ABSTRACT
Cell wall deficient bacterial L-forms are induced by exposure to cell wall-targeting antibiotics and immune effectors such as lysozyme. L-forms of different bacteria (including Escherichia coli) have been reported in human infections, but whether this is a normal adaptive strategy or simply an artifact of antibiotic treatment in certain bacterial species remains unclear. Here we show that members of a representative, diverse set of pathogenic E. coli readily proliferate as L-forms in supratherapeutic concentrations of the broad-spectrum antibiotic meropenem. We report that they are completely resistant to antibiotics targeting any penicillin-binding proteins in this state, including PBP1A/1B, PBP2, PBP3, PBP4, and PBP5/6. Importantly, we observed that reversion to the cell-walled state occurs efficiently, less than 20 h after antibiotic cessation, with few or no changes in DNA sequence. We defined for the first time a logarithmic L-form growth phase with a doubling time of 80 to 190 min, followed by a stationary phase in late cultures. We further demonstrated that L-forms are metabolically active and remain normally susceptible to antibiotics that affect DNA torsion and ribosomal function. Our findings provide insights into the biology of L-forms and help us understand the risk of β-lactam failure in persistent infections in which L-forms may be common.
IMPORTANCE Bacterial L-forms require specialized culture techniques and are neither widely reported nor well understood in human infections. To date, most of the studies have been conducted on Gram-positive and stable L-form bacteria, which usually require mutagenesis or long-term passages for their generation. Here, using an adapted osmoprotective growth media, we provide evidence that pathogenic E. coli can efficiently switch to L-forms and back to a cell-walled state, proliferating aerobically in supratherapeutic concentrations of antibiotics targeting cell walls with few or no changes in their DNA sequences. Our work demonstrates that L-form switching is an effective adaptive strategy in stressful environments and can be expected to limit the efficacy of β-lactam for many important infections.
KEYWORDS: L-forms, β-lactams, refractory infections, Escherichia coli, antibiotic resistance
OBSERVATION
Bacteria grow at exponential rates under optimal growth conditions when tested in research or diagnostic laboratories (1, 2). However, optimal conditions are rare in nature, where bacteria survive by adjusting their physiology and reducing their growth rates when stressed or starved (3, to ,6).
In infection, bacterial growth rates vary significantly and depend primarily on nutrient availability and the host immune response (7). Antibiotics may kill these bacteria rapidly but sometimes fail to eradicate a small subpopulation that can cause chronic or relapsing infections (8, 9). These cells may survive through genetic adaption to grow normally in the presence of the antibiotics (10) or adapt to tolerate the antibiotic stress and begin normal growth again once conditions improve (11).
Bacterial persisters are specific subpopulations with enhanced tolerance to antibiotics (12). They are growth-arrested bacteria with reduced metabolism that can restart normal growth after stress and have been implicated in antibiotic treatment failure and infection recurrence (12, to ,14).
First reported in the 1930s, researchers have recently revisited cell wall-deficient or L-form bacteria with modern molecular biology tools (15, to ,21). Metabolically active L-forms have been recently described in macrophages and the urine of patients with recurrent urinary tract infections (UTIs) (20, 21). L-forms may be induced by exposure to cell wall-targeting antibiotics under osmoprotective conditions (17), although some L-forms may tolerate relatively low-osmolality environments (20). Kawai et al. (15) showed that immune effectors such as lysozyme could rescue Gram-positive bacterial viability and protect it from β-lactam attack by switching into the L-form state (15). L-forms are metabolically active but divide more slowly than exponential-phase cell walled bacteria (CWB) in a manner that is completely independent of the FtsZ-based cell division machinery thought to be essential for normal fission in CWB (16, 17, 22). Without cell walls, L-forms are completely resistant to antibiotics targeting the cell wall (e.g., β-lactams) (5, 8). Unlike nondividing persister cells, L-forms thrive in the presence of powerful cell walls targeting antibiotics (17, 21), but their cell cycle and growth dynamics have not been well defined.
To date, most studies have been conducted on Gram-positive and stable L-form bacteria, which usually require mutagenesis or long-term serial passages for generation (23). In this study, we show that the L-form is a normal reversible growth state in the archetypal pathogen E. coli and define its lag, logarithmic, and stationary phases.
L-form switching is a common physiological response to cell wall targeting antibiotics in clinical E. coli isolates.
