Abstract
Aims
The aims of the Critical Care Cardiology Trials Network (CCCTN) are to develop a registry to investigate the epidemiology of cardiac critical illness and to establish a multicentre research network to conduct randomised clinical trials (RCTs) in patients with cardiac critical illness.
Methods and results
The CCCTN was founded in 2017 with 16 centres and has grown to a research network of over 40 academic and clinical centres in the United States and Canada. Each centre enters data for consecutive cardiac intensive care unit (CICU) admissions for at least 2 months of each calendar year. More than 20 000 unique CICU admissions are now included in the CCCTN Registry. To date, scientific observations from the CCCTN Registry include description of variations in care, the epidemiology and outcomes of all CICU patients, as well as subsets of patients with specific disease states, such as shock, heart failure, renal dysfunction, and respiratory failure. The CCCTN has also characterised utilization patterns, including use of mechanical circulatory support in response to changes in the heart transplantation allocation system, and the use and impact of multidisciplinary shock teams. Over years of multicentre collaboration, the CCCTN has established a robust research network to facilitate multicentre registry-based randomised trials in patients with cardiac critical illness.
Conclusion
The CCCTN is a large, prospective registry dedicated to describing processes-of-care and expanding clinical knowledge in cardiac critical illness. The CCCTN will serve as an investigational platform from which to conduct randomised controlled trials in this important patient population.
Keywords: Cardiac ICU, Critical care, Epidemiology, Cohort
Introduction
A dedicated coronary care unit (CCU) was initially designed to care for patients after acute myocardial infarction to facilitate early defibrillation of ventricular arrythmias.1 Subsequently, acute care cardiology focused intensive care units have evolved from largely disease-specific units into comprehensive cardiovascular intensive care units (CICUs). These ‘CICUs’ provide care for a diverse patient population with a high burden of acute and chronic comorbidities and multisystem organ failure in addition to primary critical cardiac illness.1 Care of patients with critical cardiac illness has become increasingly important because of the escalating burden of critical illness among patients with cardiac disease. Among patients with chronic cardiovascular disease, a large and growing proportion require Intensive care unit (ICU) care including mechanical circulatory support,2 advanced respiratory therapies3,4 and renal replacement therapy.5 Therefore, understanding the epidemiology, processes of care, quality benchmarking, and outcomes of CICU patients is important to improve cardiovascular care. There are several knowledge gaps impeding further progress in the field. First, the available evidence largely consists of CICU cohorts from single academic centres6 and retrospective cohort studies using administrative and claims data.4,7,8 Data from multicentre, prospective cohorts with pre-specified and validated exposures and outcomes would provide more robust epidemiologic data. Second, there is a lack of evidence from randomised controlled trials in care of this patient population. Consequently, many of the best practices to treat critically ill patients admitted to CICUs have been extrapolated from general medical or surgical ICU studies.9,10
Aim
The aims of the Critical Care Cardiology Trials Network (CCCTN) include (1) the development of a registry to investigate the epidemiology of cardiac critical illness, including processes-of-care, risk prediction, description of associated outcomes, and quality benchmarking, and (2) establishing a multicentre research network to conduct randomised clinical trials to improve the care of patients with cardiac critical illness.
Funding
The Thrombolysis in Myocardial Infarction (TIMI) Study Group,11 funds database hosting, data management, case report form development and maintenance,12,13 and statistical support, and leads the Executive and Steering Committees (Figure 1). At each participating site, local site investigators leverage internal resources and/or obtain funding through grants or philanthropy in order to facilitate data entry, respond to data queries, lead scientific projects, and implement quality improvement initiatives. Randomised trials are anticipated to be funded by an array of mechanisms including national organizations, government funders, specialty organizations, and industry to enable the conduct of pragmatic trials embedded in routine care.
Figure 1.
