Abstract
Antibiotic-treated mice orally inoculated with one of three Candida albicans strains (including two mutant strains) or indigenous Candida pelliculosa showed levels of candidal gastrointestinal colonization that were strain specific. However, regardless of strain, the numbers of viable candida were intermediate to high in the stomach, were consistently lowest in the upper small intestine, and increased progressively down the intestinal tract.
In humans, Candida albicans is a member of the indigenous flora of the digestive tract, but it is also a potential pathogen and a frequent cause of complicating systemic infection in immunosuppressed patients, trauma patients, postsurgical patients, diabetics, premature infants, and patients infected with human immunodeficiency virus type 1 5, 7, 20. Risk factors include neutropenia; use of vascular catheters, broad-spectrum antibiotics, and total parenteral nutrition; hemodialysis; oral mucosal colonization; abdominal surgery; prematurity; damage to the intestinal mucosa; burns; and chronic corticosteroid therapy 5, 20. Candida species number among the most common nosocomial pathogens in the United States, and C. albicans accounts for the majority of all fungal isolates 20. Increased intestinal colonization is generally accepted as a major risk factor that predisposes high-risk patients to systemic candidiasis 5.
The mouse has often been used in studies designed to clarify the pathogenesis of systemic candidiasis, making it important to understand the ability of C. albicans to colonize the mouse gastrointestinal (GI) tract. The concentration of intestinal C. albicans has often been correlated with the incidence of systemic infection, and several investigators (including ourselves) have assumed that the cecum is a representative site that can be used to monitor mouse GI colonization by C. albicans 2, 6, 12–16, although the reasons for this choice have largely been anecdotal.
Due to recent advances in genetic manipulation of C. albicans, it is now possible to sequentially disrupt both copies of a single gene in this diploid organism by use of the ura-blaster technique 1, 8, 11. This technique uses a hisG-URA3-hisG cassette to disrupt one copy of the gene in question, resulting in a heterozygous disruptant carrying the URA3 marker. Spontaneous excision of URA3 and recombination of the hisG repeats results in a heterozygous mutant lacking the URA3 marker. A second round of disruption with this heterozygote results in a homozygous disruptant carrying the URA3 marker in one allele. Mutant strains generated by this technique are being used in a variety of in vitro and in vivo studies designed to clarify the role(s) of specific genes in C. albicans pathogenesis. To our knowledge, there have been no studies that have clarified the comparative abilities of wild-type and mutant strains generated by the ura-blaster method to colonize the mouse GI tract. This may be important because strains with ura-blaster-mediated genetic disruptions likely have altered orotidine 5′-monophosphate decarboxylase enzyme activity 19. This enzyme is encoded by the URA3 gene, and there is evidence that the activity of this enzyme may affect virulence in C. albicans 17, 19. Thus, C. albicans strains with altered orotidine 5′-monophosphate decarboxylase activity may have an altered ability to colonize the mouse GI tract. Furthermore, the additional genetic manipulations associated with gene disruption may also affect colonization.