It is unclear whether L-forms result from a single process or the final endpoint of a diverse set of processes (17). To characterize the L-form physiology, we developed a double-layer osmoprotective semisolid agar medium to support the aerobic growth of L-forms by modifying the existing L-form medium (LFA) (24). We used this to assess L-form growth (Fig. 1A and B) in 45 genetically distinct E. coli isolates from 19 clinically important sequence types (Table S1, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd) in the presence of high concentrations of the β-lactam-like carbapenem antibiotic meropenem (100 mg/liter). This corresponds to a 100-fold increase of the usual minimal inhibitory concentration (MIC) for this organism and is well above the tested MIC for all of these isolates. L-forms developed quickly and proliferated aerobically in most tested strains (~80%). The majority of L-form cultures (~90%) also quickly reverted to normal rod-shaped CWB within 20 h of meropenem withdrawal (Table S1).
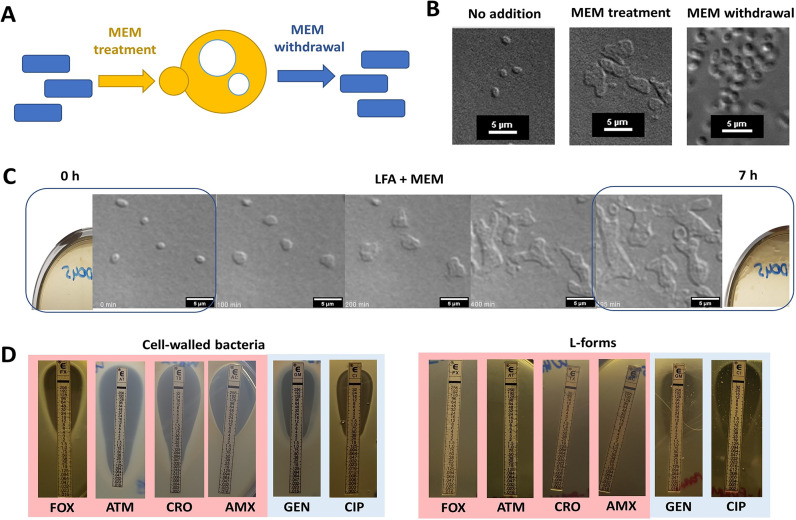
FIG 1.
Meropenem promotes L-form growth from the walled state under aerobic conditions. (A) Model illustrating how pathogenic E. coli can switch in and out of the L-form state in response to the antibiotic challenge. (B) E. coli L-form strain WH62 switch in the presence of meropenem and reversion to the cell walled state after meropenem withdrawal. (C) Time-lapse DIC microscopy of WH62 L-forms; individual micrograph frames are extracted from Movie S1, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd. (D) Susceptibility of E. coli L-forms to β-lactams, aminoglycosides, and fluoroquinolones by standard Etest. MEM, meropenem; FOX, cefoxitin; ATM, aztreonam; CRO, ceftriaxone; AMX, amoxicillin; GEN, gentamicin; CIP, ciprofloxacin.
Time-lapse differential interference contrast (DIC) microscopy revealed that bacterial cells quickly lost their regular shape in the presence of high-dose meropenem (MEM) in LFA and increased their surface area about 4-fold (3.95 ± 1.17), dividing as L-forms within ~5 h (Fig. 1B and C). This asymmetrical scission process yields a morphologically heterogeneous population of E. coli cells (Movie S1, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd).
These meropenem-induced L-forms were resistant to growth inhibition by β-lactam and β-lactam-like cell wall-active antibiotics such as ceftriaxone (targeting D, d-transpeptidase [DDT] activity of penicillin-binding protein [PBP] 1A/1B), meropenem (PBP2 and L, d-transpeptidase [LDT]), aztreonam (PBP3), amoxicillin (PBP4), and cefoxitin (PBP5/6) (25, 26) (Fig. 1D, right). A range of β-lactam antibiotics, lysozyme, and macrophages have been shown to induce L-forms (20, 21), but we found that induction of E. coli L-forms was more efficient in the presence of meropenem than ceftriaxone, cefepime, or ampicillin. Remarkably, several studies have shown that the essential requirement for PBPs can be fully bypassed by LDTs, replacing the canonical 4–3 cross-links with 3–3 cross-links and leading to broad-spectrum β-lactam resistance (27, 28). These unusual and often overlooked 3–3 cross-links are present in a smaller but significant proportion of the bacterial cell wall (e.g., 3 to 15% in E. coli cells, mostly depending on their genetic makeup and growth phase) (29, 30), and might explain incomplete induction of the L-forms in the presence of ampicillin and cephalosporins observed in this study. On the other hand, efficient L-form switching was evident in the presence of meropenem and might be attributable to its dual action and rapid inactivation of both D,d-transpeptidase (PB2) and L,d-transpeptidase in E. coli (31, 32).