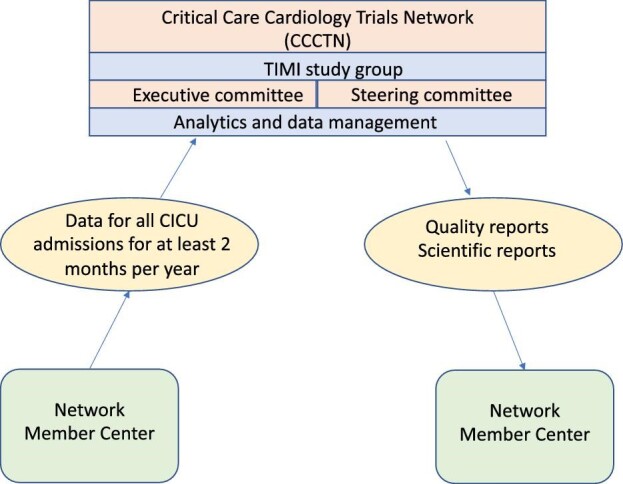
Schematic of the Cardiology Critical Care Trials Network.
Quality of care interventions
Improving the care of CICU patients is at the core of the CCCTN mission. Such improvement involves avoiding ICU complications,14 some of which are unique to CICU patients, and ensuring clinical excellence. Knowledge gaps in this area include a lack of CICU-specific benchmarks.15 To improve the quality of CICU care at member centres, the CCCTN has developed a quarterly quality report delivered to all participating centres. The report was developed using quantitative and qualitative surveys of member centres to identify the data points most relevant to practice. The report provides individual sites with their own summary data with reference to aggregate data across the network. (i.e. benchmarking).
General data provided include CICU admission and discharge volume, length of stay, and mortality, as well as the severity of illness summarised using the Sequential Organ Failure Assessment (SOFA) score.16 Process-of-care metrics include utilization of temporary mechanical circulatory support, respiratory support, pulmonary artery catheterisation and other invasive haemodynamic monitoring, vasopressors, and renal replacement therapy. Stratified data are provided for clinically important subgroups such as cardiogenic shock, acute coronary syndrome, heart failure, and patients receiving advanced ICU therapies such as mechanical ventilation and mechanical circulatory support and targeted temperature management. Site-specific data are compared with network-wide benchmarks stratified by SOFA score and Society for Cardiovascular Angiography and Interventions (SCAI) stage.17,18 The report is assessed semi-annually at CCCTN investigator meetings of all site principal investigators. It is planned that the quality report will evolve in response to CCCTN member needs and facilitates the design and implementation of prospective quality improvement projects.
Setting
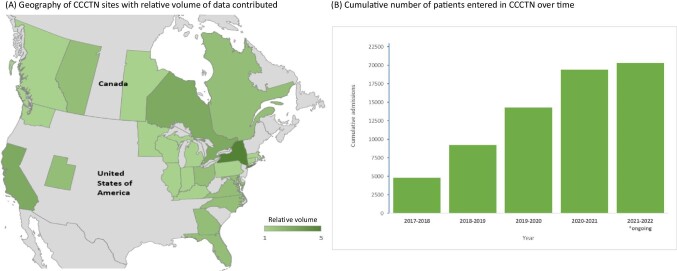
The CCCTN was initiated in 2017 in response to the knowledge gaps detailed previously. The initial CCCTN was comprised of 16 CICUs in the United States and Canada.19 The founding centres were referral centres, self-identified as ‘Level 1 CICUs’1,capable of providing comprehensive cardiac care. These founding centres were selected based on outreach, track record of investigator collaboration, feasibility to provide high volumes of high quality data and self-identified scientific interest. Since 2017, the Network has expanded to over 40 academic and clinical centers.20 Candidate centres complete a site survey that assesses their CICU structure, research experience, and infrastructure to support the registry. At its inception, the registry began by invitation targeted to centres with high-intensity staffing models and dedicated CICU leadership. As the registry has expanded, most new centres are added through their expression of interest and assessment of their potential for sustained commitment to reliable data capture in the registry. A geographic map of centres with relative volume contributed is displayed in Figure 2, panel A. To increase generalisability both geographically and regarding mixtures of academic and non-academic sites, targeted site recruitment was undertaken in subsequent years.
