ABSTRACT
Multidrug-resistant (MDR) Pseudomonas aeruginosa infections are associated with poor patient outcomes due to complex co-resistance patterns. We described common co-resistance patterns, clinical characteristics, and associated outcomes in patients admitted with an MDR P. aeruginosa. This national, multicenter, retrospective cohort study within the Veterans Affairs included adults hospitalized with a MDR P. aeruginosa infection (January 2015–December 2020) per Centers for Disease Control definition. Clinical outcomes were compared among those with differing MDR P. aeruginosa co-resistance: resistant to carbapenems and extended-spectrum cephalosporins and piperacillin-tazobactam (CARB/ESC/PT) versus without CARB/ESC/PT resistance; resistant to carbapenems and extended-spectrum cephalosporins and fluoroquinolone (CARB/ESC/FQ) versus without CARB/ESC/FQ resistance. We included 3,763 hospitalized patients. Co-resistance to CARB/ESC/PT was observed in 42.7%, and to CARB/ESC/FQ in 40.7%. The lowest co-resistance rates were observed with ceftolozane-tazobactam (6.2%, n = 6/97; 12.5%, n = 10/80, respectively) and ceftazidime-avibactam (5.2%, n = 5/97; 12.5%, n = 10/80, respectively). Overall, 14.2% of patients died during hospitalization, 59.7% had an extended length of stay, and 14.9% had reinfection with hospitalization. Outcomes were similar between patients with MDR P. aeruginosa strains with and without co-resistance to CARB/ESC/PT and CARB/ESC/FQ. Among a national cohort of patients hospitalized with MDR P. aeruginosa infections, co-resistance to three classes of standard of care antibiotics, such as carbapenem, extended-spectrum cephalosporins, and piperacillin-tazobactam or fluoroquinolones, exceeded 40% in our study population, posing great concerns for selecting appropriate empirical therapy. Clinical outcomes were poor for all patients, regardless of different co-resistance patterns. New treatment options are needed for hospitalized patients with suspected or confirmed MDR P. aeruginosa infections.
IMPORTANCE We studied antibiotic co-resistance patterns in a national group of hospitalized patients with infections due to multidrug-resistant (MDR) Pseudomonas aeruginosa, a type of bacteria that resists treatment to at least three classes of antibiotics. Co-resistance to antibiotic classes most typically used for treatment was common, which makes selecting appropriate antibiotics to successfully treat the infections difficult. Outcomes, including death, were poor for all patients in our study, regardless of the different patterns of co-resistance to common antibiotic classes. New antibiotics are needed to help treat hospitalized patients with MDR P. aeruginosa infections.
KEYWORDS: antibiotic resistance, antipseudomonal antibiotics, co-resistance, Pseudomonas aeruginosa, multidrug resistance
Pseudomonas aeruginosa infections are associated with high mortality, prolonged hospital stays, and increased health care costs (1). The poor clinical outcomes associated with these infections are mainly due to their multidrug-resistant (MDR) nature, limiting effective antibiotic treatment options (1). P. aeruginosa has the remarkable ability to evade the effects of many commonly used antibiotics such as carbapenems, cefepime, piperacillin-tazobactam, and fluoroquinolones (2, 3). Often, these multiple resistance mechanisms co-occur, conferring MDR P. aeruginosa strains, which often have complex co-resistance patterns (4). Up to 25% of clinical P. aeruginosa isolates worldwide are MDR (5). Our previous research has demonstrated high rates of P. aeruginosa across the national Veterans Affairs (VA) Healthcare System (6). Among over 20,000 clinical P. aeruginosa isolates collected from inpatient settings, 24% were resistant to at least one of the following antibiotic classes: carbapenems, extended-spectrum cephalosporins, piperacillin-tazobactam, or fluoroquinolones. The prevalence of MDR P. aeruginosa in inpatient settings was 22%.
P. aeruginosa resistance is already well defined, and mortality is highest among patients infected with MDR P. aeruginosa strains. We, therefore, set out to determine co-resistance patterns among MDR P. aeruginosa, and subsequent patient outcomes. Co-resistance has previously been defined as antibiotic resistance in a subset of isolates already resistant to other antibiotics and provides a different means for evaluating MDR organisms, and ultimately identifying novel empirical treatment options (7). Evaluating co-resistance sheds light on the challenges clinicians face when multiple classes of antibiotics are no longer treatment options. Despite the clinical importance of MDR P. aeruginosa, few studies offer comprehensive evaluations of co-resistance to commonly used antibiotics among hospitalized patients with MDR P. aeruginosa infections (7). This study examined antibiotic co-resistance among hospitalized patients with MDR P. aeruginosa infections and assessed the demographics, comorbidities, clinical characteristics, and outcomes of the study population. We then explored the impact of common co-resistance patterns on clinical outcomes.
RESULTS
Overall, 3,763 hospitalized patients with MDR P. aeruginosa infection were identified over the study period. The most common sources of infection were urine (41.4%) and respiratory (31.8%). Demographics, comorbidities, and clinical characteristics of the study population are shown in Table 1. Patients were older (mean age 69.4 years), males (97.8%), with a high comorbidity burden (median Charlson score 4), and more than half (51.6%) spent time in intensive care during the hospitalization. Healthcare exposures in the 90 days prior to admission were common, with 47.4% having a previous hospital stay, 18.1% intensive care stay, and 7.0% nursing home stay. Antibiotic exposures in the 90 days before admission were also common, with 63.1% exposed to antibiotics previously. Almost a quarter (22.8%) of patients had a prior P. aeruginosa culture 90 days before admission.