Carbapenem susceptibility of CWB was identical before and after L-form transition (Table S2) despite the absolute carbapenem and β-lactam resistance of their L-form state. The widely used fluoroquinolone antibiotic ciprofloxacin, targeting DNA gyrase and topoisomerase enzymes, remained a potent inhibitor of L-form growth with an MIC of <0.25 mg/L (33) in a modified Etest; aminoglycoside susceptibility was also retained in L-forms developed from gentamicin (aminoglycoside)-susceptible CWB (MIC <2 mg/L) (Fig. 1D, left).
To characterize growth rate and metabolic activity, we used semisolid agar in a 24-well plate supplemented with MEM (100 mg/L) and 2,3,5-triphenyltetrazolium chloride (TTC) as a redox indicator, measuring optical density at 540 nm (OD540) of E. coli J53 (a well-characterized E. coli K-12 derivative) (34) and WH62 (clinical isolate) (35).
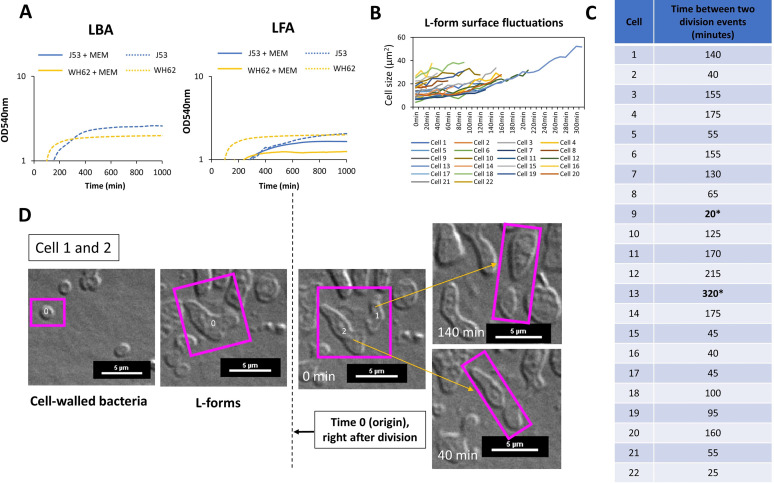
Both grew more slowly as L-forms than as CWB, with an initial lag phase (Fig. 2A) of ~80 and ~190 min for WH62 and J53, respectively, as L-forms developed from CWB in osmoprotective LFA with MEM. Growth rates increased after this lag phase, while CWB controls were completely inhibited in nonosmoprotective media (Fig. 2A, left). L-form population growth then appeared to enter a stationary phase after ~500 min of incubation, with evidently reduced metabolic activity (Movie S1, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd, and Fig. 2A). Time-lapse microscopy revealed heterogeneous growth during the L-form exponential phase in which shape deformation and an increase of cell surface area preceded asymmetrical scission and the emergence of new progeny (Fig. 2B). Imaging revealed a wide variation in the periodicity of CWB to L-form switching around an average of 114 (±75 min) in 22 independent cells (e.g., Fig. 2C and D; Data set S1, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd), generally consistent with the observed lag phase determined for L-form populations overall (Fig. 2A). Intracellular vesicles were evident after prolonged incubation in aerobic conditions (>16h) (Fig. S2).
FIG 2.
WH62 E. coli L-form growth kinetics and proliferation rates in LFA medium supplemented with the antibiotic meropenem. (A) Growth curves of J53 (ST10) and WH62 (ST127) L-forms. (B) Bacterial cell area fluctuations between division measured in 22 cells. (C) Length of L-form cell cycle measured in 22 different L-form cells. (D) Mode of cell division of L-forms, division by budding; see also Movie S1, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd. Time 0 (origin) indicates the first division event in the L-form state.