Figure 2.
(A) Geography of Cardiology Critical Care Trials Network sites with relative volume of data contributed; (B) Cumulative number of patients entered in Cardiology Critical Care Trials Network over time.
Population, start points, and consent
The study population of the CCCTN includes consecutive patients admitted to the CICU at each centre over a minimum of 2 months during each annual collection cycle. Sites can elect to contribute data year-round for all patients admitted to the CICU or for the subgroup of patients with shock. Study entry is at the time of arrival to the CCCTN hospital, via the emergency department, catheterisation lab, hospital ward or direct admission to CICU from inter-hospital transfer. Patients exit the study at time of in-hospital death or hospital discharge. The Institutional Review Board (IRB) at each participating institution provides study approval; given that no intervention is instituted in the registry and that no protected health information is included in the dataset, waiver of informed consent is granted at each participating centre. For future randomised trials, IRB approval of the individual trial protocols with appropriate consent procedures will be sought.
Baseline and follow-up data
Data on clinical presentation, demographics, laboratory data and haemodynamics, ICU care provided, as well as granular details related to various forms of shock, cardiac arrest, mechanical circulatory support, mechanical ventilation, respiratory support, and outcomes are recorded for each patient. Data collected are summarised in Table 1 and includes admission and demographic data, indication(s) for CICU admission, comorbidities, organ failure data, laboratory data, granular data on CICU care and CICU and hospital disposition, and vital status. As of this writing, >20 000 CICU patients are included in the registry database (Figure 2, panel B).
Table 1.
Categories of data included in CCCTN for each CICU admission
| Category | Data description |
|---|---|
| Presentation | ICU and hospital admission time, source of hospital admission (e.g. ED, referring hospital) |
| Demographics | Age, sex, race, weight, height |
| Primary diagnosis | Clinical diagnosis and indication for ICU admission |
| Past medical history | Cardiovascular and non-cardiovascular comorbidities |
| Cardiac arrest | Details as to type of cardiac arrest, clinical data, use of targeted temperature management |
| Shock | Shock type, stage, etiology, clinical management, use of vasopressors, invasive haemodynamics, and mechanical support |
| ICU therapeutics | ICU specific therapies used during the CICU stay, e.g. advanced respiratory support, renal replacement therapy |
| Mechanical circulatory support | Clinical use, sequence of devices, timing, and complications |
| Advanced respiratory support | Clinical use, timing, type of respiratory support, etiology of respiratory failure and outcomes |
| Laboratory evaluation | Selected admission and follow-up lab data |
| SOFA score | Components and summary SOFA score to assess severity of illness |
| Discharge | Timing of ICU and hospital discharge, vital status, and discharge disposition |
Data capture and storage
A standardised Case Report Form (CRF) with branching logic and decision support was designed, tested, and implemented using the REDCap platform.13 Data do not include any protected health information, and dates are scaled to relative dates based on arrival to the CICU as study time 0. All data are housed securely at the analytic centre of the TIMI Study Group at Brigham and Women's Hospital. The CRF is re-evaluated each annual cycle and updated to add new modules requested by consensus of the investigators and retire modules deemed of diminished scientific value and/or usefulness for quality improvement.
Data quality
Data quality is assured by automated decision support embedded in the REDCap database, structured validation rules, query resolution with the local critical care cardiologist and CICU directors as needed, and individual centralised case review by random selection with escalation to 100% review of a site's cases if quality concerns arise. A minimum of 20% of cases are randomly selected for review. 100% of cases are centrally reviewed for new centres in the network, or where there are staffing changes from the previous year. In 2021, the central team reviewed an average of 70% of the cases. There is also education for all site personnel, including a codebook of variable definitions, standardised in-person and video training for all investigators and asynchronous practice in a ‘playground’ for simulated data entry. The mean number of data queries per patient is 0.3 with a range of 0.15–1.79 queries per patient across sites.
