Abstract
Aim
To investigate temporal trends in inpatient vs. outpatient diagnosis of new-onset heart failure (HF) and the subsequent risk of death and hospitalization.
Methods and results
Using nationwide registers, 192 581 patients with a first diagnosis of HF (1997–2017) were included. We computed incidences of HF, age-standardized mortality rates, and absolute risks (ARs) of death and hospitalization (accounting for competing risk of death) to understand the importance of the diagnosis setting in relation to subsequent mortality and hospitalization. The overall incidence of HF was approximately the same (170/100 000 persons) every year during 1997–2017. However, in 1997, 77% of all first diagnoses of HF were made during a hospitalization, whereas the proportion was 39% in 2017. As inpatient diagnoses decreased, outpatient diagnoses increased from 23% to 61%. Outpatients had lower mortality and hospitalization rates than inpatients throughout the study period, although the 1-year age-standardized mortality rate decreased for each inpatient (24 to 14/100-person) and outpatient (11 to 7/100-person). One-year and five-year AR of death decreased by 11.1% and 17.0%, respectively, for all HF patients, while the risk of hospitalization for HF did not decrease significantly (1.13% and 0.96%, respectively).
Conclusion
Between 1997 and 2017, HF changed from being primarily diagnosed during hospitalization to being mostly diagnosed in the outpatient setting. Outpatients had much lower mortality rates than inpatients throughout the study period. Despite a significant decrease in mortality risk for all HF patients, neither inpatients nor outpatients experienced a reduction in the risk of an HF hospitalization.
Keywords: Heart failure, Inpatient, Outpatient, Temporal trend, Incidence, Prognosis
Introduction
Since the beginning of the new millennium, major improvements in the treatment and diagnosis of heart failure (HF) have been made.1 However, 17–45% of patients admitted to a hospital with newly presenting HF die within 1 year2 and survival rates in certain subgroups are still worse than those for several common cancers (e.g. colon, breast, or prostate cancer).3,4
A survey organized by the European Society of Cardiology showed that patients newly diagnosed with HF in the outpatient setting had significantly better 1-year survival than patients first diagnosed with HF after a hospital admission.5 This may be because patients admitted to a hospital are older, suffer from more comorbidities, and have a longer duration of HF, more severe HF, or a combination of these. In the BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOlSTAT-CHF) study, patients enrolled in hospitals were sicker than those recruited in outpatient clinics. However, these patients were not patients with a first presentation of HF and it is therefore not known whether there are similar differences between outpatients and inpatients with newly diagnosed HF.4 There has also been a recent recognition and increased awareness of HF as an important clinical challenge globally. Development of specialist clinics and services and wider availability of echocardiography and natriuretic peptides may have led to earlier diagnosis and treatment in the community and, possibly, reduced mortality at a population level and even reduced rates of hospital admission.
To examine trends in the place of first diagnosis (outpatient or in hospital), HF treatment, and outcomes (death and hospital admission), we studied all newly presenting cases of HF in Denmark between 1997 and 2017. We hypothesized that the first diagnosis of HF would be made more often in outpatient clinics (compared with in hospital), that the use of life-saving therapy would improve, and that rates of death and hospitalization due to worsening HF would decrease over the period studied.
This study suggests that patients presenting with new-onset HF as inpatients and outpatients are two different patient types. Our findings show that HF patients diagnosed in an outpatient setting have a much better prognosis than inpatients. Consequently, inpatients need close follow-up after HF hospitalization, due to the significant impact of the vulnerable phase following hospitalization. The long-term prognostic impact of the diagnosis setting needs to be studied further; however, this study suggests that diagnosing an increasing number of HF patients in the outpatient setting could improve management and prognosis.
Methods
The Danish healthcare system
Denmark has a nationwide public healthcare system. All citizens are registered with a primary care physician and virtually all inpatient care is delivered in public hospitals. Consequently, the national records document almost all outpatient and inpatient episodes of care and each episode is linked to individual patients through a unique patient identification number, which also allows linkage to prescriptions and deaths. It is recommended that patients with any clinical suspicion of HF be either referred to an outpatient clinic at a public hospital for echocardiography or acutely admitted at a public hospital if considered necessary at the discretion of the primary physician. The Supplementary material online, Figure S1, illustrates how the Danish healthcare system is structured.
Data sources
The data for this study were obtained from four Danish nationwide registers: the National Patient Register contains data on all in- and outpatient hospital contacts, coded with one primary diagnosis at discharge according to the International Classification of Diseases 10 (ICD-10) and one or more secondary diagnoses if relevant.6 The National Prescription Register contains data on every medical prescription that has been collected at Danish pharmacies.7 The National Cause of Death Register contains data regarding the date of death, including primary and underlying causes of death.8 The Central Population Register holds information on sex, date of birth, and migration.9 According to Danish regulations, register-based retrospective studies do not require ethical approval. The Danish personal identification system enables the cross-linking of data between registers. Thus, patients can only be lost to follow-up in case of emigration.
Study population
All in- and outpatients with a first-ever diagnosis of HF between 1997 and 2017 were identified. An inpatient first diagnosis of HF was defined as a patient hospitalized for HF as the primary diagnosis (= ‘admission for acute decompensated HF as initial HF diagnosis’), with no prior diagnosis of HF (either as a primary or secondary coding) and where hospitalization was defined as at least an overnight stay. The Danish National Patient Register was incepted in 1978 and we were, therefore, able to ensure that patients did not have any prior diagnosis of HF between 1978 and the time of inclusion. An outpatient first diagnosis of HF was defined as the first record of HF in an outpatient clinic—be it a primary or secondary diagnosis, with the same ‘look-back’ period to exclude any primary or secondary coding for HF. The Danish patient registers do not contain records of consultations with general practitioners and an outpatient diagnosis of HF, therefore, refers to a new onset of HF diagnosed by a cardiologist in an outpatient clinic. The ICD-10 codes used to define HF in this study have been validated previously (ICD-10—I50: HF; I42: cardiomyopathy; I11.0: hypertensive heart disease with congestive HF; or J81.9: pulmonary oedema).10–12 Patients were included if they were between 18 and 99 years old. We excluded patients who had either emigrated from Denmark or immigrated to Denmark less than 3 years prior to new-onset HF to ensure that we only found first-time cases of HF. Patients were included from the date of the first diagnosis of HF and followed until death, emigration, or 31 December 2018—whichever came first. This ensured that all patients had a potential minimum of 1 year of follow-up since the last day of inclusion was 31 December 2017.
