Abstract
Serodiagnostic tests are widely available for tick-borne diseases. We evaluated a cell-free antigen of the human granulocytic ehrlichiosis agent. Immunofluorescence assay (IFA) with this antigen is as efficient as with the MRL kit and allows a one-step IFA with other cell-free antigens that is useful when testing sera from patients bitten by ticks.
Human granulocytic ehrlichiosis (HGE) is caused by an obligate intracellular bacterium closely related to Ehrlichia phagocytophila 9. The bacteria are transmitted by Ixodes ticks 6 and share these vectors with Borrelia burgdorferi and Babesia microti in the United States and with B. burgdorferi, Babesia canis, Rickettsia helvetica, and the tick-borne encephalitis virus group in Europe 4.
Serology is currently the most widely available diagnostic laboratory test 5, 12, 15. These tests use intracellular antigens fixed onto slides and preserved at −80°C or are packed in light-protected paper. In our laboratory we systematically screen sera referred to us from patients bitten by ticks with a single test that includes several cell-free rickettsial antigens such as Rickettsia conorii, R. helvetica, R. massiliae, R. slovaca, R. africae, Bartonella quintana, Bartonella henselae, and Francisella tularensis. We compared immunofluorescence assay (IFA) with cell-free antigen of the HGE agent to the intracellular antigen from the same strain and to a commercially available IFA test (MRL Diagnostic, Cypress, Calif.).
The Webster strain of the HGE agent was propagated in HL-60 cells (ATCC CL240) 8. Intracellular ehrlichiae in HL-60 cells were preloaded onto slides, fixed with acetone, and stored at −80°C. A kit using the same type of preparation but with another HGE agent isolate and packed in light-protected paper was also tested (MRL kit). Our cell-free purified antigen was obtained by differential centrifugation of 100% infected HL-60 cells and stored at −80°C. Positive-control antibodies were prepared in rabbits. For the MRL kit, immunoglobulin G (IgG) and IgM were tested separately at a screening dilution of 1:32 for IgG and 1:20 for IgM. For the other antigens, three dilutions (1:16, 1:32, 1:64) of sera were used. When the serum titer was greater than 1:32, it was retested for IgG and IgM separately. Separate individuals reviewed stained slides twice blindly. When discrepant results were observed, the test was repeated.
A case was defined by the Centers for Disease Control and Prevention surveillance definition for human ehrlichiosis 5. Noncases were defined as either asymptomatic unexposed blood donors or acutely ill patients with a different proven etiologic diagnosis.
In order to evaluate the different tests we compared the predictive values of each in relationship to the expected prevalence of the disease. For that we use Bayes' theorem: positive predictive value (PPV) = SE × PR/(SE × PR) + (1 − SP) × (1 − PR), and negative predictive value (NPV) = SPE × (1 − PR)/SPE × (1 − PR) + (1 − SE) × PR, where PR is the prevalence of the disease in the concerned population, SPE is specificity, and SE is sensitivity. PR of ehrlichiosis varies from 2.5 to 5.8 cases per 10,000 members of the population 3, 11. Graphs and statistical analysis were obtained using Microsoft Excel 7 and Epi info 6.0.
We tested 30 sera from case patients and 137 sera from noncase patients. The SEN of the test was significantly greater with the MRL antigen than the intracellular (IC) antigen (P = 0.0098233 and P = 0.0099243) (Table 1).
TABLE 1.
