Abstract
Background and aims
There is limited data on temporal trends of cardiovascular hospitalizations and outcomes amongst cancer patients. We describe the distribution, trends of admissions, and in-hospital mortality associated with key cardiovascular diseases among cancer patients in the USA between 2004 and 2017.
Methods
Using the Nationwide Inpatient Sample we, identified admissions with five cardiovascular diseases of interest: acute myocardial infarction (AMI), pulmonary embolism (PE), ischaemic stroke, heart failure, atrial fibrillation (AF) or atrial flutter, and intracranial haemorrhage. Patients were stratified by cancer status and type. We estimated crude annual rates of hospitalizations and annual in-hospital all-cause mortality rates.
Results
From >42.5 million hospitalizations with a primary cardiovascular diagnosis, 1.9 million (4.5%) had a concurrent record of cancer. Between 2004 and 2017, cardiovascular admission rates increased by 23.2% in patients with cancer, whilst decreasing by 10.9% in patients without cancer. The admission rate increased among cancer patients across all admission causes and cancer types except prostate cancer. Patients with haematological (9.7–13.5), lung (7.4–8.9), and GI cancer (4.6–6.3) had the highest crude rates of cardiovascular hospitalizations per 100 000 US population. Heart failure was the most common reason for cardiovascular admission in patients across all cancer types, except GI cancer (crude admission rates of 13.6–16.6 per 100 000 US population for patients with cancer).
Conclusions
In contrast to declining trends in patients without cancer, primary cardiovascular admissions in patients with cancer is increasing. The highest admission rates are in patients with haematological cancer, and the most common cause of admission is heart failure.
Keywords: Cardio- oncology, trends, prognosis
Introduction
Improved detection and treatment strategies have increased the life expectancy of cancer patients.1 Patients with a history of cancer have a higher risk of cardiovascular disease (CVD) compared with the general population.2 As many more cancer patients live to an older age, CVD is emerging as an important and dominant cause of disability and premature death in this patient cohort.3
It is expected that patients with past or active cancer will be increasingly encountered across all cardiac subspecialties. These patients have unique cardiotoxic exposures (chemo-, radio-, and immune-therapies) and disease susceptibilities (e.g. thromboembolism).4 Optimizing the cardiovascular care of cancer patients has growing relevance for the entire cardiovascular community.5
To understand the cardiovascular care needs of patients with cancer, there is a need for high quality data from large populations. Cardiovascular hospitalizations are an important indicator of disease burden and service use. Previous analyses of national-level cardiovascular admission data have demonstrated greater cardiovascular mortality risk in cancer patients and differential pattern of CVDs by primary cancer site.6–8 However, existing literature does not evaluate temporal trends in cardiovascular hospitalizations, which have important implications for healthcare resource utilization and costs in cancer patients.
Using the National Inpatient Sample (NIS) database, we aimed to describe the distribution and trends of admissions and in-hospital mortality associated with key CVDs among patients with cancer, by CVD type and primary cancer site.
Methods
Data source and study population
The NIS is the largest all-payer inpatient health care database in the United States of America (USA), developed by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ).9 The NIS dataset contains hospital information on between 7 and 8 million yearly hospital discharges from 2004 onwards. Since 2012, the NIS samples discharges from all hospitals participating in HUCP, approximating a 20% stratified sample of all discharges from community hospitals in the USA. The NIS reports diagnostic data using the International Classification of Diseases, Ninth Edition (ICD-9) until September 2015, updating to International Classification of Diseases, Tenth Edition (ICD-10) codes from October 2015.
In this study, we analysed included all adults (≥18 years) admitted with a principal diagnosis of one of the defined CVDs of interest, covering the period from January 2004 to December 2017. Sampling weights were used to calculate the estimated total discharges as specified by AHRQ. As we used deidentified and publicly available data, this study was exempt from Institutional Review Board evaluation.
Ascertainment of cardiovascular disease and cancer status
Admission diagnoses were grouped into the following CVD categories: acute myocardial infarction (AMI), pulmonary embolism (PE), ischaemic stroke, heart failure, atrial fibrillation (AF) or atrial flutter, and intracranial haemorrhage. We identified patients with a record of any cancer, which we then stratified by cancer type (five most common cancers: haematological, lung, gastrointestinal, prostate, breast, and other). Admission categories, cancer types, as well as other patient characteristics, were extracted using ICD-9 and ICD-10 codes provided in Supplementary material online, Table S1.
Statistical analysis
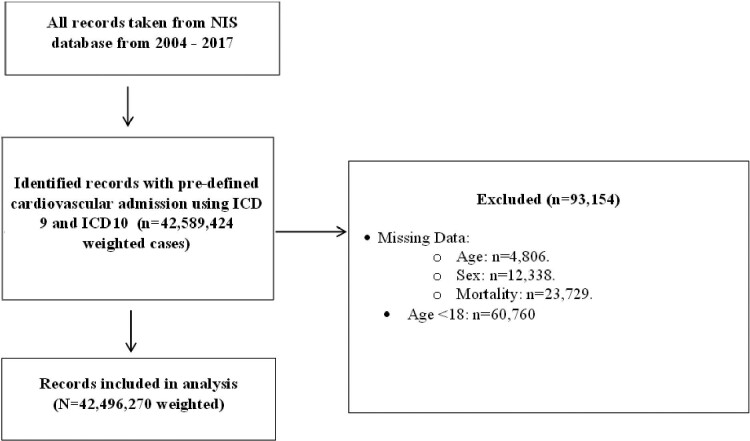
All statistical analyses were performed on IBM SPSS version 26 and Stata MP version 17.0. Information on patient demographics was recorded for each hospital discharge, including age, sex, race, admission day (weekday or weekend), expected primary payer, and median household income according to ZIP code. Records with missing data for age, sex, and mortality status were excluded from the analysis (Figure1 for study flow diagram).
Figure 1.
Flow diagram of study sample selection. NIS, national inpatient sample; ICD, international classification of disease.
We estimated the crude annual rates of hospitalization among patients with cancer per 100 000 US adults10 for the six different admission causes and cancer types. We also described the annual in-hospital all-cause mortality rates. For comparison, we also report all results in the entire sample, including patients admitted with a primary CVD diagnosis with or without a record of cancer. Continuous variables are presented as a median (25th percentile and 75th percentile) due to skewed data. Categorical data are presented as frequencies and percentages. Categorical variables were compared using the Pearson Chi-square test, while continuous variables were compared using the Kruskal–Wallis test. We used multivariable logistic regression to estimate the association of cancer types (exposure of interest) with in-hospital mortality. Associations were examined separately for each CVD category. We Hierarchical multilevel modelling was used to account for clustering/nesting of observations, by adjusting for the hospital clustering. We further adjusted for the following covariates: sex, age, race, hospital location, hospital size, hospital location/teaching status, weekend and elective admission, median zip income, expected primary payer, valvular disease, hypertension, diabetes mellitus, peripheral vascular disease, chronic lung disease, chronic renal failure, obesity, anaemia, coagulopathy, chronic liver disease. Statistical significance was set at the 2-tailed 0.05 level without any multiplicity adjustment.