Baseline characteristics
Information on patient age and nursing home status was defined at the time of their respective first-time diagnosis of HF (baseline). Prior history of comorbidity was defined in the same way and included: ischaemic heart disease (IHD), myocardial infarction (MI), coronary intervention, atrial fibrillation (AF), essential hypertension, stroke, diabetes mellitus (DM), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), and cancer (except non-melanoma skin cancer). All comorbidities were defined based on ICD-10 codes reported prior to the first HF diagnosis. The ICD-10 codes used to define these are reported in the Supplementary material online, Table S2, and have previously been validated with high positive predictive values.6,10 Medication at baseline was defined as a redeemed prescription up to 6 months prior to the first-time diagnosis of HF and included the following: beta blockers (BBs), renin–angiotensin-system inhibitors (RASi), diuretics, mineralocorticoid receptor antagonists (MRAs), statins, digoxin, anti-diabetic drugs, and anti-coagulants.
Outcomes
The outcomes of interest were all-cause mortality and HF hospitalization. HF hospitalization was defined as the first hospitalization due to HF after first diagnosis of new-onset HF. This would refer to the first HF hospitalization for outpatients and, in theory, the first HF rehospitalization for inpatients. An HF hospitalization required a primary diagnosis of HF and an overnight hospital stay to exclude admission for minor medical procedures that did not reflect worsening HF.
Statistical analysis
Patients were characterized into five age groups: <60 years, 60–69 years, 70–79 years, 80–89 years, and 90–99 years. A non-stratified comparative analysis was carried out to display temporal trends in diagnosis by clinical setting (inpatient or outpatient), plotting the incidence of HF between 1997 and 2017. Thereafter, 1-year and 5-year age-standardized mortality rates were computed and the temporal trends during 1997–2017 were plotted, both of which were standardized according to the Danish population in 2017. Then, the time interval was divided into four subintervals—1997–2001, 2002–06, 2007–11, and 2012–17—and cumulative incidence curves were made to estimate 1-year and 5-year absolute risks of death for each subinterval. Afterwards, the temporal trend in HF hospitalization between 1997 and 2017 was examined using cumulative incidence plots of HF hospitalization within 30 days of new-onset HF (an HF hospitalization within 30 days might be considered inseparable from the index event) and from 31 to 1826 days (= 5 years), separately, stratified in the four subintervals of the study period. Multivariate Cox proportional hazard models were applied to test for a potential interaction between incidence rates of outcomes (death or a HF hospitalization), patient type (in- vs. outpatients), and time periods. Hazard ratios were estimated between inpatients and outpatients, the latter being the reference group, and then presented as a forest plot. Adjustment for sex, age, and history of IHD, AF, stroke, and diabetes, at the time of new-onset HF, was performed. Model assumptions, such as linearity of continuous variables, the proportional hazard assumption, and lack of interactions, were tested and found valid unless otherwise stated. Additionally, temporal trends in treatment were also analysed, examining use of BBs, RASi, MRAs, and loop diuretics either 6 months prior to new-onset HF or 90 days after diagnosis. Only for this analysis, patients who did not survive the first 90 days were excluded.
Supplementary analyses
A comparison of the incidence of HF in the in- and outpatient settings was made between several patient subgroups: <70 years vs. ≥70 years; male vs. female; DM vs. no DM; IHD vs. no IHD; AF vs. no AF; COPD vs. no COPD; and essential hypertension vs. no essential hypertension. To understand the temporal trend in age at the time of new-onset HF, we portrayed the temporal trend in the median age of inpatients, outpatients, and all patients at the time of new-onset HF. As a sensitivity analysis, we calculated age-standardized mortality and HF hospitalization rates for each of the following five subgroups separately: prior history of no comorbidity vs. cardiovascular comorbidity vs. non-cardiovascular comorbidity, and male vs. female. Cardiovascular comorbidities were IHD, AF, and stroke, while non-cardiovascular comorbidities were DM, COPD, and cancer. Additionally, the cumulative incidence of death was computed for the first 30 days and 31–1826 (= 5 years) days after new-onset HF, respectively, and stratified in the four subintervals of the study period. The temporal trends in death within 30 and 31–1826 days were calculated to support the understanding of the temporal trend in the hospitalization for HF pattern. Finally, outcome data for the whole HF cohort independent of the location of diagnosis were calculated and presented.