TABLE 1.
Clinical outcomes among hospitalized patients with MDR Pseudomonas aeruginosa infection stratified by common co-resistance patternsa
| Clinical outcomes | All (N = 3,763) | Strain with carbapenem, extended-spectrum cephalosporin, and piperacillin/tazobactam co-resistanceb (N = 1,419) | Strain without carbapenem, extended-spectrum cephalosporin, and piperacillin/tazobactam co-resistance (thus susceptible to at least one of these antibiotic classes)b (N = 1,901) | Adjusted odds ratio, (95% confidence interval) | Strain with carbapenem, extended-spectrum cephalosporin, and fluoroquinolone co-resistancec (N = 1,446) | Strain without carbapenem, extended-spectrum cephalosporin, and fluoroquinolone co-resistance (thus susceptible to at least one of these antibiotic classes)c (N = 2,104) | Adjusted odds ratio, (95% confidence interval) |
|---|---|---|---|---|---|---|---|
| Inpatient mortality | 533 (14.2%) | 251 (17.7%) | 219 (11.5%) | 1.07 (0.84–1.37)d | 222 (15.4%) | 287 (13.6%) | 1.0 (0.80–1.25)e |
| Extended length of hospital stay greater than the median | 2,246 (59.7%) | 946 (66.7%) | 1,030 (54.2%) | 0.91 (0.75–1.10)f | 899 (62.2%) | 1,212 (57.6%) | 0.82 (0.69–1.00) g |
| MDR P. aeruginosa reinfection with hospitalization within one yr of discharge | 560 (14.9%) | 195 (13.7%) | 293 (15.4%) | 0.93 (0.76–1.13)h | 242 (16.7%) | 286 (13.6%) | 1.31(1.08–1.59) i |
Bold indicates significant in multivariable analysis. Outcome data are presented for common resistance patterns observed among our population of hospitalized patients infected with MDR P. aeruginosa.
MDR P. aeruginosa strains with a carbapenem, extended-spectrum cephalosporin, and piperacillin-tazobactam co-resistance were defined those that tested intermediate or resistant to at least one drug in each of the following categories: carbapenems (imipenem, meropenem, or doripenem), extended-spectrum cephalosporins (cefepime or ceftazidime), and piperacillin-tazobactam (piperacillin, piperacillin-tazobactam). Strains without carbapenem, extended-spectrum cephalosporin, and piperacillin-tazobactam co-resistance were defined as those that tested susceptible to at least one drug in at least 1 of the following categories: carbapenems (imipenem, meropenem, or doripenem), extended-spectrum cephalosporins (cefepime or ceftazidime), and piperacillin-tazobactam (piperacillin, piperacillin-tazobactam). Data not available for 443 patients as two or fewer of these antibiotic classes were tested for susceptibility.
MDR P. aeruginosa strains with a carbapenem, extended-spectrum cephalosporin, and fluoroquinolone co-resistance were defined as those that tested intermediate or resistant to at least one drug in each of the following categories: carbapenems (imipenem, meropenem, or doripenem), extended-spectrum cephalosporins (cefepime or ceftazidime), and fluoroquinolones (ciprofloxacin, levofloxacin). Strains without carbapenem, extended-spectrum cephalosporin, and fluoroquinolone co-resistance were defined as those that tested susceptible to at least one drug in at least one of the following categories: carbapenems (imipenem, meropenem, or doripenem), extended-spectrum cephalosporins (cefepime or ceftazidime), and fluoroquinolones (ciprofloxacin, levofloxacin). Data not available for 213 patients as two or fewer of these antibiotic classes were tested for susceptibility.
Adjusted for aminoglycoside, extended-spectrum cephalosporin, and polymyxin treatment; current complication of surgical procedures or medical care, respiratory failure, and shock; congestive heart failure and malignancy comorbidities; septicemia and urinary tract infection diagnosis during admission; Elixhauser score, intensive care unit treatment during current admission, and initial susceptible treatment.
Adjusted for aminoglycoside, carbapenem, ceftazidime-avibactam, extended-spectrum cephalosporin, and polymyxin treatment; current respiratory failure and myocardial infarction; septicemia and urinary tract infection diagnosis during admission; liver disease, intensive care unit treatment during recent admission, age, skin and soft tissue culture source, and hospital-acquired infection.
Adjusted for aminoglycoside, ceftolozane-tazobactam, extended-spectrum cephalosporin, and polymyxin treatment; current complication of surgical procedures or medical care, fever, respiratory failure, and shock; pneumonia and ulcer diagnosis during admission; Elixhauser score, intensive care unit treatment during current admission, P. aeruginosa culture in the 90 days prior to admission, antibiotics in the 90 days before admission, community admission, Hispanic ethnicity, white race, hospital-acquired infection, and initial susceptible treatment.
Adjusted for aminoglycoside, carbapenem, ceftazidime-avibactam, ceftolozane-tazobactam, extended-spectrum cephalosporin, fluoroquinolone, and polymyxin treatment; current fever and respiratory failure; cerebrovascular disease, liver disease, and peripheral vascular disease comorbidities; pneumonia, septicemia, and ulcer diagnosis during admission; intensive care unit treatment during current admission, antibiotics in the 90 days prior to admission, community admission, age, Hispanic ethnicity, urine source, hospital-acquired infection, and initial susceptible treatment.