Herein we compare the abilities of three exogenous strains of C. albicans and one indigenous Candida species, namely, Candida pelliculosa, to colonize the esophagus, stomach, upper and lower small intestine, cecum, and colon of antibiotic-treated mice. Candida species cannot be consistently recovered from the mouse intestinal flora, and C. albicans is not a member of the mouse normal flora 4, 21. C. albicans CAF2 and CAG3 were generated by the ura-blaster technique. C. albicans CAF2 (INT1/INT URA3/ura3::imm434) was obtained from W. A. Fonzi, Georgetown University, Washington, D.C. 8. Construction of C. albicans CAG3 (int1::hisG/int1::hisG-URA3-hisG ura3::imm434/ura3::imm434) has been described previously 10, and characteristics of CAF2 and CAG3 have been published elsewhere 3, 9, 10. Compared to CAG3 (int1/int1), CAF2 (INT1/INT1) demonstrates extensive hyphal development on agar media known to stimulate filamentation, shows increased adhesion to cultured epithelial cells (HeLa cells), and causes increased mortality in intravenously inoculated mice. The strain with the null mutation, CAG3 (int1/int1), has markedly reduced hyphal formation on agar media, shows minimal adherence to HeLa cells, and causes minimal mortality in intravenously inoculated mice. CAF2 has orotidine 5′-monophosphate decarboxylase activity typical of wild-type C. albicans, while CAG3 has markedly reduced activity 19. C. albicans 315 is a clinical isolate from the blood of a human, and this strain demonstrates filamentation typical of the species, i.e., germ tubes, pseudohyphae, and hyphae. C. pelliculosa M33 is an indigenous yeast isolated from the cecum of the same mouse strain used in this study. C. pelliculosa is capable of forming pseudohyphae but is not capable of forming germ tubes or true hyphae. Stock cultures of all Candida strains were maintained at −80°C in Sabouraud's dextrose broth (Difco Laboratories, Detroit, Mich.) supplemented with 15% glycerol. For oral inoculation into mice, stock cultures were plated on minimal medium agar 9 supplemented with 2% dextrose, incubated at 30°C for 48 h, and then inoculated into minimal medium dextrose broth, incubated at 30°C with shaking for 18 h, washed, and resuspended in sterile saline. The yeast concentration was determined with a hemocytometer and was verified by quantitative culture on Sabouraud's dextrose agar incubated 48 h at 30°C. All strains grew exclusively as blastoconidia (budding yeast) under these conditions.
Six-week-old female Swiss Webster mice (weight, 18 to 22 g) were purchased from Harlan Sprague-Dawley, Indianapolis, Id. Experiments were performed according to National Institutes of Health guidelines on the use of experimental animals, and all protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Mice were pretreated for 3 days with drinking water containing 1 mg of bacitracin (Sigma Chemical Co., St. Louis, Mo.) per ml, 2 mg of streptomycin sulfate (Sigma) per ml, and 0.1 mg of gentamicin sulfate (Sigma) per ml and were then orally inoculated with a feeding needle (No. 9921; Popper & Sons, Inc., New Hyde Park, N.Y.) with 107 C. albicans 315, CAF2, or CAG3 or C. pelliculosa suspended in 0.1 ml of sterile saline. Antibiotic treatment was continued for the duration of the experiment. Mice were killed 3 days later. To eliminate cross-contamination among the various treatment groups, mice were housed in cages with filter tops and were handled by specially trained personnel. These conditions have been shown to eliminate cross-contamination of inoculated strains among mouse treatment groups 16.
Mice were killed by cervical dislocation, and their tissues were aseptically excised for quantitative analysis of aerobic and facultative microbial flora. Tissues included the esophagus (entire), stomach, upper small intestine (stomach to ligament of Treitz), lower small intestine (ligament of Treitz to ileal-cecal junction), cecum, and colon. The tissues were weighed, homogenized, serially diluted, plated on agar media, and incubated for 24 to 48 h as described previously 2, 16. Agar media included MacConkey agar for selective isolation of gram-negative aerobic bacteria and colistin-nalidixic acid agar for selective isolation of aerobic gram-positive bacteria and yeast. We have shown in previous work that yeast grow equally well on colistin-naladixic acid agar and Sabouraud's dextrose agar 16, and use of both MacConkey and colistin-naladixic acid agars provides optimum recovery of both bacteria and yeast 18. Microbes were identified by standard techniques 18.
Excluding the esophageal segment, microbes were enumerated as the log10 number of viable organism per gram (wet weight) of tissue with contents, and the lower limit of detection was 3.0 log10 per gram of tissue. Esophageal microbes were enumerated as the total number per tissue, and the lower detection limit was 10 microbes per tissue. For statistical analysis, values below the lower detection limit were assigned a value equal to the lower detection limit. Data were analyzed by analysis of variance followed by Fisher's test for significant difference. Statistical analyses were performed with StatView (version 5.0; Abacus Concepts, Berkeley, Calif.), and significance was set at a P value of <0.05. Similar data were pooled from each of two experiments with four mice per treatment group, for a total of eight mice per treatment group.