Results
Baseline characteristics
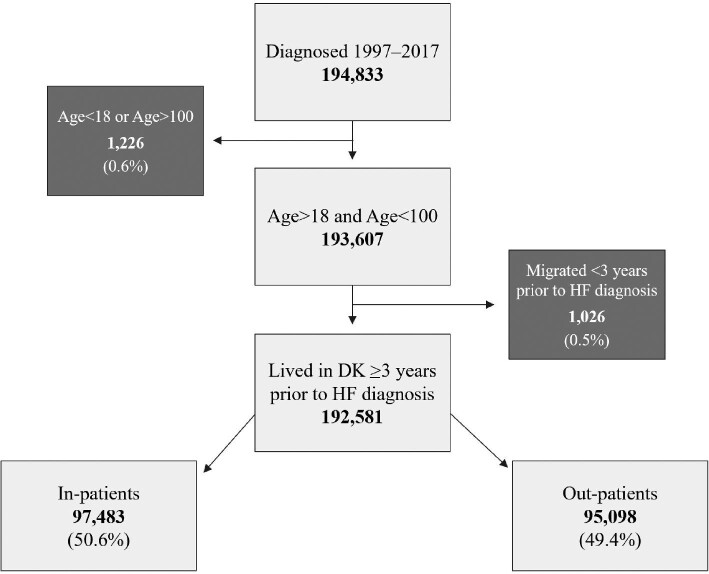
The selection of people for this study is illustrated in Figure 1. We included 192 581 patients with a first diagnosis of HF, with 50.6% identified in the inpatient setting and 49.4% as outpatients (Figure 1). Inpatients were 5 years older than outpatients and were less likely to be women (53.7% vs. 61.2%). Inpatients had a higher prevalence of comorbidity than outpatients, with the exception of coronary heart disease and coronary intervention, which were increasing and more common in outpatients throughout the study (52.6% vs. 41.2%). Inpatients were less likely to already be on cardiovascular medication at the time of HF diagnosis, except for loop diuretics (Table 1). The temporal trends in patient characteristics suggest a decreasing median age of patients presenting with new-onset HF, increasing prevalence of comorbidity, and an increasing percentage of patients initiated on cardiovascular medication at the time of HF diagnosis, still excluding loop diuretics (Supplementary material online, Table S1).
Figure 1.
Flowchart depicting the inclusion and exclusion criteria for the study population in this study. Light grey boxes indicate inclusion, whereas dark grey boxes indicate exclusion.
Table 1.
Baseline characteristics comparing out- and inpatients
| Outpatients (n = 95 098) | Inpatients (n = 97 483) | P-value | |
|---|---|---|---|
| Age (median)a | 72.2 [62.9,79.9] | 77.9 [68.3, 85.0] | <0.0001 |
| Sex (females)a | 58 215 (61.2) | 52 336 (53.7) | <0.0001 |
| Age group | <0.0001 | ||
| <60 | 18 451 (19.4) | 12 020 (12.3) | |
| 60–69 | 22 750 (23.9) | 15 695 (16.1) | |
| 70–79 | 30 419 (32.0) | 28 046 (28.8) | |
| 80–89 | 20 331 (21.4) | 32 063 (32.9) | |
| 90–99a | 3147 (3.3) | 9659 (9.9) | |
| In nursing homea | 3557 (3.7) | 7229 (7.4) | < 0.0001 |
| Comorbidity | |||
| IHDa | 49 987 (52.6) | 40 178 (41.2) | < 0.0001 |
| Previous MIa | 26 942 (28.3) | 18 338 (18.8) | < 0.0001 |
| Coronary interventiona | 14 044 (14.8) | 5124 (5.3) | < 0.0001 |
| Atrial fibrillationa | 17 247 (18.1) | 19 754 (20.2) | < 0.0001 |
| Essential hypertensiona | 36 106 (38.0) | 29 108 (29.9) | < 0.0001 |
| Strokea | 10 936 (11.5) | 13 325 (13.7) | < 0.0001 |
| Diabetesa | 16 499 (17.3) | 17 560 (18.0) | 0.0001 |
| COPDa | 9025 (9.5) | 10 413 (10.7) | < 0.0001 |
| Cancer | 9731 (10.2) | 10 526 (10.8) | 0.7994 |
| Baseline medicine | |||
| RASia | 49 803 (52.4) | 32 753 (33.6) | <0.0001 |
| Beta blockersa | 42 607 (44.8) | 26 720 (27.4) | <0.0001 |
| MRAsa | 11 142 (11.7) | 8796 (9.0) | <0.0001 |
| Loop diureticsa | 42 651 (44.8) | 44 947 (46.1) | <0.0001 |
| Diuretic combinationa | 7006 (7.4) | 4848 (5.0) | <0.0001 |
| Digoxina | 14 485 (15.2) | 16 302 (16.7) | <0.0001 |
| DM medication | 14 054 (14.8) | 14 716 (15.1) | 0.0514 |
| Anticoagulantsa | 20 652 (21.7) | 13 547 (13.9) | <0.0001 |
a p-value < 1e-04.
Incidence of HF during 1997–2017
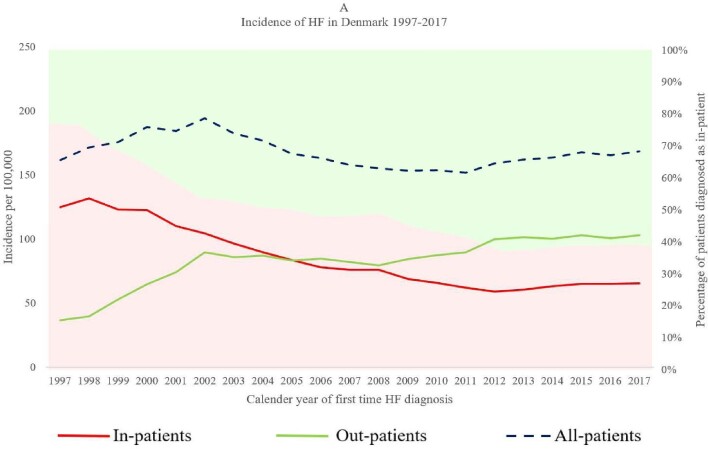
The first diagnosis of HF usually occurred in an inpatient setting before 2005, but after 2005 more first HF diagnoses were made in outpatient clinics than during hospital admission. As inpatient diagnoses decreased from 77% in 1997 to 39% in 2017, outpatient diagnoses increased from 23% in 1997 to 61% in 2017 (Figure 2). Although the place of diagnosis changed over time, the overall incidence of new-onset HF in Denmark did not change between 1997 and 2017. This change was observed in all important subgroups including +/− IHD, +/− type 2 diabetes, and +/− AF (Supplementary material online, Table S3).