Results
Baseline characteristics
Between 2004 and 2017, a total of 42 496 270 weighted cases of patients with any of the six CV admission causes of AMI, PE, ischaemic stroke, heart failure, AF or atrial flutter, and intracranial haemorrhage were identified and included in the analysis (Figure1). Of these, 1895 823 cases (4.5%) had an active cancer diagnosis. Haematological malignancy was the most common cancer type (26.1%, 495 914) followed by lung (18.7%, 354 349), gastrointestinal (12.4%, 235 795), prostate (11.6%, 220 873), and breast (6.7%, 126 617) malignancies. Other cancer types were present in 24.4% of cases (462 275 patients).
Patients with cancer were older and had a higher prevalence of valvular disease, anaemia, and coagulopathy, and a lower prevalence of hypertension, diabetes mellitus, and obesity compared with patients without cancer. Among the specific cancer types, patients with prostate cancer were, on average, the oldest (median 79 years) and had the highest prevalence of hypertension (68.9%) and peripheral artery disease (10.8%). Patients with haematological malignancies had the greatest burden of valvular heart disease (7.2%), chronic renal failure (27.9%), anaemia (33.1%), and coagulopathies (10.9%) (Table1). Baseline characteristics of patients with specific causes of admission are presented in Supplementary material online, Tables S2–S7.
Table 1.
Demographics, record characteristics, and comorbidities of patients stratified by cancer type
| No malignancy | Haematological | Lung | GI | Prostate | Breast | Other | P-value | |
|---|---|---|---|---|---|---|---|---|
| Number of weighted records | 40 600 447 | 495 914 | 354 349 | 235 795 | 220 873 | 126 617 | 462 275 | |
| Age (years), median (IQR) | 72 (60, 82) | 76 (67, 83) | 72 (64, 79) | 72 (63, 81) | 79 (72, 85) | 72 (62, 81) | 72 (63, 81) | <0.001 |
| Females, % | 49.1% | 44.9% | 45.6% | 43.2% | 0% | 98.7% | 49.7% | <0.001 |
| Race | ||||||||
| White | 73.9% | 79.6% | 80.5% | 74.1% | 73.5% | 71.5% | 79.2% | <0.001 |
| Black | 14.0% | 11.4% | 11.9% | 14% | 16.5% | 18% | 10.9% | |
| Hispanic | 6.0%7.1% | 5.2% | 3.5% | 6.6% | 5.8% | 6.1% | 5.7% | |
| Asian/Pacific Islander | 2.0% | 1.4% | 1.8% | 2.6% | 1.6% | 1.8% | 1.7% | |
| Native American | 0.5% | 0.3% | 0.4% | 0.3% | 0.3% | 0.4% | 0.4% | |
| Other | 2.5% | 2.1% | 1.8% | 2.4% | 2.2% | 2.3% | 2.1% | |
| Hospital location | ||||||||
| Northeast | 19.6% | 21.7% | 20.8% | 22.2% | 22.1% | 20.7% | 22% | <0.001 |
| Midwest | 23.6% | 25.2% | 25.4% | 24.2% | 23.9% | 25.6% | 23.9% | |
| South | 40.4% | 36.7% | 39.9% | 36.7% | 34.5% | 37% | 36.1% | |
| West | 16.5% | 16.4% | 13.9% | 16.8% | 19.5% | 16.7% | 18% | |
| Hospital size | ||||||||
| Small | 13.1% | 13.5% | 13.4% | 13.6% | 14.2% | 14.2% | 12.9% | <0.001 |
| Medium | 25.1% | 25.5% | 26% | 25.7% | 26.5% | 27.1% | 24.9% | |
| Large | 61.8% | 61% | 60.6% | 60.6% | 59.3% | 58.7% | 62.2% | |
| Hospital location/teaching status | ||||||||
| Rural | 13.1% | 11% | 12.8% | 11.2% | 13.2% | 11.7% | 10.5% | <0.001 |
| Urban non-teaching | 41.0% | 35.3% | 37.3% | 35.3% | 37.9% | 35.2% | 34.1% | |
| Teaching | 45.8% | 53.7% | 49.8% | 53.5% | 48.9% | 53% | 55.4% | |
| Weekend admission | 22.8% | 22.3% | 22.2% | 22.2% | 22.4% | 21.5% | 22% | <0.001 |
| Elective admission | 10.8% | 9.6% | 7.7% | 8.2% | 10.2% | 8.7% | 8.7% | <0.001 |
| Median income | ||||||||
| 1st quartile | 29.4% | 24.6% | 28.4% | 27.3% | 26.9% | 27.7% | 25.6% | <0.001 |
| 2nd quartile | 26.8% | 25.8% | 26.8% | 25.6% | 25.5% | 25.9% | 25.3% | |
| 3rd quartile | 23.1% | 25.2% | 24.1% | 24.1% | 24% | 24.2% | 24.7% | |
| 4th quartile | 20.8% | 24.4% | 20.7% | 23% | 23.6% | 22.2% | 24.4% | |
| Expected primary payer | ||||||||
| Medicare | 66.5% | 77.7% | 71.7% | 69.4% | 83.5% | 69.5% | 69.7% | <0.001 |
| Medicaid | 6.6% | 3.9% | 6.2% | 6.4% | 2.3% | 7.5% | 6.1% | |
| Private | 20.4% | 15.7% | 18.5% | 20.5% | 11.7% | 20.1% | 20.6% | |
| Uninsured | 3.9% | 1.1% | 1.4% | 1.6% | 0.9% | 1.3% | 1.6% | |
| No charge | 0.4% | 0.1% | 0.1% | 0.2% | 0.1% | 0.1% | 0.2% | |
| Other | 2.1% | 1.5% | 2.1% | 1.9% | 1.6% | 1.4% | 1.8% | |
| Valvular disease | 3.8% | 7.2% | 4.5% | 5.4% | 6.5% | 6.6% | 6% | <0.001 |
| Hypertension | 69.9% | 64.9% | 59.3% | 61.6% | 68.9% | 65.5% | 62.6% | <0.001 |
| Diabetes mellitus | 34.2% | 30.6% | 24.7% | 32.8% | 30.1% | 30.3% | 27.8% | <0.001 |
| Peripheral vascular disease | 9.7% | 8.2% | 10.6% | 6.9% | 10.8% | 6% | 7.6% | <0.001 |
| Chronic lung disease | 24.8% | 24.2% | 53% | 21.3% | 23.4% | 22.5% | 23.4% | <0.001 |
| Chronic renal failure | 19.3% | 27.9% | 13.9% | 16% | 24.5% | 15.7% | 20.4% | <0.001 |
| Obesity | 11.7% | 8.5% | 5.4% | 7% | 6.7% | 12.4% | 8.9% | <0.001 |
| Anaemia | 17.7% | 33.1% | 28.4% | 36.6% | 29.1% | 27.6% | 30% | <0.001 |
| Coagulopathy | 3.8% | 10.9% | 7.5% | 8.7% | 5.5% | 5.3% | 6.9% | <0.001 |
| Chronic liver disease | 1.7% | 1.7% | 1.2% | 5.3% | 1.1% | 1.2% | 1.5% | <0.001 |
| Length of stay, days, median (IQR) | 3 (2.6) | 4 (2.7) | 4 (2.7) | 4 (2.7) | 4 (2.6) | 4 (2.6) | 4 (2.7) | <0.001 |
| Total charge, $, median (IQR) | 23 361 (12 691, 46 079) | 27 333 (14 780, 53 821) | 27 164 (14 995, 21 030) | 28 184 (15 078, 54 715) | 24 587 (13 229, 48 483) | 25 076 (13 978, 46 622) | 28 030 (15 205, 53 830) | <0.001 |
| Discharge home | 56.7% | 50.1% | 43% | 43.8% | 48.8% | 51.1% | 44.6% | <0.001 |
| In-hospital mortality | 4.2% | 6.3% | 9.6% | 8.3% | 5.7% | 5% | 7.8% | <0.001 |
GI, Gastrointestinal; IQR, interquartile range.