No microbial flora, other than the inoculated candidal strain, was detected in any tissue analyzed. C. albicans 315, CAF2, and CAG3 were recovered from esophageal tissues of some, but not all, mice, and C. pelliculosa was not detected in the esophagus (Table 1). Sporadic recovery from esophageal tissue suggested that this was not a site of active colonization, and the recovered candida likely reflected transient microbes ingested by these coprophagic animals.
TABLE 1.
Recovery of candida from esophageal tissue of antibiotic-treated mice orally inoculated with C. albicans 315, CAF2, or CAG3 or C. pelliculosa
| Candida species or strain | No. of mice with esophageal candida/ total no. mice (%) | Avg ± SE no. of viable candida/ esophagusa |
|---|---|---|
| C. albicans 315 | 3/8 (38) | 440 ± 266 |
| C. albicans CAF2 | 3/8 (38) | 17 ± 7 |
| C. albicans CAG3 | 2/8 (25) | 10 ± 0 |
| C. pelliculosa | 0/8 (0) | Not applicable |
Average for mice with detectable yeast.
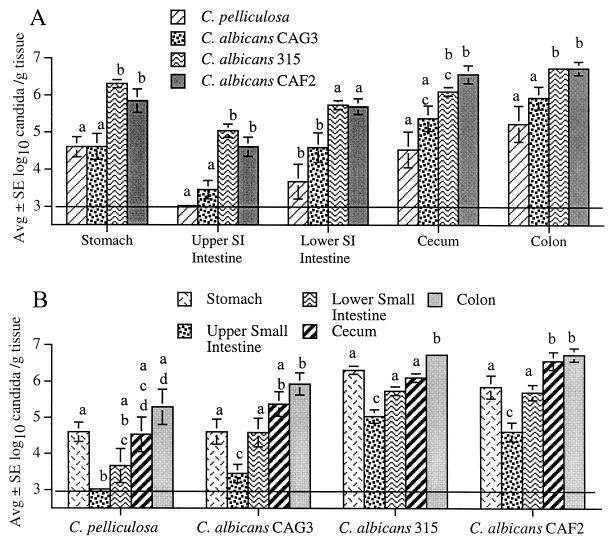
Analysis of individual GI segments (Fig. 1A) indicated that colonization was similarly high for the wild-type strain C. albicans 315 and CAF2, the parent strain (INT1/INT1) used to produce the strain with the null mutation, strain CAG3 (int1/int1). Colonization was similar for C. albicans CAG3 and the indigenous C. pelliculosa strain, and both strains were recovered from GI segments in lower numbers compared to the numbers for C. albicans 315 and CAF2 (Fig. 1A). Analysis of individual candidal strains revealed a similar colonization pattern for each strain (Fig. 1B). The numbers of candida were consistently lowest in the upper small intestine and increased progressively down the intestinal tract, with the highest numbers recovered from the colon. Interestingly, the numbers of candida recovered from the stomach were typically similar to the relatively high numbers recovered from the lower small intestine or cecum (Fig. 1B). This pattern of intestinal colonization was similar to that reported for a mouse isolate of Torulopsis (Candida) pintolopesii 22.
FIG. 1.
Numbers of viable C. albicans 315, CAF2, and CAG3 and C. pelliculosa recovered from mouse GI segments. (A) Within a given GI segment, the numbers of C. albicans 315 and CAF2 were consistently similar to each other and higher than the numbers of C. albicans CAG3 and C. pelliculosa, with exception of the cecum, where the numbers of strain 315 were not statistically different from those of strain CAG3. (B) For individual strains, candidal colonization was always lowest in the upper small intestine and highest in the colon, and with the exception of C. albicans 315, colonization in the cecum and colon was not statistically different. Bars with the same letters are not different from each other; bars with different letters are statistically different (typically P < 0.01). The horizontal line indicates the lower limit of assay detection.