Figure 2.
Temporal trend in the incidence of new-onset heart failure in Denmark during 1997–2017 for three patient types: inpatients, outpatients, and all patients. The primary y-axis portrays the incidence of heart failure for the three patient types, depicted by three different graphs (red = inpatients, green = outpatients, and blue = all patients). The secondary y-axis reflects the percentage of heart failure cases diagnosed as inpatients (red shaded area) and outpatients (green shaded area).
Temporal trends in mortality risk
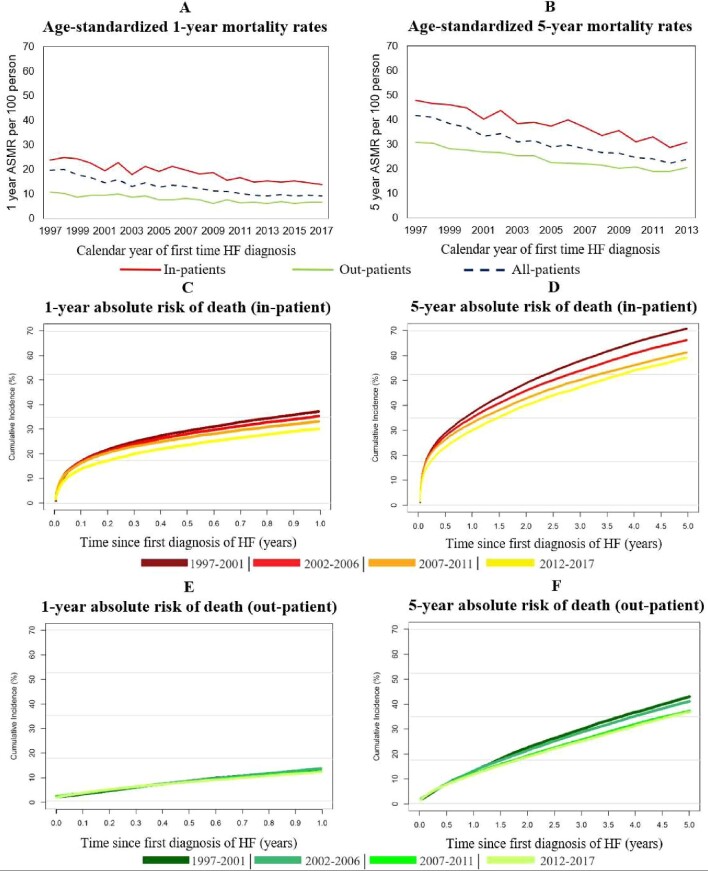
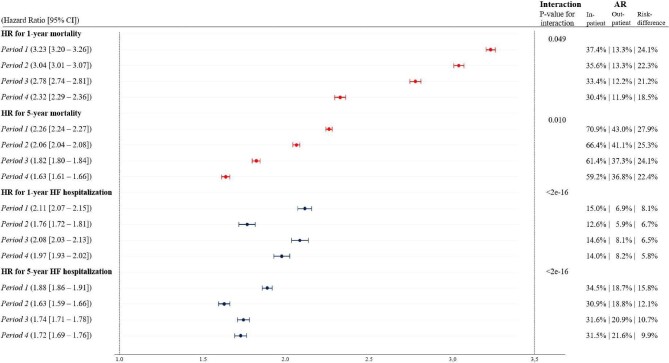
The overall rate of mortality decreased from 1997 to 2017 (Figure 3C, D). The age-standardized 1-year and 5-year mortality rates for both in- and outpatients decreased significantly during the period of study (Figure 3A, B). Both 1-year and 5-year age-standardized mortality rates were significantly lower for outpatients (11/100-person in 1997 and 7/100-person in 2017) than inpatients (24/100-person in 1997 and 14/100-person in 2017). The sensitivity analysis also showed that following stratification by comorbidty and sex, outpatients still had significantly lower age-standardized mortality rates than inpatients. The 1-year and 5-year absolute risk of death decreased by 11.1% (from 30.1% to 19.0%) and 17.0% (from 62.4% to 45.4%), respectively, for all HF patients between 1997 and 2017 (Supplementary material online, Figure S4). Statistical interaction between patient type, rate of death, and time period was observed indicating that the association between rate of death and being diagnosed with HF as an inpatient became less significant over time (Figure5).
Figure 3.
(A–F) Temporal trends in mortality after new-onset heart failure for inpatients and outpatients. (A) The temporal trend in the age-standardized 1-year mortality rate of the previously described patient types. (B) The temporal trend in the age-standardized 5-year mortality rate. Both were standardized to the population in Denmark in 2017. (C–F) The 1-year and 5-year absolute risk of death for inpatients and outpatients, respectively, comparing the following time periods: 1997–2001, 2002–06, 2007–11, and 2012–17.
Figure 5.
Adjusted hazard ratios of 1-year and 5-year mortality and heart failure hospitalization, respectively, for inpatients vs. outpatients (reference). The forest plot shows the hazard ratios of 1-year and 5-year mortality (in red) and heart failure hospitalization (in blue), respectively, for inpatients vs. outpatients—outpatients being the reference group. Note that hazard ratios for 1-year and 5-year heart failure hospitalization were estimated for inpatients/outpatients who were rehospitalized/hospitalized after 30 days of follow-up. They should therefore be considered hazard ratios for 31 days to 1 year and 31 days to 5 years of heart failure hospitalization. Moreover, the P-values for interaction show whether there was an interaction between patient type and time period for 1- and 5-year mortality and heart failure hospitalization. The absolute risk for inpatients and outpatients were estimated as crude risks (event/at risk). Hazard ratios were adjusted for age, sex, and history of comorbidity (ischaemic heart disease, atrial fibrillation, stroke, and diabetes) at the time of new-onset heart failure. The time periods were defined as follows—period 1: 1997–2001; period 2: 2002–06; period 3: 2007–11; and period 4: 2012–17. AR, absolute risk; CI, confidence interval; HF, heart failure; HR, hazard ratio.