Due to rounding numbers may not add up to 100.0%.
Overall admission trends
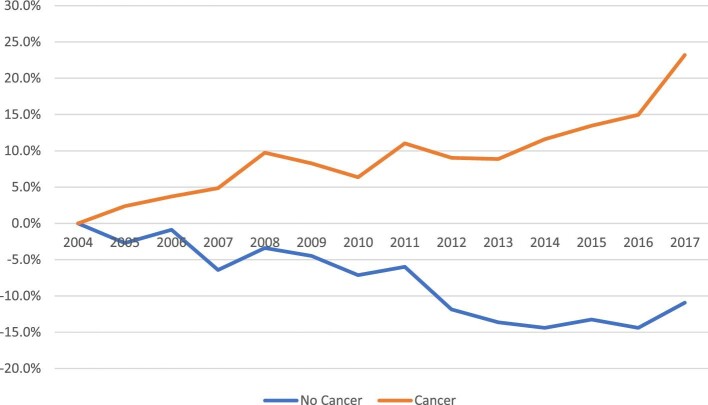
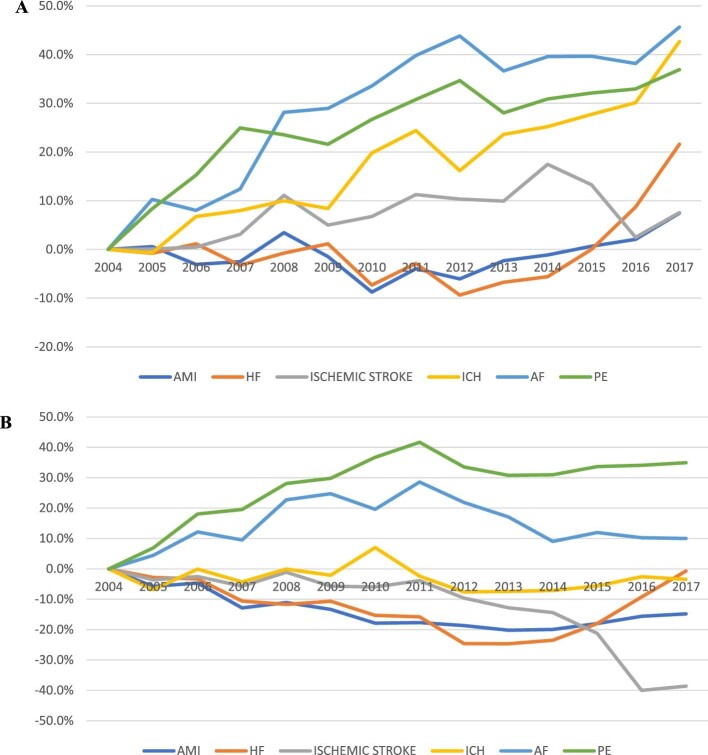
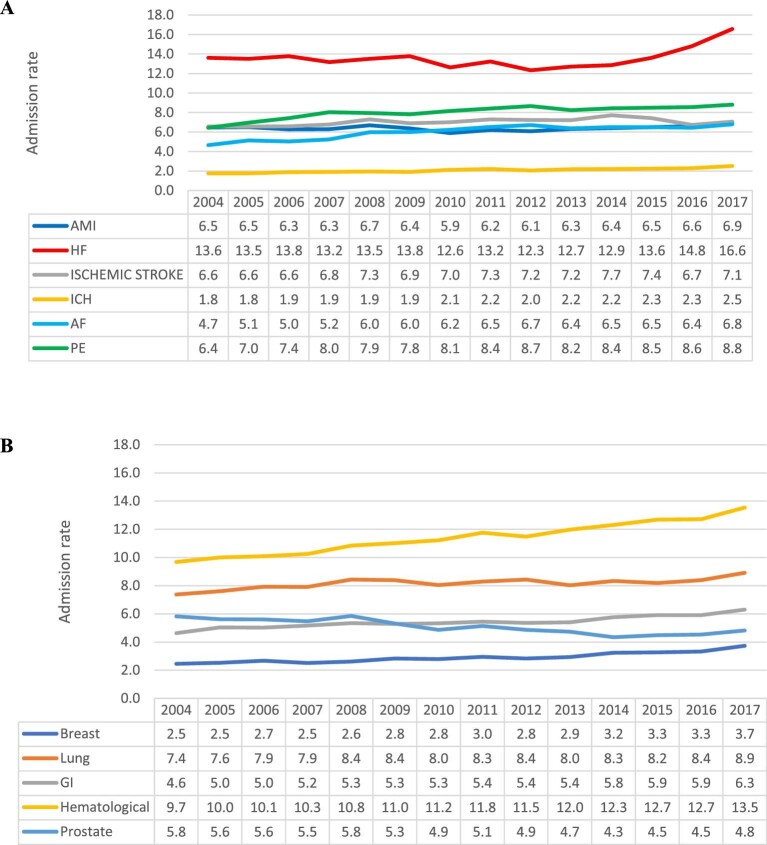
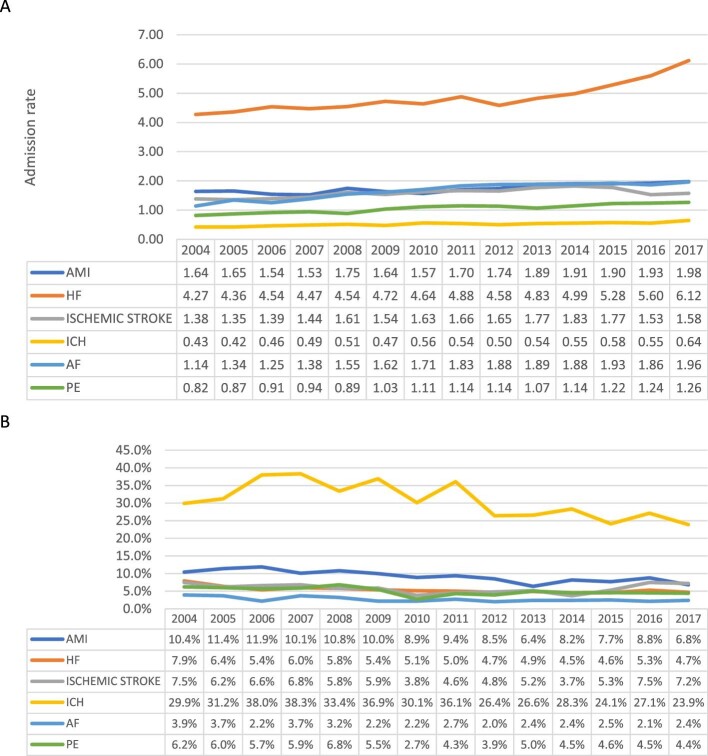
Among patients with a cancer diagnosis, we observed an overall 23.2% increase in the overall CV crude admission rate between 2004 and 2017 (Figure2). The crude admission rate increased for all six admission causes between 7% (acute MI and ischaemic stroke) and 46% (AF) (Figure3A). Among patients with cancer, heart failure was the most common cause of admission throughout the entire study period, with the annual admission rate increasing during the study period from 13.6 (95% CI 13.5–13.7) to 16.6 (95% CI 16.4–16.7) per 100 000 US population (P for trend 0.02). In 2017, PE was the second most common cause of admission, with the admission rate increasing from 6.4 (95% CI 6.3–6.5) to 8.8 (95%CI 8.7–8.9) per 100 000 US populations (P for trend < 0.001). ICH was the least common reason for admission but showed a significant increase from 2004 to 2017 [1.8 (95% CI 1.7–1.8) to 2.5 (95% CI 2.5–2.6) per 100 000 US population, P for trend < 0.001] (Figure4A).