The genomes of four genetically distinct (Fig. S1 and Table S3, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd) CWB revertants (i.e., after transition to L-form and back) differed from parent CWB strains (i.e., before the transition to L-form) in only one of the four pairs tested (B36_rev compared to E. coli B36), in which single nucleotide variants arose mainly in loci encoding surface-presented molecules, including common bacteriophage receptors (e.g., capsule) (Table S4, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd; accession number: PRJNA764821).
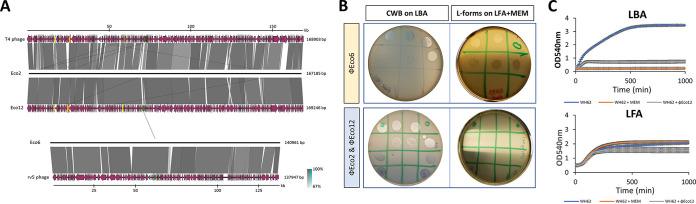
Predating viruses (bacteriophages or phages) are a common threat to bacterial populations and are now increasingly used for therapy, including of E. coli infection and often in combination with β-lactam antibiotics (2, 36). We therefore investigated predation of established L-forms by representatives of the ubiquitous and therapeutically important T4-like myoviruses (vB_EcoM_OMNI2, Eco2; and vB_EcoM_OMNI12, Eco 12) and of the less common V5-like phages (vB_EcoM_OMNI6, Eco6) (Fig. 3A). While Eco6 lysed L-forms and CWB equally (Fig. 3B, top), T4-like Eco2 and Eco12 failed to propagate in L-forms of strains in which they are obligately lytic of CWB forms (Fig. 3B, bottom), and L-form growth was unaffected (Fig. 3C). We could not induce L-form switching by exposing CWB to T4-like phages in LFA (Eco12, on three separate occasions), although this has been described for other bacteria (37, 38).
FIG 3.
Effects of the L-form switch on susceptibility to myoviruses. (A) Comparative analysis of phage genomes. Schematics show the genomic organization of phages vB_EcoM_OMNI-2 (Eco2), 6 (Eco6), and 12 (Eco12; GenBank OL362041) compared to available reference genomes (T4-like phage NC_000866.4 and V5-like phage DQ832317.1) (left). (B) Phage susceptibility of E. coli L-forms using standard spot assay and modified LFA; WH62 and JIE4799 meropenem-induced L-forms displaying resistance (no lysis) to T4-like phages (Eco2 and Eco12) (bottom right) and sensitivity to V5-like phages (Eco6) (top right), respectively. Control involved cell-walled counterpart on standard LBA without meropenem (left top and bottom) lysed by all three phages. (C) Growth curves of walled bacteria (WH62 on LBA) and L-forms (L-WH62 on LFA supplemented with meropenem) in the presence of Eco12 phage at MOI 1.
β-lactam antibiotics remain one of the most commonly prescribed drug classes (39, 40) but often fail in severe and refractory infections despite demonstrated efficacy in vitro (41, 42). L-forms may be an important contributor to bacterial virulence and to the failure of antibiotic treatment with β-lactams and related antibiotic classes.
Our data indicate that L-forms are an effective and probably ancient stress response that appears to be the norm in E. coli populations, exhibiting nonbinary cell growth with well-defined lag, log, and stationary phases.
Bacterial strains.
The potential for L-from growth was tested in a wide range of E. coli strains (n = 45), including a multidrug-resistant dominant clone sequence type (ST) 131 (clade A, B, and C). The testing also involved strains belonging to other clinically important STs (n = 19), which are listed in Table S1. A total of 15 genetically distinct strains were selected for further testing, which included testing of meropenem susceptibility in revertants and phage susceptibility (Table S2, posted at https://figshare.com/s/09b4bbc18c62c1d6aadd).
Growth conditions.