Temporal trends in the risk of first HF rehospitalization for inpatients and HF hospitalization for outpatients
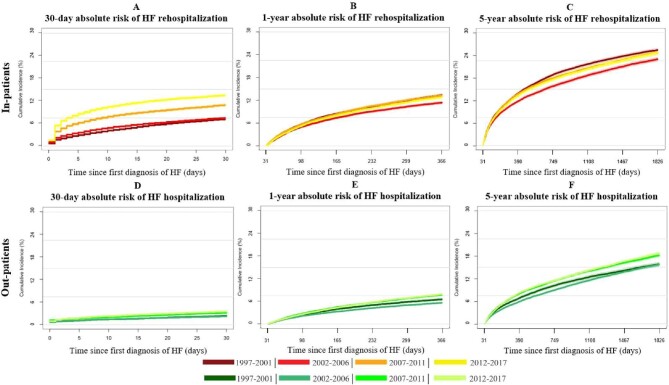
The absolute risk of the first rehospitalization for HF was higher for inpatients than the absolute risk of first HF hospitalization for outpatients (Figure 4A–F). During the first 30 days of follow-up, the absolute risk of rehospitalization for inpatients increased from 7.2% to 13.5%, while the risk of HF hospitalization for outpatients increased from 2.5% to 3.6% (Figure 4A, D). During 1997–2017, among patients who survived the first 30 days of follow-up, the 1-year and 5-year absolute risk of rehospitalization for inpatients decreased by 0.6% (from 13.4% to 12.8%) and 0.7% (from 25.4% to 24.7%), respectively (Figure 4B, C). For outpatients, the absolute risk of HF hospitalization within 1 year and 5 years of follow-up increased by 1.3% (from 6.6% to 7.9%) and 3.0% (from 15.8% to 18.7%), respectively, during 1997–2017 (Figure 4E, F). No clinical meaningful statistical interaction between patient type, rate of HF hospitalizations, and time period was observed and during the whole period, diagnosis of HF as an inpatient was associated with an increased rate of HF hospitalization (Figure 5).
Figure 4.
(A–F) Absolute risks of first heart failure rehospitalization for inpatients and first heart failure hospitalization for outpatients after new-onset heart failure. The graphs portray the absolute risk of heart failure rehospitalization and the absolute risk of heart failure hospitalization within 30 days (left) and 5 years (right) of follow-up. Note that 1-year and 5-year absolute risks were only estimated for patients who survived 31 days without HF hospitalization. (A–C) Absolute risk of HF rehospitalization for inpatients (red). (D–F) Absolute risk for HF hospitalization for outpatients (green).
Temporal trends in treatment
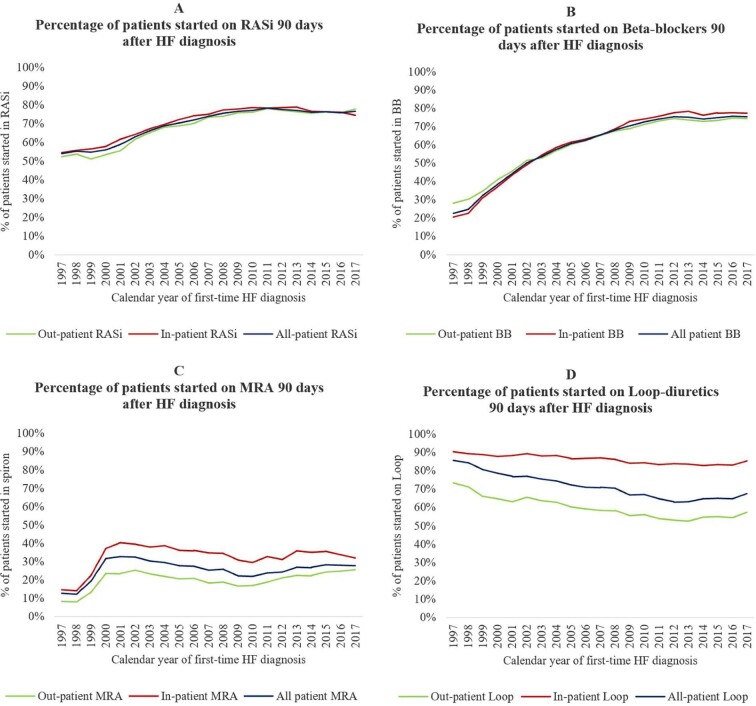
Figure 6 shows how the initiation of HF-relevant treatment increased significantly between 1997 and 2017. The percentage of patients receiving RASi (Figure 6A) and BBs (Figure 6B) within 90 days of first HF diagnosis increased markedly over time for all patients (e.g. for a RASi from 54% in 1997 to 77% in 2017 and a BB from 21% in 1997 to 75% in 2017). The initiation of MRAs (Figure 6C) peaked in 2001 without any major changes following (2001: 32%; 2017: 28%). Despite increased initiation of RASi, BBs, and MRAs, the initiation of loop diuretics (Figure 6D) following new-onset HF decreased during 1997–2017 (1997: 86%; 2017: 68%). In 2017, 85% of inpatients redeemed a prescription of loop diuretics, whereas the number was only 57% for outpatients. The overall percentage of patients initiated on relevant HF medication within 90 days of HF diagnosis followed the same pattern for in- and outpatients (Figure 6A–C).