Figure 2.
% change in crude CV admission rates in patients with vs. without cancer.
Figure 3.
% change in crude CV admission rates by admission cause among patients with (A) and without (B) cancer.
Figure 4.
Trends in cardiovascular admissions of cancer patients by cause of admission (A) and cancer type (B), per 100 000 US population.
In contrast, during the same study period, in patients without cancer, we observed a 10.9% overall decrease in the overall CV crude admission rate (Figure2). During this period, we observed >30% increase in the PE crude admission rate and a 10% increase in the AF crude admission rate. The crude admission rate of the other CVD decreased between 0.7% (heart failure) and 38% (ischaemic stroke) (Figure3B). Heart failure was the most common admission cause during the entire study period, with the admission rate decreasing from 352.2 (95% CI 351.5–352.9) to 349.8 (95% CI 349.2–350.4) per 100 000 US population (P for trend < 0.001). Ischaemic stroke was the second most common admission cause in 2004 but showed the most significant decrease during the study period and was the third most common in 2017 [250.8 (95% CI 250.2–251.3) to 154.0 (95% CI 153.6–154.4) per 100 000 US population, P for trend < 0.001]. AMI was the third most common cause in 2004, and the second in 2017 despite a significant drop in the crude admission rate [230.7 (95% CI 230.1–231.2) to 196.5 (95% CI 196.0–197.0) per 100 000 US population, P for trend < 0.001]. PE was the least common cause of admission in 2004 and showed the most significant increase during the study period [34.9%, 36.2 (95% CI 36.0–36.5) to 48.9 (95% CI 48.7–19.2) per 100 000 US population, P for trend < 0.001]. In 2017, PE was the fifth common cause of admission, and ICH was the least common cause of admission (37.6, 95% CI per 100 000 US population).
We observed an overall steady increase in primary cardiovascular admission rates across all cancer types, expect prostate cancer (Figure4B). Patients with haematological cancer had the highest crude rate of cardiovascular admissions throughout the study period, they also had the greatest increase in admissions from 9.7 (95% CI 9.6–9.8) in 2004 to 13.5 (95% CI 13.4–13.7) in 2017 per 100 000 US population (P for trend < 0.001). Lung cancer patients had the second highest rate of cardiovascular admissions also with a significant increasing trend [7.4 (95% CI 7.3–7.5) to 8.9 (95% CI 8.8–9.0) per 100 000 US population, P for trend < 0.001]. Similar significant increasing trends in rate of cardiovascular admissions was observed in patients with gastrointestinal [4.6 (95% CI 4.6–4.7) to 6.3 (95% CI 6.2–6.4) per 100 000 US population, P for trend < 0.001] and breast [2.5 (95% CI 2.4–2.5) to 3.7 (95% CI 3.7–3.8) per 100 000 US population, P for trend < 0.001] cancers; in patients with prostate cancer, CV admission rate decreased from 5.8 (95% CI 5.7–5.9) to 4.8 (95% CI 4.7–4.9) per 100 000 US population (P for trend < 0.001).
In-hospital mortality trends across cancer types
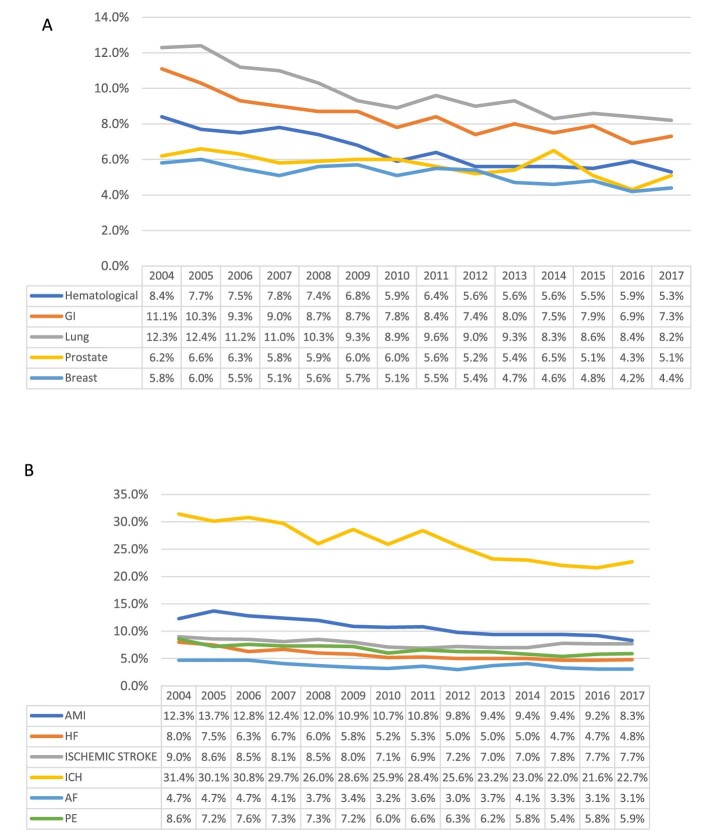
In-hospital mortality rates were higher among patients with cancer compared with patients without cancer (5–9.6% vs. 4.2%, Table1). Following adjustment, compared with other cancer types, the adjusted odds ratio for mortality was highest in patients with cancer admitted with AF, AMI, ischaemic stroke, HF, and PE [OR (95% CI) of 4.43 (3.67–5.35), 2.31 (2.02–2.63), 2.29 (2.01–2.61), 2.24 (1.97–2.54), and 2.36 (2.07–2.68), respectively]. Among patients admitted with ICH, the highest odds ratio for mortality was observed in patients with haematological malignancies (1.42 95% CI (1.23–1.63), Supplementary material online, Table S8. We examined in-hospital mortality amongst patients with current cancer admitted with any of the cardiovascular diagnosis, observing a statistically significant decline in mortality across all cancer types (Figure5A). The highest mortality rates in patients admitted with a primary cardiovascular diagnosis and cancer were observed in patients with lung cancer (decrease from 12.3% in 2004 to 8.2% in 2017, P for trend < 0.001), followed by gastrointestinal cancer (11.1–7.3%, P for trend < 0.001). During most of the study period, the lowest mortality rates in patients admitted with a primary cardiovascular diagnosis and cancer were observed among patients with prostate (6.2–5.1%, P for trend 0.003) and breast cancers (5.8–4.4%, P for trend < 0.001).