E. coli isolates were grown on Brilliance GBS Agar/Oxoid (Thermo Fisher Scientific) and in Lysogeny broth (LB-Miller, Becton, Dickinson, France). Bacterial L-forms were induced in osmoprotective LFA as described previously (24). When necessary, antibiotics and supplements were added at the following concentrations: meropenem or MEM (100 μg/mL) and 2,3,5-triphenyl-2H-tetrazolium chloride or TTC (5%). To assess L-forms switching in 45 genetically distinct E. coli clinical strains, we used a modified double-layer osmoprotective LFA (24). Briefly, 300μL of a bacterial suspension was grown to exponential phase at 37°C, with shaking at 225 rpm (revolutions per minute) and MEM was added to 4 mL top agar, gently homogenized, and poured into 90-mm petri dish previously prepared with 10 mL bottom agar. The plates were gently swirled, dried for 10 min at room temperature, and then inverted and incubated at 37°C overnight. The control included (i) growth of typical cell-walled bacteria (CWB) on standard hypotonic Luria-Bertani agar (positive control; LBA); (ii) growth inhibition of cell-walled forms by high dose of cell wall-targeting antibiotics, in standard hypotonic LBA (negative control; LBA+MEM) (Fig. 4). Reversion to the cell wall state was demonstrated by plating out L-forms on both LFA and LBA (<10% survived) without antibiotics.
Fig 4.
The double-layer method was used to test the L-form switching in pathogenic strains of E. coli.
Antibiotic susceptibility assays.
MICs of meropenem in revertants was assessed using microbroth-dilution protocol as previously described and interpreted according to the Clinical and Laboratory Standard Institute (CLSI) (1). The Etest (bioMérieux, USA) was conducted according to the manufacturer’s instructions on Mueller-Hinton agar (MHA) for walled bacteria and on LFA for L-forms with certain modifications. Bacterial lawns of WH62 and J53 were prepared using the double-layer method described above. After applying the Etest strip, the plates were incubated for 24 h aerobically. The MIC of the antibiotic was read directly from the scale printed on the Etest strip at the point of intersection between the bacterial growth zone and the strip. Susceptibility to antibiotics (amoxicillin, ceftriaxone, cefepime, and cefoxitin) was determined according to internationally accepted CLSI breakpoints (1). An E. coli ATCC25922 was used as a control.
Microscopic imaging and growth kinetics.
Sample preparation for time-lapse differential interference contrast (DIC) microscopy has been done as previously described with a slight modification (43). Instead of a standard imaging medium, LFA supplemented with meropenem was used. DIC microscopy images were acquired at 37°C on a Nikon Eclipse Ti-E motorized inverted microscope with Perfect Focus using a Nikon 100 × 1.45 pathogenic isolates of E. coli PlanApo Lambda Objective (Nikon Instruments). Images were captured using a Nikon DS-Qi2 monochrome camera at 5-min intervals for up to 16 h using the NIS-Elements software (Laboratory Imaging s.r.o.). DIC illumination was achieved using Nomarski prisms. Pictures and videos were prepared for publication using Huygens Professional version 19.04 (Scientific Volume Imaging, The Netherlands, http://svi.nl) and ImageJ (http://rsb.info.nih.gov/ij) (44).
Isolation of Escherichia coli specific phages.
Bacteriophages vB_EcoM_OMNI2, vB_EcoM_OMNI6, and vB_EcoM_OMNI12 targeting pathogenic isolates of E. coli were isolated from sewage and pond water samples respectively collected in the Greater Sydney District (Sydney, NSW, Australia) during 2019. Specimens were clarified by filtration through 0.45-μm and 0.22-μm filters. Isolation of bacteriophages was performed using an enrichment procedure (45) where single plaques were picked and purified as previously described (46). High-titer stocks were prepared by propagating bacteriophages over several double-layer plates washed in SM buffer (50 mM Tris-HCl, 8 mM MgSO4, 100 mM NaCl, pH 7.4), filtered through a 0.22-μm filter and precipitated with NaCl and PEG8000 (46). The concentration as plaque-forming units per mL (PFU/mL) was determined by spotting 10 μL of 10-fold serial dilutions onto a double layer of the target bacteria (46). High-titer (≥1,010 PFU/mL) bacteriophage stocks were stored at 4°C.
Phage susceptibility.
Phage-susceptibility testing was performed using a traditional plaque or a double-layer agar method as previously described (46). When testing phage susceptibility in L-forms, instead of standard LBA medium, LFA supplemented with meropenem was used. Inhibition of cell-walled and L-form bacterial growth was determined as described previously (24), with a modification that included LFA supplemented with meropenem to support L-form growth.
Data availability.