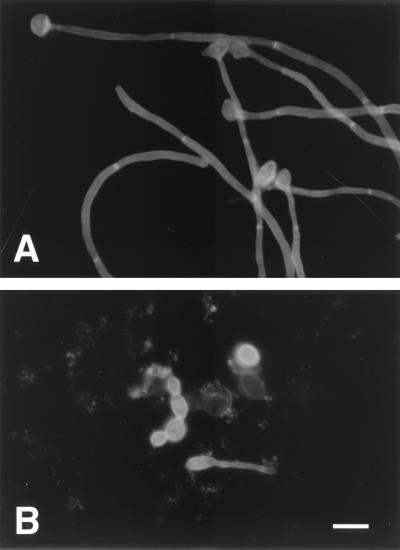
To visualize in vivo morphology, cecal contents were analyzed from all mice. Cecal contents were rinsed from the tissue with a minimal volume (1 to 2 ml) of sterile saline, and a minimal volume (100 to 200 μl) was stained with calcofluor according to the manufacturer's directions (Fungi-Fluor kit; Polysciences, Inc., Warrington, Pa.) (Fig. 2). The remaining tissue and contents were used for quantitative culture as described above. Specimens were examined under an epifluorescence microscope. Fungal elements (100 from each of eight mice per treatment group) were identified as either yeast or filamentous forms, the latter defined as an outgowth four times the width of the mother cell or a chain of four or more elongated yeast cells. The C. pelliculosa and C. albicans CAG3, CAF2, and 315 strains showed 8.3% ± 3.7%, 3.7% ± 1.5%, 14.3% ± 4.3%, and 40.0% ± 15.4% (average ± standard error) filamentation, respectively. Filamentation of C. albicans 315 was increased compared to that for each of the other strains (P < 0.01 by analysis of variance with Fisher's post hoc test). Phillips and Balish 21 reported that C. albicans was 100% yeast forms in stomach contents from conventional mice but 40% filamentous forms in stomach contents from germfree mice that had been monoassociated with C. albicans. In the present study, the morphology of cecal C. albicans appeared to be more similar to that reported for monoassociated mice than for conventionally reared mice. This seemed reasonable because, in the present study, none of the mice had detectable competing aerobic or facultative bacterial flora, and the inoculated candidal strain was the only microbe recovered from the GI tract.
FIG. 2.
Calcofluor staining of cecal contents of mice colonized with C. albicans CAF2 (A) and CAG3 (B), showing both yeast and filamentous forms in vivo. Bar, 14 μm.
In summary, although some candidal strains colonized the mouse GI tract in greater or lesser numbers, the pattern of colonization was similar from strain to strain. This consistency included exogenous C. albicans and indigenous C. pelliculosa, as well as C. albicans subjected to manipulations associated with the ura-blaster technique of mutagenesis. Either the stomach, lower small intestine, cecum, or colon could be used to monitor and/or compare colonization by different candidal strains, including strains with altered orotidine 5′-monophosphate decarboxylase activity associated with ura-blaster mutagenesis.
Acknowledgments
This work was supported by Public Health Service grant GM59221 from the National Institutes of Health, as well as grant 9906100 from the March of Dimes Birth Defects Foundation.