Figure 5.
Trends in in-hospital mortality (%) amongst cardiovascular admissions of cancer patients by cancer type (A) and cause of admission (B).
Among patients with cancer, there was a general declining in-hospital mortality trend across all the cardiovascular admissions considered (Figure5B). The highest in-hospital mortality was observed in patients admitted with ICH, ranging from (31.4–22.7%, P for trend < 0.001), followed by AMI (12.3–8.3%, P for trend < 0.001). The lowest mortality rate was in those admitted with AF/flutter (4.7–3.1%, P for trend = 0.001). Among patients without cancer, we observed similar overall declines in-hospital mortality rate trends across all admission causes, except for ischaemic stroke (mortality rate increased from 3.6% to 4%, Supplementary material online, Figure S1).
Haematological cancer
There was an overall increasing crude rate of primary cardiovascular admissions in patients with haematological cancer across all CVDs studied. The most common admission reason throughout the entire study period was heart failure, which also showed the steepest increase of all cardiovascular conditions considered [4.27 (95% CI 4.20–4.32) to 6.12 (95% CI 6.03–6.2) per 100 000 US population, P for trend < 0.005]. The remaining CVDs showed less prominent increasing trends (Figure6A). In 2017, the highest rates of in-hospital death were observed in those admitted with ICH (23.9%), ischaemic stroke (7.2%), and AMI (6.8%). These were followed by heart failure (4.7%) and PE (4.4%). The lowest mortality rates were observed in those admitted with AF/flutter (2.4%). Patients admitted with heart failure had the second highest mortality rate in 2004 (7.9%), which dropped to the fourth highest in 2017 (4.7%) (Figure6B).
Figure 6.
Trends in CV causes of admission per 100 000 US population (A) and in-hospital mortality % (B) of patients with haematological malignancy.
Lung cancer
The most common causes of primary cardiovascular admission in 2017 in lung cancer patients were heart failure [2.4 (95% CI 2.35–2.45)], PE [2.03 (95% CI 1.99–2.08)], and AF [1.50 (95% CI 1.46–1.54)]—all per 100 000 US population. In 2004, AMI was the second most common cause of primary cardiovascular admission [1.27 (95% CI 1.23–1.31) per 100 000 US population] but dropped to fifth most common in 2017 [1.2 (95% CI 1.16–1.24)], above ICH [0.40 (95% CI 0.38–0.43)], which was the least common cause of admission in lung cancer patients (Supplementary material online, Figure S2A). The mortality rate from all cardiovascular admissions was reduced over the study period. In 2017, the highest in-hospital mortality in lung cancer patients was observed in those admitted with ICH (19.3%), AMI (11.9%), and ischaemic stroke (10.9%). In 2004, the highest in-hospital mortality was observed in the same three conditions (Supplementary material online, Figure S2B).
Gastrointestinal cancer
The top three causes of primary cardiovascular admissions in patients with gastrointestinal cancer in 2017 were PE [1.69 (95% CI 1.65–1.73)], heart failure [1.62 (95% CI 1.58–1.67)], and ischaemic stroke [1.09 (95% CI 1.06–1.13)]—all per 100 000 US population. AF/flutter and ICH were the least common causes of admission. There was an increase of all primary cardiovascular admissions amongst patients with gastrointestinal cancer. The relative distribution of admission causes was generally unchanged during the study period (Supplementary material online, Figure S3A). In 2017, the highest in-hospital mortality was observed in admissions with ICH (28.1%), AMI (9.3%), and ischaemic stroke (9.3%). AF/flutter (3.9%) and heart failure (4.3%) admissions had the lowest associated in-hospital mortality (Supplementary material online, Figure S3B).
Prostate cancer
In 2017, the most common causes of primary cardiovascular admission in prostate cancer patients were heart failure [1.86 (95% CI 1.81–1.9)], AMI [0.90 (95% CI 0.86–0.93)], and ischaemic stroke [0.72 (95% CI 0.69–0.75)]—all per 100 000 US population. Contrary to other cancer types, there was an overall declining trend in cardiovascular admissions. Heart failure admissions declined steadily from 2004 to 2014 [2.25 (95% CI 2.19–2.3) to 1.37 (95% CI 1.33–1.41) per 100 000 US population] then had an upward trend from 2014 to 2017 [1.37 (95% CI 1.33–1.41) to 1.86 (95% CI 1.81–1.9) per 100 000 US population]. Ischaemic stroke admissions had a steady decline throughout the study period [1.18 (95% CI 1.14–1.22) to 0.72 (95% CI 0.69–0.75) per 100 000 US population]. The remaining conditions were either unchanged or showed a minor declining trend, except for PE, which showed a small increase [0.42 (95% CI 0.4–0.45) to 0.48 (95% CI 0.46–0.51) per 100 000 US population] (Supplementary material online, Figure S4A). There was a generally declining pattern of in-hospital mortality amongst prostate cancer patients admitted with cardiovascular conditions. The highest in-hospital mortality in 2017 was related to admissions with ICH (25%), AMI (6.4%), and PE (5.4%). Notably, we observed a trend for higher in-hospital mortality amongst patients admitted with heart failure in 2017 than in 2004 (4.4% vs. 5.3%, P for trend NS) (Supplementary material online, Figure S4B).