SEN and SPE of IFA using different sources of granulocytic ehrlichial antigena
| Antigen source | Antibody tested and cutoff titer | No. of:
|
SEN | No. of:
|
SPC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FN | TN | BD | MSF | AQF | BE | EBV | CMV | CQF | SLE | Total | ||||
| PA | IgG or IgM > 32 | 25 | 5 | 0.833 | 127 | 2 | 1 | 0 | 7 | 0 | 0 | 0 | 0 | 10 | 0.927 |
| IgG > 32 | 24 | 6 | 0.800 | 127 | 2 | 1 | 0 | 7 | 0 | 0 | 0 | 0 | 10 | 0.927 | |
| IgG > 16 | 25 | 5 | 0.833 | 121 | 8 | 1 | 0 | 7 | 0 | 0 | 0 | 0 | 16 | 0.883 | |
| IgG > 8 | 25 | 5 | 0.833 | 120 | 9 | 1 | 0 | 7 | 0 | 0 | 0 | 0 | 17 | 0.875 | |
| IgM > 32 | 8 | 22 | 0.266 | 137 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| IgM > 16 | 12 | 18 | 0.400 | 129 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0.941 | |
| IgM > 8 | 19 | 11 | 0.633 | 117 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0.854 | |
| IC | IgG or IgM > 32 | 20 | 10 | 0.666** | 124 | 3 | 0 | 0 | 6 | 0 | 1 | 2 | 1 | 13 | 0.905 |
| IgG > 32 | 17 | 13 | 0.566* | 124 | 3 | 0 | 0 | 6 | 0 | 1 | 2 | 1 | 13 | 0.905 | |
| IgG > 16 | 23 | 7 | 0.766 | 121 | 6 | 0 | 0 | 6 | 0 | 1 | 2 | 1 | 16 | 0.883 | |
| IgG > 8 | 23 | 7 | 0.766 | 117 | 10 | 0 | 0 | 6 | 0 | 1 | 2 | 1 | 20 | 0.854 | |
| IgM > 32 | 11 | 19 | 0.366 | 136 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.992 | |
| IgM > 16 | 17 | 13 | 0.566 | 131 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 6 | 0.956 | |
| IgM > 8 | 20 | 10 | 0.666 | 121 | 15 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 16 | 0.883 | |
| MRL | IgG > 32 or IgM > 20 | 28 | 2 | 0.933** | 126 | 2 | 1 | 1 | 7 | 0 | 0 | 0 | 0 | 11 | 0.919 |
| IgG > 32 | 26 | 4 | 0.866* | 127 | 1 | 1 | 1 | 7 | 0 | 0 | 0 | 0 | 10 | 0.927 | |
| IgG > 16 | 27 | 3 | 0.900 | 123 | 5 | 1 | 1 | 7 | 0 | 0 | 0 | 0 | 14 | 0.897 | |
| IgG > 8 | 27 | 3 | 0.900 | 116 | 12 | 1 | 1 | 7 | 0 | 0 | 0 | 0 | 21 | 0.846 | |
| IgM > 20 | 10 | 20 | 0.333 | 135 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.985 | |
Sera used were from 30 proven HGE cases and 137 control sera from Blood Donors (BD) (n = 96), Mediterranean (MSF) spotted fever (n = 10) (AQF) acute Q fever (n = 10), Bartonella endocarditis (BE) (n = 10), Epstein-Barr virus (EBV) (n = 2), cytomegalovirus (CMV) (n = 3), (CQF) Chronic Q fever (n = 3), and systemic lupus erythematosus (SLE) (n = 3). Symbols: *, Difference is significant with P = 0.0098233 (chi-square test); **, Difference is significant with P = 0.0099243 (chi-square test). Abbreviations: TP, true positive; TN, true negative; FP, false positive; FN, false negative.
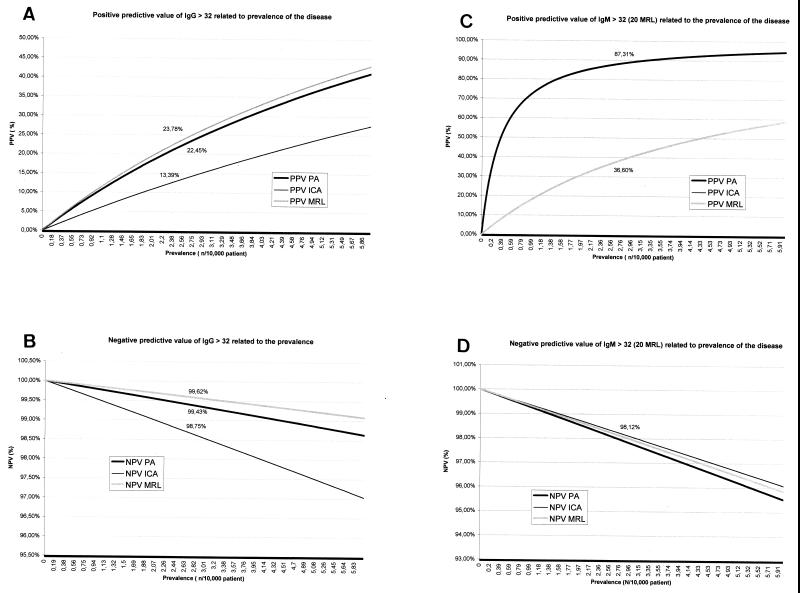
Serological cross-reactions occurred mostly with IgG in patients with endocarditis (Table 2). At least 6 of 10 sera from patients with Bartonella spp. endocarditis cross-reacted with all Ehrlichia antigens tested. In most cases IgG titers were elevated. The PPV as well as the NPV at an IgG cutoff of >32 were comparable between MRL and purified antigen (PA) and better than with IC antigen, whatever the PR of the disease (Fig. 1A and B). The PPV was better with both PA and IC antigens at an IgM cutoff of >32 than by the MRL test at an IgM cutoff of >20 (Fig. 1C). However, the predictive value of a negative IgM test was similar for the three antigen preparations (Fig. 1D).