All data generated or analyzed during this study are included in this article and its supplemental material (posted at https://figshare.com/s/09b4bbc18c62c1d6aadd). Whole-genome sequencing data are available on NCBI under the BioProject accession number PRJNA764821 and GenBank number OL362041.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Sally Partridge for advice on experimental design. We thank We Nouri Ben Zakour for her help with phage genome sequencing data and staff at the Pathogen Genomics Unit, Westmead Hospital for their technical advice and sequencing support. The authors acknowledge the technical and scientific assistance of Sydney Microscopy and Microanalysis, the University of Sydney node of Microscopy Australia.
A.P.F. is supported by the Office for Health and Medical Research (New South Wales, Australia) Phage Therapy Fellowship. This work was funded by a National Health Medical Research Council (Australian Government) Investigator Grant (Iredell_APP1197534).
A.P.F. and J.I. conceived the study and designed the main experimental plan. A.P.F., J.I., J.E., and K.M. analyzed the data. A.P.F. and J.I. wrote the paper. A.P.F. developed a double-layer plaque and microtiter assay that supports the L-forms growth in vitro. A.P.F., D.M.-M., and N.F.-R. designed the L-form microscopy experiments. N.F.-R. and A.P.F. performed imaging experiments and data image processing. C.V. analyzed whole-genome sequencing data of E. coli and revertants. A.P.F. performed all experiments. All authors were involved in reviewing and editing the final manuscript.
We declare no competing interests.
Contributor Information
Aleksandra Petrovic Fabijan, Email: aleksandra.petrovicfabijan@sydney.edu.au.
Jonathan Iredell, Email: jonathan.iredell@sydney.edu.au.
Gyanu Lamichhane, Johns Hopkins University School of Medicine.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing, 31st ed. CLSI, Berwyn, PA. [Google Scholar]
- 2.Petrovic Fabijan A, Lin RCY, Ho J, Maddocks S, Ben Zakour NL, Iredell JR, Westmead Bacteriophage Therapy Team . 2020. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol 5:465–472. doi: 10.1038/s41564-019-0634-z. [DOI] [PubMed] [Google Scholar]
- 3.Poole K. 2012. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother 67:2069–2089. doi: 10.1093/jac/dks196. [DOI] [PubMed] [Google Scholar]
- 4.Kolter R, Siegele DA, Tormo A. 1993. The stationary phase of the bacterial life cycle. Annu Rev Microbiol 47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 5.Gibson B, Wilson DJ, Feil E, Eyre-Walker A. 2018. The distribution of bacterial doubling times in the wild. Proc Biol Sci 285:20180789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergkessel M, Basta DW, Newman DK. 2016. The physiology of growth arrest: uniting molecular and environmental microbiology. Nat Rev Microbiol 14:549–562. doi: 10.1038/nrmicro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haugan MS, Charbon G, Frimodt-Moller N, Lobner-Olesen A. 2018. Chromosome replication as a measure of bacterial growth rate during Escherichia coli infection in the mouse peritonitis model. Sci Rep 8:14961. doi: 10.1038/s41598-018-33264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 9.Barrett TC, Mok WWK, Murawski AM, Brynildsen MP. 2019. Enhanced antibiotic resistance development from fluoroquinolone persisters after a single exposure to antibiotic. Nat Commun 10:1177. doi: 10.1038/s41467-019-09058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munita JM, Arias CA. 2016. Mechanisms of antibiotic resistance. Microbiol Spectr 4(2). doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y. 2014. Persisters, persistent infections and the Yin-Yang model. Emerg Microbes Infec 3:1–10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3913823/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van den Bergh B, Fauvart M, Michiels J. 2017. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol Rev 41:219–251. doi: 10.1093/femsre/fux001. [DOI] [PubMed] [Google Scholar]