Breast cancer
The most common causes of primary cardiovascular admission in breast cancer patients in 2017 were heart failure [1.29 (95% CI 1.25–1.33)], PE [0.78 (95% CI 0.57–0.81)], and ischaemic stroke [0.56 (95% CI 0.53–0.58)]—all per 100 000 US population. There was a general increase in all six cardiovascular causes of admission, which appeared most marked for heart failure [0.88 (95% CI 0.85–0.92) to 1.29 (95% CI 1.25–1.33) per 100 000 US population, P for trend 0.05] and least so for ICH [0.08 (95% CI 0.07–0.09) to 0.13 (95% CI 0.12–0.14) per 100 000 US population, P for trend 0.03]. The highest in-hospital mortality in 2017 was observed in patients admitted with ICH (25.3%), AMI (6.8%), and ischaemic stroke (5.5%) (Supplementary material online, Figure S5A). Contrary to trends across other malignancies, the mortality rates for most conditions appeared mostly unchanged over the study period. Amongst breast cancer patients, the greatest declines in in-hospital mortality were in patients admitted with heart failure (6.2–3.6%, P for trend 0.05) and PE (4.9–3.5%, P for trend 0.05) (Supplementary material online, Figure S5B).
Discussion
Summary of findings
We analysed >42 million cardiovascular admissions between 2004 and 2017, of which 4.5% were in patients with cancer. Cardiovascular admission rates increased by 23.2% in patients with cancer, whilst decreasing by 10.9% amongst patients with no record of cancer. During the study period, the crude admission rate increased among cancer patients across all admission causes. Among patients with no record of cancer, the admission rates increased for PE and AF and decreased for the other causes. The overall trends were of increasing cardiovascular admissions across all cancer types, with the exception of prostate cancer. Patients with haematological, lung and GI cancer had the highest rates of cardiovascular hospitalizations compared with other cancer types. Heart failure was the most common reason for cardiovascular admission in patients with no cancer and across all cancer types except for GI cancer, where it was the second most common. Among patients with cancer, the crude admission rate for heart failure was highest in patients with haematological, lung, and prostate cancers. PE was relatively common among patients with cancer and was the second most common cause of admission in patients with lung and breast cancer and the most common cause in those with GI cancers. AMI was the second most common CV admission cause in haematological and prostate cancer patients, and ischaemic stroke was the third most common cause of cardiovascular admission across all cancers, except for lung cancer (where it was the fourth most common).
In-hospital mortality related to cardiovascular admissions in cancer patients was higher among patients with cancer compared with patients without cancer. The in-hospital mortality rate declined during the study period, with similar trends across all cancers. Among patients without cancer, the mortality rate increased for ischaemic stroke and decreased for the other admission causes.
In summary, overall, we observed increasing rates of hospital admissions in patients with cancer and a corresponding decline in in-hospital mortality over the 13-year period of the study. There were differential trends and disease distributions across cancer types. Our findings highlight the cardiovascular care of cancer patients as a growing priority.
Comparison with existing work
CVD and cancer remain the top two reasons of mortality in the USA,11 and understanding their further interplay is of much importance. In a study using routine health data from England, including >100 000 adults, Strongman et al.3 report that in older cancer survivors, cardiovascular mortality overtakes mortality from the primary cancer. Whilst mortality data provide an unambiguous endpoint to understand epidemiologic trends, they provide an incomplete picture in relation to period of illness, severity of disease, and service use. This information is essential to understand how clinical practice and services may need to be adapted to meet the potential growing needs of patients with cancer.
Few researchers have examined cardiovascular admissions of cancer patients in large populations. In a study of 834 900 primary cancer hospitalizations from the USA, Tuzovic et al.12 report that the subset of patients with comorbid heart failure (64 740, 7.2%) had significantly higher inpatient mortality and hospitalizations costs than those without recorded heart failure. Similarly, in a study of 5.9 million cardiovascular hospitalizations, Matetic et al.6 report greater mortality risk in patients with record of cancer compared with non-cancer patients. Interestingly, higher proportions of admissions in small and rural hospitals were observed in heart failure admissions. Increased heart failure mortality in rural areas has been previously described.13 Generally, rural areas have reduced health care provider supply, greater distance to health care centres, decreased physician density, and with a higher reliance on generalists.14 Contemporary heart treatment, specifically in cancer patients, requires a higher level of expertise that may be lacking in rural areas.
In a nationwide study of heart failure admissions from the USA, Ram et al.15 report an increasing prevalence of concurrent cancer diagnosis amongst heart failure admissions over the study period between 2003 and 2014. They additionally report higher baseline in-hospital mortality amongst heart failure admissions with lung, colorectal, or prostate cancer, compared with non-cancer controls. In the early 2000’s, the HF mortality was in decline in the USA. A previous study16 reported a reverse in this trend with an increase in the HF mortality starting in the second decade of the century. This is in accordance with our findings of increasing HF morbidity in patients with and without cancer, which mostly attributed to the ageing population.
Consistent with these observations, in a study of 163 881 women with breast cancer from the Dutch nationwide cohort study between 1996 and 2005, Beddeke et al.17 report temporal trends of increased cardiovascular hospitalization rates and reduced cardiovascular mortality over the study period. Another study among >90 000 women demonstrated a higher risk of HF among breast cancer survivors compared with women without breast cancer, and the risk persisted across LVEF phenotypes.18
While heart failure was the most common CV cause of admission among patients with cancer, we observed an increase in admission rates in other CVD. The increase in AF and PE admission rates were observed in patients without cancer and can be attributed to the increasing age and prevalence of cardiometabolic risk factors19 and the increasing availability and use of diagnostic scans,20 respectively. The increase in ICH was observed only among cancer patients and may be attributed to CNS metastases, intrinsic properties of the neoplasm, and the increased use of anticoagulation in patients with cancer.21 The increase in atherosclerotic conditions as AMI and ischaemic stroke admissions may be related to the pathophysiology of cancer as well as its treatment.
Our analysis significantly extends prior observations by demonstrating temporal trends of cardiovascular admissions in cancer patients over a 13-year period stratified by five common cancers and key CVDs. Our findings support previous observations in demonstrating the increasing importance of CVD as a cause of ill health in cancer patients, as indicated by increasing cardiovascular admissions. In line with Beddeke et al.17 and general population trends,22 we observed declining in-hospital mortality in primary cardiovascular admissions of cancer patients.
Among patients admitted with the studied CVD and cancer, haematological malignancies were the most common. This cohort had the highest rate of heart failure hospitalization across all the cancer types. AMI and ischaemic stroke were also important, both in relation to admission rates and associated in-hospital mortality. These observations, and the higher and increasing rates of CV admission in patients with haematological cancer, likely reflect the constellation of cardiotoxic therapies to which haematological cancers are exposed as well as pathological processes related to haematological cancers themselves. Indeed, many of the chemotherapeutic and targeted agents used in the treatment of haematological cancer patients have a known high risk of cardiotoxicity.23–25 Additionally, mediastinal radiotherapy, which is often used in the treatment of lymphomas, has increasingly recognized pro-atherogenic consequences,26 augmenting the risk of both heart failure and atherosclerotic conditions such as AMI and ischaemic stroke. Both cancer treatments and biological properties of haematological malignancies can propagate a prothrombotic state,27 perhaps contributing to AMI and ischaemic stroke risk. In addition, the increasing CV admission rate may be partly attributed to the incidence of haematological malignancies. Non-Hodgkin lymphoma (NHL) is the most common malignancy worldwide, the increased incidence and decreased mortality of patients with NHL,28 as with other haematological malignancies, will likely increase the burden of CV morbidity in this population.