Adjusted for congestive heart failure and malignancy comorbidities; fluoroquinolone treatment, Elixhauser score, P. aeruginosa culture in the 90 days before admission, urinary tract infection diagnosis during admission; and respiratory culture source.
Adjusted for fluoroquinolone treatment, intensive care unit treatment during current admission, antibiotics in the 90 days before admission, urinary tract infection diagnosis during admission, age, and initial susceptible treatment.
TABLE 2.
Definitions of resistance in Pseudomonas aeruginosa
| Resistance phenotype | Definition |
|---|---|
| Carbapenem-resistant | Any isolate that tested either (I) or (R) to at least 1 of these: imipenem, meropenem, or doripenem |
| Extended-spectrum cephalosporin-resistant | Any isolate that tested (R) to at least 1 of these: cefepime or ceftazidime |
| Piperacillin-tazobactam-resistant | Any isolate that tested (R) to at least 1 of these: piperacillin, piperacillin-tazobactam |
| Fluoroquinolone-resistant | Any isolate that tested (R) to at least 1 of these: ciprofloxacin, levofloxacin |
| Aminoglycoside-resistant | Any isolate that tested (R) to at least 1 of these: amikacin, gentamicin, tobramycin |
| Multidrug-resistant (MDR) | Any isolate that tested either (I) or (R) to at least 1 drug in at least 3 of these categories: Extended-spectrum cephalosporins (cefepime, ceftazidime) Fluoroquinolones (ciprofloxacin, levofloxacin) Aminoglycosides (amikacin, gentamicin, tobramycin) Carbapenems (imipenem, meropenem, doripenem) Piperacillin Group (piperacillin, piperacillin-tazobactam) |
Resistance and co-resistance.
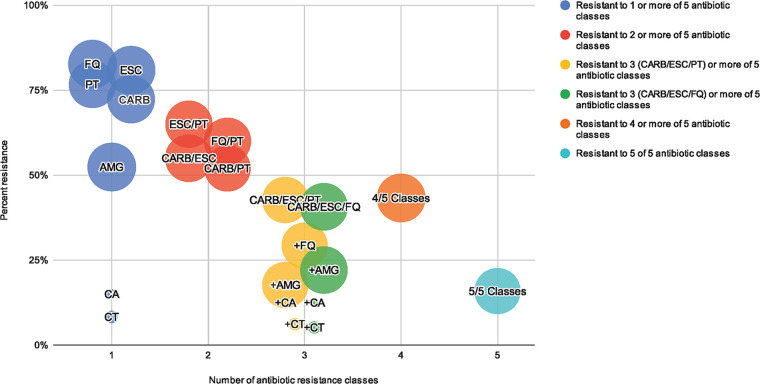
Resistance and co-resistance rates are shown in Fig. 1 and by culture source in Table S1 in the supplemental material. Individual resistance to commonly used antibiotics was high among MDR P. aeruginosa index isolates, including carbapenems (72.3%, n = 2,600/3,596), extended-spectrum cephalosporins (80.9%, n = 3,028/3,742), piperacillin-tazobactam (76.7%, n = 2,681/3,496), and fluroquinolones (82.7%, n = 3,082/3,726). Ceftolozane-tazobactam (8.3%, n = 8/97) and ceftazidime-avibactam (15.0%, n = 12/80) resistance rates were low, however few index MDR P. aeruginosa isolates were tested for susceptibility to these agents.
FIG 1.
Resistance and co-resistance in MDR Pseudomonas aeruginosa. Bubbles represent percent resistance or co-resistance for the antibiotic class/classes indicated among multidrug-resistant (MDR, tested either intermediate or resistant to at least one antibiotic in at least three categories [AMG, CARB, ESC, FQ, PT]) Pseudomonas aeruginosa isolates. Colors represent antibiotic class/classes of resistance. Yellow bubbles represent co-resistance to CARB/ESC/PT and additional classes (as indicated with the + sign). Green bubbles represent co-resistance to CARB/ESC/FQ and additional classes (as indicated with the + sign). Size of bubbles represent the number of isolates tested. AMG = aminoglycosides (amikacin, gentamicin, tobramycin); CA = ceftazidime-avibactam; CARB = carbapenems (imipenem, meropenem, doripenem); CT = ceftolozane-tazobactam; ESC = extended-spectrum cephalosporins (cefepime, ceftazidime); FQ = fluoroquinolone (ciprofloxacin, levofloxacin); PT = piperacillin-tazobactam.
Co-resistance to carbapenems and extended-spectrum cephalosporins with piperacillin-tazobactam (CARB/ESC/PT) was 42.7% (n = 1,419/3,320) and with fluoroquinolones (CARB/ESC/FQ) was 40.7% (n = 1,446/3,550). Lower rates of co-resistances were observed with the addition of aminoglycoside resistance to each of these patterns of co-resistance (17.7% CARB/ESC/PT, n = 585/3,307; and 22.1% CARB/ESC/FQ, n = 783/3,53). The lowest rates of co-resistance were observed with the addition of ceftolozane-tazobactam (6.2% CARB/ESC/PT, n = 6/97; 12.5% CARB/ESC/FQ, n = 10/80) and ceftazidime-avibactam (5.2% CARB/ESC/PT, n = 5/97; 12.5% CARB/ESC/FQ, n = 10/80) to each of these co-resistance patterns. Resistance to at least 4 and 5 classes were observed in 43.3% (n = 1,618/3,739) and in 18.2% (n = 522/3,281) of index isolates.