Figure 6.
(A–D) Temporal trends in the percentage of heart failure patients started on renin–angiotensin-system inhibitors, beta blockers, mineralocorticoid receptor antagonists, and loop diuretics 90 days after new-onset heart failure. HF, heart failure; RASi, renin–angiotensin-system inhibitors; MRAs, mineralocorticoid receptor antagonists.
Discussion
Main findings
In this study, we investigated the temporal trends in the first diagnosis of HF, according to the clinical setting, between 1997 and 2017 in Denmark. We also examined trends in subsequent mortality and hospitalization rates, as well as trends in the pharmacological management of HF. While the overall incidence of HF did not change, the place of diagnosis changed, with more cases identified in the outpatient setting and fewer patients diagnosed in a hospital. During the period of study, there was also a significant decrease in the mortality rate among patients newly diagnosed with HF. Outpatients had lower event rates than inpatients throughout the study period—regardless of age, sex, and comorbidity—although mortality rates in the two groups become more alike over time. Treatment with evidence-based therapy shown to improve survival improved over the study period in both inpatients and outpatients. However, a declining risk of hospitalization for HF was not observed in either group.
Temporal trends in inpatient vs. outpatient diagnosis
To our knowledge, this is the first study to evaluate the temporal patterns of inpatient and outpatient diagnosis of new-onset HF and the subsequent risk of death and hospitalization over a 20-year period in a nationwide public healthcare system. The overall incidence of HF in the Frammingham Heart Study and the Cardiovascular Health Study has previously been studied and no significant changes were reported during 1990–2009.13 Previous Danish studies have focused on the incidence of new-onset HF over time and the impact of age and comorbidities.14,15 These, along with other studies conducted in the UK, point towards a decreasing incidence of new-onset HF, and an increased burden of comorbidities among patients newly diagnosed with HF.16,17 Despite a fluctuating incidence of new-onset HF, we did not find a decreasing incidence of HF between 1997 and 2017. However, several new developments have been introduced over the last two decades, including earlier identification and better management of left ventricular dysfunction and HF as a result of education and more widespread availability of echocardiography and natriuretic peptide measurement,18,19 novel pharmacological therapies for HF,1 and a focus on organized multidisciplinary, specialized outpatient care.20 Interestingly, we found that rather than resulting in a higher incidence of HF, these approaches led to a shift in where the first diagnosis of HF took place, from the inpatient setting to the outpatient setting, since 2005. We also observed that the initiation of neurohormonal blockade drugs within 90 days of new-onset HF improved remarkably over the whole study period, while the initiation of MRAs mainly increased between 1997 and 2000. As reported in other studies, mentioned earlier, we also found that patients newly diagnosed with HF had an increasing prevalence of comorbidities, although the median age of patients with new-onset HF decreased. Thus, it seems that the increased focus on HF in the last two decades has resulted in earlier detection of HF in outpatient clinics, increased surveillance of cardiac and extracardiac chronic diseases, and improved early initiation of therapy in patients with new-onset HF.
Temporal trends in mortality risk
Outpatients were 5 years younger than inpatients when presenting with new-onset HF (Table 1), which could explain some of the improvement in prognosis, although, following age standardization, outpatients still fared much better than inpatients. Previous studies comparing outpatients and inpatients also found that outpatients had lower mortality rates.4,21 Looking at baseline characteristics, it seems that inpatients are older, show an increasing prevalence of comorbidity over time, and almost 1 out of 10 patients diagnosed as inpatients between 2012 and 2017 were situated in nursing homes (Supplementary material online, Table S1). However, the stratified age-standardized mortality rates showed that even patients with prior history of both cardiovascular and non-cardiovascular comorbidities had much lower mortality rates in the outpatient group compared with inpatients (Supplementary material online, Figure S5). These findings suggest that as well as presenting with acutely decompensated HF, it seems that hospitalized patients have longer-standing and more advanced HF compared with outpatients, even when newly diagnosed. Conversely, outpatients may present at an earlier stage of disease progression. It is also possible that some outpatients had left ventricular dysfunction without clinical evidence of HF, e.g. patients after MI or patients with transient HF caused by AF with a rapid ventricular rate. This can to some extent be supported by the decreasing percentages of patients redeeming loop diuretics within 90 days of being diagnosed with new-onset HF. HF patients with an outpatient diagnosis could, therefore, include both HF stage B and C according to American terminology.22
The significant decrease in the mortality risk of HF patients is presumably due to improvement in the level of care over time and enhanced initiation of HF treatment following new-onset HF. Inpatients had a greater decrease in mortality risk compared with outpatients. This can possibly be explained by a combination of increased diagnosis of vulnerable HF patients as outpatients, increased initiation of HF treatment within 90 days of new-onset HF, and improvement in the level of care over time. Note also that the fraction of HF patients with e.g. cancer and type 2 diabetes increased over time (Supplementary material online, Table S1), and increased surveillance of extracardiac chronic diseases and early detection of HF in these high-risk subgroups may also explain our results.
Temporal trends in risk of HF hospitalization
Despite earlier identification of HF and better treatment, HF hospitalization rates after diagnosis did not decrease. Looking at the temporal trends in the absolute risk of HF hospitalization, both groups were increasingly hospitalized within 30 days, while the long-term absolute risks of HF hospitalization show no clear trend over time, although hospitalization risks in the later years were higher than earlier years. The increasing risk of HF hospitalization could be explained by a decreasing mortality rate that keeps vulnerable patients alive and more hospitalizations therefore potentially occur or it could be that an HF hospitalization reflects other than the progression of HF, e.g. a strategy of care that has not changed over time.23 Still, the risk of hospitalization for HF in Denmark was low compared with the risk of HF hospitalization in the rest of Europe and the USA.24,25 Nevertheless, inpatients had higher absolute risks of HF rehospitalization compared with the risk of having a first HF hospitalization as an outpatient during days 31–1826 (5 years) of follow-up—supporting the concept that inpatients are sicker, frailer, and potentially have more severe HF.