We observed a decrease in-hospital CV mortality trend in patients with haematological malignancy. This is in accordance with our previous work that reported a decreasing trend of age-adjusted cardiovascular mortality in patients with haematological malignancies.29 This may reflect improvement in the treatment of CV diseases, adoption of cardio-oncology guidelines and services, and improved awareness of the increased risk of this population.
Lung cancer was the second most common cancer in our cohort. The most common cause of cardiovascular admission in lung cancer patients was heart failure—likely similarly driven by a combination of chemo- and radiotherapy-related cardiotoxicity.30,31 The second and third most common causes of admission were PE and AF/flutter. Whilst all malignancies are associated with a higher risk of venous thromboembolism due to the prothrombotic effects of cancer,32 it is possible that local tumour effects further augment this risk in lung cancer patients. Similarly, the high rate of AF/flutter admissions in lung cancer patients may be a response to pulmonary pathology, which may act indirectly through increasing right heart pressures or directly through compression or irritation of the atrial chambers, which may precipitate atrial arrhythmias.
There was a similar increasing trend of cardiovascular admissions and declining associated in-hospital mortality trends in patients who also had record of GI cancer. The most common causes of admission were PE, heart failure, and ischaemic stroke. These likely reflect both the demography and cancer treatment exposures in this patient cohort. Many GI cancer therapies, such as fluorouracil-based chemotherapy agents33 and trastuzumab,34 have well-established cardio-toxicity risks.
We observed both a declining trend in cardiovascular admission and in-hospital mortality rates among patients with prostate cancer admitted with the studied CVD. The declining trend of admission rates in this group was similar to the trend in patients without cancer, and in contrary to patients with other cancer types. These observations may reflect the greater cardiotoxicity of therapies used in treatments of other cancers. It is also possible that there is a discrepancy in the frequency with which cancers are recorded as a comorbidity, with a more frequent omission of prostate cancer as a concurrent diagnosis in cardiovascular admissions than other cancer types. This is conceivable given the direct implications of some cancers for CVD treatments (e.g. antithrombin therapy decisions in haematological cancer patients, arterial access in breast cancer patients).
The crude rate of cardiovascular admissions was comparatively low in breast cancer patients compared with other cancer types. This likely reflects the younger age of this cancer cohort and the almost complete dominance of this cancer type by women. The most common cause of admission was heart failure, which as with lung and haematological cancers likely reflects the adverse effects of both cardiotoxic therapeutic agents, such as HER-2 targeted therapies35,36 and anthracycline chemotherapy,37,38 as well as mediastinal radiotherapy.
Strengths and limitations
The NIS permitted characterization of temporal trends in cardiovascular hospitalizations in patients with a record of cancer, by cancer type, and CVD in a very large nationally representative sample. The recording of diseases using ICD codes permitted standardized ascertainment of CVD and cancer status. However, we are unable to verify the accuracy or completeness of recorded diagnoses. Another limitation is our inability to determine the date of cancer diagnosis. We know that risk of CVD is altered according to time from first cancer diagnosis and incorporation of such data may provide a more informative illustration of the cardiovascular care needs of cancer patients. It should also be noted that the NIS, as an inpatients database, cannot be used to assess the proportions of CV disease or CV admission among the entire population of cancer patients. Furthermore, given that the date of first cancer diagnosis is not available, we cannot perform time-to-event analyses to assess the association of cancer exposure with incident CVD. Finally, we do not have information on specific cancer treatments and, as such, cannot make inferences about the association of such exposures with the rates of cardiovascular admission and in-hospital mortality observed in our analysis.
Conclusions
In this study, we describe the distribution and trends of primary cardiovascular hospitalizations in patients with and without a concurrent record of cancer, considering differential trends by CVD and five common cancer sites. Our main findings are an overall increasing trend of primary cardiovascular admissions in patients with concurrent cancer and a decreasing trend of in-hospital mortality related to these admissions. Our study illustrates the increasing cardiovascular healthcare needs of cancer patients and the growing importance of awareness of related issues by the general cardiologist. Further studies are needed in order to explore the underlying reasons for these trends. Given the specific considerations required for cardiovascular care of these cancer patients, our findings provide insight for planning of clinical services as well as clinical training.
Supplementary Material
Acknowledgement
None.
Contributor Information
Ofer Kobo, Keele Cardiovascular Research Group, Centre for Prognosis Research, Keele University, Keele, Newcastle ST5 5BG, UK; Department of Cardiology, Hillel Yaffe Medical Center, Hadera 38100, Israel.
Zahra Raisi-Estabragh, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Centre for Advanced Cardiovascular Imaging, Queen Mary University London, ondon E1 4NS, UK; Barts Heart Centre, St Bartholomew's Hospital, Barts Health NHS Trust, West Smithfield, London EC1A 7BE, UK.
Sofie Gevaert, Department of Cardiology, Ghent University Hospital, Ghent University, 9000 Ghent, Belgium.
Jamal S Rana, Department of Cardiology, Permanente Medical Group, Oakland, CA 94612, USA; Department of Medicine, University of California San Francisco, San Francisco, CA 94143, USA.
Harriette G C Van Spall, Department of Medicine, Department of Health Research Methods, Evidence, and Impact, Population Health Research Institute, Research Institute of St. Joe's, McMaster University, Hamilton, ON L8S 4L8, Canada.
Ariel Roguin, Department of Cardiology, Hillel Yaffe Medical Center, Hadera 38100, Israel.
Steffen E Petersen, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Centre for Advanced Cardiovascular Imaging, Queen Mary University London, London E1 4NS, UK; Barts Heart Centre, St Bartholomew's Hospital, Barts Health NHS Trust, West Smithfield, London EC1A 7BE, UK; Health Data Research UK, London NW1 2BE, UK; Alan Turing Institute, London NW1 2DB, UK.
Bonnie Ky, Division of Cardiology, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA; Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Mamas A Mamas, Keele Cardiovascular Research Group, Centre for Prognosis Research, Keele University, Keele, Newcastle ST5 5BG, UK; Institute of Population Health, University of Manchester, Manchester M13 9PL, UK.
Funding
National Institute for Health Research (NIHR) Integrated Aca-demic Training programme; British Heart Foundation Clinical Re-search Training Fellowship (no. FS/17/81/33 318); National Institutefor Health Research (NIHR) Biomedical Research Centre at Barts;Health Data Research UK, UK Research and Innovation, Depart-ment of Health and Social Care (England).
Conflict of interest
none declared.
Data availability
The data underlying this article were provided by HCUP under licence/by permission. Data will be shared on request to the corresponding author with permission of HCUP.