The highest rate of co-resistance to CARB/ESC/PT was observed from index respiratory isolates (48.9%, n = 517/1,057) and CARB/ESC/FQ from urine isolates (48.6%, n = 629/1,468).
Clinical outcomes.
Clinical outcomes are shown in Table 2. Overall, 14.2% of patients with MDR P. aeruginosa infection died during the hospitalization and 14.9% had reinfection within 1 year of discharge. The median length of stay from culture to discharge was 11 days (IQR 5–28). Inpatient mortality (17.7% versus 11.5%) and extended length of stay (66.7% versus 54.2%) were significantly higher among those infected with MDR P. aeruginosa strains with CARB/ESC/PT co-resistance versus without CARB/ESC/PT co-resistance. MDR P. aeruginosa reinfection (16.7% versus 13.6%) and extended length of stay (62.2% versus 57.6%) were significantly higher among those infected with MDR P. aeruginosa strains with CARB/ESC/FQ co-resistance versus without CARB/ESC/FQ co-resistance. However, in multivariable-adjusted analyses, outcomes were generally similar between patients infected with MDR P. aeruginosa strains with co-resistance patterns to CARB/ESC/PT and CARB/ESC/FQ versus without these co-resistance patterns. The odds of MDR P. aeruginosa reinfection were significantly higher among patients infected with strains with CARB/ESC/FQ co-resistance versus without CARB/ESC/FQ co-resistance (adjusted odds ratio [OR] 1.31, 95% confidence interval [CI] 1.08–1.59).
DISCUSSION
Among hospitalized patients with MDR P. aeruginosa infection, we identified high rates of antibiotic co-resistance to carbapenems and extended-spectrum cephalosporins, plus piperacillin-tazobactam resistance or fluroquinolone resistance. By examining co-resistance, instead of just individual antibiotic resistance rates, our study provides a more robust evaluation of resistance patterns in MDR P. aeruginosa. It brings further attention to the limited effective therapy options that clinicians have in their armamentarium to treat these complex infections.
The most commonly recommended agents for the empirical treatment of patients with suspected or documented MDR P. aeruginosa infections include one of the following antipseudomonal beta-lactam backbone agents: meropenem, imipenem, piperacillin-tazobactam, cefepime, and ceftazidime (2, 8–10). However, less than 50% of MDR P. aeruginosa isolates retain activity against these agents (2). In our study of hospitalized patients with MDR P. aeruginosa infections, observed susceptibility to these agents was even lower than previous reports, with only 27.7% demonstrating susceptibility to carbapenems, 19.1% to extended-spectrum cephalosporins, and 23.3% to piperacillin-tazobactam. A more concerning finding of our work was that of patients infected with MDR P. aeruginosa, almost half (42.7%) were co-resistant to all three antipseudomonal beta-lactam backbone agents (carbapenems, extended-spectrum cephalosporins, and piperacillin-tazobactam). Previous work has also shown this concerning co-resistance pattern is common among MDR P. aeruginosa isolates, with imipenem, cefepime, piperacillin-tazobactam, and aztreonam co-resistance, and ciprofloxacin demonstrating susceptibility, being the third most common pattern (11.6%) observed among 750 MDR P. aeruginosa isolates collected at 26 U.S. clinical laboratories between 2015 and 2017 (11).
Fluoroquinolones or aminoglycosides may be used as empirical therapy in combination with one or more of the antipseudomonal beta-lactam backbone agents when MDR P. aeruginosa is suspected (2, 10, 12). We also observed high co-resistance to carbapenems, extended-spectrum cephalosporins, and fluoroquinolones (40.7%). In addition, we observed co-resistance to carbapenems, extended-spectrum cephalosporins, piperacillin-tazobactam, and fluoroquinolones in 29.3%, leaving very few effective treatment options in patients infected with MDR P. aeruginosa strains co-resistant to these agents. In previous work, imipenem, cefepime, piperacillin-tazobactam, ciprofloxacin, and aztreonam resistance was the most common pattern (27.9%) observed among 750 MDR P. aeruginosa isolates (11). We observed lower individual antimicrobial resistance and co-resistance rates with aminoglycosides. These findings are supported by previous work which found that amikacin retained the highest percent susceptibility (96%) among MDR P. aeruginosa isolates from 26 clinical laboratories in the United States (11). Another previous multicenter retrospective study of 205 patients with MDR P. aeruginosa infections observed high aminoglycoside susceptibility rates (78.6% amikacin, 57.7% gentamicin, and 74.3% tobramycin) (13). Despite retaining activity, the role of aminoglycosides in the treatment of MDR P. aeruginosa infections remains limited due to toxicity and inferior efficacy and effectiveness as monotherapy (14–16).