TABLE 2.
Serological cross-reaction with serum from patients with other documented diseases
| Antigen source | Population tested (n)a | No. of specimens IgG positive/total no. tested | IgG titer(s) | No. of specimens IgM positive/total no. tested | IgM titer |
|---|---|---|---|---|---|
| PA | Acute MSF (10) | 1/10 | 64 | 0 | <32 |
| Acute Q fever (10) | 0 | <32 | 0 | <32 | |
| Acute CMV infection (3) | 0 | <32 | 0 | <32 | |
| Bartonella sp. endocarditis (10) | 7/10 | 2,048, 1,024, 8,192, 256, 128, 512, 1,024 | 0 | <32 | |
| Q fever endocarditis (3) | 0 | <32 | 0 | <32 | |
| Systemic lupus erythematous (3) | 0 | <32 | 0 | <32 | |
| IC | Acute MSF (10) | 0 | <32 | 0 | <32 |
| Acute Q fever (10) | 0 | <32 | 0 | <32 | |
| Acute CMV infection (3) | 1/3 | 64 | 0 | <32 | |
| Bartonella sp. endocarditis (10) | 6/10 | 1,024, 1,024, 4,096, 128, 64, 128 | 1/10 | 64 | |
| Q fever endocarditis (3) | 2/3 | 256, 512 | 0 | <32 | |
| Systemic lupus erythematous (3) | 1/3 | 64 | 0 | <32 | |
| MRL | Acute MSF (10) | 1/10 | 128 | 0 | <20 |
| Acute Q fever (10) | 1/10 | 256 | 0 | <20 | |
| Acute CMV infection (3) | 0 | <32 | 0 | <20 | |
| Bartonella sp. endocarditis (10) | 7/10 | 512, 512, 2,048, 64, 256, 128, 256 | 0 | <20 | |
| Q fever endocarditis (3) | 0 | <32 | 0 | <20 | |
| Systemic lupus erythematous (3) | 0 | <32 | 0 | <20 |
MSF, Mediterranean spotted fever; CMV, cytomegalovirus.
FIG. 1.
PPVs (A and C) and NPVs (B and D) of HGE screening using IgG (A and B)and IgM (C and D).
The SE of tests using different strains of intracellular antigen varied between 60 and 100%, although the differences were not statistically significant 15. We found the MRL test for IgG and IgM or IgG alone to be more sensitive than IFA with intracellular HGE agent Webster strain (P = 0.009). The MRL IFA test uses a human-derived isolate of the HGE agent (HGE1 strain) obtained from J. L. Goodman (Department of Medicine, University of Minnesota Academic Health Center) that is genetically very close to the human Webster strain. The discovery that isolates of the HGE agent and Ehrlichia equi are antigenically diverse suggests that differences in SE and SPE may exist 1, 2, 10, 13. Serological cross-reactions occurred mostly with Bartonella endocarditis. Endocarditis is often characterized by very high specific antibody titers and by frequent lower-titer serological cross-reactions (4a).
Whatever the PR of the disease both PA and MRL tests have good PPVs and NPVs. The comparatively lower predictive value of an IgM-positive test with MRL antigens is likely due to the fact that the cutoff used is lower than that with other antigens, leading to lower SPE. The good predictive value of a negative test with all of the antigens and regardless whether tested for IgG or IgM indicates that a negative result in our population is unlikely to occur in a case patient.
