuka K, Suzuki M, Yamamoto N, Sagara D, Nishiyama M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg 2007; 45:1016–1021. [DOI] [PubMed] [Google Scholar]
- 34. Suami H, Heydon-White A, Mackie H, Czerniec S, Koelmeyer L, Boyages J. A new indocyanine green fluorescence lymphography protocol for identification of the lymphatic drainage pathway for patients with breast cancer-related lymphoedema. BMC Cancer 2019; 19:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamamoto T, Narushima M, Doi K, Oshima A, Ogata F, Mihara M, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: The generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011; 127:1979–1986. [DOI] [PubMed] [Google Scholar]
- 36. Mazzei MA, Gentili F, Mazzei FG, Gennaro P, Guerrieri D, Nigri A, et al. High-resolution MR lymphangiography for planning lymphaticovenous anastomosis treatment: A single-centre experience. Radiol Med 2017; 122:918–927. [DOI] [PubMed] [Google Scholar]
- 37. Arkill KP, Moger J, Winlove CP. The structure and mechanical properties of collecting lymphatic vessels: An investigation using multimodal nonlinear microscopy. J Anat 2010; 216:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasselhof V, Sperling A, Buttler K, Ströbel, P, Becker J, Aung T, et al. Morphological and molecular characterization of human dermal lymphatic collectors. PLoS One 2016; 11:e0164964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol 2009; 7:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. To KHT, Gui P, Li M, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. T-type, but not L-type, voltage-gated calcium channels are dispensable for lymphatic pacemaking and spontaneous contractions. Sci Rep 2020;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vittet D. Lymphatic collecting vessel maturation and valve morphogenesis. Microvasc Res 2014; 96:31–37. [DOI] [PubMed] [Google Scholar]
- 42. Betterman KL, Harvey NL. The lymphatic vasculature: Development and role in shaping immunity. Immunol Rev 2016; 271:276–292. [DOI] [PubMed] [Google Scholar]
- 43. Petrova TV, Karpane T, Norrmén C, Mellor R, Tamakoshi T, Finegold D, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 2004; 10:974–981. [DOI] [PubMed] [Google Scholar]
- 44. Kazenwadel J, Betterman KL, Chong CE, Stokes PH, Lee YK, Secker GA, et al. GATA2 is required for lymphatic vessel valve development and maintenance. J Clin Invest 2015; 125:2979–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nonomura K, Lukacs V, Sweet DT, Goddard LM, Kanie A, Whitwam T, Ranade SS, Fujimori T, Kahn ML, Patapoutian A. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc Natl Acad Sci U S A 2018; 115:12817–12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun 2015; 6:8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hägerling R, Pollmann C, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell 2012; 22:430–445. [DOI] [PubMed] [Google Scholar]
- 48. Finegold DN, Baty CJ, Knickelbein KZ, Perschke S, Noon SE, Campbell D, et al. Connexin 47 mutations increase risk for secondary lymphedema following breast cancer treatment. Clin Cancer Res 2012; 18:2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ohhashi T, Fukushima S, Azuma T. Vasa vasorum within the media of bovine mesenteric lymphatics. Proc Soc Exp Biol Med 1977; 154:52–56. [DOI] [PubMed] [Google Scholar]
- 50. Aglianò M, Sacchi G, Weber E, Pucci AM, Comparini L. Vasa vasorum of superficial collecting lymphatics of human thigh. Lymphology 1997; 30:116–121. [PubMed] [Google Scholar]
- 51. Zhao SB, Belhoul-Fakir H, Jansen S, Hamzah J, Mishani S, Lawrence Brown M, et al. Major gaps in human evidence for structure and function of the vasa vasora limit our understanding of the link with atherosclerosis. J Anat 2021; 238:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mihara M, Hara H, Hayashi Y, Narushima M, Yamamoto T, Todokoro T, et al. Pathological steps of cancer-related lymphedema: Histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 2012; 7:e41126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ogata F, Fujiu K, Koshima I, Nagai R, Manabe I. Phenotypic modulation of smooth muscle cells in lymphoedema. Br J Dermatol 2015; 172:1286–1293. [DOI] [PubMed] [Google Scholar]
- 54. Yang Y, Cha B, Motawe ZY, Srinivasan RS, Scallan JP. VE-cadherin is required for lymphatic valve formation and maintenance. Cell Rep 2019; 28:2397–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jannaway M, Scallan JP. VE-cadherin and vesicles differentially regulate lymphatic vascular permeability to solutes of various sizes. Front Physiol 2021; 12:687563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barone V, Borghini A, Tedone Clemente E, Aglianò M, Gabriele G, Gennaro P, et al. New insights into the pathophysiology of primary and secondary lymphedema: histopathological studies on human lymphatic collecting vessels. Lymphat Res Biol 2020; 18:502–509. [DOI] [PubMed] [Google Scholar]
- 57. Tashiro K, Feng J, Wu SH, Mashiko T, Kanayama K, Narushima M, et al. Pathological changes of adipose tissue in secondary lymphoedema. Br J Dermatol 2017; 177:158–167. [DOI] [PubMed] [Google Scholar]
- 58. Iyer D, Jannaway M, Yang Y, P Scallan J. Lymphatic valves and lymph flow in cancer-related lymphedema. Cancers (Basel) 2020; 12:2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Olszewski WL. Contractility patterns of normal and pathologically changed human lymphatics. Ann N Y Acad Sci 2002; 979:52–63. [DOI] [PubMed] [Google Scholar]
- 60. Castorena-Gonzalez JA, Staiculescu MC, Foote C, Martinez-Lemus LA. Mechanisms of the inward remodeling process in resistance vessels: Is the actin cytoskeleton involved? Microcirculation 2014; 21:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Asano S, Mikami T, Matsubara S, Maegawa J, Wakui H, Tamura K, et al. Preliminary report: The relevance of tumor necrosis factor-α in acquired primary lymphedema-A histopathological investigation. Lymphat Res Biol 2020; 18:232–238. [DOI] [PubMed] [Google Scholar]
- 62. Chakraborty S, Gurusamy M, Zawieja DC, Muthuchamy M. Lymphatic filariasis: Perspectives on lymphatic remodeling and contractile dysfunction in filarial disease pathogenesis. Microcirculation 2013; 20:349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Babu S, Nutman TB. Immunopathogenesis of lymphatic filarial disease. Semin Immunopathol 2012; 34:847–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sheik Y, Qureshi SF, Mohhammed B, Nallari P. FOXC2 and FLT4 gene variants in lymphatic filariasis. Lymphat Res Biol 2015; 13:112–119. [DOI] [PubMed] [Google Scholar]
- 65. Mehrara BJ, Greene AK. Lymphedema and obesity: Is there a link? Plast Reconstr Surg 2014; 134:154e–160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Greene AK, Grant FD, Slavin SA. Lower-extremity lymphedema and elevated body-mass index. N Engl J Med 2012; 366:2136–2137. [DOI] [PubMed] [Google Scholar]
- 67. Kataru RP, Park HJ, Baik JE, Li C, Shin J, Mehrara BJ. Regulation of lymphatic function in obesity. Front Physiol 2020; 11:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D, Mehrara BJ. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 2013; 8:e70703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, Joseph WJ, Mehrara BJ. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol 2014; 307:H165–H172. [DOI] [PMC free article] [PubMed] [Google Scholar]