Treatment decisions become increasingly complicated for MDR P. aeruginosa co-resistant to several antibiotic classes, leading to delays in the initiation of susceptible therapies and the need for other less commonly used antibiotic treatments or the use of antibiotic treatments with known toxicities or poor safety profiles (1). Our study found that patients infected with MDR P. aeruginosa strains with CARB/ESC/PT and CARB/ESC/FQ co-resistance patterns generally had similar outcomes compared to those without these co-resistance patterns. This is the first work to compare outcomes between patients infected with MDR P. aeruginosa strains with and without specific co-resistance patterns. Previous work has generally focused on comparing outcomes among those with MDR P. aeruginosa strains and those with multidrug-susceptible strains (1). These previous studies have demonstrated significant increases in morbidity, mortality, surgical interventions, length of hospital stay, chronic care for treatment of infection, and treatment costs in patients with infections due to drug-resistant versus drug-susceptible P. aeruginosa strains (17–21). For example, one study found a significantly higher crude mortality rate in patients with MDR P. aeruginosa infections compared with multidrug-susceptible P. aeruginosa (54.5% vs 16.2%; P < 0.05) in univariate analysis, however the impact of MDR was not evaluated in multivariable analysis (22). In contrast to these findings, outcomes were generally similar between those infected with and without specific co-resistance patterns. However, MDR P. aeruginosa reinfection was significantly higher among those infected with strains demonstrating CARB/ESC/FQ co-resistance. Previous work has shown that most recurrent episodes of P. aeruginosa pneumonia in ventilated patients occur due to persistence of strains present in a prior infection rather than reinfection to a new one (23). In addition, prompt initiation of appropriate antibiotic therapy is a fundamental principle in treating serious infections due to MDR P. aeruginosa (2). Delays in appropriate therapy may contribute to infection and treatment failure persistence (1). Therefore, our finding may be related to delay in time to appropriate therapy, persistence of infection, and/or treatment failure among patients infected with CARB/ESC/FQ co-resistant strains of MDR P. aeruginosa.
Because of complex co-resistance patterns to older antipseudomonal antibiotics, newer agents with activity against MDR P. aeruginosa, such as ceftazidime-avibactam and ceftolozane-tazobactam, are increasingly being used for the treatment of serious infections (2). While few patients in our study had infections with MDR P. aeruginosa isolates that were tested for susceptibility to these agents, we did observe low rates (≤15%) of individual resistance and co-resistance to ceftazidime-avibactam and ceftolozane-tazobactam. Our study may have important implications for antimicrobial stewardship programs that are weighing empirical use of newer versus older antipseudomonal agents, namely, meropenem, imipenem, piperacillin-tazobactam, cefepime, and ceftazidime (24). It may be important to initiate therapy with newer agents in patients with known risk factors for MDR P. aeruginosa, such as previous infection or colonization with P. aeruginosa, ICU stays, and previous health care and antibiotic exposure, which were prevalent among our study population (18, 25, 26). Future studies are needed to identify patient populations that would benefit clinically from empirical or earlier initiation of newer antipseudomonal antibiotics compared to older combination therapies.
The limitations of our study are as follows. Antimicrobial susceptibility testing across the VA Healthcare System is not uniform and the methodology used to test susceptibility at each site may differ. Isolates that did not have reported MICs were analyzed using the laboratory’s interpretation, which may or may not reflect the most up-to-date breakpoints and definitions. Further, we may have overestimated antibiotic resistance by including isolates that are intermediate into our resistance category. However, this aligns with the phenotypic definition of MDR P. aeruginosa used by the Centers for Disease Control (27). Moreover, different laboratories may have different antibiotics selected for routine susceptibility testing, leading to different denominators based on which antibiotics were tested for susceptibility. Few isolates were tested for susceptibility to newer agents including, ceftazidime-avibactam and ceftolozane-tazobactam. There are other newer antipseudomonal agents, including cefiderocol, imipenem/relebactam, and meropenem-vaborbactam, that were even more recently approved than ceftazidime-avibactam and ceftolozane-tazobactam and not included in our study. Another limitation is that we did not assess clinical symptoms and could not discern between colonization versus true clinical MDR P. aeruginosa infection. However, our definition of infection did require antipseudomonal treatment in addition to a positive culture. We measured all-cause mortality as cause of death was not available. Finally, the generalizability of our study which included a mostly older male Veteran population to the general population is limited.
Conclusion.
Among a national cohort of patients hospitalized with MDR P. aeruginosa infections, co-resistance to three classes of standard of care antibiotics, such as carbapenem, extended-spectrum cephalosporins, and piperacillin-tazobactam or fluoroquinolones, exceeded 40% in our study population, posing great concerns for selecting appropriate empirical therapy. Clinical outcomes were poor for all patients, regardless of different co-resistance patterns. New treatment options are needed for hospitalized patients with suspected or confirmed MDR P. aeruginosa infections.
MATERIALS AND METHODS
This national, multicenter, retrospective cohort study was conducted among patients receiving care in the VA Healthcare System. We used data from national VA data sets, including inpatient admissions, inpatient and outpatient care, diagnoses, procedures, vital status, microbiology, and pharmacy. The study was approved by the Institutional Review Board (IRB) and the Research and Development (R&D) Committee of the Providence Veterans Affairs Medical Center prior to initiation. This research was conducted with a waiver of informed consent from the Providence VA Medical Center IRB.