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendel C M, Kinneberg K M, Jechorek R P, Erlandsen S L, Sahar D E, Wells C L. The Candida albicans INT1 gene facilitates cecal colonization in endotoxin-treated mice. Shock. 2000;13:453–458. doi: 10.1097/00024382-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bendel C M, Kinneberg K M, Jechorek R P, Gale C A, Erlandsen S L, Hostetter M K, Wells C L. Systemic infection following intravenous inoculation of mice with Candida albicans INT1 mutant strains. Mol Genet Metab. 1999;67:343–351. doi: 10.1006/mgme.1999.2875. [DOI] [PubMed] [Google Scholar]
- 4.Brown J F, Balish E. Gastrointestinal microecology of BALB/c nude mice. Appl Environ Microbiol. 1978;36:144–159. doi: 10.1128/aem.36.1.144-159.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole G T, Halawa A A, Anaissie E J. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin Infect Dis. 1996;22(Suppl. 2):S73–S88. doi: 10.1093/clinids/22.supplement_2.s73. [DOI] [PubMed] [Google Scholar]
- 6.Ekenna O, Sherertz R J. Factors affecting colonization and dissemination of Candida albicans from the gastrointestinal tract of mice. Infect Immun. 1987;55:1558–1563. doi: 10.1128/iai.55.7.1558-1563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eubanks P J, de Virgilio C, Klein S, Bongard F. Candida sepsis in surgical patients. Am J Surg. 1993;166:617–620. doi: 10.1016/s0002-9610(05)80666-x. [DOI] [PubMed] [Google Scholar]
- 8.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gale C, Finkel D, Tao N, Meinke M, McClellan M, Olson J, Kendrick K, Hostetter M. Cloning and expression of a gene encoding an integrin-like protein in Candida albicans. Proc Natl Acad Sci USA. 1996;93:357–361. doi: 10.1073/pnas.93.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale C A, Bendel C M, McClellan M A, Hauser M, Becker J M, Berman J A, Hostetter M K. Linkage of adhesion, filamentous growth and virulence in Candida albicans to a single gene INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 11.Gow N A R, Robbins P W, Lester J W, Brown A J P, Fonzi W A, Chapman T, Kinsman O S. A hyphal-specific chitin synthase gene (CHS2) is not essential for growth, dimorphism, or virulence of Candida albicans. Proc Natl Acad Sci USA. 1994;91:6216–6220. doi: 10.1073/pnas.91.13.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helstrom P B, Balish E. Effect of oral tetracycline, the microbial flora, and the athymic state on gastrointestinal colonization and infection of BALB/c mice with Candida albicans. Infect Immun. 1979;23:764–774. doi: 10.1128/iai.23.3.764-774.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy M J, Volz P A. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985;49:654–663. doi: 10.1128/iai.49.3.654-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy M J, Volz P A, Edwards C A, Yancey R J. Mechanisms of association of Candida albicans with intestinal mucosa. J Med Microbiol. 1987;24:333–341. doi: 10.1099/00222615-24-4-333. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy M J, Volz P A. Effect of various antibiotics on gastrointestinal colonization and dissemination by Candida albicans. Sabouraudia J Med Vet Mycol. 1985;32:265–273. doi: 10.1080/00362178585380391. [DOI] [PubMed] [Google Scholar]
- 16.Kinnenberg K M, Bendel C M, Jechorek R P, Cebelinski E A, Gale C A, Berman J G, Erlandsen S L, Hostetter M K, Wells C L. Effect of INT1 gene on Candida albicans murine intestinal colonization. J Surg Res. 1999;87:245–251. doi: 10.1006/jsre.1999.5755. [DOI] [PubMed] [Google Scholar]
- 17.Kirsch D R, Whitney R R. Pathogenicity of Candida albicans auxotrophic mutants in experimental infections. Infect Immun. 1991;59:3297–3300. doi: 10.1128/iai.59.9.3297-3300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koneman E W, Allen S D, Janda W M, Schreckenberger P C, Winn W C, editors. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia, Pa: Lippincott; 1997. [Google Scholar]
- 19.Lay J, Henry J K, Clifford J, Koltin Y, Bulawa C, Becker J. Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect Immun. 1998;66:5301–5306. doi: 10.1128/iai.66.11.5301-5306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller M A. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin Infect Dis. 1996;22(Suppl. 2):S89–S94. doi: 10.1093/clinids/22.supplement_2.s89. [DOI] [PubMed] [Google Scholar]
- 21.Phillips A W, Balish E. Growth and invasiveness of Candida albicans in the germ-free and conventional mouse after oral challenge. Appl Microbiol. 1966;14:737–741. doi: 10.1128/am.14.5.737-741.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suegara N, Siegel J, Savage D. Ecological determinants in microbial colonization of the murine gastrointestinal tract: adherence of Torulopsis pintolopesii to epithelial surfaces. Infect Immun. 1979;25:139–145. doi: 10.1128/iai.25.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]