- 13.Wu N, He L, Cui P, Wang W, Yuan Y, Liu S, Xu T, Zhang S, Wu J, Zhang W, Zhang Y. 2015. Ranking of persister genes in the same Escherichia coli genetic background demonstrates varying importance of individual persister genes in tolerance to different antibiotics. Front Microbiol 6:1003. doi: 10.3389/fmicb.2015.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood TK, Knabel SJ, Kwan BW. 2013. Bacterial persister cell formation and dormancy. Appl Environ Microbiol 79:7116–7121. doi: 10.1128/AEM.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai Y, Mercier R, Wu LJ, Domínguez-Cuevas P, Oshima T, Errington J. 2015. Cell growth of wall-free L-form bacteria is limited by oxidative damage. Curr Biol 25:1613–1618. doi: 10.1016/j.cub.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercier R, Kawai Y, Errington J. 2013. Excess membrane synthesis drives a primitive mode of cell proliferation. Cell 152:997–1007. doi: 10.1016/j.cell.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Mercier R, Kawai Y, Errington J. 2014. General principles for the formation and proliferation of a wall-free (L-form) state in bacteria. Elife 3:e04629. doi: 10.7554/eLife.04629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Errington J, Mickiewicz K, Kawai Y, Wu LJ. 2016. L-form bacteria, chronic diseases and the origins of life. Philos Trans R Soc Lond B Biol Sci 371:20150494. doi: 10.1098/rstb.2015.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leaver M, Dominguez-Cuevas P, Coxhead JM, Daniel RA, Errington J. 2009. Erratum: life without a wall or division machine in Bacillus subtilis. Nature 460:538. doi: 10.1038/nature08232. [DOI] [PubMed] [Google Scholar]
- 20.Kawai Y, Mickiewicz K, Errington J. 2018. Lysozyme counteracts β-lactam antibiotics by promoting the emergence of L-form bacteria. Cell 172:1038–1049.e10. doi: 10.1016/j.cell.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mickiewicz KM, Kawai Y, Drage L, Gomes MC, Davison F, Pickard R, Hall J, Mostowy S, Aldridge PD, Errington J. 2019. Possible role of L-form switching in recurrent urinary tract infection. Nat Commun 10:4379. doi: 10.1038/s41467-019-12359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai K, Lutkenhaus J. 1991. ftsZ is an essential cell-division gene in Escherichia coli. J Bacteriol 173:3500–3506. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glover WA, Yang YQ, Zhang Y. 2009. Insights into the molecular basis of L-form formation and survival in Escherichia coli. PLoS One 4:e7316. doi: 10.1371/journal.pone.0007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davison F, Chapman J, Mickiewicz K. 2020. Isolation of L-form bacteria from urine using filtration method. J Vis Exp 160:e61380. doi: 10.3791/61380. [DOI] [PubMed] [Google Scholar]
- 25.Fontana R, Cornaglia G, Ligozzi M, Mazzariol A. 2000. The final goal: penicillin-binding proteins and the target of cephalosporins. Clin Microbiol Infect 6(Suppl 3):34–40. doi: 10.1111/j.1469-0691.2000.tb02038.x. [DOI] [PubMed] [Google Scholar]
- 26.Li W-J, Li D-F, Hu Y-L, Zhang X-E, Bi L-J, Wang D-C. 2013. Crystal structure of L,D-transpeptidase LdtMt2 in complex with meropenem reveals the mechanism of carbapenem against Mycobacterium tuberculosis. Cell Res 23:728–731. doi: 10.1038/cr.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisabarro AG, de Pedro MA, Vazquez D. 1985. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol 161:238–242. doi: 10.1128/jb.161.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mainardi J-L, Fourgeaud M, Hugonnet J-E, Dubost L, Brouard J-P, Ouazzani J, Rice LB, Gutmann L, Arthur M. 2005. A novel peptidoglycan cross-linking enzyme for a beta-lactam-resistant transpeptidation pathway. J Biol Chem 280:38146–38152. doi: 10.1074/jbc.M507384200. [DOI] [PubMed] [Google Scholar]
- 29.Tuomanen E, Cozens R. 1987. Changes in peptidoglycan composition and penicillin-binding proteins in slowly growing Escherichia coli. J Bacteriol 169:5308–5310. doi: 10.1128/jb.169.11.5308-5310.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glauner B, Holtje JV, Schwarz U. 1988. The composition of the murein of Escherichia coli. J Biol Chem 263:10088–10095. doi: 10.1016/S0021-9258(19)81481-3. [DOI] [PubMed] [Google Scholar]