Patients were included if they were 18 years of age or older and hospitalized in a VA medical center with an MDR P. aeruginosa infection between 1 Jan. 2015 and 31 Dec. 2020. MDR P. aeruginosa infection was defined as a positive MDR P. aeruginosa culture from any source and administration of at least one antipseudomonal treatment (piperacillin-tazobactam, piperacillin, cefepime, ceftazidime, imipenem, doripenem, meropenem, ciprofloxacin, levofloxacin, gentamicin, tobramycin, amikacin, aztreonam, colistin, polymyxin B, ceftolozane-tazobactam, and ceftazidime-avibactam) during admission. Per the Centers for Disease Control phenotype analytical definitions for classifying antibiotic resistance categories (Table 3), MDR P. aeruginosa was defined as a P. aeruginosa isolate that demonstrated intermediate (I) or resistant (R) antimicrobial susceptibility to at least one antibiotic in at least three of the following drug classes: aminoglycosides (amikacin, gentamicin, tobramycin), carbapenems (imipenem, meropenem, doripenem), extended-spectrum cephalosporins (cefepime, ceftazidime), fluoroquinolones (ciprofloxacin, levofloxacin), and piperacillin-tazobactam (piperacillin, piperacillin-tazobactam) (27). For patients with multiple MDR P. aeruginosa infection admissions during the study period, only the first was included.
TABLE 3.
Demographics, comorbidities, clinical characteristics, and outcomes of hospitalized patients with MDR Pseudomonas aeruginosa infection
| Demographics | N (3,763 unique patients) |
|---|---|
| Age (yr), mean (SD) | 69.4 (12.0) |
| Male | 3,680 (97.8%) |
| White Black Asian |
2,537 (67.4%) 951 (25.3%) 19 (0.5%) |
| Hispanic | 320 (8.5%) |
| Married | 1,766 (46.9%) |
| Admission source Home/community Long-term care Another hospital Other/unknown |
3,092 (82.2%) 389 (10.3%) 161 (4.3%) 121 (3.2%) |
| Intensive care unit during current admission | 1,942 (51.6%) |
| Charlson score, Median (interquartile range) | 4 (2–6) |
| Elixhauser score, Median (interquartile range) | 5 (4–7) |
| Current medical problems Respiratory failure Fever of unknown origin Acute renal failure Shock Complications of surgical procedures or medical care |
1,815 (48.2%) 576 (15.3%) 1,531 (40.7%) 829 (22.0%) 1,209 (32.1%) |
| Comorbidities during admission Myocardial infarction Congestive heart failure Peripheral vascular disease Cerebrovascular disease Dementia Chronic pulmonary disease Liver disease Diabetes Malignancy |
382 (10.2%) 1,199 (31.9%) 720 (19.1%) 659 (17.5%) 494 (13.1%) 1,620 (43.1%) 341 (9.1%) 1,741(46.3%) 675 (17.9%) |
| Healthcare exposures in the 90 days prior to admission Hospitalization Nursing home Intensive care |
1,784 (47.4%) 264 (7.0%) 682 (18.1%) |
| Antibiotics in the 90 days prior to admission | 2,374 (63.1%) |
| P. aeruginosa culture in the 90 days prior to admission | 859 (22.8%) |
| Hospital-acquired infectiona | 472 (12.5%) |
| Infection diagnoses during admissionb Bacterial infection of an unspecified site Skin and subcutaneous infection Ulcers Intraabdominal infection Urinary tract infection Osteomyelitis Chronic osteomyelitis Pneumonia Septicemia |
2,587 (68.8%) 674 (17.9%) 1,755 (46.6%) 421 (11.2%) 2,107 (56.0%) 726 (19.3%) 439 (11.7%) 1,676 (44.5%) 1,924 (51.1%) |
| Initial susceptible treatmentc | 1,259 (33.5%) |
Hospital-acquired infection was defined as a culture taken >2 days after admission and no previous P. aeruginosa culture in the 90 days prior to admission.
Infection diagnoses during admission are not mutually exclusive. Patients may have had more than one infection diagnosis during the admission.
Initial susceptible treatment was defined as receipt of at least one susceptible antibiotic treatment within 48 h of the index culture date.
The first MDR P. aeruginosa culture collected during the admission was used to identify the index P. aeruginosa isolate. If the patient had multiple MDR P. aeruginosa cultures collected on the same day from different culture sources, then the culture from the most sterile source (cerebrospinal fluid > blood > bone and joint > catheter tip > intraabdominal > respiratory > skin and soft tissue > urine > other > unknown) was selected as the index isolate.
Resistance and co-resistance.
Individual antimicrobial resistance and co-resistance to multiple classes of antibiotics of the index MDR P. aeruginosa isolate were evaluated. The following five classes of antibiotics were assessed for resistance and co-resistance: aminoglycosides, carbapenems, extended-spectrum cephalosporins, fluoroquinolones, and piperacillin-tazobactam. Resistance and co-resistance to ceftolozane-tazobactam and ceftazidime-avibactam were also evaluated. Resistance and co-resistance rates were stratified by culture source. MICs were used to determine susceptibility results based on Clinical and Laboratory Standards Institute breakpoints for susceptibility where available. Interpretations of the clinical laboratory performing the testing were used where MICs were unavailable. Resistance was defined as nonsusceptibility (I or R) for this study (27).
Demographics, comorbidities, and clinical characteristics.