Access to data
Access to the analyses occurs through a collaborative process between the study Executive Committee and site investigators who are encouraged to submit proposals; the analyses are prioritised based on scientific impact, feasibility, and collective interest.
Conclusions
The published scientific findings to date from CCCTN are displayed in Table 2. These include descriptions of utilisation patterns, epidemiology, indications for ICU level care and treatments, and outcomes of all CICU patients19 as well as patients with specific disease states, including shock and heart failure,18,21–23 renal dysfunction,24 respiratory failure,25 and COVID-19.26 CCCTN has characterised the CICU use of palliative care20 and mechanical support overall21 and in response to changes in the heart transplantation allocation system,27 and the impact of multidisciplinary shock teams in shock care and outcomes.28 Finally, CCCTN has investigated novel risk prediction scores.29
Table 2.
Scientific findings from the CCCTN
| Manuscript | Main findings |
|---|---|
| Epidemiology of shock in Contemporary Cardiac Intensive Care Units22 | Profiles of shock in contemporary CICU's are diverse. Cardiogenic shock (CS) related to acute myocardial infarction now represents only one-third of all CS cases, whereas many CS cases occur in the context of advanced heart failure. |
| Demographics, care patterns, and outcomes of patients admitted to Cardiac Intensive Care Units19 | Respiratory failure and shock are the most common indications for CICU admission, and an important minority of CICU admissions are solely for observation and monitoring. |
| Clinical practice patterns in temporary mechanical circulatory support for shock21 | There is substantial hospital-level variation in use of temporary mechanical circulatory support for shock in the contemporary CICU, not fully explained by disease severity. |
| Incidence, underlying conditions, and outcomes of patients receiving acute renal replacement therapies in the CICU24 | Requirement for renal replacement therapy in the CICU is associated with >40% in-hospital mortality. |
| Use of temporary mechanical circulatory support for management of cardiogenic shock before and after the UNOS donor heart allocation system changes27 | Subsequent to changes in the UNOS donor heart allocation system, use of mechanical circulatory support increased in transplant center CICUs but not in other CICUs. |
| Advanced respiratory support in the Contemporary Cardiac ICU25 | One-third of CICU admissions require respiratory support for a diverse array of underlying causes; such patients are at high risk of adverse outcomes including re-intubation, CICU readmission, and death. |
| The range of cardiogenic shock survival by clinical stage18 | There is a stepwise gradient of survival across SCAI shock stages in the contemporary CICU, with implications for shock phenotyping, quality benchmarking, and randomised trial design. |
| Management and outcomes of cardiogenic shock in cardiac ICUs with vs. without shock teams28 | CICUs that utilize multidisciplinary shock teams were more likely to use invasive haemodynamic monitoring and advanced mechanical support, and had lower ICU mortality for shock patients. |
| De novo vs. acute-on-chronic presentations of heart failure-related cardiogenic shock23 | Patients with de novo heart failure-related cardiogenic shock had more severe presentations and worse outcomes than those with cardiogenic shock due to acute-on-chronic heart failure. |
| End-of-life care in the cardiac intensive care unit20 |
In the contemporary CICU, two-thirds of deaths were preceded by comfort measures; however, utilization of palliative care services was infrequent. |
| Epidemiology of acute heart failure in critically ill patients with COVID-1926 |
Of COVID-19 ICU admissions, 9% manifest acute heart failure and such patients have more myocardial injury and greater elevation in biomarkers of haemodynamic stress. |
| A pragmatic lab-based tool for risk assessment in cardiac critical care29 | A risk score based on lab markers accurately stratifies CICU mortality risk. |
The CCCTN Registry is currently in its fifth year of enrollment and is expanding with additional centres. The CCCTN is amongst the largest and most detailed multicentre registries in cardiac critical illness, and is based on individual clinical case review rather than administrative data. Future goals include incorporation of a biorepository for stored specimens, enabling deeper phenotyping and risk assessment beyond clinical data alone as well as incorporation of longer term follow-up to ascertain outcomes beyond the date of hospital discharge, which would require new infrastructure and funding sources. Infrastructure innovations for enhanced interoperability with other datasets and automated data collection using the electronic medical record are underway. The quality and benchmarking missions of the CCCTN will continue to expand with more detailed quality reports to member centres. Finally, the future mission of the CCCTN includes the execution of randomised clinical trials and qualitative research leveraging the robust investigational infrastructure and site network developed over the past 5 years.