References
- 1. Cancer Statistics for the UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk.
- 2. Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon ARet al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet North Am Ed 2019;394:1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strongman H, Gadd S, Matthews AA, Mansfield KE, Stanway S, Lyon AR. Does cardiovascular mortality overtake cancer mortality during cancer survivorship?: an English Retrospective Cohort Study. Cardio Oncol 2022;4:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi Met al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 5. Tan LL, Lyon AR. Cardio-oncology for the general cardiologist. Heart 2021;107:1254–1266. [DOI] [PubMed] [Google Scholar]
- 6. Matetic A, Mohamed M, Miller RJH, Kolman L, Lopez-Mattei J, Cheung WYet al. Impact of cancer diagnosis on causes and outcomes of 5.9 million US patients with cardiovascular admissions. Int J Cardiol 2021;341:76–83. [DOI] [PubMed] [Google Scholar]
- 7. Raisi-Estabragh Z, Kobo O, Freeman P, Petersen SE, Kolman L, Miller RJHet al. Temporal trends in disease-specific causes of cardiovascular mortality amongst patients with cancer in the USA between 1999 to 2019. Eur Hear Journal Qual Care Clin Outcomes 2022; doi: 10.1093/ehjqcco/qcac016. Published online ahead of print 18 April 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobo O, Moledina SM, Raisi-Estabragh Z, Shanmuganathan JWD, Chieffo A, Al Ayoubi Fet al. Emergency department cardiovascular disease encounters and associated mortality in patients with cancer: a study of 20.6 million records from the USA. Int J Cardiol 2022;363:210–217. [DOI] [PubMed] [Google Scholar]
- 9. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp (13 September 2021).
- 10. World Bank Open Data | Data. https://data.worldbank.org/
- 11. Rana JS, Khan SS, Lloyd-Jones DM, Sidney S. Changes in mortality in top 10 causes of death from 2011 to 2018. J Gen Intern Med 2021;36:2517–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuzovic M, Yang EH, Sevag Packard RR, Ganz PA, Fonarow GC, Ziaeian B. National outcomes in hospitalized patients with cancer and comorbid heart failure. J Card Fail 2019;25:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pierce JB, Shah NS, Petito LC, Pool L, Lloyd-Jones DM, Feinglass Jet al. Trends in heart failure-related cardiovascular mortality in rural versus urban United States counties, 2011-2018: a cross-sectional study. PLoS One 2021;16:e0246813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verdejo HE, Ferreccio C, Castro PF. Heart failure in rural communities. Heart Fail Clin 2015;11:515–522. [DOI] [PubMed] [Google Scholar]
- 15. Ram P, Tiu A, Lo KB, Parikh K, Shah M. Trends in the prevalence of malignancy among patients admitted with acute heart failure and associated outcomes: a nationwide population-based study. Heart Fail Rev 2019;24:989–995. [DOI] [PubMed] [Google Scholar]
- 16. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GCet al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buddeke J, Gernaat SAM, Bots ML, van den Bongard D H J G, Grobbee D E, Vaartjes Iet al. Trends in the risk of cardiovascular disease in women with breast cancer in a Dutch nationwide cohort study. BMJ Open 2019;9:e022664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kwan ML, Cheng RK, Iribarren C, Shen H, Laurent CA, Roh JMet al. Risk of heart failure with preserved versus reduced ejection fraction in women with breast cancer. Breast Cancer Res Treat 2022;193:669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017;120:1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stein PD, Beemath A, Olson RE. Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients. Am J Cardiol 2005;95:1525–1526. [DOI] [PubMed] [Google Scholar]
- 21. Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep 2012;14:373–381. [DOI] [PubMed] [Google Scholar]
- 22. Timmis (Chair Writing Group) A; Vardas P, Townsend N, Torbica A, Katus H, De Smedt Det al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022; 43: 716–799. [DOI] [PubMed] [Google Scholar]
- 23. Oliveira GH, Al-Kindi SG, Caimi PF, Lazarus HM. Maximizing anthracycline tolerability in hematologic malignancies: treat to each heart's content. Blood Rev 2016;30:169–178. [DOI] [PubMed] [Google Scholar]
- 24. Johnson SA. Anthracycline-induced cardiotoxicity in adult hematologic malignancies. Semin Oncol 2006;33:22–27. [DOI] [PubMed] [Google Scholar]
- 25. Aleman BMP, Van Den Belt-Dusebout AW, De Bruin ML, Van ‘t Veer MB, Baaijens MHA, De Boer JPet al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878–1886. [DOI] [PubMed] [Google Scholar]
- 26. Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol 2013;61:2319–2328. [DOI] [PubMed] [Google Scholar]
- 27. Falanga A, Vignoli A, Marchetti M. Coagulation in hematological malignancies. Cancer Investigation 2009;27:7–16. [Google Scholar]
- 28. Thandra KC, Barsouk A, Saginala K, Padala SA, Barsouk A, Rawla P. Epidemiology of non-Hodgkin's lymphoma. Med Sci (Basel, Switzerland) 2021;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kobo O, Khattak S, Lopez-Mattei J, Van Spall H G C, Graham M, Cheng RKet al. Trends in cardiovascular mortality of cancer patients in the US over two decades 1999–2019. Int J Clin Pract 2021;75:e14841. [DOI] [PubMed] [Google Scholar]
- 30. Hardy D, Liu CC, Cormier JN, Xia R, Du XL. Cardiac toxicity in association with chemotherapy and radiation therapy in a large cohort of older patients with non-small-cell lung cancer. Ann Oncol 2010;21:1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vojtíšek R. Cardiac toxicity of lung cancer radiotherapy. Reports Pract Oncol Radiother J Gt Cancer Cent Pozn Polish Soc Radiat Oncol 2020;25:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost 2017;117:57–65. [DOI] [PubMed] [Google Scholar]
- 33. Jin X, Bai Y, Gao L, Wu S. Incidence of and risk factors for cardiotoxicity after fluorouracil-based chemotherapy in locally advanced or metastatic gastric cancer patients. Cancer Chemother Pharmacol 2019;84:599–607. [DOI] [PubMed] [Google Scholar]
- 34. Park JS, Youn JC, Shim CY, Hong GR, Lee CK, Kim JHet al. Cardiotoxicity of trastuzumab in patients with HER2-positive gastric cancer. Oncotarget 2017;8:61837–61845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde Aet al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 36. Florido R, Smith KL, Cuomo KK, Russell SD. Cardiotoxicity from human epidermal growth factor receptor-2 (HER2) targeted therapies. J Am Heart Assoc 2017;6:e006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 2007;25:3808–3815. [DOI] [PubMed] [Google Scholar]
- 38. Barrett-Lee PJ, Dixon JM, Farrell C, Jones A, Leonard R, Murray Net al. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol 2009;20:816–827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by HCUP under licence/by permission. Data will be shared on request to the corresponding author with permission of HCUP.