Demographics, comorbidities, and clinical characteristics were described among our cohort of hospitalized patients with MDR P. aeruginosa infection. Current medical problems and comorbidities were identified using the International Classification of Diseases, Ninth or Tenth Revision (ICD-9 or ICD-10) diagnosis and procedure codes during the admission. Previous P. aeruginosa cultures, health care exposures, and antibiotic exposures were evaluated 90 days before admission.
Clinical outcomes.
Clinical outcomes evaluated included inpatient mortality (defined as death due to any cause from hospital admission to discharge), extended length of stay (defined as length of hospital stay from culture collection greater than the median), and reinfection with hospitalization (defined as a subsequent admission with an MDR P. aeruginosa culture and antipseudomonal treatment within 1 year of discharge). For patients that died during the admission, the date of death was considered the end of the hospital stay. An extended length of stay has previously been defined as a length of stay greater than the typical length of stay of the population (28). We assessed reinfection with hospitalization up to 1 year of discharge, as previous work has shown that further episodes of P. aeruginosa typically occur within several months to years of the initial episode (29).
Statistical analysis.
Descriptive statistics in the form of counts and percentages were used for describing resistance and co-resistance rates. Resistance and co-resistance rates were calculated among patients with index MDR P. aeruginosa isolates that were tested for susceptibility to that specific antibiotic/class or co-resistance pattern; hence the denominator changes for different individual antimicrobial resistance and co-resistance patterns.
To determine whether clinical outcomes vary between patients with differing MDR P. aeruginosa resistance patterns, we built six logistic regression models to compare clinical outcomes (dependent variable: inpatient mortality, extended length of stay, and reinfection with hospitalization) among patients with and without two common resistance patterns identified in our study population (independent variables of interest). The first resistance pattern assessed was MDR P. aeruginosa strains with and without co-resistance to carbapenems and extended-spectrum cephalosporins and piperacillin-tazobactam (with CARB/ESC/PT resistance versus susceptible to CARB, ESC, and/or PT). The second resistance pattern assessed was MDR P. aeruginosa strains with and without co-resistance to carbapenems and extended-spectrum cephalosporins and fluoroquinolones (with CARB/ESC/FQ resistance versus susceptible to CARB, ESC, and/or FQ). Confounders significantly associated with co-resistance and clinical outcomes were controlled for in the adjusted logistic regression models (other independent variables included in models which are listed in the footnote of Table 2). Backwards, automatic, stepwise, unconditional logistic regression (initial selection P value < 0.1, retained in model P value < 0.05) was used to calculate adjusted ORs and 95% CIs. Analyses were completed with SAS (Version 9.2).
Data availability.
The study data may be made available upon reasonable request and approval by the Department of Veterans Affairs.
Supplementary Material
ACKNOWLEDGMENTS
The views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs. This material is based upon work supported, in part, by the Office of Research and Development, Department of Veterans Affairs.
K.L.L. has received research funding from AbbVie, Gilead, Merck, Pfizer and Shionogi, and has been a consultant/advisor for Ferring, Merck, Pfizer and Paratek. A.R.C. has received research funding from AbbVie, Gilead, Merck, and Shionogi and has been a speaker/advisor for Merck. L.A.P. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA.
This work was funded, in part, by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA.
Conception and design of the study: H.J.A., A.R.C., L.A.P., K.L.L. Data generation: H.J.A., A.R.C., V.L. Analysis and/or interpretation of the data: H.J.A., A.R.C., E.C.P., V.L., L.A.P., K.L.L. Preparation or critical revision of the manuscript: H.J.A., A.R.C., E.C.P., V.L., L.A.P., K.L.L.
Footnotes
Supplemental material is available online only.
Contributor Information
Aisling R. Caffrey, Email: Aisling_Caffrey@uri.edu.
Kerry L. LaPlante, Email: KerryLaPlante@uri.edu.
Sen Pei, Columbia University.