The absolute risk of death and hospitalization within 30 days of new-onset HF was markedly lower in outpatients compared with inpatients. This could be explained by the longer-standing HF for inpatients and that patients presenting with end-stage HF are more likely to manifest as inpatients. Yet, it is striking that the 30-day absolute risk of rehospitalization for inpatients newly diagnosed with HF was similar to the risk of rehospitalization during 31 days to 1 year. Several studies have shown that HF hospitalizations are associated with acceleration of the progression of HF and that patients hospitalized for HF are at high risk of death and readmission in the first 2–3 months post-discharge.25,26 Since outpatients are diagnosed in outpatient clinics and initiated on HF treatment earlier in the course of their disease, the progression of HF might be halted enough to delay overnight hospitalizations and the trajectories associated with HF hospitalizations.26 Therefore, our findings underscore that inpatients are at higher risk of being rehospitalized after new-onset HF and that despite improvements in the management of HF, the risk of HF hospitalization did not decrease over time.
Strengths and limitations
The risk of inclusion and selection bias was minimal due to the nationwide study design. The main strength of this study is the sample size and the data completeness of a nationwide cohort with HF with at least 1 year of follow-up. The HF diagnosis has been acknowledged as suitable to identify large groups of patients with HF in the Danish registers.21 Patients could not be lost to follow-up unless they emigrated (2.8%).
To assess the management of HF in terms of treatment, we looked at redeemed prescriptions within the first 90 days following HF diagnosis. However, it was only certain that patients redeemed their prescription; thus, adherence bias might be present. Further, to minimize the potential impact of lead-time bias,27 when comparing outpatients with inpatients, we made age-standardized analyses.
Data on treatment with devices are not included in this study since only a few patients were treated with those within 3 months of onset of HF.28 Future studies should focus on the lack of implementation of these, the impact of risk factors, and their impact on prognosis to deepen the understanding of outpatients and inpatients according to important subgroups. Also, the registers do not contain information on prognostic factors such as left ventricular ejection fraction, functional class, renal function, blood pressure, and biomarkers; hence, we are limited to only describing management and outcomes based on hospital administrative codes.
Therefore, unmeasured and residual confounding cannot be excluded, and the strength of the association between patient type and rate of outcomes should be interpreted in that context. However, knowledge of these important clinical variables would not have changed the location of diagnosis of HF and the following 1- and 5-year risks of outcomes. It has previously been shown that a prior hospitalization—not necessarily an index event—is associated with a poor outcome in a fully adjusted model.29 Still, we were unable to differentiate between HF with preserved and reduced left ventricular ejection fraction and how the incidence and location of diagnosis of these HF phenotypes have changed over time. Older age and female sex are frequent in patients with HF with preserved ejection fraction, and these important subgroups showed the same pattern as the main result (Supplementary material online, Table S3). Finally, how and whether outpatient management of worsening HF has changed over time—and whether this has influenced hospitalization for HF—cannot be deduced from our results.28,30
Clinical perspectives
This study suggests that patients presenting with new-onset HF as inpatients and outpatients are two different patient types. Our findings show that HF patients diagnosed in an outpatient setting have a much better prognosis than inpatients regardless of age, sex, and comorbidity, and are likely presenting with HF at an earlier stage of disease than inpatients. Consequently, inpatients need close follow-up after HF hospitalization, due to the significant impact of the vulnerable phase following hospitalization. The long-term prognostic impact of the diagnosis setting needs to be studied further; however, this study suggests that diagnosing an increasing number of HF patients in the outpatient setting could improve management and prognosis.
Conclusions
The overall incidence of HF in Denmark from 1997 to 2017 was unchanged. However, the location of diagnosis changed, with more HF cases being identified in the outpatient setting and fewer patients being diagnosed in the hospital. There was also a significant decrease in the mortality rate among patients newly diagnosed with HF—probably explained by the earlier diagnosis as outpatients and increased initiation of evidence-based therapy. However, a reduced risk of hospitalization for HF following initial diagnosis was not observed. Outpatients had lower event rates than inpatients throughout the study period—regardless of age, sex, and comorbidity—although mortality rates become more alike over time. These findings may have important implications for risk stratification in trials and clinical practice.
Supplementary Material
Contributor Information
Anojhaan Arulmurugananthavadivel, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark.
Anders Holt, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark.
Saaima Parveen, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark.
Morten Lamberts, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark.
Gunnar H Gislason, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark; The Danish Heart Foundation, 1120 Copenhagen, Denmark.
Christian Torp-Pedersen, Department of Cardiology, Aalborg University Hospital, 9100 Aalborg; Department of Cardiology, Nordsjællands Hospital, 3400 Hillerød, Denmark; Department of Biostatistics, University of Copenhagen, 1014 Copenhagen, Denmark.
Christian Madelaire, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark.
Charlotte Andersson, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark; Section of Cardiovascular Medicine, Boston Medical Center, Boston University, 02118 Boston, MA, USA.
Deewa Zahir, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark.
Jawad H Butt, Department of Cardiology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark.
Mark C Petrie, Department of Cardiology, Glasgow Royal Infirmary, G4 0SF Glasgow, UK; British Heart Foundation Cardiovascular Research Centre, University of Glasgow, G12 8TA Glasgow, UK.
John McMurray, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, G12 8TA Glasgow, UK.
Emil L Fosbol, Department of Cardiology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark.
Lars Kober, Department of Cardiology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark.