In conclusion, the CCCTN Registry is a cardiac critical care registry dedicated to improving the care and expanding clinical science for patients with cardiac critical illness. The CCCTN Registry already has contributed significantly to CICU care through scientific findings and quality initiatives. The CCCTN aims to provide impactful investigational infrastructure to conduct randomised controlled trials in this important patient population.
Contributor Information
Thomas S Metkus, Divisions of Cardiology and Cardiac Surgery, Departments of Medicine and Surgery, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Vivian M Baird-Zars, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA.
Carlos E Alfonso, Division of Cardiology, Department of Medicine; University of Miami Hospital & Clinics, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Carlos L Alviar, Leon H. Charney Division of Cardiology, NYU Langone Medical Center, New York 10016 NY, USA.
Christopher F Barnett, Division of Cardiology, Department of Medicine, University of California San Francisco, San Francisco, CA 94143, USA.
Gregory W Barsness, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN 55902, USA.
David D Berg, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA.
Mia Bertic, University of Toronto Etobicoke,Toronto ON, Canada.
Erin A Bohula, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA.
James Burke, Lehigh Valley Heart Institute, Allentown, PA 18103, USA.
Barry Burstein, University of Toronto, Toronto, ON, Canada.
Sunit-Preet Chaudhry, StVincent Heart Center, Indianapolis, IN 46260, USA.
Howard A Cooper, Westchester Medical Center and New York Medical College, Valhalla NY 10901, USA.
Lori B Daniels, Division of Cardiovascular Medicine La Jolla, UCSD, San Diego, CA 92037, USA.
Christopher B Fordyce, UBC Centre for Cardiovascular Innovation, Cardiovascular Health Program, UBC Centre for Health Evaluation & Outcomes Sciences, University of British Columbia, Vancouver, BC, Canada.
Shahab Ghafghazi, Division of Cardiovascular Medicine, University of Louisville, Louisville, KY 40202, USA.
Michael Goldfarb, Division of Cardiology, Jewish General Hospital, McGill University, Montréal, QC, Canada.
Jason N Katz, Division of Cardiology, Duke University School of Medicine, Durham, NC 27710, USA.
Ellen C Keeley, Division of Cardiology, Department of Medicine, University of Florida, Gainesville, FL 32610, USA.
Norma M Keller, Department of Medicine at NYU Grossman School of Medicine, Bellevue Hospital, New York NY 10016, USA.
Benjamin Kenigsberg, Departments of Cardiology and Critical Care Medicine, MedStar Washington Hospital Center, Washington DC, WA 20010, USA.
Michael C Kontos, Division of Cardiology, Virginia Commonwealth University, Richmond, VA 23219, USA.
Younghoon Kwon, Division of Cardiology, University of Washington, Seattle, WA 98104, USA.
Patrick R Lawler, Peter Munk Cardiac Centre, Toronto General Hospital, University of Toronto, Toronto ON, Canada.
Evan Leibner, Department of Emergency Medicine, Icahn School of Medicine at Mount Sinai, The Mount Sinai Hospital, New York, NY 10029, USA.
Shuangbo Liu, Max Rady College of Medicine St. Boniface Hospital Winnipeg, Manitoba, Canada.
Venu Menon, Cardiovascular Medicine, Cleveland Clinic Foundation, Cleveland, OH 44195, USA.