- 31.Hugonnet J-E, Mengin-Lecreulx D, Monton A, den Blaauwen T, Carbonnelle E, Veckerlé C, Brun YV, van Nieuwenhze M, Bouchier C, Tu K, Rice LB, Arthur M. 2016. Factors essential for L,D-transpeptidase-mediated peptidoglycan cross-linking and β-lactam resistance in Escherichia coli. Elife 5:e19469. doi: 10.7554/eLife.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aliashkevich A, Cava F. 2021. LD-transpeptidases: the great unknown among the peptidoglycan cross-linkers. FEBS J 289:4718–4730. doi: 10.1111/febs.16066. [DOI] [PubMed] [Google Scholar]
- 33.European Committee on Antimicrobial Susceptibility Testing. 2020. Breakpoint tables for interpretation of MICs and zone diameters, version 10.0. EUCAST, Copenhagen, Denmark. [Google Scholar]
- 34.Yi H, Cho YJ, Yong D, Chun J. 2012. Genome sequence of Escherichia coli J53, a reference strain for genetic studies. J Bacteriol 194:3742–3743. doi: 10.1128/JB.00641-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agyekum A, Fajardo-Lubián A, Ai X, Ginn AN, Zong Z, Guo X, Turnidge J, Partridge SR, Iredell JR. 2016. Predictability of phenotype in relation to common β-lactam resistance mechanisms in Escherichia coli and Klebsiella pneumoniae. J Clin Microbiol 54:1243–1250. doi: 10.1128/JCM.02153-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan EM, Alkawareek MY, Donnelly RF, Gilmore BF. 2012. Synergistic phage–antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol Med Microbiol 65:395–398. doi: 10.1111/j.1574-695X.2012.00977.x. [DOI] [PubMed] [Google Scholar]
- 37.Ongenae V, Sidi Mabrouk A, Crooijmans M, Rozen D, Briegel A, Claessen D. 2021. Reversible bacteriophage resistance by shedding the bacterial cell wall. bioRxiv. doi: 10.1101/2021.11.17.468999. [DOI] [PMC free article] [PubMed]
- 38.Ongenae V, Mabrouk AS, Crooijmans M, Rozen D, Briegel A, Claessen D. 2022. Reversible bacteriophage resistance by shedding the bacterial cell wall. Open Biol 12:210379. doi: 10.1098/rsob.210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Australian Commission on Safety and Quality in Health Care. 2021. AURA 2021: fourth Australian report on antimicrobial use and resistance in human health. Australian Commission on Safety and Quality in Health Care, Sydney, Australia. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-2021. [Google Scholar]
- 40.CDC, U.S. Department of Health and Human Services. 2021. 2021 update: antibiotic use in the United States: progress and opportunities. CDC, Atlanta, GA. https://www.cdc.gov/antibiotic-use/pdfs/stewardship-report-2021-h.pdf [Google Scholar]
- 41.Paterson DL, Stewart AG, Young B, Lye DC, Davis JS, Schneider K, Yilmaz M, Dinleyici R, Runnegar N, Henderson A, Archuleta S, Kalimuddin S, Forde BM, Chatfield MD, Bauer MJ, Lipman J, Harris-Brown T, Harris PNA, for the MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN) . 2021. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections caused by AmpC β-lactamase-producing Enterobacter spp., Citrobacter freundii, Morganella morganii, Providencia spp., or Serratia marcescens: a pilot multicenter randomized controlled trial (MERINO-2). Open Forum Infect Dis 8:ofab387. doi: 10.1093/ofid/ofab387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Jong IG, Beilharz K, Kuipers OP, Veening JW. 2011. Live cell imaging of Bacillus subtilis and Streptococcus pneumoniae using automated time-lapse microscopy. J Vis Exp 53:e3145. doi: 10.3791/3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knezevic P, Kostanjsek R, Obreht D, Petrovic O. 2009. Isolation of Pseudomonas aeruginosa specific phages with broad activity spectra. Curr Microbiol 59:173–180. doi: 10.1007/s00284-009-9417-8. [DOI] [PubMed] [Google Scholar]
- 46.Petrovic A, Kostanjsek R, Rakhely G, Knezevic P. 2017. The first Siphoviridae family bacteriophages infecting Bordetella bronchiseptica isolated from environment. Microb Ecol 73:368–377. doi: 10.1007/s00248-016-0847-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplemental material (posted at https://figshare.com/s/09b4bbc18c62c1d6aadd). Whole-genome sequencing data are available on NCBI under the BioProject accession number PRJNA764821 and GenBank number OL362041.