Morten Schou, Department of Cardiology, Herlev and Gentofte University Hospital, 2900 Copenhagen, Denmark.
Conflict of interest
L.K. reports personal fees from speakers’ honorarium from Novo, Novartis, AstraZeneca, and Boehringer, outside the submitted work. M.L. reports personal fees from BMS, Bayer, from null, outside the submitted work. All other authors have nothing to disclose.
Data availability
The data used to support the findings of this study are available from Statistics Denmark. However, these data are not publicly available, since they are subject to restrictions and are used under permission and license for the current study. Data are however available from the authors upon reasonable request and with permission of Statistics Denmark.
References
- 1. Mcmurray JJV, Pfeffer MA.. Heart failure. Lancet North Am Ed 2005;365:1877–1889. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu Set al. Heart failure: preventing disease and death worldwide. ESC Heart Fai 2014;1:4–25. [DOI] [PubMed] [Google Scholar]
- 3. Butt JH, Fosbøl EL, Gerds TA, Andersson C, McMurray JJV, Petrie MCet al. Readmission and death in patients admitted with new-onset versus worsening of chronic heart failure: insights from a nationwide cohort. Eur J Heart Fail 2020;22(10):1–9. [DOI] [PubMed] [Google Scholar]
- 4. Ferreira JP, Metra M, Mordi I, Gregson J, Ter Maaten, Tromp Jet al. Heart failure in the outpatient versus inpatient setting: findings from the BIOSTAT-CHF study. Eur J Heart Fail 2019;21:112–120. [DOI] [PubMed] [Google Scholar]
- 5. Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka Fet al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–625. [DOI] [PubMed] [Google Scholar]
- 6. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 7. Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39:26–29. [DOI] [PubMed] [Google Scholar]
- 8. Schmidt M, Pedersen L, Sørensen HT.. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 9. Sundbøll J, Adelborg K, Munch T, Frøslev T, Toft Sørensen H, Erik Bøtker H, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mard S, Nielsen FE.. Positive predictive value and impact of misdiagnosis of a heart failure diagnosis in administrative registers among patients admitted to a university hospital cardiac care unit. Clinical Epidemiology 2010;2:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madelaire C, Gustafsson F, Køber L, Torp-Pedersen C, Andersson C, Kristensen SLet al. Identification of patients with new-onset heart failure and reduced ejection fraction in Danish administrative registers. Clin Epidemiol 2020;12:589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT.. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemio 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JRet al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC: Heart Fail 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christiansen MN, Køber L, Weeke P, Vasan RS, Jeppesen JL, Smith JGet al. Age-specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation 2017;135:1214–1223. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Ulrichsen SP, Pedersen L, Bøtker HE, Sørensen HT.. Thirty-year trends in heart failure hospitalization and mortality rates and the prognostic impact of co-morbidity: a Danish nationwide cohort study. Eur J Heart Fail 2016;18:490–499. [DOI] [PubMed] [Google Scholar]
- 16. Conrad N, Judge A, Canoy D, Tran J, Pinho-Gomes AC, Millett ERCet al. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86000 individuals. JAMA Cardiol 2019;4:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo APet al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet North Am Ed 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kober L, Torp-Pedersen C, Carlsen J, Videbaek R, Egeblad H.. An echocardiographic method for selecting high risk patients shortly after acute myocardial infarction, for inclusion in multi-centre studies (as used in the TRACE study). TRAndolapril Cardiac Evaluation. Eur Heart J 1994;15:1616–1620. [DOI] [PubMed] [Google Scholar]
- 19. Galatius S, Gustafsson F, Nielsen PH, Atar D, Hildebrandt PR.. An integrated approach to diagnosis and therapeutic management of patients with systolic heart failure in the Copenhagen metropolitan area. Am Heart J 2002;144;A7–A13. [DOI] [PubMed] [Google Scholar]
- 20. Redfield MM. Heart failure—an epidemic of uncertain proportions. N Engl J Med 2002;347:1442–1444. [DOI] [PubMed] [Google Scholar]
- 21. Madelaire C, Gustafsson F, Stevenson LW, Kristensen SL, Køber L, Andersen Jet al. Favorable five-year outcomes for heart failure diagnosed in younger patients without severe comorbidity. Int J Cardiol 2020;305:106–112. [DOI] [PubMed] [Google Scholar]
- 22. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MMet al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 23. Greene SJ, Felker GM, Butler J.. Outpatient versus inpatient worsening heart failure: distinguishing biology and risk from location of care. Eur J Heart Fail 2019;21:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Butler J, Yang M, Manzi MA, Hess GP, Patel MJ, Rhodes Tet al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol 2019;73:935–944. [DOI] [PubMed] [Google Scholar]
- 25. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan Met al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 26. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M.. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015;12:220–229. [DOI] [PubMed] [Google Scholar]
- 27. Andersson TML, Rutherford MJ, Humphreys K.. Assessment of lead-time bias in estimates of relative survival for breast cancer. Cancer Epidemiol 2017;46:50–56. [DOI] [PubMed] [Google Scholar]
- 28. Madelaire C, Gustafsson F, Stevenson LW, Kristensen SL, Køber L, Andersen Jet al. One-year mortality after intensification of outpatient diuretic therapy. J Am Heart Assoc 2020;9:e016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomon SD, Claggett B, Packer M, Desai A, Zile MR, Swedberg Ket al. Efficacy of sacubitril/valsartan relative to a prior decompensation: the PARADIGM-HF trial. JACC: Heart Fail 2016;4:816–822. [DOI] [PubMed] [Google Scholar]
- 30. Okumura N, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JLet al. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Circulation 2016;133:2254–2262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from Statistics Denmark. However, these data are not publicly available, since they are subject to restrictions and are used under permission and license for the current study. Data are however available from the authors upon reasonable request and with permission of Statistics Denmark.