P Elliott Miller, Department of Cardiovascular Medicine, Yale School of Medicine, New Haven, CT 06510, USA.
L Kristin Newby, Divison of Cardiology, Duke University School of Medicine, Durham, NC 27710, USA.
Connor G O'Brien, Department of Medicine, Division of Cardiology, University of California-San Francisco School of Medicine, San Francisco, CA 94143, USA.
Alexander I Papolos, Departments of Cardiology and Critical Care Medicine, MedStar Washington Hospital Center, Washington DC, WA 20010, USA.
Matthew J Pierce, Department of Cardiology, Zucker School of Medicine at Hofstra/Northwell, Long Island, NY 11549, USA.
Rajnish Prasad, Wellstar Cardiovascular Medicine, Marietta, GA 30060, USA.
Barbara Pisani, Atrium Wake Forest Baptist Winston-Salem, NC 27157, USA.
Brian J Potter, Centre Hospitalier de l'Université de Montréal, Montreal, QC, Canada.
Robert O Roswell, Lenox Hospital, Northwell Health, New York, NY 10075, USA.
Shashank S Sinha, Inova Heart and Vascular Institute, Inova Fairfax Medical Center, Falls Church, VA 22042, USA.
Kevin S Shah, University of Utah Health Sciences Center, Salt Lake City, UT 84132, USA.
Timothy D Smith, The Christ Hospital and Lindner Institute for Research and Education Cincinnati, OH 45219, USA.
R Jeffrey Snell, Rush University Medical Center, Chicago IL 60612, USA.
Derek So, University of Ottawa Heart Institute, Ottawa, ON, Canada.
Michael A Solomon, University of Toronto, Toronto, ON, Canada.
Bradley W Ternus, Division of Cardiology, Department of Internal Medicine, University of Wisconsin, Madison, WI 53792, USA.
Jeffrey J Teuteberg, Division of Cardiovascular Medicine, Stanford University Medical Center, Palo Alto, CA 94305, USA.
Sean van Diepen, Division of Cardiology, Department of Critical Care Medicine, Department of Medicine, University of Alberta, Edmonton, AB, Canada.
Sammy Zakaria, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
David A Morrow, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA.
Funding
Dr TSM received salary support from the National Institutes of Health-funded Institutional Career Development Core at Johns Hopkins (project number 5KL2TR003099-02). Y.K. reports support from NIH R01 HL158765 NIH R21 HL150502 NIH R21 AG070576. Dr MAS receives research support from the National Institutes of Health Clinical Center intramural research funds. Dr S.V.D. receives funding from the Sir John A MacDonald Fund for Innovation.
Conflict of interest: T.S.M. reports royalties for a textbook publication for McGraw-Hill publishing, unrelated to this subject matter, consulting unrelated to this subject matter for TelaDoc/BestDoctors Inc and Oakstone/EBIX, honorarium from Nova BioMedical and expert witness for defense. L.B.D. reports consulting fees from Quidel, Roche, and Siemens. C.B.F. reports grants from Bayer and Pfizer, consulting fees from Bayer, Novo Nordisk, Boehringer Ingelheim, Sanofi, Amgen, Novartis, Pendopharm, support to attend meetings from Novo Nordisk and leadership role for Heartflow. J.N.K. reports grant from Abbott. M.C.K. reports leadership roles including Chair, NCDR CP-MI committee (unpaid) VCSQI, board member (unpaid) VHAC steering committee (unpaid) ACC.org ACS editor (paid) NCDR accreditation committee (unpaid). Y.K. reports consulting fees from Caretaker Medical and DSMB service for trial: IMPact of Real-time Predictive Monitoring in Acute Care Cardiology Trial (PM-IMPACCT). L.K.N. reports grants/contracts from NIH, BioKier, NCDHHS, Roche Diagnostics, consulting fees from Medtronic and CSL, honoraria from Alabama ACC Boston University WCVI University of Arizona Sarver Heart Center, DSMB service for MESA OSMB (NHLBI), and leadership roles for David H Murdock Research Institute (unpaid) AstraZeneca Healthcare Foundation (unpaid). R.P. reports honoraria and DSMB service for Abiomed. B.P. reports travel from Abbott and owning stock in Abbott, Amgen, Medtronic, GlaxoSmith Kline, Bristol Meyers Squibb. T.D.S. reports consulting fees and honoraria from Boston Scientific and Abiomed and honoraria from Macquet. M.A.S. reports leadership roles including American College of Cardiology (ACC)—Co-chair of the Critical Care Cardiology Leadership Council