REFERENCES
- 1.Hirsch EB, Tam VH. 2010. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res 10:441–451. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Donnell JN, Bidell MR, Lodise TP. 2020. Approach to the treatment of patients with serious multidrug-resistant Pseudomonas aeruginosa infections. Pharmacotherapy 40:952–969. doi: 10.1002/phar.2449. [DOI] [PubMed] [Google Scholar]
- 3.Abdelraouf K, Tam V. 2017. Pseudomonas, p 899–922. In Mayers D, Sobel J, Ouellette M, Kaye K, Marchaim D (ed), Antimicrobial drug resistance. Springer, Cham, France. doi: 10.1007/978-3-319-47266-9_9. [DOI] [Google Scholar]
- 4.Pachori P, Gothalwal R, Gandhi P. 2019. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis 6:109–119. doi: 10.1016/j.gendis.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shortridge D, Gales AC, Streit JM, Huband MD, Tsakris A, Jones RN. 2019. Geographic and temporal patterns of antimicrobial resistance in Pseudomonas aeruginosa over 20 years from the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect Dis 6:S63–S68. doi: 10.1093/ofid/ofy343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appaneal HJ, Caffrey AR, Jiang L, Dosa D, Mermel LA, LaPlante KL. 2018. Antibiotic resistance rates for Pseudomonas aeruginosa clinical respiratory and bloodstream isolates among the Veterans Affairs Healthcare System from 2009 to 2013. Diagn Microbiol Infect Dis 90:311–315. doi: 10.1016/j.diagmicrobio.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong PH, von Krosigk M, Roscoe DL, Lau TT, Yousefi M, Bowie WR. 2014. Antimicrobial co-resistance patterns of gram-negative bacilli isolated from bloodstream infections: a longitudinal epidemiological study from 2002–2011. BMC Infect Dis 14:393. doi: 10.1186/1471-2334-14-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamma PD, Cosgrove SE, Maragakis LL. 2012. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mensa J, Barberán J, Soriano A, Llinares P, Marco F, Cantón R, Bou G, González Del Castillo J, Maseda E, Azanza JR, Pasquau J, García-Vidal C, Reguera JM, Sousa D, Gómez J, Montejo M, Borges M, Torres A, Alvarez-Lerma F, Salavert M, Zaragoza R, Oliver A. 2018. Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: guidelines by the Spanish Society of Chemotherapy. Rev Esp Quimioter 31:78–100. [PMC free article] [PubMed] [Google Scholar]
- 10.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlowsky JA, Lob SH, Young K, Motyl MR, Sahm DF. 2019. Activity of imipenem-relebactam against multidrug-resistant Pseudomonas aeruginosa from the United States – SMART 2015–2017. Diagn Microbiol Infect Dis 95:212–215. doi: 10.1016/j.diagmicrobio.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher JC, Satlin MJ, Elabor A, Saraiya N, McCreary EK, Molnar E, El-Beyrouty C, Jones BM, Dixit D, Heil EL, Claeys KC, Hiles J, Vyas NM, Bland CM, Suh J, Biason K, McCoy D, King MA, Richards L, Harrington N, Guo Y, Chaudhry S, Lu X, Yu D. 2018. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa Infections: a multicenter study. Open Forum Infect Dis 5:ofy280. doi: 10.1093/ofid/ofy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidal L, Gafter-Gvili A, Borok S, Fraser A, Leibovici L, Paul M. 2007. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 60:247–257. doi: 10.1093/jac/dkm193. [DOI] [PubMed] [Google Scholar]
- 15.Leibovici L, Paul M, Poznanski O, Drucker M, Samra Z, Konigsberger H, Pitlik SD. 1997. Monotherapy versus beta-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother 41:1127–1133. doi: 10.1128/AAC.41.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phe K, Bowers DR, Babic JT, Tam VH. 2019. Outcomes of empiric aminoglycoside monotherapy for Pseudomonas aeruginosa bacteremia. Diagn Microbiol Infect Dis 93:346–348. doi: 10.1016/j.diagmicrobio.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. 2006. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother 50:43–48. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmeli Y, Troillet N, Karchmer AW, Samore MH. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch Intern Med 159:1127–1132. doi: 10.1001/archinte.159.10.1127. [DOI] [PubMed] [Google Scholar]
- 20.Dimatatac EL, Alejandria MM, Montalban C, Pineda C, Ang C, Delino R. 2003. Clinical outcomes and costs of care of antibiotic resistant Pseudomonas aeruginosa infections. Philipp J Microbiol Infect Dis 32:159–167. [Google Scholar]
- 21.Gasink LB, Fishman NO, Weiner MG, Nachamkin I, Bilker WB, Lautenbach E. 2006. Fluoroquinolone-resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact. Am J Med 119:526.e19-25–526.e25. doi: 10.1016/j.amjmed.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Cao B, Wang H, Sun H, Zhu Y, Chen M. 2004. Risk factors and clinical outcomes of nosocomial multi-drug resistant Pseudomonas aeruginosa infections. J Hosp Infect 57:112–118. doi: 10.1016/j.jhin.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Rello J, Mariscal D, March F, Jubert P, Sanchez F, Valles J, Coll P. 1998. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: relapse or reinfection? Am J Respir Crit Care Med 157:912–916. doi: 10.1164/ajrccm.157.3.9703014. [DOI] [PubMed] [Google Scholar]
- 24.Lodise TP, Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, Lomaestro B, McGregor JC. 2007. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother 51:3510–3515. doi: 10.1128/AAC.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micek ST, Wunderink RG, Kollef MH, Chen C, Rello J, Chastre J, Antonelli M, Welte T, Clair B, Ostermann H, Calbo E, Torres A, Menichetti F, Schramm GE, Menon V. 2015. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care 19:219. doi: 10.1186/s13054-015-0926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross AE, Van Schooneveld TC, Olsen KM, Rupp ME, Bui TH, Forsung E, Kalil AC. 2014. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother 58:5262–5268. doi: 10.1128/AAC.02582-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control. 2020. Antimicrobial resistant phenotype definitions 2020. https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/aur/ar-phenotype-definitions-508.pdf.
- 28.Brasel KJ, Lim HJ, Nirula R, Weigelt JA. 2007. Length of stay: an appropriate quality measure? Arch Surg 142:461–465; discussion 5–6. doi: 10.1001/archsurg.142.5.461. [DOI] [PubMed] [Google Scholar]
- 29.Palser S, Smith S, Nash EF, Agarwal A, Smyth AR. 2019. Treatments for preventing recurrence of infection with Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev 12:CD012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download spectrum.02336-22-s0001.pdf, PDF file, 0.08 MB (86.6KB, pdf)
Data Availability Statement
The study data may be made available upon reasonable request and approval by the Department of Veterans Affairs.