Unlike cells infected with monocytic ehrlichiae, granulocytic ehrlichiae grown in immature HL-60 cells clumped together when frozen and thawed. Consequently, antigen slides for serological diagnosis of granulocytic ehrlichiosis are prepared with freshly infected cells, fixed, and preserved as antigen slides either frozen or in light-protected paper 7, 12, 15. Micro immunofluorescence diagnosis of other intracellular rickettsial infections can be made with cell-free antigens 14. IFA testing with cell-free antigens is as efficient and predictive as commercially prepared serologic kits, storage is easier, and it allows performance of a one-step IFA using several cell-free antigens of interest when testing sera from patients with tick bites. Elevated IgG titers in a patient with a clinical and epidemiological history not compatible with ehrlichiosis might suggest Bartonella endocarditis.
Acknowledgments
We thank Jane Markley from MRL laboratory for her support with the Ehrlichia IFA diagnostic kits, and Johan Bakken for assistance with identifying patients.
REFERENCES
- 1.Aguero-Rosenfeld M E, Kalantarpour F, Baluch M, Horowitz H W, McKenna D F, Raffali J T, Hsieh T, Wu J, Dumler J S, Wormser G P. Serology of culture-confirmed cases of human granulocytic ehrlichiosis. J Clin Microbiol. 2000;38:635–638. doi: 10.1128/jcm.38.2.635-638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asanovich K M, Bakken J S, Madigan J E, Aguero-Rosenfeld M, Wormser G P, Dumler J S. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J Infect Dis. 1997;176:1029–1034. doi: 10.1086/516529. [DOI] [PubMed] [Google Scholar]
- 3.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 4.Brouqui P. Ehrlichiosis in Europe. In: Raoult D, Brouqui P, editors. Rickettsiae and Rickettsial diseases at the turn of the third millenium. Paris, France: Elsevier; 1999. pp. 220–232. [Google Scholar]
- 4a.Brouqui, P., and D. Raoult. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev., in press. [DOI] [PMC free article] [PubMed]
- 5.Comer J A, Nicholson W L, Olson J G, Childs J E. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J Clin Microbiol. 1999;37:558–564. doi: 10.1128/jcm.37.3.558-564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumler J S, Bakken J S. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 7.Fingerle V, Goodman J L, Johnson R C, Kurtti T J, Munderloh U G, Wilske B. Human granulocytic ehrlichiosis in southern Germany: increased seroprevalence in high-risk groups. J Clin Microbiol. 1997;35:3244–3247. doi: 10.1128/jcm.35.12.3244-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. . (Erratum, 335:361, 1996.) [DOI] [PubMed] [Google Scholar]
- 9.Macleod J. Preliminary studies in the tick transmission of loupin ill.II: a study of the recation of sheep to tick infestation. Vet J. 1932;88:276–284. [Google Scholar]
- 10.Magnarelli L A, IJdo J W, Dumler J S, Heimer R, Fikrig E. Reactivity of human sera to different strains of granulocytic ehrlichiae in immunodiagnostic assays. J Infect Dis. 1998;178:1835–1838. doi: 10.1086/314516. [DOI] [PubMed] [Google Scholar]
- 11.McQuiston J H, Paddock C D, Holman R C, Childs J E. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–642. doi: 10.3201/eid0505.990504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson W L, Comer J A, Summer J W, Gingrich-Baker C, Coughlin R T, Magnarelli L A, Olson J G, Childs J E. An indirect immunofluorescence assay using a cell culture-derived antigen for detection of antibodies to the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1510–1516. doi: 10.1128/jcm.35.6.1510-1516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrovec M, Lotric F S, Zupanc T A, Strle F, Brouqui P, Roux V, Dumler J S. Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–1559. doi: 10.1128/jcm.35.6.1556-1559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tissot-Dupont H, Brouqui P, Faugere B, Raoult D. Prevalence of antibodies to Coxiella burnetti, Rickettsia conorii, and Rickettsia typhi in seven African countries. Clin Infect Dis. 1995;21:1126–1133. doi: 10.1093/clinids/21.5.1126. [DOI] [PubMed] [Google Scholar]
- 15.Walls J J, Aguero-Rosenfeld M, Bakken J S, Goodman J L, Hossain D, Johnson R C, Dumler J S. Inter- and intralaboratory comparison of Ehrlichia equi and human granulocytic ehrlichiosis (HGE) agent strains for serodiagnosis of HGE by the immunofluorescent-antibody test. J Clin Microbiol. 1999;37:2968–2973. doi: 10.1128/jcm.37.9.2968-2973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]