Chair of the ACC Credentialing and Member Services Committee. J.J.T. reports consulting fees from Abiomed, Abbott, CareDx, Takeda, and honoraria from Medtronic, CareDx, Paragonix, Cytokinetics. All other authors report no conflicts of interest.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Morrow DA, Fang JC, Fintel DJ, Granger CB, Katz JN, Kushner FGet al. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation 2012;126:1408–1428. [DOI] [PubMed] [Google Scholar]
- 2. Vallabhajosyula S, Dewaswala N, Sundaragiri PR, Bhopalwala HM, Cheungpasitporn W, Doshi Ret al. Cardiogenic shock complicating ST-segment elevation myocardial infarction: An 18-year analysis of temporal trends, epidemiology, management and outcomes. Shock 2021;57:360–369. [DOI] [PubMed] [Google Scholar]
- 3. Metkus TS, Stephens RS, Schulman S, Hsu S, Morrow DA, Eid SM.. Utilization and outcomes of early respiratory support in 6.5 million acute heart failure hospitalizations. Eur Heart J Qual Care Clin Outcomes 2019;6:72–80. [DOI] [PubMed] [Google Scholar]
- 4. Metkus TS, Albaeni A, Chandra-Strobos N, Eid SM.. Incidence and prognostic impact of respiratory support in patients with ST-segment Elevation Myocardial Infarction. Am J Cardiol 2017;119:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallabhajosyula S, Dunlay SM, Barsness GW, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PRet al. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS One 2019;14:e0222894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watson RA, Bohula EA, Gilliland TC, Sanchez PA, Berg DD, Morrow DA.. Editor's choice-prospective registry of cardiac critical illness in a modern tertiary care cardiac intensive care unit. Eur Heart J Acute Cardiovasc Care 2019;8:755–761. [DOI] [PubMed] [Google Scholar]
- 7. Sinha SS, Sjoding MW, Sukul D, Prescott HC, Iwashyna TJ, Gurm HSet al. Changes in primary noncardiac diagnoses over time among elderly cardiac intensive care unit patients in the United States. Circ Cardiovasc Qual Outcomes 2017;10:e003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Na SJ, Chung CR, Jeon K, Park CM, Suh GY, Ahn JHet al. Association between presence of a cardiac intensivist and mortality in an adult cardiac care unit. J Am Coll Cardiol 2016;68:2637–2648. [DOI] [PubMed] [Google Scholar]
- 9. van Diepen S, Granger CB, Jacka M, Gilchrist IC, Morrow DA, Katz JN.. The unmet need for addressing cardiac issues in intensive care research. Crit Care Med 2015;43:128–134. [DOI] [PubMed] [Google Scholar]
- 10. Fordyce CB, Katz JN, Alviar CL, Arslanian-Engoren C, Bohula EA, Geller BJet al. prevention of complications in the cardiac intensive care unit: A scientific statement from the American Heart Association. Circulation 2020;142:e379–e406. [DOI] [PubMed] [Google Scholar]
- 11. Sabatine MS, Braunwald E.. Thrombolysis in myocardial infarction (timi) study group: jacc focus seminar 2/8. J Am Coll Cardiol 2021;77:2822–2845. [DOI] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal Let al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Diepen S, Sligl WI, Washam JB, Gilchrist IC, Arora RC, Katz JN.. Prevention of critical care complications in the coronary intensive care unit: protocols, bundles, and insights from intensive care studies. Can J Cardiol 2017;33:101–109. [DOI] [PubMed] [Google Scholar]
- 15. Metkus TS, Lindsley J, Fair L, Riley S, Berry S, Sahetya Set al. Quality of heart failure care in the intensive care unit. J Card Fail 2021;27:1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining Het al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 17. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CSet al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol 2019;74:2117–2128. [DOI] [PubMed] [Google Scholar]
- 18. Lawler PR, Berg DD, Park JG, Katz JN, Baird-Zars VM, Barsness GWet al. Critical care cardiology trials network I. The range of cardiogenic shock survival by clinical stage: Data from the critical care cardiology trials network registry. Crit Care Med 2021;49:1293–1302. [DOI] [PubMed] [Google Scholar]
- 19. Bohula EA, Katz JN, van Diepen S, Alviar CL, Baird-Zars VM, Park JGet al. Critical care cardiology trials network. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: the critical care cardiology trials network prospective North American multicentre registry of cardiac critical illness. JAMA Cardiol 2019;4:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fagundes A, Berg DD, Bohula EA, Baird-Zars VM, Barnett CF, Carnicelli APet al. End-of-life care in the cardiac intensive care unit: a contemporary view from the Critical Care Cardiology Trials Network (CCCTN) registry. Eur Heart J Acute Cardiovasc Care 2022;11:190–197. [DOI] [PubMed] [Google Scholar]
- 21. Berg DD, Barnett CF, Kenigsberg BB, Papolos A, Alviar CL, Baird-Zars VMet al. Clinical practice patterns in temporary mechanical circulatory support for shock in the Critical Care Cardiology Trials Network (CCCTN) Registry. Circ Heart Fail 2019;12:e006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VMet al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 2019;12:e005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhatt AS, Berg DD, Bohula EA, Alviar CL, Baird-Zars VM, Barnett CFet al. De novo vs acute-on-chronic presentations of heart failure-related cardiogenic shock: insights from the critical care cardiology trials network registry. J Card Fail 2021;27:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Diepen S, Tymchak W, Bohula EA, Park JG, Daniels LB, Phreaner Net al. Critical care cardiology trials network I. Incidence, underlying conditions, and outcomes of patients receiving acute renal replacement therapies in tertiary cardiac intensive care units: An analysis from the critical care cardiology trials network registry. Am Heart J 2020;222:8–14. [DOI] [PubMed] [Google Scholar]
- 25. Metkus TS, Miller PE, Alviar CL, Baird-Zars VM, Bohula EA, Cremer PCet al. et al. Advanced respiratory support in the contemporary cardiac ICU. Crit Care Explor 2020;2:e0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berg DD, Alviar CL, Bhatt AS, Baird-Zars VM, Barnett CF, Daniels LB. Epidemiology of acute heart failure in critically ill patients with COVID-19: an analysis from the critical care cardiology trials network. J Card Fail 2022,;28:675–681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varshney AS, Berg DD, Katz JN, Baird-Zars VM, Bohula EA, Carnicelli APet al. Critical care cardiology trials network I. Use of temporary mechanical circulatory support for management of cardiogenic shock before and after the united network for organ sharing donor heart allocation system changes. JAMA Cardiol 2020;5:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papolos AI, Kenigsberg BB, Berg DD, Alviar CL, Bohula E, Burke JAet al. Critical care cardiology trials network I. Management and outcomes of cardiogenic shock in cardiac ICUs with versus without shock teams. J Am Coll Cardiol 2021;78:1309–1317. [DOI] [PubMed] [Google Scholar]
- 29. Patel SM, Jentzer JC, Alviar CL, Baird-Zars VM, Barsness GW, Berg DDet al. A pragmatic lab-based tool for risk assessment in cardiac critical care: data from the Critical Care Cardiology Trials Network (CCCTN) Registry. Eur Heart J Acute Cardiovasc Care 2022;11:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.